Abstract
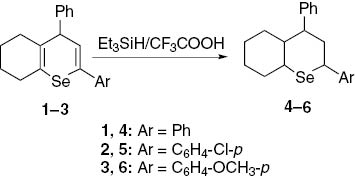
The reaction of 2-aryl-4-phenyl-5,6,7,8-tetrahydro-4H-selenochromenes 1–3 with trifluoroacetic acid and triethylsilane furnished a novel class of compounds, 2-aryl-4-phenyloctahydroselenochromenes.
Introduction
Selenium is an important ultramicroelement and its shortage in animal and people nutrition can lead to a variety of diseases [1], [2], [3]. Selenium-containing compounds have a wide spectrum of practical significancy [4], [5]. Previously, we have synthesized 2-aryl-4-phenyl-5,6,7,8-tetrahydro-4H-selenochromenes 1–3 [6]. In continuation of that work, we now report reduction of compounds 1–3 that does not lead to selenium elimination [7], [8] and furnishes the corresponding 2,4-diphenyloctahydroselenochromenes 4–6. The reaction is carried out in the presence of triethylsilane and trifluoroacetic acid (Scheme 1).
The diphenyloctahydroselenochromene 4 is composed of three isomers (Figure 1) that give three well-separated signals with different retention times (21.01, 21.58 and 22.05 min on a chromatogram and show identical molecular ion peaks at m/z 356 for isotope 80Se in their mass spectra. It can be suggested that these are geometrical isomers that differ in the configuration at the ring junction but exhibit identical, most stable pseudo-equatorial conformation of the aryl substituents. Nevertheless, the given stereochemistry of 4a–c is tentative and, at the present time, it is not possible to assign a physical characteristic, such as retention time, to a particular isomer.

The suggested isomers 4a–c, 5a–d, 6a.
In a similar way, a 4-chlorophenyl derivative is a mixture of four geometrical stereoisomers 5a, 5b, 5c, 5d. Accordingly, the signals with different retention times (25.51, 26.22, 26.44 and 27.20 min) can be observed in the chromatogram and the mass spectra show identical molecular ion peak at m/z 390 for isotope 80Se in each case.
The compound 2-(4-methoxyphenyl)-4-phenyloctahydroselenochromene (6) has only one isomer tentatively assigned the structure 6a (Figure 1). Only one signal with retention time of 26.63 min can be observed in the chromatogram and the mass spectrum shows a molecular ion peak at m/z 386 for isotope 80Se.
Experimental
A mixture of a 2-aryl-4-phenyl-5,6,7,8-tetrahydro-4H-selenochromene (1–3, 0.001 mol), triethylsilane (0.3 mL) and trifluoroacetic acid (0.37 mL), prepared in that order, was stirred biefly in the air and treated with diethyl ether (10 mL). The resultant homogeneous solution was washed with water to the neutral reaction and concentrated in the air to give the 2-aryl-4-phenyloctahydroselenochromene 4–6. This synthesis was monitored by a capillary gas-liquid partition chromatography using mass-selective detector Agilent 5973: Tinjector=200°C, tinitial=3 min, Tinitial=50°C, Tend=280°C, gradient ΔT=10°C/min, carrier gas – helium, ν=1 mL/min. The 1H NMR spectra were recorded in deuteriochloroform on a Bruker AV instrument operating at 600 MHz.
2,4-Diphenyloctahydroselenochromene (4)
This compound was obtained in 79% yield as a brown powder; mp 115–117°C; 1H NMR: δ 1.08–1.17 (m, 2H), 1.27 (m, 1H), 1.53 (dd, 1H, J = 7.1 Hz and 6.0 Hz), 1.62 (m, 2H), 1.86 (m, 1H), 1.92–1.99 (m, 4H), 2.58 (q, 1H, J = 8.0 Hz), 3.10–3.21 (m, 1H), 7.24–7.40 (m, 10H); MS (EI): m/z 356 (M+). Anal. Calcd for C21H24Se: C, 70.79; H, 6.74. Found: C, 71.25; H, 7.17.
2-(4-Chlorophenyl)-4-phenyloctahydroselenochromene (5)
This compound was obtained in 73% yield as brown powder; mp 42–44°C; 1H NMR: δ 1.04–1.15 (m, 2H), 1.25 (m, 1H), 1.46 (m, 1H), 1.49 (m, 4H), 1.72 (m, 2H), 1.86 (m, 1H), 2.55 (q, 1H, J = 16.0 Hz), 2.90 (m, 1H), 7.18–7.36 (m, 9H); MS (EI): m/z 390 (M+). Anal. Calcd for C21H23SeCl: C, 64.62; H, 5.90. Found: C, 65.03; H, 6.46.
2-(4-Methoxyphenyl)-4-phenyloctahydroselenochromene (6)
This compound was obtained in 84% yield as a brown powder; mp 100–102°C; 1H NMR: δ 1.14 (m, 2H), 1.19–1.30 (m, 1H), 1.42 (m, 1H), 1.65 (m, 2H), 1.77–2.2 (m, 4H), 2.08 (m, 1H), 2.40 (m, 1H), 2.96–3.30 (m, 1H), 3.77 (s, 3H), 6.51 (d, 1H, J = 8.0 Hz), 6.99 (d, 1H, J = 8.0 Hz), 7.15–7.43 (m, 7H); MS (EI): m/z 386 (M+). Anal. Calcd for C22H26SeO: C, 68.39; H, 6.74. Found: C, 68.95; H, 7.23.
Supplementary material: The mass spectra of all isomers of compounds 4–6 observed by gas chromatography are given in the online supplementary material.
References
[1] Stadtman, T. C. Tracing the role of a trace element in protein function. PLoS Biology. 2005, 3, 2077–2079.10.1371/journal.pbio.0030421Suche in Google Scholar PubMed PubMed Central
[2] Yu, F.; Zhang, Y.; Wang, B.; Long, J.; Huang, D.; Liu, J. Effect of selenium exposure on the immunological function in mice. Huanjing Yu Zhiye Yixue. 2006, 23, 38–40.Suche in Google Scholar
[3] Al-Saleh, I.; Billedo, G.; El-Doush, I.; El-Din, M. G.; Yosef, G. Selenium and vitamins status in Saudi children. Clinica Chimica Acta. 2006, 368, 99–109.10.1016/j.cca.2005.12.025Suche in Google Scholar PubMed
[4] Dong, M; Fu, S.; Liu, S.; Xu, J.; Huang, C. One-pot synthesis of CdSe quantum dots in aqueous solution for biological labeling. J. Chin. Chem. Soc. 2013, 60, 1328–1332.10.1002/jccs.201300260Suche in Google Scholar
[5] Wei-jun, F.; Mei, Z.; Guang-long, Z. Synthesis of 3,3-disubstituted oxindoles by organoselenium-induced radical cyclizations of N-arylacrylamides. Heterocycl. Commun.2015, 21, 9–12.10.1515/hc-2014-0195Suche in Google Scholar
[6] Direnko, D. Y.; Drevko, Y. B.; Drevko, B. I. The synthesis of new organoselenium heterocyclic compounds: 2-aryl-4-phenyl-5,6,7,8-tetrahydro-4H-selenochromenes. J. Chin. Chem. Soc. 2015, 62, 1068–1071.10.1002/chin.201619169Suche in Google Scholar
[7] Blinohvatov, A. F.; Markovceva, O. V.; Nefedova, N. A.; Kharchenko, V. G.; Parnes, Z. N. The ionic hydrogenation of the sim-octahydroselenoksanten. Chem. Heterocycl. Compd. 1981, 4, 564.10.1002/chin.198137249Suche in Google Scholar
[8] Drevko, B. I.; Almaeva, A. F.; Isaev, I. N.; Mandych, V. G.; Uchaeva, I. M. A ionic hydrogenation of aryl-substituted 4H-selenopyrans. Chem. Heterocycl. Compd. 2009, 45, 123–124.10.1007/s10593-009-0239-1Suche in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/hc-2016-0076) offers supplementary material, available to authorized users.
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines
Artikel in diesem Heft
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines

