Abstract
A batch cylindrical photocatalytic reactor, externally irradiated by 1–6 UV fluorescent lamps and containing a stirred slurry of polycrystalline TiO2, was modeled by coupling a modified Langmuir–Hinshelwood kinetics together with a two-dimensional light intensity field. The radiation field has been determined on the main assumptions of diffuse radiation, isotropic scattering and negligible backward reflected photon flow. The model has been applied to the photocatalytic oxidation of organic substrates which do not undergo homogeneous photochemical degradation. The model is characterized by the following four parameters: the kinetic constants of substrate adsorption, desorption and degradation and the exponent of the power law expressing the kinetics dependence on the light intensity. The model constants may be determined by applying a simple least-squares best fitting procedure.
Appendix A: Geometrical derivation of r1and r2as a function of θ, r0and R
The coordinate system used was indicated by (x, y) in Figure 6.

Geometric scheme referred to reactor and lamp (grey).
![[28]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq28.png)
![[29]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq29.png)
Substituting eq. [29] in eq. [28]:
![[30]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq30.png)
Developing the square present in eq. [30] and substituting 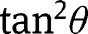 ith
ith 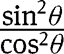 :
:
![[31]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq31.png)
Substituting 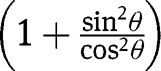 with
with 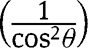 in eq. [31]:
in eq. [31]:
![[32]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq32.png)
By solving eq. [32] with respect to 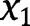 :
:
![[33]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq33.png)
By considering the following equation coming from Pythagorean theorem applied to the down-right triangle and by applying eq. [29]:
![[34]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq34.png)
By substituting eq. [33] in eq. [34]:
![[35]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq35.png)
Finally:
![[36]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq36.png)
whereas by applying the plus sign in eq. [33] one gets the relationship for r2:
![[37]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq37.png)
Appendix B: Geometrical derivation of rII, rIII, rIV, rV, rVI, θ2, θ3, θ4, θ5 and θ6

Geometric scheme referred to lamp 1 reference system.
![[38]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq38.png)
![[39]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq39.png)
![[40]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq40.png)
![[41]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq41.png)
![[42]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq42.png)
![[43]](/document/doi/10.1515/ijcre-2012-0049/asset/graphic/ijcre-2012-0049_eq43.png)
Notations
| A [m2] | catalyst surface area (specific surface area multiplied by weight) |
| b [dimensionless] | parameter defined by eq. [8] |
| Ccat [g m–3] | catalyst concentration |
| Ci [mmol m–3] | concentration of I-intermediate |
| CSub [mmol m–3] | substrate concentration |
| CSub,0 [mmol m–3] | substrate initial concentration |
| e [dimensionless] | Napierian extinction coefficient |
| I [W m–2] | light radiation flux coming from one lamp in the positive propagation direction |
| I0 [W m–2] | light radiation flux coming from one lamp at boundary medium (between light source and reacting suspension) |
| I0,r1 [W m–2] | light radiation flux coming from one lamp at boundary medium as a function of r1 |
| Iλ [W m–2] | local radiation intensity |
| J [W m–2] | radiation flux in the opposite propagation direction |
| k [m–1] | absorption coefficient |
| k* [m2 g–1] | absorption coefficient per unit concentration |
| k′ [mmol m–2 h–1] | absolute kinetic constant (not depending on light intensity) |
| k1″ [mmol m–2 h–1] = k′′θOx | L.-H. surface pseudo-first-order kinetic rate constant |
| k2″ [mmol m–2 h–1] | L.-H. second-order kinetic rate constant |
| kads [m3mmol–1] | substrate adsorption kinetic constant |
| kdes [m3mmol–1] | substrate desorption kinetic constant |
| KSub [m3mmol–1] | reagent equilibrium adsorption constant |
| Ki [m3mmol–1] | intermediate products equilibrium adsorption constant |
| kobs [m h–1] | observed pseudo-first-order disappearance rate |
| l [m] | light path length; coordinate along which propagation occurs |
| NSub [mmol] | phenol moles present in the liquid phase |
| r [m] | distance between light source and a reactant element, i.e. cylindrical radial coordinate |
| r0 [m] | smaller distance between light source and external reactor wall |
| r1 [m] = (r–l) | distance between light source 1 and circumference points nearer to the light source (see Figures 2 and 6) |
| r2 [m] | distance between light source 1 and circumference points farer from the light source (see Figures 2 and 7) |
| rII, rIII, rIV, rV, rVI [m] | distance between light source II, III, IV, V, VI and circumference points nearer to the light source (see Appendix B) |
| rSub [mmol m–2 h–1] | volumetric averaged surface reaction rate |
| R [m] | reactor radius |
| R∞ [dimensionless] | diffuse reflectance |
| s [m–1] | scattering coefficient |
| s* [m2 g–1] | scattering coefficient per unit concentration |
| S [m2] | cross-sectional area of the photoreactor |
| t [h] | reaction time |
| V [m3] | reaction volume |
| α [dimensionless] | parameter varying from 0.5 to 1 (present as an exponent in eq. [18]) |
| θ [rad] | cylindrical angular coordinate referred to lamp 1 |
| θ2, θ3, θ4, θ5, θ6[rad] | cylindrical angular coordinate referred to lamp 2, 3, 4, 5, 6 |
| θ* [dimensionless] | fractional site coverage |
| θ0 [rad] | boundary cylindrical angular to be considered |
| θOx [dimensionless] | oxygen fractional site coverage |
| θSub [dimensionless] | reagent fractional site coverage |
| λ [m] | radiation wavelength |
References
1 Cabrera MI, Alfano OM, Cassano AE. Novel reactor for photocatalytic kinetic studies. Ind Eng Chem Res 1994;33:3031–42.10.1021/ie00036a019Suche in Google Scholar
2 Li Puma G, Brucato A. Dimensionless analysis of slurry photocatalytic reactors using two-flux and six-flux radiation absorption-scattering models. Catal Today 2007;122:78–90.10.1016/j.cattod.2007.01.027Suche in Google Scholar
3 Colina-Márquez J, Machuca-Martínez F, Li Puma G. Photocatalytic mineralization of commercial herbicides in a pilot-scale solar CPC reactor: photoreactor modeling and reaction kinetics constants independent of radiation field. Environ Sci Technol 2009;43:8953–60.10.1021/es902004bSuche in Google Scholar
4 Camera-Roda G, Santarelli F. A rational approach to the design of photocatalytic reactors. Ind Eng Chem Res 2007;46:7637–44.10.1021/ie070302aSuche in Google Scholar
5 Ray AK. Design, modelling and experimentation of a new large-scale photocatalytic reactor for water treatment. Chem Eng Sci 1999;54:3113–25.10.1016/S0009-2509(98)00507-7Suche in Google Scholar
6 Li D, Xiong K, Li W, Yang Z, Liu C, Feng X, Lu X. Comparative study in liquid-phase heterogeneous photocatalysis: model for photoreactor scale-up. Ind Eng Chem Res 2010;49:8397–405.10.1021/ie100277gSuche in Google Scholar
7 Arancibia-Bulnes CA, Bandala ER, Estrada CA. Radiation absorption and rate constants for carbarylphotocatalytic degradation in a solar collector. Catal Today 2002;76:149–59.10.1016/S0920-5861(02)00215-8Suche in Google Scholar
8 Salaices M, Serrano B, De Lasa HI. Experimental evaluation of photon absorption in an aqueous TiO2 slurry reactor. Chem Eng J 2002;90:219–29.10.1016/S1385-8947(02)00037-2Suche in Google Scholar
9 Taranto J, Frochot D, Pichat P. Modeling and optimizing irradiance on planar, folded, and honeycomb shapes to maximize photocatalytic air purification. Catal Today 2007;122:66–77.10.1016/j.cattod.2007.01.031Suche in Google Scholar
10 Augugliaro V, Camera-Roda G, Loddo V, Palmisano G, Palmisano L, Parrino F, Puma MA. Synthesis of vanillin in water by TiO2 photocatalysis. Appl Catal B: Environ 2012;111–112:555–61.10.1016/j.apcatb.2011.11.007Suche in Google Scholar
11 Yoshihawa N, Kimura T, Kawase Y. Oxidative degradation of nonionic surfactants with TiO2photocatalyst in a bubbling column reactor. Can J Chem Eng 2003;81:719–24.10.1002/cjce.5450810351Suche in Google Scholar
12 Tokumura M, Znad HT, Kawase Y. Modeling of an external light irradiation slurry photoreactor: UV light or sunlight-photoassisted Fenton discoloration of azo-dye Orange II with natural mineral tourmaline powder. Chem Eng Sci 2006;61:6361–71.10.1016/j.ces.2006.05.038Suche in Google Scholar
13 Cassano AE, Alfano OM. Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal Today 2000;58:167–97.10.1016/S0920-5861(00)00251-0Suche in Google Scholar
14 Brandi RJ, Citroni MA, Alfano OM, Cassano AE. Absolute quantum yields in photocatalytic slurry reactors. Chem Eng Sci 2003;58:979–85.10.1016/S0009-2509(02)00638-3Suche in Google Scholar
15 Modest MF. Radiative heat transfer. New York: McGraw-Hill, 1993.Suche in Google Scholar
16 Kortüm G. Reflectance spectroscopy: principles, methods, applications. New York: Springer, 1969.10.1007/978-3-642-88071-1Suche in Google Scholar
17 Yurdakal S, Loddo V, Augugliaro V, Berber H, Palmisano G, Palmisano L. Photodegradation of pharmaceutical drugs in aqueous TiO2 suspensions: mechanism and kinetics. Catal Today 2007;129:9–15.10.1016/j.cattod.2007.06.044Suche in Google Scholar
18 Augugliaro V, Loddo V, Palmisano L, Schiavello M. Performance of heterogeneous photocatalytic systems: influence of operational variables on photoactivity of aqueous suspension of TiO2. J Catal 1995;153:32–40.10.1006/jcat.1995.1105Suche in Google Scholar
19 Turchi C, Ollis DF. Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 1990;122:178–92.10.1016/0021-9517(90)90269-PSuche in Google Scholar
20 Mills A, Wang J, Ollis DF. Dependence of the kinetics of liquid-phase photocatalysed reactions on oxygen concentration and light intensity. J Catal 2006;243:1–6.10.1016/j.jcat.2006.06.025Suche in Google Scholar
21 Fox MA, Dulay MT. Heterogeneous photocatalysis. Chem Rev 1993;93:341–57.10.1021/cr00017a016Suche in Google Scholar
22 Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chem Rev 1995;95:69–96.10.1021/cr00033a004Suche in Google Scholar
23 Mills A, Le Hunte S. An overview of semiconductor photocatalysis. J Photochem Photobiol A: Chem 1997;108:1–35.10.1016/S1010-6030(97)00118-4Suche in Google Scholar
24 Ollis DF. Kinetics of liquid phase photocatalyzed reactions: an illuminating approach. J Phys Chem B 2005;109:2439–44.10.1021/jp040236fSuche in Google Scholar
25 Mills A, Wang J. The kinetics of semiconductor photocatalysis; light intensity effects. Z Phys 1999;213:49–58.10.1524/zpch.1999.213.Part_1.049Suche in Google Scholar
26 Emeline AV, Ryabchuk V, Serpone N. Factors affecting the efficiency of a photocatalyzed process in aqueous metal-oxide dispersions – prospect of distinguishing between two kinetic models. J Photochem Photobiol A: Chem 2000;133:89–97.10.1016/S1010-6030(00)00225-2Suche in Google Scholar
27 Martyanov I, Savinov E. Photocatalytic steady-state methylviologen oxidation in air-saturated TiO2 aqueous suspension: initial photonic efficiency and initial oxidation rate as a function of methylviologen concentration and light intensity. J Photochem Photobiol A: Chem 2000;134:219–26.10.1016/S1010-6030(00)00254-9Suche in Google Scholar
28 Xu Y, Langford CH. Variation of Langmuir adsorption constant determined for TiO2-photocatalyzed degradation of acetophenone under different light intensity. J Photochem Photobiol A: Chem 2000;133:67–71.10.1016/S1010-6030(00)00220-3Suche in Google Scholar
29 Emeline AV, Ryabchuk V, Serpone N. Dogmas and misconceptions in heterogeneous photocatalysis. Some enlightened reflections. J Phys Chem B 2005;109:18515–21.10.1021/jp0523367Suche in Google Scholar PubMed
30 Loddo V, Addamo M, Augugliaro V, Palmisano L, Schiavello M, Garrone E. Optical properties and quantum yield determination in photocatalytic suspensions. AIChE J 2006;52:2565–74.10.1002/aic.10883Suche in Google Scholar
©2013 by Walter de Gruyter Berlin / Boston
Artikel in diesem Heft
- Masthead
- Masthead
- Editorial
- In Honor of Alberto E. Cassano: Researcher, Engineer, and Academic
- Articles
- From Ideal Reactor Concepts to Reality: The Novel Drum Reactor for Photocatalytic Wastewater Treatment
- Synthesis, Characterization, and Comparison of Sol–Gel TiO2 Immobilized Photocatalysts
- Determination of Kinetic Parameter in a Unified Kinetic Model for the Photodegradation of Phenol by Using Nonlinear Regression and the Genetic Algorithm
- Mass Transfer and Conservation from a Finite Source to an Infinite Media
- Modelling and Simulation of Gas–liquid Hydrodynamics in a Rectangular Air-lift Reactor
- Two-Dimensional Modeling of an Externally Irradiated Slurry Photoreactor
- Role of Aspect Ratio and Joule Heating within the Fluid Region Near a Cylindrical Electrode in Electrokinetic Remediation: A Numerical Solution based on the Boundary Layer Model
- Solar Water Disinfection Using NF-codoped TiO2 Photocatalysis: Estimation of Scaling-up Parameters
- A Simple and Semi-Empirical Model to Predict THMs Generation in Water Facilities Including pH Effects
- On the Standardization of the Photocatalytic Gas/Solid Tests
- Microalgae Technology: A Patent Survey
- Influence of Physical and Optical Parameters on 2,4-Dichlorophenol Degradation
- Factors Capable of Modifying the Response of Pseudomonas aeruginosa to the Inactivation Induced by Heterogeneous Photocatalysis
- Enhanced Antibacterial Activity of CeO2 Nanoparticles by Surfactants
- Determination of Photochemical, Electrochemical and Photoelectrochemical Efficiencies in a Photoelectrocatalytic Reactor
- Correlations between Molecular Descriptors from Various Volatile Organic Compounds and Photocatalytic Oxidation Kinetic Constants
- Role of Joule Heating in Electro-Assisted Processes: A Boundary Layer Approach for Rectangular Electrodes
Artikel in diesem Heft
- Masthead
- Masthead
- Editorial
- In Honor of Alberto E. Cassano: Researcher, Engineer, and Academic
- Articles
- From Ideal Reactor Concepts to Reality: The Novel Drum Reactor for Photocatalytic Wastewater Treatment
- Synthesis, Characterization, and Comparison of Sol–Gel TiO2 Immobilized Photocatalysts
- Determination of Kinetic Parameter in a Unified Kinetic Model for the Photodegradation of Phenol by Using Nonlinear Regression and the Genetic Algorithm
- Mass Transfer and Conservation from a Finite Source to an Infinite Media
- Modelling and Simulation of Gas–liquid Hydrodynamics in a Rectangular Air-lift Reactor
- Two-Dimensional Modeling of an Externally Irradiated Slurry Photoreactor
- Role of Aspect Ratio and Joule Heating within the Fluid Region Near a Cylindrical Electrode in Electrokinetic Remediation: A Numerical Solution based on the Boundary Layer Model
- Solar Water Disinfection Using NF-codoped TiO2 Photocatalysis: Estimation of Scaling-up Parameters
- A Simple and Semi-Empirical Model to Predict THMs Generation in Water Facilities Including pH Effects
- On the Standardization of the Photocatalytic Gas/Solid Tests
- Microalgae Technology: A Patent Survey
- Influence of Physical and Optical Parameters on 2,4-Dichlorophenol Degradation
- Factors Capable of Modifying the Response of Pseudomonas aeruginosa to the Inactivation Induced by Heterogeneous Photocatalysis
- Enhanced Antibacterial Activity of CeO2 Nanoparticles by Surfactants
- Determination of Photochemical, Electrochemical and Photoelectrochemical Efficiencies in a Photoelectrocatalytic Reactor
- Correlations between Molecular Descriptors from Various Volatile Organic Compounds and Photocatalytic Oxidation Kinetic Constants
- Role of Joule Heating in Electro-Assisted Processes: A Boundary Layer Approach for Rectangular Electrodes

