Abstract
Intrapartum fetal surveillance aims to predict significant fetal hypoxia and institute timely intervention to avoid fetal injury, and do so without unnecessary operative delivery of fetuses at no risk of intrapartum hypoxia. However, the configuration and application of current clinical guidelines inadvertently undermine these aims because of persistent failure to incorporate increased understanding of fetal cardiovascular physiology and adaptations to oxygen deprivation, advances in signal acquisition/processing, and related technologies. Consequently, the field on intrapartum fetal surveillance is stuck in rudimentary counts of the fetal R–R intervals and visual assessment of very common, but nonspecific fetal heart decelerations and fetal heart rate variability. The present authors argue that the time has come to move away from classifications of static morphological appearances of FHR decelerations, which do not assist the thinking clinician in understanding how the fetus defends itself and compensates for intrapartum hypoxic ischaemic insults or the patterns that suggest progressive loss of compensation. We also reappraise some of the controversial aspects of intrapartum fetal surveillance in modern obstetric practice, the current state of flux in training and certification, and contemplate the future of the field particularly in the context of the emerging role of artificial intelligence.
Introduction
The purpose of intrapartum fetal surveillance is to predict significant fetal hypoxia and intervene timeously before the fetus incurs injury. A second important, but understated aim of intrapartum fetal monitoring, is avoidance of emergency operative delivery of fetuses at no risk of intrapartum hypoxia. Unnecessary operative delivery carries significant risks for both mother and baby particularly in advanced stages of labour and adds to healthcare costs [1, 2]. For example, in 2008, an estimated 6·20 million unnecessary Caesarean sections were done worldwide at an estimated cost of $2·32 billion, whilst the cost of needed but unmet Caesarean sections globally at the time was about $432 million [3]. Our profession should reflect on these inequities and what they mean for our values and practice, even locally. Compared to other clinical specialities, intrapartum fetal monitoring has seen less application of advances in technology. Although modern fetal heart rate (FHR) monitors look better, and have more features including interactivity, intrapartum fetal monitoring is still rooted in rudimentary counts of the fetal R–R intervals, visual assessment of very common, but nonspecific decelerations and FHR variability. The presumption is that these parameters somehow define fetal compromise without emphasis on the critical temporal relationships between them. This approach is embedded in our clinical guidelines, training, and practice despite 50 years of increased understanding of the behaviour and regulation of the fetal cardiovascular system during labour, and its adaptations to oxygen deprivation [4, 5]. Other intrinsic but visually unseen features of the FHR have not been studied or tested sufficiently for clinical utility.
It is time to move away from classifications of static morphological appearances of FHR decelerations into descriptive categories as these do not assist the clinician in understanding how the fetus defends itself and compensates for intrapartum hypoxic ischaemic insults or grasp the patterns that suggest progressive loss of compensation. In this review the authors reappraise some of the controversial aspects of intrapartum fetal surveillance in modern obstetric practice including intermittent auscultation (IA), the admission test (AT), electronic fetal monitoring (EFM), physiology of FHR decelerations, fetal blood sample (FBS), fetal ST-segment analysis (STAN), and the current state of training and certification.
The silence of current guidelines on relevant pathways to fetal injury
In 2014 the Royal College of Obstetricians and Gynaecologists (RCOG) launched the National quality improvement programme “Each Baby Counts (EBC)” with the aim of halving the number of babies who die or suffer severe disability because of avoidable incidents during term labour, by the year 2020. Since these incidents are rare, it is unlikely that common themes and trends will be obvious in single centre reviews, hence a national level analysis is warranted. The EBC reports found that for many of the babies, different care might have resulted in a different outcome [6, 7]. This is not dissimilar to the findings of the report of the 4th Confidential Enquiries into Stillbirths and Deaths in Infancy (CESDI) [8], which preceded the EBC initiative, and other national reviews and audits including the Perinatal Mortality Review Tool (PMRT), and the more recent report of Ockenden independent review of maternity services at the Shrewsbury and Telford Hospital NHS Trust [9]. The findings are also consistent with the results of a secondary analysis of the INFANT trial [10] and with medicolegal claims [11]. Collectively, these reports highlight the very complex nature of maternity care. For example, analysis of the EBC reviews identified over 3,800 intricately related critical contributory factors, with an average of six contributory factors for each baby. This suggests that we need complex and highly nuanced solutions to the issue of intrapartum fetal injury. The EBC report identified fetal monitoring, human factors, and neonatal care as problematic areas, and acknowledged the complicated interrelatedness of these domains. Therefore, improving fetal monitoring skills without cohesive maternity teams, with the knowledge and expertise to appraise “the full clinical picture” will be futile and ineffective.
In practice the “full clinical picture” is a nebulous and ill-defined obstetric “slogan” or “sound bite”. It often refers to a mixture of complex clinical scenarios associated with fetal damage such as maternal fever, chorioamnionitis, fetal inflammatory response (FSIR) and its synergistic interaction with hypoxia, use of uterotonic agents, meconium staining, and maternal disease, to name a few. Undiagnosed developmental disorders of the placenta have a role too. These scenarios may operate outside of the hypoxia pathway but cause direct fetal neurologic damage or sensitise the fetus to amplified injury if exposed to hypoxia ischaemia. Current national guidelines do not provide unambiguous or comprehensive algorithms for intrapartum FHR interpretation with recommendations for managing these scenarios. It is unlikely that consistent and effective response to abnormal FHR patterns in these situations will happen without the development of such algorithms. Unfortunately, the mechanisms involved, the magnitude of the risks, and the nature of the interactions between these complex factors are poorly understood at the present time. The profession needs to acknowledge that many more babies probably suffer injury from these noxious factors than from de novo intrapartum fetal hypoxia, and we need to invest the necessary resources in understanding the mechanisms and pathways to strengthen our practice guidelines.
The regulation and relevance of FHR decelerations to fetal wellbeing
Fetal heart rate decelerations characterised by rapid but transient falls in the FHR are reflex cardiovascular responses to brief interruption of oxygen delivery to the fetus. They are almost always associated with uterine contractions, which may or may not be clinically apparent. The specific reflex, which mediates FHR decelerations is surprisingly controversial probably because of the persistence of some unsupported historical hypotheses. The proposal that variable decelerations are a reflex response to abrupt changes in blood volume and arterial pressure secondary to umbilical cord compression is regularly cited and taught in fetal monitoring modules but is not supported by experimental or clinical evidence. The FHR in the near-term fetal sheep decelerated only when umbilical cord compression reduced blood flow by 50% or more [12], and no significant changes in arterial blood pressure were observed in response to cord compression [12, 13]. Therefore, the baroreflex could not have been triggered during partial occlusion of the umbilical cord. By contrast, rapid and complete cord occlusion elicits two separate increases in fetal arterial pressures. Firstly, a small and transient increase in arterial pressure, which is within normal baseline arterial pressure fluctuations, and thought to be due to the removal of the low resistance vascular bed of the placenta. This is unlikely to trigger the baroreflex. Secondly, a much larger and sustained increase in arterial pressure, which takes 2–3 min to fully develop and occurs after the onset of FHR deceleration [12, 14, 15]. Given the time required for the increase in arterial pressure to kick-in it could not have contributed to the initial fall in FHR, Figure 1 reproduced with permission of the publisher, Wiley. Critically, this second increase in arterial pressure is mediated by increased sympathetic nervous system activity leading to peripheral vasoconstriction [12, 16, 17]. In contrast the baroreflex response is inhibitory to the sympathetic system and leads to peripheral vasodilatation [18]. Therefore, the baroreflex cannot possibly explain the intense peripheral vasoconstriction during acute umbilical cord occlusion.
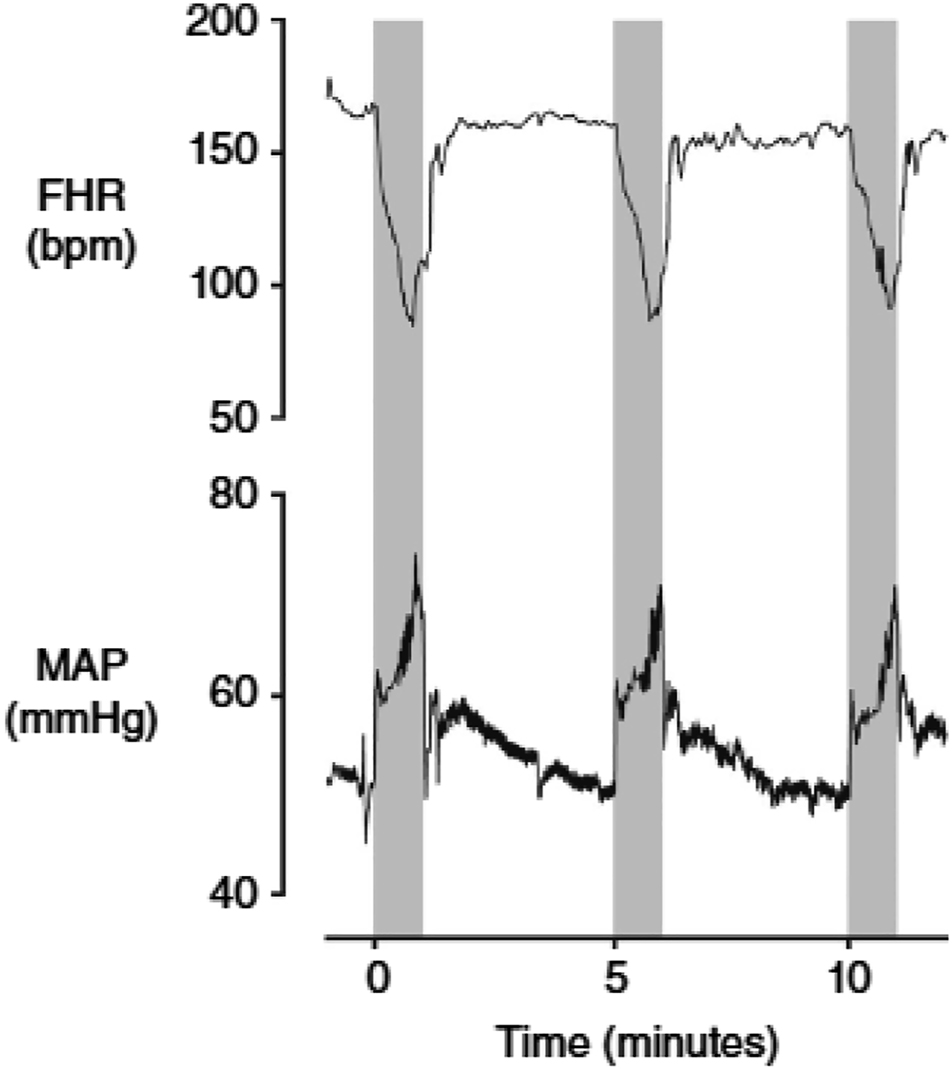
Fetal heart rate (FHR, beats min–1 (bpm)) and mean arterial pressure (MAP, mmHg) during three successive 1 min complete umbilical cord occlusions, repeated every 5 min in a near-term fetal sheep (0.85 of gestation). The periods of occlusion are shown in grey. Additionally the release of occlusion is not associated with the development of hypotension below baseline levels. The first occlusion shown here is the 18th in a series of 49 occlusions; at this time arterial pH was 7.358, with a lactate of 1.6 mmol l–1. Data are 1 s averages. Source: Lear CA, Galinsky R, Wassink G, Yamaguchi K, Davidson JO, Westgate JA. et al. The myths and physiology surrounding intrapartum decelerations: the critical role of the peripheral chemoreflex. J Physiol 2016;594:4711–25.
This is unequivocal evidence that the peripheral chemoreflex is active during fetal life and readily explains majority of intrapartum decelerations independent of any umbilical cord compression or occlusion. The fetus relies on continuous placental and umbilical blood flow for oxygen delivery. However, increases in intrauterine pressure during contractions may reduce maternal uterine artery blood velocity by up to 73% [19], umbilical artery blood flow during variable decelerations in the human [20], and during second stage contractions in the bovine fetus [21]. Uterine contractions result in impaired fetal arterial oxygenation, regardless of direct or indirect umbilical cord compression [22]. The key efferent arms of the chemoreflex are the rapid vagally-mediated FHR deceleration, and sympathetic nervous system-mediated peripheral vasoconstriction by release of vasoactive mediators [14, 23], including adrenal catecholamines [16, 17] vasopressin [24], and neuropeptide Y [25]. The immediate fall in cardiac output due to the FHR deceleration is offset by sympathetic-mediated peripheral vasoconstriction. The combined effect of these responses maintains/increases arterial pressure and consequently blood flow to the “central organs” including the brain, heart, and adrenals [23].
Taken together the evidence is compelling that the peripheral chemoreflex and not the baroreflex is the key mediator of intrapartum FHR decelerations independent of umbilical cord compression or occlusion. The recent attempt to classify FHR decelerations into two categories namely, those caused by “baroreceptors” and those caused by “chemoreceptors” based on their morphological appearance and timing of onset is misguided and misleading, and contrary to available experimental evidence. The reader is referred to our recent detailed review of the subject [26].
Electronic fetal monitoring (EFM) with the cardiotocograph (CTG)
The most recent update of Cochrane systematic reviews comparing continuous CTG with and without fetal blood sampling (FBS) with no fetal monitoring, intermittent auscultation (IA), and intermittent CTG included 13 trials involving over 37,000 women [27]. There were no studies comparing continuous CTG with no fetal monitoring. One trial (4,044 women) compared continuous CTG with intermittent CTG, and the other trials compared continuous CTG with IA. The results showed that continuous CTG did not significantly improve overall perinatal death rate compared to IA (RR 0.86, 95%CI, 0.59–1.23, n=33,513, 11 trials), but continuous CTG was associated with 50% reduction in neonatal seizure rates (RR 0.50, 95% CI 0.31–0.80, n=32,386, 9 trials). No differences in cord blood acidosis (RR 0.92, 95% CI 0.27–3.11, n=2,494, 2 trials, or cerebral palsy rates (RR 1.75, 95% CI 0.84–3.63, n=13,252, 2 trials) were observed. Continuous CTG was associated with an increase in Caesarean sections (RR 1.63, 95% CI 1.29–2.07, n=18,861, 11 trials) and instrumental vaginal births (RR 1.15, 95% CI 1.01–1.33, n=18,615, 10 trials). Compared to intermittent CTG, continuous CTG made no difference to Caesarean section rates (RR 1.29, 95% CI 0.84–1.97, n=4,044, 1 trial) or instrumental births (RR 1.16, 95% CI 0.92–1.46, n=4,044, 1 trial). The evidence was assessed using GRADE and most outcomes were graded as low-quality evidence (perinatal death, cerebral palsy, Caesarean section, and instrumental vaginal births) for design limitations, inconsistency, and imprecision of results. The remaining outcomes were graded as moderate quality (neonatal seizures), and very low quality (cord blood acidosis), due to similar concerns.
The review authors concluded that CTG during labour was associated with reduced rates of neonatal seizures, but no differences in cerebral palsy, infant mortality, or other measures of neonatal wellbeing: and that continuous CTG was associated with increased rates of Caesarean sections and instrumental vaginal births. Freedom of movement, easy change of birthing positions, or use of the birthing pool to help with comfort during labour are difficult, if not impossible, with continuous CTG. In addition, continuous CTG monitoring inadvertently consumes a disproportionate amount of the caregiver’s time for interpretation with less focus on the woman’s needs in labour. The review authors considered whether future RCTs should measure efficacy or effectiveness, and long-term effects of operative births for women and babies. We know that the links between antenatal or intrapartum events, neonatal seizures, and long-term neurodevelopmental outcomes, are yet to be fully mapped out. Obstetricians and midwives should discuss womens’ needs with them including their wishes about intrapartum fetal monitoring.
Pathophysiological framework for intrapartum FHR interpretation
Since a normal CTG likely, but not invariably, suggests fetal neurological integrity, normoxia, absence of significant acidosis or acidaemia, low risk of intrapartum asphyxia, and fetal capability to react and defend itself against intrapartum hypoxia, the authors pioneered and introduced the approach of monitoring babies in labour by focusing on the type of hypoxia, which might develop during labour from a previously normal CTG namely, acute, subacute, and gradually developing hypoxia. The general characteristics of these hypoxia subtypes and the algorithm for their clinical application are reviewed in detail elsewhere [4, 5]. They can be applied as a stand alone approach to FHR monitoring or as an adjunct to existing clinical guidelines to improve the quality of CTG interpretation and recognition of patterns, which suggest fetal decompensation and risk of injury.
The first and most critical step in the application of fetal physiology to CTG interpretation is the identification and management of the baby in the latent phase or early labour with unexplained baseline FHR tachycardia, with or without decelerations, reduced or normal (even increased) FHR variability, particularly in association with meconium staining of the amniotic fluid. Babies with this CTG pattern characteristically have altered behavioural state and do not exhibit alternating periods of reduced vs. increased FHR variability, ± accelerations, so called fetal cycling activity. Fetal cycling activity is a key behavioural state of the normal neurologically intact term or near-term fetus without significant hypoxia or acidaemia. Consideration should be given to pre-existing feto-placental infection, chronic hypoxia, meconium aspiration syndrome, feto-maternal haemo antecedent brain injury, intracranial haemorrhage, maternal systemic disease, drugs including recreational substances, and chromosomal abnormalities. This pattern is not specific for chronic hypoxia as have been erroneously suggested by some. Senior staff involvement should be sought early and delivery by Caesarean section considered depending on the clinical situation. Obstetricians should also beware that rapid fetal decompensation may occur without warning. The outcome may still be unfavourable even with early delivery, and attributable to the underlying disorder but intrapartum exacerbation of the pre-existing insult will be avoided. Although many other maternity units have adopted this approach to CTG interpretation plans are underway to test its clinical utility systematically and more widely.
The role of fetal scalp blood sampling
A fetal blood sample (FBS) is indicated if the CTG is interpreted as pathological. It needs to be performed bearing in mind the whole clinical context and the woman should be fully informed. Evidence from systematic review of 6 studies showed that the rates of Caesarean section and instrumental vaginal birth were higher in women who were monitored with CTG plus fetal blood sampling (FBS) compared to women monitored with intermittent auscultation (IA) only. The rates of resuscitation, neonatal seizures, and Apgar score <7 at 5 min were lower in babies born to women who were monitored with CTG plus FBS compared to babies born to women monitored with IA or CTG only. Neonatal acidaemia was lower in women monitored with CTG plus fetal blood sampling compared to monitoring with CTG alone, but there was no difference when compared to women monitored with IA. No difference was found between the 2 groups in the incidence of cerebral palsy [27]. Since fetal scalp pH estimation is undertaken when the CTG was classified as pathological, it follows that the accuracy of CTG interpretation is critical to the performance of FBS. If the label “pathological CTG” is assigned followed by FBS in babies who are not at genuine risk of intrapartum hypoxia the usefulness of FBS, and pH estimation will be degraded. It is likely that this was the case in at least some of the studies included in the systematic review. We know that the need for FBS to confirm fetal acidosis in a pathological CTG is significantly reduced with the recognition of CTG features, which are associated with acidaemia. It is therefore unsurprising that the correlations between fetal scalp pH, the CTG, and perinatal outcomes have been poor. Clinicians should beware that in the presence of chorioamnionitis, or significant meconium, FBS results may be falsely reassuring. There is no difference in acid base status between infected babies and their non-infected peers however, infected neonates have significantly lower 5 min Apgar scores compared to their uninfected counterparts [28].
Intelligent Intermittent Auscultation (IIA)
Systematic reviews and meta-analyses of trials comparing continuous electronic FHR monitoring using the cardiotocograph (CTG) to IA show that CTG monitoring was associated with increased risk of Caesarean section without improvement in perinatal outcomes in women at low risk of intrapartum hypoxia [27]. Therefore, the National Institute for Health and Clinical Excellence (NICE) recommends the use of intermittent auscultation for fetal surveillance during labour in women without pregnancy complications, that is, low-risk women. However, there is no evidence for the ideal device to use, the timing, duration, and frequency of IA. The guidance for the conduct of IA is to firstly palpate and document the maternal heart rate (MHR) and listen to the FHR for 60 s after a uterine contraction every 15 min in the first stage of labour and every 5 min in the second stage. www.nice.org.uk/guidance/cg190/chapter/recommendations#monitoring-during-labour.
This is to detect late or complicated variable decelerations, which are associated with the development of fetal acidosis. The concept of Intelligent Intermittent Auscultation (IIA) has been proposed to improve the detection of healthy FHR accelerations, decelerations after a contraction, overshoots, and early recourse to Continuous Electronic Fetal Monitoring (CEFM) if indicated. In practice the midwife listens to and counts the FHR for 60 s in six lots of 15 s each and examines the similarities, variations, and timing of the numbers to deduce the occurrence of decelerations or accelerations https://doi.org/10.1186/1471-2393-14-184. The reader is referred to Chapter 3 of the book “Intrapartum Surveillance” (in press) by the same authors for detailed discussion of the practice of IIA. Some midwives may find this approach cumbersome in practice. More recently a structured variation of IIA has been developed (ISIA) to include assessment of antenatal factors, findings on abdominal examination, uterine contractions, fetal movements, and FHR, to further improve selection of suitability to IA [29]. Recording of fetal movements and determination of FHR increase associated with fetal movements are the two key concepts incorporated into the ISIA framework [29].
Admission test/cardiotocograph (CTG)
Admission test (AT) was introduced and widely practised in the 1980s as a screening test administered in early labour for the detection of pre-existing or subsequent intrapartum “fetal distress”. It was based on the findings of an observational study, which showed that AT predicted 5-min Apgar score <7, new-born acidaemia, and operative delivery for fetal distress [30]. However, systematic review and meta-analysis of four RCTs comparing AT to intermittent auscultation found no evidence of benefit for the use of the admission CTG in low-risk women on admission in labour [31]. In addition, admission CTG increases the caesarean section rate in low-risk women by a non-statistically significant 20% without evidence of benefit [31]. The data lacked power to detect possible important differences in perinatal mortality. In practice the CTG is a very complex tool and is affected by many factors, some of which are mediated via non-hypoxia pathways making the AT insensitive and the fetus may decompensate later in labour. Furthermore, injury from an antenatal insult may still be evolving or the fetus may have recovered metabolically and with good cardiovascular function, but with a residual neurologic injury. Phelan et al. showed that about half of brain-damaged fetuses may present with a normal CTG in early labour and nearly 10% of them maintained a normal CTG throughout labour [32, 33]. It is likely that AT performs poorly as a screening test in part because it relies on visual analysis and interpretation of the CTG by human eye and individual experience without consideration or inclusion of the clinical factors such as those identified in CESDI, EBC, INFANT study, and the recent Ockenden report [6], [7], [8], [9]. To address this gap we recently used routinely collected clinical data in a large tertiary hospital to investigate whether infants with “severe compromise” at birth exhibited FHR abnormalities in their CTGs during the first hour of admission in labour [34]. We found that FHR tachycardia, non-reactive CTG pattern, reduced long- and short-term variability, decelerative capacity, and absence of accelerations, were significantly higher amongst babies with severe compromise during the first hour of admission, compared to those without severe compromise. Furthermore, thick meconium, fever, and small for gestational age were more common in severely compromised infants [34]. There is ongoing work to re-appraise the role of the admission test using an algorithm, which incorporates objective tools such as the Oxford System (OxSys) for CTG analysis based on “big” datasets, and important clinical factors.
Fetal ECG–ST waveform analysis
Fetal electrocardiogram (FECG) waveform analysis (STAN) was introduced in the 1990s to reduce perinatal morbidity and mortality. Animal experimental data have shown that key components of fetal adaptation to stressors including hypoxia include catecholamine surge and preferential increase in blood flow and oxygen delivery to the myocardium [35]. The catecholamine surge results in mobilisation of stored myocardial glycogen, a shift in glucose and K+ ions, leading to increased T-wave amplitude [36]. The STAN technology relies on computerised analysis of changes in the ST segment of the FECG as they relate to metabolic events in the fetal myocardium during hypoxia. The tool is applicable and validated for fetuses who are 36 completed weeks of gestation or more. Significant ST events, when judged along with the CTG, indicate the need for intervention, which may include alleviation of a cause of abnormal FHR pattern such as oxytocin overstimulation or maternal hypotension, or delivery of the baby. If the ST event occurred in the active phase of the second stage of labour, delivery within 20 min is recommended. In the already compromised fetus with lack of FHR variability and reactivity or a persistently prolonged deceleration immediate delivery is warranted. In the authors’ experience successful use of STAN requires regular and universal staff usage regardless of time of day, ongoing cycles of training, retraining, case reviews with particular focus on physiological CTG interpretation, availability of a central monitoring display unit, and audit. The methodology is not currently recommended by the UK National Institute for Health and Care Excellence (NICE).
The STAN methodology was developed and validated using the first FIGO 1987 guidelines on CTG classification and the 2007 revision of the STAN guidelines. However, a new FIGO classification system for CTG interpretation came into force in 2015 [37], raising the question whether this new classification system is applicable to STAN. Research using existing databases have identified CTG patterns, which are associated with ST events and adverse outcomes but not covered by existing CTG classifications. The investigators concluded that any CTG classification system may be used, provided a more physiologically based interpretation is applied for CTG assessment in relation to ST events [38, 39]. Earlier studies on STAN showed promising results from some studies [40, 41] but inconsistent results in others [42]. This disparity prompted computerisation of ECG analysis and calculation of rise in fetal T/QRS ratio from its own baseline instead of fixed values applicable to all fetuses.
Several meta-analyses on the role of STAN monitoring have been published albeit with variable selection criteria, inclusion or not, of revised data from primary trials, and end points including Caesarean delivery, metabolic acidosis, neonatal encephalopathy or death, low Apgar score, and intubation for ventilation support. The results have unsurprisingly been inconsistent [43], [44], [45], [46]. However, Blix et al. used corrected datasets, the most appropriate methodology, and analyses, and concluded that CTG plus ST monitoring reduced operative vaginal delivery rates by 8% (RR, 0.92; 95% CI, 0.86–0.99) and metabolic acidosis rates by 36% (OR, 0.64, 95% CI, 0.46–0.88) [45]. This is in contrast with the non-significant reductions in metabolic acidosis reported in the Cochrane database of systematic reviews and meta-analyses, 28% (RR, 0.72, 95% CI, 0.43–1.20) [43], for example. In the authors’ opinion the balance of current evidence suggests that ST segment analysis significantly reduced metabolic acidosis, operative deliveries, and FBS rates. Maternity units considering the introduction of the STAN methodology for intrapartum fetal surveillance should invest in intensive and mandatory staff training, competency testing, and retraining, particularly in fetal and labour physiology, labours complicated by infection, meconium, and undertake regular case reviews, acquire central monitoring systems, and data archiving systems to support practice and audit.
Current state of training in intrapartum fetal surveillance
The teaching and practice of classifying the baseline FHR, variability, accelerations, and decelerations, as though they are independent indicators of fetal health is flawed [4, 5], hence the predictable doubt over the efficacy and utility of CTG training programmes [47]. There are currently no systematic or validated modules for mandatory training and testing of clinicians’ knowledge and competence in intrapartum fetal monitoring before they take charge of intrapartum fetal monitoring in the UK and in most other developed countries [48]. Moreover, we do not know what the optimum number of hours, the content, and frequency of training are, what the best format of instruction, or the design of a necessary mandatory test that is fit for purpose [48]. Although we administer mandatory tests of CTG knowledge and competency at St. George’s University Hospital, London, with the pass mark set arbitrarily at 85%, we are conscious that testing clinicians without establishing the validity of the tests first is less than ideal [49]. Obstetricians and Midwives who do not make the required 85% pass mark are assigned to more senior obstetricians or midwives for further training and case reviews on the Central monitoring, and archiving systems.
Appropriate CTG interpretation occurs in a complex environment including organisational setup and structure, human factors, and communication. Therefore, this environmental context, communication, emergency response, and guidelines should be tested in studies of educational interventions [50].
The future of intrapartum fetal surveillance
The future of intrapartum FHR monitoring will see a total shift away from the current classification of snapshots/static FHR features such as baseline rate, variability, and decelerations as a basis for estimating the risk of intrapartum hypoxia, towards trend analysis of fetal physiological adaptation to evolving/pre-existing hypoxia and other stressors, as the appropriate template for the definition of intrapartum hypoxia and decompensation. Field trials evaluating this approach will be invaluable. However, visual interpretation of FHR signals is still subjective and suffers from significant inter- and intra-observer variability [51, 52], hence computer-aided diagnostic systems based on artificial intelligence (AI) technology will be the leading tools to assist obstetricians and midwives in making more objective decisions in future. Whether these systems can effectively capture and quantify the role played by noxious factors associated with adverse perinatal outcome such as fever, meconium, chorioamnionitis, FSIRS and synergistic interaction with hypoxia, fetal strokes, FGR, maternal disease, placental disorders, excessive moulding etc., depends on the success of mathematical interrogation of very large datasets, and improved risk prediction through the development of machine learning.
Machine learning is the process through which computer algorithms develop the ability to recognise patterns, continuously learn from data, make predictions based on the data, and make corrections without specific human programming to do so. Deep learning algorithms’ neural networks are constructed to replicate the structure of the human brain with learning capabilities to get better at a task over time without human feedback. These systems are already being tested and applied in the field of intrapartum fetal surveillance [53], [54], [55], [56].
Initial attempts at CTG analysis using AI failed because the algorithms simply mimicked human methods of CTG analysis such as recognition of baseline FHR, variability, and decelerations and therefore were no different from adding a “second evaluator with similar instructions” [57]. Systematic reviews of AI for CTG interpretation found no reduction in adverse perinatal outcomes including neonatal acidosis, seizures, death, unnecessary interventions, or neonatal intensive care admissions, suggesting the need for the capability to analyse features, which are not obvious to the human for these systems to be effective [57], [58], [59].
The INFANT system uses numerical algorithms and neural networks to extract and quantify FHR features typically interpreted by the human in current practice such as baseline rate, variability, accelerations, decelerations, and their temporal relationships with contractions; clinical information, such as cervical dilation, analgesia, FBS; and risk factors including FGR, abruption, and meconium; to interpret the data and provide decision support, but not recommendations for clinical actions [60]. A multicentre RCT of the INFANT system on 47,000 participants found no improvement in clinical outcomes, despite correctly and effectively detecting FHR abnormalities [61], suggesting that substandard care was not due to failure to identify abnormal CTG but the consequence of poor clinical management decisions made after the identification CTG abnormalities. The trial decision-support system did not include clinical information relating to labour itself such as the duration and progress, leading investigators to suggest that including this information in the decision support system might have improved decisions to escalate. Another AI-based system Omniview-SisPorto 3.5 analyses and classifies the CTG into four categories based on FIGO guidelines and provide alerts. Results of an RCT of the system on 7,320 participants found no reduction in neonatal acidosis and obstetric interventions between the intervention and control arms [62]. A small RCT of quantitative cardiotocography (qCTG) decision-support system, which computes FHR, FHR micro-fluctuations, and decelerations, including 720 participants found reduced incidence of hypoxia, acidaemia, and operative deliveries in the intervention arm compared to the control arm [63]. Alternative AI-based CTG interpretation systems applying feature engineering theory from other domains or the use of phase-rectified signal averaging (PRSA) to compute the mean decelerative capacity of FHR have shown encouraging results [64], but yet to be validated in large RCTs. It is highly likely that these systems will complement, if not replace existing human interpretation of the intrapartum FHR over time.
Conclusions
The field of intrapartum fetal surveillance is finally on the verge of aligning its principles and practices with advances and developments in fetal and labour physiology, data science, and artificial intelligence. Modern computer systems use advanced machine learning methods and include a wider range of analyses, are immune to human factors such as bias, poor communication, tiredness, distraction, etc., and are available round the clock. The impact of this realignment will be profound, particularly in the more controversial aspects of the field. CTG interpretation is a core and essential skill, which requires the development of quality-controlled training modules ideally embedded in appropriate organisational, human factors, team dynamics, and clinical context. The development of the right training modules is a pressing priority and should be strengthened with validated tests to provide reassurance that practitioners who have undergone training and certification can be relied upon to provide a minimum standard of safe and good quality care.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Nelson, KB, Dambrosia, JM, Ting, TY, Grether, JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med 1996;334:613–8. https://doi.org/10.1056/nejm199603073341001.Suche in Google Scholar PubMed
2. Parer, JT, King, T. Fetal heart rate monitoring: is it salvageable? Am J Obstet Gynecol 2000;182:982–7. https://doi.org/10.1016/s0002-9378(00)70358-9.Suche in Google Scholar PubMed
3. Gibbons, L, Belizán, JM, Lauer, JA, Betrán, AP, Merialdi, M, Althabe, F. The global numbers and costs of additionally needed and unnecessary caesarean sections performed per year: overuse as a barrier to universal coverage. In: World Health Report 2010; Background paper 30. Geneva: World Health Organization; 2010.Suche in Google Scholar
4. Ugwumadu, A. Understanding cardiotocographic patterns associated with intrapartum fetal hypoxia and neurologic injury. Best Pract Res Clin Obstet Gynaecol 2013;27:509–36. https://doi.org/10.1016/j.bpobgyn.2013.04.002.Suche in Google Scholar PubMed
5. Ugwumadu, A. Are we (mis)guided by current guidelines on intrapartum fetal heart rate monitoring? Case for a more physiological approach to interpretation. BJOG 2014;121:1063–70. https://doi.org/10.1111/1471-0528.12900.Suche in Google Scholar PubMed
6. Royal College of Obstetricians and Gynaecologists Each baby counts: 2018 progress report. London: RCOG; 2018.Suche in Google Scholar
7. The Royal College of Obstetricians and Gynaecologists Each baby counts: 2019 progress report. London: RCOG; 2020. Available from: www.rcog.org.uk/en/guidelines-research-services/audit-quality-improvement/each-baby-counts/reports-updates.Suche in Google Scholar
8. Maternal and Child Health Research Consortium. Confidential enquiry into stillbirths and deaths in infancy: 4th annual report, 1 January–31 December 1995. London: Royal College of Obstetricians and Gynaecologists; 1997.Suche in Google Scholar
9. Ockenden report - final March 30, 2022, findings, conclusions, and essential actions from the independent review of maternity services at the Shrewsbury and Telford hospital NHS trust. Available from: https://www.ockendenmaternityreview.org.uk.Suche in Google Scholar
10. Steer, PJ, Kovar, I, McKenzie, C, Griffin, M, Linsell, L. Computerised analysis of intrapartum fetal heart rate patterns and adverse outcomes in the INFANT trial. BJOG 2019;126:1354–61. https://doi.org/10.1111/1471-0528.15535.Suche in Google Scholar PubMed
11. Berglund, S, Grunewald, C, Pettersson, H, Cnattingius, S. Severe asphyxia due to delivery-related malpractice in Sweden 1990–2005. BJOG 2008;115:316–23. https://doi.org/10.1111/j.1471-0528.2007.01602.x.Suche in Google Scholar PubMed PubMed Central
12. Itskovitz, J, LaGamma, EF, Rudolph, AM. Heart rate and blood pressure responses to umbilical cord compression in fetal lambs with special reference to the mechanism of variable deceleration. Am J Obstet Gynecol 1983;147:451–7. https://doi.org/10.1097/00132582-198406000-00011.Suche in Google Scholar
13. Giussani, DA, Unno, N, Jenkins, SL, Wentworth, RA, Derks, JB, Collins, JH, et al.. Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late-gestation sheep fetus. Am J Physiol 1997;273:H2351–60. https://doi.org/10.1152/ajpheart.1997.273.5.h2351.Suche in Google Scholar
14. Booth, LC, Malpas, SC, Barrett, CJ, Guild, SJ, Gunn, AJ, Bennet, L. Renal sympathetic nerve activity during asphyxia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 2012;303:R30–8. https://doi.org/10.1152/ajpregu.00063.2012.Suche in Google Scholar PubMed
15. Bennet, L, Westgate, JA, Lui, YC, Wassink, G, Gunn, AJ. Fetal acidosis and hypotension during repeated umbilical cord occlusions are associated with enhanced chemoreflex responses in near-term fetal sheep. J Appl Physiol 2005;99:1477–82. https://doi.org/10.1152/japplphysiol.00431.2005.Suche in Google Scholar PubMed
16. Giussani, DA, Spencer, JA, Moore, PJ, Bennet, L, Hanson, MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 1993;461:431–49. https://doi.org/10.1113/jphysiol.1993.sp019521.Suche in Google Scholar PubMed PubMed Central
17. Galinsky, R, Jensen, EC, Bennet, L, Mitchell, CJ, Gunn, ER, Wassink, G, et al.. Sustained sympathetic nervous system support of arterial blood pressure during repeated brief umbilical cord occlusions in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol 2014;306:R787–95. https://doi.org/10.1152/ajpregu.00001.2014.Suche in Google Scholar PubMed
18. Charkoudian, N, Wallin, BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 2014;4:825–50.10.1002/cphy.c130038Suche in Google Scholar PubMed
19. Janbu, T, Nesheim, BI. Uterine artery blood velocities during contractions in pregnancy and labour related to intrauterine pressure. Br J Obstet Gynaecol 1987;94:1150–5. https://doi.org/10.1111/j.1471-0528.1987.tb02314.x.Suche in Google Scholar PubMed
20. Sakai, M, Kozuma, S, Okai, T, Kagawa, H, Ryo, E, Taketani, Y. Doppler blood flow velocity waveforms of the umbilical artery during variable decelerations in labor. Int J Gynaecol Obstet 1997;59:207–11. https://doi.org/10.1016/s0020-7292(97)00129-x.Suche in Google Scholar PubMed
21. Bleul, U, Lejeune, B, Schwantag, S, Kahn, W. Ultrasonic transit-time measurement of blood flow in the umbilical arteries and veins in the bovine fetus during stage II of labor. Theriogenology 2007;67:1123–33. https://doi.org/10.1016/j.theriogenology.2006.12.007.Suche in Google Scholar PubMed
22. Aldrich, CJ, D’Antona, D, Spencer, JA, Delpy, DT, Reynolds, EO, Wyatt, JS. Fetal heart rate changes and cerebral oxygenation measured by near-infrared spectroscopy during the first stage of labour. Eur J Obstet Gynecol Reprod Biol 1996;64:189–95. https://doi.org/10.1016/0301-2115(95)02284-8.Suche in Google Scholar PubMed
23. Giussani, DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 2016;594:1215–30. https://doi.org/10.1113/jp271099.Suche in Google Scholar PubMed PubMed Central
24. Perez, R, Espinoza, M, Riquelme, R, Parer, JT, Llanos, AJ. Arginine vasopressin mediates cardiovascular responses to hypoxemia in fetal sheep. Am J Physiol 1989;256:R1011–8. https://doi.org/10.1152/ajpregu.1989.256.5.r1011.Suche in Google Scholar PubMed
25. Fletcher, AJ, Edwards, CM, Gardner, DS, Fowden, AL, Giussani, DA. Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology 2000;141:3976–82. https://doi.org/10.1210/endo.141.11.7770.Suche in Google Scholar PubMed
26. Lear, CA, Wassink, G, Westgate, JA, Nijhuis, JG, Ugwumadu, A, Galinsky, R, et al.. The peripheral chemoreflex: indefatigable guardian of fetal physiological adaptation to labour. J Physiol 2018;596:5611–23. https://doi.org/10.1113/jp274937.Suche in Google Scholar PubMed PubMed Central
27. Alfirevic, Z, Devane, D, Gyte, GMI, Cuthbert, A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour Cochrane. Database Syst Rev 2017;2:CD006066.10.1002/14651858.CD006066.pub3Suche in Google Scholar PubMed PubMed Central
28. Maberry, MC, Ramin, SM, Gilstrap, LC, Leveno, KJ, Dax, JS. Intrapartum asphyxia in pregnancies complicated by intra-amniotic infection. Obstet Gynecol 1990;76:351–4.Suche in Google Scholar
29. Maude, RM, Skinner, JP, Foureur, MJ. Intelligent Structured Intermittent Auscultation (ISIA): evaluation of a decision-making framework for fetal heart monitoring of low-risk women. BMC Pregnancy Childbirth 2014;14:184. https://doi.org/10.1186/1471-2393-14-184.Suche in Google Scholar PubMed PubMed Central
30. Ingemarsson, I, Arulkumaran, S, Ingemarsson, E, Tambyraja, RL, Ratnam, SS. Admission test: a screening test for fetal distress in labor. Obstet Gynecol 1986;8:800–6.Suche in Google Scholar
31. Devane, D, Lalor, JG, Daly, S, McGuire, W, Cuthbert, A, Smith, V. Cardiotocography versus intermittent auscultation of fetal heart on admission to labour ward for assessment of fetal wellbeing. Cochrane Database Syst Rev 2017;CD005122. https://doi.org/10.1002/14651858.cd005122.pub5.Suche in Google Scholar
32. Phelan, JP, Ahn, MO. Perinatal observations in forty-eight neurologically impaired term infants. Am J Obstet Gynecol 1994;171:424–31. https://doi.org/10.1016/0002-9378(94)90278-x.Suche in Google Scholar PubMed
33. Phelan, JP, Ahn, MO. Fetal heart rate observations in 300 term brain-damaged infants. J Matern Fetal Invest 1998;8:1–5.Suche in Google Scholar
34. Lovers, AAK, Ugwumadu, A, Georgieva, A. Cardiotocography and clinical risk factors in early term labor: a retrospective cohort study using computerized analysis with Oxford system. Front Pediatr 2022;16:784439. https://doi.org/10.3389/fped.2022.784439.Suche in Google Scholar PubMed PubMed Central
35. Rosen, KG, Dagbjartsson, A, Henriksson, BA, Lagercrantz, H, Kjellmer, I. The relationship between circulating catecholamine and ST waveform in the fetal lamb electrocardiogram during hypoxia. Am J Obstet Gynecol 1984;149:190–5. https://doi.org/10.1016/0002-9378(84)90197-2.Suche in Google Scholar PubMed
36. Hökegård, KH, Eriksson, BO, Kjellmer, I, Magno, R, Rosén, KG. Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand 1981;113:1–7. https://doi.org/10.1111/j.1748-1716.1981.tb06853.x.Suche in Google Scholar PubMed
37. Visser, GH, Ayres-de-Campos, D. FIGO consensus guidelines on intrapartum fetal monitoring: adjunctive technologies. Int J Gynaecol Obstet 2015;131:25–9. https://doi.org/10.1016/j.ijgo.2015.06.021.Suche in Google Scholar PubMed
38. Rosen, KG, Noren, H, Carlsson, A. FHR patterns that become significant in connection with ST waveform changes and metabolic acidosis at birth. J Matern Fetal Neonatal Med 2019;32:3288–93.10.1080/14767058.2018.1462326Suche in Google Scholar PubMed
39. Olofsson, P, Norén, H, Carlsson, A. New FIGO and Swedish intrapartum cardiotocography classification systems incorporated in the fetal ECG ST analysis (STAN) interpretation algorithm: agreements and discrepancies in cardiotocography classification and evaluation of significant ST events. Acta Obstet Gynecol Scand 2018;97:219–28. https://doi.org/10.1111/aogs.13277.Suche in Google Scholar PubMed PubMed Central
40. Lilja, H, Arulkumaran, S, Lindecrantz, K, Rattan, SS, Rosen, KG. Fetal ECG during labour; a presentation of a microprocessor-based system. J Biomed Eng 1988;10:348–50. https://doi.org/10.1016/0141-5425(88)90066-0.Suche in Google Scholar PubMed
41. Arulkumaran, S, Lilja, H, Lindecrantz, K, Ratnam, SS, Thavarasah, AS, Rosen, KG. Fetal ECG waveform analysis should improve fetal surveillance in labour. J Perinat Med 1990;187:13–22. https://doi.org/10.1515/jpme.1990.18.1.13.Suche in Google Scholar PubMed
42. MacLachlan, NA, Harding, K, Spencer, JAD, Arulkumaran, S. Fetal heart rate, fetal acidaemia and the T/QRS ratio of the fetal ECG in labour. Br J Obstet Gynaecol 1992;99:26–31.10.1111/j.1471-0528.1992.tb14387.xSuche in Google Scholar PubMed
43. Neilson, JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev 2015;12:CD000116.10.1002/14651858.CD000116.pub5Suche in Google Scholar PubMed PubMed Central
44. Saccone, G, Schuit, E, Amer-Wahlin, I, Xodo, S, Berghella, V. Electrocardiogram ST analysis during labor: a systematic review and meta-analysis of randomized controlled trials. Obstet Gynecol 2016;127:127–35. https://doi.org/10.1097/aog.0000000000001198.Suche in Google Scholar
45. Blix, E, Brurberg, KG, Reierth, E, Reinar, LM, Oian, P. ST waveform analysis versus cardiotocography alone for intrapartum fetal monitoring: a systematic review and meta-analysis of randomized trials. Acta Obstet Gynecol Scand 2016;95:16–27. https://doi.org/10.1111/aogs.12828.Suche in Google Scholar PubMed
46. Wetterslev, J, Jakobsen, JC, Gluud, C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0315-7.Suche in Google Scholar PubMed PubMed Central
47. Pehrson, C, Sorenson, JL, Amer-Wahlin, I. Evaluation and impact of cardiotocography training schemes: a systematic review. BJOG 2011;118:926–35.10.1111/j.1471-0528.2011.03021.xSuche in Google Scholar PubMed
48. Ugwumadu, A, Steer, P, Parer, B, Carbone, B, Vayssiere, C, Maso, G, et al.. Time to optimise and enforce training in interpretation of intrapartum cardiotocograph. BJOG 2016;123:866–9. https://doi.org/10.1111/1471-0528.13846.Suche in Google Scholar PubMed
49. Downing, SM. Validity: on the meaningful interpretation of assessment data. Med Educ 2003;37:830–7. https://doi.org/10.1046/j.1365-2923.2003.01594.x.Suche in Google Scholar PubMed
50. Pettker, CM, Thung, SF, Raab, CA, Donohue, KP, Copel, JA, Lockwood, CJ, et al.. A comprehensive obstetrics patient safety program improves safety climate and culture. Am J Obstet Gynecol 2011;204:216. https://doi.org/10.1016/j.ajog.2010.11.004.Suche in Google Scholar PubMed
51. Costa Santos, C, Costa Pereira, A, Bernardes, J. Agreement studies in obstetrics and gynaecology: inappropriateness, controversies, and consequences. Br J Obstet Gynaecol 2005;112:667–9. https://doi.org/10.1111/j.1471-0528.2004.00505.x.Suche in Google Scholar PubMed
52. Hruban, L, Spilka, J, Ek, VC, Jank, P, Huptych, M. Agreement on intrapartum cardiotocogram recordings between expert obstetricians. J Eval Clin Pract 2015;21:694–702. https://doi.org/10.1111/jep.12368.Suche in Google Scholar PubMed
53. O’Sullivan, ME, Considine, EC, O’Riordan, M, Marnane, WP, Rennie, JM, Boylan, GB. Challenges of developing robust AI for intrapartum fetal heart rate monitoring. Front Artif Intell 2021;26:765210.10.3389/frai.2021.765210Suche in Google Scholar PubMed PubMed Central
54. Zhao, Z, Deng, Y, Zhang, Y, Zhang, Y, Zhang, X, Shao, L. DeepFHR: intelligent prediction of fetal Acidemia using fetal heart rate signals based on convolutional neural network. BMC Med Inf Decis Making 2019;19:286. https://doi.org/10.1186/s12911-019-1007-5.Suche in Google Scholar PubMed PubMed Central
55. Esteban-Escaño, J, Castán, B, Castán, S, Chóliz-Ezquerro, M, Asensio, C, Laliena, AR, et al.. Machine learning algorithm to predict acidemia using electronic fetal monitoring recording parameters. Entropy 2021;24:68. https://doi.org/10.3390/e24010068.Suche in Google Scholar PubMed PubMed Central
56. Gold, N, Herry, CL, Wang, X, Frasch, MG. Fetal cardiovascular decompensation during labor predicted from the individual heart rate tracing: a machine learning approach in near-term fetal sheep model. Front Pediatr 2021;9:593889. https://doi.org/10.3389/fped.2021.593889.Suche in Google Scholar PubMed PubMed Central
57. Balayla, J, Shrem, G. Use of artificial intelligence (AI) in the interpretation of intrapartum fetal heart rate (FHR) tracings: a systematic review and meta-analysis. Arch Gynecol Obstet 2019;300:7–14. https://doi.org/10.1007/s00404-019-05151-7.Suche in Google Scholar PubMed
58. Campanile, M, D’Alessandro, P, Della Corte, L, Saccone, G, Tagliaferri, S, Arduino, B, et al.. Intrapartum cardiotocography with and without computer analysis: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med 2018;33:2284–90. https://doi.org/10.1080/14767058.2018.1542676.Suche in Google Scholar PubMed
59. Garcia-Canadilla, P, Sanchez-Martinez, S, Crispi, F, Bijnens, B. Machine learning in fetal cardiology: what to expect. Fetal Diagn Ther 2020;47:363–72. https://doi.org/10.1159/000505021.Suche in Google Scholar PubMed
60. Keith, RDF, Greene, KR. Development, evaluation and validation of an intelligent system for the management of labour. Bailliere Clin Obstet Gynaecol 1994;8:583–605. https://doi.org/10.1016/s0950-3552(05)80200-7.Suche in Google Scholar PubMed
61. Brocklehurst, P, Field, D, Greene, K, Juszczak, E, Keith, R, Kenyon, S, et al.. Computerised interpretation of fetal heart rate during labour (INFANT): a randomised controlled trial. Lancet 2017;389:1719–29. https://doi.org/10.1016/s0140-6736(17)30568-8.Suche in Google Scholar PubMed PubMed Central
62. Nunes, I, Ayres-de-Campos, D, Ugwumadu, A, Amin, P, Banfield, P, Nicoll, A, et al.. Central fetal monitoring with and without computer analysis: a randomized controlled trial. Fetal monitoring and alert (FM-ALERT) study group. Obstet Gynecol 2017;129:83–90.10.1097/AOG.0000000000001799Suche in Google Scholar PubMed
63. Ignatov, PN, Lutomski, JE. Quantitative cardiotocography to improve fetal assessment during labor: a preliminary randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2016;205:91–7. https://doi.org/10.1016/j.ejogrb.2016.08.023.Suche in Google Scholar PubMed
64. Georgieva, A, Papageorghiou, A, Payne, S, Moulden, M, Redman, C. Phase-rectified signal averaging for intrapartum electronic fetal heart rate monitoring is related to acidaemia at birth. BJOG An Int J Obstet Gynaecol 2014;121:889–94. https://doi.org/10.1111/1471-0528.12568.Suche in Google Scholar PubMed
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment
Artikel in diesem Heft
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment


