Abstract
For newly born babies, especially those in need of intervention at birth, actions taken during the first minute after birth, the so-called “Golden Minute”, can have important implications for long-term outcomes. Both delivery room handling, including identification of maternal and infant risk factors and provision of effective resuscitation interventions, and antenatal care decisions regarding antenatal steroid administration and mode of delivery, are important and can affect outcomes. Anticipating risk factors for neonates at high risk of requiring resuscitation can decrease time to resuscitation and improve the prognosis. Following a review of maternal and fetal risk factors affecting newborn resuscitation, we summarize the current recommendations for delivery room handling of the newborn. This includes recommendations and rationale for the use of delayed cord clamping and cord milking, heart rate assessment [including the use of electrocardiogram (ECG) electrodes in the delivery room], role of suctioning in newborn resuscitation, and the impact of various ventilatory modes. Oxygenation should be monitored by pulse oximetry. Effects of oxygen and surfactant on subsequent pulmonary outcomes, and recommendations for provisions of appropriate thermoregulatory support are discussed. Regular teaching of delivery room handling should be mandatory.
Introduction
Delivery room handling of the newborn covers all procedures carried out on the newborn immediately following birth, including heart rate assessment, suctioning, ventilation/sustained inflation, provision of positive end-expiratory pressure (PEEP), cord clamping, oxygen supplementation and heat loss prevention. This critical time period was first called “the golden minutes” by Vento et al. in 2009 [1]. The following year, the International Liaison Committee on Resuscitation (ILCOR) emphasized the importance of the first minute of life using the term the Golden Minute [2]. Unfortunately, ILCOR did not define when the “Golden Minute” starts, and there has been a wide variation in practice as to when the clock should be started. Therefore, we have previously emphasized the importance of a common definition with international agreement about when the Golden Minute begins [3]. A baby is born when the whole body is out, and that is when the clock is started and the Golden Minute begins.
During the first 30 s, the baby should be dried and kept warm. For babies <28 weeks of gestational age (GA), this includes being wrapped in plastic without drying, and placement under a radiant warmer. Neonates should be stimulated to breathe by rubbing the chest or the spine preferably in a caudocranial direction, which may lead to extension of the spine contributing to opening of the lungs. The infant should also be positioned correctly to open the airway, and the heart rate and breathing rate should have been recorded within this timeframe. In the next 30 s, respiratory support should be established if needed and, if available, a pulse oximetry probe should be placed. While these recommendations are important to optimize newborn outcomes, it might be unrealistic to reach all these goals in such a short period of time. A recent study found that the median time to start auscultation of the heart is 62 s (inter-quartile range 40–79 s) and the first heart rate is available after 70 s (57–89). Similarly, premature babies were placed in a plastic bag at 62 s (40–79) while pulse oximetry data were obtained at 78 s (64–95) [4].
Maternal and fetal factors affecting delivery room handling
Although the intrauterine to extrauterine transition is complex, the majority of newborn infants do not require resuscitation at birth [5], [6], [7]. According to ILCOR, 85% of babies born at term initiate spontaneous respirations within 10–30 s, 10% respond to drying and stimulation, 3% initiate respirations after positive pressure ventilation, 2% will be intubated to support respiratory function, and only 0.1% will require chest compressions and/or epinephrine [8]. The need for bag and mask ventilation varies but typically is about 3–5% of all newborns in European countries and probably higher in low-income areas [9], [10]. The need for resuscitation is higher in premature infants and is highly dependent on the degree of immaturity. European data from the e-newborn database show that more than 90% of infants <29 weeks need resuscitation in the delivery room with a peak of 97% at 25 weeks of GA and decreasing to 56% for infants at 32 weeks of GA [11]. In those cases when resuscitation is needed, the presence of medical staff trained in neonatal stabilization is required. The ability to anticipate or predict the need for advanced resuscitation is useful in order to provide appropriate care to the patients who need it and to conserve expensive resources for those who do not [5]. An early prospective, self-reported audit by Mitchell et al. evaluated the frequency of resuscitative interventions within clinical settings across Canada and found that 76% of resuscitations were not anticipated [7]. Historically, it was believed that the need for newborn resuscitation could not be predicted, but this view has been challenged by various studies that have identified antenatal and intra-partum risk factors associated with a higher need for resuscitation [12], [13]. For example, chorioamnionitis, preeclampsia, multiple gestation, maternal body mass index (BMI) before or at the beginning of pregnancy and certain maternal diseases are important risk factors that affect neonatal outcome [5], [14]. Several additional risk factors have been described by Mitchell et al. and Almudeer et al. (Tables 1 and 2, respectively) [7], [15]. Antenatal management decisions can also have significant impact on neonatal outcomes. For example, the use of antenatal steroids for babies less than 34 weeks of gestation reduces morbidity and mortality, while C-section increases morbidity compared to vaginal delivery all the way up to term gestation [16]. We have recently demonstrated in a population-based study that high placental weight (4th quartile vs. 2nd and 3rd quartile combined) increased the risk of neonatal death for infants born in gestational weeks 29–36 [17]. Finally, gender and fetal weight are also important considerations.
Risk factors identified by Mitchell et al. as associated with the need for resuscitation in the delivery room (Ref. [7]).
| Maternal factors | Fetal factors | Perinatal factors |
|---|---|---|
| Group B Streptococcus Gestational diabetes Maternal smoking Narcotic administration to mother Placenta previa Pregnancy-induced hypertension Uterine rupture | Breech Congenital anomalies Fetal distress Meconium-stained fluid Multiple gestation Post-dates Prematurity Twin-to-twin transfusion | Induction of labor Placental abruption Precipitous delivery Prolapsed cord |
Ante- and intrapartum risk factors identified by Almudeer et al. associated with intubation rates (Ref. [15]).
| Risk factors associated with increased intubation rates | Risk factors not associated with increased intubation rates |
|---|---|
| Maternal factors Chorioamnionitis Drug therapy during pregnancy General anesthesia to mother Maternal neurologic disease Polyhydramnios Fetal factors Fetal anemia Fetal anomaly Fetal distress Fetal hydrops Meconium-stained fluid Prematurity (gestational age <37 weeks) Small for gestational age (SGA) Perinatal factors Cesarean delivery Intrapartum hemorrhage Placental abruption Prolapsed cord | Maternal factors Maternal sepsis Narcotic administration within 4 h of birth Fetal factors Multiple gestation Perinatal factors Instrumentation at the time of delivery Rupture of membranes >24 h |
Cord clamping
In healthy term infants 2/3 of the fetoplacental blood volume is in the infant and 1/3 in the placenta/umbilical cord at birth [18]. Approximately 70–80% of this volume is transfused to the infant in the first minute, while prolonging cord clamping beyond that provides a relatively modest volume gain with another 15–20% of fetoplacental volume transfused after one additional minute [19]. Delayed cord clamping is now widely recommended in uncomplicated deliveries with benefits that include increased iron stores and reduction in the risk of iron deficiency anemia in infants [20]. Some studies have demonstrated an increased need for phototherapy [19], [20] although this was not found in a recent study from Nepal [21]. However, the definition of “delayed clamping” varies between 30 and 60 s and might explain conflicting results on the need for phototherapy.
A randomized study from Australia – the Australian Placental Transfusion Study (APTS) – found that for premature infants <30 weeks of GA, delayed cord clamping of 60 s compared to 10 s reduced mortality by 30% [22]. This finding was confirmed in a large meta-analysis and systematic review in infants <37 weeks of GA [23]. However, these studies in premature infants did not find any reduction in intraventricular hemorrhage (IVH) following delayed cord clamping in contrast to data from a previous Cochrane review [24].
Cord milking has been found to have equivalent efficacy to delayed cord clamping. In this procedure, the cord is grasped and the cord blood repeatedly pushed in the direction of the baby, for instance 3 times at a speed of 10 cm/s [25]. Katheria et al. found that cord milking compared to delayed cord clamping increased cognitive and language scores in infants at 23–31 weeks of gestation [26]. However, recent data from the same authors indicate a four-fold increased risk of IVH in the most premature babies following this procedure [27].
Experimental data as well as older clinical data seem to demonstrate that so-called physiologic cord clamping is associated with a better outcome. In this procedure, the cord is not clamped until the child has taken his or her first breath, leading to a more stable hemodynamic state [28], [29]. If cord clamping occurs after ventilation is initiated, the traditionally observed increase in carotid artery pressure and cerebral blood flow is greatly mitigated, while pulmonary blood flow is increased, leading to improved hemodynamic stability [28], [30].
A study from Nepal in newborn infants between 34 and 41 weeks of GA showed that in infants not in need of ventilation at birth, the oxygen saturation was 18% higher at 1min, 13% higher at 5min and 10% higher at 10min in babies who had cord clamping delayed by 180 s compared to the early clamping group. The heart rate was 9 and 3 beats lower at 1and 5min, respectively, in the delayed group compared to the early group. Time to first breath and regular breathing was established earlier in babies who had cord clamping at 180s or more [31]. A study by Katheria et al. demonstrated the feasibility of resuscitation with an intact cord; however, the benefits of this procedure remain to be proven [32]. In babies who required resuscitation at birth, a significantly higher oxygen saturation (SpO2) (90.4% vs. 85.4%) was demonstrated 10 min after birth in the intact cord group compared to the early cord clamping group. The heart rate was lower in the intact cord group at 1 and 5 min and slightly higher at 10 min (all significant findings). Apgar scores were higher at 1, 5 and 10 min [33]. Special trollies are now available to be put beside the mother’s bed enabling resuscitation with an intact cord [34].
Finally, a study from Vain et al. indicates that the position of the baby in relation to the placenta has no or minimal effect on the volume of blood transfused from the placenta to the neonate after vaginal deliveries. This means the newborn can be quickly and safely placed on the mother’s abdomen to provide skin contact even with an intact cord [35]. More studies on this topic are needed [36].
Heart rate assessment
In both term and preterm delivery, heart rate is the most important clinical parameter in the assessment of the infant immediately after birth. The infant’s pulse rate identifies those in need of resuscitation and indicates their response to treatment efforts [8]. A recent observational study by Kapadia et al. in preterm infants <32 weeks of GA showed that neonates who did not reach a heart rate of 100 bpm by 5 min of life were at an increased risk of death. In addition, the association between the duration of bradycardia at birth and mortality was almost linear with 5% mortality if bradycardia occurred at 1 min vs. 20% if it lasted the first 5 min of life [37].
There has been considerable discussion concerning which method should be used to best evaluate heart rate. Auscultation and umbilical palpation are methods of heart rate assessment that are inexpensive, independent of technology and easy to perform. Auscultation offers the added advantage of assessing both heart rate and ventilation with one measure. However, in the setting of newborn resuscitation, both auscultation and umbilical palpation have been shown to be inaccurate and liable to disturbance, reporting a lower heart rate compared to electrocardiogram (ECG) [38]. Pulse oximetry has the advantage of measuring saturation and pulse rate both simultaneously and continuously; however, it is sensitive to poor tissue perfusion and may underestimate the heart rate in the first minutes of life [39].
ECG monitoring in the delivery room is achievable in both preterm and term infants, and displays heart rate earlier and more accurately than pulse oximetry [40], [41], [42], [43]. At present, ECG electrodes are not universally available in delivery rooms, and there may be limitations to the use of ECG in extremely preterm infants due to the fragility of their skin. Recent updates in international resuscitation guidelines emphasize that although the use of 3-lead ECG may be useful for rapid and accurate heart rate measurement, pulse oximetry is still necessary in order to evaluate oxygenation and titrate oxygen supplementation [8].
New ECG devices have been developed that allow rapid placement of ECG sensors over the abdomen. The device gives the first signal after 6 s; however, 38% of the signals were invalid [44]. Various other new technologies for heart rate measurement employing digital technology such as phonocardiography and wireless or wearable sensors have also been proposed, yet so far translation into routine clinical use has not occurred [38]. Until more evidence is available, we suggest that auscultation is still the gold standard for heart rate assessment at birth [45].
Suctioning
According to ILCOR 2010, routine intrapartum oropharyngeal and nasopharyngeal suctioning for infants born with clear or meconium-stained amniotic fluid is no longer recommended [2]. In fact, it has been demonstrated that routine oropharyngeal suctioning of term infants born vaginally significantly reduces oxygen saturation for the first 6 min after birth [46]. Suctioning may activate the vagus nerve and induce bradycardia and apnea. It may also inflict pain and cause lesions in the mucosa, increasing risk of infections. Routine suctioning is therefore not recommended. Wiping the face, nose and mouth with a towel in most cases is sufficient, provided there is no airway obstruction [47]. If suctioning is required based on clinical assessment, always suction the mouth before the nose (m before n) to reduce the risk of aspiration. The Neonatal Resuscitation Program (NRP) guidelines recommend clearing the airway with a bulb syringe or a suction catheter if airway obstruction is evident or positive ventilation is required [13]. If suctioning is required and done mechanically, the negative pressure should be limited to 100 mm Hg [13]. In addition, suctioning the stomach is not needed routinely after C-section despite previous widespread practice. This practice opens the esophagus leading to more air leaks into the stomach when positive pressure ventilation with bag and mask is needed.
Stimulation
Neonatal resuscitation guidelines universally state that tactile stimulation should be the initial step to help establish a regular breathing pattern in term and preterm babies after birth [8], [48]. However, the concept of stimulation is poorly defined, and there is very little data to guide clinicians with respect to how this intervention is best performed. Rigorous evaluations of its effectiveness are also lacking but it has been speculated that in a low-resource setting, stimulation alone may reduce perinatal mortality by 10% [49].
Preterm babies are reported to receive tactile stimulation less frequently than term babies; however, clinical practice varies widely between centers, particularly in terms of how stimulation is provided [50], [51]. Factors potentially influencing the use of tactile stimulation in preterm stabilization include focus on early respiratory support, the recommendation to place babies within polyethylene bags without drying and the concern for the fragility of more immature babies’ skin. A small, recently published randomized study compared repetitive stimulation of the soles of the feet of preterm babies (GA 27–32 weeks) with standard care (tactile stimulation at the discretion of the physician). Compared to standard care, babies receiving repetitive stimulation had a non-significant increase in respiratory minute volume after birth, and significantly better oxygenation at a lower FiO2 at the time of transfer to the neonatal intensive care unit (NICU) [52]. Further studies are needed to elucidate the potential benefit of tactile stimulation on preterm ventilation after birth.
Ventilation practices within delivery room resuscitation
Approximately 3–5% of newborns require assisted ventilation at the time of birth and can become severely depressed unless effective ventilation is provided rapidly [53]. Ventilation can be adequately provided through several modes of delivery. Various studies in preterm neonates have identified early continuous positive airway pressure (CPAP) use in the immediate postnatal period as beneficial in decreasing the need for and/or the duration of mechanical ventilation and the need for surfactant without evidence of worsening bronchopulmonary dysplasia (BPD) [54], [55], [56], [57]. In a Cochrane review meta-analysis, prophylactic CPAP was compared with supportive care (defined as oxygen therapy delivered by head box or standard nasal cannula) or mechanical ventilation. When comparing prophylactic CPAP with mechanical ventilation, there was moderate evidence to suggest a statistically significant and clinically important reduction in BPD development. Prophylactic CPAP was also shown to decrease the need for mechanical ventilation and the need for surfactant [56]. More recent studies have also shown that early CPAP in the delivery room decreases the need for intubation and is associated with fewer days on mechanical ventilation, fewer surfactant doses, fewer resuscitations and lower oxygen needs [54], [57]. The European Resuscitation Guidelines reiterate that the ideal CPAP level is unknown but that the majority of studies use levels between 6 cm and 9 cm H2O [48]. The textbook of neonatal resuscitation recommends using a CPAP level of 5–6 cm H2O initially [13].
Additional studies evaluated the effectiveness of sustained lung inflation with CPAP vs. intermittent lung inflation with bag mask ventilation in the immediate post-natal period with mixed results. While one study found that sustained lung inflation is more effective for improvement of short-term respiratory outcomes [58], a meta-analysis of eight separate trials demonstrated that sustained lung inflation in preterm infants was not superior to intermittent ventilation for reducing mortality in the delivery room or during hospitalization, although it was associated with a decrease in the duration of mechanical ventilation [59]. At this time, sustained lung inflation should only be attempted in clinical trials as further studies are needed to determine its safety profile [48].
An additional Cochrane review comparing neonatal resuscitation using laryngeal mask airway (LMA) vs. bag mask ventilation and endotracheal intubation identified LMA as effective in obtaining adequate ventilation within the time frame consistent with current resuscitation guidelines in infants greater than 34 weeks of GA or birth weight ≥1500 g. LMA was associated with a reduced need for endotracheal intubation over bag mask ventilation. Newborns resuscitated with LMA were less likely to require admission to the NICU. There is lack of evidence to support the use of LMA in more premature infants [53].
Additionally, there are multiple interfaces available for use when non-invasive respiratory support is required during stabilization. When providing CPAP, the T-piece resuscitator is superior to the self-inflating bag because it allows for measurement of PEEP delivery. Oxygen delivery (heated and humidified) can be provided by both high-flow nasal cannula (HFNC) and CPAP, but HFNC has been associated with higher rates of respiratory failure requiring escalation in support to CPAP to avoid intubation [48]. A recent Cochrane meta-analysis reviewed HFNC vs. CPAP as well as HFNC vs. non-invasive positive pressure ventilation (NIPPV) as primary support after birth. There was no difference in treatment failure in the HFNC vs. CPAP group, but the studies included had small numbers of infants and no extremely preterm infants (<28 weeks). In the second analysis of HFNC vs. NIPPV, infants receiving HFNC remained on non-invasive support for a longer time period [60].
Oxygenation in the delivery room
Resuscitation of term and late preterm infants with room air compared to 100% oxygen reduces mortality [61], [62]. In 2010, ILCOR recommended that term or near-term infants in need of artificial ventilation in the delivery room should be given room air instead of 100% O2 [2]. These recommendations were confirmed in 2015 [8]. For premature infants, the question is more challenging. Recent meta-analyses have shown that for infants with GA between 28 and 31 weeks, an initial FiO2 of 21–30% is appropriate. For infants <28 weeks, supplementing oxygen (for instance 30%) is recommended [63], [64], [65]. The Torpido trial showed increased mortality for premature babies <28 weeks of GA resuscitated with room air compared to 100% O2 [66]. For all infants, FiO2 should be titrated based on their SpO2. However, the optimal development of SpO2 in the first few minutes of life is not known. Very recent data have shown that babies <32 weeks of GA who do not reach an SpO2 of 80% within 5 min of life have an increased risk of death and severe IVH, with significantly reduced cognitive and motor scores at 2 years of age [67]. Although it is presently not known what that cause and effect relationship is, it is recommended that an SpO2 of 80% be reached within 5 min of birth [65].
Surfactant in delivery room
There are several considerations regarding surfactant therapy, including optimal timing and mode of delivery as well as the best surfactant preparation. Today, in the era of antenatal steroids, it has been established that prophylactic intubation and surfactant instillation for all babies increases the risk of BPD [68]. A Cochrane review summarized evidence for early rescue therapy vs. the INSURE (intubation surfactant extubation) technique [69]. There was no difference in mortality between the two methods. However, the need for ventilation and the development of air leaks and BPD at 28 days were significantly reduced by the INSURE method. In addition, the need for surfactant was decreased.
Recently, surfactant has been introduced by new techniques such as the less-invasive surfactant application (LISA) or the minimal surfactant therapy (MIST) [70], [71], [72]. A meta-analysis summarizing five studies showed that LISA is superior to intubation or INSURE at reducing the need for ventilation and decreasing rates of BPD. Death and/or BPD at 36 weeks was also reduced by 25% [73]. If surfactant is required, earlier application will likely result in better outcomes. The recently updated European guidelines state that natural surfactants are broadly equivalent and should be used [48]; however, promising clinical data using synthetic surfactant with surfactant protein B and C analogs have been recently published [74], [75]. Hopefully, such compounds will replace natural surfactants, resulting in reduced cost and decreased need for animal-derived products.
Thermal control
The aim of thermal control in the delivery room should be to maintain a rectal temperature between 36.5 and 37.5°C [76]. All newborns, but particularly preterm babies, are vulnerable to hypothermia after birth [77]. Low admission temperature is a predictor of poor outcome across all GAs, yet it is not known whether hypothermia is causally related to patient outcomes or simply a marker of disease severity [8]. In asphyxiated infants, avoiding hyperthermia is of particular concern, as elevated temperatures have been shown to be associated with increased risk of death or disability in these babies [78].
Various strategies exist to prevent hypothermia in newborns, and these are frequently used in combination, making the specific contribution of each element difficult to ascertain. Standard measures to prevent heat loss typically include drying and wrapping the baby in pre-warmed towels, and, if available, the placement of the infant under a radiant warmer [79]. While ensuring an adequate ambient temperature in the delivery room is recommended by international guidelines, it may be difficult to achieve in practice [8], [80]. In babies delivered by cesarean section, increasing the temperature in the operating room from 20 to 23°C has been shown to significantly reduce the proportion of hypothermic infants after birth [81].
Adequate thermal control in preterm and low-birth-weight infants requires additional interventions, particularly in the most premature infants. The benefit of using occlusive plastic wrapping or bags in conserving heat loss by evaporation has been demonstrated in a number of studies, yet there is insufficient evidence to support an impact on long-term outcomes [79]. Skin-to-skin contact between mother and child immediately after birth has been shown to reduce the risk of hypothermia compared to using conventional incubators, and may have the added benefit of increased physiological stability for the child [82]. Other authors have demonstrated that skin-to-skin can be safely used even in extremely preterm infants, and more studies of kangaroo mother care in the delivery room setting are currently underway [83], [84]. Reducing heat loss through the respiratory system may be another important strategy in improving thermal control in preterm babies. A recent meta-analysis showed that initiating respiratory support in preterm infants with heated, humidified gas instead of cold dry gas significantly decreases the proportion of hypothermic infants upon admission [85].
Discussion
Optimal delivery room handling is of great importance for neonatal outcomes. Figure 1 summarizes some of the factors that may be particularly significant. The handling of the baby during the first few minutes of life in the delivery room may have consequences for the newborn and the family potentially affecting them the rest of their lives. An evidence-based approach therefore is of utmost importance. Since the first oxygen trials were carried out in the delivery room more than 25 years ago [86], [87], a number of other issues related to newborn care at the time of birth have been studied in detail. In fact, delivery room handling research has exploded in recent decades [88], and almost all issues summarized in this article have recently been assessed by randomized studies.
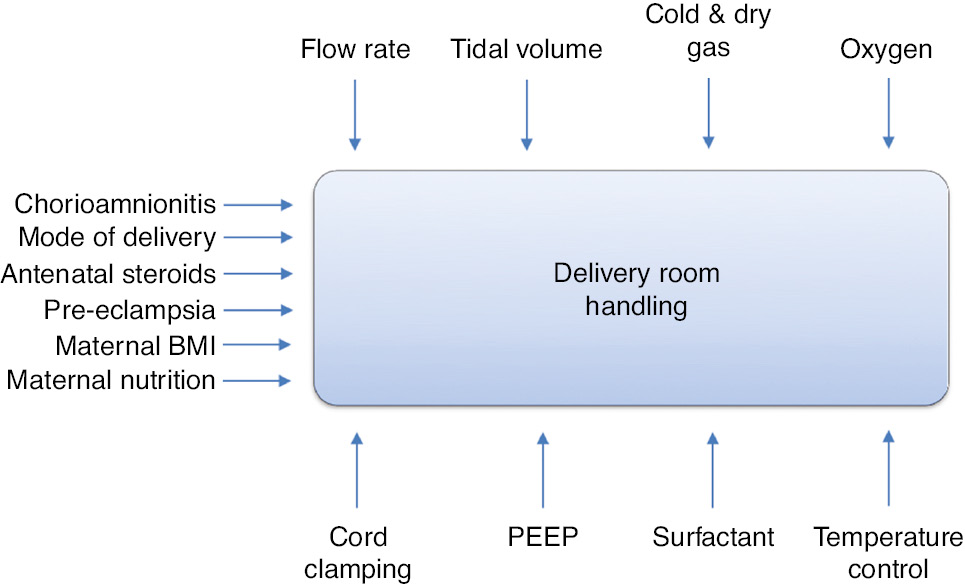
Factors influencing the Golden Minute(s) and outcome of delivery room handling.
The recently updated European guidelines [48] for delivery room management of preterm infants at risk for respiratory distress syndrome emphasize many of the points discussed earlier. This includes delayed cord clamping for at least 60 s, utilizing a blender to control the fraction of inspired oxygen with initial use of 30% FiO2 for babies <28 weeks’ gestation, and 21–30% for those at 28–31 weeks with adjustments guided by pulse oximetry. In spontaneously breathing babies, stabilization with CPAP of 6–9 cm H2O via mask or nasal prongs is emphasized without the use of sustained inflation. Intubation should be reserved for babies who do not respond to positive pressure ventilation via face mask. Indications for administration of surfactant therapy have changed since the turn of the millennium. Thermal control is emphasized with recommendations to apply plastic bags or occlusive wrapping under a radiant warmer during stabilization in the delivery suite for babies <28 weeks’ gestation to reduce the risk of hypothermia. The importance of checklists for the resuscitation procedure, teaching and monitoring of response to resuscitation should be emphasized as well.
And finally, while clinical guidelines provide some clarity on appropriate delivery room interventions for term and preterm infants, future research needs to focus on areas where we still lack adequate knowledge to guide resuscitation decisions. This includes intact cord resuscitation, synthetic surfactant use, development of new devices to assist in delivery room management, and further defining goal oxygenation targets and their timing for preterm neonates, to name a few.
Correction Note
Correction added after online publication December 21, 2019: Mistakenly this article was previously published online ahead of print containing a wrong name for 1 author: Asta Maria Lang. The correct name is: Astri Maria Lang.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work has received support from NIH grant K08HL124295.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Vento M, Cheung PY, Aguar M. The first golden minutes of the extremely-low-gestational-age neonate: a gentle approach. Neonatology 2009;95:286–98.10.1159/000178770Search in Google Scholar PubMed
2. Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2010;122:S516–38.10.1161/CIRCULATIONAHA.110.971127Search in Google Scholar PubMed
3. Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology 2015;107:365–71.10.1159/000381159Search in Google Scholar PubMed
4. McCarthy LK, Morley CJ, Davis PG, Kamlin CO, O’Donnell CP. Timing of interventions in the delivery room: does reality compare with neonatal resuscitation guidelines? J Pediatr 2013;163:1553–7.e1.10.1016/j.jpeds.2013.06.007Search in Google Scholar PubMed
5. Aziz K, Chadwick M, Baker M, Andrews W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation 2008;79:444–52.10.1016/j.resuscitation.2008.08.004Search in Google Scholar PubMed
6. Berazategui JP, Aguilar A, Escobedo M, Dannaway D, Guinsburg R, de Almeida MF, et al. Risk factors for advanced resuscitation in term and near-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed 2017;102:F44–50.10.1136/archdischild-2015-309525Search in Google Scholar PubMed
7. Mitchell A, Niday P, Boulton J, Chance G, Dulberg C. A prospective clinical audit of neonatal resuscitation practices in Canada. Adv Neonatal Care 2002;2:316–26.10.1053/adnc.2002.36831Search in Google Scholar PubMed
8. Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;132:S204–41.10.1161/CIR.0000000000000276Search in Google Scholar PubMed
9. Niles DE, Cines C, Insley E, Foglia EE, Elci OU, Skare C, et al. Incidence and characteristics of positive pressure ventilation delivered to newborns in a us tertiary academic hospital. Resuscitation 2017;115:102–9.10.1016/j.resuscitation.2017.03.035Search in Google Scholar PubMed
10. Skare C, Boldingh AM, Nakstad B, Calisch TE, Niles DE, Nadkarni VM, et al. Ventilation fraction during the first 30s of neonatal resuscitation. Resuscitation 2016;107:25–30.10.1016/j.resuscitation.2016.07.231Search in Google Scholar PubMed
11. Haumont D, NguyenBa C, Modi N. Enewborn: the information technology revolution and challenges for neonatal networks. Neonatology 2017;111:388–97.10.1159/000464267Search in Google Scholar
12. Sawyer T, Lee HC, Aziz K. Anticipation and preparation for every delivery room resuscitation. Semin Fetal Neonatal Med 2018;23:312–20.10.1016/j.siny.2018.06.004Search in Google Scholar
13. Weiner GM, Zaichkin J, Kattwinkel J, editors. Textbook of neonatal resuscitation, 7th ed. Elk Grove Village, IL: American Academy of Pediatrics, American Heart Association; 2016.10.1542/9781610020251Search in Google Scholar
14. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. J Am Med Assoc 2014;311:1536–46.10.1001/jama.2014.2269Search in Google Scholar
15. Almudeer A, McMillan D, O’Connell C, El-Naggar W. Do we need an intubation-skilled person at all high-risk deliveries? J Pediatr 2016;171:55–9.10.1016/j.jpeds.2015.11.049Search in Google Scholar
16. Alfirevic Z, Milan SJ, Livio S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev 2013;9:CD000078.10.1002/14651858.CD000078.pub2Search in Google Scholar
17. Dypvik J, Larsen S, Haavaldsen C, Saugstad OD, Eskild A. Placental weight and risk of neonatal death. JAMA Pediatr 2019. DOI: 10.1001/jamapediatrics.2019.4556.Search in Google Scholar
18. Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet 1969;2:871–3.10.1016/S0140-6736(69)92328-9Search in Google Scholar
19. Katheria AC, Lakshminrusimha S, Rabe H, McAdams R, Mercer JS. Placental transfusion: a review. J Perinatol 2017;37:105–11.10.1038/jp.2016.151Search in Google Scholar PubMed PubMed Central
20. McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evid Based Child Health 2014;9:303–97.10.1002/ebch.1971Search in Google Scholar PubMed
21. Rana N, Ranneberg LJ, Malqvist M, Kc A, Andersson O. Delayed cord clamping was not associated with an increased risk of hyperbilirubinaemia on the day of birth or jaundice in the first 4 weeks. Acta Paediatr 2019;00:1–7.10.1111/apa.14913Search in Google Scholar PubMed
22. Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med 2017;377:2445–55.10.1056/NEJMoa1711281Search in Google Scholar PubMed
23. Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol 2018;218:1–18.10.1016/j.ajog.2017.10.231Search in Google Scholar PubMed
24. Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012;9:CD003248.10.1002/14651858.CD003248.pub3Search in Google Scholar PubMed
25. Katheria AC. Umbilical cord milking: a review. Front Pediatr 2018;6:335.10.3389/fped.2018.00335Search in Google Scholar PubMed PubMed Central
26. Katheria A, Garey D, Truong G, Akshoomoff N, Steen J, Maldonado M, et al. A randomized clinical trial of umbilical cord milking vs delayed cord clamping in preterm infants: neurodevelopmental outcomes at 22–26 months of corrected age. J Pediatr 2018;194:76–80.10.1016/j.jpeds.2017.10.037Search in Google Scholar PubMed
27. Katheria A, Reister F, Hummler H, Essers J, Mendler M, Truong GST, et al. Pediatric Academic Societies; April 28, 2019; Baltimore, MD, 2019.Search in Google Scholar
28. Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol 2013;591:2113–26.10.1113/jphysiol.2012.250084Search in Google Scholar PubMed PubMed Central
29. Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed 2015;100:F355–60.10.1136/archdischild-2013-305703Search in Google Scholar PubMed
30. Polglase GR, Dawson JA, Kluckow M, Gill AW, Davis PG, Te Pas AB, et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS One 2015;10:e0117504.10.1371/journal.pone.0117504Search in Google Scholar PubMed PubMed Central
31. Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth - randomized clinical trial. Matern Health Neonatol Perinatol 2019;5:7.10.1186/s40748-019-0103-ySearch in Google Scholar PubMed PubMed Central
32. Katheria AC. Neonatal resuscitation with an intact cord: current and ongoing trials. Children (Basel) 2019;6:60.10.3390/children6040060Search in Google Scholar
33. Andersson O, Rana N, Ewald U, Malqvist M, Stripple G, Basnet O, et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (nepcord iii) – a randomized clinical trial. Matern Health Neonatol Perinatol 2019;5:15.10.1186/s40748-019-0110-zSearch in Google Scholar
34. Hutchon D, Bettles N. Motherside care of the term neonate at birth. Matern Health Neonatol Perinatol 2016;2:5.10.1186/s40748-016-0034-9Search in Google Scholar
35. Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet 2014;384:235–40.10.1016/S0140-6736(14)60197-5Search in Google Scholar
36. Raju TN. Delayed cord clamping: does gravity matter? Lancet 2014;384:213–4.10.1016/S0140-6736(14)60411-6Search in Google Scholar
37. Kapadia V, Oei JL, Saugstad OD, Rabi Y, Finer NN, Tarnow-Mordi W, et al. Bradyprem study: heart rate is most vital of vital signs during resuscitation of preterms. Toronto, Canada: Pediatric Academic Societies; 2018.Search in Google Scholar
38. Phillipos E, Solevag AL, Pichler G, Aziz K, van Os S, O’Reilly M, et al. Heart rate assessment immediately after birth. Neonatology 2016;109:130–8.10.1159/000441940Search in Google Scholar PubMed
39. van Vonderen JJ, Hooper SB, Kroese JK, Roest AA, Narayen IC, van Zwet EW, et al. Pulse oximetry measures a lower heart rate at birth compared with electrocardiography. J Pediatr 2015;166:49–53.10.1016/j.jpeds.2014.09.015Search in Google Scholar PubMed
40. Katheria A, Rich W, Finer N. Electrocardiogram provides a continuous heart rate faster than oximetry during neonatal resuscitation. Pediatrics 2012;130:e1177–81.10.1542/peds.2012-0784Search in Google Scholar PubMed
41. Mizumoto H, Tomotaki S, Shibata H, Ueda K, Akashi R, Uchio H, et al. Electrocardiogram shows reliable heart rates much earlier than pulse oximetry during neonatal resuscitation. Pediatr Int 2012;54:205–7.10.1111/j.1442-200X.2011.03506.xSearch in Google Scholar PubMed
42. Kamlin CO, Dawson JA, O’Donnell CP, Morley CJ, Donath SM, Sekhon J, et al. Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J Pediatr 2008;152:756–60.10.1016/j.jpeds.2008.01.002Search in Google Scholar PubMed
43. Iglesias B, Rodriguez MJ, Aleo E, Criado E, Herranz G, Moro M, et al. Pulse oximetry versus electrocardiogram for heart rate assessment during resuscitation of the preterm infant. An Pediatr 2016;84:271–7.10.1016/j.anpede.2015.08.017Search in Google Scholar
44. Linde JE, Schulz J, Perlman JM, Oymar K, Francis F, Eilevstjonn J, et al. Normal newborn heart rate in the first five minutes of life assessed by dry-electrode electrocardiography. Neonatology 2016;110:231–7.10.1159/000445930Search in Google Scholar
45. Saugstad OD, Soll RF. Assessing heart rate at birth: auscultation is still the gold standard. Neonatology 2016;110:238–40.10.1159/000446527Search in Google Scholar
46. Carrasco M, Martell M, Estol PC. Oronasopharyngeal suction at birth: effects on arterial oxygen saturation. J Pediatr 1997;130:832–4.10.1016/S0022-3476(97)80031-5Search in Google Scholar
47. Kelleher J, Bhat R, Salas AA, Addis D, Mills EC, Mallick H, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet 2013;382:326–30.10.1016/S0140-6736(13)60775-8Search in Google Scholar
48. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 2019;115:432–50.10.1159/000499361Search in Google Scholar PubMed PubMed Central
49. Lee AC, Cousens S, Wall SN, Niermeyer S, Darmstadt GL, Carlo WA, et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and delphi estimation of mortality effect. BMC Public Health 2011;11(Suppl 3):S12.10.1186/1471-2458-11-S3-S12Search in Google Scholar PubMed PubMed Central
50. Gaertner VD, Flemmer SA, Lorenz L, Davis PG, Kamlin COF. Physical stimulation of newborn infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 2018;103:F132–6.10.1136/archdischild-2016-312311Search in Google Scholar PubMed
51. van Henten TMA, Dekker J, Te Pas AB, Zivanovic S, Hooper SB, Roehr CC. Tactile stimulation in the delivery room: do we practice what we preach? Arch Dis Child Fetal Neonatal Ed 2019;104:F661–2.10.1136/archdischild-2018-316344Search in Google Scholar PubMed
52. Dekker J, Hooper SB, Martherus T, Cramer SJE, van Geloven N, Te Pas AB. Repetitive versus standard tactile stimulation of preterm infants at birth – a randomized controlled trial. Resuscitation 2018;127:37–43.10.1016/j.resuscitation.2018.03.030Search in Google Scholar PubMed
53. Qureshi MJ, Kumar M. Laryngeal mask airway versus bag-mask ventilation or endotracheal intubation for neonatal resuscitation. Cochrane Database Syst Rev 2018;3:CD003314.10.1002/14651858.CD003314.pub3Search in Google Scholar PubMed PubMed Central
54. Abelenda VLB, Valente TCO, Marinho CL, Lopes AJ. Effects of underwater bubble cpap on very-low-birth-weight preterm newborns in the delivery room and after transport to the neonatal intensive care unit. J Child Health Care 2018;22:216–27.10.1177/1367493517752500Search in Google Scholar PubMed
55. Ramanathan R. Optimal ventilatory strategies and surfactant to protect the preterm lungs. Neonatology 2008;93:302–8.10.1159/000121456Search in Google Scholar PubMed
56. Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev 2016;6:CD001243.10.1002/14651858.CD001243.pub3Search in Google Scholar PubMed
57. Govindaswami B, Nudelman M, Narasimhan SR, Huang A, Misra S, Urquidez G, et al. Eliminating risk of intubation in very preterm infants with noninvasive cardiorespiratory support in the delivery room and neonatal intensive care unit. Biomed Res Int 2019;2019:5984305.10.1155/2019/5984305Search in Google Scholar PubMed PubMed Central
58. El-Chimi MS, Awad HA, El-Gammasy TM, El-Farghali OG, Sallam MT, Shinkar DM. Sustained versus intermittent lung inflation for resuscitation of preterm infants: a randomized controlled trial. J Matern Fetal Neonatal Med 2017;30:1273–8.10.1080/14767058.2016.1210598Search in Google Scholar PubMed
59. Bruschettini M, O’Donnell CP, Davis PG, Morley CJ, Moja L, Zappettini S, et al. Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes. Cochrane Database Syst Rev 2017;7:CD004953.10.1002/14651858.CD004953.pub3Search in Google Scholar PubMed PubMed Central
60. Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev 2016;2:CD006405.10.1002/14651858.CD006405.pub3Search in Google Scholar PubMed
61. Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008;94:176–82.10.1159/000143397Search in Google Scholar PubMed
62. Welsford M, Nishiyama C, Shortt C, Isayama T, Dawson JA, Weiner G, et al. Room air for initiating term newborn resuscitation: a systematic review with meta-analysis. Pediatrics 2019;143:e20181828.10.1542/peds.2018-1825Search in Google Scholar PubMed
63. Oei JL, Vento M, Rabi Y, Wright I, Finer N, Rich W, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;102:F24–30.10.1136/archdischild-2016-310435Search in Google Scholar PubMed
64. Castillo M, Tehranzadeh J, Becerra J, Mnaymneh W. Case report 408: malignant fibrous histiocytoma of innominate bones and femur (multicentric). Skeletal Radiol 1987;16:74–7.10.1007/BF00349934Search in Google Scholar PubMed
65. Oei JL, Saugstad OD, Vento M. Oxygen and preterm infant resuscitation: what else do we need to know? Curr Opin Pediatr 2018;30:192–8.10.1097/MOP.0000000000000610Search in Google Scholar PubMed
66. Oei JL, Saugstad OD, Lui K, Wright IM, Smyth JP, Craven P, et al. Targeted oxygen in the resuscitation of preterm infants, a randomized clinical trial. Pediatrics 2017;139:e20161452.10.1542/peds.2016-1452Search in Google Scholar PubMed
67. Thamrin V, Saugstad OD, Tarnow-Mordi W, Wang YA, Lui K, Wright IM, et al. Preterm infant outcomes after randomization to initial resuscitation with fio2 0.21 or 1.0. J Pediatr 2018;201:55–61.e1.10.1016/j.jpeds.2018.05.053Search in Google Scholar PubMed
68. Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012;3:CD000510.10.1002/14651858.CD000510.pub2Search in Google Scholar PubMed
69. Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. Selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007;4:CD003063.10.1002/14651858.CD003063.pub2Search in Google Scholar PubMed
70. Klebermass-Schrehof K, Wald M, Schwindt J, Grill A, Prusa AR, Haiden N, et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 2013;103:252–8.10.1159/000346521Search in Google Scholar PubMed
71. Kribs A. Minimally invasive surfactant therapy and noninvasive respiratory support. Clin Perinatol 2016;43:755–71.10.1016/j.clp.2016.07.010Search in Google Scholar PubMed
72. Dargaville PA, Ali SKM, Jackson HD, Williams C, De Paoli AG. Impact of minimally invasive surfactant therapy in preterm infants at 29–32 weeks gestation. Neonatology 2018;113:7–14.10.1159/000480066Search in Google Scholar PubMed
73. Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;102:F17–23.10.1136/archdischild-2015-310299Search in Google Scholar PubMed
74. Johansson J, Curstedt T. Synthetic surfactants with sp-b and sp-c analogues to enable worldwide treatment of neonatal respiratory distress syndrome and other lung diseases. J Intern Med 2019;285:165–86.10.1111/joim.12845Search in Google Scholar PubMed
75. Sweet DG, Turner MA, Stranak Z, Plavka R, Clarke P, Stenson BJ, et al. A first-in-human clinical study of a new sp-b and sp-c enriched synthetic surfactant (chf5633) in preterm babies with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed 2017;102:F497–503.10.1136/archdischild-2017-312722Search in Google Scholar PubMed PubMed Central
76. Trevisanuto D, Testoni D, de Almeida MFB. Maintaining normothermia: why and how? Semin Fetal Neonatal Med 2018;23:333–9.10.1016/j.siny.2018.03.009Search in Google Scholar PubMed
77. NICU by the numbers: despite decreases, nearly 4 in 10 infants are cold when admitted to nicu.: Vermont Oxford Network; 2017 [Available from: https://public.vtoxford.org/nicu-by-the-numbers/despite-decreases-nearly-4-in-10-infants-are-cold-when-admitted-to-the-nicu/.Search in Google Scholar
78. Laptook A, Tyson J, Shankaran S, McDonald S, Ehrenkranz R, Fanaroff A, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics 2008;122:491–9.10.1542/peds.2007-1673Search in Google Scholar PubMed PubMed Central
79. McCall EM, Alderdice F, Halliday HL, Vohra S, Johnston L. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2018;2:Cd004210.10.1002/14651858.CD004210.pub5Search in Google Scholar PubMed PubMed Central
80. Reilly MC, Vohra S, Rac VE, Dunn M, Ferrelli K, Kiss A, et al. Randomized trial of occlusive wrap for heat loss prevention in preterm infants. J Pediatr 2015;166:262–8.e2.10.1016/j.jpeds.2014.09.068Search in Google Scholar PubMed
81. Duryea EL, Nelson DB, Wyckoff MH, Grant EN, Tao W, Sadana N, et al. The impact of ambient operating room temperature on neonatal and maternal hypothermia and associated morbidities: a randomized controlled trial. Am J Obstet Gynecol 2016;214:505.e1–7.10.1016/j.ajog.2016.01.190Search in Google Scholar PubMed
82. Bergman NJ, Linley LL, Fawcus SR. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr 2004;93:779–85.10.1111/j.1651-2227.2004.tb03018.xSearch in Google Scholar PubMed
83. Kristoffersen L, Stoen R, Rygh H, Sognnaes M, Follestad T, Mohn HS, et al. Early skin-to-skin contact or incubator for very preterm infants: study protocol for a randomized controlled trial. Trials 2016;17:593.10.1186/s13063-016-1730-5Search in Google Scholar PubMed PubMed Central
84. Karlsson V, Heinemann AB, Sjors G, Nykvist KH, Agren J. Early skin-to-skin care in extremely preterm infants: thermal balance and care environment. J Pediatr 2012;161:422–6.10.1016/j.jpeds.2012.02.034Search in Google Scholar PubMed
85. Meyer MP, Owen LS, Te Pas AB. Use of heated humidified gases for early stabilization of preterm infants: a meta-analysis. Front Pediatr 2018;6:319.10.3389/fped.2018.00319Search in Google Scholar PubMed PubMed Central
86. Ramji S, Ahuja S, Thirupuram S, Rootwelt T, Rooth G, Saugstad OD. Resuscitation of asphyxic newborn infants with room air or 100% oxygen. Pediatr Res 1993;34:809–12.10.1203/00006450-199312000-00023Search in Google Scholar PubMed
87. Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the resair 2 study. Pediatrics 1998;102:e1.10.1542/peds.102.1.e1Search in Google Scholar PubMed
88. Morley CJ. Monitoring neonatal resuscitation: why is it needed? Neonatology 2018;113:387–92.10.1159/000487614Search in Google Scholar PubMed
©2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- 10.1515/jpm-2020-frontmatter1
- Review
- Delivery room handling of the newborn
- Original Articles – Obstetrics
- Examining the validity of a predictive model for vaginal birth after cesarean
- Correlation between endometrial thickness and perinatal outcome for pregnancies achieved through assisted reproduction technology
- Significance of the routine first-trimester antenatal screening program for aneuploidy in the assessment of the risk of placenta accreta spectrum disorders
- A decade’s experience in primipara, term, singleton, vertex parturients with a sustained low rate of CD
- Survey of alongside midwifery-led care in North Rhine-Westfalia, Germany
- Correlation between aneuploidy pregnancy and the concentration of various hormones and vascular endothelial factor in follicular fluid as well as the number of acquired oocytes
- Bacteriuria in pregnancy varies with the ambiance: a retrospective observational study at a tertiary hospital in Doha, Qatar
- Microarray findings in pregnancies with oligohydramnios – a retrospective cohort study and literature review
- Lifestyle characteristics of parental electronic cigarette and marijuana users: healthy or not?
- Influence of maternal HIV infection on fetal thymus size
- Original Articles – Fetus
- Clinical outcome of prenatally suspected cardiac rhabdomyomas of the fetus
- Original Articles – Newborns
- Prolonged ventilation and postnatal growth of preterm infants
- Letter to the Editor
- Neonatal sepsis associated with Lactobacillus supplementation
Articles in the same Issue
- 10.1515/jpm-2020-frontmatter1
- Review
- Delivery room handling of the newborn
- Original Articles – Obstetrics
- Examining the validity of a predictive model for vaginal birth after cesarean
- Correlation between endometrial thickness and perinatal outcome for pregnancies achieved through assisted reproduction technology
- Significance of the routine first-trimester antenatal screening program for aneuploidy in the assessment of the risk of placenta accreta spectrum disorders
- A decade’s experience in primipara, term, singleton, vertex parturients with a sustained low rate of CD
- Survey of alongside midwifery-led care in North Rhine-Westfalia, Germany
- Correlation between aneuploidy pregnancy and the concentration of various hormones and vascular endothelial factor in follicular fluid as well as the number of acquired oocytes
- Bacteriuria in pregnancy varies with the ambiance: a retrospective observational study at a tertiary hospital in Doha, Qatar
- Microarray findings in pregnancies with oligohydramnios – a retrospective cohort study and literature review
- Lifestyle characteristics of parental electronic cigarette and marijuana users: healthy or not?
- Influence of maternal HIV infection on fetal thymus size
- Original Articles – Fetus
- Clinical outcome of prenatally suspected cardiac rhabdomyomas of the fetus
- Original Articles – Newborns
- Prolonged ventilation and postnatal growth of preterm infants
- Letter to the Editor
- Neonatal sepsis associated with Lactobacillus supplementation

