Abstract
The interpretation of marker experiments in studying the formation mechanism and defect structure of higher oxides during oxidation of lower oxides, showing rather high deviations from stoichiometry, has been discussed. It has been shown that correct results can be obtained only if the concentration of point defects in the substrate is taken into account. Theoretical considerations presented in this paper have been illustrated by the experimental results obtained during sulphidation of highly disordered Co1-yS to form CoS2, as well as Ni1-yS to form NiS2.
Introduction
Marker method has been applied for the first time by Pfeil [1] about 80 years ago in studying the mechanism of scale formation on iron and this method is continuously used not only in studying the high temperature corrosion, but also in determining the predominant disorder in oxides and sulphides of transition metals. This method consists in the deposition of a small amount of substance on the substrate surface, called marker, which does not react with the oxidant and substrate as well as with reaction product and after terminating the oxidation reaction to determine its location in the system substrate–product. If the marker is found at the substrate–product interface (in the case of metal oxidation, it is metal–scale interface), such a location of the marker means univocally that the reaction product was growing by the outward diffusion of cations and consequently that the cation sublattice is predominantly disordered. If, in turn, the marker is located on the surface of reaction product, it means that the product layer was growing by the inward diffusion of the oxidant. If, finally, the marker is found in the interior of the reaction product layer, it suggests that both sublattices of the compound forming the reaction product are disordered to a comparable degree. However, from an energetic point of view such a situation is found very rarely, because predominant disorder is generally observed in one sublattice only, and most frequently, this is cation sublattice. A specific situation is observed in the case when a marker is found in the interior of the scale but the inner layer, showing the same phase composition as the outer one, is porous. In this particular case, the location of the marker does not indicate that both sublattices are disordered, but that the formation of the inner porous layer beneath the marker is a result of secondary processes, the mechanism of which is described in the literature as “dissociative mechanism of scale growth” [2–8].
Theoretical considerations
A separate and important problem constitutes the application of marker method in studying the type of dominating defects in higher oxides and sulphides of transition metals, the formation of which proceeds not on the metal surface, but on the surface of lower oxides or sulphides [9, 10]. The application of marker method in this particular case is much more difficult and needs separate interpretation. These difficulties constitute the main reason of scant information in the literature concerning defect structure, and in particular predominant disorder in higher oxides and sulphides of transition metals.
If in the discussed case the markers are deposited not on the surface of metal, but on the surface of lower oxide or sulphide (MeaXb) and after terminating the reaction they are found on the surface of reaction product (MecXd), it clearly suggests that – like in the case of pure metal oxidation – the reaction product was growing owing to the inward diffusion of oxidant X (Figure 1(a)). If, on the other hand, the reaction product is being formed as a result of the outward diffusion of cations, the markers – in contrast to the case of pure metal oxidation – will be found not at the substrate–product interface, but in the interior of the reaction product layer (Figure 1(b)), because this layer is formed not only on the outer surface, but also at the substrate–product interface due to the substrate decomposition. The formation of the product (MecXd) beneath the markers, as a result of substrate (MeaXb) decomposition, may be described by the following equation:

Marker position in the higher oxide layer (MecXd), grown on the surface of the lower oxide (MeaXb) as a result of (a) inward diffusion of oxidant; (b) outward diffusion of oxidant
It follows from this formula that for 1 mole of reaction product being formed as a result of substrate decomposition the appropriate number of cations liberated in this process diffuses outwards to the outer surface, where they react with oxidant. This second reaction can be demonstrated as follows:
From these two relationships the ratio (f) of both product layers formed in decomposition process and outward diffusion of cations can be calculated:
The ratio f results from dividing the coefficient before the chemical formula of reaction product forming the outer layer by the coefficient located before the chemical formula of reaction product forming the inner layer (e.i. by 1).
There are a number of cases in which the substrate (oxide or sulphide) is highly disordered. Such a situation is observed among others during the formation of the product of sulphidation of several metals as well as of oxidation of iron to form wustite, Fe1-yO [11, 12], and cobalt, to form Co1-yO [13, 14]. The approach to this problem needs specific treatment, both in experimental procedure and in the interpretation of the marker results. These aspects are discussed in the present paper.
The aim of the experimental part of this work was the explanation of the predominant disorder in the higher cobalt sulphide, CoS2, growing on the surface of the lower cobalt sulphide, Co1-yS, as well as in the higher nickel sulphide, NiS2, growing on the surface of the lower nickel sulphide, Ni1-yS, characterized by extremely high deviation from stoichiometry, y, and thereby point defect concentration, of the order of 0.1 mole of defects per 1 mole of the sulphide [15, 16]. Analogous high deviations from stoichiometry are observed in iron sulphide [17] and oxide [11, 12] (Fe1-yS and Fe1-yO), copper sulphide (Cu2-yS) [18], etc.
Materials and experimental procedure
Substrates in the present study constitute flat, rectangular samples of Co1-yS and Ni1-yS sulphides, obtained as a result of completely sulphidized metal samples (15 mm × 10 mm × 0.5 mm) at sulphur pressures lower than the dissociation pressure of CoS2 and NiS2, respectively. High-purity cobalt and nickel have been used as starting materials, containing trace amounts of impurities summarized in Table 1.
Concentration of impurities in Co and Ni metals
| Metal | Impurities (mass %) | ||||||||||
| Ag | Al | Ca | Cr | Cu | Fe | Mg | Mn | Ni | Si | Sn | |
| Co | 0.0002 | 0.0001 | 0.0001 | <0.0001 | 0.0003 | 0.0007 | 0.0001 | <0.0001 | 0.0002 | 0.0005 | 0.0001 |
| Ni | 0.0001 | 0.0001 | 0.0001 | <0.0001 | 0.0003 | 0.0015 | <0.0001 | <0.0001 | – | 0.0002 | <0.0001 |
In order to get homogeneous Co1-yS and Ni1-yS sulphides, the sulphidation of pure metals has been carried out in the apparatus described elsewhere [19]. Experimental conditions have been chosen in such a way that only the Co1-yS or Ni1-yS sulphides were thermodynamically stable. These conditions have been marked in Figures 2 and 3, describing the fragment of phase diagrams of Co-S and Ni-S systems. Because of very high concentration of defects (cation vacancies) in Co1-yS and Ni1-yS, the sulphidation process of both metals proceeded very quickly and consequently after about 24 hours the metal samples were completely sulphidized. However, the most important problem consists in having homogeneous distribution (without gradient) of the defect concentration in the whole sulphide specimen. The homogenization process of such specimens has been carried out during about 24 hours for a number of different sulphur vapour pressures and temperatures in order to get different defect concentration in homogenized specimens. The aim of this procedure will be explained in the next paragraph.

The part of phase diagram of the Co-S system, illustrating temperature–pressure ranges of both: Co1-yS pretreatment (A) and CoS2 formation during marker experiments (B)

The part of phase diagram of the Ni-S system, illustrating temperature–pressure ranges of both: Ni1-yS pretreatment (A) and NiS2 formation during marker experiments (B)
The samples obtained in this way have been marked by gold. As the sulphidation rate of Co1-yS and Ni1-yS substrates proceeds very slowly, the thickness of CoS2 and Ni1-yS products is even after hundreds of hours extremely thin. As the markers have to be at least one order of magnitude thinner than the sulphidation product layer, the gold marker film was vacuum evaporated onto the surface of the Co1-yS and Ni1-yS substrates through the copper mesh. Consequently, small islands of gold were obtained, spread over the specimen surface. The samples marked in this way were then treated at temperatures presented in Figures 2 and 3 by grey (A) polygon during 24 hours, under sulphur vapour pressure lower than the dissociation pressures of CoS2 or NiS2. In the next step, the marked samples were sulphidized under conditions illustrated in Figures 2 and 3 by grey (B) polygon to form CoS2 or NiS2, respectively. After terminating these reactions, a part of the specimens has been electrochemically covered by nickel in order to prevent the spallation of the sample during metallographic procedure. Subsequently, the cross section of the samples has been made in order to determine the position of the marker islands in the interior of CoS2 orNiS2 layers, using scanning electron microscope.
Results and discussion
All transition metal oxides and sulphides show deviations from stoichiometry, resulting from easiness of cation valency changes. A number of transition metal sulphides, and in particular Co1-yS, Ni1-yS, Fe1-yS, show dramatically high deviations from stoichiometry, y, resulting from the presence of cation vacancies of the order of 0.1 mole of cation vacancies per mole of the sulphide. If such a high concentration of point defects is not taken into account in marker experiments, it may be a reason for erroneous conclusions. In contrast to metals, the marker experiment in the case of the oxidation of the lower oxides and sulphide, showing high nonstoichiometry, it is necessary to homogenize the substrate in order to establish homogeneous distribution of point defects, without their concentration gradient. This homogenization process may be carried out under different oxidant pressures, being however always lower or equal to the dissociation pressure of the higher oxide. As a result of homogenization at different oxidant pressures, the concentration of point defects in the substrate becomes different, assuming that the thermodynamic equilibrium has been reached during homogenization. Substrate samples prepared in this way have been marked by gold using the procedure described previously. The oxidation or sulphidation process of the marked substrate, homogenized under lower oxidant pressure, begins to oxidize after raising the oxidant pressure above the dissociation pressure of the reaction product. In the very beginning of the reaction, extremely thin product of the higher oxide starts to develop, but in the same time substrate sample is “oxidized”, resulting from increase of the defect concentration. This process in the case of CoS and NiS monosulphides can be described using Kröger–Vink notation of defects [20] by the following quasi-chemical equations:
It follows from these relationships that the reactions of these monosulphides with sulphur result not only in the increase in the defect concentration (i.e. doubly ionized cation vacancies and electron holes) but also in the increase of the mass of the sample. These processes proceed orders of magnitude faster than the formation of higher sulphides. As the substrate layer is growing by the outward diffusion of cations, the marker located before the reaction on the surface of the specimen becomes overgrown by the substrate sulphide, as shown in Figure 4. The thickness of the layer above the marker depends on the substrate thickness and on the difference between the sulphur pressures under which the substrate has been homogenized and that of the sulphidation reaction. The higher is this difference and the initial substrate thickness, the thicker is the substrate layer above the marker. As the time of oxidation increases, the thickness of this layer reaches a constant value. This constant value depends on the difference of defect concentration corresponding to the dissociation pressure of the product and oxidant pressure applied during homogenization and on the initial thickness of the specimen substrate. The layer of the higher sulphide, growing extremely slowly, proceeds as a result of the consumption of the substrate layer (lower sulphide) above the marker. Thus, with longer and longer sulphidation time, the thickness of the lower sulphide layer (MeX) above the markers is decreasing due to its sulphidation and finally this layer disappears completely, as schematically shown in Figure 5. Consequently, the markers will be found at the substrate–product interface. Further sulphidation will result in the formation of the product above and below the marker, because the outward diffusion of cations will result on the one hand on the formation of the product due to the decomposition of the substrate (eq. (1)) and on the other hand on the formation of the product at the outer surface (eq. (2)). Thus, markers will be found in the interior of the higher oxide (sulphide) layer, as schematically depicted in Figure 5.

The influence of homogenization of Co1-yS sulphide at T = 973 K and under sulphur vapour pressure
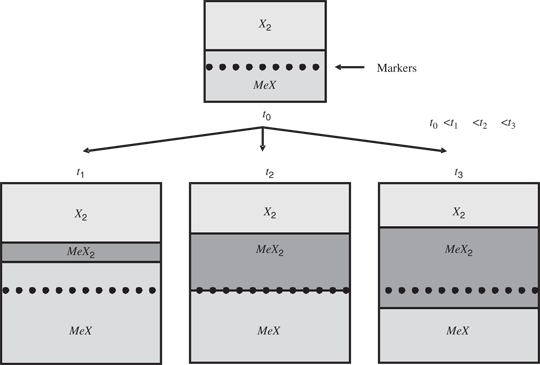
Schematic representation of the positions of markers in the highly nonstoichiometric MeX substrate, homogenized under oxidant pressure much lower than the dissociation pressure of higher oxide MeX2 (where t1, t2 and t3 denote reaction times)
From these considerations it follows that if the marker experiment is carried out with substrate homogenized under sulphur pressure lower than the dissociation pressure of the product of its sulphidation, the position of the markers will depend on the number of factors described above, and consequently, any rational conclusions concerning the mechanism of the formation of the higher sulphide cannot be formulated.
Taking into account the above conclusion, the marker experiment must be carried out with the substrate homogenized under sulphur pressure equal to the dissociation pressure of the higher sulphide. In this situation, namely, after introducing the sulphur to the reaction chamber, the substrate does not consume oxidant to change nonstoichiometry, because the concentration of defects (and thereby the nonstoichiometry) has already reached the maximum value during the homogenization process (Figure 6). Thus, on the surface of marked substrate only the higher sulphide layer begins to grow. If the product is growing by the outward diffusion of cations, the markers will be found in the interior of the higher sulphide layer in the position described by eq. (3) (Figure 1(b)). On the other hand, if the oxidation product is growing by the inward diffusion of sulphur, markers will be located at the outer surface of the product layer (Figure 1(a)).

The influence of homogenization of Co1-yS sulphide at T = 973 K and under sulphur vapour pressure
It follows from these remarks that rational results of marker experiment can only be obtained if the markers before the reaction are located on the lower sulphide (or oxide) surface, homogenized at the dissociation pressure of the product. The homogenization of substrate at oxidant pressure exactly equal to the dissociation pressure of the higher sulphide (or oxide) constitutes a rather difficult experimental problem. This requirement is particularly difficult to be realized in the case of sulphides, because to get the sulphur partial pressure on strictly defined level is not an easy experimental problem. These difficulties can be overcome by the homogenization of given lower sulphide or oxide substrates at oxidant pressure slightly higher than the dissociation pressure of the reaction product. Under these conditions, the higher sulphide (or oxide) starts to develop as a rule very slowly and after a short period of time a substrate will be homogenized under dissociation pressure of higher sulphide (or oxide). During this period of time, the thickness of the outer sulphide layer is extremely small, which may not be taken into account in marker experiments. The surface of the sample prepared in this way may then be marked and after long period of sulphidation, when the product layer thickness is at least two orders of magnitude higher than the initial thickness developed before marker deposition, the marker position is determined. The above described procedure makes the homogenization much more simple, assuring in the same time the concentration of defects in the substrate on maximal constant level.
Considering different aspects of marker experiments in studying the defect structure in higher oxides and sulphides, it is necessary to take into account the influence of nonstoichiometry on the location of marker in the interior of the reaction product. This situation is well illustrated by microphotograph, showing the cross section of Ni1-yS substrate, after sulphidation at sulphur vapour pressure higher than the dissociation pressure of NiS2 disulphide (Figure 7). As can be seen, a compact NiS2 layer is formed on the surface of Ni1-yS substrate (marked before the reaction by gold). The position of the markers in the reaction product is clearly visible, but not exactly in the middle of the thickness of the NiS2 product, which is expected from theoretical analysis of eqs (1)–(3). There may be two reasons of such a location of markers in NiS2 product. First, thicker inner layer of NiS2 beneath the markers may be the result of small participation of inward diffusion of sulphur through point defects or grain boundaries of the reaction product. This conclusion can be a priori ruled out, because the NiS2 product is coarse grained and the sulphidation experiments carried out with the use of radioactive sulphur isotope 35S have demonstrated [21, 22] that the inward grain boundary and lattice diffusion of sulphur does not participate in the formation of the sulphide scale on this metal. The second possibility of the explanation of such position of markers is as follows. If, namely, NiS2 sulphide would grow on the surface of strictly stoichiometric NiS by the outward diffusion of cations only, the markers should be found exactly in the middle of NiS2 product (see Figure 1(b)). This conclusion follows directly from the application of eqs (1)–(3) to the process of NiS2 formation on strictly stoichiometric NiS substrate:

Microphotograph of metallographic cross section of NiS2 layer, formed on NiS surface during sulphidation of NiS “marked” by gold islands
Such a situation would be observed in the case if the nonstoichiometry of the substrate could be neglected. In reality, nickel sulphide, Ni1-yS, shows extremely high deviation from stoichiometry, y, equal to 0.15 at the NiS/NiS2 interface. Considering this fact, the general equations (1)–(3) assume the following form:
From these relationships it follows that during decomposition of 2 mole of Ni0.85S, under the marker 1 mole of NiS2 is formed and owing to the outward diffusion of cations above the markers 0.7 mole of NiS2 develops. It is then obvious that in this case, the markers will not be located in the middle of NiS2 layer but closer to the outer surface of NiS2, in agreement with microphotograph presented in Figure 7. It should finally be pointed out that if the markers would be found at the surface of the reaction product layer, it suggests univocally that the product has been growing by the inward lattice or intergranular diffusion. In this particular case, the nonstoichiometry and thereby defect concentration has not any relation with the matter transport through the reaction product.
Conclusions
From the above discussion it follows clearly that the rational results of marker experiments in the case of the formation of the higher sulphide (or oxide) on lower ones need a number of introductory experiments in order to get rational explanation of defect structure. In particular, the problem of homogenization of the starting material is extremely important. In fact, the homogenization process of the substrate must be made under oxidant pressure equal or slightly higher than the dissociation pressure of the higher oxide to be formed. In addition, the nonstoichiometry of the substrate, as well as the product must carefully be taken into account in order to foresee the rational location of markers in the interior of the product layer. Under these conditions only, the position of the markers in the reaction product allows rational conclusions to be formulated.
Funding statement: Funding: This work was supported by The National Centre for Research and Development in Poland no NN507 295939.
References
1. PfeilLB. The Oxidation of Iron and Steel at High Temperatures. J Iron Steel Inst1929;119:501–47.Search in Google Scholar
2. MrowecS, WerberT. Verwendung der radioaktiven isotope desoxydators in den untersuchungen des mechanismus der hochtemperaturoxydation von metallen und legierungen. Corrosion Sci1965;5:717–27.10.1016/S0010-938X(65)80028-2Search in Google Scholar
3. MrowecS. On the mechanism of high temperature oxidation of metals and alloys. Corrosion Sci1967;7:563–78.10.1016/0010-938X(67)80033-7Search in Google Scholar
4. MrowecS. Sulphidation of Alloys at High Temperatures. J Mater Sci1976;2:99–112.Search in Google Scholar
5. VermaSK, RaynaudGM, RappRA. Hot-stage scanning electron-microscope for high-temperature insitu oxidation studies. Oxid Met1981;15:471–83.10.1007/BF00603538Search in Google Scholar
6. MrowecS. The influence of secondary processes on the mechanism of scales growth on metals and alloys. In: Transactions of the Japan Institute of Metals (JIMS-3), 1983, 69.Search in Google Scholar
7. RappRA. The high-temperature oxidation of metals forming cation-diffusing scales. Metall Trans A1984;15:765–82.10.1007/BF02644552Search in Google Scholar
8. MrowecS. Dissociative mechanism of scales growth on metals and alloys. High Temp Mater Processes2005;24:375–94.10.1515/HTMP.2005.24.6.375Search in Google Scholar
9. PrzybylskiK, SmeltzerWW. High-temperature oxidation mechanism of CoO to Co3O4. J Electrochem Soc1981;128:897–902.10.1149/1.2127528Search in Google Scholar
10. GrzesikZ, MigdalskaM, MrowecS. Oxidation mechanism of Cu2O and defect structure of CuO at high temperatures. High Temp Mater Proc2011;30:277–87.10.1515/htmp.2011.046Search in Google Scholar
11. SwaroopB, WagnerJB. On vacancy concentrations of wustite (FeOx) near p to n transition. Trans Met Soc AIME1967;239:1215–18.Search in Google Scholar
12. TakayamaE, KimizukaN. Thermodynamic properties and subphases of wustite field determined by means of thermogravimetric method in the temperature range of 1100°–1300°C. J Electrochem Soc1980;127:970–76.10.1149/1.2129797Search in Google Scholar
13. DieckmannR. Cobaltous oxide point defect structure and non-stoichiometry, electrical conductivity, cobalt tracer diffusion. Z Physik Chem Neue Folge1977;107:189–210.10.1524/zpch.1977.107.2.189Search in Google Scholar
14. GesmundoF, VianiF, Petot-ErvasG, PetotC, BuscagliaV. Defect structure of pure and doped CoO in advances in ceramics. In: CatlowCRA, MackrodtWC, editors. Westerville, OH: The American Ceramic Society, 1987:83, vol. 23.Search in Google Scholar
15. RauH. Range of homogeneity and defect energetics in Co1-xS. J Phys Chem1976;37:931–34.10.1016/0022-3697(76)90034-2Search in Google Scholar
16. RauH. Ranege of homogeneity and defect interaction in high temperature nickel sulphide Ni1-xS. J Phys Chem Solids1975;36:1199–204.10.1016/0022-3697(75)90190-0Search in Google Scholar
17. RauH. Energetics of defect formation and interaction in pyrrhotite Fe1-xS and its homogeneity range. J Phys Chem Solids1976;37:425–29.10.1016/0022-3697(76)90024-XSearch in Google Scholar
18. RauH. Homogeneity Range of Cubic High Temperature Cuprous Sulfide (Digenite). J Phys Chem Solids1974;35:1415–24.10.1016/S0022-3697(74)80247-7Search in Google Scholar
19. GrzesikZ, MrowecS, WalecT, DąbekJ. New microthermogravimetric apparatus – Kinetics of metal sulphidation and transport properties of transition metal sulphides. J Thermal Anal Cal2000;59:985–97.10.1023/A:1010199030780Search in Google Scholar
20. KrögerFA. The chemistry of imperfect crystals. Amsterdam and New York: North-Holland Publ. Co., 1964.Search in Google Scholar
21. BruckmanA, MrowecS, WerberT. Issledovanija stepeni ucastija sery v reakcionnoj difuzii pri obrazovanii sulfidnoj okaliny na nikele. Fizika Metall Metalloved1965;20:702–7.Search in Google Scholar
22. BruckmanA, MrowecS, WerberT. Über den Mechanismus der Hochtemperaturoxidation von Metallen und Legierungen. Z Phys Chem1966;231:375–81.10.1515/zpch-1966-23142Search in Google Scholar
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- Research Articles
- The Effect of Aging Heat Treatment on the Microstructure and Mechanical Properties of 10Cr20Ni25Mo1.5NbN Austenitic Steel
- Weldability Characteristics of Sintered Hot-Forged AISI 4135 Steel Produced through P/M Route by Using Pulsed Current Gas Tungsten Arc Welding
- Marker Method in Studying the Defect Structure in Products of the Oxidation of Highly Disordered Substrates
- Research on the Semi-Solid Compressive Deformation Behavior of Ti-7Cu Alloy
- Numerical Prediction of the Thermodynamic Properties of Ternary Al-Ni-Pd Alloys
- Study on Control of Inclusion Compositions in Tire Cord Steel by Low Basicity Top Slag
- An Improved Arrhenius Constitutive Model and Three-Dimensional Processing Map of a Solution-Treated Ni-Based Superalloy
- Reaction between Steel-Making Slag and Carbonaceous Materials While Mixing with High Density Polyethylene
- Mechanism Research on Melting Loss of Coppery Tuyere Small Sleeve in Blast Furnace
- Research on Fracture Toughness of Flattened Brazilian Disc Specimen after High Temperature
- Plasma-Augmented Fluidized Bed Gasification of Sub-bituminous Coal in CO2–O2 Atmospheres
- Structure and Properties of the Aluminide Coatings on the Inconel 625 Superalloy
- Dynamic Transmission Performances of Alumina and Mullite Refractory Ceramics in Microwave High-Temperature Heating
Articles in the same Issue
- Frontmatter
- Research Articles
- The Effect of Aging Heat Treatment on the Microstructure and Mechanical Properties of 10Cr20Ni25Mo1.5NbN Austenitic Steel
- Weldability Characteristics of Sintered Hot-Forged AISI 4135 Steel Produced through P/M Route by Using Pulsed Current Gas Tungsten Arc Welding
- Marker Method in Studying the Defect Structure in Products of the Oxidation of Highly Disordered Substrates
- Research on the Semi-Solid Compressive Deformation Behavior of Ti-7Cu Alloy
- Numerical Prediction of the Thermodynamic Properties of Ternary Al-Ni-Pd Alloys
- Study on Control of Inclusion Compositions in Tire Cord Steel by Low Basicity Top Slag
- An Improved Arrhenius Constitutive Model and Three-Dimensional Processing Map of a Solution-Treated Ni-Based Superalloy
- Reaction between Steel-Making Slag and Carbonaceous Materials While Mixing with High Density Polyethylene
- Mechanism Research on Melting Loss of Coppery Tuyere Small Sleeve in Blast Furnace
- Research on Fracture Toughness of Flattened Brazilian Disc Specimen after High Temperature
- Plasma-Augmented Fluidized Bed Gasification of Sub-bituminous Coal in CO2–O2 Atmospheres
- Structure and Properties of the Aluminide Coatings on the Inconel 625 Superalloy
- Dynamic Transmission Performances of Alumina and Mullite Refractory Ceramics in Microwave High-Temperature Heating

