Abstract
Triazoles act as important pharmacophores in showing biological activity such as antibacterial, antifungal, antitumour/anticancer, anti-inflammatory activities. Literature review suggests that triazoles have been maximally used in carrying research related activities in reference to biological evaluation as compared to other nitrogen containing five membered heterocycles like tetrazoles, pentazoles, pyrazoles, and imidazoles. The first compound of this class was discovered by Janseen Group in 1960s. The microbes act counteractively towards antibiotics which in turn challenge the efficacy of the drugs and thus create room for the progression of more potent avant-garde drugs. Thus, the synthesis of hybrid molecules has been accelerated from last two decades as the hybrids possess more potency, vigour, and adequacy than its constituting pharmacophores. So, this review represents a condensed report of the research carried out in relation to synthetical procedures and assessment of the antibacterial and antifungal activity of triazoles.
1 Introduction
Heterocyclic organic chemistry is among the most prominent branches of medicinal chemistry. Heterocyclic compounds can be of different types depending upon the heteroatom (atom other than carbon) it contains which can be oxygen, sulphur, nitrogen, and others. As per the statistics, above 85% of biologically effective molecules possess a heterocycle [1]. Scientists on daily basis isolate various heterocyclic compounds and study them for drug discovery. More than 70% of the drugs used today have heterocycle in them, and out of it, more than 60% drugs have nitrogen in their scaffold [2]. The Scopus database reports suggest that the most frequently studied 5-membered heterocycles in medicinal chemistry are triazoles, particularly 1,2,3-triazoles [3]. The annual report of GARDP 2022 suggests that drug resistant microbes are causing death of 1.3 billion people every year. The main reason of this drug resistance is that bacteria form biofilms against the antibiotics [4]. So, the process of development of new more potent drug is an incessant process and one of the most propitious accessions to this target is achievable through the molecular hybridisation approach [5,6].
1.1 Properties of triazoles
Triazoles are the 5-segment heterocycles comprising two C atoms, three N atoms, and two double bonds [7]. The two isomeric forms of this molecule are 1,2,3-triazole and 1,2,4-triazole depending upon the position of nitrogen [8]. 1,2,3-triazole (Figure 1) exist in the form of colourless, hygroscopic crystals with melting point (m.p.) 24°C and boiling point (b.p.) 209°C and 1,2,4-triazole (Figure 2) exist in the form of water-soluble colourless crystals with m.p. 121°C and b.p. 260°C [9]. These compounds show exciting features like good solubility and effective H-bonding and π-stacking [10]. Extensive literature is available which proves that triazoles are favourable scaffolds in the genre of medicinal chemistry in virtue of their therapeutic activity such as anti-inflammatory, antioxidant, antibacterial, antitumour, antifungal, antiviral, antitubercular and antidepressant [11].

1,2,3-triazole.

1,2,4-triazole.
2 Most commonly adopted synthetic strategies of action adopted for the formation of 1,2,3-triazoles
Some of the common methodical procedures involving the formation of 1,2,3-triazole nucleus are copper catalysed azide-alkyne cycloaddition (CuAAC), ruthenium catalysed azide-alkyne cycloaddition RuAAC, and Huisgen azide-alkyne 1,3-dipolar cycloaddition.
CuAAC – Himo et al. contended that the reactants (azide, alkyne) undergo addition in cyclic form catalysed by copper forming a part of “Click Chemistry” [12].
Proposed catalytic cycle – Worrell et al. illustrated the mechanistic representation of desired derivative catalysed by Cu. Alkyne collaborates with Cu forming a π-complex. Azide then comes into play and upon interacting with the π-complex, forms a σ-complex. Cu is ejected forming the desired derivative [13].

RuAAC – Boren et al. contended that azide and alkyne undergo addition in cyclic form catalysed by Ruthenium [14].
Proposed catalytic cycle – Azide interacts with alkyne in the presence of ruthenium catalyst via 1,3-dipolar mechanism to form π-complex which then rearranges to form σ-complex. Latter ejects ruthenium catalyst to generate the desired derivative of triazole.

Huisgen azide-alkyne 1,3-dipolar cycloaddition – Kalyani Keerthi (2020) synthesised triazole moiety from azide and alkyne which undergo Huisgen cycloaddition regioselectively [15].
Proposed catalytic cycle – Reactants undergo 2s + 4s cycloaddition which is akin to Diels–Alder reaction. The following representation shows the mechanism of above reaction.
As we all know that time is a preeminent asset in the research, so the methods which involve less consumption of time are worthy of adoption while performing the research. There are two types of approach of synthesis - Green methodical approach of synthesis and Conventional approach of synthesis. Some Green approaches of synthesis are as follows:
Moorhouse and Moses (2008) initiated a microwave irradiated process of formation of substituted product and azides are derived via one-pot azidation reaction of anilines [16].
Nasrollahzadeh et al. adopted a clean and green method of synthesis of the molecule under consideration substituted at first as well as fourth position with the use of copper nanoparticles (NPs). The conditions are mild, and Cu NPs act as heterogenous catalyst and is obtained from the leaves of Otostegia persica [17].
Boominathan et al. adopted a new green method to synthesise 1,2,3-triazole derivatives. A base of TiO2 supported Au NPs is formed which is mixed with ethyl propiolate, phenylacylazide, and water and then heated on a water bath for 20–30 min to get our desired product [18].
Singh et al. evolved a green method which takes only 2 min to synthesise 1,2,3-triazole derivatives employing microwave irradiation and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-water catalyst at room temperature. The catalytic exercise of DBU increases substantially in water [19].
Some synthetic procedures used for the formation of triazoles which take time of less than 3 h to complete are listed as under.
Shu et al. proposed that N-tosylhydrazones undergo coupling cyclisation reaction with sodium azide mediated by molecular iodine to form 1,2,3-triazole nucleus [20].
Huang et al. proposed that 1-aminopyridinium iodide, methyl ketone, and p-toluenesulfonyl hydrazine undergo [2 + 2 + 1] cyclisation mediated by iodine to form 1,2,3-triazole nucleus held down by the case of azide-free and metal-free plight [21].
Quan et al. proposed that nitroolefins and sodium azide undergo 1,3-dipolar cycloadddition reaction mediated by p-TsOH which results in the rapid fabrication of the desired molecule in good yield [22].
Yamada et al. proposed that the catalyst exhibited in the picture enables the effective cycloaddition of alkynes and azides to provide excellent yield of product [23].
Shao et al. proposed a synthesis which is a Cu catalysed azide alkyne cycloaddition promoted jointly by acid-base with crisp reaction time [24].
Liu et al. recommended that the substituted triazole nucleus is formed by cycloaddition of alkyne and azide using Grignard Reagent [25].
Kim et al. suggested that this method is applicable to both aliphatic and aromatic substrates and 1,2,3-triazoles are synthesised in aqueous and ambient conditions [26].
Khalili et al. proposed the cyclisation of non-terminal alkynes/terminal alkynes and azide using CuAl2O4 nanoparticles in less time under aqueous conditions [27].
Ferrini et al. proposed a ruthenium catalysed cycloaddition synthesis. Reaction of aryl/alkyl azides along with N-Boc-arylynamide is regioselective reaction and with N-Boc-alkylynamide, formation of mixture of regioisomers takes place [28].
Cheng et al. suggested a solitary vessel fabrication of disubstituted 1,2,3-triazoles at first as well as fourth position [29].
Vadivelu et al. adopted a method of selective formation of the befitting molecule having −NO2 substitution at its fourth position. The method executes the synthesis of product in soaring yield, forming non-toxic by-product and the catalyst is recyclable and non-toxic [30].
2.1 Synthetic strategies of action for designing 1,2,4-triazoles
Some Green approaches of synthesis are as follows:
Venigalla et al. designed an innovative and productive method to synthesise 1,2,4-triazole derivatives with magnificent yield in which hydrazine carboxamide reacts with the substituted benzaldehyde in one pot containing ethanol as a solvent. This pot is treated with ultrasonic waves at room temperature. This method does not need any catalyst. The product is formed in less than 6 min [31].
Shelke et al. planned a method which involves microwave irradiated formation of 1,2,4-triazole using hydrazines and formamide, which does not require any catalyst [32].
Nakka et al. planned an environmentally congenial methodical procedure used in the designing of 3,4,5-trisubstituted-1,2,4-triazoles using PEG (which is recyclable) and ceric ammonium nitrate as a catalyst. This method is a commercially feasible method [33].
Jatangi et al. steered an environmentally congenial and beneficial approach used in the formation of substituted nucleus. It is mediated by iodine under mild conditions. This method illustrates great substrate endurance [34].
Some conventional methods which take less than 3 h are listed below: (Tables 1 and 2)
Tseng et al. designed a method that enables the formation of 1,3,5-trisubstituted product in good harvest by virtue of 1,3-dipolar cycloaddition [35].
Ma et al. proposed a method that enables a regiospecific synthesis of broad range of aryl and cyano substituted product using a 3-component Cu enables [3 + 2] annulation reaction [36].
Zhao et al. planned a method that gives access to the formation of disubstituted nucleus in good yield under tepid conditions [37].
Wong et al. designed a method in which 1,3,4-oxadiazolium hexafluorophosphate salt reacts with cyanamide to form desired product in satisfactory yield [38].
Bechara et al. planned the activation of triflic anhydride trailed by cyclodehydration induced by microwave irradiation. It forms the basis of single canister formation of 3,4,5-trisubstituted-1,2,4-triazoles [39].
Detailed comparative analysis of all the schemes mentioned above
| Scheme | Yield (%) | Benefit | Reference |
|---|---|---|---|
| Scheme 1 | 84–94 | Conducive and decent procedure | [12] |
| Scheme 2 | 75–93 | Entire utilisation of starting materials procuring of regioselectivity | [14] |
| Scheme 3 | ∼99 | Smooth product segregation stereospecific product | [15] |
| Scheme 4 | 80–99 | MW radiations substantially augment the rate of reaction | [16] |
| Scheme 5 | 86–93 | Budgetary, harmless, and efficacious procedure | [17] |
| Scheme 6 | 86–97 | NPs lift up the rate of reaction, regioisomer formation is accomplished | [18] |
| Scheme 7 | 82–94 | Magnificent salvageable reaction medium | [19] |
| Scheme 8 | 46–85 | Metal-free blueprint, swift procedure | [20] |
| Scheme 9 | 69 | Contribution pointing to pragmatic and rational approach to preclude IDO | [21] |
| Scheme 10 | 63–96 | Correspondent to infamous 1,3-dipolar cycloaddition | [22] |
| Scheme 11 | 94–99 | Efficacious catalyst | [23] |
| Scheme 12 | 90–98 | Benign for usage in non-aqueous solvents | [24] |
| Scheme 13 | 58–83 | Tepid conditions, regioselective product | [25] |
| Scheme 14 | ∼91 | Viably smooth, reaction advances in aqueous and non-aqueous solvents at room temperature | [26] |
| Scheme 15 | 79–96 | Reducing agent is not requisite, segregation of catalyst by plain filtration | [27] |
| Scheme 16 | 79–90 | Regiocontrolled product | [28] |
| Scheme 17 | 73–86 | Uncomplicated method, effortlessly accessible substrates | [29] |
| Scheme 18 | 77–98 | Non-lethal by-products, detainment of −NO2 group | [30] |
| Scheme 19 | 94–98 | Crisp reaction time, green procedure | [31] |
| Scheme 20 | 54–81 | Single pot synthesis, nominal side reactions | [32] |
| Scheme 21 | 88–96 | Crisp reaction time, ambient conditions | [33] |
| Scheme 22 | 82–92 | Reaction assuages the concern of green chemistry | [34] |
| Scheme 23 | 53–91 | Method is adapted for distinct aldehydes | [35] |
| Scheme 24 | 40–84 | Tepid reaction conditions | [36] |
| Scheme 25 | ∼91 | Competent and diverse synthesis | [37] |
| Scheme 26 | 62–88 | Secure and ascendible synthesis | [38] |
| Scheme 27 | 81–85 | Judicious and productive synthesis | [39] |

Designing of 1,2,3-triazole nucleus under balmy conditions.

Formation of 1,2,3-triazole nucleus using ruthenium catalyst.

Formation of desired molecule in the ubiquity of heat.

Formation of 1,2,3-triazole using microwave radiations.

Formation of 1,2,3-triazole nucleus using Cu NPs.

Formation of 1,2,3-triazole nucleus using Au NPs.
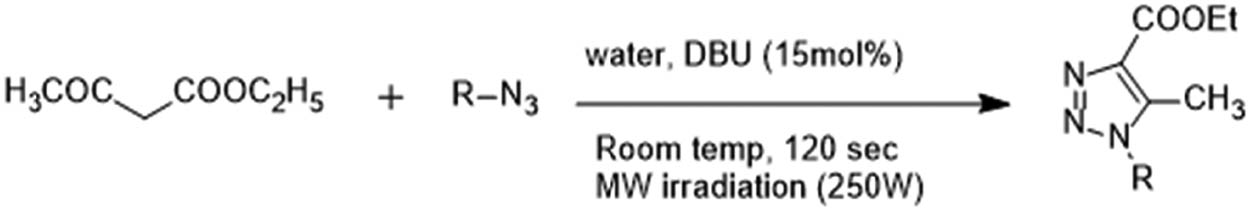
Formation of 1,2,3-triazole using DBU-water catalyst and microwave radiations.

Formation of 1,2,3-triazole using DMSO, iodine, and methane sulfonic acid.

Formation of 1,2,3-triazole using DMSO and iodine.

Formation of 1,2,3-triazole nucleus using DMF and p-toluene sulfonic acid.

Formation of desired nucleus using sodium nitride, sodium ascorbate, water, butanol, and catalyst.

Formation of 1,2,3-triazole using acid and base.

Formation of 1,2,3-triazole using Grignard reagent, Phenyl nitride, THF, and NH4Cl.

Formation of 1,2,3-triazole using aqueous caesium carbonate.

Synthesis of 1,2,3-triazole using Nanoparticles.

Formation of 1,2,3-triazole employing ruthenium catalyst.

Formation of 1,2,3-triazole having copper iodide, sodium ascorbate, DBU, and DMSO in the vessel.

Formation of 1,2,3-triazole using ball milling method.

Synthesis of 1,2,4-triazole derivative using ultrasonic waves.

Formation of 1,2,4-triazole using microwave radiations.
![Scheme 21
Formation of 1,2,4-triazole derivative with poly(ethylene glycol) [PEG].](/document/doi/10.1515/hc-2022-0174/asset/graphic/j_hc-2022-0174_fig_073.jpg)
Formation of 1,2,4-triazole derivative with poly(ethylene glycol) [PEG].

Formation of 1,2,4-triazole using mild conditions.

Formation of 1,2,4-triazole derivative using reflux.

Formation of 1,2,4-triazole adopting the use of copper bromide and potassium carbonate.

Formation of 1,2,4-triazole using methyl alcohol and sulphuric acid.

Formation of 1,2,4-triazoles in one pot using microwave radiations.

Formation of 1,2,4-triazole using DCE, trifluoromethanesulfonic anhydride, and microwave radiations.
Commercially used antimycobacterial drugs having triazoles
| S. No. | Name of drug | Structure | Activity |
|---|---|---|---|
| 1. | Tazobactam [40] |
 |
Antibacterial |
| 2. | Fluconazole [41] |
 |
Antifungal |
| 3. | Ravuconazole [42] |
 |
Antifungal |
| 4. | Cefatrizine [43] |
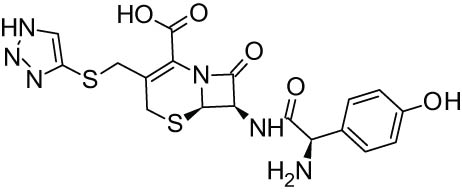 |
Antibacterial |
| 5. | Itraconazole [44] |
 |
Antifungal |
| 6. | Posaconazole [45] |
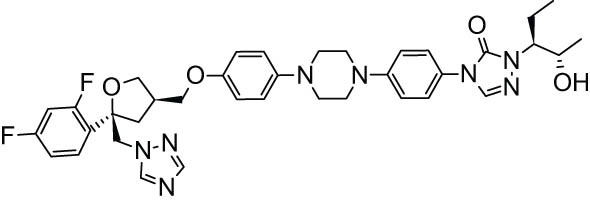 |
Antifungal |
| 7. | Voriconazole [46] |
 |
Antifungal |
| 8. | I-A09 (under clinical evaluation) [47] |
 |
Antitubercular |
3 Laboratory tests used to test antimycobacterial activity
Microbial resistance is augmenting per diem which urges the progression of more efficient antimicrobial drugs. The methods generally used for testing the activity are disk diffusion method, agar or broth dilution, cross streak, poisoned food technique, flow cytofluorometric test, time-kill test, ATP bioluminescence assay, and antimicrobial gradient method [48].
Minimum inhibitory concentration (MIC) values are compared with the reference standards. More the MIC value, the less effective the drug is and lesser the MIC value, the more effective the drug is.
4 Literature review
This work reviews various pharmacological activities shown by triazoles.
4.1 Antimycobacterial activity
Patel et al. synthesised a parent moiety in which benzothiazole ring was clubbed with 1,2,4-triazole (both separately have antimicrobial properties) and studied their antimicrobial activity using microdilution method. Analogues carrying halogen, nitro, and methyl group as substituents acted as promising antibacterial agents and antifungal activity is shown promisingly by analogues having methyl substituent. Compounds showing promising antibacterial activity showed comparable antitubercular activity too. One compound (Compound 1) having Cl at fourth position shows better antitubercular activity out of all the synthesised compounds [49]. Figure 3 shows the pictorial representation.

Compound 1 showing better antibacterial and antitubercular activity than other synthesised compounds.
Singh and Kumar Singh (2010) synthesised 1, 2, 4- triazole (substituted with aryl and pyridyl groups at fourth and fifth positions, respectively) molecules and studied their bactericidal activity. Desired compounds are formed with 70–85% yield. Significant bactericidal activity is shown by the newly formed compounds (Compounds 2a, 2b, and 2c). TLC was used to check the purity of the formed compounds [50]. Figure 4 shows the pictorial representation.

Compounds 2a, 2b, and 2c showing significant antibacterial activity.
Patil et al. synthesised compounds shown in Figure 5 and tested the compounds for antibacterial and antifungal activity. Satisfactory yields were obtained. MIC of targeted compounds was compared with reference standards, and it was established that synthesised compounds (Compounds 3a and 3b) exhibited poor antifungal activity but significant antibacterial activity against the strains mentioned above [51].

Compounds 3a and 3b showing convincing antibacterial activity.
Zhang et al. synthesised propionates and acetates and studied their antifungal activity using mycelial growth rate method. Benzimidazole and 1,2,4-triazole moieties have been found to hog antifungal and antibacterial activities. Targeted compounds were found to be more operative against B. cinerea than S. sclerotiorum when compared with EC50 values of Carbendazim. No significant difference in EC50 values of acetates (4a) and propionates (4b) was found when compared, which means both class of compounds (4a and 4b) showed similar fungicidal activity [52]. Figure 6 shows the pictorial representation.

Class of compounds 4a (acetates) and 4b (propionates) showing good antibacterial activity.
Ouahrouch et al. contended that the targeted compounds were synthesised using Cu-AAC reaction with the assistance of microwave radiations. Kremsner and Kappe noted that various organic synthesis could be performed efficiently by irradiating the substrates with microwaves [53]. Antifungal activity was studied against phytopathogenic fungi Fusarium oxysporum (Foa) and Verticillium dahliae (VD). Standard antibacterial drugs used for comparison were Ciprofloxacin and Linezolid and positive control used for comparing antifungal activity was PELT (a fungicide which is a precursor of benzimidazole having 70% of methyl thiophanate). MICs were tested for the targeted compounds, and all showed less antibacterial activity than the standard drugs. All the title compounds (5a–h) except a compound carrying CF3 group showed less antifungal activity and the reason might be attributed to the good lipophilicity of CF3 group which might increase absorption and thus enhanced its activity [54]. Figure 7 and Chart 1 shows the synthesised compounds and their activity.

The structure of formed compounds with various substituents.
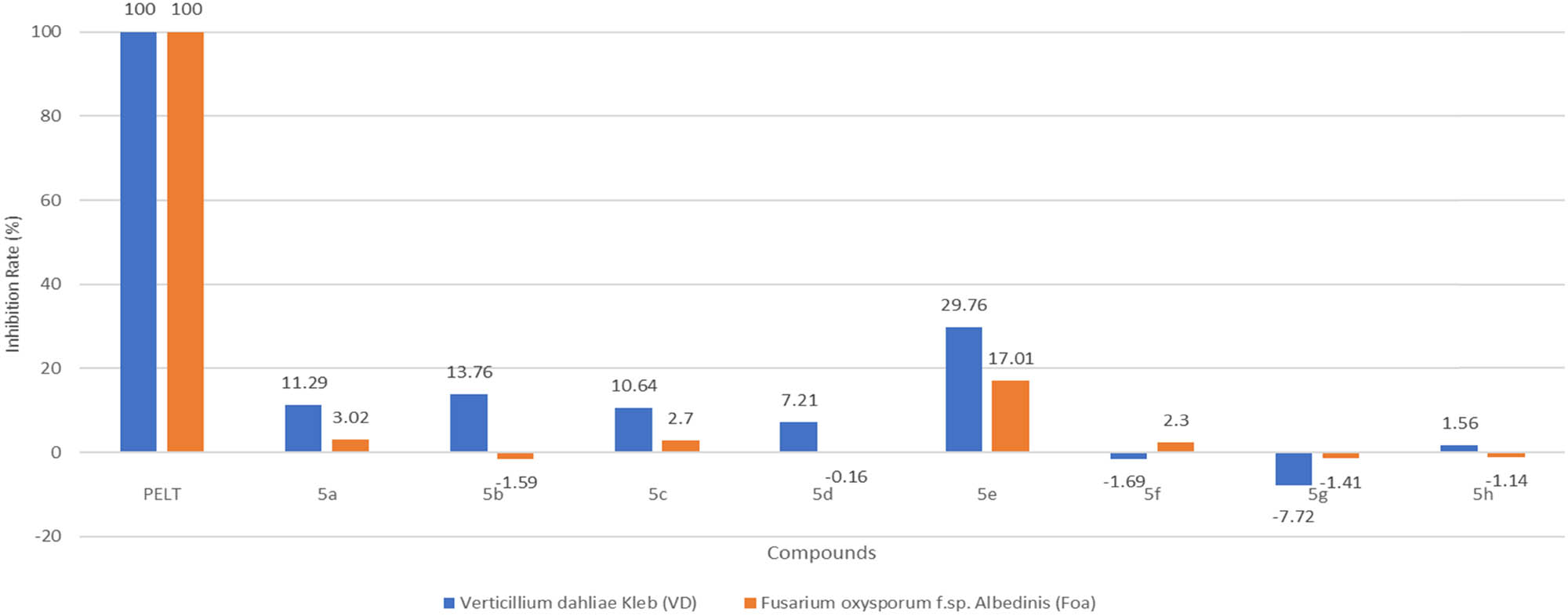
Paralleling of inhibition rate of compounds 5a–h on eighth day against VD and Foa at 20 µg/mL.
Shi & Zhou synthesised triazole derivatives of coumarin by clubbing 1,2,4-triazolyl ring with coumarin in order to increase their efficiency against microbes and two-fold serial dilution technique was adopted to gauge antimicrobial activity. Antibacterial and antifungal activity were shown more promisingly by coumarin bis-triazoles as compared to their mono-triazole analogues. With increment in the length of aliphatic chain of substituent, the antimicrobial activity decreased because increase in chain decreases the water solubility of the compounds and water-soluble alkyl triazole hydrochloride precursors of coumarin show stronger antimicrobial efficacy than less water soluble aralkyl triazole precursors which is also supported by literature [55]. Figure 8 and Charts 2–4 show the graphical representations.

Structure of the synthesised compounds.

Paralleling of MIC of coumarin triazoles, coumarin bis-triazoles, and their corresponding hydrochlorides with reference standard against Gram-positive bacteria.

Comparison of MIC of coumarin triazoles, coumarin bis-triazoles, and their corresponding hydrochlorides with reference standard against Gram-negative bacteria.

Comparison of MIC of coumarin triazoles, coumarin bis-triazoles, and their corresponding hydrochlorides with reference standard against different fungal strains.
Afreen et al. performed synthesis of compounds 10–12 and their anti-TB activity was analysed. Yields of products were found to be in the range of 62–82%. TLC was used to check the purity of compounds and solvent system used for TLC is CH3OH:CHCl3:CH3COC2H5 = 1:2:7. MIC (µg/mL) was analysed and parelleled with the reference drug and one compound exhibited MIC 12.5 µg/mL and Streptomycin exhibited MIC 6.25 µg/mL [56]. MIC values of compounds 10–12 are shown graphically in Chart 5 and structures in Figure 9.

Comparison of MIC values of different synthesised compounds with reference standards.

Figure showing structure of the above discussed compounds.
Seelam et al. synthesised new heterocyclic compounds in which different moieties like pyrazole, 1,2,4-triazole, thiazole, and isoxazole were clubbed together as all separately possess biological activity. Antimycobacterial and Antitubercular activities of the targeted compounds were studied. The targeted compounds were endowed to possess property against Gram positive bacteria than the negative one. MIC was studied for comparing the activity. Potentiality of electron withdrawing groups cause an accretion in bactericidal property of compounds. All the synthesised compounds showed modest antifungal activity. The tested compounds showed propitious antitubercular activity [57]. Figure 10 (compounds 13 and 14) shows the graphical representation.

Pictorial representation showing increase in antibacterial activity on the grounds of potentiality of electron withdrawing groups.
Slivka et al. synthesised [1,3]thiazolo[3,2-b][1,2,4] triazol-7-ium salts via regioselective halocyclisation. Bromine was found to be a better stereoselective electrophilic reagent than Iodine and Iodine monobromide as almost 90% E-isomer of product was formed using bromine and mixture of E- and Z- isomers were formed using Iodine and Iodine monobromide. The compounds were synthesised by electrophilic heterocyclisation and their regioselectivity and stereoselectivity were analysed. Use of tellurium tetrahalogenides resulted in the synthesis of blend of geometrical isomers of the salts. Non-selective halogenated products were formed with less non-polar solvents. Use of selenium tetrahalides in place of tellurium counterparts led to formation of products i.e. amorphous selenium and other oily products that posed a challenge in purification and it complied with the literature which states that selenium containing products are unstable. Out of several derivatives, compound 15 shown in Figure 11 showed antimicrobial activity [58].

Picture showing the structure of formed compound.
Marepu et al. synthesised new moiety in which 1,2,3-triazole was merged with pyridine/pyrimidine and studied their antimycobacterial activity. Targeted compounds were synthesised regioselectively using Buchwald’s strategy. Promising antibacterial activity was shown by two of the triazolopyridines when compared with Streptomycin. Triazolopyrimidines did not work as desired so they possess future scope of study as further improvement can be done in these molecules to make them more effective antimycobacterial agents [59]. Figure 12 (compounds 16 and 17) shows the pictorial representation.

Compound 16 and 17 showing good antibacterial activity.
Santosh et al. contended to orchestrate and design an exclusive streak of 1,2,3-triazole-chalcone scaffolds for studying their antibacterial activity. α, β-unsaturated carbonyl group of moiety was further modified to pyrazole, pyrimidine, isooxazoline, and cyanopyridine as these groups separately have biological activity as per literature. Fluoro-substituted chalcone and nitro-substituted pyrazoles showed good antibacterial activity as both possessed inhibitory activity against Chorismate synthase. Substituted pyrimidine and cyanosubstituted pyridine showed good DPPH radical scavenging activity. Title compounds also possessed great binding affinity for DNA [60]. Figure 13 (compounds 18a and b) shows the pictorial representation of activity.

Compounds showing good antibacterial activity and good DPPH radical scavenging activity.
Singh et al. reported that formation of SBTBS was welded by Cu(i) catalysed click silylation reaction (a single step reaction) from SBTBOTS as individually all three, i.e. 1,2,3-triazole, Schiff base and silatrane show antibacterial activity. Yield was found to be greater than 90%. Literature says that trigonal bipyramidal structure of silatrane causes increase in electronegativity and dipole moment which facilitates their chemisorption in cell membrane by H-bonding with equatorial oxygen atom and dipole-dipole interactions and the fact of showing specific chemical properties by silatranes might be attributed to the transannular N–Si dative bond. Literature also supports that 2-hydroxy-1-naphthaldehyde act as versatile fluorescent chemosensor, so, clubbing it with 1,2,3-triazole bridged silatrane and primary amine presents a coherent fluorophore unit. All the formed compounds were tested for antibacterial activity. The synthesised bridged silatranes were established to be more stable towards hydrolysis as compared to their triethoxysilane analogues. Out of all compounds, the entity having methoxy substituent, compound 19 (Figure 14) was endowed to acquire highest counter-bacterial property, even comparable to the reference standard used. The results showed more effective affinity towards Gram +ve bacteria as paralleled to its −ve counterpart because latter possess an extra membrane [61].

Structure of the synthesised compound.
Paneth et al. orchestrated a streak of innovative Mannich bases containing fluorophenyl moiety and Broth microdilution process was mobilised to study in vitro activity against bacterial −ve and +ve strains. 90% yield was obtained for targeted compounds.The derivatives with single chlorine group on phenylpiperidine showed the maximum activity. Presence of two chlorine atoms on phenylpiperidine substituent increased the activity against Gram −ve bacteria by two-fold but decreased the activity against Gram +ve bacteria. Furanopiperazine derivative exhibited a considerable reduction in activity as paralleled to phenylpiperazine derivatives. The ubiquity of phenyl group at piperazine’s fourth position attached to the synthesised compound 20 (Figure 15) was established to be a necessary state of affairs to display counter-bacterial property. Nearly all the targeted compounds showed less activity than the reference standards used [62].

Structure of the targeted compound.
Nalawade et al. reported that title compounds were synthesised after clubbing thiazole, pyrazole, and 1,2,3-triazole ring together and their counter-microbial property was studied. Literature survey suggests that clubbed thiazole-pyrazole possess antitubercular, antimicrobial and anti-inflammatory activity; the clubbed thiazole-triazole possess antitubercular and antimicrobial activity; the clubbed triazole-pyrazole possess anticancer, antibacterial and antifungal activity which is comparable to antibacterial drug Streptomycin and antifungal drug Fluconazole. Moderate antibacterial activity was shown by the title compounds against Gram +ve bacteria. Promising activity was shown by compounds against fungi under testing which concluded that azole derivatives might have blocked ergosterol biosynthesis by impeding the activity of cytochrome P450 enzyme which is required for formation of ergosterol from lanosterol where ergosterol, being a considerable part and parcel of fungal membrane, helps in maintaining the functions of the fungal cell. To confirm the mechanism, molecular docking analysis was done. The targeted compounds 21a–i shown in Figure 16 exhibited paralleled activity with standard drugs under testing [63].

Structure of the formed compounds.
Agisho et al. reported that 3,5-disubstituted-1,2,4-triazoles and 1,3,5-trisubstituted–1,2,4-triazoles were synthesised in which tert-butyl hydroperoxide is used as an oxidant and tetra N-butylammonium iodide as catalyst. Yields obtained were more than 70%. The exact mechanism is unclear yet and provides a demand for future scope of study though a radical pathway had been suggested and radical trapping method was used to confirm the mechanism. The synthesised compounds 22a–d shown in Figure 17 exhibited bactericidal activity more against E. coli than S. aureus [64].

Structure of the targeted compounds.
Bangalore et al. says that usnic acid obtained from lichens was clubbed with 1,2,3-triazoles and further modifications were done in the clubbed structure on which antitubercular and antibacterial activity were studied. The desired products were obtained in average yield, i.e. >40%. It was proved that the presence of two fluorine atoms was beneficial for antitubercular property. Presence of more or less fluorine atoms decreased the activity. Structure activity relationship studies implied that potency of the molecules augmented due to the ubiquity of fluorine atoms as compared to chlorine atoms. Though p-halogen is favourable, but the deciding factor is the m-halogen. Antitubercular activity had not been shown by the compounds having methoxy substituent whereas the same compound showed good antibacterial activity. Cyclic sulphonamide moiety was proved to be a complementing feature to usnic acid enamine. Tricyclic systems with structural modification might be effective in showing the activity. Compounds with difluorophenylacyl and biphenylacyl substituents can be further used for future drug development [65]. The synthesised compound 23 is shown in Figure 18.

Picture showing targeted compound with MIC value.
Maddila et al. synthesised an array of unique pyrano[2,3-d]-pyrimidine via cycloaddition of alkynyl pyranomyrimidinone with differently substituted aryl azides. Titled compounds got tested for bactericidal and fungicidal properties. ZoI of titled compounds was studied and paralleled with the reference standards. Presence of e− withdrawing groups, i.e. fluoro and nitro, on benzene moiety affixed to 1,2,3-triazole of pyranomyrimidine ring (compounds 24 and 24a, shown in Figure 19) caused increase in antibacterial and antifungal activities. The ubiquity of e− donating groups on benzene moiety affixed to 1,2,3-triazole of pyranomyrimidine ring caused decrease in antibacterial activity [66].

Structure of the targeted compounds.
Pradeep Kumar et al. synthesised unique 1,2,3-triazole based imidazole derivatives. The titled compounds were synthesised using CuAAC reaction and compounds were obtained with satisfactory yields. The compounds containing amino and amido substitution were proved to be good anti-TB agents and cytotoxicity studies proved that the titled compounds showed low toxicity. But these hybrid compounds 25 and 25a (Figure 20) could be further modified by trying different substitutions to get more potent molecules [67].

Structure of the targeted compounds.
Negi and Rawat (2022) synthesised 21 novel hybrid compounds in vitro having thymol and 1,2,3-triazole and certified against germ causing tuberculosis, i.e. Mycobacterium tuberculosis and 13 compounds were found to be effective against H37Rv strain of bacteria at 50 µg/mL (Figure 21). The reference standards used are isoniazid, ethambutol, and rifampicin whose MIC99 are 0.2, 0.5, and 0.25 µg/mL respectively. Further accrual can be done to increase the potency of the synthesised molecules [68].

Structure of the synthesised compounds.
Basheen (2022) synthesised innovative class of compounds of hydrazones and bis-hydrazones possessing 1,2,3-triazole component. Yields are above 80%. The characterisation was done adopting distinctive spectroscopic approach. The synthesised compounds 28a–c (Figure 22) show balanced activity against bacteria [69].

Structure of the desired compounds.
Reddyrajula et al. synthesised 36 hybrid molecules comprising phenothiazine, 1,2,3-triazole using avenue of molecular hybridisation and tested against H37Rv strain of Mycobacterium tuberculosis and out of these synthesised compounds, 19 compounds display symbolic antibacterial activity with MIC 1.6 µg/mL. The reference drugs used are pyrazinamide and isoniazid. The synthesised compounds have good oral bioavailability and are non-toxic. The studies advocated that ubiquity of e− attracting moieties like –NO2, –CN, –F on phenyl ring affixed to 1,2,3-triazole heightens pharmacological property of synthesised compounds [70]. Figure 23 shows the pictorial representation of the strategy.

Strategy showing formation of the desired compounds.
4.2 Anti-inflammatory activity
Paprocka et al. contended that a plethora of novel 1,2,4-triazole compounds along with methacrylic acid got synthesised and out of all the targeted compounds, the compound 29 depicted in Figure 24 showed proportionate anti-inflammatory activity with ibuprofen [71].

Compound showing unrivalled anti-inflammatory activity.
Haider et al. synthesised novel series of compounds which were bis-heterocycles possessing 1,2,3-triazole moiety and found that out of all the synthesised compounds, the compound 30 shown in the Figure 25 came out as a compelling anti-inflammatory agent and the logic for this activity is attributed to the presence of one extra π-π bond [72].

Compound showing incomparable anti-inflammatory activity.
Shafi et al. prepared a series of compounds which are bis-heterocycles bearing 1,2,3-triazole moeity and discovered a compound 31 (Figure 26) exhibiting good anti-inflammatory activity as Ibuprofen and biochemical cyclooxygenase activity [73].

Compound showing excellent anti-inflammatory activity.
Almasirad et al. proposed the orchestration of hybrid compounds containing imidazole and 1,2,4-triazole moiety. Their anti-inflammatory profiles were tested. They found out that two compounds 32 and 33 depicted in Figure 27 showed unmatched anti-inflammatory activity [74].

Compounds showing distinguished anti-inflammatory activity.
4.3 Anti-convulsant activity
Song et al. synthesised the hybrid compounds possessing 1,2,4-triazole and certified for anticonvulsant property using seizure models in mice. The compound 34 in Figure 28 proved to be a roseate anticonvulsant agent showing ED50 values (effective dosage of a medicine that produces 50% of the achievable response) of 23.7 and 18.9 mg/kg [75].

Compound showing promising anti-convulsant activity.
Mahdavi et al. synthesised two novel compounds and tested them for anticonvulsant activity by measuring ED50 values and Diazepam was used as a reference standard. It was found that out of two synthesised compounds 35 and 36 (Figure 29), one of them (compound 36) showed comparable activity with the reference standard [76] (Chart 6).

Novel synthesised compounds.

Graphical comparison of the anticonvulsant activity of the synthesised compounds with the reference standard using ED50 values. Smaller the ED50 values, more potent the drug is.
Wang et al. formed a library of various molecules possessing 1,2,4-triazole and tested for anticonvulsant property adopting MES and scPTZ studies. Also, the neurotoxicity of the targeted compounds was detected by Rotarod test. It was found that a compound 37 (Figure 30) showed outstanding anticonvulsant activity which was comparable to the clinical drugs such as Ethosuximide and Carbamazepine [77].

A potent anticonvulsant compound.
Dehestani et al. orchestrated a library of phenacyl-1,2,4-triazole hydrazones via hybridisation approach. The above mentioned compounds were checked for the anticonvulsant property adopting MES and PTZ tests. A compound 38 (Figure 31) emerged out to be a strong anticonvulsant agent [78].

The synthesised compound showing remarkable anticonvulsant activity.
4.4 Anticancer activity
Ma et al. synthesised hybrids containing pyrimidine and 1,2,3-triazole and checked for anticancer property. A compound 39 (Figure 32) was found to act like a futuristic clinical anticancer drug with IC50 value ranging from 1.42–6.52 µM [79].

A potent anticancer drug.
Aouad et al. prepared di- and tri-substituted 1,2,3-triazoles clubbed with piperazine and benzothiazole with and without copper(i) catalyst. The series of compounds formed were endowed to possess activity against cancer cells chosen to test anticancer property. The compound 40 (Figure 33) emerged out to be a star compound in showing anticancer activity [80].
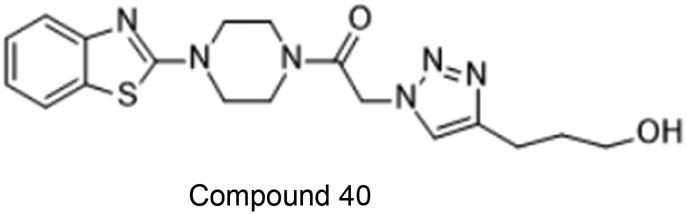
This compound shows best anticancer activity out of all the synthesised compounds.
Saftic et al. synthesised a library of novel derivatives and a derivative having smallest substituent at fourth carbon of triazole (Compound 41, Figure 34) was endowed being active out of all synthesised ones against cancer cell lines [81].

A compound showing anticancer activity.
Duan et al. performed the formation of array of unique hybrids having 1,2,3-triazole and thiocarbamate and tested against cancerous cells. Two compounds (42 and 43, Figure 35) were established as more active against cancerous cells as compared to other synthesised compounds having IC50 value ranging from 0.73–11.61 µM [82].
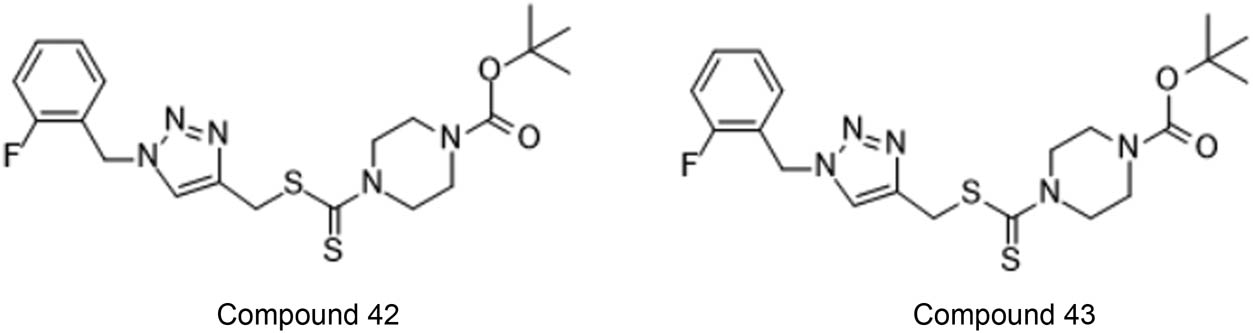
Compounds which emerged as active against the chosen cancerous cells.
4.5 Antioxidant activity
Vieira Veloso et al. synthesised 30 glucal based mono- and bis-1,2,3-triazoles. They found out that a compound 44 (Figure 36) had antioxidant properties and it reduced the oxidative stress by acting on red blood corpuscles which in turn helps in the treatment of sickle cell disease [83].

A mono triazole compound possessing antioxidant activity.
AlNeyadi et al. synthesised various triazole derivatives. These molecules are checked for antioxidant property using DPPH (a common method to test the antioxidant activity) and found a compound 45 (Figure 37), which possessed antioxidant activity with IC50 value of 5.84 µg/mL [84].

A triazole-thiol derivative possessing antioxidant activity.
Shaikh et al. synthesised derivatives possessing 1,2,3-triazole and coumarin and assessed various biological properties. The antioxidant activity was tested using DPPH radical scavenging activity and a compound 46 (Figure 38) was found, which had good antioxidant activity [85].

Coumarin based 1,2,3-triazole compound having antioxidant properties.
Kaushik and Chahal synthesised a library of various hybrids possessing 1,2,3-triazole and coumarin moiety and found that two compounds 47 and 48 (Figure 39) possessed good antioxidant properties and four compounds 49–52 (Figure 40) possessed antimalarial activity [86].

Compounds showing antioxidant activity.

Compounds bearing antimalarial properties.
4.6 Antiviral activity
He et al. synthesised two strings of various compounds and tested for antiviral activity counter to influenza A and influenza B virus. It was established that one compound 53 (Figure 41) retained good activity against influenza A virus and three compounds 54–56 (Figure 42) retained valuable activity against influenza B virus [87].

Structure of compound which was found active against influenza A virus.

Structure of compounds which were found to be active against influenza B virus.
El-Sayed et al. synthesised a unique string of compound suspended with 1,2,3-triazole, pyridine, and carbohydrate moiety. Their antiviral property was checked against H5N1 strain of influenza virus. It was found that a compound 57 (Figure 43) possessed better antiviral activity out of all the synthesised compounds [88].

Structure of an antiviral compound.
Witkowski et al. synthesised ribonucleoside suspended 1,2,4-triazole moieties. Their antiviral activity was checked against different virus. It was found that compounds 58–60 (Figure 44 and Chart 7) showed antiviral activity [89].

Compound 53 is active against parainfluenza, herpes simplex, and rhino virus, Compound 54 is active against herpes simplex virus, and Compound 55 is active against herpes simplex, parainfluenza, and rhino virus.

Graphical representation of activity of the synthesised compounds against different virus.
De Lourdes et al. synthesised various hybrid compounds of 1,2,3-triazole. Their antiviral activity was paralleled with reference standard Ribavirin. A compound 61 (Figure 45) was endowed to have activity commensurable to the master reference with IC50 value of 14 µM for influenza A virus and 3.8 µM for HIV-1 virus [90].

A compound showing good antiviral activity against HIV-1 and influenza A.
5 Conclusion
Triazoles can be synthesised by various time-saving and energy-saving methods. Nitrogen compounds being versatile elements provide a huge platform to be incorporated with other elements to form various therapeutic agents. The groups which show biological activity are benzothiazole, coumarin, benzimidazole, pyridine, pyrazole, imidazole, pyrimidine, thiazole, chalcone, silatrane, phenothiazine, hydrazine, thymol, benzoxazole, benzothiazole, and glycoside which can be clubbed either together or with 1,2,3-triazole and 1,2,4-triazole with some modifications to form a new moiety which act as more potent remedial agent.
Acknowledgements
School of Basic and Applied Sciences of K. R. Mangalam University, Sohna, Gurugram is acknowledged for guiding us and providing us adequate sources to prepare the review.
-
Funding information: Authors state no funding involved.
-
Author contributions: Akshi Goyal – Conceptualization Data curation, Formal analysis, writing of First draft. Meena Bhandari – Supervision, Revision, Editing, Verification.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Jampilek J. Heterocycles in medicinal chemistry. Molecules. 2019;24(21):10–3.10.3390/molecules24213839Search in Google Scholar PubMed PubMed Central
[2] Haider S. Heterocycles, back bone of drug design. Phytochem Biochem. 2017;1(1):2017.Search in Google Scholar
[3] Bozorov K, Zhao J, Aisa HA. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg Med Chem. 2019;27(16):3511–31.10.1016/j.bmc.2019.07.005Search in Google Scholar PubMed PubMed Central
[4] Qi L, Li H, Zhang C, Liang B, Li J, Wang L, et al. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiology. 2016;7:483.10.3389/fmicb.2016.00483Search in Google Scholar PubMed PubMed Central
[5] Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Fraga CAM. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem. 2007;14(17):1829–52.10.2174/092986707781058805Search in Google Scholar PubMed
[6] Sunil RJ, Pal S, Jayashree A. Molecular hybridization-an emanating tool in drug design. Med Chem. 2019;9(6):93–5.Search in Google Scholar
[7] Singh G, Singh L, Ishar MPS. 2-(N-Methylanilino)-3-formylchromone – a versatile synthon for incorporation of chromone moiety in a variety of heterocyclic systems and macrocycles through reactions with bifunctional nucleophiles. Tetrahedron. 2002;58(39):7883–90.10.1016/S0040-4020(02)00908-0Search in Google Scholar
[8] (a) Martens S, Mithöfer A. Flavones and flavone synthases. Phytochemistry. 2005;66(20):2399–407; (b) Kuroda M, Uchida S, Watanabe K, Mimaki Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry. 2009;70(2):288–93.Search in Google Scholar
[9] Sahu JK, Ganguly S, Kaushik A. Triazoles: A valuable insight into recent developments and biological activities. Chin J Nat Med. 2013;11(5):456–65.10.1016/S1875-5364(13)60084-9Search in Google Scholar PubMed
[10] Bonandi E, Christodoulou MS, Fumagalli G, Perdicchia D, Rastelli G, Passarella D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discovery Today. 2017;22(10):1572–81.10.1016/j.drudis.2017.05.014Search in Google Scholar PubMed
[11] Dixit D, Verma PK, Marwaha RK. A review on ‘triazoles’: their chemistry, synthesis and pharmacological potentials. J Iran Chem Soc. 2021;18(10):2535–65.10.1007/s13738-021-02231-xSearch in Google Scholar
[12] Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, et al. Copper (I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc. 2005;127(1):210–6.10.1021/ja0471525Search in Google Scholar PubMed
[13] Worrell BT, Malik JA, Fokin VV. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science. 2013;340(6131):457–60.10.1126/science.1229506Search in Google Scholar PubMed PubMed Central
[14] Boren BC, Narayan S, Rasmussen LK, Zhang L, Zhao H, Lin Z, et al. Ruthenium-catalyzed azide−alkyne cycloaddition: Scope and mechanism. J Am Chem Soc. 2008;130(28):8923–30.10.1021/ja0749993Search in Google Scholar PubMed
[15] Kalyan Keerthi P. Synthesis of 1, 2, 3-triazoles by click chemistry. Western Illinois University ProQuest Dissertations Publishing; 2020.Search in Google Scholar
[16] Moorhouse AD, Moses JE. Microwave enhancement of a ‘one-pot’ tandem azidation-‘click’ cycloaddition of anilines. Synlett. 2008;2008(14):2089–92.10.1055/s-2008-1078019Search in Google Scholar
[17] Nasrollahzadeh M, Sajadi SM, Mirzaei Y. An efficient one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles at room temperature by green synthesized Cu NPs using Otostegia persica leaf extract. J Colloid Interface Sci. 2016;468:156–62.10.1016/j.jcis.2016.01.050Search in Google Scholar PubMed
[18] Boominathan M, Pugazhenthiran N, Nagaraj M, Muthusubramanian S, Murugesan S, Bhuvanesh N. Nanoporous titania-supported gold nanoparticle-catalyzed green synthesis of 1,2,3-triazoles in aqueous medium. ACS Sustainable Chem Eng. 2013;1(11):1405–11.10.1021/sc400147rSearch in Google Scholar
[19] Singh H, Sindhu J, Khurana JM. Synthesis of biologically as well as industrially important 1,4,5-trisubstituted-1,2,3-triazoles using a highly efficient, green and recyclable DBU-H2O catalytic system. RSC Adv. 2013;3(44):22360–6.10.1039/c3ra44440fSearch in Google Scholar
[20] Shu W-M, Zhang X-F, Zhang X-X, Li M, Wang A-J, Wu A-X. Metal-Free Cascade [4 + 1] cyclization Access to 4-Aryl-NH-1,2,3-triazoles from N-tosylhydrazones and sodium azide. J Org Chem. 2019;84(22):14919–25.10.1021/acs.joc.9b02250Search in Google Scholar PubMed
[21] Huang C, Geng X, Zhao P, Zhou Y, Yu X-X, Wang L-S, et al. Direct synthesis of 4-Aryl-1,2,3-triazoles via I2-promoted cyclization under metal- and azide-free conditions. J Org Chem. 2021;86(19):13664–72.10.1021/acs.joc.1c01702Search in Google Scholar PubMed
[22] Quan XJ, Ren ZH, Wang YY, Guan ZH. p-Toluenesulfonic acid mediated 1, 3-dipolar cycloaddition of nitroolefins with NaN3 for synthesis of 4-aryl-NH-1, 2, 3-triazoles. Org Lett. 2014;16(21):5728–31.10.1021/ol5027975Search in Google Scholar PubMed
[23] Yamada YM, Sarkar SM, Uozumi Y. Amphiphilic self-assembled polymeric copper catalyst to parts per million levels: click chemistry. J Am Chem Soc. 2012;134(22):9285–90.10.1021/ja3036543Search in Google Scholar PubMed
[24] Shao C, Wang X, Zhang Q, Luo S, Zhao J, Hu Y. Acid–base jointly promoted copper (I)-catalyzed azide–alkyne cycloaddition. J Org Chem. 2011;76(16):6832–6.10.1021/jo200869aSearch in Google Scholar PubMed
[25] Liu D, Gao W, Dai Q, Zhang X. Triazole-based monophosphines for suzuki−miyaura coupling and amination reactions of aryl chlorides. Org Lett. 2005;7(22):4907–10.10.1021/ol051844wSearch in Google Scholar PubMed
[26] Kim WG, Kang ME, Lee JB, Jeon MH, Lee S, Lee J, et al. Nickel-catalyzed azide–alkyne cycloaddition to access 1, 5-disubstituted 1, 2, 3-triazoles in air and water. J Am Chem Soc. 2017;139(35):12121–4.10.1021/jacs.7b06338Search in Google Scholar PubMed
[27] Khalili D, Kavoosi L, Khalafi-Nezhad A. Copper aluminate spinel in click chemistry: An efficient heterogeneous nanocatalyst for the highly regioselective synthesis of triazoles in water. Synlett. 2019;30(19):2136–42. 10.1055/s-0039-1690719 Search in Google Scholar
[28] Ferrini S, Chandanshive JZ, Lena S, Comes Franchini M, Giannini G, Tafi A, et al. Ruthenium-catalyzed synthesis of 5-amino-1, 2, 3-triazole-4-carboxylates for triazole-based scaffolds: beyond the Dimroth rearrangement. J Org Chem. 2015;80(5):2562–72.10.1021/jo502577eSearch in Google Scholar PubMed
[29] Cheng X, Yang Y, Kuang C, Yang Q. Copper (I) iodide catalyzed synthesis of 1, 4-disubstituted 1, 2, 3-triazoles from anti-3-aryl-2, 3-dibromopropanoic acids and organic azides. Synthesis. 2011;2011(18):2907–12.10.1055/s-0030-1261030Search in Google Scholar
[30] Vadivelu M, Raheem AA, Raj JP, Elangovan J, Karthikeyan K, Praveen C. Mechanochemical access to functional clickates with nitro-retentive selectivity via organocatalyzed oxidative azide-olefin cycloaddition. Org Lett. 2022;24(15):2798–2803.10.1021/acs.orglett.2c00621Search in Google Scholar PubMed
[31] Venigalla LS, Maddila S, Jonnalagadda SB. Facile, efficient, catalyst-free, ultrasound-assisted one-pot green synthesis of triazole derivatives. J Iran Chem Soc. 2020;17(7):1539–44.10.1007/s13738-020-01887-1Search in Google Scholar
[32] Shelke GM, Rao VK, Jha M, Cameron TS, Kumar A. Microwave-assisted catalyst-free synthesis of substituted 1, 2, 4-triazoles. Synlett. 2015;26(03):404–7.10.1055/s-0034-1379734Search in Google Scholar
[33] Nakka M, Tadikonda R, Rayavarapu S, Sarakula P, Vidavalur S. A simple and efficient synthesis of 3, 4, 5-trisubstituted/N-fused 1, 2, 4-triazoles via ceric ammonium nitrate catalyzed oxidative cyclization of amidrazones with aldehydes using polyethylene glycol as a recyclable reaction medium. Synthesis. 2015;47(04):517–25.10.1055/s-0034-1378909Search in Google Scholar
[34] Jatangi N, Tumula N, Palakodety RK, Nakka M. I2-mediated oxidative C–N and N–S bond formation in water: a metal-free synthesis of 4, 5-disubstituted/n-fused 3-amino-1, 2, 4-triazoles and 3-substituted 5-amino-1, 2, 4-thiadiazoles. J Org Chem. 2018;83(10):5715–23.10.1021/acs.joc.8b00753Search in Google Scholar PubMed
[35] Tseng WC, Wang LY, Wu TS, Wong FF. “One-flask” synthesis to 3,5-disubstituted 1,2,4-triazoles from aldehydes with hydrazonoyl hydrochlorides via 1,3-dipolar cycloaddition. Tetrahedron. 2011;67(29):5339–45. 10.1016/j.tet.2011.05.003 Search in Google Scholar
[36] Zhou LN, Feng FF, Cheung CW, Ma JA. Cu-enabled [3 + 2] annulation of in situ formed nitrile ylides with aryldiazonium salts: access to 5-cyano-1, 2, 4-triazoles. Org Lett. 2021;23(3):739–44.10.1021/acs.orglett.0c03960Search in Google Scholar PubMed
[37] Zhao F, Singh T, Xiao Y, Su W, Yang D, Jia C, et al. Divergent synthesis of substituted amino-1, 2, 4-triazole derivatives. Synthesis. 2021;53(11):1901–10.10.1055/a-1477-4630Search in Google Scholar
[38] Wong B, Stumpf A, Carrera D, Gu C, Zhang H. A Safe Synthesis of 1, 5-Disubstituted 3-Amino-1H-1, 2, 4-triazoles from 1, 3, 4-oxadiazolium Hexafluorophosphates. Synthesis. 2013;45(08):1083–93.10.1055/s-0032-1316877Search in Google Scholar
[39] Bechara WS, Khazhieva IS, Rodriguez E, Charette AB. One-pot synthesis of 3, 4, 5-trisubstituted 1, 2, 4-triazoles via the addition of hydrazides to activated secondary amides. Org Lett. 2015;17(5):1184–7.10.1021/acs.orglett.5b00128Search in Google Scholar PubMed
[40] Bush K, Macalintal C, Rasmussen BA, Lee VJ, Yang Y. Kinetic interactions of tazobactam with β-lactamases from all major structural classes. Antimicrob Agents Chemother. 1993;37(4):851–8.10.1128/AAC.37.4.851Search in Google Scholar PubMed PubMed Central
[41] Richardson K, Cooper K, Marriott MS, Tarbit MH, Troke F, Whittle PJ. Discovery of fluconazole, a novel antifungal agent. Rev Infect Dis. 1990;12(April):S267–71.10.1093/clinids/12.Supplement_3.S267Search in Google Scholar PubMed
[42] Spósito PÁ, Mazzeti AL, Faria de CO, Aurbina J, Pound-Lana G, Bahia MT, et al. Ravuconazole self-emulsifying delivery system: In vitro activity against Trypanosoma cruzi amastigotes and in vivo toxicity. Int J Nanomed. 2017;12:3785–99.10.2147/IJN.S133708Search in Google Scholar PubMed PubMed Central
[43] Dunn GL, Hoover JRE, Berges DA, Taggart JJ, Davis LD, Dietz EM, et al. Orally active 7-phenylglycyl cephalosporins structure-activity studies related to cefatrizine (SK&F 60771). J Antibiotics. 1976;29(1):65–80.10.7164/antibiotics.29.65Search in Google Scholar PubMed
[44] Bailey EM, Pharm D, Krakovsky DJ, Pharm D, Rybak MJ, Pharm D. The triazole antifungal agents: a review of itraconazole and fluconazole. 1990;10(2):146–53.10.1002/j.1875-9114.1990.tb02561.xSearch in Google Scholar
[45] Molina J, Martins-filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. Activities of the triazole derivative SCH 56592 (Posaconazole) against drug-resistant strains of the protozoan parasite trypanosoma (schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. 2000;44(1):150–5.10.1128/AAC.44.1.150-155.2000Search in Google Scholar PubMed PubMed Central
[46] Sabo JA, Abdel-Rahman SM. Voriconazole: a new triazole antifungal. Ann Pharmacother. 2000;34(9):1032–43.10.1345/aph.19237Search in Google Scholar PubMed
[47] Smit FJ, Seldon R, Aucamp J, Jordaan A, Warner DF, Da DDN. Chemistry synthesis and antimycobacterial activity of disubstituted benzyltriazoles. Med Chem Res. 2019;28:2279–93.10.1007/s00044-019-02458-7Search in Google Scholar
[48] Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71–9.10.1016/j.jpha.2015.11.005Search in Google Scholar PubMed PubMed Central
[49] Patel NB, Khan IH, Rajani SD. Pharmacological evaluation and characterizations of newly synthesized 1,2,4-triazoles. Eur J Med Chem. 2010;45(9):4293–9.10.1016/j.ejmech.2010.06.031Search in Google Scholar PubMed
[50] Singh RJ, Kumar Singh D. Novel syntheses of some 1, 2, 4-triazoles as potent bacteriocidal agents. 2010;7(1):37–40.10.1155/2010/168651Search in Google Scholar
[51] Patil BS, Krishnamurthy G, Naik HB, Latthe PR, Ghate M. Synthesis, characterization and antimicrobial studies of 2-(4-methoxy-phenyl)-5-methyl-4-(2-arylsulfanyl-ethyl)-2,4-dihydro-[1,2,4] triazolo-3-ones and their corresponding sulfones. Eur J Med Chem. 2010;45(8):3329–34.10.1016/j.ejmech.2010.04.016Search in Google Scholar PubMed
[52] Zhang PZ, Zhou SF, Li TR, Jiang L. Efficient synthesis and in vitro antifungal activity of 1H-benzimidazol-1-yl acetates/propionates containing 1H-1,2,4-triazole moiety. Chin Chem Lett. 2012;23(12):1381–4.10.1016/j.cclet.2012.10.024Search in Google Scholar
[53] Kremsner JM, Kappe CO. Microwave-assisted organic synthesis in near-critical water at 300°C - A proof-of-concept study. Eur J Org Chem. 2005;17:3672–9.10.1002/ejoc.200500324Search in Google Scholar
[54] Ouahrouch A, Ighachane H, Taourirte M, Engels JW, Sedra MH, Lazrek HB. Benzimidazole-1,2,3-triazole hybrid molecules: Synthesis and evaluation for antibacterial/antifungal activity. Arch Der Pharmazie. 2014;347(10):748–55.10.1002/ardp.201400142Search in Google Scholar PubMed PubMed Central
[55] Shi Y, Zhou CH. Synthesis and evaluation of a class of new Coumarin triazole derivatives as potential antimicrobial agents. Bioorg Med Chem Lett. 2011;21(3):956–60.10.1016/j.bmcl.2010.12.059Search in Google Scholar PubMed
[56] Afreen F, Chakraborty R, Thakur A. Synthesis of a triazole derivative and evaluation of their antituberculer activity. Int J Pharm Chem. 2015;5:352–8.Search in Google Scholar
[57] Seelam N, ShrivastavaS., P, Prasanthi S, Gupta S. Synthesis and in vitro study of some fused 1,2,4-triazole derivatives as antimycobacterial agents. J Saudi Chem Soc. 2016;20(4):411–8.10.1016/j.jscs.2012.11.011Search in Google Scholar
[58] Slivka M, Korol N, Pantyo V, Baumer V, Lendel V. Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4H-1,2,4-triazoles and their antimicrobial activity. Heterocycl Commun. 2017;23(2):109–13.10.1515/hc-2016-0233Search in Google Scholar
[59] Marepu N, Yeturu S, Pal M. 1,2,3-Triazole fused with pyridine/pyrimidine as new template for antimicrobial agents: Regioselective synthesis and identification of potent N-heteroarenes. Bioorg Med Chem Lett. 2018;28(20):3302–6.10.1016/j.bmcl.2018.09.021Search in Google Scholar PubMed
[60] Santosh R, Selvam MK, Kanekar SU, Nagaraja GK. Synthesis, characterization, antibacterial and antioxidant studies of some heterocyclic compounds from triazole-linked chalcone derivatives. ChemistrySelect. 2018;3(23):6338–43.10.1002/slct.201800905Search in Google Scholar
[61] Singh G, Singh J, Singh A, Singh J, Kumar M, Gupta K, et al. Synthesis, characterization and antibacterial studies of schiff based 1,2,3-triazole bridged silatranes. J Organomet Chem. 2018;871:21–7.10.1016/j.jorganchem.2018.06.024Search in Google Scholar
[62] Paneth A, Trotsko N, Popiołek Ł, Grzegorczyk A, Krzanowski T, Janowska S, et al. Synthesis and antibacterial evaluation of mannich bases derived from 1,2,4-triazole. Chem Biodivers. 2019;16(10):e1900377.10.1002/cbdv.201900377Search in Google Scholar PubMed
[63] Nalawade J, Shinde A, Chavan A, Patil S, Suryavanshi M, Modak M, et al. Synthesis of new thiazolyl-pyrazolyl-1,2,3-triazole derivatives as potential antimicrobial agents. Eur J Med Chem. 2019;179:649–59.10.1016/j.ejmech.2019.06.074Search in Google Scholar PubMed
[64] Agisho HA, Esatu H, Hairat S, Zaki M. TBHP/TBAI-mediated simple and efficient synthesis of 3,5-disubstituted and 1,3,5-trisubstituted 1H-1,2,4-triazoles via oxidative decarbonylation of aromatic aldehydes and testing for antibacterial activities. Tetrahedron Lett. 2020;61(24):151989.10.1016/j.tetlet.2020.151989Search in Google Scholar
[65] Bangalore PK, Vagolu SK, Bollikanda RK, Veeragoni DK, Choudante PC, Misra S, et al. Usnic acid enaminone-coupled 1,2,3-triazoles as antibacterial and antitubercular agents. J Nat Products. 2020;83(1):26–35.10.1021/acs.jnatprod.9b00475Search in Google Scholar PubMed
[66] Maddila S, Nagaraju K, Jonnalagadda SB. Synthesis and antimicrobial evaluation of novel pyrano[2,3-d]-pyrimidine bearing 1,2,3-triazoles. Chem Data Collect. 2020;28:100486.10.1016/j.cdc.2020.100486Search in Google Scholar
[67] Pradeep Kumar CB, Prathibha BS, Prasad KNN, Raghu MS, Prashanth MK, Jayanna BK, et al. Click synthesis of 1,2,3-triazole based imidazoles: Antitubercular evaluation, molecular docking and HSA binding studies. Bioorg Med Chem Lett. 2021;36(November 2020):1–8.10.1016/j.bmcl.2021.127810Search in Google Scholar PubMed
[68] Negi B, Rawat DS. Synthesis, characterization, and antimycobacterial activity of novel thymol- triazole hybrids. Indian J Heterocycl Chem. 2022;28(1):113–24.Search in Google Scholar
[69] Baashen MA. Synthesis and antibacterial evaluation of novel hydrazones and bis-hydrazones containing 1,2,3-triazole moiety. J Taibah Univ Sci. 2022;16(1):1157–64.10.1080/16583655.2022.2151297Search in Google Scholar
[70] Reddyrajula R, Dalimba U, Madan Kumar S. Molecular hybridization approach for phenothiazine incorporated 1,2,3-triazole hybrids as promising antimicrobial agents: Design, synthesis, molecular docking and in silico ADME studies. Eur J Med Chem. 2019;168:263–82.10.1016/j.ejmech.2019.02.010Search in Google Scholar PubMed
[71] Paprocka R, Wiese M, Eljaszewicz A, Helmin-Basa A, Gzella A, Modzelewska-Banachiewicz B, et al. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorg Med Chem Lett. 2015;25(13):2664–7.10.1016/j.bmcl.2015.04.079Search in Google Scholar PubMed
[72] Haider S, Alam MS, Hamid H, Shafi S, Nargotra A, Mahajan P, et al. Synthesis of novel triazole based benzoxazolinones: Their TNF-α based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur J Med Chem. 2013;70:579–88.10.1016/j.ejmech.2013.10.032Search in Google Scholar PubMed
[73] Shafi S, Mahboob Alam M, Mulakayala N, Mulakayala C, Vanaja G, Kalle AM, et al. Synthesis of novel 2-mercapto benzothiazole and 1,2,3-triazole based bis-heterocycles: Their anti-inflammatory and anti-nociceptive activities. Eur J Med Chem. 2012;49:324–33.10.1016/j.ejmech.2012.01.032Search in Google Scholar PubMed
[74] Almasirad A, Mousavi Z, Tajik M, Assarzadeh MJ, Shafiee A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. DARU J Pharm Sci. 2014;22:1–8.10.1186/2008-2231-22-22Search in Google Scholar PubMed PubMed Central
[75] Song MX, Wang ZY, He SH, Yu SW, Chen SL, Guo DF, et al. Synthesis and evaluation of the anticonvulsant activities of 4-(2-(alkylthio)benzo[d]oxazol-5-yl)-2,4-dihydro-3H-1,2,4-triazol-3-ones. Molecules. 2018;23(4):1–12.10.3390/molecules23040756Search in Google Scholar PubMed PubMed Central
[76] Mahdavi M, Akbarzadeh T, Sheibani V, Abbasi M. Triazoles with Anticonvulsant Activity. Iran J Pharm Res. 2010;9(3):265–9.Search in Google Scholar
[77] Wang S, Liu H, Wang X, Lei K, Li G, Li X, et al. Synthesis and evaluation of anticonvulsant activities of 7-phenyl-4,5,6,7-tetrahydrothieno[3,2-b]pyridine derivatives. Arch Der Pharmazie. 2019;352:10.10.1002/ardp.201900106Search in Google Scholar PubMed
[78] Dehestani L, Ahangar N, Hashemi SM, Irannejad H, Honarchian Masihi P, Shakiba A, et al. Design, synthesis, in vivo and in silico evaluation of phenacyl triazole hydrazones as new anticonvulsant agents. Bioorg Chem. 2018;78:119–29.10.1016/j.bioorg.2018.03.001Search in Google Scholar PubMed
[79] Ma LY, Pang LP, Wang B, Zhang M, Hu B, Xue DQ, et al. Design and synthesis of novel 1,2,3-triazole-pyrimidine hybrids as potential anticancer agents. Eur J Med Chem. 2014;86:368–80.10.1016/j.ejmech.2014.08.010Search in Google Scholar PubMed
[80] Aouad MR, Soliman MA, Alharbi MO, Bardaweel SK, Sahu PK, Ali AA, et al. Design, synthesis and anticancer screening of novel benzothiazole-piperazine-1,2,3-triazole hybrids. Molecules. 2018;23(11):1–14.10.3390/molecules23112788Search in Google Scholar PubMed PubMed Central
[81] Saftić D, Žinić B, Glavaš-Obrovac L, Studzińska M, Paradowska E, Leśnikowski ZJ. Synthesis and in vitro evaluation of antiviral and cytostatic properties of novel 8-triazolyl acyclovir derivatives. Nucleosides, Nucleotides Nucleic Acids. 2018;37(7):397–414.10.1080/15257770.2018.1485932Search in Google Scholar PubMed
[82] Duan YC, Ma YC, Zhang E, Shi XJ, Wang MM, Ye XW, et al. Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur J Med Chem. 2013;62:11–9.10.1016/j.ejmech.2012.12.046Search in Google Scholar PubMed
[83] Vieira Veloso R, Shamim A, Lamarrey Y, Stefani HA, Mozer Sciani J. Antioxidant and anti-sickling activity of glucal-based triazoles compounds – An in vitro and in silico study. Bioorg Chem. 2021;109(October 2020):104709.10.1016/j.bioorg.2021.104709Search in Google Scholar PubMed
[84] AlNeyadi SS, Amer N, Thomas TG, Al Ajeil R, Breitener P, Munawar N. Synthesis, characterization, and antioxidant activity of some 2-methoxyphenols derivatives. Heterocycl Commun. 2020;26(1):112–22.10.1515/hc-2020-0112Search in Google Scholar
[85] Shaikh MH, Subhedar DD, Shingate BB, Kalam Khan FA, Sangshetti JN, Khedkar VM, et al. Synthesis, biological evaluation and molecular docking of novel coumarin incorporated triazoles as antitubercular, antioxidant and antimicrobial agents. Med Chem Res. 2016;25(4):790–804.10.1007/s00044-016-1519-9Search in Google Scholar
[86] Kaushik CP, Chahal M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1,4-disubstituted 1,2,3-triazoles. Monatshefte Fur Chem. 2021;152(8):1001–12.10.1007/s00706-021-02821-8Search in Google Scholar
[87] He YW, Dong CZ, Zhao JY, Ma LL, Li YH, Aisa HA. 1,2,3-Triazole-containing derivatives of rupestonic acid: Click-chemical synthesis and antiviral activities against influenza viruses. Eur J Med Chem. 2014;76:245–55.10.1016/j.ejmech.2014.02.029Search in Google Scholar PubMed
[88] El-Sayed WA, Khalaf HS, Mohamed SF, Hussien HA, Kutkat OM, Amr AE. Synthesis and antiviral activity of 1,2,3-triazole glycosides based substituted pyridine via click cycloaddition. Russian J Gen Chem. 2017;87(10):2444–53.10.1134/S1070363217100279Search in Google Scholar
[89] Witkowski JT, Robins RK, Khare GP, Sidwell RW. Synthesis and antiviral activity of 1,2,4-triazole-3-thiocarboxamide and 1,2,4-triazole-3-carboxamidine ribonucleosides. J Med Chem. 1973;16(8):935–7.10.1021/jm00266a014Search in Google Scholar PubMed
[90] De Lourdes G, Ferreira M, Pinheiro LCS, Santos-Filho OA, Peçanha MDS, Sacramento CQ, et al. Design, synthesis, and antiviral activity of new 1H-1,2,3-triazole nucleoside ribavirin analogs. Med Chem Res. 2014;23(3):1501–11.10.1007/s00044-013-0762-6Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids

