Abstract
This work presents a study focused on the development of a simple useful tool to predict the generation of trihalomethanes in drinking water purification systems, using two precursors and trichloromethane as model compounds through a simple chlorine decay model. This work proposed a semiempirical model without adjustable power parameters where fast and slow stages and the effect of pH were included. Despite that the model is not based in a complete kinetic scheme, using the proposed equations it is possible to predict the simultaneous evolution of chlorine and TCM with a set of linear kinetics parameters which characterize the system and will be obtained using simple routine laboratory measurements. The results show that both TCM formation and chlorine decay are strongly dependent on the chemical nature of the model precursor. Although resorcinol and phenol have different reactivity with chlorine and represent different functional groups which are present in natural compounds, the TCM generation appears to be properly described in both cases by the total chlorine consumption. Considering that during the potabilization processes the pH changes, the study of the effects of this variable is very important to achieve the minimization of THMs generation. The pH has a significant effect on the time evolution of chlorine-substituted hydroxybenzene intermediates and therefore on the TCM formation, since the properties of the reacting species are directly affected by the reaction medium for their participation in the different reaction paths. The study of the distribution and selectivity of the intermediate species allowed explaining the results obtained for the kinetics of formation of TCM. The results suggest that in order to understand the effect of pH, the nature of oxidation of HOCl and ClO‒, should be considered simultaneously with the electronegative nature of the precursor compounds. Finally, in terms of minimizing the generation of THM it is important to consider the potential impact of pH changes within the water treatment process and supply and the stages where chlorination may be carried out.
Acknowledgements
Thanks are given to Universidad Nacional del Litoral and CONICET for financial help and the doctoral fellowships of M.B.G. The technical assistance of Silvina Addona and Juan C. Andini is gratefully appreciated.
Notation
| TCM | Trichloromethane |
| THM | Trihalomethane |
| NOM | Natural organic matter |
| R | Resorcinol |
| P | Phenol |
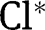 | Total concentration of chlorine oxidative species (HOCl+OCl–) |
| ClPs | Chloro-phenol intermediates |
| ClRs | Chloro-resorcinol intermediates |
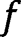 | Fraction of the 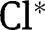 attributed to the rapid reaction attributed to the rapid reaction |
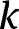 | Apparent rate constant |
| MCP | 2-monochlorophenol plus 4-monochlorophenol |
| DCP | 2,6-dichlorophenol plus 2,4-dichlorophenol |
| TCP | 2,4,6-trichlorophenol |
| TPs | Phenol plus Chloro-phenol intermediates |
| Greek Letters | |
| η | virtual “yield” of 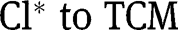 |
| Subscripts | |
| TCM | Relative to trichloromethane |
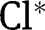 | Relative to total concentration of chlorine oxidative species (HOCl+OCl–) |
| r | Denotes rapid reaction |
| s | Denotes slow reaction |
| Superscripts | |
| 0 | Represents initial condition |
References
1. Rook JJ. Formation of haloforms during chlorination of natural waters. Water Treatment Examination 1974;23:234–43.Search in Google Scholar
2. Rook JJ. Chlorination reactions of fulvic acids in natural waters. Environ Sci Technol 1977;11:478–82.10.1021/es60128a014Search in Google Scholar
3. Rebenne LM, Gonzalez AC, Olson TM. Aqueous chlorination kinetics and mechanism of substituted dihydroxybenzenes. Environ Sci Technol 1996;30:2235–42.10.1021/es950607tSearch in Google Scholar
4. Arnold WA, Bolotin J, Gunten UV, Hofstetter T. Evaluation of functional groups responsible for chloroform formation during water chlorination using compound specific isotope analysis. Environ Sci Technol 2008;42:7778–85.10.1021/es800399aSearch in Google Scholar
5. Cimetiere N, Dossier-Berne F, De Laat J. Monochloramination of resorcinol: mechanism and kinetic modeling. Environ Sci Technol 2009;43:9380–5.10.1021/es901425nSearch in Google Scholar
6. Korshin GV, Benjamin MM, Chang HS, Gallard H. Examination of NOM chlorination reactions by conventional and stop-flow differential absorbance spectroscopy. Environ Sci Technol 2007;41:2776–81.10.1021/es062268hSearch in Google Scholar
7. Gallard H, von Gunten U. Chlorination of natural organic matter: kinetics of chlorination and of THM formation. Water Res 2002;36:65–74.10.1016/S0043-1354(01)00187-7Search in Google Scholar
8. Clark RM. Chlorine demand and TTHM formation kinetics: a second-order model. J Environ Eng 1998;124:16–24.10.1061/(ASCE)0733-9372(1998)124:1(16)Search in Google Scholar
9. Gang D, Clevenger TE, Banerji SK. Relationship of chlorine decay and THMs formation to NOM size. J Hazardous Mater 2003;96:1–12.10.1016/S0304-3894(02)00164-4Search in Google Scholar
10. Gallard H, von Gunten U. Chlorination of phenols: kinetics and formation of chloroform. Environ Sci Technol 2002;36:884–90.10.1021/es010076aSearch in Google Scholar PubMed
11. Chang EE, Chiang PC, Chao SH, Lin YL. Relationship between chlorine consumption and chlorination by-products formation for model compounds. Chemosphere 2006;64:1196–203.10.1016/j.chemosphere.2005.11.036Search in Google Scholar PubMed
12. Brown D, Bridgeman J, West JR. Predicting chlorine decay and THM formation in water supply systems. Rev Environ Sci Biotechnol 2011;10:79–99.10.1007/s11157-011-9229-8Search in Google Scholar
13. Montgomery DC. Design and analysis of experiments, 7th ed. John Wiley & Sons, Inc., 2009.Search in Google Scholar
14. Gilliard M. Analysis, modeling and experimental verification of the kinetics of trihalomethanes (THMs) formation in water disinfection processes. PhD Thesis, 2012.Search in Google Scholar
15. ASTM D 6520–06. Standard practice for the Solid Phase Micro Extraction (SPME) of water and its headspace for the analysis of volatile and semi-volatile organic compounds, 2006.Search in Google Scholar
16. Hua F, West JR, Barker RA, Foster CF. Modelling of chlorine decay in municipal water supplies. Water Res 1999;33:2735–46.10.1016/S0043-1354(98)00519-3Search in Google Scholar
17. Courtis BJ, West JR, Bridgeman J. Chlorine demand-based predictive modeling of THM formation in water distribution networks. Urban Water J 2009;6:407–15.10.1080/15730620903038461Search in Google Scholar
18. Noack MG, Doerr RL. Reactions of chlorine, chlorine dioxide and mixture thereof with humic acid: an interim report. In: Jolley RL et al., editors, Water chlorination environmental impact and health effects, vol 2. Ann Arbor, MI: Ann Arbor Science, 1978:49–58.Search in Google Scholar
19. Qualls RG, Johnson JD. Kinetics of the short-term consumption of chlorine by fulvic acid. Environ Sci Technol 1983;17:692–8.10.1021/es00117a013Search in Google Scholar PubMed
20. Ge F, Zhu L, Wang J. Distribution of chlorination products of phenols under various pHs in water disinfection. Desalination 2008;225156–66.Search in Google Scholar
21. Lee GF, Morris JC. Kinetics of chlorination of phenol-chlorophenolic tastes and odors, Int. J. Air Wat. Poll. 1962;6:419–31.Search in Google Scholar
©2013 by Walter de Gruyter Berlin / Boston
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- In Honor of Alberto E. Cassano: Researcher, Engineer, and Academic
- Articles
- From Ideal Reactor Concepts to Reality: The Novel Drum Reactor for Photocatalytic Wastewater Treatment
- Synthesis, Characterization, and Comparison of Sol–Gel TiO2 Immobilized Photocatalysts
- Determination of Kinetic Parameter in a Unified Kinetic Model for the Photodegradation of Phenol by Using Nonlinear Regression and the Genetic Algorithm
- Mass Transfer and Conservation from a Finite Source to an Infinite Media
- Modelling and Simulation of Gas–liquid Hydrodynamics in a Rectangular Air-lift Reactor
- Two-Dimensional Modeling of an Externally Irradiated Slurry Photoreactor
- Role of Aspect Ratio and Joule Heating within the Fluid Region Near a Cylindrical Electrode in Electrokinetic Remediation: A Numerical Solution based on the Boundary Layer Model
- Solar Water Disinfection Using NF-codoped TiO2 Photocatalysis: Estimation of Scaling-up Parameters
- A Simple and Semi-Empirical Model to Predict THMs Generation in Water Facilities Including pH Effects
- On the Standardization of the Photocatalytic Gas/Solid Tests
- Microalgae Technology: A Patent Survey
- Influence of Physical and Optical Parameters on 2,4-Dichlorophenol Degradation
- Factors Capable of Modifying the Response of Pseudomonas aeruginosa to the Inactivation Induced by Heterogeneous Photocatalysis
- Enhanced Antibacterial Activity of CeO2 Nanoparticles by Surfactants
- Determination of Photochemical, Electrochemical and Photoelectrochemical Efficiencies in a Photoelectrocatalytic Reactor
- Correlations between Molecular Descriptors from Various Volatile Organic Compounds and Photocatalytic Oxidation Kinetic Constants
- Role of Joule Heating in Electro-Assisted Processes: A Boundary Layer Approach for Rectangular Electrodes
Articles in the same Issue
- Masthead
- Masthead
- Editorial
- In Honor of Alberto E. Cassano: Researcher, Engineer, and Academic
- Articles
- From Ideal Reactor Concepts to Reality: The Novel Drum Reactor for Photocatalytic Wastewater Treatment
- Synthesis, Characterization, and Comparison of Sol–Gel TiO2 Immobilized Photocatalysts
- Determination of Kinetic Parameter in a Unified Kinetic Model for the Photodegradation of Phenol by Using Nonlinear Regression and the Genetic Algorithm
- Mass Transfer and Conservation from a Finite Source to an Infinite Media
- Modelling and Simulation of Gas–liquid Hydrodynamics in a Rectangular Air-lift Reactor
- Two-Dimensional Modeling of an Externally Irradiated Slurry Photoreactor
- Role of Aspect Ratio and Joule Heating within the Fluid Region Near a Cylindrical Electrode in Electrokinetic Remediation: A Numerical Solution based on the Boundary Layer Model
- Solar Water Disinfection Using NF-codoped TiO2 Photocatalysis: Estimation of Scaling-up Parameters
- A Simple and Semi-Empirical Model to Predict THMs Generation in Water Facilities Including pH Effects
- On the Standardization of the Photocatalytic Gas/Solid Tests
- Microalgae Technology: A Patent Survey
- Influence of Physical and Optical Parameters on 2,4-Dichlorophenol Degradation
- Factors Capable of Modifying the Response of Pseudomonas aeruginosa to the Inactivation Induced by Heterogeneous Photocatalysis
- Enhanced Antibacterial Activity of CeO2 Nanoparticles by Surfactants
- Determination of Photochemical, Electrochemical and Photoelectrochemical Efficiencies in a Photoelectrocatalytic Reactor
- Correlations between Molecular Descriptors from Various Volatile Organic Compounds and Photocatalytic Oxidation Kinetic Constants
- Role of Joule Heating in Electro-Assisted Processes: A Boundary Layer Approach for Rectangular Electrodes


