Abstract
Readily prepared copper nanoparticles are an effective catalyst for 1,3-dipolar cycloaddition of carbohydrate azide and a variety of alkynes that furnishes the corresponding 1,2,3-triazole-sucrose derivatives in excellent yields. Products were screened for their antimycobacterial activity against Mycobacterium tuberculosisH37Rv strain. Two compounds, 3b,c, demonstrate significant growth inhibitory activity against the bacterial strain with a MIC of 3.125 mg/mL. The presence of an amide group on the 1,2,3-triazole ring enhances the inhibition activity of the molecules. The active compounds are not toxic to a normal cell line which signifies the lack of general cellular toxicity of these compounds.
Introduction
Tuberculosis caused by Mycobacterium tuberculosis still remains the leading cause of worldwide deaths among infectious diseases. The World Health Organization reported that more than one-third of the world’s population was infected with tuberculosis, which resulted in an estimated 1.5 million deaths worldwide in 2013 [1]. The long duration of therapy generally is due to the nonconformity of the treatment and to the extensively drug-resistant tuberculosis, which is highly lethal, extremely expensive and complicated to treat, posing new challenges for the prevention, treatment and control of tuberculosis [2].
Ferreria and coworkers [3] have reported that 1,4-disubstituted 1,2,3-triazole derivatives A and B (Figure 1) exhibit good inhibitory activities against MTB H37Rv. Similarly, 1,2,3-triazoles C show inhibitory activities against H37Rv [4].
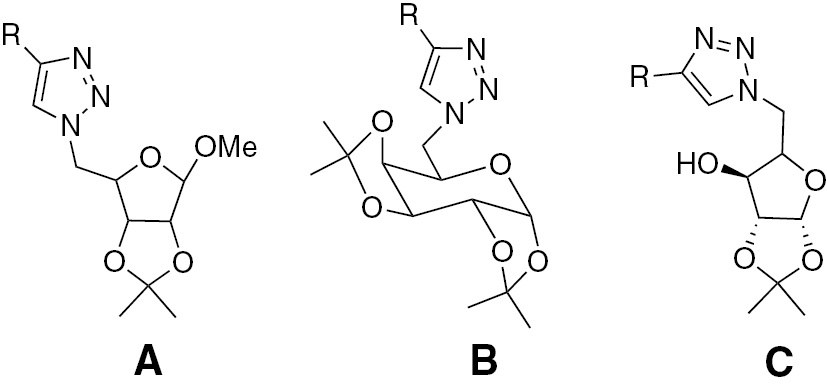
1,2,3-Triazoles A–C with antitubercular activity.
The catalyzed azide-alkyne cycloaddition reaction has found extensive application in the preparation of carbohydrate derivatives [5]. This method, developed by Meldal [6] and Sharpless [7], has became an important tool in organic synthesis due to a regioselective formation of 1,4-disubstituted 1,2,3-triazoles [8]. Moreover, the use of water as a solvent makes this reaction very attractive [9]. Recently, significant attention also has been paid to the use of copper nanoparticles (CuNPs) as catalysts in ‘CuAAC’ reactions in the synthesis of 1,2,3-triazoles. However, most of these reactions require organic solvents and high loading of CuNPs [10], [11], [12]. We wish to present herein the applicability of a copper nanoparticles for the click synthesis of new 1,2,3-triazole derivatives of carbohydrates in water.
Results and discussion
The cycloaddition of 1-prop-2-ynyl-pyrrolidine-2,5-dione 1a with carbohydrate azide 2 [13] in acetonitrile afforded the desired triazole 3a under copper catalysis conditions (Scheme 1). The CuNPs was prepared by reducing CuSO4 with hydrazine according to literature procedures [14], [15]. The use of CuNPs and CuBr gave similar results. Then, the replacement of acetonitrile by water as a solvent substantially affected the yield (80%) of the reaction catalyzed by CuNPs which was completed (TLC) within 15 min, while the CuBr catalyzed reaction gave 3a in 60% yield under similar conditions. Encouraged by the efficiency of the reaction protocol described above, the scope of the reaction was examined with alkynes 1b–e. Corresponding triazoles 3b–e were obtained in good yields.
All the target molecules 3b–e were screened against M. tuberculosis H37RV (ATCC27294) using agar dilution method [16]. Their antimycobacterial activity was evaluated in terms of minimum inhibitory concentration (MIC) values. The MIC values of these compounds are in the range of 3.125–25 mg/mL. As can be seen from Figure 2, compounds 3a–c show potent anti-tubercular activity with MIC of 3.125 μg/mL each. The MIC values of these three compounds are comparable with that of the standard drug, ethambutol. Compound 3e shows moderate inhibition activity with MIC of 12.5 mg/mL.
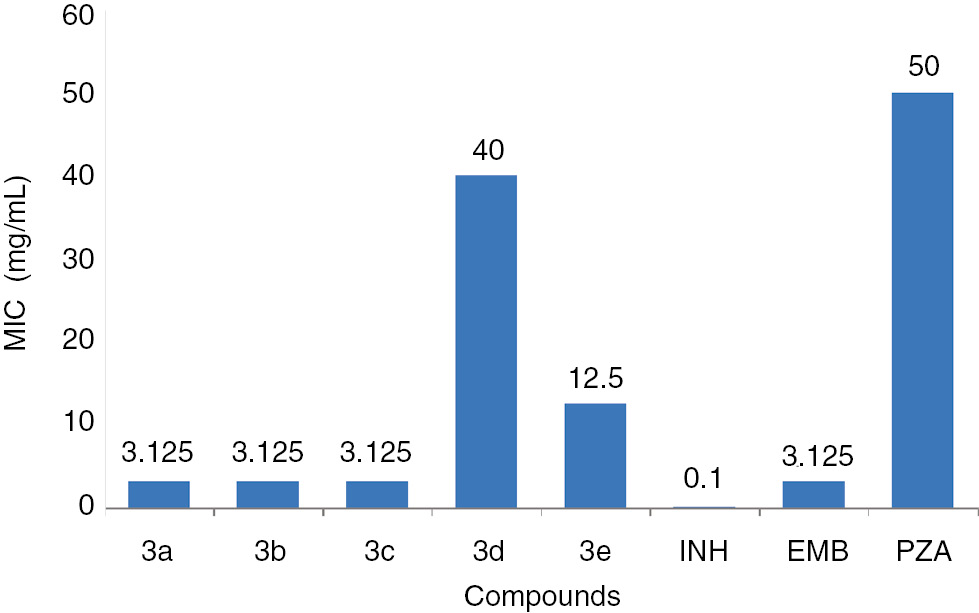
The antitubercular activity of 3a–e against M. tuberculosis H37RV (INH: isoniazid; EMB: ethambutol; PZA: pyrazinamide).
Conclusion
The 1,3-dipolar cycloaddition reaction, the so-called click reaction, between a terminal alkyne and a carbohydrate azide is catalyzed by copper nanoparticles. Water works well as a green solvent for this reaction.
Experimental
Flash chromatography was performed using silica gel Merck 60 (particle size 0.040–0.063 mm). All anhydrous reactions were performed under nitrogen using anhydrous solvents. NMR spectra were obtained on a Bruker AC 300 spectrometer operating at 300 MHz for 1H and at 75 MHz for 13C. Melting points were determined on a Buchi-510 capillary melting point apparatus. Chemical shifts are given in parts per million relative to tetramethylsilane (TMS). The spectra were recorded in CDCl3 as solvent at room temperature. Elemental analysis was recorded on a Perkin-Elmer 240B microanalyzer. Mass spectra were recorded on a Finnigan LCQ DECA XP plus spectrometer.
Synthesis of triazoles 3
To a solution azide 1 (160 mg, 0.5 mmol, 1 eq) in acetonitrile (12 mL) was added alkyne 2 (0.88 mmol), CuSO4·5H2O (12 mg, 0.05 mmol) and hydrazine hydrate (25 mg, 0.5 mmol). The mixture was microwave-irradiated at 100 W for 5 min. Reaction was monitored by TLC. After completion of the reaction, the mixture was passed through a celite pad and the filtrate was concentrated under a reduced pressure. The residue of 3 was purified on a silica gel column eluting with ethyl acetate.
1-((1-(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-Tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)pyrrolidine-2,5-dione (3a)
Yield 80%; white solid; mp 76°C; [α]22D +45° (c 1, CH2Cl2); Rf 0.4 (AcOEt); 1H NMR: δ 7.72 (s, 1H, H5), 5.49 (d, JH3a-8b = 4.8, 1H, H3a), 4.85–4.72 (m, 1H, H6), 4.63–53 (m, 2H, H8a, NCH), 4.43–4.92 (m, 2H, H8b, NCH), 4.16–4.14 (m, 2H, H5′, H5a), 2.73 (s, 4H, H7), 1.47 (s, 3H, CH3), 1.38 (s, 3H, CH3), 1.34 (s, 3H, CH3), 1.28 (s, 3H, CH3); 13C NMR: δ 175.3 (CO), 140.7 (C4), 113.3 (C5), 108.9 (Cisop), 108.1 (Cisop), 95.3 (C3a), 70.3 (C5a), 69.9 (C8a), 69.5 (C8b), 66.2 (C5′), 49.5 (CNH), 32.8 (C6), 27.2 (C7), 25.0, 24.9, 23.9, 23.5 (CH3). HRMS. Calcd for C18H24N4O7: m/z 408.1645. Found: m/z 408.1644. Anal. Calcd for C18H24N4O7: C, 52.94; H, 5.92; N, 13.72. Found: C, 52.92; H, 5.96; N, 13.70.
1-((1-(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-Tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)pyrrolidin-2-one (3b)
Yield 70%; white solid; mp 113°C; [α]22D +40° (c 1, CH2Cl2); Rf 0.3 (AcOEt); 1H NMR: δ 7.61 (s, 1H, H5), 5.42 (d, JH3a-8b = 4.8, 1H, H3a), 4.57–4.48 (m, 3H, H8a, NCH, H6), 4.43–4.32 (m, 1H, NCH), 4.25 (dd, JH8b-8a = 2.4, JH8b-3a = 4.8, 1H, H8b), 4.11–4.08 (m, 2H, H5′, H5a), 3.38 (t, 2H, H7), 2.32 (t, JH9-8 = 8.1, 2H, H9), 1.96–1.86 (m, 1H, H8), 1.41 (s, 3H, CH3), 1.31(s, 3H, CH3), 1.28 (s, 3H, CH3), 1.21 (s, 3H, CH3); 13C NMR: δ 174.7 (CO), 140.7 (C4), 123.7 (C5), 109.9 (Cisop), 109.0 (Cisop), 96.2 (C3a), 71.2 (C5a), 70.8 (C8a), 70.4 (C8b), 67.2 (C5′), 50.5 (CNH), 47.0 (C7), 37.8 (C6), 30.8 (C9), 25.9, 25.9, 24.8, 24.4 (CH3), 17.8 (C8). HRMS. Calcd for C18H26N4O6: m/z 394.1852. Found: m/z 394.1855. Anal. Calcd for C18H26N4O6: C, 54.81; H, 6.64; N, 14.20. Found: C, 54.84; H, 6.68; N, 14.18.
1-((1-(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-Tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)piperidine-2,6-dione (3c)
Yield 75%; white solid; mp 147°C; [α]22D +71° (c 1, CH2Cl2); Rf 0.2 (cyclohexane/AcOEt 3:7); 1H NMR: δ 7.69 (s, 1H, H5), 5.52 (d, JH3a-H8b = 5.1, 1H, H3a), 5.16–5.01 (m, 2H, H6), 4.65–4.55 (m, 2H, H8a, NCH), 4.42–4.34 (m, 1H, NCH), 4.31 (dd, JH8b-8a = 2.7, JH8b-3a = 5.1, 1H, H8b), 4.18–4.15 (m, 2H, H5′, H5a), 2.66 (t, JH5-H6 = 6.6, 4H, H7), 1.97–1.93 (m, 2H, H8), 1.49 (s, 3H, CH3), 1.40 (s, 3H, CH3), 1.36 (s, 3H, CH3), 1.30 (s, 3H, CH3); 13C NMR: δ 171.5 (CO), 142.6 (C4), 123.8 (C5), 109.4 (Cisop), 108.5 (Cisop), 95.7 (C3a), 70.6 (C5a), 70.2 (C8a), 69.5 (C8b), 66.6 (C5′), 49.9 (CNH), 33.9 (C6), 32.2 (C7), 29.2, 25.5, 24.4, 23.9 (CH3), 16.5 (C8). HRMS. Calcd for C19H26N4O7: m/z 422.1802. Found: m/z 422.1804. Anal. Calcd for C19H26N4O7: C, 54.02; H, 6.20; N, 13.26. Found: C, 54.04; H, 6.17; N, 13.23.
4-(Pyrrolidin-1-ylmethyl)-1-(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)methyl)-1H-1,2,3-triazole (3d)
Yield 77%; white solid; mp 110°C; [α]22D +65° (c 1, CH2Cl2); Rf 0.05 (AcOEt); 1H NMR: δ 7.67 (s, 1H, H5), 5.52 (d, JH3a-H8b = 5.1, 1H, H3a), 4.65–4.59 (m, 2H, H8a, NCH), 4.47–4.40 (m, 1H, NCH), 4.33 (dd, JH8b-8a = 2.4, JH8b-3a = 5.1, 1H, H8b), 4.19–4.15 (m, 2H, H5′, H5a), 3.82 (d, JH6-H5 = 1.8, 2H, H6), 2.59–2.56 (m, 4H, H7), 1.83–1.77 (m, 4H, H8), 1.50 (s, 3H, CH3), 1.39 (s, 3H, CH3), 1.36 (s, 3H, CH3), 1.30 (s, 3H, CH3); 13C NMR: δ 145.1 (C4), 123.5 (C5), 109.8 (Cisop), 109.0 (Cisop), 96.2 (C3a), 71.2 (C5a), 70.8 (C8a), 70.4 (C8b), 67.3 (C5′), 53.6 (C7), 50.5 (C6), 50.4 (CNH), 25.9, 25.9, 24.8, 24.4 (CH3), 23.5 (C8). HRMS. Calcd for C18H28N4O5: m/z 308.2060. Found: m/z 308.2064. Anal. Calcd for C18H28N4O5: C, 56.83; H, 7.42; N, 14.73. Found: C, 56.87; H, 7.39; N, 14.69.
1-((1-(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-Tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)piperidine 3e
Yield 72%; white solid; mp 130°C; [α]22D +36° (c 1, CH2Cl2); Rf 0.1 (AcOEt); 1H NMR: δ 7.69 (s, 1H, H5), 5.51 (d, JH3a-H8b = 4.8, 1H, H3a), 4.64–4.61 (m, 2H, H8a, NCH), 4.48–4.41 (m, 1H, NCH), 4.32 (dd, JH8b-8a = 2.4, JH8b-3a = 4.8, 1H, H8b), 4.18–4.16 (m, 2H, H5′, H5a), 2.47 (s, 2H, H6), 1.58–1.56 (m, 22H, H7, H8, H9, 4 CH3); 13C NMR: δ 144.5 (C4), 123.9 (C5), 109.8 (Cisop), 108.9 (Cisop), 96.2 (C3a), 71.2 (C5a), 70.8 (C8a), 70.5 (C8b), 67.4 (C5′), 53.2 (C6), 50.5 (NCH), 26.6, 24.8, 24.4 (CH3), 29.6, 25.9, 24.1 (C7, C8, C9). HRMS. Calcd for C19H30N4O5: m/z 394.2216. Found: m/z 394.2219. Anal. Calcd for C19H30N4O5: C, 57.85; H, 7.67; N, 14.20. Found: C, 57.82; H, 7.72; N, 14.24.
Antitubercular studies
A standard methodology, recommended by the National Committee for Clinical Laboratory Standards, USA, for the determination of MIC, was used. The assays were conducted in triplicate.
References
[1] Global tuberculosis control: WHO report, 2014.Search in Google Scholar
[2] Young, D. B.; Perkins, M. D.; Duncan, K.; Barry, C. E. Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Invest. 2008, 118, 1255–1265.10.1172/JCI34614Search in Google Scholar
[3] Sabrina, B. F.; Ana, C. R. S.; Mariana, F. C. C.; Emerson, S. L.; Carlos, R. K.; Floriano, P. S.; Vitor, F. F. Synthesis, biological activity, and molecular modeling studies of 1H-1,2,3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J. Med. Chem.2010, 53, 2364–2375.10.1021/jm901265hSearch in Google Scholar
[4] Biswajit, K. S.; Amit, K. Y.; Brijesh, K. A.; Gaikwa, S. K. S.; Vinita, C.; Rama, P. T. Preparation and reactions of sugar azides with alkynes: synthesis of sugar triazoles as antitubercular agents. Carbohydrate Res.2008, 343, 1153–1162.10.1016/j.carres.2008.02.013Search in Google Scholar
[5] Ge, X.; Qian, C.; Chen, X. Synthesis of novel carbohydrate-based valine-derived formamide organocatalysts by CuAAC click chemistry and their application in asymmetric reduction of imines with trichlorosilane. Tetrahedron Asym.2014, 25, 1450–1455.10.1016/j.tetasy.2014.10.003Search in Google Scholar
[6] Tornøe, C. W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064.10.1021/jo011148jSearch in Google Scholar
[7] Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4Search in Google Scholar
[8] Ali, A. A.; Chetia, M.; Saikia, B.; Saikia, P. J.; Sarma, D. AgN(CN)2/DIPEA/H2O-EG: a highly efficient catalytic system for synthesis of 1,4-disubstituted-1,2,3 triazoles at room temperature. Tetrahedron Lett. 2015, 56, 5892–5895.10.1016/j.tetlet.2015.09.025Search in Google Scholar
[9] Hajlaoui, K.; Ben Hamadi, N.; Msaddek, M. Copper nanoparticles cycloaddition of terminal acetylenes with carbohydrate azide. Catal. Lett. 2015, 145, 1246–1250.10.1007/s10562-015-1521-8Search in Google Scholar
[10] Kumar, A.; Aerry, S.; Saxena, A.; Deb, A.; Mozumdar, S. Copper nanoparticulates in Guar-gum: a recyclable catalytic system for the Huisgen [3 + 2]-cycloaddition of azides and alkynes without additives under ambient conditions. Green Chem. 2012, 14, 1298–1301.10.1039/c2gc35070jSearch in Google Scholar
[11] Hudson, R.; Li, C.-J.; Moores, A. Magnetic copper–iron nanoparticles as simple heterogeneous catalysts for the azide–alkyne click reaction in water. Green Chem. 2012, 14, 622–624.10.1039/c2gc16421cSearch in Google Scholar
[12] Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org. Biomol. Chem. 2011, 9, 6385–6395.10.1039/c1ob05735aSearch in Google Scholar PubMed
[13] Ben Hamadi, N.; Msaddek, M. Synthesis and reactivity of N-sugar-maleimides: an access to novel highly substituted enantiopure pyrazolines. Tetrahedron: Asymmetry2012, 23, 1689–1693.10.1016/j.tetasy.2012.11.005Search in Google Scholar
[14] Zhu, H. T.; Zhang, C. Y.; Yin, Y. S. Novel synthesis of copper nanoparticles: influence of the synthesis conditions on the particle size. Nanotechnology2005, 16, 3079–3083.10.1088/0957-4484/16/12/059Search in Google Scholar
[15] Huangdi, F.; Yuan, L.; Shengji, L.; Erik, V. V. E.; Gonghua, S. Nano Cu-catalyzed efficient and selective reduction of nitroarenes under combined microwave and ultrasound irradiation. Sustainable Chem. Proc.2014, 2, 14.10.1186/2043-7129-2-14Search in Google Scholar
[16] Jurupula, R.; Nagabhushana, N.; Udayakumar, D.; Perumal, Y.; Dharmarajan, S. One-pot synthesis of new triazole–imidazo[2,1-b][1,3,4]thiadiazole hybrids via click chemistry and evaluation of their antitubercular activity. Bioorg. Med. Chem. Lett.2015, 25, 4169–4173.10.1016/j.bmcl.2015.08.009Search in Google Scholar PubMed
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines
Articles in the same Issue
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines


