Abstract
The Chloranthaceae is a small family with only four genera (Ascarina, Chloranthus, Hedyosmum, Sarcandra), of which nearly 70 species are distributed around the world. Chemical constituents in Chloranthaceae plants, especially sesquiterpenes, have attracted a great deal of attention in recent 5 years. Many characteristic constituents of this family may be responsible for anti-microbial, anti-tumor and other activities. In order to provide information for the future research, the structures and biological activities of the known constituents from the plants of Chloranthaceae have been reviewed in this article.
Introduction
The Chloranthaceae is a small family with only four genera (Ascarina, Chloranthus, Hedyosmum, Sarcandra), nearly 70 species which are distributed throughout tropics and subtropical zones of South America, East Asia and Pacific. Genus Ascarina consists of about 12 species, found in the Australian region, the Pacific Islands and Madagascar. The genus Chloranthus consists of 15 species, mainly distributed in eastern Asia, and all species can be found in China. Genus Hedyosmum is mainly distributed in tropical America, and consists of 41 species. The last genus Sarcandra consists of three species. Of those plants in Chloranthaceae family, there are three genera (Chloranthus, Hedyosmum, Sarcandra), 16 species and five varieties distributed in China. Many species of Chloranthaceae have been used as herbal medicines which show varied medicinal features. In order to provide information for the further research work, this article reviews the structures and biological activities of the known constituents from the plants of Chloranthaceae.
Chemical constituents
Of over 70 Chloranthaceae species, there are 21 species have been studied about chemical constituents. In the genus of Ascarina, only one species Ascarina lucida was reported about the isolation of 15 flavonoids. Plants in genus Chloranthus have achieved wide and deep studies. Among the 15 species, 14 have been investigated for their chemical constituents as follows, Chloranthus japonicus, 63 compounds isolated and elucidated; Chloranthus serratus, 48 compounds; Chloranthus henryi, 48 compounds; Chloranthus multistachys, 46 compounds; Chloranthus spicatus, 41 compounds; Chloranthus anhuiensis, 22 compounds; Chloranthus elatior, 18 compounds; Chloranthus sessilifolius, 17 compounds; Chloranthus fortune, 13 compounds; Chloranthus angustifolius, eight compounds; Chloranthus glaber, seven compounds; Chloranthus holostegius, six compounds; Chloranthus tianmushanensis, two compounds; and Chloranthus erectus, one compound. Of the 41 species of genus Hedyosmum, only four species have been studied for chemical constituents. These are Hedyosmum orientale from which nine compounds have been isolated; Hedyosmum brasiliense, seven compounds; Hedyosmum angustifolium, four compounds; and Hedyosmum arborescens, one compound isolated. The species Sarcandra glabra and Sarcandra hannanensis have been analyzed. S. glabra is used as traditional herbal medicine in China for the remedy of influenza, pneumonia, rheumatoid arthritis and bacillary dysentery. In total, 100 constituents have been isolated from the whole plants of S. glabra during the period of 2005–2015. It ranks the first in the numbers of isolated compounds in all 21 species of Chloranthaceae. S. hannanensis is the unique species only distributed in the province of Hainan, China. There are 18 compounds isolated from this species to date.
The chemical constituents of Chloranthaceae plants include terpenoids, coumarins, lignans, flavonoids and some other compounds. Their structures (compounds 1–504) are shown in Figures 1–7, and their names and the corresponding plant sources are collected in Tables 1–7. Possible biogenetic pathway of selected sesquiterpenes in the plants of Chloranthaceae are shown in Schemes 1–3.

Sesquiterpenoid structures.
Diterpenoid and triterpenoid structures.
Coumarin structures.
Lignan structures.
Phenylpropionic acid and other phenylpropanoid structures.
Flavonoid structures.
Structures of other compounds.
Sesquiterpenes.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| Eudesmanes | ||||
| 1 | Neoacolamone | Roots | C. serratus | [1] |
| 2 | 7α-Hydroxy-neoacolamone (7α-Hydroxy-eudesm-4-en-6-one) | Roots | C. serratus | [1] |
| Flowers | C. spicatus | [6] | ||
| 3 | Ccolamone | Roots | C. serratus | [1] |
| 4 | Chlorantene B | Whole plants | C. serratus | [2] |
| 5 | Chlorantene C (4β-Hydroxy-8,12-epoxyeudesma-7,11-diene-1,6-dione) | Whole plants | C. serratus | [2] |
| Leaves and stems | C. henryi | [15] | ||
| Whole plants | C. multistachys | [36] | ||
| 6 | Chlorantene D | Whole plants | C. serratus | [2] |
| Whole plants | C. multistachys | [36] | ||
| 7 | Chlorantene G | Whole plants | C. serratus | [2] |
| 8 | Serralactone A (Sarcandralactone B) (1β-Hydroxyeudesma-3,7(11)-dien-12,8α-olide) | Whole plants | C. serratus | [3] |
| Whole plants | S. glabra | [5] | ||
| 9 | Serralactone B | Whole plants | C. serratus | [3] |
| 10 | Serralactone C | Whole plants | C. serratus | [3] |
| 11 | Serralactone D | Whole plants | C. serratus | [3] |
| 12 | Neolitacumone B | Whole plants | C. serratus | [3] |
| Whole plants | C. japonicus | [28] | ||
| Whole plants | C. elatior | [11] | ||
| Whole plants | C. spicatus | [10] | ||
| Whole plants | S. glabra | [5] | ||
| 13 | Cyperusol C | Whole plants | C. serratus | [3] |
| 14 | Eudesm-4(15)-ene-1β,7,11-triol | Whole plants | C. serratus | [3] |
| 15 | Eudesm-3-ene-1β,7,11-triol | Whole plants | C. serratus | [3] |
| Whole plants | S. glabra | [5] | ||
| 16 | 4β-Hydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | Whole plants | C. serratus | [4] |
| 17 | 4β,8β-Dihydroxy-5α(H)-eudesm-7(11)-en-8,12-olide | Whole plants | C. serratus | [4] |
| Whole plants | C. elatior | [11] | ||
| 18 | Eudesma-4(15),7(11),9-trien-12-olide | Flowers | C. spicatus | [6] |
| 19 | 1β,4β-Dihydroxy-5α,8β(H)-eudesm-7(11)Z-en-8,12-olide | Aerial part | C. spicatus | [7] |
| Whole plants | C. multistachys | [115] | ||
| Aerial part | C. elatior | [19] | ||
| 20 | 1β,4α-Dihydroxy-5α,8β(H)-eudesm-7(11)Z-en-8,12-olide | Aerial part | C. spicatus | [7] |
| Whole plant | C. multistachys | [115] | ||
| Aerial part | C. elatior | [19] | ||
| 21 | Homalomenol A | Aerial part | C. spicatus | [7] |
| 22 | Oplodiol | Aerial part | C. spicatus | [7] |
| Whole plants | C. serratus | [3] | ||
| 23 | 5α,7α (H)-6,8-Cycloeudesma-1β,4β-diol | Aerial part | C. spicatus | [7] |
| Whole plants | C. spicatus | [10] | ||
| 24 | 4α-Hydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | Roots | C. spicatus | [8] |
| Whole plants | C. elatior | [11] | ||
| Whole plants | C. serratus | [4] | ||
| 25 | 4α-Hydroxy-5α,8α(H)-eudesm-7(11)-en-8,12-olide | Roots | C. spicatus | [8] |
| Whole plants | C. elatior | [11] | ||
| 26 | 4α,8β-Dihydroxy-5α(H)-eudesm-7(11)-en-8,12-olide (multislactone A) | Roots | C. spicatus | [8] |
| Whole plants | C. elatior | [11] | ||
| Whole plants | C. serratus | [4] | ||
| Whole plants | C. multistachys | [9] | ||
| Roots | C. angustifolius | [41] | ||
| Aerial part | C. angustifolius | [42] | ||
| 27 | 4α-Hydroxy-5α(H)-8β-methoxy-eudesm-7(11)-en-8,12-olide | Roots | C. spicatus | [8] |
| 28 | Chlospicate A | Whole plants | C. spicatus | [10] |
| 29 | Chlospicate B (chlorelactone A) | Whole plants | C. spicatus | [10] |
| Whole plants | C. elatior | [11] | ||
| 30 | 5-Eudesmene-1β,4α-diol | Whole plants | C. spicatus | [10] |
| Aerial part | C. elatior | [19] | ||
| 31 | Curcolonol | Roots | C. henryi | [12] |
| Whole plants | C. multistachys | [90] | ||
| Roots | C. anhuiensis | [39] | ||
| Roots | C. angustifolius | [41] | ||
| 32 | Zedoarofuran | Roots | C. henryi | [12] |
| Whole plants | C. multistachys | [36] | ||
| 33 | 1α-Hydroxy-8,12-epoxyeudesma-4,7,11-triene-6,9-dione | Leaves and stems | C. henryi | [13] |
| 34 | 7α-8-Oxoeudesm-4(14)-en-12-oic acid | Leaves and stems | C. henryi | [14] |
| 35 | 1α-Methoxy-8,12-epoxyeudesma-4,7,11-trien-6-one | Leaves and stems | C. henryi | [3] |
| 36 | 11,12,13-Trihydroxyeudesma-4(15),8-dien-9-one | Leaves and stems | C. henryi | [3] |
| 37 | 1α-Hydroxy-8,12-epoxyeudesma-4,7,11-triene-3,6-dione | Roots | C. henryi | [16] |
| 38 | Curcolone | Roots | C. henryi | [16] |
| 39 | Endesm-4(15)-en-7α,11-diol | Roots | C. henryi | [16] |
| 40 | (5S,6R,8S,10R)-6-Hydroxyeudesma-4(15),7(11)-diene-12,8-olide | Whole plant | C. henryi | [17] |
| 41 | 6α-Hydroxyeudesma-4(15),7(11),8(9)-triene-12,8-olide | Whole plant | C. henryi | [17] |
| 42 | 8,12-Epoxy-1β-hydroxyeudesma-4(15),7,11-trien-6-one | Whole plant | C. henryi | [17] |
| 43 | Chlorelactone B (9α-Hydroxy-5α,8β(H)-2-oxoeudesma-3,7(11)-dien-12,8α-olide) | Whole plants | C. elatior | [11] |
| 44 | Chloranthalic acid | Whole plants | C. elatior | [11] |
| 45 | Chlorantholide A (2-Oxoeudesma-3,7(11),8-trien-12,8-olide) | Whole plants | C. elatior | [18] |
| 46 | Chlorantholide B (2-Oxoeudesma-3,7(11)-dien-12,8α-olide) | Whole plants | C. elatior | [18] |
| 47 | Chlorantholide C (2-Oxoeudesma-3,7(11)-dien-12,8β-olide) | Whole plants | C. elatior | [18] |
| 48 | Chlorantholide D (8β-Hydroxy-2-oxoeudesma-3,7(11)-dien-12,8-olide) | Whole plants | C. elatior | [18] |
| 49 | Chlorantholide E (8β,9α-Dihydroxy-2-oxoeudesma-3,7(11)-dien-12,8-olide) | Whole plants | C. elatior | [18] |
| 50 | Chlorantholide F (5α,8β-Dihydroxy-2-oxoeudesma-3,7(11)-dien-12,8-olide) | Whole plants | C. elatior | [18] |
| 51 | (1R,4R,5R,8S,10R)-1-Hydroxy-4-methoxy-eudesm-7(11)-en-12,8-olide | Aerial parts | C. elatior | [19] |
| 52 | Chloranthone A | Aerial parts | C. elatior | [20] |
| 53 | Chloranthone B | Aerial parts | C. elatior | [20] |
| 54 | Chloranthone C | Aerial parts | C. elatior | [20] |
| 55 | Chloranthone D | Aerial parts | C. elatior | [20] |
| 56 | 8β-Hydroxyeudesma-4(15),7(11)-dien-12,8-olide (codonolactone, atractylenolide III, atractylenolide β) | Whole plants | C. japonicus | [21] |
| Roots | C. henryi | [13] | ||
| Roots | C. anhuiensis | [39] | ||
| Roots | C. fortunei | [44] | ||
| Leaves | C. tianmushanensis | [86] | ||
| Whole plants | C. multistachys | [9] | ||
| Whole plants | C. spicatus | [10] | ||
| Whole plants | C. serratus | [4] | ||
| Whole plants | S. glabra | [31] | ||
| 57 | Shizukafuranol | Whole plants | C. japonicus | [22] |
| 58 | Shizukolidol | Whole plants | C. japonicus | [22] |
| Whole plants | C. henryi | [23] | ||
| Roots | C. spicatus | [8] | ||
| Whole plants | C. elatior | [18] | ||
| 59 | 5α-(Cinnamoyloxy)-8,12-epoxy-3-methoxy-7βH,8αH-eudesma-3, 11-dien-6-one | Roots | C. japonicus | [24] |
| 60 | 8β-(Cinnamoyloxy)eudesma-4(14),7(11)-dien-12,8-olide | Roots | C. japonicus | [24] |
| 61 | 8,12-Epoxy-1α-hydroxy-4αH,5αH-eudesma-7,11-diene-6,9-dione | Roots | C. japonicus | [24] |
| 62 | 8,12-Epoxy-1α-methoxy-4αH,5αH-eudesma-7,11-diene-6,9-dione | Roots | C. japonicus | [24] |
| 63 | Chlorajapolide I (4β-Hydroxy-8β-ethyoxyl-7(11)-eneudesm-8,12-olide) | Aerial part | C. japonicus | [25] |
| 64 | 3,4,8α-Trimethyl-4α,7,8,8α-tetrahydro-4a-naphto [2,3-b] furan-9-one | Aerial part | C. japonicus | [26] |
| 65 | Chloraeudolide | Whole plants | C. japonicus | [27] |
| Whole plants | C. multistachys | [38] | ||
| 66 | Chlojaponilactone A (8β-Droxyeudesm-3, 7(11)-dien-12, 8α-olide) | Whole plants | C. japonicus | [28] |
| 67 | Sarcaglaboside A (1β,5α,8βH-Eudesman-4(15),7(11)-dien-8α,12-olide-1-O-β-D-glucopyranoside) | Whole plants | S. glabra | [29] |
| Whole plants | C. spicatus | [72] | ||
| Whole plants | C. japonicus | [52] | ||
| 68 | Sarcaglaboside B (1β,5α,8βH-Eudesman-2,4(15),7(11)-trien-8α,12-olide-1-O-β-D-glucopyranoside) | Whole plants | S. glabra | [29] |
| 69 | Sarcaglaboside H (4α-Hydroxy-5α,8βH-eudesman-7(11)-en-8α,12-olide-15-O-β-D-glucopyranoside) | Whole plants | S. glabra | [30] |
| 70 | 8β,9α-Dihydroxyeudesman-4(15),7(11)-dien-8α,12-olide | Whole plants | S. glabra | [31] |
| 71 | Glabranol B (1β,4α,7β,11-Tetrahydroxyeudesmane) | Aerial parts | S. glabra | [32] |
| 72 | 1α,8α,9α-Trihydroxyeudesman-3(4),7(11)-dien-8β,12-olide | Whole plants | S. glabra | [33] |
| 73 | Atractylenolide IV | Whole plants | S. glabra | [34] |
| 74 | Sarcandralactone E | Whole plants | S. glabra | [35] |
| 75 | Chlomultin B | Whole plants | C. multistachys | [36] |
| Whole plant | C. henryi | [17] | ||
| 76 | 1β,8β-Dihydroxyeudesman-3,7(11)-dien-8α,12-olide | Whole plants | C. multistachys | [36] |
| 77 | Lasianthuslactone A | Whole plants | C. multistachys | [9] |
| Whole plants | C. serratus | [4] | ||
| 78 | ent-(3R)-3-Hydroxyatractylenolide III | Whole plants | C. multistachys | [38] |
| 79 | Multistalactone A (4R,5R,6S,8R,10S)-6,8-Dihydroxy-4,15-epoxy-eudes-7(11)-en-12,8-olide) | Whole plants | C. multistachys | [38] |
| 80 | Multistalactone B (4S,5R,8R,10R)-4-Hydroxy-1-oxoeudesm-7(11)-en-12,8-olide) | Whole plants | C. multistachys | [38] |
| 81 | Multistalactone C (4R,5R,8S,10R)-4-Hydroxy-1-oxoeudesm-7(11)-en-12,8-olide | Whole plants | C. multistachys | [38] |
| 82 | (3R)-3-Hydroxyatractylenolide III | Roots | C. anhuiensis | [39] |
| 83 | 8β-Hydroxy-1-oxoeudesma-3,7(11)-dien-12,8α-olide | Roots | C. anhuiensis | [39] |
| 84 | 5α-Hydroxyeudesma-4(15),7(11),8(9)-trien-8,12-olide | Roots | C. anhuiensis | [39] |
| 85 | 1-Oxoeudesm-7(11)-en-8,12-olide | Roots | C. anhuiensis | [39] |
| Whole plants | C. henryi | [17] | ||
| 86 | Anhuienoside A | Leaves | C. anhuiensis | [40] |
| 87 | 9α-Hydroxycurcolonol | Roots | C. angustifolius | [41] |
| 88 | 3α-Hydroxy-4-deoxy-5-dehydrocurcolonol | Roots | C. angustifolius | [41] |
| 89 | 4β,7β,11-Enantioeudesmantriol | Aerial parts | C. angustifolius | [42] |
| 90 | Atractylenolide II (8βH-Eudesma-4(14),7(11)-dien-12,8-olide) | Leaves | C. glaber | [43] |
| Leaves and stems | C. henryi | [13] | ||
| 91 | Atractylenolactam | Roots | C. fortunei | [44] |
| 92 | Chloranerectuslactone V | Leaves | C. erectus | [45] |
| 93 | 9α-Hydroxyasterolide | Aerial part | H. orientale | [37] |
| 94 | 1α-Acetoxyeudesma-3,7(11)-dien-8,12-olide | Leaves | H. brasiliense | [46] |
| Lindenranes | ||||
| 95 | Shizukanolide A (Shizukanolide) | Aerial parts | C. japonicus | [48] |
| Roots | C. japonicus | [65] | ||
| Roots | C. glaber | [56] | ||
| Whole plants | C. henryi | [23] | ||
| Whole plants | S. glabra | [93] | ||
| 96 | Chloranthalactone C (13-deoxyshizukanolide H) | Whole plants | C. japonicus | [21] |
| Roots | C. holostegius | [95] | ||
| Roots | C. serratus | [1] | ||
| Roots | C. fortunei | [44] | ||
| Aerial part | C. fortunei | [57] | ||
| 97 | Chloranthalactone D | Whole plants | C. japonicus | [21] |
| 98 | Chloranthalactone E | Whole plants | C. japonicus | [21] |
| Leaves | C. glaber | [43] | ||
| Whole plants | S. glabra | [31] | ||
| Roots | C. japonicus | [49] | ||
| 99 | Shizukanolide C | Roots | C. japonicus | [49] |
| Whole plants | C. japonicus | [28] | ||
| Aerial part | C. fortunei | [57] | ||
| Whole plants | C. spicatus | [72] | ||
| 100 | Shizukanolide D | Roots | C. japonicus | [50] |
| 101 | 9-Hydroxy heterogorgiolide | Aerial part | C. japonicus | [51] |
| Leaves | C. erectus | [45] | ||
| Whole plants | S. glabra | [30] | ||
| 102 | Yinxiancaoside A (Sarcaglaboside G) | Whole plants | C. japonicus | [52] |
| Whole plants | S. glabra | [30] | ||
| 103 | Chlorajapolide A ((1α,3α,6β,8β)-6-Hydroxy-15-al-1H-lindan-4,7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 104 | Chlorajapolide B ((1α,3α,6β,8β)-6,15-Epoxy-15-hydroxy-1H-lindan-4,7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 105 | Chlorajapolide C ((1α,3α,8β)-15-Hydroxy-1H-lindan-4,7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 106 | Chlorajapolide D ((1α,3α,5α,8β)-4α,15-Dihydroxy-1H-lindan-4,7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 107 | Chlorajapolide E ((1α,3α,4β,9β)-8β-Methoxy-9α-hydroxy-15-acetyl-1H-lindan-4,7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 108 | Chlorajaposide ((1α,3α-8β-Glucopyranosyl-1H-lindan-4(15),7(11)-dien-12,8α-olide) | Whole plants | C. japonicus | [27] |
| 109 | Chlorajapolide F | Aerial part | C. japonicus | [25] |
| 110 | Chlorajapolide G (chlojaponilactone E) | Aerial part | C. japonicus | [25] |
| Whole plants | C. japonicus | [53] | ||
| 111 | Chlorajapolide H | Aerial part | C. japonicus | [25] |
| 112 | Chlojaponilactone B | Whole plants | C. japonicus | [53] |
| 113 | Chlojaponilactone C | Whole plants | C. japonicus | [53] |
| 114 | Chlojaponilactone D | Whole plants | C. japonicus | [53] |
| 115 | Chloranthalactone E 8-O-β-D-glucopyranoside (8β,9α-Dihydroxy-5α,9βH-lindan-4(15),7(13)-dien-8α,12-olide-8β-O-β-D-glucopyranoside) | Whole plants | S. glabra | [29] |
| 116 | 8β,9α-Dihydroxylindan-4(5),7(11)-dien-8α,12-olide | Whole plants | S. glabra | [31] |
| 117 | Sarcaglaboside F (8β,9β-Epoxy-4α-hydroxy-5αH-lindan-7(11)-en-8α,12-olide-15-O-β-D-glucopyranoside) | Whole plants | S. glabra | [30] |
| 118 | Sarcandralactone A | Whole plants | S. glabra | [5] |
| 119 | Glabranol A (8α,9α,15-Trihydroxylinden-4,7(11)-dien-12,8β-olide) | Whole plants | S. glabra | [32] |
| 120 | 4α-Hydroxy-5αH-lindan-8 (9)-en-8, 12-olide | Whole plants | S. glabra | [54] |
| 121 | Sarcandralactone C | Whole plants | S. glabra | [35] |
| 122 | Sarcandralactone D | Whole plants | S. glabra | [35] |
| 123 | Chloranthalactone A = shizukanolide B (Dehydro-shizukanolide, 8,9-Dehydroshizukanolide) | Roots | C. glaber | [55] |
| Aerial parts | C. japonicus | [48] | ||
| Leaves | C. glaber | [43] | ||
| Flowers | C. spicatus | [6] | ||
| Whole plants | C. henryi | [23] | ||
| Roots | C. japonicus | [65] | ||
| Leaves | C. tianmushanensis | [86] | ||
| barks | H. angustifolium | [60] | ||
| 124 | Chloranthalactone B | Roots | C. glaber | [55] |
| Leaves | C. glaber | [43] | ||
| Whole plants | C. japonicus | [21] | ||
| Whole plants | S. glabra | [30] | ||
| Aerial part | C. japonicus | [25] | ||
| Leaves | C. erectus | [45] | ||
| 125 | Chloranthalactone F | Leaves | C. glaber | [43] |
| 126 | Chloranoside A (Shizukanolide E 15-O-β-glucoside) | Whole plants | C. glaber | [56] |
| Whole plants | C. japonicus | [52] | ||
| Aerial part | C. fortunei | [57] | ||
| Whole plants | C. spicatus | [72] | ||
| Whole plants | S. glabra | [29] | ||
| 127 | Chloranoside B (Shizukanolide F 15-O-β-glucoside) | Whole plants | C. glaber | [56] |
| 128 | Shizukanolide G | Aerial part | C. fortunei | [57] |
| 129 | Shizukanolide H | Aerial part | C. fortunei | [57] |
| Whole plants | C. japonicus | [28] | ||
| Whole plants | S. glabra | [35] | ||
| 130 | Chlorafortulide | Whole plants | C. fortunei | [58] |
| 131 | Shizukanolide E | Roots | C. serratus | [50] |
| Roots | C. henryi | [12] | ||
| Whole plants | S. glabra | [100] | ||
| 132 | Shizukanolide F | Roots | C. serratus | [50] |
| Aerial part | C. fortunei | [57] | ||
| Whole plants | C. spicatus | [72] | ||
| Whole plants | S. glabra | [34] | ||
| 133 | 13-Hydroxy-8,9-dehydroshizukanolide (onoseriolide) | Stems and Leaves | H. brasiliense | [59] |
| Aerial part | H. orientale | [37] | ||
| barks | H. angustifolium | [60] | ||
| Leaves | H. brasiliense | [46] | ||
| 134 | Oxyonoseriolide | barks | H. angustifolium | [60] |
| 135 | Hedyosmone | barks | H. angustifolium | [60] |
| Guaianes | ||||
| 136 | Hedyosumin A (7α,10α-Epoxy-3-oxo-1αH-guaia-4(5),11(13)-dien-8α,12-olide) | Aerial part | H. orientale | [37] |
| 137 | Hedyosumin B (7α,10α-Epoxy-3-oxo-1,11αH-guaia-4(5)-en-8α,12-olide) | Aerial part | H. orientale | [37] |
| 138 | Hedyosumin C (3β-Hydroxy-7α,10α-epoxy-1,11αH-guaia-4(5)-en-8α,12-olide) | Aerial part | H. orientale | [37] |
| 139 | Hedyosumin D (13-Acetoxy-1α,5αH-guaia-3,7(11),10(15)-trien-8α, 12-olide) | Aerial part | H. orientale | [37] |
| 140 | Hedyosumin E (1α,5α,8βH-Guaia-3,7(11)-dien-8,12-olide-10-O-β-D-glucopyranoside) | Aerial part | H. orientale | [37] |
| 141 | 10α-Hydroxy-1,5αH-guaia-3,7(11)-dien-8α,12-olide | Aerial part | H. orientale | [37] |
| 142 | Hedyosmum F | Aerial part | H. orientale | [61] |
| 143 | Chlomultin A | Whole plants | C. multistachys | [36] |
| 144 | (1R,4S,5R,8S,10S)-Zedoalactone A | Whole plants | C. multistachys | [38] |
| 145 | Multistalactone D (chlospicate C) | Whole plants | C. multistachys | [38] |
| Whole plants | C. spicatus | [10] | ||
| 146 | Multistalactone E | Whole plants | C. multistachys | [38] |
| 147 | Multistalactone F | Whole plants | C. multistachys | [38] |
| 148 | Podoandin | Leaves | H. brasiliense | [62] |
| 149 | 1,2-Epoxy-10α-hydroxy-podoandin | Leaves | H. brasiliense | [62] |
| 150 | 1-Hydroxy-10,15-methylenepodoandin | Leaves | H. brasiliense | [62] |
| 151 | 7,10-Epoxy-1,5-guaia-3,11-dien-8,12-olide (7,10-Epoxy-hedyosminolide) | Leaves | H. arborescens | [63] |
| Aerial part | H. orientale | [61] | ||
| 152 | Chlorantene A | Whole plants | C. serratus | [2] |
| 153 | (1S,4S,5S,8R,10S)-4,10-Dihydroxyguai-7(11)-en-12,8-olide (zedoalactone A) | Whole plants | C. serratus | [3] |
| Aerial part | C. elatior | [19] | ||
| 154 | 12-Oxochloraniolide A | Whole plants | C. henryi | [17] |
| 155 | (7S,1(10)Z)-4,5-Secoguaia-1(10),11-diene-4,5-dione | Whole plants | C. henryi | [17] |
| 156 | Chloraniolide A | Roots | C. anhuiensis | [39] |
| 157 | Chlospicate D | Whole plants | C. spicatus | [10] |
| 158 | Zedoalactone E (1βH,5βH,8βH-4α,10α-Dihydroxyguai-7(11)-en-12,8-olide) | Aerial part | C. elatior | [19] |
| Germacranes | ||||
| 159 | Acoragermacrone | Roots | C. serratus | [1] |
| 160 | Aederone | Roots | C. serratus | [1] |
| 161 | Furanodienone | Roots | C. serratus | [1] |
| Whole plants | C. japonicus | [22] | ||
| Roots | C. angustifolius. | [41] | ||
| Whole plants | S. glabra | [93] | ||
| 162 | Chlorantene E | Whole plants | C. serratus | [2] |
| 163 | (1E,4Z)-8-Hydroxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone | Leaves and stems | C. henryi | [3] |
| 164 | 8-Methoxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone | Leaves and stems | C. henryi | [3] |
| 165 | 15-Hydroxy-11βH-8-oxogermacra-1(10),4-dieno-12,6α-lacton | Leaves and stems | C. henryi | [3] |
| 166 | Zederone epoxide | Whole plants | C. henryi | [17] |
| 167 | (1S,4S,5S,10S)-1,10 : 4,5-Diepoxygermacrone | Whole plants | C. henryi | [17] |
| 168 | Germacra-5E,10(14)-dien-1β,4β-diol | Whole plants | C. spicatus | [10] |
| 169 | 4α,5α-Epoxy-1(10),7(11)-Dienegermacr-8α,12-olide | Whole plants | C. spicatus | [10] |
| 170 | Isofuranodiene | Roots | C. japonicus | [65] |
| Roots | C. serratus | [1] | ||
| Leaves | C. tianmushanensis | [86] | ||
| 171 | Glechomanolide | Roots | C. japonicus | [65] |
| Leaves | C. tianmushanensis | [86] | ||
| Whole plants | C. serratus | [3] | ||
| 172 | Chloranthatone | Roots | C. fortunei | [44] |
| 173 | 1β,10α,4α,5β-Diepoxy-6β-hydroxyglechoman-8α,12-olide | Whole plants | C. multistachys | [122] |
| 174 | Sarcaglaboside E (1E,4Z)-8βH-Germacra-1,4,7(11)-trien-8α,12-olide-15-O-[β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside] | Whole plants | S. glabra | [29] |
| Cadinanes | ||||
| 175 | (11β)-8,11-Dihydroxycadina-6,8,10-trien-12-oic acid γ-lactone | Leaves and stems | C. henryi | [14] |
| Whole plants | C. henryi | [17] | ||
| 176 | (4α,11β)-8,11-Dihydroxycadina-6,8,10-trien-12-oic acid γ-lactone | Leaves and stems | C. henryi | [14] |
| Whole plants | C. henryi | [17] | ||
| 177 | (8α)-6,8-Dihydroxycadina-7(11),10(15)-dien-12-oic acid γ-lactone | Leaves and stems | C. henryi | [14] |
| 178 | (4α)-8-Hydroxy-12-norcardina-6,8,10-trien-11-one | Whole plants | C. henryi | [17] |
| 179 | (-)-Dihydropyrocurzerenone | Whole plants | C. serratus | [66] |
| 180 | Pyrocurzerenone | Whole plants | C. serratus | [66] |
| 181 | 6α,8α,10α-Trihydroxycardina-4(15),7(11)-dien-12-oic acid γ-lactone | Whole plants | C. serratus | [4] |
| 182 | Chlomultin C | Whole plants | C. multistachys | [36] |
| 183 | Chlomultin D | Whole plants | C. multistachys | [36] |
| 184 | Furanocadina-1(10),6,8-triene-4-ol | Whole plants | C. multistachys | [36] |
| Whole plants | C. serratus | [4] | ||
| Eremophilanes | ||||
| 185 | (-)-Istanbulin A | Leaves | S. glabra | [67] |
| 186 | (3R,4S,5R,10S,11S)-3-Hydroxy-8-oxo-6-eremophilen-12-oic acid | Leaves | C. anhuiensis | [40] |
| 187 | Anhuienol | Leaves | C. anhuiensis | [40] |
| 188 | (3R,4S,5R,6R,8R,10S)-3,6,8-Trihydroxy-7(11)-eremophilen-12,8-olide | Leaves | C. anhuiensis | [40] |
| 189 | 3α,6α-Dihydroxy-8αH-7(11)-eremophilen-12,8-olide | Leaves | C. anhuiensis | [40] |
| 190 | 6αH,8αH-7(11)-Eremophilen-12,8:15,6-diolide | Leaves | C. anhuiensis | [40] |
| 191 | Istanbulin F (1β,8-Dihydroxyeremophila-3,7(11)-dien-8,12-olide) | Roots | C. anhuiensis | [39] |
| 192 | 10α-Hydroxy-1-oxoeremophila-7(11),8(9)-diene-8,12-olide | Whole plants | C. japonicus | [28] |
| 193 | Tsoongianolide D | Whole plants | C. japonicus | [53] |
| 194 | Tsoongianolide E | Whole plants | C. japonicus | [53] |
| Aromadendranes | ||||
| 195 | Aromadendrane-4α,10β-diol | Leaves | C. glaber | [43] |
| 196 | 4β,10α-Dihydroxyaromadendrane ((+)-Alloromadendrane-4β,10α-diol) | Aerial part | C. spicatus | [7] |
| Whole plants | C. spicatus | [10] | ||
| Aerial part | C. elatior | [19] | ||
| 197 | Spathulenol | Aerial part | C. spicatus | [7] |
| Aerial part | H. orientale | [37] | ||
| Barks | H. angustifolium | [60] | ||
| Whole plants | S. glabra | [5] | ||
| Whole plants | C. elatior | [11] | ||
| 198 | Aromadendrane-4β,10β-diol | Aerial part | H. orientale | [37] |
| 199 | 1αH,5βH,6αH,7αH-4β,10β,15-Trihydroxyaromadendrane | Aerial part | C. elatior | [19] |
| 200 | Aromadendrane-4β,10α,15-triol | Aerial part | C. elatior | [19] |
| Elemanes | ||||
| 201 | Isogermafurenolide | Flowers | C. spicatus | [6] |
| 202 | Sarcaglaboside C (5α,8βH-Eleman-1,3,7(11)-trien-8α,12-olide-15-O-β-D-glucopyranoside) | Whole plants | S. glabra | [29] |
| 203 | Sarcaglaboside D (5α,8βH-Eleman-1,3,7(11)-trien-8α,12-olide-15-O-[β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside] | Whole plants | S. glabra | [29] |
| 204 | Chlorantene F | Whole plants | C. serratus | [2] |
| 205 | 15-Hydroxy-isogermafurenolide | Leaves | H. brasiliense | [46] |
| Other sesquiterpenes | ||||
| 206 | Shizukaacoradienol | Whole plants | C. japonicus | [22] |
| Roots | C. fortunei | [44] | ||
| 207 | Oplopanone | Aerial parts | C. spicatus | [7] |
| Whole plants | C. spicatus | [10] | ||
| 208 | Dayejijiol | Whole plants | C. henryi | [23] |
| 209 | Pisumionoside (3S,5S,6R,7E)-3,5,6-Trihydroxy-9-oxo-megastigm-7-ene-3-O-β-glucopyranoside) | Whole plants | C. japonicus | [52] |
| 210 | Yinxiancaoside B (3β,5α,6β,7E)-3-O-β-Glucopyranosyl-3,5,6,9-tetrahydroxymegastigm-7-ene-9-O-β-glucopyranoside | Whole plants | C. japonicus | [52] |
| 211 | 11-Hydroxydrim-8,12-en-14-oic acid | Roots | C. henryi | [16] |
| 212 | Acrostalic acid | Roots | C. anhuiensis | [39] |
| 213 | Chlospicate E | Whole plants | C. spicatus | [10] |
| 214 | Homalomenol C | Whole plants | C. spicatus | [10] |
| 215 | (1S,4S,5S,6R,7R,10S)-1,4-Dihydroxymaaliane | Aerial part | C. elatior | [19] |
| Sesquiterpene polymers | ||||
| 216 | Shizukaol A | Roots | C. japonicus | [68] |
| Roots | C. serratus | [81] | ||
| Roots | C. fortunei | [83] | ||
| 217 | Shizukaol E | Roots | C. japonicus | [69] |
| Whole plants | C. spicatus | [72] | ||
| Roots | C. fortunei | [83] | ||
| Whole plants | S. glabra | [5] | ||
| 218 | Shizukaol F | Roots | C. japonicus | [69] |
| Whole plants | C. multistachys | [90] | ||
| Aerial part | C. fortunei | [57] | ||
| Roots | C. spicatus | [76] | ||
| Roots | C. fortunei | [83] | ||
| Aerial part | C. angustifolius | [42] | ||
| 219 | Shizukaol G | Roots | C. japonicus | [69] |
| Roots | C. spicatus | [8] | ||
| Whole plants | S. glabra | [5] | ||
| Whole plants | C. fortunei | [58] | ||
| Seeds | S. glabra | [87] | ||
| 220 | Shizukaol H | Roots | C. japonicus | [69] |
| Whole plants | C. spicatus | [10] | ||
| Whole plants | S. glabra | [35] | ||
| 221 | Shizukaol I | Roots | C. japonicus | [69] |
| Roots | C. fortunei | [83] | ||
| 222 | Tishizukaol A | Roots | C. japonicus | [70] |
| 223 | Shizukaol J | Roots | C. japonicus | [70] |
| Roots | C. fortunei | [83] | ||
| 224 | Yinxiancaol | Roots | C. japonicus | [24] |
| Aerial part | C. fortunei | [57] | ||
| 225 | Chlorajaponol | Whole plants | C. japonicus | [27] |
| 226 | Chlorajaponilide A | Whole plants | C. japonicus | [71] |
| 227 | Chlorajaponilide B | Whole plants | C. japonicus | [71] |
| 228 | Chlorajaponilide C | Whole plants | C. japonicus | [71] |
| 229 | Chlorajaponilide D | Whole plants | C. japonicus | [71] |
| 230 | Chlorajaponilide E | Whole plants | C. japonicus | [71] |
| Whole plants | S. glabra | [35] | ||
| 231 | Chloramultilide B | Whole plants | C. spicatus | [72] |
| Aerial part | C. fortunei | [57] | ||
| 232 | Chloramultilide C (henriol A) | Whole plants | C. spicatus | [72] |
| Roots | C. henryi | [73] | ||
| Whole plants | C. multistachys | [79] | ||
| Whole plants | C. elatior | [11] | ||
| Whole plants | C. serratus | [4] | ||
| Roots | C. angustifolius | [41] | ||
| Aerial part | C. angustifolius | [42] | ||
| 233 | Chloramultilide D (henriol B) | Whole plants | C. spicatus | [72] |
| Roots | C. henryi | [73] | ||
| Whole plants | C. multistachys | [79] | ||
| 234 | Spicachlorantin A | Roots | C. spicatus | [74] |
| Whole plants | C. serratus | [4] | ||
| Whole plants | C. henryi | [17] | ||
| Roots | C. angustifolius. | [41] | ||
| 235 | Spicachlorantin B | Roots | C. spicatus | [74] |
| Whole plants | C. multistachys | [79] | ||
| Whole plants | C. japonicus | [71] | ||
| Whole plants | C. henryi | [17] | ||
| Aerial part | C. angustifolius | [42] | ||
| 236 | Spicachlorantin C | Roots | C. spicatus | [75] |
| Whole plants | C. serratus | [4] | ||
| 237 | Spicachlorantin D | Roots | C. spicatus | [75] |
| 238 | Spicachlorantin E | Roots | C. spicatus | [75] |
| 239 | Spicachlorantin F | Roots | C. spicatus | [75] |
| Whole plants | S. glabra | [35] | ||
| 240 | Spicachlorantin G | Roots | C. spicatus | [76] |
| Whole plants | C. henryi | [17] | ||
| 241 | Spicachlorantin H | Roots | C. spicatus | [76] |
| 242 | Spicachlorantin I | Roots | C. spicatus | [76] |
| 243 | Spicachlorantin J | Roots | C. spicatus | [76] |
| 244 | Chloramultilide A | Whole plants | C. multistachys | [77] |
| Whole plants | C. spicatus | [72] | ||
| Roots | C. spicatus | [74] | ||
| Whole plants | C. serratus | [4] | ||
| Whole plants | C. henryi | [17] | ||
| Roots | C. angustifolius. | [41] | ||
| 245 | Multistalide A | Whole plants | C. multistachys | [78] |
| 246 | Multistalide B | Whole plants | C. multistachys | [78] |
| 247 | Chloramultiol A | Whole plants | C. multistachys | [79] |
| 248 | Chloramultiol B | Whole plants | C. multistachys | [79] |
| 249 | Chloramultiol C | Whole plants | C. multistachys | [79] |
| 250 | Chloramultiol D | Whole plants | C. multistachys | [79] |
| 251 | Chloramultiol E | Whole plants | C. multistachys | [79] |
| 252 | Chloramultiol F | Whole plants | C. multistachys | [79] |
| 253 | Chloramultiol G | Whole plants | C. multistachys | [38] |
| 254 | Shizukaol B (henriol C) | Roots | C. serratus | [80] |
| Roots | C. japonicus | [24] | ||
| Aerial part | C. japonicus | [25] | ||
| Roots | C. henryi | [73] | ||
| Roots | C. fortunei | [83] | ||
| Roots | C. spicatus | [8] | ||
| Whole plants | C. spicatus | [10] | ||
| Whole plants | S. glabra | [5] | ||
| Roots | C. angustifolius | [41] | ||
| Aerial part | C. angustifolius | [42] | ||
| Seeds | S. glabra | [87] | ||
| 255 | Shizukaol C | Roots | C. serratus | [80] |
| Roots | C. henryi | [73] | ||
| Whole plants | C. multistachys | [79] | ||
| Roots | C. spicatus | [8] | ||
| Whole plants | C. spicatus | [10] | ||
| Whole plants | S. glabra | [5] | ||
| Whole plants | C. fortunei | [58] | ||
| Aerial part | C. japonicus | [25] | ||
| Seeds | S. glabra | [87] | ||
| Aerial part | C. angustifolius | [42] | ||
| 256 | Shizukaol D | Roots | C. serratus | [80] |
| Roots | C. fortunei | [83] | ||
| Whole plants | C. multistachys | [79] | ||
| Roots | C. spicatus | [76] | ||
| Aerial part | C. japonicus | [25] | ||
| Whole plants | S. glabra | [35] | ||
| 257 | Cycloshizukaol A | Roots | C. serratus | [81] |
| Whole plants | C. multistachys | [90] | ||
| Aerial part | C. fortunei | [57] | ||
| Roots | C. spicatus | [8] | ||
| Roots | C. japonicus | [124] | ||
| Whole plants | C. japonicus | [71] | ||
| Whole plants | S. glabra | [5] | ||
| 258 | Serratustones A | Whole plants | C. serratus | [82] |
| 259 | Serratustones B | Whole plants | C. serratus | [82] |
| 260 | 8α-Ethoxy-spicachlorantin A | Whole plants | C. serratus | [4] |
| 261 | 8α-Hydroxy-chloramultiol F | Whole plants | C. serratus | [4] |
| 262 | Shizukaol K | Roots | C. fortunei | [83] |
| 263 | Shizukaol L | Roots | C. fortunei | [83] |
| 264 | Shizukaol M | Roots | C. fortunei | [83] |
| 265 | Shizukaol N | Roots | C. fortunei | [83] |
| Seeds | S. glabra | [87] | ||
| 266 | Shizukaol O | Roots | C. fortunei | [83] |
| Whole plants | C. fortunei | [58] | ||
| Aerial part | C. japonicus | [25] | ||
| 267 | 13′-Acetylshizukaol C (chlorahololide D, henriol D) | Roots | C. fortunei | [83] |
| Whole plants | C. fortunei | [58] | ||
| Whole plants | C. holostegius | [85] | ||
| Roots | C. henryi | [73] | ||
| Stems and Roots | C. henryi | [118] | ||
| Roots | C. spicatus | [8] | ||
| Whole plants | S. glabra | [35] | ||
| 268 | Shizukaol P | Aerial part | C. fortunei | [57] |
| Roots | C. spicatus | [76] | ||
| 269 | 9-O-β-Glucopyranosylcycloshizukaol A | Aerial part | C. fortunei | [57] |
| 270 | Chlorahololide A | Whole plants | C. holostegius | [84] |
| 271 | Chlorahololide B | Whole plants | C. holostegius | [84] |
| Whole plants | C. spicatus | [72] | ||
| Whole plants | C. japonicus | [71] | ||
| 272 | Chlorahololide C | Whole plants | C. holostegius | [85] |
| Whole plants | C. japonicus | [71] | ||
| 273 | Chlorahololide E | Whole plants | C. holostegius | [85] |
| 274 | Chlorahololide F | Whole plants | C. holostegius | [85] |
| Whole plants | S. glabra | [5] | ||
| 275 | Tianmushanol | Leaves | C. tianmushanensis | [86] |
| Roots | C. angustifolius | [41] | ||
| 276 | 8-O-Methyltianmushanol | Leaves | C. tianmushanensis | [86] |
| Roots | C. angustifolius | [41] | ||
| 277 | Sarcandrolide A (13′-deoxyshizukaol C) | Whole plants | S. glabra | [5] |
| Seeds | S. glabra | [87] | ||
| 278 | Sarcandrolide B | Whole plants | S. glabra | [5] |
| 279 | Sarcandrolide C (2′′′-O-acetylshizukaol G) | Whole plants | S. glabra | [5] |
| 280 | Sarcandrolide D | Whole plants | S. glabra | [5] |
| 281 | Sarcandrolide E | Whole plants | S. glabra | [5] |
| 282 | Sarcandrolide F | Whole plants | S. glabra | [35] |
| 283 | Sarcandrolide G | Whole plants | S. glabra | [35] |
| 284 | Sarcandrolide H | Whole plants | S. glabra | [35] |
| 285 | Sarcandrolide I | Whole plants | S. glabra | [35] |
| 286 | Sarcandrolide J | Whole plants | S. glabra | [35] |
| 287 | Sarglabolide A | Seeds | S. glabra | [87] |
| 288 | Sarglabolide B | Seeds | S. glabra | [87] |
| 289 | Sarglabolide C | Seeds | S. glabra | [87] |
| 290 | Sarglabolide D | Seeds | S. glabra | [87] |
| 291 | Sarglabolide E | Seeds | S. glabra | [87] |
| 292 | Sarglabolide F | Seeds | S. glabra | [87] |
| 293 | Sarglabolide G | Seeds | S. glabra | [87] |
| 294 | Sarglabolide H | Seeds | S. glabra | [87] |
| 295 | Sarglabolide I | Seeds | S. glabra | [87] |
| 296 | Sarglabolide J | Seeds | S. glabra | [87] |
| 297 | Sarglabolide K | Seeds | S. glabra | [87] |
| 298 | Sarcanolide A | Whole plants | S. hainanensis | [88] |
| 299 | Sarcanolide B | Whole plants | S. hainanensis | [88] |
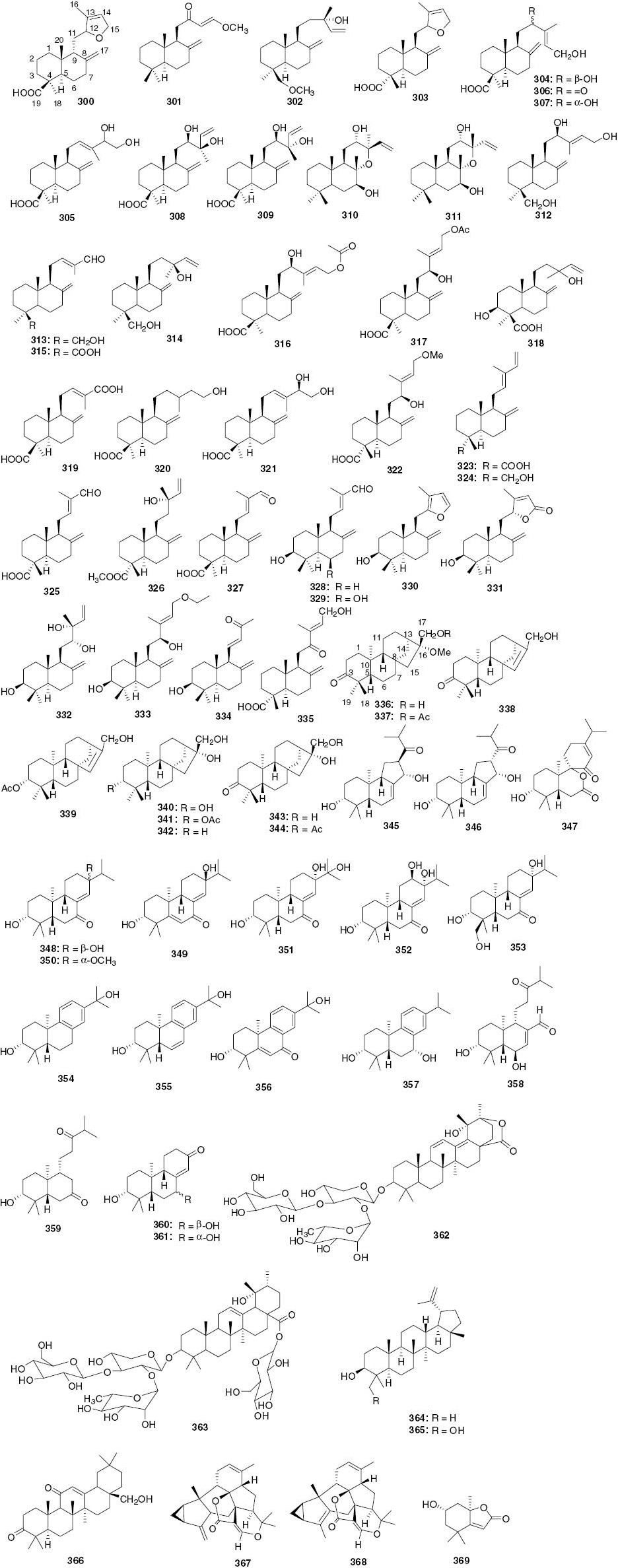
Diterpenoids and triterpenoids.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| Labdanes | ||||
| 300 | 12,15-Epoxy-5αH,9βH-labda-8(17),13-dien-19-oic acid | Leaves and stems | C. henryi | [13] |
| 301 | 14-Methoxy-15,16-dinor-5αH,9αH-labda-13(E),8(17)-dien-12-one | Leaves and stems | C. henryi | [13] |
| 302 | (13S)-13-Hydroxy-19-methoxy-5αH-8(17),14-labdadien | Leaves and stems | C. henryi | [23] |
| 303 | 12,15-Epoxylabda-8(20),13-dien-18-oic acid | Leaves and stems | C. henryi | [14] |
| 304 | Henrilabdane A (12 (R),15-Dihydroxylabda-8(17),13E-dien-19-oic acid) | Roots | C. henryi | [73] |
| Roots | C. henryi | [16] | ||
| Roots | C. anhuiensis | [39] | ||
| Whole plants | C. multistachys | [9] | ||
| 305 | Henrilabdane B (9S,14,15-Dihydroxylabda-8(17),12E-dien-19-oic acid) | Roots | C. henryi | [73] |
| 306 | Henrilabdane C (12-oxo-15-Hydroxylabda-8(17),13E-dien-19-oic acid) | Roots | C. henryi | [73] |
| Roots | C. henryi | [16] | ||
| Roots | C. anhuiensis | [39] | ||
| Whole plants | C. multistachys | [9] | ||
| 307 | 12(S),15-Dihydroxylabda-8(17),13E-dien-19-oic acid | Roots | C. henryi | [73] |
| Roots | C. anhuiensis | [39] | ||
| Whole plants | C. multistachys | [9] | ||
| 308 | 12(R),13(S)-Dihydroxylabda-8(17),14-dien-19-oic acid | Roots | C. henryi | [73] |
| 309 | 12(R),13(R)-Dihydroxylabda-8(17),14-dien-19-oic acid | Roots | C. henryi | [73] |
| 310 | 7β,12α-Dihydroxy-13-epi-manoyl oxide | Roots | C. henryi | [16] |
| 311 | 7β,12α-Dihydroxymanoyl oxide | Roots | C. henryi | [16] |
| 312 | (12R)-Labda-8(17),13E-dien-12,15,19-triol | Roots | C. henryi | [16] |
| 313 | 15-Nor-14-oxolabda-8(17),12E-dien-19-ol | Roots | C. henryi | [16] |
| 314 | 13-Epitorulosol | Roots | C. henryi | [16] |
| 315 | 15-Nor-14-xolabda-8(17),12E-dien-19-oic acid | Roots | C. henryi | [16] |
| 316 | (12R,13E)-15-Acetoxy-12-hydroxylabda-8(20),13-dien-19-oic acid | Roots | C. anhuiensis | [39] |
| 317 | (12S,13E)-15-Acetoxy-12-hydroxylabda-8(20),13-dien-19-oic acid | Roots | C. anhuiensis | [39] |
| 318 | 3β,13-Dihydroxylabda-8(20),14-dien-19-oic acid | Roots | C. anhuiensis | [39] |
| 319 | (12E)-15-Norlabda-8(20),12-dien-13,19-dioic acid | Roots | C. anhuiensis | [39] |
| 320 | 13,14-Dihydrogen-isocupressic acid | Roots | C. anhuiensis | [39] |
| 321 | (12E,14R)-14,15-Dihydroxylabda-8(20),12-dien-19-oic acid | Roots | C. anhuiensis | [39] |
| 322 | (12S*,13E)-12-Hydroxy-15-methoxylabda-8(17),13-dien-18-oic acid | Roots | C. spicatus | [8] |
| 323 | Labdan-8(17),12,14-trien-18-oic acid | Roots | C. spicatus | [8] |
| 324 | Labdan-8(17),12,14-trien-18-ol | Roots | C. spicatus | [8] |
| 325 | (12E)-15-Nor-14-oxolabda-8(17),12-diene-18-oic acid | Roots | C. spicatus | [8] |
| 326 | 13β-Hydroxylabda-8(17),14-dien-18-oic acid methyl ester | Roots | C. spicatus | [8] |
| 327 | 15-Norlabda-8(20),12E-diene-14-carboxalde-19-oic acid | Whole plants | C. spicatus | [10] |
| 328 | 3β-Hydroxy-15-nor-14-oxo-8(17),12-labdadien-14-al | Whole plants | C. serratus | [4] |
| 329 | 3β,6β-Dihydroxy-15-nor-14-oxo-8(17),12-labdadien-14-al | Whole plants | C. serratus | [4] |
| 330 | Serralabdane A | Whole plants | C. serratus | [89] |
| 331 | Serralabdane B | Whole plants | C. serratus | [89] |
| 332 | Serralabdane C | Whole plants | C. serratus | [89] |
| 333 | Serralabdane D | Whole plants | C. serratus | [89] |
| 334 | Serralabdane E | Whole plants | C. serratus | [89] |
| 335 | Elatiorlabdane | Whole plants | C. elatior | [11] |
| Kauranes | ||||
| 336 | ent-17-Hydroxyl-16β-methoxyl-kauran-3-one | Whole plants | C. multistachys | [90] |
| 337 | ent-17-Acetoxyl-16β-methoxyl-kauran-3-one | Whole plants | C. multistachys | [90] |
| 338 | ent-17-Hydroxyl-kaur-15-en-3-one | Whole plants | C. multistachys | [90] |
| 339 | ent-3β-Acetoxyl-kaur-15-en-16β, 17-diol | Whole plants | C. multistachys | [90] |
| 340 | ent-Kauran-3β, 16β, 17-triol | Whole plants | C. multistachys | [90] |
| 341 | ent-3β-Acetoxyl-kauran-16β, 17-diol | Whole plants | C. multistachys | [90] |
| 342 | ent-Kauran-16β, 17-diol | Whole plants | C. multistachys | [90] |
| 343 | Abbeokutone | Whole plants | C. multistachys | [90] |
| 344 | ent-17α-Acetyl-16β-hydroxyl-kauran-3-one | Whole plants | C. multistachys | [90] |
| Ent-abietanes | ||||
| 345 | Sessilifol A | Whole plants | C. sessilifolius | [91] |
| 346 | Sessilifol B | Whole plants | C. sessilifolius | [91] |
| 347 | Sessilifol C | Whole plants | C. sessilifolius | [91] |
| 348 | Sessilifol D | Whole plants | C. sessilifolius | [91] |
| 349 | Sessilifol E | Whole plants | C. sessilifolius | [91] |
| 350 | Sessilifol F | Whole plants | C. sessilifolius | [91] |
| 351 | Sessilifol G | Whole plants | C. sessilifolius | [91] |
| 352 | Sessilifol H | Whole plants | C. sessilifolius | [91] |
| 353 | Sessilifol I | Whole plants | C. sessilifolius | [91] |
| 354 | Sessilifol J | Whole plants | C. sessilifolius | [91] |
| 355 | Sessilifol K | Whole plants | C. sessilifolius | [91] |
| 356 | Sessilifol L | Whole plants | C. sessilifolius | [91] |
| 357 | Sessilifol M | Whole plants | C. sessilifolius | [91] |
| 358 | Sessilifol N | Whole plants | C. sessilifolius | [91] |
| Norditerpenoids | ||||
| 359 | Sessilifol O | Whole plants | C. sessilifolius | [91] |
| 360 | Sessilifol P | Whole plants | C. sessilifolius | [91] |
| 361 | Sessilifol Q | Whole plants | C. sessilifolius | [91] |
| Triterpenoids and other terpenoids | ||||
| 362 | Sarcandroside A (3β,19α,20β-Trihydroxyurs-11,13(18)-diene-28,20β-lactone-3-O-β-D-glucopyranosyl (1→3)-[α-L-rhamnopyranosyl (1→2)]-β-D-xylopyranoside) | Whole plants | S. glabra | [92] |
| 363 | Sarcandroside B (3-O-β-D-Glucopyranosyl (1→3)-[α-L-rhamnopyranosyl (1→2)]-β-D-xylopyranosyl-pomolic acid 28-O-β-D-glucopyranosyl ester) | Whole plants | S. glabra | [92] |
| 364 | Lupeol | Whole plants | S. glabra | [93] |
| 365 | 24-Hydroxylupeol | Whole plants | S. glabra | [93] |
| 366 | 28-Hydroxyolean-12-ene-3,11-dione | Leaves and stems | C. henryi | [14] |
| 367 | Bolivianine | trunk bark | H. angustifolium | [94] |
| 368 | Isobolivianine | trunk bark | H. angustifolium | [94] |
| 369 | Loliolide | Aerial part | C. japonicus | [25] |
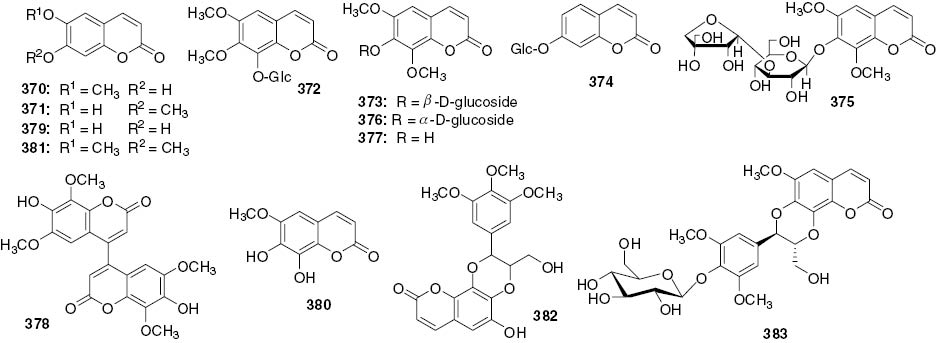
Coumarins.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| 370 | Scopoletin | Whole plants | C. Japonicus | [22] |
| Whole plants | S. glabra | [7] | ||
| Aerial parts | H. brasiliense | [62] | ||
| 371 | Isoscopoletin | Whole plants | C. Japonicus | [22] |
| Whole plants | S. glabra | [100] | ||
| 372 | Fraxidin-8-O-β-D-glucoside | Roots | C. holostegius | [95] |
| 373 | Skimmin (isofraxidin-7-O-β-D-glucoside) | Roots | C. henryi | [12] |
| Whole plants | S. glabra | [109] | ||
| Whole plants | S. hainanensis | [111] | ||
| 374 | Calucanthoside (Umbelliferone 7-O-β-D-glucoside) | Roots | C. henryi | [12] |
| 375 | Chloracoumarin (6,8-Gimethoxy-7-O-[β-D-apiofunanosyl(1→3)-β-D-glucopyranosyl ]-2H-benzopyran-2-one) | Roots | C. henryi | [12] |
| 376 | Eleutheroside B1 (Isofraxidin 7-O-α-D-glucopyranoside) | Whole plants | S. glabra | [93] |
| 377 | Isofraxidin | Whole plants | S. glabra | [96] |
| 378 | 4, 4′-Biisofraxidin | Whole plants | S. glabra | [97] |
| 379 | Esculetin | Whole plants | S. glabra | [97] |
| 380 | Fraxitin | Whole plants | S. glabra | [97] |
| 381 | Scoparone | Whole plants | S. glabra | [97] |
| 382 | Hemidesmin-1 | Whole plants | S. glabra | [98] |
| 383 | Yinxiancaoside C (Cleomiscosin C-4-O-β-D-glucopyranoside) | Whole plants | C. japonicus | [99] |
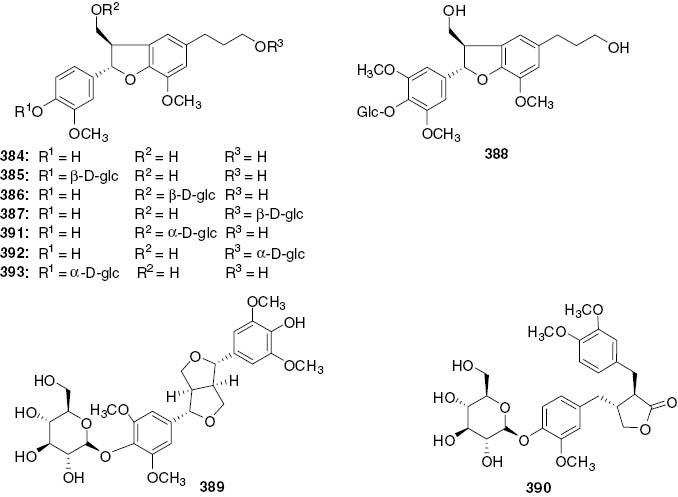
Lignans.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| 384 | (7S, 8R)-Dihydrodehydrodiconiferyl alcohol | Whole plants | C. japonicus | [99] |
| Whole plants | C. multistachys | [115] | ||
| Whole plants | S. glabra | [98] | ||
| 385 | (7S, 8R)-Urolignoside | Whole plants | C. japonicus | [99] |
| Leaves | H. brasiliense | [46] | ||
| 386 | (7S, 8R)-Dihydrodehydrodiconiferyl alcohol-9-O-β-D-glu copyranoside | Whole plants | C. japonicus | [99] |
| 387 | (7S, 8R)-Dihydrodehydrodiconiferyl alcohol-9′-O-β-D-glu copyranoside | Whole plants | C. japonicus | [99] |
| 388 | (7S, 8R)-5-Methoxydihydrodehydrodiconiferyl alcohol-4-O-β-D-glucopyranoside | Whole plants | C. japonicus | [99] |
| Whole plant | S. glabra | [101] | ||
| Leaves | H. brasiliense | [46] | ||
| 389 | Syringaresinol monoside | Whole plants | S. glabra | [100] |
| 390 | Styraxjaponoside B | Whole plants | S. glabra | [100] |
| 391 | (-)-(7S, 8R)-Dihydrodehydrodiconiferyl alcohol 9-O-α-D-glucopyranoside | Whole plant | S. glabra | [101] |
| 392 | (-)-(7S, 8R)-Dihydrodehydrodiconiferyl alcohol 9′-O-α-D-glucopyranoside | Whole plant | S. glabra | [101] |
| 393 | (-)-(7S, 8R)-Dihydrodehydrodiconiferyl alcohol 4-O-α-D-glucopyranoside | Whole plant | S. glabra | [101] |
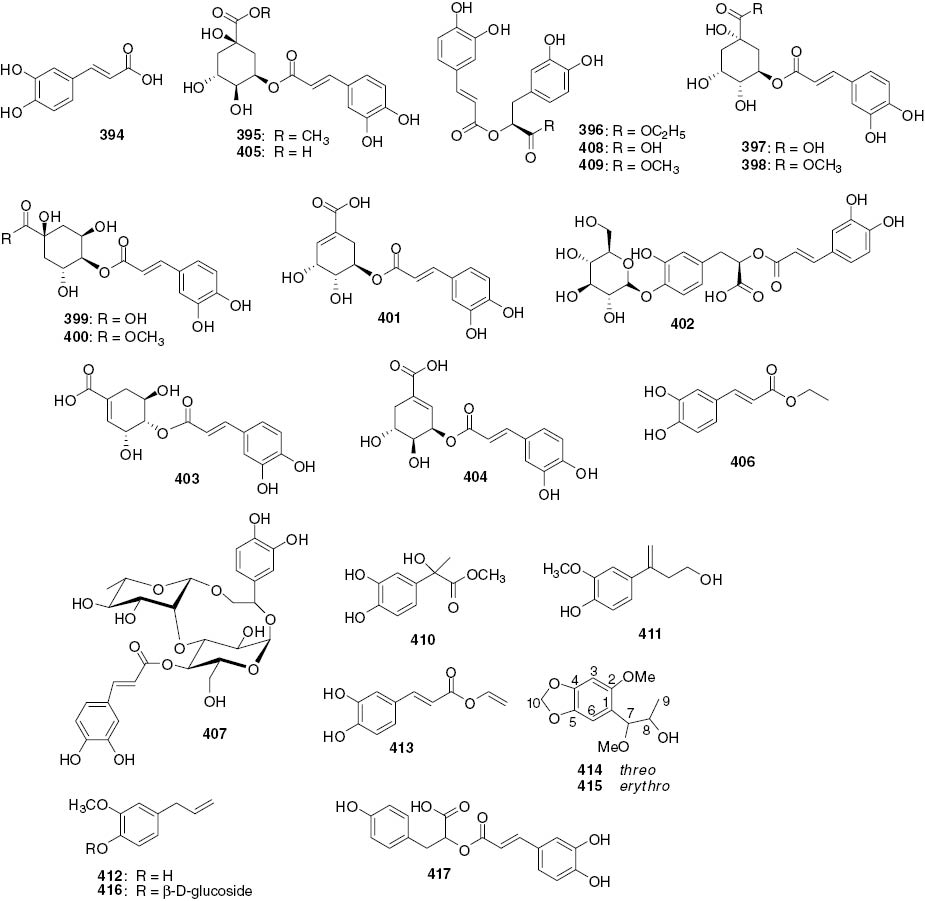
Phenylpropionic acids and other phenylpropanoids.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| 394 | Caffeic acid | Whole plants | S. glabra | [96] |
| 395 | 5-O-Caffeoyl quinic acid Me ester | Whole plants | S. glabra | [102] |
| 396 | Ethyl rosmarinate | Whole plants | S. glabra | [98] |
| 397 | 3-O-Caffeoylquinic acid (chlorogenic acid) | Whole plants | S. glabra | [103] |
| 398 | 3-O-Caffeoylquinic acid Me ester | Whole plants | S. glabra | [103] |
| 399 | 4-O-Caffeoylquinic acid | Whole plants | S. glabra | [103] |
| 400 | 4-O-Caffeoylquinic acid Me ester | Whole plants | S. glabra | [103] |
| 401 | 5-O-Caffeoyl shikimic acid | Whole plants | S. glabra | [100] |
| 402 | Rosmarinic acid-4-O-glucoside | Whole plants | S. glabra | [104] |
| 403 | 4-O-Caffeoylshikimic acid | Whole plants | S. glabra | [105] |
| 404 | 3-O-Caffeoylshikimic acid | Whole plants | S. glabra | [105] |
| 405 | 5-O-Caffeoylquinic acid | Whole plants | S. glabra | [105] |
| 406 | Caffeic acid ethyl ester | Whole plants | S. glabra | [105] |
| 407 | Anhuienoside B | Leaves | C. anhuiensis | [40] |
| 408 | Rosmarinic acid | Whole plants | C. multistachys | [38] |
| Whole plants | S. glabra | [96] | ||
| Leaves | H. brasiliense | [46] | ||
| 409 | Methyl rosmarinate (Rosmarinic acid Me ester) | Whole plants | C. multistachys | [38] |
| Whole plants | S. glabra | [96] | ||
| 410 | Isorinic acid | Leaves | H. brasiliense | [46] |
| 411 | Methyl 3, 4-dihydroxyphenyllactate | Whole plants | S. glabra | [96] |
| 412 | β-Hydroxypropiovanillone | Whole plants | S. glabra | [104] |
| 413 | Caryophyllic acid | Whole plants | S. glabra | [105] |
| 414 | Vinyl caffeate | Whole plants | S. glabra | [105] |
| 415 | Threo-1-(1-methoxy-2-hydroxypropyl)-2-methoxy-4,5-methylenedioxybenzene | Whole plants | C. serratus | [2] |
| 416 | Erythro-1-(1-methoxy-2-hydroxypropyl)-2-methoxy-4,5-methylenedioxybenzene | Whole plants | C. serratus | [2] |
| 417 | Citrusin C | Whole plants | C. multistachys | [38] |
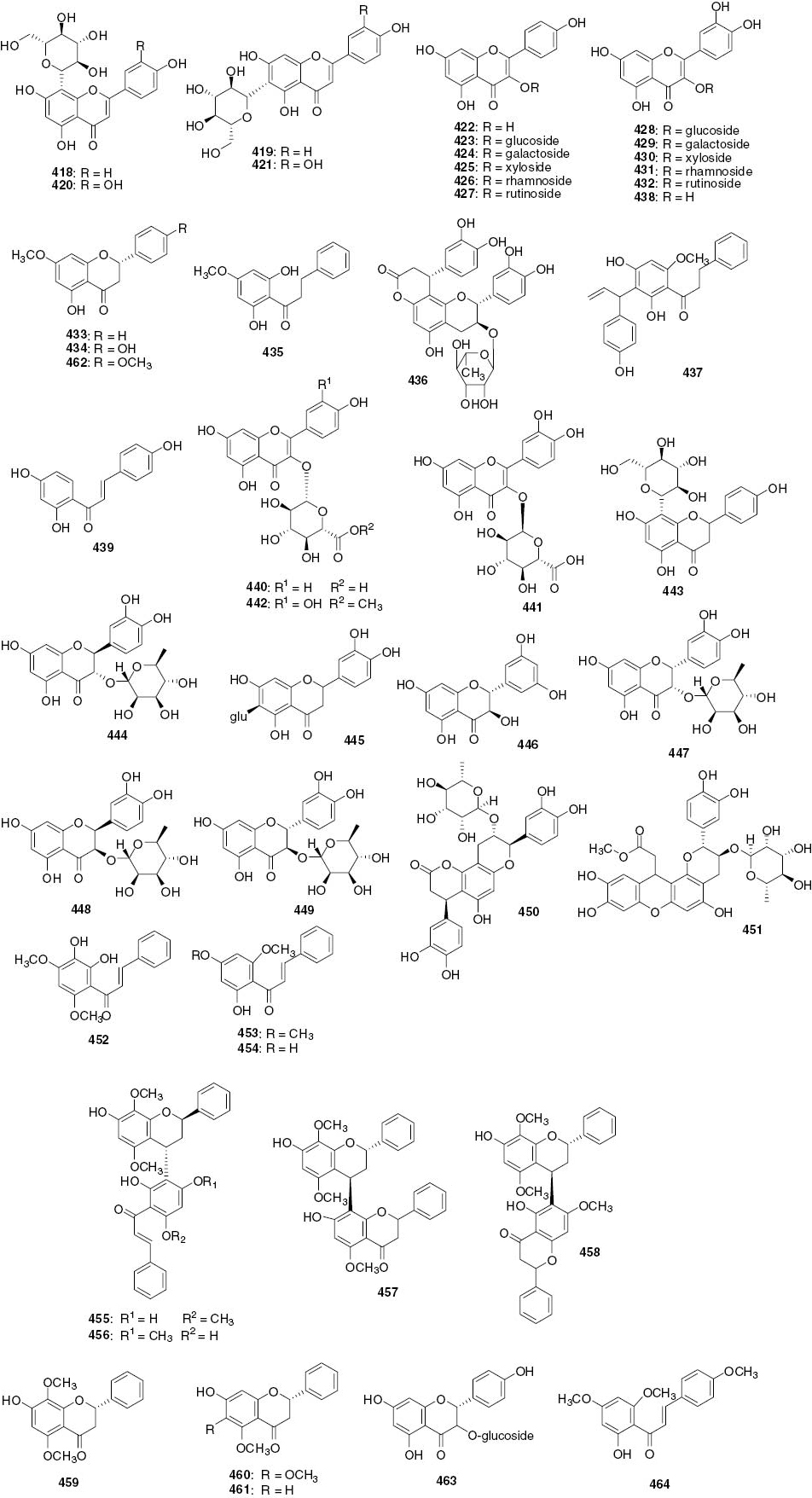
Flavonoids.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| 418 | Vitexin (apigenin 8-C-β-D-glucoside) | Leaves | A. lucida | [106] |
| 419 | Isovitexin (apigenin 6-C-β-D-glucoside) | Leaves | A. lucida | [106] |
| 420 | Orientin | Leaves | A. lucida | [106] |
| 421 | Isoorientin | Leaves | A. lucida | [106] |
| 422 | Kaempferol | Leaves | A. lucida | [106] |
| Whole plants | S. glabra | [109] | ||
| Whole plants | S. hainanensis | [112] | ||
| 423 | Kaempferol 3-O-β-D-glucoside | Leaves | A. lucida | [106] |
| Whole plants | S. hainanensis | [112] | ||
| 424 | Kaempferol 3-O-β-D-galactoside | Leaves | A. lucida | [106] |
| 425 | Kaempferol 3-O-β-D-xyloside | Leaves | A. lucida | [106] |
| 426 | Kaempferol 3-O-a-L-rhamnoside | Leaves | A. lucida | [106] |
| 427 | Kaempferol 3-O-rutinoside | Leaves | A. lucida | [106] |
| 428 | Quercetin 3-O-β-D-glucoside | Leaves | A. lucida | [106] |
| 429 | Quercetin 3-O-β-D-galactoside | Leaves | A. lucida | [106] |
| 430 | Quercetin 3-O-β-D-xyloside | Leaves | A. lucida | [106] |
| 431 | Quercetin 3-O-a-L-rhamnoside | Leaves | A. lucida | [106] |
| Whole plants | S. glabra | [114] | ||
| 432 | Quercetin 3-O-β-D-rutinoside (Rutin) | Leaves | A. lucida | [106] |
| Whole plants | S. glabra | [114] | ||
| 433 | Pinostrobin | Whole plants | S. glabra | [93] |
| Whole plants | S. hainanensis | [111] | ||
| 434 | 7-Methylnaringenin | Whole plants | S. glabra | [93] |
| 435 | 2′,6′-Dihydroxy-4′-methoxydihydrochalcone | Whole plants | S. glabra | [93] |
| 436 | Glabraoside A | Whole plants | S. glabra | [107] |
| 437 | 3′-(7″-Allylphrnyl)-2′,4′,4″-trihydroxy-6′-methoxydihydrochalcone | Whole plants | S. glabra | [107] |
| 438 | Quercetin | Whole plants | S. glabra | [108] |
| 439 | Isoliquiritigenin | Whole plants | S. glabra | [108] |
| 440 | Kaempferol-3-O-β-D-glucuronide | Whole plants | S. glabra | [102] |
| Leaves | H. brasiliense | [46] | ||
| 441 | Quercetin-3-O-α-D-glucuronide | Whole plants | S. glabra | [102] |
| 442 | Quercetin-3-O-β-D-glucuronopyranoside Me ester | Whole plants | S. glabra | [102] |
| 443 | 5, 7, 4′-Trihydroxy-8-C-β-D-glucopyranosyl flavanone | Whole plants | S. glabra | [102] |
| 444 | Neoastilbin | Whole plants | S. glabra | [102] |
| 445 | 5, 7, 3′, 4′-Tetrahydroxy-6-C-β-D-glucopyranosyl flavanone | Whole plants | S. glabra | [109] |
| 446 | (+)-3,3′,5,5′,7-Pentahydroxyflavanone | Whole plants | S. glabra | [98] |
| 447 | Isoastilbin | Whole plants | S. glabra | [100] |
| 448 | Neoisoastilbin | Whole plants | S. glabra | [100] |
| 449 | Astilbin | Whole plants | S. glabra | [100] |
| 450 | Glabraoside C | Whole plants | S. glabra | [33] |
| 451 | Glabraoside D | Whole plants | S. glabra | [33] |
| 452 | 2′,3′-Dihydroxy-4′,6′-dimethoxychalcone | Whole plants | S. hainanensis | [110] |
| 453 | 2′-Hydroxy-4′,6′-dimethoxychalcone | Whole plants | S. hainanensis | [110] |
| 454 | Cardamonin | Whole plants | S. hainanensis | [110] |
| 455 | Sarcandrone A | Whole plants | S. hainanensis | [111] |
| 456 | Sarcandrone B | Whole plants | S. hainanensis | [111] |
| 457 | Sarcandrone C | Whole plants | S. hainanensis | [112] |
| 458 | Sarcandrone D | Whole plants | S. hainanensis | [112] |
| 459 | 7-Hydroxy-5,8-dimethoxyflavanone | Whole plants | S. hainanensis | [111] |
| 460 | 7-Hydroxy-5,6-dimethoxyflavanone | Whole plants | S. hainanensis | [112] |
| 461 | 7-Hydroxy-5-methoxyflavanone | Whole plants | S. hainanensis | [112] |
| 462 | Naringenin-4′,7-dimethyl ether | Whole plants | S. hainanensis | [112] |
| 463 | 3,4′,5,7-Tetrahydroxyflavanone-3-O-glucoside | Whole plants | S. hainanensis | [112] |
| 464 | 2-Hydroxy-4,4′,6′-trimethoxychalcone | Whole plants | C. multistachys | [38] |
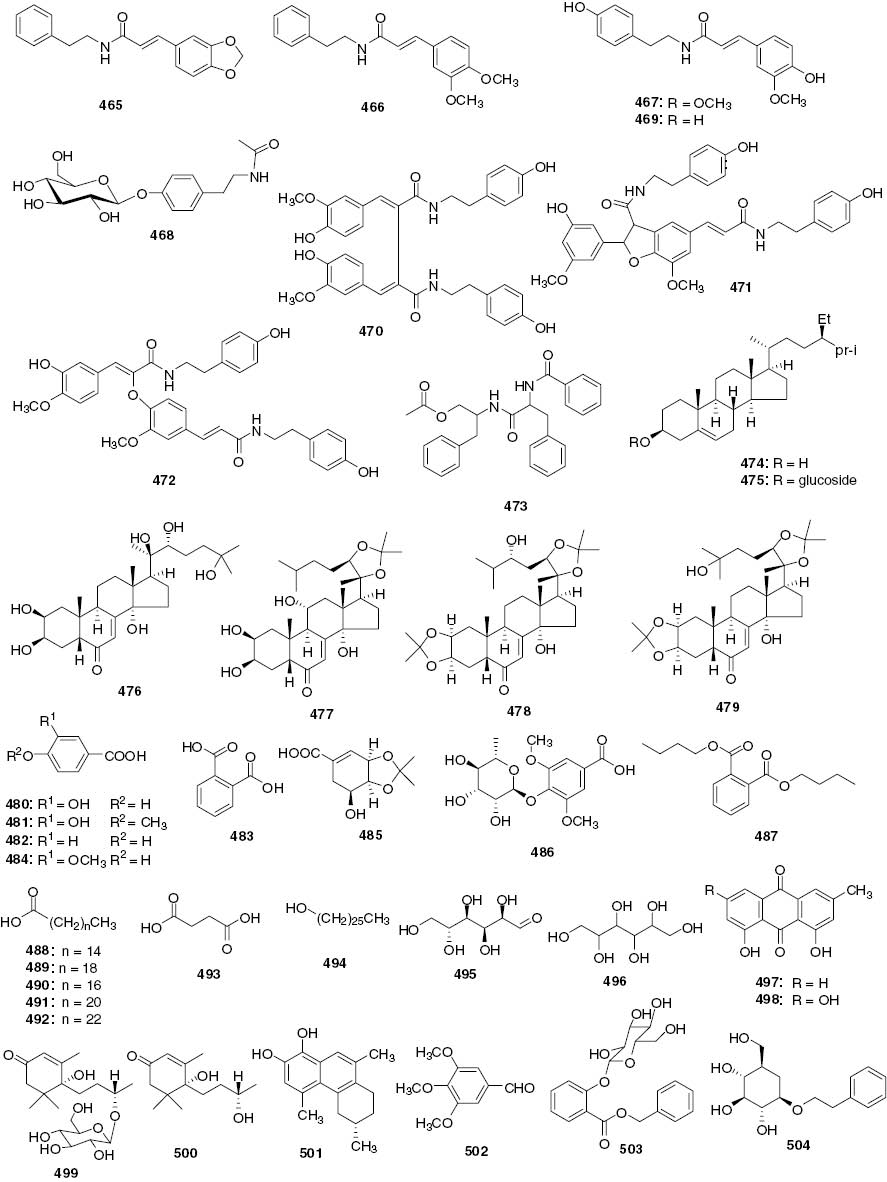
Other compounds.
| No. | Name | Part | Source | References |
|---|---|---|---|---|
| 465 | N-β-phenethyl-3-(3,4-methylenedioxyphenyl) propenamide | Whole plants | C. serratus | [113] |
| 466 | N-β-phenethyl-3-(3,4-dimethoxyphenyl) propenamide | Whole plants | C. serratus | [113] |
| 467 | N-trans-feruloyltyramine | Whole plants | S. glabra | [98] |
| Aerial parts | C. angustifolius | [42] | ||
| 468 | N-acetyltyramine 1-O-β-D-glucoside | Leaves | C. anhuiensis | [40] |
| 469 | N-p-trans-coumaroyltyramine | Aerial parts | C. angustifolius | [42] |
| 470 | Cannabisin G | Aerial parts | C. angustifolius | [42] |
| 471 | Thoreliamide A | Aerial parts | C. angustifolius | [42] |
| 472 | Cannabisin F | Aerial parts | C. angustifolius | [42] |
| 473 | Aurantiamide acetate | Aerial parts | C. angustifolius | [42] |
| 474 | β-Sitosterol | Roots | C. henryi | [12] |
| Whole plants | S. glabra | [93] | ||
| Whole plants | S. hainanensis | [110] | ||
| 475 | Daucosterol | Roots | C. henryi | [12] |
| Whole plants | S. glabra | [108] | ||
| 476 | β-Ecdysterone | Whole plants | C. multistachys | [38] |
| 477 | Ajugasterone C-20,22-acetonide | Whole plants | C. multistachys | [38] |
| 478 | 24-Epi-pterosterone-2,3,20,22-diacetonide | Whole plants | C. multistachys | [38] |
| 479 | 20-Hydroxyecdysterone-2,3:20,22-diacetonide | Whole plants | C. multistachys | [38] |
| 480 | 3,4-Dihydroxybenzonic acid (protocatechuic acid) | Whole plants | S. glabra | [93] |
| 481 | Isovanillic acid | Whole plants | S. glabra | [104] |
| 482 | p-Hydroxybenzoic acid | Whole plants | S. glabra | [114] |
| 483 | o-Phthalic acid | Whole plants | S. glabra | [114] |
| 484 | 3-Methoxy-4-hydroxybenzoic acid | Whole plants | S. glabra | [105] |
| 485 | Dibutyl phthalate | Whole plants | S. glabra | [96] |
| 486 | 3,4-O-Isopropylidene shikimic acid | Whole plants | C. multistachys | [38] |
| 487 | Syringic acid-4-O-α-L-rhamnopyranoside | Whole plants | C. multistachys | [115] |
| 488 | Palmitic acid | Whole plants | S. glabra | [93] |
| Whole plants | S. hainanensis | [110] | ||
| 489 | Icosanoic acid | Whole plants | S. hainanensis | [110] |
| 490 | Octadecanoic acid | Whole plants | S. hainanensis | [110] |
| 491 | Docosanoic acid | Whole plants | S. glabra | [114] |
| 492 | Tetracosanoic acid | Whole plants | S. glabra | [114] |
| 493 | Succinic acid | Whole plants | S. glabra | [114] |
| 494 | Hexacosanol | Whole plants | S. glabra | [108] |
| 495 | Glucose | Whole plants | S. glabra | [108] |
| 496 | Hexitol | Whole plants | S. glabra | [108] |
| 497 | Chrysophanol | Whole plants | S. hainanensis | [110] |
| 498 | Emodin | Whole plants | S. hainanensis | [110] |
| 499 | Dihydrovomifoliol-O-β-D-glucopyranoside | Whole plants | S. glabra | [30] |
| 500 | Dihydrovomifoliol | Whole plants | S. glabra | [34] |
| 501 | Henryin A ((S)-4,6,9-Trimethyl-5,6,7,8-tetrahydrophenan-threne-1,2-diol) | Leaves | C. henryi | [117] |
| 502 | 3,4,5-Trimethoxybenzaldehyde | Leaves | C. anhuiensis | [40] |
| 503 | Benzyl 2-β-glucopyranosyloxybenzoate | Whole plants | S. glabra | [101] |
| 504 | Phenethyl-8-O-β-D-glucopyranoside | Whole plants | C. multistachys | [38] |
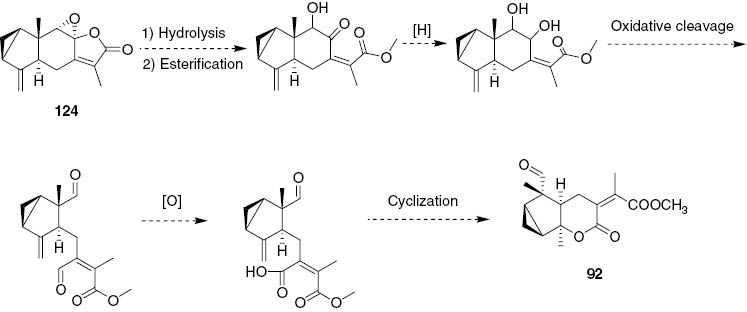
Plausible transformation of 124 into 92.
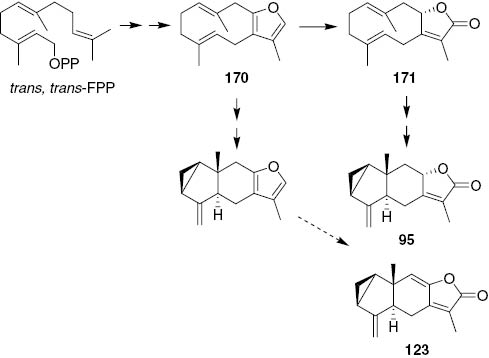
Possible biogenetic pathway of shizukanolides.
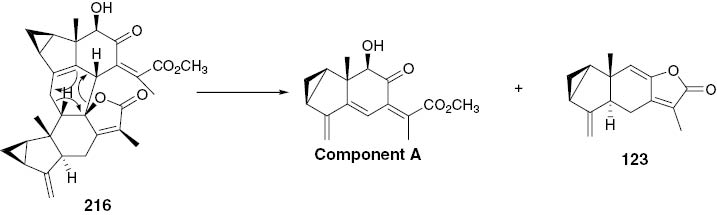
Pyrolysis of compound 216.
Sesquiterpenoids
Plants of Chloranthaceae are rich in sesquiterpenes of eudesmane, lindenane, guaiane, germacrane, cadinane, and aromadendrane-type compounds.
Eudesmanes
Eudesmanes are the main constituents in the genus of Chloranthus, mainly found in the species C. serratus (1–17), C. spicatus (18–30), C. Henryi (31–42), C. elatior (43–55), C. japonicus (56–66), C. multistachy (75–81), C. anhuiensis (82–86), C. angustifolius (87–89), C. glaber (90), C. fortunei (91), and C. erectus (92). In the genus of Sarcandra, eight eudesmanes (67–74) have been isolated from S. glabra. As for the genus of Hedyosmum, only two eudesmanes (93 and 94) have been found, one in H. orientale and second in H. brasiliense. Totally 94 eudesmanes have been reported to date. In 1985, the first three eudesmanes 1–3 were isolated from the roots of C. serratus [1]. Compounds 4–7 [2], 8–15 [3] and 16–17 [4] were isolated from the whole plants of C. serratus. Compound 4 possesses a nitro group at C-1 which is an uncommon substituent of eudesmane-type sesquiterpenes. Compound 8, named serralactone A, was first isolated from the whole plants of C. serratus in 2009. But in 2010 it was isolated again from the whole plants of S. glabra, mistakenly reported as a new sesquiterpene and named sarcandralactone B [5]. Thus, serralactone A and sarcandralactone B show virtually identical HR-MS, 1H-NMR and 13C-NMR spectra.
Chloranthus spicatus plants grown in Vietnam mainly produce flowers for scenting tea. Phytochemical investigation of this plant have led to compounds 2 and 18, which have been isolated for the first time as minor constituents of the essential oil from the flowers of C. spicatus [6]. Compounds 19–23 have been isolated from a polar extract of the aerial parts of C. spicatus [7]. Among them, compounds 19 and 20 differ in absolute stereochemistry at C-4, and compound 23 is a cycloeudesmane-type sesquiterpene. Compounds 24–27 have been isolated from the roots of C. spicatus [8]. Compound 25 has an eudesmane-type backbone with the same substitution pattern as compound 24, but differs in the stereochemistry of the H-C fragment (R = H), as shown in Figure 1. Compound 26 was first isolated from the roots of C. spicatus in 2010 and, mistakenly, also reported as a new compound from the whole plants of C. multistachys in 2012 [9] and named multislactone A. Compound 27 is an O-methylated derivative of 26. In 2012, Yang and co-workers re-examined the data of C. spicatus in order to explore the chemical differences between aerial parts and whole plants. As a result, two new compounds 28, 29, and one known compound 30 were isolated [10]. Compound 29 was reported as a new sesquiterpenoid both in the whole plants of C. spicatus and C. elaior in the same year [11].
The species C. henryi has long been used as a folk medicine for dispelling pathogenic wind, removing dampness, and for promoting blood circulation. In 2005, compounds 31–32 were isolated from the roots of C. henryi for the first time [12] and compounds 33 [13] and 34 [14] were isolated from leaves and stems of C. henryi. The structure of 33 was elucidated by spectroscopic methods. Compound 34 has an uncertain configuration at C-11. Compounds 5, 35 and 36 have been isolated from leaves and stems of C. henryi [15]. Compounds 37–39 have been isolated from roots of C. henryi [16]. Compounds 40–42 have been isolated from the whole plant of C. henryi [17]. Compound 41 shows a significant anti-neuroinflammatory effect by inhibiting nitric-oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine BV-2 microglial cells with relatively low cytotoxicity.
The species C. elatior is a perennial plant that grows in the Southwest of China. In 2012, two compounds 43 and 44 were isolated from the whole plants of C. elatior [11]. Six 2-oxoeudesm-7(11)-en-12,8-olide derivatives, named chlorantholides A–F (45–50), were isolated from the ethanol extract of C. Elatior [18]. These six new compounds all have an α,β-unsaturated carbonyl group at C-2 position, and their 13C NMR chemical shifts are in the range of δC 196.8–198.6, which are quite different from the chemical shift of C-12 at about δC 170.6–174.2. The structure of 83 (chlorantholide D) was also revised in this report. In 2013, compound 51 with a methoxy group rather than a tertiary hydroxy group at C-4 was isolated from the ethanol extract of the aerial parts of C. elatior [19]. In 2014, four novel naturally occurring diastereoisomers of dinor-eudesmenes with a degraded five-membered ring B, compounds 52–55, were isolated from the aerial parts of C. elatior. Their biosynthesis apparently involves a series of oxidation, degradation, and rearrangement reactions [20].
To date, 14 eudesmane-type sesquiterpenes have been isolated from C. japonicus. The first one, compound 56, was isolated from the whole plants of C. japonicus in 1980 [21]. Two additional compounds 57 and 58 were isolated in 1984 [22], [23]. Chemical investigation of the roots of C. japonicus have resulted in the isolation and characterization of four new eudesmane-type sesquiterpenes 59–62 including two new sesquiterpene cinnamates [24]. The structure elucidation of 59–62 has been conducted by using spectroscopic methods including comparison of the 1H- and 13C-NMR data of 59 with those of other eudesmane-type sesquiterpenes. The 13C-atom signal at δ 169.3 in the 13C-NMR spectrum of 59 has been assigned to the carbonyl of the cinnamoyl group. The (E)-configuration of the cinnamoyl group is fully consistent with the coupling constant of 16.2 Hz observed in the doublets for the two olefinic H-atoms at δ 8.09 and δ 6.95. Compounds 63 and 64 have been isolated from the aerial parts of C. japonicus [25]. Compound 64, a novel sesquiterpene furan compound, shows antifungal activity [26]. A new sesquiterpene lactone 65 has been isolated from an ethyl acetate-soluble partition of the ethanol extract of the whole plants of C. japonicus [27]. Compound 66 is a new eudesmane-type sesquiterpenoid lactone isolated from the whole plant of C. japonicus [28].
The species Sarcandra glabra, belonging to the genus Sarcandra of Chloranthaceae, grows mainly in the southern part of China and Japan. The whole plant has been used as an antibacterial and antitumor agent in China. The first three new eudesmanolide glycosides, 67, 68 [29] and 69 [30], have been isolated from the whole plant of S. glabra; their sugar moiety has been determined as D-glucose. A new sesquiterpene lactone 70 has been isolated from the whole plant of S. glabra [31]. Phytochemical study of the ethanol extract of S. glabra has resulted in the isolation of a new sesquiterpene 71 [32]. In 2012, as a continuation of the chemical investigation of S. glabra in the search for hepatoprotective substances, a new sesquiterpene lactone 72 was isolated [33]. Compound 73 has been isolated from the 70% aqueous acetone extract of the whole plant of S. glabra and, by far, it is the only eudesmane with a hydroxymethyl substitutent at C-4 found in plants of Chloranthaceae. In the MTT assay, compound 73 has shown little cytotoxic activity against Hela, HCT-8 and MCF-7 cancer cell lines with IC50 > 50 µg mL-1 [34]. A new sesquiterpenoid monomer 74 has been isolated from the whole plants of S. glabra [35].
The plant Chloranthus multistachys is a perennial herb distributed in wet areas of eastern Asia. Compounds 75, 76 [36] and 77 [9] have been isolated from the whole plant of C. multistachys. The structure of 76 given in the literature [36] is wrong, and the actual structure must be 76a (9α-hydroxyasterolide) [37] (Figure 1). In 2013, compounds 78–81 were isolated from the whole plant of C. multistachys [38]. Compound 79 is the only eudesmane sesquiterpenoid with an epoxide ring located between C-4 and C-15 in compounds of the Chloranthaceae family. Chloranthus anhuiensis is a species endemic to Anhui Province of China, and its chemical constituents were not investigated until 2010 when four compounds 82–85 were isolated from the roots of this plant (compound 83 was revised as 48 in 2012 [18]). Antifungal screening of the compounds conducted with the NCCLS M27-A method have showed that the compounds exhibit weak antifungal activities [39]. Compound 86 is produced as phytoalexin in the fresh leaves of C. anhuiensis in response to abiotic stress elicitation by CuCl2, and it is the fourth eudesmane glycoside isolated from plants of Chloranthaceae [40].
Plant Chloranthus angustifolius is an endemic species found in Sichuan and Hubei provinces of China. There are few reports about its chemical constituents. In 2014, two new eudesmane-type sesquiterpenes, compounds 87 and 88, were isolated from the roots of C. angustifolius [41]. The following year, compound 89 was isolated from the aerial parts of this plant [42]. Compound 89 can be used to differentiate C. angustifolius from other species of Chloranthus because of the fact that it has not been isolated from other species of this genus so far. The eudesmanolide 90 has been isolated from leaves of C. glaber [43]. Compound 91 has been isolated from the roots of C. fortune [44] and, for the first time, from the genus of Chloranthus. From the leaves of C. erectus, a new secoeudesmanolide 92 has been isolated. It has been suggested that compound 92 is formed by a series of consecutive transformations of chloranthalactone B (124) as shown in Scheme 1 [45].
Plants of the Hedyosmum genus (Chloranthaceae) are mainly distributed in the tropical area of America, and only one species, H. orientale, grows in China. Compound 93 has been isolated from the aerial parts of H. orientale. It shows moderate activities against A-549 and HL-60 tumor cell lines with the IC50 values of 3.1 and 8.8 µm, respectively [37]. The plant H. brasiliense is an aromatic and dioecious neotropical shrub endemic to Brazil; compound 94 has been isolated from its leaves for the first time [46].
Lindenanes
The distribution of lindenane sesquiterpenes in natural sources is limited, but Chloranthaceous plants have been found to be rich in unusual sesquiterpene lactones having a lindenane skeleton. These derivatives are named shizukanolides and chloranthalactones. In total, 41 compounds have been reported to date. They are mainly found in species C. japonicus (95–114), S. glabra (115–122), C. glaber (123–127), C. fortunei (128–130), C. serratus (131–132), H. brasiliense (133) and H. angustifolium (134 and 135). Because of its special 3/5/6 linear cyclic system, lindenanolides can be regarded as characteristic constituents of some Chloranthaceae plants and might be used as chemotaxonomical markers [47].
Lindenanolide 95 was isolated for the first time in 1979 from plants of Chloranthaceae from aerial parts of C. japonicus [48]. Compounds 96–98 have been found in the whole plants of C. japonicus. Compound 98 contain two hydroxy groups in addition to a lactone ring [21]. The cytotoxicities of these three lactones against mouse lymphosarcoma L-5178Y cells have been evaluated in comparison with that of helenalin. These lactones are moderately cytotoxic. In 1981, a 15-hydroxylindenanolide 99 was isolated from roots of C. japonicus. The compound does not show antifungal activity against Mucor griseocyanus AHU 6044 compared with that of chloranthalactone A, which is known to be highly active [49]. A highly oxygenated lindenanolide 100 has also been isolated from roots of C. japonicus. The compound has a relatively unique γ,δ-epoxy-α,β-unsaturated-γ-lactone moiety, which has been rarely found in natural products [50]. Compound 101, the structure of which has been revised to the eight-epimer in the literature [25], has been isolated from the aerial parts of C. japonicus. It shows mild inhibitory effects on collagen, U45519, AA and epinephrine induced platelet aggregation [51]. The third lindenane sesquiterpene glucoside 102, found in Chloranthaceae plants, has been isolated from the whole plant of C. japonicus [52]. This compound has also been reported as a new constituent of S. glabra [30]. Six new lindenanolides 103–108 have been isolated from an ethyl acetate-soluble partition of the ethanol extract of the whole plants of C. japonicus [27]. In 2012, three new compounds 109–111 were isolated from the aerial part of C. japonicus [25]. Compound 110, named chlorajapolide G, was also reported as a new lindenane-type sesquiterpenoid lactone, named chlojaponilactone E, identified in the same plant in 2013. Four lindenanoids 110 and 112–114 have been isolated from AcOEt-soluble part of the EtOH extract of whole plants of C. japonicus [53]. Compounds 112 and 113 are the only two lindenanoids with an OAc group at the C-6 position.
The lindenanoid glucosides 115 and 117 have been isolated from the whole plant of S. glabra [29], [30]. Compound 115 shows pronounced hepatoprotective activity against D-galactosamine-induced toxicity in WB-F344 rat hepatic epithelial stem-like cells. Compound 116 has also been isolated from the whole plant of S. glabra [31]. Biologically inactive sesquiterpenes 118 [5], 119 [32], 120 [54] and 121, 122 [35] have also been isolated from the whole plants of S. glabra.
In 1978, the first two lindenanes 123 and 124 were isolated from roots of C. glaber [55]. From the leaves of C. glaber, a new lindenane 125 was isolated [43]. Then, in 1994, the first two lindenane C-15 glycosides 126 and 127 were isolated [56].
In 2009, two new sesquiterpenes, 128 and 129, were isolated from the aerial part of C. fortune [57]. A novel lindenane sesquiterpene with an unprecedented 18-membered triester ring 130 was isolated from the whole plant of C. fortune [58].
Only two highly oxygenated lindenanilides 131 and 132 have been isolated from the roots of C. serratus [50]. Compound 132 is the first entry to 13-hydroxylated lindenanes in the Chloranthaceae. By now, three lindenanilides have been found in the genus of Hedyosmum. Compound 133 has been isolated from the extract of H. brasiliense [59]. The anti-leishmanial compounds 134 and 135 have been isolated from the ethyl acetate extracts of the bark of H. angustifolium [60].
Guaianes
In the Chloranthaceae family, the 8,12-guaianolides are mainly secondary metabolites. The biological activities of this type of guaianes can be correlated with the presence of an α,β methylene function conjugated to a γ-lactone. They are mainly found in the genera Chloranthus and Hedyosmum as constituents of H. orientale (136–142), C. multistachys (143–147), H. brasiliense (148–150), H. arborescens (151), C. serratus (152 and 153), C. henryi (154 and 155), C. anhuiensis (156), C. spicatus (157), and C. elatior (158). This class of compounds maybe used as chemotaxonomical markers of the above species.
Seven guaiane-type sesquiterpenoids 136–141 [37] and 142 [61] have been isolated from the aerial parts of H. orientale. Compounds 140 and 142 are the only two guaiane 10-glucosides found in Chloranthaceae plants.
There are five guaianes, 143 [36] and 144–147 [38], isolated from the whole plant of C. multistachys. Compound 145 was reported for the first time as a constituent in the whole plant of C. spicatus in 2012 [10].
From leaves of H. brasiliense, the only representative of the Chloranthaceae in Brazil, three compounds 148–150 have been isolated. These compounds do not show anti-mycobacterial activity against isoniazid-sensitive M. tuberculosis at concentrations of 1–30 μm [62].
The first 7,10-epoxy-guaianolide constituent 151 of Chloranthaceae was isolated from the leaves of H. arborescens in 2005 [63]. Together with compounds 136–138, there are four 7,10-epoxy-guaianolides identified in Chloranthaceae plants to date. The absolute configuration of this class of guaianolides, with unusual 7,10-epoxy group, has been unambiguously established by analysis of the calculated and experimental VCD spectra [64].
Compound 152 [2], a guaiane with a unique C-4 and C-10 linkage, and its analogue 153 [3], have been isolated from the whole plants of C. serratus. Isolation of three secoguaienes, 154 and 155 from the whole plant of C. henryi [17] and 156 from the roots of C. anhuiensis [39], has been reported. In 2012, two new 12,8-guaianolide-type compounds 145 and 157 were isolated from the whole plant of C. spicatus [10]. Compound 158 has been isolated from the EtOH extract of the aerial parts of C. elatior [19].
Germacranes
A total of 16 germacrane-sesquiterpene compounds (159–174) have been isolated from Chloranthaceae plants. They have been found in the species of C. serratus (159–162), C. henryi (163–167), C. spicatus (168 and 169), C. japonicus (170 and 171), C. fortunei (172), C. multistachys (173), and S. glabra (174). The characteristic feature of this class of compounds is a macro-ten-membered ring system that is sometimes fused to a furan ring.
Compounds 159–161 have been isolated from roots of C. serratus [1]. Compound 161, present in the amount of about 0.1% in fresh roots of this herb, has been speculated to be partially responsible for the insecticidal activity of this plant. Compound 162 has been isolated from the whole plants of C. serratus [2]. Compounds 163–165 have been isolated from leaves and stems of C. henryi [15] and 166 and 167 from the whole plants of C. henryi [17]. Compounds 168 and 169 have been isolated from the whole plants of C. spicatus [10]. Compounds 170 and 171, isolated from roots of C. aponicas [65], were the first two germacrane-sesquiterpene reported from Chloranthaceae plants in 1981. Their possible biogenetic pathway is shown in Scheme 2. Compound 172 has been isolated from roots of C. fortune [44] and 173 from the whole plants of C. multistachys [38]. In 2006, in the search for hepatoprotective compounds from S. glabra, a new germacrane glycoside 174 was isolated [29].
Cadinanes
Ten cadinanes (175–184) have been reported to date. These are compounds 175–178 from C. henryi [14], [17], compounds 179–181 [4], [66] from C. serratus and compounds 182–184 from the whole plant of C. multistachys [36].
By using the MTT colorimetric method, compound 177 shows antitumor activity against the Hela, A549, MCF, and K562 human-tumor cell lines, with IC50 values of 4.7, 8.9, 9.6, and 11.8 μg/mL, respectively. Compound 181 [4] is a Chinese folk medicine for the treatment of bruises, bone fractures, and rheumatoid arthritis.
Eremophilanes
In 1988, a new sesquiterpene lactone 185, the enantiomer of istanbulin A, was obtained from S. glabra leaves [67]. Some six-eremophilene derivatives 186–190 [40] and 191 [39] are produced as phytoalexin in the fresh leaves of C. anhuiensis, in response to abiotic stress elicitation by CuCl2. These are the first reports of eremophilane-type sesquiterpenes in C. anhuiensis. From the whole plants of C. japonicus, three eremophilanes 192–194 have been repored. Compound 192 has been isolated from this species for the first time [28]. Reinvestigation of the AcOEt-soluble part of the EtOH extract of whole plants of C. japonicus have afforded compounds 193 and 194 [53].
Aromadendranes
The first aromadendrane-type sesquiterpene isolated from Chloranthaceae plants was compound 195 which was found in the leaves of C. glaber in 1993 [43]. Compounds 196 and 197 were isolated from a polar extracts of the aerial parts of C. spicatus [7]. Compound 196 is the C-4, C-10 epimer of 195. From H. orientale, the only one species of Hedyosmum genus growing in China, a non-cytotoxic compound 198 has been isolated [37]. It is the C-4 epimer of compound 195 and C-10 epimer of 196. Investigation to the ethanol extract of the aerial parts of C. elatior has resulted in finding two aromadendranes 199 and 200. Compound 199 is the C-10 epimer of 200 [19].
Elemanes
Five elemane-type sesquiterpenes, 201–205, have been reported as constituents of Chloranthaceae plants. Compound 201 has been isolated for the first from the essential oil of the flowers of C. spicatus [6]. Two new elemanolide glycosides 202 and 203 have been isolated from the whole plant of S. glabra [29]. Hepatoprotective activity against D-galactosamine-induced toxicity of these two new compounds have been examined in WB-F344 cells. This has been the first report of hepatoprotective activity from a Sarcandra species. In 2008, compound 204 was isolated from the whole plants of C. serratus as a new sesquiterpenoid [2]. It shows moderate activity against Helicobacter pylori-SS1 in 13 microorganisms with MICs of 25–50 µg/mL. In the course of study of the hot tea infusion from the fresh leaves of H. brasiliense, a new secondary metabolite 205 has been identified [46].
Other sesquiterpenes
At present, only compound 206 with acorane skeleton has been found in Chloranthaceae plants. It has been isolated from the whole plants of C. japonicus [22]. Compound 207, isolated from aerial parts of C. spicatus, has a special skeleton with a trans 5/6 connectivity [7]. A new sesquiterpene with a novel bicarbocyclic framework 208 has been isolated from C. henryi. This compound exhibits anti-tumor activitiy against Hela and K562 human tumor cell lines [23]. Two sesquiterpene glucosides 209 and 210 have been isolated from the whole plant of C. japonicus [52]. Compound 210 is a rare bidesmosidic megastigmane sesquiterpene glucoside. The cytotoxic activities of compounds 209 and 210 have been tested by a MTT method but the results have showed marginal cytotoxic activities against Hepg-2, OV420, and MCF-7 cell lines. Compounds 211 from C. henryi [16] and 212 from C. anhuiensis [39] have been reported. In 2012, a new sesquiterpenoid 213, along with a known sesquiterpenoid 214, were isolated from the whole plant of C. spicatus [10]. Compound 215 is a new naturally occurring maaliane-type sesquiterpenoid isolated from the ethanol extract of the aerial parts of C. elatior [19].
Sesquiterpene polymers
Sesquiterpene polymers are characteristic constituents of the chloranthaceous plants. More than 80 sesquiterpene dimers have been isolated from this family. Excepting 258 and 259, most of them comprise the same scaffold constructed from two lindenane moieties via a [4+2] cycloaddition reaction.
The dimeric sesquiterpenes 216–299 have been isolated from genera Chloranthus and Sarcandra. The distribution of these compounds in plants is as follows, C. japonicus (216–230), C. spicatus (231–243), C. multistachys (244–253), C. serratus (254–261), C. fortunei (262–269), C. holostegius (270–274), C. tianmushanensis (275 and 276), S. glabra (277–297), and S. hainanensis (298 and 299).
Kawabata and co-workers have searched for sesquiterpenes in plants of the Chloranthaceae and they have found a series of unusual sesquiterpene lactones having a lindenane skeleton (216–223) in the roots of C. japonicus. In 1990, the first dimeric sesquiterpene 216 consisting of two lindenane units was isolated. These two lindenane units can be obtained by pyrolysis of 216 (Scheme 3) [68]. The presence of 216 in the fresh extracts suggests that this kind of dimer is natural. Its molecular formula has been deduced as C31H34O6 by EI-HR-MS. The spectrum shows a molecular ion peak at m/z 502 and two fragment ion peaks at m/z 274 and m/z 228 for the fragments discussed above. This result suggests that 216 is easily dissociated into its two components shown in Scheme 3.
In 1995, five novel lindenane dimers 217–221 were isolated from roots of C. japonicus [69]. In 1997, further investigation of the chemical constituents of C. japonicus yielded a novel lindenane trimer 222 along with a structurally related dimer 223. This is the first and the only trimeric lindenane sesquiterpene isolated from chloranthaceous plants [70]. These two compounds correspond to a basic lindenatriene which is a component of all shizukaols. In 2008 a new sesquiterpene dimer 224 with a macrocyclic tris-lactone unit attached at C-13′ and C-15′ was reported. Related compounds 225–230 were isolated in 2011 from the whole plants of C. japonicus. The MTT assay has been used to determine the cytotoxicity of 225 against a panel of eight cancer cell lines. However, this compound does not show any marked activity [27]. Experiments to find compounds with anti-HIV-1 activities from C. japonicus have led to the isolation of five new lindenane dimers 226–230 [71]. Compounds 226 and 227 represent the first examples of lindenane dimers with a C-5 hydroxy group and a C-4–C-15 double bond. Compound 230 is a rare lindenane disesquiterpenoid containing a hydroperoxy group at C-4.
Totally 13 lindenane dimers 231–243 were isolated from the whole plants and roots of C. spicatus. In 2007, an investigation on the whole plant of C. spicatus revealed three new dimeric sesquiterpenoids 231–233 [72]. Among them, 232 (named chloramultilide C) and 233(named chloramultilide D), were also reported as new compounds, named henriol A and B, that were isolated from the roots of C. henryi in 2008 [73]. They all contain a 4-hydroxy substitution. The same year the Kim group examined the methanol extracts from the roots of C. spicatus, and isolated two new lindenane sesquiterpene dimers 234 and 235 which can be considered the products of a Diels-Alder cycloaddition involving a macrocyclic trilactone consisting of a 4-hydroxy-2-methyl-but-2-enoyl group and a succinyl group by ester linkages [74]. Compounds 236–239, new lindenane-sesquiterpene dimers possessing a hydroperoxy group, have been isolated from the roots of C. spicatus. These compounds can be considered to be biogenetic precursors for the corresponding hydroxy derivatives of dimeric lindenane sesquiterpenes distributed in Chloranthus plants [75]. Further study of the roots of C. spicatus have resulted in the isolation of four new lindenane sesquiterpenoid dimers 240–243, the absolute configurations of which have been determined by CD spectroscopic analysis [76].
Compounds 244–253 have been isolated from whole plant of C. multistachys. In 2006, a highly complex sesquiterpenoid dimer 244 was found [77]. In 2010, two novel sesquiterpenoid dimers 245 and 246 were isolated from the whole plant of C. multistachys [78]. Six complex lindenane-type sesquiterpenoid dimers 247–252 have been isolated from the whole plant of C. multistachys [79]. Compounds 247 and 252 contain a unique 18-membered macrocyclic triester system. In 2013, an 8,9-seco-lindenane disesquiterpenoid 253 was isolated from whole plant tissues of C. multistachys [38]. This compound seems to be derived from the enzymatic endo-Diels-Alder cyclo-addition of two lindenanes and an enzymatic Baeyer-Villiger oxidation.
Chemical constituents investigation of roots and the whole plants of C. serratus, has revealed the presence of 254–261. In 1992, three novel dimeric sesquiterpenes 254–256 named shizukaol B, C and D, were isolated from the roots of C. serratus [80]. These compounds consist of two lindenane (modified eudesmane) units. Among them, compound 254 (named shizukaol B) was also reported as a new compound, named henriol C, isolated from the roots of C. henryi in 2008 [73]. In 1993, a novel dimeric sesquiterpene 257, having a C2-symmetric structure and the unique 12-membered ring system, was isolated from the roots of C. serratus [81]. In 2012, two dimeric sesquiterpenes, named serratustones A (258) and B (259) were isolated from the whole plants of C. serratus [82]. Serratustones A and B represent a new carbon skeleton that is formed by dimerization of an elemane and an eudesmane, and they are the first examples of nonlindenane-type sesquiterpenoid dimers from the Chloranthaceae family [82]. Compounds 260 and 261 have been isolated from the whole plant of C. serratus [4].
Compounds 262–269 have been isolated from roots and aerial part of C. fortunei. The respective dimers 262–266 are named shizukaols K-O. A previously known sesquiterpene dimer 267 has also been isolated from the roots of C. fortune [83]. In the original literature, the proposed structure of compound 265 was incorrect. The revised structure of compound 265 is given in this review. In 2009, a sesquiterpene dimer 268 and a sesquiterpene glycoside 269 were isolated from the aerial part of C. fortune [57]. In all lindenane-type sesquiterpenoid dimers reported from Chloranthaceae family to date, only two compounds have a hydroxy group at C-15, one is compound 268 and the other is 247. Compound 269 is the 9-O-β-glucoside of 257. The initially proposed structure of 269 has been revised in this review.
Two highly complex sesquiterpenoid dimers 270 and 271 have been isolated from C. holostegius and the biogenetic pathway to 270 has been proposed in the literature [84]. Further analysis of C. holostegius has revealed the presence of four additional sesquiterpenoid dimers 267 and 272–274. A suggested biogenetic pathway to 272 and 273 can be found in the original literature [85].
Investigation of the leaves of C. tianmushanensis have resulted in the isolation and characterization of two new sesquiterpene dimers 275 and 276 with a rare 18-membered triester ring system. Their proposed biogenetic pathway is described in the original literature [86].
Twenty three novel lindenane-type sesquiterpenoid dimers have been found in the genus of Sarcandra. Three species in the Sarcandra genus of the Chloranthaceae family, which are mainly distributed in the southeast of Asia, and two of them, Sarcandra glabra and S. hainanensis, have been found to contain lindenane-type sesquiterpenoid dimers. In 2010, five new dimeric sesquiterpenoids, sarcandrolides A-E (277–281), were isolated from the whole plants of S. glabra [5]. In 2013, five new sesquiterpenoid dimers, sarcandrolides F-J (282–286), were isolated from the whole plants of S. glabra [35]. Compound 282 represents the first example of a lindenane-type sesquiterpenoid dimer bearing a hydroperoxy group at C-5. In 2015, 11 new sesquiterpene dimers, (287–297), were isolated from the seeds of S. glabra [87]. Compound 287 possesses a 17-membered macrocyclic ester system, which is different from the 18-membered rings present in other reported analogues. Two novel lindenane-type sesquiterpenoid dimers 298 and 299 have been isolated from the whole plants of S. hainanensis [88]. These two compounds feature a new nonacyclic scaffold in which the bond of C-11–C-7′ imposes the five-membered lactone ring in a full β-direction [88].
Diterpenoids, triterpenoids and other terpenoids
Labdanes
Thirty six labdane-type diperpenoids have been isolated from Chloranthaceae family. These compounds are distributed in the genus of Chloranthus as follows, C. henryi (300–315), C. anhuiensis (316–321), C. spicatus (322–327), C. serratus (328–334) and C. elatior (335). In 2006, the first two labdane-type diterpenes 300 and 301 were isolated from the roots of C. henryi. Compound 301 is a bis(nor-landane) that exhibits antitumor activity against Hela and K562 human tumor cell lines [13]. Its analogues 302 and 303 isolated from C. henryi are biologically inactive [14], [23]. Further investigation of C. henryi by bioactivity-guided fractionation has led to isolation of three new diterpenoids 304–306 and three known labdane diterpenes 307–309 [73]. Among them, compounds 304 and 307 show hepatoprotective activity against D-galactosamine-induced toxicity in WB-F344 rat hepatic epithelial stem-like cells. In search for new anti-rheumatoid agents, four new diterpenoids 310–313 and two known diterpenoids 314–315 have been isolated from the roots of C. henryi [16].
In 2010, the first phytochemical investigation of the roots of C. anhuiensis led to the isolation two new labdane-type diterpenes 316 and 317 and four known compounds 318–321 [39]. In 2010, compounds 322–326 were isolated from the roots of C. spicatus [8] and compound 327 was isolated from the whole plants of C. spicatus [10].
There are seven labdane-type diterpenes isolated from the whole plant of C. serratus. In 2012, two new norditerpenoids 328 and 329 were isolated [4]. In 2013, five new biologically inactive [89] labdane diterpenes 330–334, named serralabdanes A–E, were isolated. The absolute configuration of the 12,13-diol moiety in serralabdane C (332) has been determined by using the induced circular dichroism after the addition of dimolybdenum teracetate in DMSO solution (Snatzke’s method). One new labdane-type diterpenoid, named elatiorlabdane (335) has been found in the whole plants of C. elatior [11].
Kauranes
In 2008, two new kaurane-type diterpenoids, 336 and 337, along with seven known compounds 338–344, were isolated from the whole plants of C. multistachys [90]. This is the only report on the isolation of kaurane-type diterpenoids from Chloranthaceae family.
Ent-abietanes
To date, 14 ent-abietane-type diterpenoids have been isolated from C. sessilifolius. In 2015, sessilifols A–N (345–358), were isolated from the whole plants of C. sessilifolius [91]. Sessilifols A (345) and B (346) possess an uncommon five-membered C-ring formed by oxidative cleavage of the C-13/C-14 bond in abieta-7,13-diene followed by the formation of a new C-C bond between C-12 and C-14. Sessilifol C (347) is a rare 7,8-seco-9-spiro-fused ent-abietane.
Norditerpenoids
Three new norditerpenoids 359–361 have been isolated from C. sessilifolius [91]. Sessilifol O (359) represents the first example of a naturally occurring 14-norabietane-type diterpenoid. Only five triterpenoids have been reported in the plants of Chloranthaceae family, and four of them have been isolated from S. glabra. In 2005, two new triterpenoid saponins 362–363 [92] and two known triterpenoids 364–365 [93] were isolated from the whole plants of S. glabra. A biologically inactive triterpenoid 366 has been isolated from the leaves and stems of C. henryi [14]. In 2007, a novel sesterpene 367 with an unprecedented skeleton was isolated from the trunk bark of H. angustifolium [94]. Under acidic conditions this compound undergoes isomerization to 368. A biologically inactive monoterpene 369 has been isolated from the aerial part of C. japonicus [25].
Coumarins
Fourteen coumarin derivatives have been reported to date. Their distribution in plants is as follows, C. Japonicus (370, 371, 383), C. holostegius (372), C. henryi (373–375), S. glabra (376–382). In 1984, compounds 370 and 371 were isolated from C. Japonicus [22]. In 1987, a coumarin glucoside 372 was found in the roots of C. holostegius [95]. In 2005, during the study of the chemical constituents of the roots of C. henryi, three coumarin glucosides 373–375 were obtained [12], along with a new compound 132 of a different class. Chemical investigation to the whole plants of S. glabra has led to the isolation of compounds 376 [93], 377 [96], 378–381 [97] and 382 [98]. In 2009, a new coumarinolignan glucoside 329 was isolated from the whole plant of C. japonicus. This compound is marginally cytotoxic against human hepatoma (Hepg-2), ovarian carcinoma (OV420), and breast cancer (MCF-7) cells [99].
Lignans
To date, only ten lignans 384–393 have been isolated from Chloranthaceae family, mainly found in C. japonicus and S. glabra. Compounds 384–388 belong to eupomatenoid benzofuran-type neolignans isolated from the whole plants of C. japonicus [99]. Compounds 389, 390 [100] and 391–393 [101] have been isolated from the whole plants of S. glabra.
Phenylpropionic acids and other phenylpropanoids
A total of 24 phenylpropionic acid and phenylpropanoids have been reported. They are mainly distributed in plants of S. glabra (394–406, 411–414), C. anhuiensis (407), C. multistachys (408, 409, 417), H. brasiliense (410), and C. serratus (415 and 416).
Phenylpropionic acids 394 [96], 395 [102], 396 [98], 397–400 [103], 401 [100], 402 [104] and 403–406 [105] have been isolated from the whole plants of S. glabra. In 2010, one new caffeoyl phenylethanoid diglycoside with an unusual cyclic structure, anhuienoside B (407), was isolated in the fresh leaves of C. anhuiensis [40]. Compounds 408 and 409 were isolated from the whole plants of C. multistachys [38]. Studies in 2015 led to the isolation of compound 410 from the fresh leaves of H. brasiliense [46]. Phenylpropanoids 411 [96], 412 [104] and 413, 414 [105] have been isolated from the whole plants of S. glabra. Two new phenylpropanoids 415 and 416, a pair of stereoisomers, have been isolated from the whole plants of C. serratus [2]. Compound 417, a glucoside of 413, has been isolated from whole plants of C. multistachys [38].
Flavonoids
Forty seven flavonoids have been isolated from Chloranthaceae family since 1982. They are mainly distributed in plants of A. lucida (418–432), S. glabra (433–451), S. hainanensis (452–463), and C. multistachys (464). The earliest report on the isolation of flavonoids from Chloranthaceae plants was in 1982 with four flavone C-glycosides 418–420, some flavonoid aglycones and their 3-O-mono and diglycosides 421–432 described as constituents of the leaves of A. lucida [106]. This is also the only report about chemical investigation of plants in genus Ascarina to date.
Most flavonoids 433–463 isolated from Chloranthaceae plants are mainly distributed in the plants of genus Sarcandra. Flavonoids 433–451 have been isolated from the whole plants of S. glabra. Two flavanones 433, 434 and a dihydrochalcone 435 have been obtained from the whole plants of S. glabra [93]. Compound 436, a novel phenylpropanoid-substituted catechin glycoside, and compound 437 with a rare phenylpropanoid-substituted dihydrochalcone have also been isolated from S. glabra [107]. Flavonoid 438 and chalcone 439 are two compound isolated for the first time from S. glabra [108]. In 2008, five flavonoid glycosides including 440–443 and a flavonone 3-O-glycoside 444 were isolated from the whole plants of S. glabra [102]. In the same year, a flavonone 6-C-glycoside 445 [109] and a rare flavonone 446 [98] without a 4′-substituent were isolated. Compounds 447–449, that differ in the absolute configuration at C-2 and C-3, were also isolated from S. glabra [100]. In 2012, two additional phenylpropanoid-substituted catechin glycosides 450 and 451 were isolated from the whole plant [33]. Flavonoids 452–463 have been obtained from the whole plants of S. hainanensis [110], [111], [112]. The chalcone 464, found in genus Chloranthus, has been isolated from whole plant tissues of C. multistachys [38].
Other compounds
Several amides 465–473 have been isolated from the genus of Chloranthus and Sarcandra. They are distributed in the species C. serratus [113], S. glabra [98], C. anhuiensis [40] and C. angustifolius [42]. These amide derivatives in Chloranthus and Sarcandra may serve as specific fingerprint markers for distinguishing between species within the genus.
Six steroids 474–479 are distributed in two kinds of plants, namely C. henryi and C. multistachys [38]. Compounds 480–487 are derivatives of benzoic acid. Compounds 480 [93], 481 [104], 482 and 483 [114], 484 [105] and 485 [96] have been found in the whole plants of S. glabra. The esters 486 [38] and 487 [115] have been isolated from the whole plants of C. multistachys. Other carboxylic acids and alcohols, 488 [93], 489–492 [110], 493 [114] and 494 [108] have been isolated from the whole plants of S. glabra and S. hainanensis. Monosaccharides 495 and 496 have been isolated from the whole plants of S. glabra [108]. Two anthraquinones 497 and 498 found in the whole plants of S. hainanensis [110] are the only anthraquinones in Chloranthaceae plants. The glycoside 499 [30] and its aglycone 500 [116] have been isolated from S. glabra. In 2010, a new tetrahydrophenanthrene named henryin A (501) was isolated from the leaves of C. henryi [117] and a benzaldehyde derivative 502 was isolated from the leaves of C. anhuiensis [40]. In 2012, from the whole plant of S. glabra, a new glycoside 503 was isolated [101]. The phenylethanoid glycoside 504 has been isolated from C. multistachys [38].
Biological activities
Antimicrobial activity
Compounds 4–7 have been tested for antimicrobial activity against 13 microorganisms in vitro but only compounds 6 and 7 show moderate activity against Helicobacter pylori-SS1 with MICs of 25–50 µg/mL [2]. Compounds 26, 31, 87, 88, 161, 232, 234, 244, 254, 275 and 276 have been evaluated for antimicrobial activity against five bacterial strains including Staphylococcus aureus (Sa, ATCC 29213), Escherichia coli (Ec, ATCC 25922), Salmonella typhimurium (St, ATCC 13311), Pseudomonas aeruginosa (Pa, ATCC 27853), and Klebsiella pneumoniae (Kp, ATCC 18433), as well as six fungal strains including Candida albicans (Ca, ATCC 90028), Cryptococcus neoformans (Cn, ATCC 22402), Candida glabrata (Cg, ATCC 90030), Candida krusei (Ck, ATCC 6258), Candida parapsilosis (Cp, ATCC 22019), and Aspergillus sp. (As, ATCC 293) in vitro by using a microdilution assay. Sesquiterpene dimers 232, 234, 244, 254, 275 and 276 show remarkable antifungal activity against C. albicans with MIC values ranging from 4 to 8 μg/mL. Sesquiterpenes 26, 31, 87 and 88, show moderate inhibitory activity against C. albicans with MIC values ranging from 16 to 32 μg/mL. The fungus C. neoformans is most sensitive to sesquiterpene dimer 254 with a MIC value of 8 μg/mL. These results suggest that the sesquiterpenes may be the chemical constituents responsible for the known anti-ringworm effect of C. angustifolius [41].
Compound 64 is a specific inhibitor of chitin synthase 2 of S. cerevisiae. It also exhibits antifungal activities against various human and phytopathogenic fungi such as C. albicans, Cryptococcus neoformans, Alternaria kikuchiana, Colletotrichum lagenarium, Fusarium oxysporum, Magnaporthe grisea, Botrytis cineria, Pythium ultimum and Rhizoctonia solani with MIC values of 50– 100 μg/mL [26].
Compound 231 is an antifungal agent against Candida albicans and C. parapsilosis with an MIC value of 0.068 μm [72]. Specific inhibitors of chitin synthases might serve as interesting lead compounds for the development of effective antifungal agents. Sesquiterpenes 254, 256 and 260, have been evaluated for their inhibitory effects on the release of NO from macrophages using LPS-induced RAW264.7 cells as a model system, with the respective IC50 values of 0.22, 0.15, and 7.22 μm [4]. Compound 267 is an antifungal agent with MICs of 1–32 μg/mL in vitro against Alternaria kikuchiana, Botrytis cinerea, Colletotrichum lagenarium, Magnaporthe grisea, Pythium ultimum and Phytophthora infestans. The disease-control activity of compound 267 is stronger than that of the commercially available fungicide chlorothalonil, but weaker than that of dimethomorph. Therefore, compound 267 might be an interesting lead compound to develop effective antifungal agents [118]. Essential oils from C. japonicus and C. multistachys show strong antimicrobial activity against most tested microorganisms. For both species, minimum values for inhibitory and bactericidal concentrations are 0.39–12.50 mg/mL and 0.78–50.00 mg/mL, respectively. These essential oils may be natural sources of potent antimicrobial agents for the medicinal and pharmaceutical industries [119].
Anti-inflammatory activity
Compounds 26 and 306 have been evaluated for anti-inflammatory activities by measuring the inhibition of the PAF induced release of β-glucuronidase from rat PMNs in vitro. These compounds inhibit the release of β-glucuronidase from rat PMNs induced by PAF [9]. Compounds 41, 166, 235, 240, 244 and 254 have been assayed for their inhibitory effects on NO production in LPS-activated BV-2 cells. These compounds decrease NO production with IC50 values 79.0, 68.1, 79.4, 70.4, 47.9, and 31.1 μm, respectively, without cytotoxicity [17]. Compounds 350 and 353 show moderate antineuroinflammatory activities, with IC50 values of 8.3 and 7.4 μm, respectively [91]. This is the first report on the antineuroinflammatory activity of naturally occurring ent-abietanes [91]. In 2012, compounds 96, 130, 217, 219, 254–257, 264, 266 and 267, were tested for their inhibitory effects on LPS-induced nitric oxide production in RAW264.7 cells. Shizukaols E (217), G (219), M (264), O (266), and henriol D (267) show significant anti-inflammatory activities with IC50 values of 1.90, 3.68, 1.95, 7.01, and 1.95 μm, respectively [58]. Serralabdanes A-E (330–334) have also been investigated using the same assay. Compared with IC50 values of the reference compound dexamethasone at 1.08 ± 0.15 μm, the five compounds display weak activities with IC50 values of 38.45 ± 1.02, 29.78 ± 0.92, 44.37 ± 0.58, 53.68 ± 1.52, and 47.31 ± 1.26, respectively [89]. Dimers 219, 254 and 287 can significantly inhibit NO production in LPS-induced macrophages with IC50 values at, 4.65, 2.33 and 3.04 μmol/L, respectively [87]. The pretreatment with coumarin 377 increases the survival rate following LPS stimulation in mice. The effect involves regulation of NF-κB signal which, in turn, regulates production of inflammatory cytokine TNF-α, suggesting that IF may have a therapeutic effect against LPS-induced inflammatory disease [120]. Further research has suggested that compound 377 has a protective effect against LPS-induced acute lung injury (serious lung inflammation and increased capillary permeability) [121].
Anti-tumor activity
Compound 33 shows moderate antitumor activity against human cervical squamous carcinoma (Hela) cells (IC50 = 22.2 µm) and human erythroleukemia (K562) cells (IC50 = 21.8 µm) and weak antitumor activity against Hela cells (IC50 = 89.3 µm) and K562 cells (IC50 = 78.5 µm). Compound 301 is highly active against Hela cells (IC50 = 5.6 µm) and K562 cells (IC50 = 5.9 µm) [13]. Codonolactone 56 inhibits invasion and migration of breast cancer cells in vitro. The mechanism of its inhibitory effects has been investigated and results suggest that 56 inhibits the binding of Runx2 to sequences of the mmp-13 promoter [122]. Compound 208 shows strong anti-tumor activity against Hela (IC50 = 5.6 μg/mL), and K562 (IC50 = 5.0 μg/mL). Compound 95 demonstrates moderate anti-tumor activity against Hela (IC50 = 17.2 μg/mL, and K562 (IC50 = 21.6 μg/mL) [23]. Compounds 282 and 284 exhibit cytotoxicity against the HL-60 cell line with IC50 values of 0.03 and 1.2 μm, respectively [35]. Compound 93 shows moderate activity against A549 and HL-60 with IC50 values of 3.1 and 8.8 µm, respectively [37]. Compound 177 shows remarkable antitumor activity against the Hela, A549, MCF, and K562 human-tumor cell lines, with IC50 values of 4.7, 8.9, 9.6, and 11.8 μg/mL, respectively [14]. Crude extracts of C. japonicus and some isolates have been tested for their cytotoxicity against NCI-H460 (human large cell lung cancer cell line) and SMMC-7721 (hepatocellular carcinoma) cell lines. Among them, the methanol extract, ethyl acetate fraction, and isolated shizukaols B, C, D, and O (254, 256 and 266) show marked cytotoxicities with IC50 value from 32.5 to 36.79 μm against NCI-H460, and 13.71 to 37.39 μm against SMMC-7721. Sesquiterpene dimers are presumably responsible for the cytotoxicities exhibited by C. japonicus [25]. Compound 254 derived from the ethanol extract of the roots of C. henryi exhibits cytotoxic activities against BEL-7402 and BGC-823 cell lines (IC50 = 1.4 and 3.2 μg/mL, respectively). Compound 304 shows moderate cytotoxic activities against BEL-7402, HCT-8, and BGC-823 cell lines (IC50 = 17.0, 0.54, and 5.76 μg/mL, respectively) [73]. Compounds 254, 257 and 218, isolated from C. japonicus, are potential cell adhesion inhibitors. They inhibit PMA-induced homotypic aggregation of HL-60 cells with MIC values of 34.1 nm (254), 0.9 μm (257) and 27.3 nm (218). All three compounds significantly inhibit ICAM-1 expression in HL-60 cells in a dose-dependent manner. It can be suggested that compounds 254, 257 and 218 prevent monocyte adhesion to HUVEC through the inhibition of cell adhesion molecules expression stimulated by TNF-α [123]. Sesquiterpene polymers 277–279 show inhibitory activities against the HL-60 (human leukemia) cell line with IC50 values of 3.1, 8.4, and 8.5 µm, respectively. Compounds 277 and 279 show inhibitory activities against the A-549 cell line with the respective IC50 values of 7.2 and 4.7 µm. Eleutheroside B1 (376) exhibits potent activity against BGC-823 and A2780 cancer cell lines with the IC50 value of 2.53 and 1.85 µm, respectively [101].
Anti-HIV activity
Fifteen disesquiterpenoids (216, 218, 220, 224, 226–229, 235, 254–257, 271 and 272) have been tested for their activity against HIV-1 replication. Compounds 218, 220, 254 and 255 show inhibitory effects on HIV-1 replication (C50 in the range from 0.11 to 4.05 μm) for wild-type HIV-1 and two non-nucleoside reverse transcriptase inhibitor resistant HIV-1 strains (HIVRT-K103N and HIVRT-Y181C). Among the tested compounds, shizukaol B (254) exhibits remarkable activity against HIVwt, HIVRT-K103N, and HIVRT-K103N (EC50 = 0.22, 0.47, and 0.50 μm, respectively). Compounds 218, 220, 254 and 255 show significant cytotoxity against C8166 cell line (CC50 = 0.047, 0.022, 0.020 and 0.089, respectively), and inhibitory activities against HIV-1 (EC50 = 0.0043, 0.0033, 0.0014 and 0.016 μm, respectively) [71].
Compounds 455 and 456 have been assayed for their HIV-1 integrase (IN) inhibition activities with a microplate screening method using magnetic beads. The reference drug has been baicalein (IC50 = 1.06 μm). They exhibit weak activities with IC50 values of 18.05 and 25.27 μm, respectively [111].
Additional biological activity
In the search for compounds with hepatoprotective activity from S. glabra, compounds 67, 68, 115, 126, 174, 202 and 203 have been found to be active at 10-4 min vitro, without any obvious cytotoxic effects [29]. This is the first report of hepatoprotective activity of compounds derived from a Sarcandra species [29]. Compounds 232 and 304–306 show moderate hepatoprotective activities with IC50 values of 0.19, 0.66, 0.09 and 0.18 μm, respectively [73].
The hydroalcoholic extract (HE) from H. brosiliense and compound 133 show activity in many assays of pain, such as abdominal constriction response caused by intraperitoneal injection of acetic acid, formalin-induced licking, capsaicin-induced licking, hot-plate test, and tail-flick test. HE produces remarkable inhibition of acetic acid-induced abdominal constriction in mice (ID50 = 12.7 mg/kg). In the assay of formalin-induced licking, HE inhibits the first and second phase (ID50 = 31.1 and 21.7 mg/kg, respectively). HE also has an effect on capsaicin-induced neurogenic pain (ID50 = 69.0 mg/kg). Compound 133 shows graded antinociception against acetic acid writhing and capsaicin-induced neurogenic pain [59].
The search for anti-leishmanial agents from H. angustifolium has led to the isolation of compounds 123, 133–135, and 197. Compound 133 exhibits significant activity against L. amazonensis and L. infantum with a value of IC50 of 19.8 and 20.9 µm, respectively. This is the first time of anti-leishmanial activity finding for a lindenane sesquiterpene [60].
Tyrosinase is a key enzyme for melanin biosynthesis in plants and animals. Tyrosinase inhibitors, therefore, can be clinically useful for the treatment of some dermatological disorders associated with melanin hyperpigmentation. Compounds 163 and 164 inhibit the enzyme tyrosinase with the IC50 values of 325 and 269 μm, respectively, compared to the standard tyrosinase inhibitor kojic acid (IC50 = 211 μm) [15]. Similar values have been found for 275 and 276 [86].
Six novel sesquiterpenoid dimers isolated from C. holostegius have been tested their inhibition on the delayed rectifier (IK)K+ current. Chlorahololides A (270) and B (271), exhibit potent and selective inhibition with IC50 of 10.9 and 18.6 µm, respectively, and are 56- and 96-fold more potent than the positive control, tetraethylammonium chloride (IC50 = 1.05 μm), a classical blocker of the delayed rectifier (IK)K+ current [84]. Chlorahololides C-F (272, 267, 273 and 274), show potent and selective inhibition on the delayed rectifier (IK)K+ current with the IC50 values of 3.6 ± 10.1, 2.7 ± 0.3, 27.5 ± 5.1 and 57.5 ± 6.1 μm, respectively [85].
Shizukaol D (256) can activate AMP-activated protein kinase (AMPK), which is a key sensor and regulator of intracellular energy metabolism, leading to a decrease in triglyceride and cholesterol levels in HepG2 cells. Compound 256 can also induce mitochondrial dysfunction by depolarizing the mitochondrial membrane and suppressing energy production, which may result in AMPK activation. This research suggested that 256 might be used to treat metabolic syndrome [124].
Conclusions
In total, 299 sesquiterpenes have been isolated from plants of Chloranthaceae. Eudesmanes, lindenranes and sesquiterpene polymers are present in large numbers. The lindenrane-type sesquiterpene dimers can be used as chemotaxonomical markers of genera Chloranthus and Sarcandra. Since cadinane-type sesquiterpenes have been found only in three species, C. henryi, C. serratus and C. multistachys of genus Chloranthus, this class of compounds may be explored as the chemotaxonomical markers of the above three species or genus Chloranthus. Many species have been used by local people as traditional herbal medicines. Some components isolated from the herbal medicines exhibit significant bioactivities. For example, chlorahololides C-F (272–274) represent a new class of potassium channel blockers, and their potent and selective inhibition on the delayed rectifier (IK) K+ current suggest that further investigation into this structural class is warranted [85]. On the other hand, additional work is warranted to investigate mechanisms of these interesting bioactivities. There are also many species, such as plants of genus Hedyosmum and Ascarina, that have received no or little attention from the viewpoints of chemical and biological properties.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81302664
Funding statement: The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (81302664), Key Foundation of Hebei Ministry of Education (ZD2016093), Traditional Chinese Medicine Administration of Hebei Province (2016040) and Syngenta Ltd. (2008-Hebei Medical University-Syngenta-02)
Acknowledgments
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (81302664), Key Foundation of Hebei Ministry of Education (ZD2016093), Traditional Chinese Medicine Administration of Hebei Province (2016040) and Syngenta Ltd. (2008-Hebei Medical University-Syngenta-02).
References
[1] Kawabata, J.; Fukushi, Y.; Tahara, S.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae plants. Part IV. Structures of novel sesquiterpene ketones from Chloranthus serratus (Chloranthaceae). Agric. Biol. Chem.1985, 49, 1479–1485.10.1271/bbb1961.49.1479Suche in Google Scholar
[2] Yuan, T.; Zhang, C.-R.; Yang, S.-P.; Yin, S.; Wu, W.-B.; Dong, L.; Yue, J.-M. Sesquiterpenoids and phenylpropanoids from Chloranthus serratus. J. Nat. Prod.2008, 71, 2021–2025.10.1021/np800543fSuche in Google Scholar PubMed
[3] Teng, F.; Zhong, H.-M.; Chen, C.-X.; Liu, H.-Y. Four new eudesmane sesquiterpenoid lactones from Chloranthus serratus. Helv. Chim. Acta.2009, 92, 1298–1303.10.1002/hlca.200800450Suche in Google Scholar
[4] Zhang, M.; Iinuma, M.; Wang, J.-S.; Oyama, M.; Ito, T.; Kong, L.-Y. Terpenoids from Chloranthus serratus and their anti-inflammatory activities. J. Nat. Prod.2012, 75, 694–698.10.1021/np200968pSuche in Google Scholar PubMed
[5] He, X.-F.; Yin, S.; Ji, Y.-C.; Su, Z.-S.; Geng, M.-Y.; Yue, J.-M. Sesquiterpenes and Dimeric Sesquiterpenoids from Sarcandra glabra. J. Nat. Prod.2010, 73, 45–50.10.1021/np9006469Suche in Google Scholar PubMed
[6] Tesso, H.; Koenig, W. A.; Son, P. T.; Giang, P. M. Composition of the essential oil of flowers of Chloranthus spicatus (Thunb.) Makino. Flavour Fragrance J.2006, 21, 592–597.10.1002/ffj.1528Suche in Google Scholar
[7] Yang, S.-P.; Zhang, C.-R.; Chen, H.-D.; Liao, S.-G.; Yue, J.-M. Sesquiterpenoids from Chloranthus spicatus (Thunb.) Makino. Chin. J. Chem.2007, 25, 1892–1895.10.1002/cjoc.200790349Suche in Google Scholar
[8] Xiao, Z.-Y.; Wang, X.-C.; Zhang, G.-P.; Huang, Z.-L.; Hu, L.-H. Terpenoids from Roots of Chloranthus spicatus. Helv. Chim. Acta.2010, 93, 803–810.10.1002/hlca.200900299Suche in Google Scholar
[9] Luo, Y.; Yin, X.; Xie, M. Chemical constituents and bioactivity of Chloranthus multistachys. Adv. Mater. Res. (Durnten-Zurich, Switz.)2012, 343–344, 1189–1192.10.4028/www.scientific.net/AMR.343-344.1189Suche in Google Scholar
[10] Yang, S.; Chen, H.; Yue, J. Sesquiterpenoids from Chloranthus spicatus. Chin. J. Chem.2012, 30, 1243–1248.10.1002/cjoc.201200455Suche in Google Scholar
[11] Sun, C.-L.; Yan, H.; Li, X.-H.; Zheng, X.-F.; Liu, H.-Y. Terpenoids from Chloranthus elatior. Nat. Prod. Bioprospect.2012, 2, 156–159.10.1007/s13659-012-0039-7Suche in Google Scholar
[12] Li, C.; Zhang, D.; Luo, Y. Umbelliferone 7-O-β-D-glucoside. Acta Pharm. Sinica2005, 40, 525–528.Suche in Google Scholar
[13] Wu, B.; He, S.; Wu, X.-d.; Pan, Y.-j. Bioactive terpenes from the roots of Chloranthus henryi. Planta Med.2006, 72, 1334–1338.10.1055/s-2006-947256Suche in Google Scholar PubMed
[14] Wu, B.; He, S.; Wu, X.-D.; Wu, D.-K.; Pan, Y.-J. Cadinane and eudesmane sesquiterpenoids from Chloranthus henryi. Helv. Chim. Acta2007, 90, 1586–1592.10.1002/hlca.200790166Suche in Google Scholar
[15] Wu, B.; He, S.; Wu, X.-D.; Pan, Y.-J. New tyrosinase inhibitory sesquiterpenes from Chloranthus henryi. Chem. Biodivers.2008, 5, 1298–1303.10.1002/cbdv.200890116Suche in Google Scholar PubMed
[16] Gan, X.; Ma, L.; Chen, Q.; Chen, Q.; Yu, Q.; Hu, L. Terpenoids from roots of Chloranthus henryi. Planta Med.2009, 75, 1344–1348.10.1055/s-0029-1185560Suche in Google Scholar PubMed
[17] Wang, L. J.; Xiong, J.; Liu, S. T.; Liu, X. H.; Hu, J. F. Sesquiterpenoids from Chloranthus henryi and their anti-neuroinflammatory activities. Chem Biodivers.2014, 11, 919–928.10.1002/cbdv.201300283Suche in Google Scholar PubMed
[18] Wang, F.; Zhou, D.-S.; Wei, G.-Z.; Ren, F.-C.; Liu, J.-K. Chlorantholides A-F, eudesmane-type sesquiterpene lactones from Chloranthus elatior. Phytochemistry2012, 77, 312–317.10.1016/j.phytochem.2012.02.008Suche in Google Scholar PubMed
[19] Xiong, J.; Liu, S.-T.; Tang, Y.; Wang, W.-X.; Bui, V.-B.; Zhao, Y.; Fan, H.; Yang, G.-X.; Hu, J.-F. Sesquiterpenoids from the aerial parts of Chloranthus elatior. Phytochem. Lett.2013, 6, 586–589.10.1016/j.phytol.2013.07.015Suche in Google Scholar
[20] Liu, S. T.; Xiong, J.; Tang, Y.; Wang, W. X.; Bui, V. B.; Ma, G. L.; Huang, Y.; Zhao, Y.; Yang, G. X.; Hu, J. F. Chloranthones A-D: minor and unprecedented dinor-eudesmenes from Chloranthus elatior. Chem. Biodivers.2014, 11, 904–909.10.1002/cbdv.201300247Suche in Google Scholar PubMed
[21] Uchida, M.; Koike, Y.; Kusano, G.; Kondo, Y.; Nozoe, S.; Kabuto, C.; Takemoto, T. Studies on the constituents of Chloranthus spp. III. Six sesquiterpenes from Chloranthus japonicus. Chem. Pharm. Bull.1980, 28, 92–102.10.1248/cpb.28.92Suche in Google Scholar
[22] Kawabata, J.; Fukushi, Y.; Tahara, S.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae plants. Part III. Structures of novel sesquiterpene alcohols from Chloranthus japonicus (Chloranthaceae). Agric. Biol. Chem.1984, 48, 713–717.10.1271/bbb1961.48.713Suche in Google Scholar
[23] Wu, B.; He, S.; Pan, Y. Sesquiterpenoid with new skeleton from Chloranthus henryi. Tetrahedron Lett.2007, 48, 453–456.10.1016/j.tetlet.2006.11.054Suche in Google Scholar
[24] Wu, B.; Qu, H.; Cheng, Y. New sesquiterpenes from Chloranthus japonicus. Helv. Chim. Acta2008, 91, 725–733.10.1002/hlca.200890073Suche in Google Scholar
[25] Zhang, M.; Wang, J.-S.; Wang, P.-R.; Oyama, M.; Luo, J.; Ito, T.; Iinuma, M.; Kong, L.-Y. Sesquiterpenes from the aerial part of Chloranthus japonicus and their cytotoxicities. Fitoterapia2012, 83, 1604–1609.10.1016/j.fitote.2012.09.009Suche in Google Scholar
[26] Yim, N. H.; Hwang, E. I.; Yun, B. S.; Park, K. D.; Moon, J. S.; Lee, S. H.; Sung, N. D.; Kim, S. U. Sesquiterpene furan compound CJ-01, a novel chitin synthase 2 inhibitor from Chloranthus japonicus SIEB. Biol. Pharm. Bull.2008, 31, 1041–1044.10.1248/bpb.31.1041Suche in Google Scholar
[27] Wang, Q.-H.; Kuang, H.-X.; Yang, B.-Y.; Xia, Y.-G.; Wang, J.-S.; Kong, L.-Y. Sesquiterpenes from Chloranthus japonicus. J. Nat. Prod.2011, 74, 16–20.10.1021/np100504mSuche in Google Scholar
[28] Fang, P.-L.; Liu, H.-Y.; Zhong, H.-M. A new eudesmane sesquiterpenoid lactone from Chloranthus japonicus. Zhongguo Tianran Yaowu2012, 10, 24–27.10.1016/S1875-5364(12)60005-3Suche in Google Scholar
[29] Li, Y.; Zhang, D.-M.; Li, J.-B.; Yu, S.-S.; Li, Y.; Luo, Y.-M. Hepatoprotective sesquiterpene glycosides from Sarcandra glabra. J. Nat. Prod.2006, 69, 616–620.10.1021/np050480dSuche in Google Scholar PubMed
[30] Hu, X.-r.; Yang, J.-s.; Xu, X.-d. Three novel sesquiterpene glycosides of Sarcandra glabra. Chem. Pharm. Bull.2009, 57, 418–420.10.1248/cpb.57.418Suche in Google Scholar PubMed
[31] Zhu, L.-P.; Li, Y.; Yang, J.-Z.; Zuo, L.; Zhang, D.-M. Two new sesquiterpene lactones from Sarcandra glabra. J. Asian Nat. Prod. Res.2008, 10, 541–545.10.1080/10286020801966773Suche in Google Scholar PubMed
[32] Oanh, D. T.; Ky, P. T.; Hang, T. B.; Yen, P. H.; Hanh, T. H.; Cuong, N. X.; Luong, D. V.; Van Minh, C.; Van Kiem, P. Two new sesquiterpenes from Sarcandra glabra. Nat. Prod. Commun.2010, 5, 1717–1720.10.1177/1934578X1000501102Suche in Google Scholar
[33] Wang, C.; Li, Y.; Li, C. J.; Yu, S. S.; Zhang, D. M. Three new compounds from Sarcandra glabra. Chin. Chem. Lett.2012, 23, 823–826.10.1016/j.cclet.2012.05.007Suche in Google Scholar
[34] Hu, X.-R.; Wu, H.-F.; Zhang, X.-P.; Yang, J.-S.; Dai, Z.; Lin, R.-C.; Xu, X.-D. A new sesquiterpene lactone from Sarcandra glabra. Nat. Prod. Res.2013, 27, 1197–1201.10.1080/14786419.2012.722084Suche in Google Scholar PubMed
[35] Ni, G.; Zhang, H.; Liu, H.-C.; Yang, S.-P.; Geng, M.-Y.; Yue, J.-M. Cytotoxic sesquiterpenoids from Sarcandra glabra. Tetrahedron2013, 69, 564–569.10.1016/j.tet.2012.11.023Suche in Google Scholar
[36] Zhang, S.; Su, Z.-S.; Yang, S.-P.; Yue, J.-M. Four sesquiterpenoids from Chloranthus multistachys. J. Asian Nat. Prod. Res.2010, 12, 522–528.10.1080/10286020.2010.492599Suche in Google Scholar PubMed
[37] Su, Z.-S.; Yin, S.; Zhou, Z.-W.; Wu, Y.; Ding, J.; Yue, J.-M. Sesquiterpenoids from Hedyosmum orientale. J. Nat. Prod.2008, 71, 1410–1413.10.1021/np800240vSuche in Google Scholar PubMed
[38] Liu, H.-Y.; Ran, X.-H.; Gong, N.-B.; Ni, W.; Qin, X.-J.; Hou, Y.-Y.; Lu, Y.; Chen, C.-X. Sesquiterpenoids from Chloranthus multistachys. Phytochemistry2013, 88, 112–118.10.1016/j.phytochem.2012.12.002Suche in Google Scholar PubMed
[39] Xu, Y.-J.; Tang, C.-P.; Tan, M.-J.; Ke, C.-Q.; Wu, T.; Ye, Y. Sesquiterpenoids and Diterpenoids from Chloranthus anhuiensis. Chem. Biodivers.2010, 7, 151–157.10.1002/cbdv.200800300Suche in Google Scholar PubMed
[40] Wu, B.; Gan, L.; Qu, H. An unusual stress metabolite induced by CuCl2 and other constituents from the leaves of Chloranthus anhuiensis. J. Nat. Prod.2010, 73, 1069–1074.10.1021/np100063qSuche in Google Scholar PubMed
[41] Yang, X.; Wang, C.; Yang, J.; Wan, D.; Lin, Q.; Yang, G.; Mei, Z.; Feng, Y. Antimicrobial sesquiterpenes from the Chinese medicinal plant, Chloranthus angustifolius. Tetrahedron Lett.2014, 55, 5632–5634.10.1016/j.tetlet.2014.08.052Suche in Google Scholar
[42] Liu, C.; Li, G.; Huang, R.; Li, Y.; Ding, W. Chemotaxonomic significance of sesquiterpenes and amide derivatives from Chloranthus angustifolius Oliv. Biochem. Syst. Ecol.2015, 58, 30–33.10.1016/j.bse.2014.10.011Suche in Google Scholar
[43] Takeda, Y.; Yamashita, H.; Matsumoto, T.; Terao, H. Chloranthalactone F, a sesquiterpenoid from the leaves of Chloranthus glaber. Phytochemistry1993, 33, 713–715.10.1016/0031-9422(93)85480-FSuche in Google Scholar
[44] Wang, X.-C.; Wu, W.-Q.; Ma, S.-P.; Liu, J.-H.; Hu, L.-H. A new sesquiterpenoid from the roots of Chloranthus fortunei. Chin. J. Nat. Meds2008, 6, 404–407.10.1016/S1875-5364(09)60034-0Suche in Google Scholar
[45] Huong, T. T.; Van Thong, N.; Minh, T. T.; Tram, L. H.; Anh, N. T.; Cuong, H. D.; Van Cuong, P.; Ca, D. V. Chloranerectuslactone V, a New Sesquiterpene from Chloranthus erectus Verdc. Lett. Org. Chem.2014, 11, 639–642.10.2174/157017861109140902152358Suche in Google Scholar
[46] Amoah, S. K. S.; Kouloura, E.; Dutra, L. M.; Barison, A.; Wildner, L. M.; Bazzo, M. L.; Halabalaki, M.; Skaltsounis, L. A.; Biavatti, M. W. Phytochemical analysis of the hot tea infusion of Hedyosmum brasiliense. Phytochem. Lett.2015, 13, 267–274.10.1016/j.phytol.2015.07.013Suche in Google Scholar
[47] Kawabata, J.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae plants. Part V. Distribution of lindenanolides in the Chloranthaceae. Agric. Biol. Chem.1988, 52, 2965–2966.10.1080/00021369.1988.10869122Suche in Google Scholar
[48] Kawabata, J.; Tahara, S.; Mizutani, J.; Furusaki, A.; Hashiba, N.; Matsumoto, T. Shizukanolides, two sesquiterpenoids from Chloranthus japonicus (Chloranthaceae). Agric. Biol. Chem.1979, 43, 885–887.10.1271/bbb1961.43.885Suche in Google Scholar
[49] Tahara, S.; Fukushi, Y.; Kawabata, J.; Mizutani, J. Studies on the chemical constituents of chloranthaceae plants. Part II. Lindenanolides in the root of chloranthus japonicus (Chloranthaceae). Agric. Biol. Chem.1981, 45, 1511–1512.10.1271/bbb1961.45.1511Suche in Google Scholar
[50] Kawabata, J.; Mizutani, J. Studies on the chemical constituents of Chloroanthaceae plants. Part VI. Shizukanolides D, E and F, novel lindenanolides from Chloranthus spp. (Chloranthaceae). Agric. Biol. Chem.1989, 53, 203–207.10.1271/bbb1961.53.203Suche in Google Scholar
[51] Heo, J. E.; Jin, J. L.; Lee, Y. Y.; Yun-Choi, H. S. Chemical constituents of the aerial parts of Chloranthus japonicus Sieb. Nat. Prod. Sci.2005, 11, 41–44.Suche in Google Scholar
[52] Kuang, H.; Xia, Y.; Yang, B.; Wang, Q.; Lu, S. Sesquiterpene glucosides from Chloranthus japonicus SIEB. Chem. Biodivers.2008, 5, 1736–1742.10.1002/cbdv.200890162Suche in Google Scholar PubMed
[53] Yan, H.; Li, X.-H.; Zheng, X.-F.; Sun, C.-L.; Liu, H.-Y. Chlojaponilactones B-E, four new lindenane sesquiterpenoid lactones from Chloranthus japonicus. Helv. Chim. Acta2013, 96, 1386–1391.10.1002/hlca.201200508Suche in Google Scholar
[54] Li, X.; Zhang, Y.; Yang, L.; Feng, Y.; Liu, Y.; Zeng, X. Sesquiterpenoids in sarcandra glabra. Yao Xue Xue Bao2011, 46, 1349–1351.Suche in Google Scholar
[55] Uchida, M.; Kusano, G.; Kondo, Y.; Nozoe, S.; Takemoto, T. Two new sesquiterpenoids from Chloranthus glaber Makino. Heterocycles1978, 9, 139–144.10.3987/R-1978-02-0139Suche in Google Scholar
[56] Okamura, H.; Nakashima, N.; Iwagawa, T.; Nakayama, N.; Nakatani, M. The structures of two lindenane sesquiterpene glucosides from Chloranthus glaber. Chem. Lett.1994, 1541–1542.10.1246/cl.1994.1541Suche in Google Scholar
[57] Wang, X.-C.; Wang, L.-L.; Ouyang, X.-W.; Ma, S.-P.; Liu, J.-H.; Hu, L.-H. Sesquiterpenes and dimers thereof from Chloranthus fortunei. Helv. Chim. Acta2009, 92, 313–320.10.1002/hlca.200800262Suche in Google Scholar
[58] Zhang, M.; Wang, J.-S.; Oyama, M.; Luo, J.; Guo, C.; Ito, T.; Iinuma, M.; Kong, L.-Y. Anti-inflammatory sesquiterpenes and sesquiterpene dimers from Chloranthus fortunei. J. Asian Nat. Prod. Res.2012, 14, 708–712.10.1080/10286020.2012.685724Suche in Google Scholar PubMed
[59] Trentin, A. P.; Santos, A. R.; Guedes, A.; Pizzolatti, M. G.; Yunes, R. A.; Calixto, J. B. Antinociception caused by the extract of Hedyosmum brasiliense and its active principle, the sesquiterpene lactone 13-hydroxy-8,9-dehydroshizukanolide. Planta Med.1999, 65, 517–521.10.1055/s-1999-14007Suche in Google Scholar PubMed
[60] Acebey, L.; Jullian, V.; Sereno, D.; Chevalley, S.; Estevez, Y.; Moulis, C.; Beck, S.; Valentin, A.; Gimenez, A.; Sauvain, M. Anti-leishmanial lindenane sesquiterpenes from Hedyosmum angustifolium. Planta Med.2010, 76, 365–368.10.1055/s-0029-1186192Suche in Google Scholar PubMed
[61] Rao, G.-W.; Zhan, Z.-J.; Li, C.-P.; Shan, W.-G. A novel sesquiterpenoid glucoside from Hedyosmum orientale. J. Chem. Res.2010, 34, 697–698.10.3184/030823410X12857507693364Suche in Google Scholar
[62] Amoah, S. K. S.; de Oliveira Fabio, L.; da Cruz Ana Caroline, H.; de Souza Nicole, M.; Campos Francinete, R.; Barison, A.; Biavatti Maique, W. Sesquiterpene lactones from the leaves of Hedyosmum brasiliense (Chloranthaceae). Phytochemistry2013, 87, 126–132.10.1016/j.phytochem.2012.11.018Suche in Google Scholar PubMed
[63] Bercion, S.; Couppe de K/Martin, M.-A.; Baltaze, J.-P.; Bourgeois, P. A newα-methylene γ-lactone sesquiterpene from Hedyosmum arborescens. Fitoterapia2005, 76, 620–624.10.1016/j.fitote.2005.06.006Suche in Google Scholar PubMed
[64] Bercion, S.; Buffeteau, T.; Lespade, L.; Couppe deK. Martin, M.-A. IR, VCD, 1H and 13C NMR experimental and theoretical studies of a natural guaianolide: unambiguous determination of its absolute configuration. J. Mol. Struct.2006, 791, 186–192.10.1016/j.molstruc.2006.01.030Suche in Google Scholar
[65] Kawabata, J.; Tahara, S.; Mizutani, J. Studies on the chemical constituents of chloranthaceae plants. Part I. Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae). Agric. Biol. Chem.1981, 45, 1447–1453.10.1271/bbb1961.45.1447Suche in Google Scholar
[66] Takemoto, T.; Uchida, M.; Kusano, G. Studies on the constituents of Chloranthus spp. II. The sesquiterpenes from Chloranthus serratus. Chem. Pharm. Bull.1976, 24, 531–533.10.1248/cpb.24.531Suche in Google Scholar
[67] Wang, A. Q.; Feng, S. C.; He, X.; Xu, R. S. A new sesquiterpene lactone from Sarcandra glabra. Acta Pharm. Sinica1988, 23, 64–66.Suche in Google Scholar
[68] Kawabata, J.; Fukushi, Y.; Tahara, S.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae. Part 7. Shizukaol A, a sesquiterpene dimer from Chloranthus japonicus. Phytochemistry1990, 29, 2332–2334.10.1016/0031-9422(90)83065-9Suche in Google Scholar
[69] Kawabata, J.; Fukushi, E.; Mizutani, J. Sesquiterpene dimers from Chloranthus japonicus. Phytochemistry1995, 39, 121–125.10.1016/0031-9422(94)00865-QSuche in Google Scholar
[70] Kawabata, J.; Fukushi, E.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae Plants. Part II. Sesquiterpene dimer and trimer from Chloranthus japonicus. Phytochemistry1997, 47, 231–235.10.1016/S0031-9422(97)00419-6Suche in Google Scholar
[71] Fang, P.-L.; Cao, Y.-L.; Yan, H.; Pan, L.-L.; Liu, S.-C.; Gong, N.-B.; Lu, Y.; Chen, C.-X.; Zhong, H.-M.; Guo, Y.; et al. Lindenane disesquiterpenoids with anti-HIV-1 activity from Chloranthus japonicus. J. Nat. Prod.2011, 74, 1408–1413.10.1021/np200087dSuche in Google Scholar PubMed
[72] Xu, Y.-J.; Tang, C.-P.; Ke, C.-Q.; Zhang, J.-B.; Weiss, H.-C.; Gesing, E.-R.; Ye, Y. Mono- and Di-sesquiterpenoids from Chloranthus spicatus. J. Nat. Prod.2007, 70, 1987–1990.10.1021/np070433gSuche in Google Scholar PubMed
[73] Li, C.-J.; Zhang, D.-M.; Luo, Y.-M.; Yu, S.-S.; Li, Y.; Lu, Y. Bis-sesquiterpenes and diterpenes from Chloranthus henryi. Phytochemistry2008, 69, 2867–2874.10.1016/j.phytochem.2008.08.022Suche in Google Scholar PubMed
[74] Kim, S.-Y.; Kashiwada, Y.; Kawazoe, K.; Murakami, K.; Sun, H.-D.; Li, S.-L.; Takaishi, Y. Spicachlorantins A and B, new dimeric sesquiterpenes from the roots of Chloranthus spicatus. Phytochem. Lett.2009, 2, 110–113.10.1016/j.phytol.2009.03.001Suche in Google Scholar
[75] Kim, S.-Y.; Kashiwada, Y.; Kawazoe, K.; Murakami, K.; Sun, H.-D.; Li, S.-L.; Takaishi, Y. Spicachlorantins C-F, hydroperoxy dimeric sesquiterpenes from the roots of Chloranthus spicatus. Tetrahedron Lett.2009, 50, 6032–6035.10.1016/j.tetlet.2009.08.026Suche in Google Scholar
[76] Kim, S.-Y.; Kashiwada, Y.; Kawazoe, K.; Murakami, K.; Sun, H.-D.; Li, S.-L.; Takaishi, Y. Spicachlorantins G-J, new lindenane sesquiterpenoid dimers from the roots of Chloranthus spicatus. Chem. Pharm. Bull.2011, 59, 1281–1284.10.1248/cpb.59.1281Suche in Google Scholar PubMed
[77] Yang, S.-P.; Yue, J.-M. Chloramultilide A, a highly complex sesquiterpenoid dimmer from Chloranthus multistachys. Tetrahedron Lett.2006, 47, 1129–1132.10.1016/j.tetlet.2005.12.023Suche in Google Scholar
[78] Zhang, S.; Yang, S.-P.; Yuan, T.; Lin, B.-D.; Wu, Y.; Yue, J.-M. Multistalides A and B, two novel sesquiterpenoid dimers from Chloranthus multistachys. Tetrahedron Lett.2010, 51, 764–766.10.1016/j.tetlet.2009.12.001Suche in Google Scholar
[79] Ran, X.-H.; Teng, F.; Chen, C.-X.; Wei, G.; Hao, X.-J.; Liu, H.-Y. Chloramultiols A-F, lindenane-type sesquiterpenoid dimers from Chloranthus multistachys. J. Nat. Prod.2010, 73, 972–975.10.1021/np900764nSuche in Google Scholar
[80] Kawabata, J.; Mizutani, J. Studies on the chemical constituents of Chloranthaceae plants. Part 8. Dimeric sesquiterpenoid esters from Chloranthus serratus. Phytochemistry1992, 31, 1293–1296.10.1016/0031-9422(92)80281-ISuche in Google Scholar
[81] Kawabata, J.; Fukushi, E.; Mizutani, J. Chloranthaceae plants. Part 9. Symmetric sesquiterpene dimer from Chloranthus serratus. Phytochemistry1993, 32, 1347–1349.10.1016/S0031-9422(00)95119-7Suche in Google Scholar
[82] Yuan, T.; Zhu, R.-X.; Yang, S.-P.; Zhang, H.; Zhang, C.-R.; Yue, J.-M. Serratustones A and B representing a new dimerization pattern of two types of sesquiterpenoids from Chloranthus serratus. Org. Lett.2012, 14, 3198–3201.10.1021/ol301306eSuche in Google Scholar PubMed
[83] Wang, X.-C.; Zhang, Y.-N.; Wang, L.-L.; Ma, S.-P.; Liu, J.-H.; Hu, L.-H. Lindenane sesquiterpene dimers from Chloranthus fortunei. J. Nat. Prod.2008, 71, 674–677.10.1021/np7007544Suche in Google Scholar PubMed
[84] Yang, S.-P.; Gao, Z.-B.; Wang, F.-D.; Liao, S.-G.; Chen, H.-D.; Zhang, C.-R.; Hu, G.-Y.; Yue, J.-M. Chlorahololides A and B, two potent and selective blockers of the potassium channel isolated from Chloranthus holostegius. Org. Lett.2007, 9, 903–906.10.1021/ol0700759Suche in Google Scholar PubMed
[85] Yang, S.-P.; Gao, Z.-B.; Wu, Y.; Hu, G.-Y.; Yue, J.-M. Chlorahololides C-F: a new class of potent and selective potassium channel blockers from Chloranthus holostegius. Tetrahedron2008, 64, 2027–2034.10.1016/j.tet.2007.12.057Suche in Google Scholar
[86] Wu, B.; Chen, J.; Qu, H.; Cheng, Y. Complex sesquiterpenoids with tyrosinase inhibitory activity from the leaves of Chloranthus tianmushanensis. J. Nat. Prod.2008, 71, 877–880.10.1021/np070623rSuche in Google Scholar PubMed
[87] Wang, P.; Luo, J.; Zhang, Y.-M.; Kong, L.-Y. Sesquiterpene dimers esterified with diverse small organic acids from the seeds of Sarcandra glabra. Tetrahedron2015, 71, 5362–5370.10.1016/j.tet.2015.05.112Suche in Google Scholar
[88] He, X.-F.; Zhang, S.; Zhu, R.-X.; Yang, S.-P.; Yuan, T.; Yue, J.-M. Sarcanolides A and B: Two sesquiterpenoid dimers with a nonacyclic scaffold from Sarcandra hainanensis. Tetrahedron2011, 67, 3170–3174.10.1016/j.tet.2011.03.021Suche in Google Scholar
[89] Zhang, M.; Wang, J.; Luo, J.; Wang, P.; Guo, C.; Kong, L. Labdane diterpenes from Chloranthus serratus. Fitoterapia2013, 91, 95–99.10.1016/j.fitote.2013.08.015Suche in Google Scholar PubMed
[90] Yang, S.-P.; Yue, J.-M. Terpenoids from Chloranthus multistachys. Nat. Prod. Res.2008, 22, 1163–1168.10.1080/14786410601133434Suche in Google Scholar PubMed
[91] Wang, L. J.; Xiong, J.; Liu, S. T.; Pan, L. L.; Yang, G. X.; Hu, J. F. ent-Abietane-type and related seco-/nor-diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius and their antineuroinflammatory activities. J. Nat. Prod.2015, 78, 1635–1646.10.1021/acs.jnatprod.5b00195Suche in Google Scholar PubMed
[92] Luo, Y. M.; Liu, A. H.; Zhang, D. M.; Huang, L. Q. Two new triterpenoid saponins from Sarcandra glabra. J. Asian Nat. Prod. Res.2005, 7, 829–834.10.1080/10286020410001721104Suche in Google Scholar PubMed
[93] Luo, Y.; Liu, A.; Yu, B.; Kang, L.; Huang, L. Studies on chemical constituents of Sarcandra glabra. Chin. Pharm. J.2005, 40, 1296–1298.10.2991/icimm-15.2015.43Suche in Google Scholar
[94] Acebey, L.; Sauvain, M.; Beck, S.; Moulis, C.; Gimenez, A.; Jullian, V. Bolivianine, a new sesterpene with an unusual skeleton from Hedyosmum angustifolium, and its isomer, isobolivianine. Org. Lett.2007, 9, 4693–4696.10.1021/ol7015725Suche in Google Scholar PubMed
[95] Gao, C.; Chen, Y.; Xie, J.; Zhao, S. Chemical constituents of Chloranthus holostegius (Hand-Mazz.) Pei et. Shan. Chemical J. Chin. Univs.1987, 8, 141–142.Suche in Google Scholar
[96] Huang, M.; Li, Y.; Zeng, G.; Yuan, W.; Tan, J.; Tan, G.; Zhou, Y. Chemical constituents of Sarcandra glabra. Central South Pharmacy2007, 5, 459–461.Suche in Google Scholar
[97] Xu, X.; Hu, X.; Yuan, J.; Yang, J. Studies on chemical constituents of Sarcandra glabra. China J. Chin. Mater. Medica2008, 33, 900–902.Suche in Google Scholar
[98] Zhu, L.; Li, Y.; Yang, J.; Zuo, L.; Zhang, D. Studies on chemical constituents of Sarcandra glabra. China J. Chin. Mater. Medica2008, 33, 155–157.Suche in Google Scholar
[99] Kuang, H.-x.; Xia, Y.-g.; Yang, B.-y.; Wang, Q.-h.; Lu, S.-w. Lignan constituents from Chloranthus japonicus Sieb. Arch. Pharmacal Res.2009, 32, 329–334.10.1007/s12272-009-1303-1Suche in Google Scholar PubMed
[100] Wang, C.; Zhu, L.; Yang, J.; Li, C.; Zhang, D. Chemical constituents from Sarcandra glabra. China J. Chin. Mater. Medica2010, 35, 714–717.10.4268/cjcmm20100612Suche in Google Scholar
[101] Wu, H.; Hu, X.; Zhang, X.; Chen, S.; Yang, J.; Xu, X. Benzyl 2-beta-glucopyranosyloxybenzoate, a new phenolic acid glycoside from Sarcandra glabra. Molecules2012, 17, 5212–5218.10.3390/molecules17055212Suche in Google Scholar PubMed PubMed Central
[102] Huang, M.; Zeng, G.; Tan, J.; Li, Y.; Tan, G.; Zhou, Y. Studies on flavonoid glycosides of sarcandra glabra. China J. Chin. Mater. Medica2008, 33, 1700–1702.Suche in Google Scholar
[103] Li, B.; Huang, M.-j.; Li, Y.-l.; Zeng, G.-y.; Tan, J.-b.; Zhou, Y.-j. Antioxidant constituents of Sarcandra glabra (Thunb.) Nakai. Shenyang Yaoke Daxue Xuebao2009, 26, 900–903, 910.Suche in Google Scholar
[104] Duan, Y.; Dai, Y.; Gao, H.; Ye, W.; Yao, X. Chemical constituents of Sarcandra glabra. Chin. Trad. Herb. Drugs2010, 41, 29–32.Suche in Google Scholar
[105] Li, X.; Zhang, Y.; Yang, L.; Feng, Y.; Liu, Y.; Zeng, X. Studies of phenolic acid constituents from the whole plant of Sarcandra glabra. Trad. Chin. Drug Res. Clini. Pharm.2012, 23, 295–298.Suche in Google Scholar
[106] Soltis, D. E.; Bohm, B. A. Flavonoids of Ascarina lucida. J. Nat. Prod.1982, 45, 415–417.10.1021/np50022a009Suche in Google Scholar
[107] Li, Y.; Zhang, D. M.; Yu, S. S.; Li, J. B.; Luo, Y. M. A novel phenylpropanoid-substituted catechin glycoside and a new dihydrochalcone from Sarcandra glabra. Chin. Chem. Lett.2006, 17, 207–210.Suche in Google Scholar
[108] Zou, X.; Gao, H.; Wu, B.; Wang, Y.; Wang, S.; Yang, S.; Wu, L. Chemical components of Herba Sarcandrae. Chin. Trad. Herb. Drugs2007, 38, 354–356.Suche in Google Scholar
[109] Yuan, K.; Zhu, J.; Si, J.; Cai, H.; Ding, X.; Pan, Y. Studies on chemical constituents and antibacterial activity from n-butanol extract of Sarcandra glabra. China J. Chin. Mater. Medica2008, 33, 1843–1846.Suche in Google Scholar
[110] Cao, C.; Xu, L.; Chen, K.; Peng, Y.; Xiao, P. Chemical study on petroleum ether portion of Sarcandra hainanensis. China J. Chin. Mater. Medica2009, 34, 1009–1010.Suche in Google Scholar
[111] Cao, C.-M.; Peng, Y.; Xu, L.-J.; Wang, Y.-J.; Yang, J.-S.; Xiao, P.-G. Two flavonoid dimers from Sarcandra hainanensis (Pei) Swamy et Bailey. Chem. Pharm. Bull.2009, 57, 743–746.10.1248/cpb.57.743Suche in Google Scholar PubMed
[112] Cao, C.-M.; Xu, L.-J.; Peng, Y.; Shi, Q.-W.; Xiao, P.-G. Two new flavan-flavanones from Sarcandra hainanensis. Chem. Pharm. Bull.2010, 58, 1395–1398.10.1248/cpb.58.1395Suche in Google Scholar PubMed
[113] Takemoto, T.; Uchida, M.; Koike, K.; Hoshina, Y.; Kusano, G. Constituents of Chloranthus species. I. Structures of two new amides from Chloranthus serratus and the isolation of isofraxidin from C. japonicus. Chem. Pharm. Bull.1975, 23, 1161–1163.10.1248/cpb.23.1161Suche in Google Scholar
[114] Tong, S.; Huang, J.; Wang, B.; Yan, J. Chemical constituents of Sarcandra glabra. Zhongcaoyao2010, 41, 198–201.Suche in Google Scholar
[115] Ran, X.; Ni, W.; Wei, G.; Chen, C.; Liu, H. A new phenolic glycoside from Chloranthus multistachys (Chloranthaceae). Acta Botanica Yunnanica2010, 32, 83–86.10.3724/SP.J.1143.2010.00083Suche in Google Scholar
[116] Hu, X.-R.; Wu, H.-F.; Zhang, X.-P.; Yang, J.-S.; Dai, Z.; Lin, R.-C.; Xu, X.-D. A new sesquiterpene lactone from Sarcandra glabra. Nat. Prod. Res.2013, 27, 1197–1201.10.1080/14786419.2012.722084Suche in Google Scholar PubMed
[117] Fu, H. Z.; Luo, Y. M.; Liu, H.; Zhang, D. M.; Ji, T. F. A new tetrahydrophenanthrene from Chloranthus henryi. Chin. Chem. Lett.2010, 21, 206–208.10.1016/j.cclet.2009.10.005Suche in Google Scholar
[118] Lee, Y. M.; Moon, J. S.; Yun, B.-S.; Park, K. D.; Choi, G. J.; Kim, J.-C.; Lee, S. H.; Kim, S. U. Antifungal activity of CHE-23C, a dimeric sesquiterpene from Chloranthus henryi. J. Agric. Food Chem.2009, 57, 5750–5755.10.1021/jf900674ySuche in Google Scholar PubMed
[119] Kang, J.-f.; Zhang, Y.; Du, Y.-l.; Wang, Z.-z. Chemical composition and antimicrobial activity of the essential oils from Chloranthus japonicus Sieb. and Chloranthus multistachys Pei. Z. Naturforsch., C J. Biosci.2010, 65, 660–666.10.1515/znc-2010-11-1205Suche in Google Scholar PubMed
[120] Liu, L.; Mu, Q.; Li, W.; Xing, W.; Zhang, H.; Fan, T.; Yao, H.; He, L. Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes. Immunobiology2015, 220, 406–413.10.1016/j.imbio.2014.10.007Suche in Google Scholar PubMed
[121] Niu, X.; Wang, Y.; Li, W.; Mu, Q.; Li, H.; Yao, H.; Zhang, H. Protective effects of Isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol.2015, 24, 432–439.10.1016/j.intimp.2014.12.041Suche in Google Scholar PubMed
[122] Wang, W.; Chen, B.; Tu, X.; Tan, S.; Liu, Z.; Fu, J.; Zou, R.; Lu, H. Codonolactone, a sesquiterpene lactone isolated from Chloranthus henryi Hemsl, inhibits breast cancer cell invasion, migration and metastasis by downregulating the transcriptional activity of Runx2. Int. J. Oncol.2014, 45, 1891–1900.10.3892/ijo.2014.2643Suche in Google Scholar PubMed
[123] Kwon, O. E.; Lee, H. S.; Lee, S. W.; Bae, K.; Kim, K.; Hayashi, M.; Rho, M.-C.; Kim, Y.-K. Dimeric sesquiterpenoids isolated from Chloranthus japonicus inhibited the expression of cell adhesion molecules. J. Ethnopharmacol.2006, 104, 270–277.10.1016/j.jep.2005.09.018Suche in Google Scholar PubMed
[124] Hu, R.; Yan, H.; Hao, X.; Liu, H.; Wu, J. Shizukaol D isolated from Chloranthus japonicas inhibits AMPK-dependent lipid content in hepatic cells by inducing mitochondrial dysfunction. PLoS One2013, 8, e73527.10.1371/journal.pone.0073527Suche in Google Scholar PubMed PubMed Central
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines
Artikel in diesem Heft
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines

