Abstract
A simple method for the synthesis of the title compounds by an efficient in-situ reduction and cyclization reactions of aromatic aldehydes, tert-butyl 2,4-dioxopiperidine-1-carboxylate and 5-nitro-1H-indazole or 6-nitro-1H-indazole was developed.
Introduction
Naphthyridines are an important class of heterocycles due to their inherent biological activities [1, 2, 3, 4, 5]. Substituted indazoles are also pharmaceutically important [6]. Previously, we introduced a three-component reaction of 1H-indol-6-amine, aromatic aldehyde and cyclic 1,3-dicarbonyl compound under catalyst-free conditions [7, 8, 9]. Compared with amino compounds, nitro derivatives are more stable, easier to obtain, and they can be easily reduced to the amines [10, 11]. The reduction is often accomplished with the use of iron [Fe(0)]. This reagent is one of the most abundant, nontoxic, inexpensive and environmentally friendly transition metals [12, 13, 14]. In view of the importance of the previously detailed structures, in this report we describe synthesis of indazolo[5,4-b] [1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives starting from 5-nitro-1H-indazole or 6-nitro-1H-indazole using Fe(0) as a reducing agent via a one-pot multicomponent protocol [15, 16, 17].
Results and discussion
Initially, 4-chlorobenzaldehyde (1a), tert-butyl 2,4-dioxopiperidine-1-carboxylate (2) and 6-nitro-1H-indazole (3) were allowed to react to optimize the reaction conditions (Scheme 1). When the reaction was performed in the presence of two equivalents of Fe(0), water(1 mL) and acetic acid (1 mL) in ethanol at 80°C, we were pleased to obtain the expected product 4a in 58% yield. Then, the reaction was tested in ethanol under different ratios of 3 and Fe(0). With three equivalents of Fe(0), the yield was increased to 88%. The effects of the amounts of water and acetic acid were also evaluated. The best results were obtained in the presence of 1 mL of water and 1 mL of acetic acid using ethanol as solvent. Ethanol was the optimal solvent in comparison to tetrahydrofuran, methanol, dioxane and toluene. With the optimized reaction conditions in hand, other aromatic aldehydes 1b-j were applied in this synthesis to furnish products 4a-j in good yields (Scheme 1). It was found that under the optimized conditions the treatment of 5-nitro-1H-indazole (5), instead of the isomer 3, with 1 and 2 furnished the expected products 6 (Scheme 2). The structures of all products were characterized by IR, NMR and HRMS spectral methods.
![Scheme 1 Synthesis of indazolo[6,7-b][1,6]naphthyridine derivatives 4a-4j.](/document/doi/10.1515/hc-2019-0006/asset/graphic/j_hc-2019-0006_fig_001.jpg)
Synthesis of indazolo[6,7-b][1,6]naphthyridine derivatives 4a-4j.
![Scheme 2 Synthesis of indazolo[5,4-b][1,6]naphthyridine derivatives 6a-6j.](/document/doi/10.1515/hc-2019-0006/asset/graphic/j_hc-2019-0006_fig_002.jpg)
Synthesis of indazolo[5,4-b][1,6]naphthyridine derivatives 6a-6j.
A possible mechanism is shown in Scheme 3 using substrate 3 as an example. First, the nitro compound 3 is reduced to 6-aminoindazole A. At the same time, the Knoevenagel product B is generated by the reaction of substrates 1 and 2. Then, 6-aminoindazole A undergoes reaction with the intermediate product B to obtain the intermediate C. Then, intramolecular cyclization of C generates the intermediate product D which is the final precursor to the observed product 4.
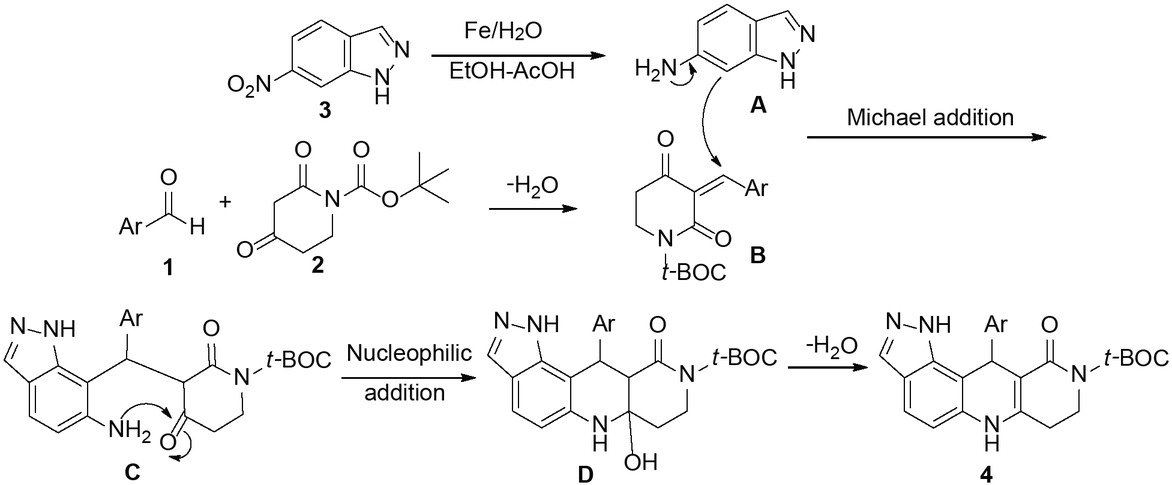
The proposed mechanism.
Conclusion
A simple and efficient method was developed for the synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives directly form nitro compounds by in situ reduction and cyclization reaction in Fe/H2O medium.
Experimental
Melting points were determined in open capillaries and are uncorrected. IR spectra were recorded on a Tensor 27 spectrometer in KBr pellets. 1H NMR (400 MHz) and 13C NMR (125 MHz) spectra were obtained in DMSO-d6 with Me4Si as internal standard using Bruker-400 and Bruker-500 spectrometers. ESI-HRMS analyses were carried out using a Bruker-micro-TOF-Q-MS analyzer.
General procedure for the synthesis of 4 and 6
A dry 25-mL round-bottom flask was charged with aromatic aldehyde 1 (1 mmol), tert-butyl 2,4-dioxopiperidine-1-carboxylate 2 (1.0 mmol), 6-nitro-1H-indazole 3 or 5-nitro-1H-indazole 5 (1.0 mmol), powdered iron (3 mmol), EtOH (5 mL), H2O (1 mL), and AcOH (1 mL). The mixture was stirred at 80°C for 8-10 h until TLC analysis revealed that the reaction was completed and then treated with brine (10 mL). The mixture was extracted with EtOAc (3 × 15 mL). The organic layers were combined and washed thoroughly with brine, dried with Na2SO4, and filtered through Celite. Following removal of the solvent in vacuo, the residue was purified by crystallization from DMF to give the pure product 4 or 6.
tert-Butyl 11-(4-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4a)
Yield 88%; white powder; mp 240-241°C; IR: ν 3427, 3316, 2936, 1741, 1626, 1606, 1533, 1494, 1398, 1213, 1159, 1047, 949, 851, 774, 703 cm-1; 1H NMR: δH 12.90 (s, 1H, NH), 9.80 (s, 1H, NH), 7.94 (s, 1H, ArH), 7.53 (d, J = 8.4 Hz, 1H, ArH), 7.40 (d, J = 8.0 Hz, 2H, ArH), 7.23 (d, J = 8.0 Hz, 2H, ArH), 6.85 (d, J = 8.4 Hz, 1H, ArH), 5.56 (s, 1H, CH), 4.01-3.98 (m, 1H, CH2), 3.50-3.44 (m, 1H, CH2), 2.82-2.78 (m, 1H, CH2), 2.66-2.62 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: δC 164.1, 152.5, 148.6, 145.8, 138.5, 134.1, 134.0, 130.6, 129.3, 127.8, 119.9, 119.6, 111.3, 104.7, 100.0, 80.9, 42.1, 35.8, 27.7, 26.3. HRMS. Calcd for C24H22N4O3Cl, [M - H]-: m/z 449.1386. Found: m/z 449.1384.
tert-Butyl 11-(4-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4b)
Yield 90%; white powder; mp: 242-243°C; IR: n 3310, 3061, 1735, 1633, 1599, 1536, 1494, 1387, 1285, 1154, 1061, 947, 830, 775, 702 cm-1; 1H NMR: δH 12.89 (s, 1H, NH), 9.79 (s, 1H, NH), 7.93 (s, 1H, ArH), 7.53 (d, J = 8.4 Hz, 1H, ArH), 7.37-7.33 (m, 4H, ArH), 6.84 (d, J = 8.0 Hz, 1H, ArH), 5.54 (s, 1H, CH), 4.01-3.98 (m, 1H, CH2), 3.51-3.43 (m, 1H, CH2), 2.82-2.75 (m, 1H, CH2), 2.66-2.62 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: δC 164.6, 153.0, 149.1, 146.7, 139.0, 134.6, 134.5, 131.3, 130.3, 120.4, 120.1, 119.6, 111.8, 105.2, 100.5, 81.4, 42.6, 36.4, 28.3, 26.9. HRMS. Calcd for C24H22N4O3Br, [M - H]-: m/z 493.0881. Found: m/z 493.0885.
tert-Butyl 11-(4-fluorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4c)
Yield 81%; white powder; mp 243-244°C; IR: n 3471, 3307, 2980, 1738, 1670, 1535, 1503, 1461, 1369, 1248, 1141, 1050, 946, 850, 743, 710 cm-1; 1H NMR: δH 12.88 (s, 1H, NH), 9.76 (s, 1H, NH), 7.93 (s, 1H, ArH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.41-7.37 (m, 2H, ArH), 6.99-6.95 (m, 2H, ArH), 6.82 (d, J = 8.4 Hz, 1H, ArH), 5.54 (s, 1H, CH), 4.01-3.95 (m, 1H, CH2), 3.48-3.40 (m, 1H, CH2), 2.81-2.72 (m, 1H, CH2), 2.66-2.59 (m, 1H, CH2), 1.41 (s, 9H, 3CH3); 13C NMR: δC 164.6, 161.1(d, JF-C = 192.4 Hz), 153.0, 149.0, 143.6, 139.1, 134.6, 134.4, 129.7 (d, JF-C = 6.3 Hz), 120.4, 120.0, 115.0 (d, JF-C = 16.7 Hz), 111.8, 105.6, 100.8, 81.3, 42.6, 36.1, 28.2, 26.9. HRMS. Calcd for C24H22N4O3F, [M - H]-: m/z 433.1681. Found: m/z 433.1680.
tert-Butyl 11-(4-nitrophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4d)
Yield 84%; yellow powder; mp 235-236°C; IR: n 3310, 3217, 2977, 1737, 1665, 1638, 1540, 1509, 1393, 1214, 1138, 1049, 943, 852, 781, 710 cm-1; 1H NMR: δH 12.97 (s, 1H, NH), 9.90 (s, 1H, NH), 8.07 (d, J = 8.8 Hz, 2H, ArH), 7.95 (s, 1H, ArH), 7.64 (d, J = 8.4 Hz, 2H, ArH), 7.57 (d, J = 8.4 Hz, 1H, ArH), 6.88 (d, J = 8.8 Hz, 1H, ArH), 5.72 (s, 1H, CH), 4.01-3.96 (m, 1H, CH2), 3.53-3.42 (m, 1H, CH2), 2.85-2.76 (m, 1H, CH2), 2.70-2.62 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: dC 164.5, 154.5, 152.9, 149.5, 146.3, 139.1, 134.8, 134.6, 129.2, 123.8, 120.54, 120.46, 111.9, 104.3, 99.9, 81.4, 42.5, 37.1, 28.2, 26.9. HRMS. Calcd for C24H22N5O5, [M - H]-: m/z 460.1626. Found: m/z 460.1635.
tert-Butyl 11-(3-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4e)
Yield 84%; white powder; mp: 227-228°C; IR: n 3450, 3291, 2977, 1738, 1667, 1626, 1536, 1496, 1446, 1369, 1212, 1138, 1048, 949, 850, 776, 731 cm-1; 1H NMR: dH 12.93 (s, 1H, NH), 9.83 (s, 1H, NH), 7.94 (s, 1H, ArH), 7.56-7.52 (m, 2H, ArH), 7.27 (d, J = 7.6 Hz, 1H, ArH), 7.22-7.18 (m, 1H, ArH), 7.14-7.11 (m, 1H, ArH), 6.86 (d, J = 8.8 Hz, 1H, ArH), 5.55 (s, 1H, CH), 4.02-3.97 (m, 1H, CH2), 3.51-3.44 (m, 1H, CH2), 2.83-2.76 (m, 1H, CH2), 2.70-2.64 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: dC 164.6, 152.9, 149.6, 149.2, 139.0, 134.7, 134.5, 133.0, 130.4, 127.8, 126.7, 126.5, 120.4, 120.2, 111.8, 105.0, 100.3, 81.4, 42.6, 36.7, 28.3, 26.8. HRMS. Calcd for C24H22N4O3Cl, [M - H]-: m/z 449.1386. Found: m/z 449.1404.
tert-Butyl 11-(3-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4f)
Yield 83%; white powder; mp: 229-230°C; IR: n 3450, 3279, 2972, 1745, 1625, 1537, 1498, 1467, 1367, 1216, 1158, 1049, 945, 815, 775, 712 cm-1; 1H NMR: dH 12.94 (s, 1H, NH), 9.83 (s, 1H, NH), 7.94 (s, 1H, ArH), 7.66 (s, 1H, ArH), 7.54 (d, J = 8.4 Hz, 1H, ArH), 7.32-7.25 (m, 2H, ArH), 7.17-7.12 (m, 1H, ArH), 6.86 (d, J = 8.4 Hz, 1H, ArH), 5.54 (s, 1H, CH), 4.02-3.90 (m, 1H, CH2), 3.51-3.43 (m, 1H, CH2), 2.83-2.76 (m, 1H, CH2), 2.70-2.65 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: dC 164.5, 152.9, 149.9, 149.2, 139.0, 134.7, 134.5, 130.8, 130.7, 129.4, 127.1, 121.8, 120.4, 120.2, 111.8, 105.0, 100.4, 81.4, 42.6, 36.8, 28.3, 26.8. HRMS. Calcd for C24H22N4O3Br, [M - H]-: m/z 493.0881. Found: m/z 493.0893.
tert-Butyl 11-(3,4-dichlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4g)
Yield 89%; white powder; mp: 250-251°C; IR: n 3301, 2978, 1737, 1630, 1601, 1540, 1496, 1392, 1285, 1215, 1155, 1049, 975, 871, 774, 701 cm-1; 1H NMR: dH 12.94 (s, 1H, NH), 9.86 (s, 1H, NH), 7.96 (s, 1H, ArH), 7.71 (d, J = 8.4 Hz, 1H, ArH), 7.56 (d, J = 8.8 Hz, 1H, ArH), 7.44 (d, J = 8.4 Hz, 1H, ArH), 7.25 (dd, J = 8.4 Hz, J’ = 2.0 Hz, 1H, ArH), 6.86 (d, J = 8.4 Hz, 1H, ArH), 5.55 (s, 1H, CH), 4.01-3.95 (m, 1H, CH2), 3.54-3.45 (m, 1H, CH2), 2.82-2.74 (m, 1H, CH2), 2.70-2.63 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: dC 164.5, 152.9, 149.3, 148.1, 139.0, 134.7, 134.5, 130.9, 130.8, 129.9, 129.1, 128.4, 120.4, 111.8, 104.5, 100.0, 81.4, 42.6, 36.4, 28.2, 26.8. HRMS. Calcd for C24H21N4O3Cl2, [M - H]-: m/z 483.0996. Found: m/z 483.0994.
tert-Butyl 11-(2-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4h)
Yield 86%; white powder; mp: 280-281°C; IR: n 3414, 3319, 3101, 2974, 1745, 1627, 1531, 1494, 1469, 1398, 1321, 1210, 1141, 1048, 943, 872, 782, 704 cm-1; 1H NMR: dH 11.97 (s, 1H, NH), 9.89 (s, 1H, NH), 7.90 (s, 1H, ArH), 7.86 (d, J = 7.6 Hz, 1H, ArH), 7.50 (d, J = 8.4 Hz, 1H, ArH), 7.24-7.19 (m, 2H, ArH), 7.13-7.09 (m, 1H, ArH), 6.77 (d, J = 8.4 Hz, 1H, ArH), 5.80 (s, 1H, CH), 4.01-3.96 (m, 1H, CH2), 3.45-3.37 (m, 1H, CH2), 2.80-2.71 (m, 1H, CH2), 2.59-2.48 (m, 1H, CH2), 1.40 (s, 9H, 3CH3); 13C NMR: dC 164.3, 152.8, 149.7, 142.2, 139.4, 135.3, 134.9, 133.6, 132.8, 130.5, 128.5, 127.0, 120.44, 120.37, 111.7, 103.4, 98.1, 81.3, 42.6, 38.1, 28.3, 26.9. HRMS. Calcd for C24H22N4O3Cl, [M - H]-: m/z 449.1386. Found: m/z 449.1386.
tert-Butyl 10-oxo-11-(thiophen-2-yl)-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4i)
Yield 89%; white powder; mp > 300°C; IR: n 3203, 3079, 2975, 1699, 1651, 1604, 1545, 1504, 1453, 1388, 1324, 1216, 1156, 970, 850, 767, 710 cm-1; 1H NMR: dH 13.08 (s, 1H, NH), 9.88 (s, 1H, NH), 7.97 (s, 1H, ArH), 7.54 (d, J = 8.4 Hz, 1H, ArH), 7.17-7.15 (m, 1H, ArH), 6.97 (d, J = 3.2 Hz, 1H, ArH), 6.83-6.78 (m, 2H, ArH), 5.94 (s, 1H, CH), 4.08-4.03 (m, 1H, CH2), 3.49-3.41 (m, 1H, CH2), 2.86-2.76 (m, 1H, CH2), 2.67-2.62 (m, 1H, CH2), 1.44 (s, 9H, 3CH3); 13C NMR: dC 164.6, 153.0, 151.5, 148.9, 139.0,134.5, 134.3, 126.8, 124.3, 123.8, 120.4, 120.1, 111.7, 105.2, 100.4, 81.4, 42.6, 32.0, 28.3, 26.9. HRMS. Calcd for C22H21N4O3S, [M - H]-: m/z 421.1340. Found: m/z 421.1331.
tert-Butyl 11-(benzo[d][1,3]dioxol-5-yl)-10-oxo-7,8,10,11-tetrahydro-1H-indazolo [6,7-b][1,6]naphthyridine-9(6H)-carboxylate (4j)
Yield 87%; white powder; mp 247-248°C; IR: n 3296, 3224, 2971, 1710, 1673, 1625, 1538, 1503, 1443, 1327, 1287, 1166, 1049, 946, 811, 776, 703 cm-1; 1H NMR: dH 12.86 (s, 1H, NH), 9.74 (s, 1H, NH), 7.93 (s, 1H, ArH), 7.51 (d, J = 8.4 Hz, 1H, ArH), 6.98 (s, 1H, ArH), 6.89-6.78 (m, 2H, ArH), 6.70 (d, J = 7.2 Hz, 1H, ArH), 5.88 (d, J = 7.2 Hz, 2H, CH2), 5.46 (s, 1H, CH), 4.01-3.98 (m, 1H, CH2), 3.50-3.44 (m, 1H, CH2), 2.81-2.78 (m, 1H, CH2), 2.67-2.63 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: dC 164.6, 153.0, 148.7, 147.3, 145.9, 141.7, 139.1, 134.5, 134.3, 120.9, 120.3, 119.8, 111.8, 108.7, 108.2, 106.0, 101.1, 101.0, 81.3,42.6, 36.5, 28.3, 26.9. HRMS. Calcd for C25H23N4O5, [M - H]-: m/z 459.1674. Found: m/z 459.1667.
tert-Butyl 11-(4-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6a)
Yield 87%; white powder; mp 221-222°C; IR: n 3297, 3079, 2977, 2933, 2885, 1719, 1681, 1605, 1543, 1492, 1401, 1369, 1331, 1253, 1213, 1142, 1092, 1049, 949, 846, 805, 775, 703 cm-1; 1H NMR: dH 13.01 (s, 1H, NH), 9.67 (s, 1H, NH), 8.01 (s, 1H, ArH), 7.37-7.34 (m, 3H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 7.07 (d, J = 8.4 Hz, 1H, ArH), 5.52 (s, 1H, CH), 4.02-3.96 (m, 1H, CH2), 3.60-3.50 (m, 1H, CH2), 2.82-2.75 (m, 1H, CH2), 2.64-2.59 (m, 1H, CH2), 1.41 (s, 9H, 3CH3); 13C NMR: dC 164.7, 153.1, 148.9, 147.2, 137.9, 132.1, 130.9, 130.0, 129.3, 128.4, 122.1, 117.2, 114.3, 110.0, 99.1, 81.3, 42.6, 39.0, 28.3, 27.0. HRMS. Calcd for C24H22N4O3Cl, [M - H]-: m/z 449.1386. Found: m/z 449.1384.
tert-Butyl 11-(4-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6b)
Yield 90%; white powder; mp 223-225°C; IR: n 3296, 3077, 2977, 2933, 2885, 1719, 1680, 1605, 1543, 1492, 1401, 1369, 1331, 1254, 1212, 1142, 1049, 949, 845, 804, 775, 738, 714 cm-1; 1H NMR: dH 12.97 (s, 1H, NH), 9.63 (s, 1H, NH), 7.98 (s, 1H, ArH), 7.35-7.32 (m, 3H, ArH), 7.28 (d, J = 8.4 Hz, 2H, ArH), 7.05 (d, J = 8.8 Hz, 1H, ArH), 5.49 (s, 1H, CH), 4.00-3.95 (m, 1H, CH2), 3.46-3.39 (m, 1H, CH2), 2.80-2.74 (m, 1H, CH2), 2.62-2.58 (m, 1H, CH2), 1.39 (s, 9H, 3CH3); 13C NMR: dC 164.7, 153.1, 148.9, 147.7, 137.9, 132.1, 131.3, 130.4, 129.3, 122.1, 119.4, 117.2, 114.2, 110.0, 99.1, 81.3, 42.6, 39.1, 28.3, 27.0. HRMS. Calcd for C24H22N4O3Br [M - H]-: m/z 493.0881. Found: m/z 493.0873.
tert-Butyl 11-(3-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6c)
Yield 86%; white powder; mp 287-288°C; IR: n 3278, 3085, 2978, 2934, 2886, 1720, 1682, 1609, 1546, 1492, 1402, 1370, 1329, 1251, 1212, 1140, 1049, 947, 894, 841, 808, 777, 732 cm-1; 1H NMR: dH 13.02 (s, 1H, NH), 9.68 (s, 1H, NH), 8.06 (s, 1H, ArH), 7.37 (d, J = 8.0 Hz, 2H, ArH), 7.30 (d, J = 7.6 Hz, 1H, ArH), 7.23-7.19 (m, 1H, ArH), 7.13-7.07 (m, 2H, ArH), 5.55 (s, 1H, CH), 4.01-3.98 (m, 1H, CH2), 3.49-3.44 (m, 1H, CH2), 2.81-2.78 (m, 1H, CH2), 2.68-2.63 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: dC 164.7, 153.0, 150.7, 149.1, 137.9, 133.2, 132.2, 130.3, 129.3, 127.8, 126.9, 126.3, 122.1, 117.2, 114.0, 110.1, 99.0, 81.3, 42.6, 39.3, 28.3, 26.9. HRMS. Calcd for C24H22N4O3, [M - H]-: m/z 449.1386. Found: m/z 449.1387.
tert-Butyl 11-(3-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6d)
Yield 84%; white powder; mp 285-286°C; IR: n 3291, 3085, 2977, 2934, 2886, 1721, 1681, 1609, 1545, 1494, 1401, 1369, 1328, 1251, 1211, 1159, 1139, 1049, 947, 892, 840, 808, 776, 711 cm-1; 1H NMR: dH 13.01 (s, 1H, NH), 9.67 (s, 1H, NH), 8.05 (s, 1H, ArH), 7.51-7.50 (m, 1H, ArH), 7.38-7.33 (m, 2H, ArH), 7.25 (d, J = 8.0 Hz, 1H, ArH), 7.17-7.13 (m, 1H, ArH), 7.09 (d, J = 8.8 Hz, 1H, ArH), 5.54 (s, 1H, CH), 4.02-3.97 (m, 1H, CH2), 3.50-3.46 (m, 1H, CH2), 2.82-2.78 (m, 1H, CH2), 2.68-2.64 (m, 1H, CH2), 1.42 (s, 9H, 3CH3) ; 13C NMR: dC 164.7, 153.0, 150.9, 149.1, 137.9, 132.2, 130.69, 130.67, 129.3, 129.2, 127.3, 122.1, 121.9, 117.2, 114.0, 110.1, 99.0, 81.3, 42.6, 39.3, 28.3, 26.9. HRMS. Calcd for C24H22N4O3Br, [M - H]-: m/z 493.0881. Found: m/z 493.0876.
tert-Butyl 11-(3,4-dichlorophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6e)
Yield 89%; white powder; mp 256-257°C; IR: n 3297, 3080, 2978, 2934, 2886, 1720, 1680, 1606, 1544, 1493, 1467, 1401, 1370, 1329, 1253, 1213, 1143, 1049, 1031, 947, 889, 840, 805, 776, 700 cm-1; 1H NMR: dH 13.02 (s, 1H, NH), 9.69 (s, 1H, NH), 8.07 (s, 1H, ArH), 7.60 (d, J = 2.0 Hz, 1H, ArH), 7.43 (d, J = 8.4 Hz, 1H, ArH), 7.38 (d, J = 8.4 Hz, 1H, ArH), 7.29 (dd, J = 8.4 Hz, J’ = 2.0 Hz, 1H, ArH), 7.09 (d, J = 8.4 Hz, 1H, ArH), 5.58 (s, 1H, CH), 4.02-3.96 (m, 1H, CH2), 3.52-3.45 (m, 1H, CH2), 2.82-2.76 (m, 1H, CH2), 2.68-2.62 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: dC 164.7, 153.0, 149.20, 149.17, 137.9, 132.1, 131.0, 130.7, 129.9, 129.3, 128.9, 128.6, 122.0, 117.2, 113.6, 110.3, 98.7, 81.3, 42.6, 38.9, 28.3, 26.9. HRMS. Calcd for C24H21N4O3Cl2, [M - H]-: m/z 483.0996. Found: m/z 483.0992.
tert-Butyl 11-(2-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6f)
Yield 85%; white powder; mp 226-227°C; IR: n 3270, 3076, 2972, 2935, 2888, 1719, 1622, 1601, 1543, 1491, 1388, 1336, 1251, 1211, 1142, 1049, 943, 894, 851, 807, 779, 759, 742, 705 cm-1; 1H NMR: dH 13.03 (s, 1H, NH), 9.70 (s, 1H, NH), 7.95 (s, 1H, ArH), 7.41 (d, J = 7.2 Hz, 1H, ArH), 7.36 (d, J = 8.8 Hz, 1H, ArH), 7.29 (d, J = 7.6 Hz, 1H, ArH), 7.18-7.15 (m, 1H, ArH), 7.10-7.06 (m, 2H, ArH), 5.89 (s, 1H, CH), 4.00-3.97 (m, 1H, CH2), 3.46-3.44 (m, 1H, CH2), 2.81-2.77 (m, 1H, CH2), 2.64-2.60 (m, 1H, CH2), 1.41 (s, 9H, 3CH3); 13C NMR: dC 164.4, 152.8, 149.1, 145.8, 137.8, 132.0, 131.9, 131.4, 129.6, 129.4, 128.1, 127.9, 122.3, 117.4, 113.9, 110.2, 99.0, 81.2, 42.6, 37.4, 28.3, 27.0. HRMS. Calcd for C24H22N4O3Cl, [M - H]-: m/z 449.1386. Found: m/z 449.1391.
tert-Butyl 11-(2-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6g)
Yield 83%; white powder; mp 225-227°C; IR: n 3317, 3070, 2969, 2934, 2886, 1720, 1623, 1602, 1542, 1490, 1474, 1398, 1369, 1331, 1250, 1210, 1141, 1048, 942, 892, 851, 808, 781, 738, 710 cm-1; 1H NMR: dH 13.01 (s, 1H, NH), 9.68 (s, 1H, NH), 8.04 (s, 1H, ArH), 7.46 (d, J = 7.6 Hz, 1H, ArH), 7.39-7.35 (m, 2H, ArH), 7.22-7.19 (m, 1H, ArH), 7.07 (d, J = 8.8 Hz, 1H, ArH), 7.02-6.98 (m, 1H, ArH), 5.86 (s, 1H, CH), 4.01-3.95 (m, 1H, CH2), 3.48-3.41 (m, 1H, CH2), 2.81-2.76 (m, 1H, CH2), 2.65-2.59 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: dC 164.4, 152.7, 149.0, 147.6, 137.8, 132.7, 132.3, 132.2, 129.5, 128.5, 128.3, 122.3, 122.1, 117.4, 114.1, 110.2, 99.4, 81.2, 42.6, 39.5, 28.3, 27.0. HRMS. Calcd for C24H22N4O3Br, [M - H]-: m/z 493.0881. Found: m/z 493.0876.
tert-Butyl 11-(3-methoxyphenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6h)
Yield 86%; white powder; mp 281-283°C; IR: n 3303, 3078, 2972, 2888, 1717, 1679, 1606, 1543, 1492, 1476, 1399, 1369, 1335, 1262, 1148, 1049, 949, 843, 807, 769, 735, 710 cm-1; 1H NMR: dH 11.76 (s, 1H, NH), 9.94 (s, 1H, NH), 7.94 (s, 1H, ArH), 7.86 (dd, J = 8.0 Hz, J’ = 1.2 Hz, 1H, ArH), 7.53 (d, J = 8.4 Hz, 1H, ArH), 7.43-7.42 (m, 1H, ArH), 7.31-7.28 (m, 1H, ArH), 7.08-7.04 (m, 1H, ArH), 6.79 (d, J = 8.8 Hz, 1H, ArH), 5.82 (s, 1H, CH), 4.04-3.98 (m, 1H, CH2), 3.67 (s, 3H, CH3O), 3.48-3.41 (m, 1H, CH2), 2.83-2.76 (m, 1H, CH2), 2.62-2.56 (m, 1H, CH2), 1.41 (s, 9H, 3CH3); 13C NMR: dC 164.7, 159.5, 153.1, 149.8, 148.8, 137.9, 132.3, 129.4, 129.3, 122.2, 120.6, 117.1, 114.8, 114.5, 111.0, 109.7, 99.3, 81.2, 55.3, 42.6, 39.4, 28.3, 27.0. HRMS. Calcd for C25H25N4O4, [M - H]-: m/z 445.1881. Found: m/z 445.1877.
tert-Butyl 11-(3,5-dimethoxyphenyl)-10-oxo-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6i)
Yield 87%; white powder; mp 284-286°C; IR: n 3307, 3082, 2976, 2887, 1792, 1660, 1610, 1546, 1497, 1464, 1398, 1368, 1250, 1211, 1143, 1052, 949, 934, 850, 817, 779, 731 cm-1; 1H NMR: dH 12.97 (s, 1H, NH), 9.59 (s, 1H, NH), 8.11 (s, 1H, ArH), 7.34 (d, J = 8.8 Hz, 1H, ArH), 7.06 (d, J = 8.4 Hz, 1H, ArH), 6.49-6.48 (m, 2H, ArH), 6.22 (s, 1H, ArH), 5.45 (s, 1H, CH), 4.03-4.00 (m, 1H, CH2), 3.65 (s, 6H, 2CH3O), 3.50-3.43 (m, 1H, CH2), 2.82-2.77 (m, 1H, CH2), 2.67-2.62 (m, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR: dC 164.8, 160.6, 153.1, 150.6, 148.8, 137.9, 132.4, 129.3, 122.2, 117.2, 114.8, 109.7, 106.8, 99.3, 97.4, 81.3, 55.4, 42.7, 39.4, 28.2, 27.0. HRMS. Calcd for C26H27N4O5, [M - H]-: m/z 475.1987. Found: m/z 475.1990.
tert-Butyl 10-oxo-11-phenyl-7,8,10,11-tetrahydro-3H-indazolo [5,4-b][1,6]naphthyridine-9(6H)-carboxylate (6j)
Yield 85%; white powder; mp 292-294°C; IR: n 3296, 3080, 2974, 2889, 1719, 1604, 1543, 1493, 1400, 1370, 1254, 1212, 1158, 1049, 947, 845, 808, 783, 759, 709 cm-1; 1H NMR: dH 12.96 (s, 1H, NH), 9.61 (s, 1H, NH), 8.02 (s, 1H, ArH), 7.36-7.32 (m, 3H, ArH), 7.19-7.15 (m, 2H, ArH), 7.08-7.02 (m, 2H, ArH), 5.51 (s, 1H, CH), 4.03-3.99 (m, 1H, CH2), 3.48-3.41 (m, 1H, CH2), 2.83-2.73 (m, 1H, CH2), 2.66-2.61 (m, 1H, CH2), 1.42 (s, 9H, 3CH3); 13C NMR: dC 164.7, 153.1, 148.8, 148.4, 137.9, 132.2, 129.2, 128.4, 128.1, 126.3, 122.2, 117.1, 114.9, 109.7, 99.4, 81.2, 42.6, 39.5, 28.3, 27.0. HRMS. Calcd for C24H23N4O3, [M - H]-: m/z 415.1776. Found: m/z 415.1769.
Acknowledgements
We are grateful to the Natural Science Foundation for Colleges and Universities of Jiangsu Province (18KJB150024), Science Research and Development Foundation of Nanjing Medical University (2017NJMU227), Science Research and Development Foundation of the Kangda College of Nanjing Medical University (KD201822NYDKJ03, KD2017KYJJYB003, KD2017KYJJYB004) and a Qing Lan Project for financial support.
References
[1] Srivastava, S. K.; Jaggi, M.; Singh, A. T.; Madan, A. ; Rani, N. ; Vishnoi, M.; Agarwal, S. K.; Mukherjee, R.; Burman, A. C. Anticancer and anti-inflammatory activities of 1, 8-naphthyridine-3-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 6660-6664.10.1016/j.bmcl.2007.08.006Search in Google Scholar
[2] Tsuzuki, Y.; Tomita, K.; Sato, Y.; Kashimoto, S.; Chiba, K. Synthesis and structure–activity relationships of 3-substituted 1, 4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridines as novel antitumor agents. Bioorg. Med. Chem. Lett. 2004, 14, 3189-3193.10.1016/j.bmcl.2004.04.011Search in Google Scholar
[3] Huang, X. G.; Zhang, A. Q.; Chen,D. L.; Jia, Z. H.; Li, X. S. 4-Substituted 4-(1H-1,2,3-triazol-1-yl)piperidine: Novel C7 moieties of fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2010, 20, 2859-2863.10.1016/j.bmcl.2010.03.044Search in Google Scholar
[4] Johns, B. A.; Weatherhead, J. G.; Allen, S. H.; Thompson, J. B.; Garvey, E. P.; Foster, S. A.; Jeffrey, J. L.; Miller, W. H. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 1: Establishing the pharmacophore. Med. Chem. Lett. 2009, 19, 1802-1806.10.1016/j.bmcl.2009.01.090Search in Google Scholar
[5] Li, Y. W.; Liang, J.; Siu, T.; Hu, E.; Rossi, M. A.; Barnett, S. F.; Defeo-Jones, D.; Jones, R. E.; Robinson, R. G.; Leander, K.; Huber, H. E.; Mittal, S.; Cosford, N.; Prasit, P. Allosteric inhibitors of Akt1 and Akt2: discovery of [1,2,4]triazolo[3, 4-f [1,6] naphthyridines with potent and balanced activity. Bioorg. Med. Chem. Lett. 2009, 19, 834-836.10.1016/j.bmcl.2008.12.017Search in Google Scholar
[6] Gaikwad, D. D.; Chapolikar, A. D.; Devkate, C. G.; Warad, K. D.; Tayade, A. P.; Pawar, R. P.; Domb, A. J. Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem. 2015, 90, 707.10.1016/j.ejmech.2014.11.029Search in Google Scholar
[7] Lu, W. Q.; Zhuang, R.; Chen, D. S.; Wang, X. S. An efficient synthesis of polycyclic heterocycles containing pyrazolo[3,4-f quinoline or benzohindazolo[6,7-b[1,6]naphthyridine under catalyst-Free conditions. Polycycl. Aromat. Comp. 2014, 34, 606-619.10.1080/10406638.2014.927774Search in Google Scholar
[8] Zhang, M. M. ; Chen, D. S.; Wang, X. S. A three-component domino reaction for efficient synthesis of functionalized pyrazolo[3,4-fquinolines under catalyst-free conditions. Res. Chem. Intermed. 2015, 41, 6339-6350.10.1007/s11164-014-1743-1Search in Google Scholar
[9] Chen, D. S.; Zhou, Y. J.; Li, Y. L.; Yao, C. S.; Wang, X. S. Combinatorial synthesis of pyrazoloquinoline and pyrazoloacridine derivatives with high regioselectivity. Comb. Chem. High. T. Scr. 2013, 16, 550-561.10.2174/1386207311316070005Search in Google Scholar
[10] Bellamy, F. D.; Ou, K. Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium. Tetrahendron Lett. 1984, 25, 839-842.10.1016/S0040-4039(01)80041-1Search in Google Scholar
[11] Corma, A.; Concepción, P.; Serna, P. A different reaction pathway for the reduction of aromatic nitro compounds on gold catalysts. Angew. Chemie 2007, 119, 7404-7407.10.1002/ange.200700823Search in Google Scholar
[12] Damavandi, S.; Sandaroos, R. Solvent-free one pot synthesis of indenoquinolinones catalyzed by iron(III) triflate. Heterocycl. Commun. 2011, 17, 121-124.10.1515/hc.2011.028Search in Google Scholar
[13] Zhang, F. F.; Gao, Q.; Zhao, J. X.; Ge, M.; Bai, Y. J. Design and synthesis of a novel rhodamine-based chemosensor and recognition study to Fe3+Heterocycl. Commun. 2016, 22, 37-42.10.1515/hc-2015-0139Search in Google Scholar
[14] Bauer, I.; Knölker, H. J. Iron catalysis in organic synthesis. Chem. Rev. 2015, 115, 3170-3387.10.1021/cr500425uSearch in Google Scholar PubMed
[15] Javahershenas, R.; Khalafy, J. A new synthesis of pyrrolo [3,2-d pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst. Heterocycl. Commun. 2018, 24, 37-41.10.1515/hc-2017-0187Search in Google Scholar
[16] Ebrahimlo, A. R. M. Efficient three-component synthesis of 5-(6-hydroxy-2, 4-dioxo-1, 2, 3, 4-tetrahydropyrimidin-5-yl)-5, 12-dihydrobenzo b pyrimido [5, 4-g[1,8] naphthyridine-2,4(1H3H- dione. Heterocycl. Commun. 2018, 24, 75-77.10.1515/hc-2017-0265Search in Google Scholar
[17] Gao, Q.; Hao, W. J.; Liu, F; Tu, S. J.; Wang, S. L.; Li, G. G.; Jiang, B. Unexpected isocyanide-based three-component bicyclization for the stereoselective synthesis of densely functionalized pyrano [3,4-cpyrroles. Chem. Commun. 2016, 52, 900-903.10.1039/C5CC08071ASearch in Google Scholar PubMed PubMed Central
© 2019 Dong-Mei Chen et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives
Articles in the same Issue
- Research Article
- Magnesium porphyrazine with peripheral methyl (3,5-dibromophenylmethyl)amino groups – synthesis and optical properties
- Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives
- Synthesis of indazolo[5,4-b][1,6]naphthyridine and indazolo[6,7-b][1,6]naphthyridine derivatives
- Zinc Chloride Catalyzed Amino Claisen Rearrangement of 1-N-Allylindolines: An Expedient Protocol for the Synthesis of Functionalized 7-Allylindolines
- Synthesis and Biological Evaluation of (E)-N’-Benzylidene-7-methyl-2-propyl-1H-benzo[d] imidazole-5-carbohydrazides as Antioxidant, Anti-inflammatory and Analgesic agents
- Efficient synthesis, reactions and spectral characterization of pyrazolo[4’,3’:4,5]thieno[3,2-d] pyrimidines and related heterocycles
- Asymmetric Mannich Reaction: Synthesis of Novel Chiral 5-(substituted aryl)-1,3,4-Thiadiazole Derivatives with Anti-Plant-Virus Potency
- Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl)carboxamides
- Remarkable electronic effect on the total stereoselectivity of the cycloaddition reaction of arylnitrile oxides with pyrrol-2-one derivatives
- Preliminary Communications
- Crystal structure and molecular docking studies of new pyrazole-4-carboxamides
- Research Article
- Synthesis of polycyclic phosphonates via an intramolecular Diels-Alder reaction of 2-benzoylbenzalaldehyde and alkenyl phosphites
- Asymmetric total synthesis of filamentous fungi related resorcylic acid lactones 7-epi-zeaenol and zeaenol
- The first in situ synthesis of 1,3-dioxan-5-one derivatives and their direct use in Claisen-Schmidt reactions
- Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives
- Combined XRD-paramagnetic 13C NMR spectroscopy of 1,2,3-triazoles for revealing copper traces in a Huisgen click-chemistry cycloaddition. A model case
- Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile
- Substrate-controlled Diastereoselective Michael Addition of Alkylidene Malonates by Grignard Reagents
- Synthesis of 1,2,3 triazole-linked benzimidazole through a copper-catalyzed click reaction
- Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides
- Facile One-pot Protocol of Derivatization Nitropyridines: Access to 3-Acetamidopyridin-2-yl 4-methylbenzenesulfonate Derivatives
- Naphthalene substituted benzo[c]coumarins: Synthesis, characterization and evaluation of antibacterial activity and cytotoxicity
- A Green Synthesis and Antibacterial Activity of N-Arylsulfonylhydrazone Compounds
- Preliminary Communications
- Facile Synthesis of Spiro[cyclohexane-1,3’-indoline]-2,2’-diones
- Research Article
- Synthesis and AChE inhibitory activity of N-glycosyl benzofuran derivatives
- [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives

