Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
Abstract
Small libraries of 1-(2-pyridyl)imidazolidin-2-one and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one derivatives were prepared from substituted pyridine or quinoline N-oxides and 2-chloro-4,5-dihydroimidazole by means of the α-ureation and α-amination reactions. The α-ureation reaction of pyridine and quinoline N-oxides was studied theoretically by means of quantum chemical calculations at the density functional theory level. The newly obtained compounds were screened for their potential in vitro cytotoxic activity against human tumor cell lines LCLC-103H, 5637 and A-427.
Introduction
N-Aryl(heteroaryl)ureas constitute an important structural component of many interesting biologically active compounds including anticancer [1–7], antiparasitic [8–10], antiviral [11], and antibacterial [12] agents, central nervous system active compounds [13, 14], anti-inflammatory [15] and plant growth regulating agents [16]. In view of the fragment based drug discovery concept [17–19], these low molecular weight chemical fragments conform to the ‘rule of three’: molecular weight ≤300, Clog p-value ≤3, the number of hydrogen bond donors, hydrogen bond acceptors and rotatable bonds ≤3, polar surface area ≤60 [20] and, therefore, can be considered as valuable building blocks of more complex lead compounds. By contrast, various biologically active cyclic ureas have been designed as the bioisosters of aryl amides [21], ureas [22], guanidines and thiones [23].
With the above information in mind, it was decided to develop a small library of N-(2-pyridyl)imidazolidin-2-one fragments to identify compounds that possess cytotoxic activity against selected human tumor cell lines. As shown in Figure 1, there are at least four possible synthetic approaches to the synthesis of N-(2-pyridyl)imidazolidine-2-ones. According to method A, a 2-aminopyridine is subjected to the reaction with 2-chloroethyl isocyanate (formation of urea derivative) followed by the treatment with base which gives rise to the imidazolidin-2-one ring formation [24, 25]. A more straightforward Goldberg-Buchwald-Nandakumar method B utilizes a 2-bromo- or 2-iodopyridine which is used for N-heteroarylation of amides, ureas and imidazolidin-2-ones in the presence of Pd or Cu catalyst [26–32]. According to method C, a corresponding ethylenediamine derivative is subjected to the reaction with carbonylation reagent such as phosgene, dialkyl carbonate or carbonyldiimidazole [24]. However, various substituted 2-aminopyridines as well as 2-bromo- and 2-iodopyridines are difficult to prepare [33], which makes the methods discussed above of limited value for the generation of libraries of azine-containing fragments. Therefore, we decided to take advantage of a poorly explored α-ureation method D that comprises the reaction of an azine (pyridine or quinoline) N-oxide with 2-chloro-4,5-dihydroimidazole [34, 35].
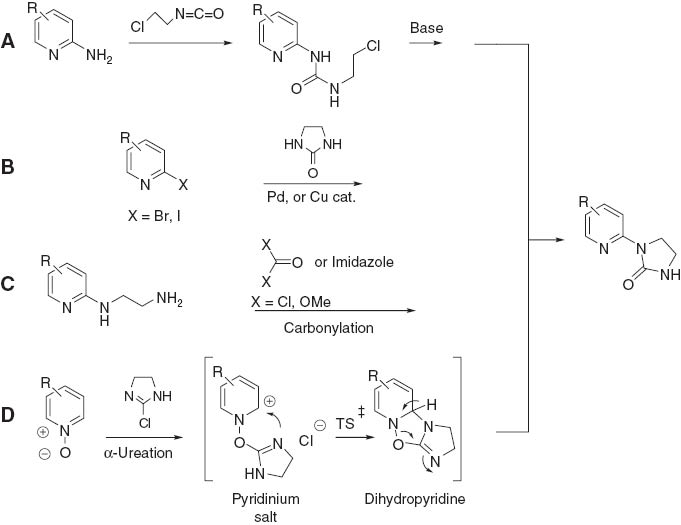
Possible routes to 1-aryl(heteroaryl)imidazolidin-2-ones.
The only products of α-ureation of pyridine N-oxides described in the literature are N-(6-methyl-2-pyridyl)-imidazolidin-2-one and 1,3-bis-(4-methyl-2-pyridyl)-imidazolidin-2-one obtained from 2-picoline N-oxide and 4-picoline N-oxide, respectively [35]. Hence, in the present paper we describe the synthesis of a small library of various substituted N-(2-pyridyl)imidazolidin-2-ones and the results of the theoretical study of the α-ureation process carried out by means of quantum chemical calculations at the density functional theory level. Preliminary data regarding cytotoxic properties of the prepared fragments against selected human tumor cell lines are also presented.
Results and discussion
Chemistry
In contrast to the previously described heteroarylation of 2-aminopyridine N-oxide [36], the α-ureation of alkyl-, alkoxy- and halo-substituted pyridine N-oxides 1 with 2-chloro-4,5-dihydroimidazole (2), a formal 2-chloroamidine derivative, led to the complex mixtures of products. As shown in Scheme 1, pyridinium salts of type A were found to be the major products that upon workup in aqueous solution give back the substrates 1 and N-(imidazolin-2-yl)imidazolidin-2-one (B). However, using preparative thin layer chromatography (chromatotron™) or column chromatography we were able to isolate the desired α-ureation products, that is, 1-monosubstituted imidazolidin-2-ones 3 and 1,3-disubstituted imidazolidin-2-ones 4 in 4–11% of isolated yield. Occasionally, from a reaction mixture we also separated 1-(2-pyridyl)-2,3,7,8-tetrahydroimidazo[1,2-b][1,3,5]-triazepin-5(6H)-ones 5 resulting from α-amination reaction of pyridine N-oxides with 1. The products isolated from each reaction mixture are presented in Table 1. In this context, it should be pointed out that a complex mixture of products was also obtained by Abramovitch and co-workers from a mechanistically related α-acylamination reaction of pyridine N-oxides with imidoyl chlorides [37, 38].

α-Ureation and α-amination of pyridine N-oxides 1 with 2-chloro-4,5-dihydroimidazole (2).
Products of α-ureation and α-amination of substituted pyridine N-oxides.
 |  |  |  | |
|---|---|---|---|---|
| 1 | R | 3 | 4 | 5 |
| a | 3-Me | a | – | – |
| b | 4-Me | b | b | – |
| c | 4-Et | c | c | c |
| d | 3,4-di-Me | d | – | d |
| e | 4-But | e | – | – |
| f | 4-Ph | f | f | – |
| g | 4-(CH2)3Ph | g | – | g |
| h | 2-OMe | h | – | – |
| i | 4-OMe | i | i | i |
| j | 2-OEt | j | – | – |
| k | 4-OEt | k | k | k |
| l | 4-OCH2Ph | – | l | l |
| m | 2-Me, 4-OMe | m | – | m |
| n | 2-OPrn | n | – | – |
| o | 4-OPrn | o | – | – |
| p | 2-OPri | p | – | – |
| q | 4-OPri | – | q | q |
| r | 2-Me, 4-OEt | r | – | – |
| s | 2-OBun | s | – | – |
| t | 4-OBun | t | – | – |
| u | 4-OCH2CH(Me)2 | u | u | u |
| v | 4-Cl | – | – | v |
| w | 4-Br | – | – | w |
Proposed mechanisms of α-ureation of azine N-oxides leading to N-heteroaryl-imidazolidin-2-ones of types 3 and 4 have been discussed previously [34], whereas a plausible route leading to the formation of α-amination products 5 is depicted in Scheme 2. We presume that the initially formed unstable pyridinium salt A undergoes α-amination reaction to give the dihydropyridine C which undergoes a spontaneous 1,5-proton shift accompanied by rearomatization of dihydropyridine moiety to generate the isocyanate derivative D. Then, the intramolecular addition of imidazolidine NH group to heterocumulene affords the final imidazo[1,2-b][1,3,5]-triazepin-5(6H)-one 5.

α-Amination of pyridine N-oxides 1 with 2-chloro-4,5-dihydroimidazole (2).
Rather low yields of α-ureation of pyridine N-oxides with 1 contrast with our finding that an analogous reaction of quinoline N-oxides 6 provides the corresponding 1-(2-quinolyl)imidazolidin-2-ones 7 in fairly good yields (Scheme 3).

α-Ureation of quinoline N-oxides.
Therefore, to rationalize the observed difference in reactivity of pyridine N-oxides and quinoline N-oxides, we calculated the α-ureation reaction energies yielding 1-(2-pyridyl)imidazolidin-2-one and 1-(2-quinolyl)imidazolidin-2-one, respectively. Quantum chemical calculations carried out at the B3LYP level clearly show that both reactions are highly exothermic (-76.4 kcal/mol and -78.8 kcal/mol, respectively), whereas the activation energy for α-ureation of quinoline N-oxide is 19.1 kcal/mol, which is lower than the barrier for α-ureation of pyridine N-oxide (25.9 kcal/mol). This may explain why the yields of pyridine α-ureation (Figure 2) products are reduced. It is worth noting that the previously investigated α-bromination of quinoline and pyridine N-oxides gave similar results, that is, 2-bromoquinolines were obtained in high yields, whereas regioselective bromination of pyridine N-oxides failed [33].
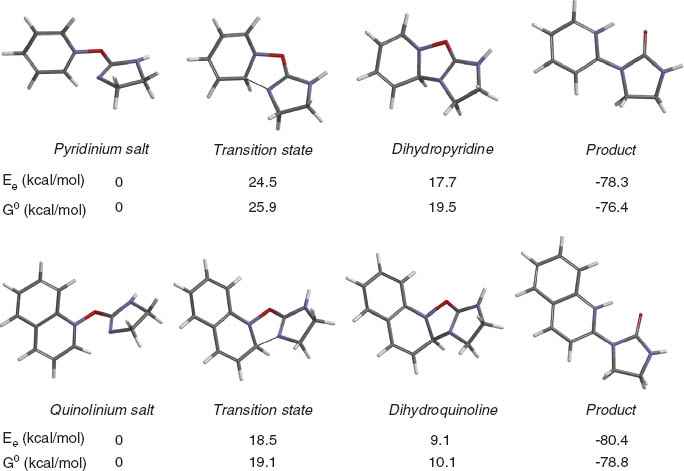
Thermodynamic reaction profile of α-ureation of pyridine N-oxide 1 (top) and quinoline N-oxide 6 (bottom) with 2-chloro-4,5-dihydroimidazole (2) in dichloromethane solution. Relative electronic energies Ee and free Gibbs energies (G0, 298.15 K) calculated for intermediates (azinium salts and dihydroazines), transition states and products at B3LYP/6-31+G* in kcal/mol.
Structures of all compounds 3–5 and 7 were confirmed by elemental analyses and IR, 1H NMR and 13C NMR spectroscopy. Moreover, the structures of 3a, 3g, 3r, 4l, 5m and 7a were analyzed by X-ray crystallography diffraction (Figure 3). It is interesting to note that 1-(azin-2-yl)imidazolidin-2-one molecules in the studied crystals adopt E configuration and are slightly non-planar due to steric interactions between the azine C3-H atoms and the imidazolidin-2-one O atom.

Views of the molecular structures of compounds 3a (top left), 3g (top center), 3r (top right), 4l (bottom left), 5m (bottom center) and 7a (bottom right) with displacement ellipsoids drawn at the 50% probability level.
Antitumor studies
Preliminary in vitro studies of new compounds for cytotoxic activity were performed on three human tumor cell lines: two non-small-cell lung cancer lines LCLC-103H and A-427, and the bladder cancer cell line 5637. Data presented in Table 2 indicate that most of the tested compounds are not active enough to inhibit the cell growth by 50% at concentration of 20 mm, which is the concentration that can typically be attained in the human body. The only active compounds are 1-monosubstituted imidazolidin-2-ones 3j and 3s, 1,3-disubstituted imidazolidin-2-one 4i and imidazo[2,1-b][1,3,5]triazepin-5(6H)-one 5w. The most sensitive to these compounds are the 5637 and A-427 cell lines, whereas the LCLC-103H cell line proved to be much less sensitive.
The relative % of cell growth (%) compared to untreated control at a concentration of 20 μm (average of three determinations).
| Cell line Comp. | LCLC-103H | 5637 | A-427 |
|---|---|---|---|
| 3a | 137.04±27.2 | 129.44±25.08 | 133.21±24.14 |
| 3c | 101.2±7.2 | 95.9±32.2 | 74.2±7.3 |
| 3g | 52.87±5.35 | 87.6±14.53 | 106.04±1.6 |
| 3h | 101.2±6.4 | 126.8±10.8 | 101.3±6.7 |
| 3j | 96.7±20.3 | 23.9±13.2 | 18.4±7.7 |
| 3r | 100.5±8.8 | 130.3±11.3 | 93.5±27.7 |
| 3s | 93.4±9.3 | 124.3±46.7 | 38.54±9.8 |
| 4i | 74.1±13.8 | 25.9±2.0 | 79.7±13.3 |
| 4l | 58.97±16.98 | 82.9±10.97 | 115.75±13.72 |
| 5i | 99.3±14.1 | 86.5±12.7 | 87.1±20.1 |
| 5q | 96.8±4.2 | 57.6±12.8 | 86.2±22.1 |
| 5w | 91.3±5.2 | 38.4±4.7 | 38.4±11.1 |
Conclusion
A small library of various substituted N-(2-pyridyl)-imidazolidin-2-ones was synthesized by α-ureation of corresponding pyridine N-oxides 1 with 2-chloro-4,5-dihydroimidazole (2). Most of these compounds and 1-(2-pyridyl)-2,3,7,8-tetrahydroimidazo[2,1-b][1,3,5]-triazepin-5(6H)-one derivatives resulting from α-amination of pyridine N-oxides with 2 were obtained for the first time. Quantum chemical calculations of the α-ureation process revealed that lower reactivity of pyridine N-oxides in comparison with quinoline N-oxides is due to thermodynamic factors. The investigated compounds exhibit a general lack of cytotoxic activity on the human cancer cell lines tested. The cell lines 5637 and A-427 are the ones moderately sensitive to imidazolidin-2-ones 3j, 3s and 4i and imidazo[2,1-b][1,3,5]triazepin-5(6H)-one 5w.
Experimental
General
All melting points were determined on a Boetius apparatus and are uncorrected. FT-IR spectra were measured by the Nicolet-380 model. Unless stated otherwise, the 1H NMR and 13C NMR spectra were recorded on a Varian Gemini instrument operating at 200 MHz and 50 MHz, respectively.
Chromatographic separations were performed on silica gel 60 PF254 containing gypsum (Merck) by use of chromatotron™ or flash column chromatography (silica gel 0.040–0.063 mm, Alfa Aesar). Analytical thin layer chromatography was performed with Merck silica gel plates and spots were visualized with UV light at 254 nm.
N-oxides 1a, 1b, 1f, 1g, 1i, 1l and 6 were acquired from commercial suppliers (Sigma-Aldrich) and used as provided; other N-oxides were prepared according to literature procedures [39–42].
The diffraction data for single crystals of compounds 3a, 3g, 3r and 7 were collected with an Oxford Diffraction Xcalibur diffractometer using Mo Kα radiation, whereas an Oxford Diffraction SuperNova diffractometer using Cu Kα radiation was used for compounds 4l and 5m. The intensity data were collected and processed using Oxford Diffraction CrysAlis Software [43]. The structures were solved by direct methods with the program SHELXS-97 [44] and refined by full-matrix least-squares method on F2 with SHELXL-97 [44]. Crystallographic data for compounds have been deposited with the Cambridge Crystallographic Data Centre, with the deposition Nos. CCDC 778723–778725, 780760, 808498 and 953311. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
All cell culture reagents were purchased from Sigma (Deisenhofen, Germany). Cancer cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Brauschweig, Germany). These included: human large cell lung carcinoma LCLC-103H, human urinary bladder carcinoma 5637 and human lung carcinoma A-427. The culture medium for all cancer cell lines was RPMI-1640 medium containing 2 g/L HCO3-, and 10% fetal calf serum. Cells were grown in 75 cm2 plastic culture flasks (Sarstedt, Nümbrecht, Germany) in a humid atmosphere of 5% CO2 at 37°C and passaged shortly before becoming confluent. Cytotoxicity determinations were based on cellular staining with crystal violet and were performed as previously described [45]. Cells were automatically counted by using a Coulter Counter Z2 (Beckman-Coulter) instrument.
General procedure for the reaction of azine N-oxides 1 and 6 with 2-chloro-4,5- dihydroimidazole (2); synthesis of 3–5, 7
To a stirred solution of 2-chloro-4,5-dihydro-1H-imidazole (2, 0.025 mol) [46] in dichloromethane (30 mL) was added appropriate pyridine N-oxide 1a–w (0.0125 mol). When the exothermic reaction had subsided, the reaction mixture was stirred at room temperature for 12 h. Then the solvent was evaporated under reduced pressure, and the oily residue was basified with 20% aqueous potassium carbonate. The product was extracted with chloroform, and the extract was dried with anhydrous MgSO4 and concentrated. The residue was separated on a chromatotron™ or flash column chromatography. In the case of the reactions of pyridine N-oxides 1v, 1w and quinoline N-oxides 6a, 6b, the only products 5v, 5w, 7a and 7b, respectively, were separated by suction after basification of the reaction mixture with K2CO3 and purified by crystallization.
1-(5-Methyl-2-pyridyl)imidazolidin-2-one (3a)
Compound 3a was purified by use of chromatotron (eluent: chloroform/ethyl acetate, 2:1, v/v). The resulting oil was mixed with anhydrous methanol and cooled to give white solid; yield 5%; mp 202–204°C ([24]; mp 200–202°C); IR: ν 3234 (NH), 1697 (CO), 1608, 1500, 1487, 1415, 1261 cm-1; 1H NMR (DMSO-d6): δ 2.21 (s, 3H, CH3), 3.38 (t, 2H, CH2), 3.95 (t, 2H, CH2), 7.10 (br s, 1H, NH), 7.51 (dd, J1 = 2 Hz, J2 = 8.5 Hz, 1H, Ar-H), 8.06 (m, 2H, Ar-H); 13C NMR (DMSO-d6): δ 17.4, 36.7, 43.8, 111.6, 126.1, 138.1, 147.2, 150.8, 158.7. Anal. Calcd for C9H11N3O: C, 61.00; H, 6.26; N, 23.71. Found: C, 60.94; H, 6.22; N, 23.43.
Crystal data for 3a: C9H11N3O, triclinic, space group P-1, a = 6.8020(8), b = 7.1679(7), c = 10.1343(9) Å, α = 99.816(8), β = 102.607(9), γ = 114.186(11)°, V = 420.70(9) Å3, Z = 2, T = 100 K, dx = 1.399 g cm-3, μ(Mo Kα) = 0.096 mm-1, 4585 data were collected up to θmax = 28.96° for a crystal with dimensions 0.3×0.3×0.2 mm3 (Rint = 0.0199, Rσ = 0.0325). Final R indices for 1479 reflections with I>2σ(I) and 123 refined parameters are: R1 = 0.0377, wR2 = 0.0977 (R1 = 0.0513, wR2 = 0.1016 for all 1953 data). CCDC 778723.
1-(4-Methyl-2-pyridyl)imidazolidin-2-one (3b)
Compound 3b was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone, 6:3:1, v/v/v); yield 11%; mp 141–143°C ([15]; mp 145–146°C); IR: ν 3234 (NH), 1703 (CO), 1602, 1559, 1481, 1413, 1261 cm-1; 1H NMR (DMSO-d6): δ 2.29 (s, 3H, CH3); 3.39 (t, 2H, CH2); 3.97 (t, 2H, CH2); 6.82 (d, J = 5 Hz, 1H, Ar-H); 7.17 (s, 1H, NH); 8.02 (s, 1H, Ar-H); 8.13 (d, J = 5 Hz, 1H, Ar-H); 13C NMR: (DMSO-d6): δ 21.2, 36.7, 43.8, 112.3, 118.7, 147.3, 147.9, 153.0, 158.6. Anal. Calcd for C9H11N3O: C, 61.00; H, 6.26; N, 23.71. Found: C, 60.88; H, 6.19; N, 23.77.
1-(4-Ethyl-2-pyridyl)imidazolidin-2-one (3c)
Compound 3c was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 4%; mp 111–113°C; IR: ν 3236 (NH), 1705 (CO), 1604, 1558, 1431, 1263 cm-1; 1H NMR (DMSO-d6): δ 1.16 (t, 3H, CH3), 2.55 (q, 2H, CH2), 3.38 (t, 2H, CH2), 3.97 (t, 2H, CH2), 6.84 (d, J = 5 Hz, 1H, Ar-H), 7.16 (s, 1H, NH), 8.05 (s, 1H, Ar-H), 8.15 (d, J = 5 Hz, 1H, Ar-H); 13C NMR (CDCl3): δ 15.0, 29.1, 37.9, 44.7, 112.6, 118.4, 147.3, 153.1, 155.2, 159.8. Anal. Calcd for C10H13N3O: C, 62.81; H, 6.85; N, 21.97. Found: C, 62.74; H, 6.81; N, 21.73.
1-(4,5-Dimethyl-2-pyridyl)imidazolidin-2-one (3d)
Compound 3d was purified by use of chromatotron (chloroform/acetone, 5:1, v/v); yield 4%; mp 170–173°C; IR: ν 3236 (NH), 1698 (CO), 1611, 1488, 1414; 1H NMR (CDCl3): δ 2.16 (s, 3H, CH3); 2.26 (s, 3H, CH3); 3.55 (t, 2H, CH2); 4.14 (t, 2H, CH2); 5.67 (br s, 1H, NH); 7.98 (s, 1H, Ar-H); 8.03 (s, 1H, Ar-H); 13C NMR (CDCl3): δ 16.32, 20.16, 37.92, 44.56, 113.77, 126.89, 147.23, 147.79, 151.36, 159.95. Anal. Calcd for C10H13N3O: C, 62.81; H, 6.85; N, 21.97. Found: C, 62.48; H, 6.70; N, 21.63.
1-(4-tert-Butyl-2-pyridyl)imidazolidin-2-one (3e)
Compound 3e was purified by use of chromatotron (eluent: chloroform/ethyl acetate, 2:1, v/v); yield 6%; mp 135–137°C; IR: ν 3261 (NH), 1715, 1701 (CO), 1599, 1548, 1252 cm-1; 1H NMR (CDCl3): δ 1.31 (s, 9H, 3×CH3), 3.56 (t, 2H, CH2), 4.18 (t, 2H, CH2), 5.36 (br s, 1H, NH), 6.94 (dd, J1 = 1.5 Hz, J2 = 5.5 Hz, 1H, Ar-H), 8.19 (d, J= 5.5 Hz, 1H, Ar-H), 8.31 (d, J = 1.5 Hz, 1H, Ar-H); 13C NMR (CDCl3): δ 31.05 (three overlapping signals), 35.5, 37.9, 44.8, 110.3, 115.9, 147.1, 153.1, 159.7, 162.3. Anal. Calcd for C12H17N3O: C, 65.73; H, 7.81; N, 19.16. Found: C, 65.61; H, 7.68; N, 18.85.
1-(4-Phenyl-2-pyridyl)imidazolidin-2-one (3f)
Compound 3f was purified by use of chromatotron (eluent: chloroform/ethyl acetate, 2:1, v/v); yield 10%; mp 221–213°C; IR: ν 3227 (NH), 1716 (CO), 1592, 1475, 1268 cm-1; 1H NMR (DMSO-d6): δ 3.42 (t, 2H, CH2), 4.03 (t, 2H, CH2), 7.26–7.29 (m, 2H, Ar-H), 7.43–7.56 (m, 3H, Ar-H+NH), 7.69 (m, 2H, Ar-H), 8.33 (d, J = 5 Hz, 1H, Ar-H), 8.49 (s, 1H, Ar-H); 13C NMR (DMSO-d6): δ 36.7, 43.9, 109.3, 115.5, 127.0 (two overlapping signals), 129.4, 129.5 (two overlapping signals), 138.2, 148.0, 148.6, 153.6, 158.7. Anal. Calcd for C14H13N3O: C, 70.28; H, 5.48; N, 17.56. Found: C, 70.19; H, 5.38; N, 17.74.
1-[4-(3-Phenylpropyl)-2-pyridyl]imidazolidin-2-one (3g)
Compound 3g was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/methanol, 13:6:1, v/v/v); yield 7%; mp 157–158°C; IR: ν 3220 (NH), 1698 (CO), 1599, 1476, 1264 cm-1; 1H NMR (DMSO-d6): δ 1.88 (m, 2H, CH2); 2.59 (m, 4H, 2×CH2); 3.39 (t, 2H, CH2); 3.97 (t, 2H, CH2); 6.85 (d, J = 5 Hz, 1H, Ar-H); 7.25 (m, 6H, Ar-H+NH); 8.05 (s, 1H, Ar-H); 8.16 (d, J = 5 Hz, 1H, Ar-H). Anal. Calcd for C17H19N3O: C, 72.57; H, 6.81; N, 14.94. Found: C, 72.41; H, 6.68; N, 14.56.
Crystal data for 3g: C17H19N3O, monoclinic, space group P21/c, a = 9.6609(2), b = 5.8864(1), c = 25.8096(6) Å, β = 98.026(2)°, V = 1453.36(5) Å3, Z = 4, T = 100 K, dx = 1.286 g cm-3, μ(Mo Kα) = 0.082 mm-1, 12 518 data were collected up to θmax = 26.37° for a crystal with dimensions 0.3×0.3×0.1 mm3 (Rint = 0.0246, Rσ = 0.0253). Final R indices for 2368 reflections with I>2σ(I) and 195 refined parameters are: R1 = 0.0319, wR2 = 0.0761 (R1 = 0.0435, wR2 = 0.0783 for all 2964 data). CCDC 953311.
1-(6-Methoxy-2-pyridyl)imidazolidin-2-one (3h)
Compound 3h was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 8%; mp 185–186°C; IR: ν 3259 (NH), 1706 (CO), 1677, 1590, 1352, 1265 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 3.40 (t, 2H, CH2), 3.83 (s, 3H, OCH3), 4.02 (t, 2H, CH2), 6.37 (d, J = 7.5 Hz, 1H, Ar-H), 7.18 (s, 1H, NH), 7.60 (t, 1H, Ar-H), 7.70 (d, J = 7.5 Hz, 1H, Ar-H); 13C NMR (DMSO-d6): δ 36.7, 43.5, 53.0, 102.0, 103.7, 140.3, 151.1, 158.4, 162.4. Anal. Calcd for C9H11N3O2: C, 55.95; H, 5.74; N, 21.75. Found: C, 55.78; H, 5.56; N, 21.66.
1-(4-Methoxy-2-pyridyl)imidazolidin-2-one (3i)
Compound 3i was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 10%; mp 133–135°C; IR: ν 3219 (NH), 1724, 1707 (CO), 1594, 1560, 1420, 1229 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 3.37 (t, 2H, CH2), 3.78 (s, 3H, OCH3), 3.96 (t, 2H, CH2), 6.59 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H), 7.20 (br s, 1H, NH), 7.79 (d, J = 2 Hz, 1H, Ar-H), 8.08 (d, J = 6 Hz, 1H, Ar-H); 13C NMR (DMSO-d6): δ 36.6, 44.0, 55.3, 96.5, 105.45, 148.6, 154.4, 158.6, 166.2. Anal. Calcd for C9H11N3O2: C, 55.95; H, 5.74; N, 21.75. Found: C, 55.85; H, 5.72; N, 21.50.
1-(6-Ethoxy-2-pyridyl)imidazolidin-2-one (3j)
Compound 3j was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 4%; mp 171–172°C; IR: ν 3261 (NH), 1697 (CO), 1675, 1589, 1457, 1267 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 1.31 (t, 3H, CH3), 3.34 (t, 2H, CH2), 3.99 (t, 2H, CH2), 4.27 (q, 2H, OCH2), 6.34 (d, J = 7.5 Hz, 1H, Ar-H), 7.16 (s, 1H, NH), 7.58 (t, 1H, Ar-H), 7.68 (d, J=7.5 Hz, 1H, Ar-H), 13C NMR (CDCl3): δ 15.2, 37.8, 44.4, 62.0, 103.4, 104.8, 140.5, 150.9, 159.8, 162.8. Anal. Calcd for C10H13N3O2: C, 57.96; H, 6.32; N, 20.28. Found: C, 57.79; H, 6.19; N, 20.18.
1-(4-Ethoxy-2-pyridyl)imidazolidin-2-one (3k)
Compound 3k was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone, 6:3:1, v/v/v); yield 4%; mp 165–167°C; IR: ν 3235 (NH), 1702 (CO), 1596, 1439, 1257, 1220 cm-1; 1H NMR (DMSO-d6): δ 1.33 (t, 3H, CH3), 3.37 (t, 2H, CH2), 4.01 (m, 4H, 2×CH2), 6.57 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H), 7.19 (s, 1H, NH), 7.77 (d, J = 2 Hz, 1H, Ar-H), 8.06 (d, J = 6 Hz, 1H, Ar-H); 13C NMR [(CD3)2SO]: δ 14.6, 36.6, 43.9, 63.5, 96.9, 105.7, 148.6, 154.4, 158.6, 165.5. Anal. Calcd for C10H13N3O2: C, 57.96; H, 6.32; N, 20.28. Found: C, 57.72; H, 6.19; N, 20.19.
1-(4-Methoxy-6-methyl-2-pyridyl)imidazolidin-2-one (3m)
Compound 3m was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone/methanol, 14:2:2:1, v/v/v/v); yield 8%; mp 187–190°C; IR: ν 3234 (NH), 1696 (CO), 1597, 1577, 1262 cm-1; 1H NMR (DMSO-d6): δ 2.32 (s, 3H, CH3); 3.34 (t, 2H, CH2); 3.38 (s, 3H, OCH3); 3.95 (t, 2H, CH2); 6.47 (d, J = 2 Hz, 1H, Ar-H); 7.14 (s, 1H, NH); 7.61 (d, J = 2 Hz, 1H, Ar-H). Anal. Calcd for C10H13N3O2: C, 57.96; H, 6.32; N, 20.28. Found: C, 57.88; H, 6.16; N, 20.12.
1-(6-Propoxy-2-pyridyl)imidazolidin-2-one (3n)
Compound 3n was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 9%; mp 154–156°C; IR: ν 3264 (NH), 1699 (CO), 1581, 1442, 1264 cm-1; 1H NMR (DMSO-d6): δ 0.95 (t, 3H, CH3); 1.73 (m, 2H, CH2); 3.38 (t, 2H, CH2); 3.98 (t, 2H, CH2); 4.17 (t, 2H, OCH2); 6.33 (d, J= 7.5 Hz, 1H, Ar-H); 7.15 (s, 1H, NH); 7.60 (m, 2H, Ar-H); 13C NMR (CDCl3): δ 11.1, 22.9, 37.8, 44.4, 67.9, 103.4, 104.7, 140.5, 150.9, 159.7, 162.9. Anal. Calcd for C11H15N3O2: C, 59.71; H, 6.83; N, 18.99. Found: C, 59.65; H, 6.71; N, 18.66.
1-(4-Propoxy-2-pyridyl)imidazolidin-2-one (3o)
Compound 3o was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 4%; mp 141–143°C; IR: ν 3232 (NH), 1701 (CO), 1593, 1468, 1433, 1270 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 0.98 (t, 3H, CH3), 1.75 (m, 2H, CH2), 3.38 (t, 2H, CH2), 3.97 (m, 4H, OCH2+CH2), 6.60 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H), 7.20 (s, 1H, NH), 7.78 (d, J = 2 Hz, 1H, Ar-H), 8.07 (d, J = 6 Hz, 1H, Ar-H). Anal. Calcd for C11H15N3O2: C, 59.71; H, 6.83; N, 18.99. Found: C, 59.57; H, 6.69; N, 18.63.
1-(6-Isopropoxy-2-pyridyl)imidazolidin-2-one (3p)
Compound 3p was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 4%; mp 160–162°C; IR: ν 3281 (NH), 1716 (CO), 1680, 1580, 1448, 1264 cm-1; 1H NMR (DMSO-d6): δ 1.28 (d, J = 6 Hz, 6H, 2×CH3), 3.38 (t, 2H, CH2), 3.98 (t, 2H, CH2), 5.13 (m, 1H, OCH), 6.28 (d, J = 7.5 Hz, 1H, Ar-H), 7.14 (s, 1H, NH), 7.59 (m, 2H, Ar-H). Anal. Calcd for C11H15N3O2: C, 59.71; H, 6.83; N, 18.99. Found: C, 59.57; H, 6.74; N, 18.65.
1-(4-Ethoxy-6-methyl-2-pyridyl)imidazolidin-2-one (3r)
Compound 3r was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone, 7:2:1, v/v/v); yield 9%; mp 193–195°C; IR: ν 3240 (NH), 1698 (CO), 1595, 1263 cm-1; 1H NMR (DMSO-d6): δ 1.31 (t, 3H, CH3), 2.30 (s, 3H, CH3), 3.35 (t, 2H, CH2), 4.00 (m, 4H, CH2+OCH2), 6.45 (d, J = 1.5 Hz, 1H, Ar-H), 7.13 (s, 1H, NH), 7.59 (d, J = 1.5 Hz, 1H, Ar-H). Anal. Calcd for C11H15N3O2: C, 59.71; H, 6.83; N, 18.99. Found: C, 59.47; H, 6.71; N, 18.74.
Crystal data for 3r: C11H15N3O2, monoclinic, space group P21/c, a = 8.6347(3), b = 13.5280(5), c = 9.5159(3) Å, β = 102.420(3)°, V = 1085.54(6) Å3, Z = 4, T = 100 K, dx = 1.354 g cm-3, μ(Mo Kα) = 0.096 mm-1, 9745 data were collected up to θmax = 29.03° for a crystal with dimensions 0.3×0.3×0.3 mm3 (Rint = 0.0215, Rσ = 0.0274). Final R indices for 1982 reflections with I>2σ(I) and 151 refined parameters are: R1 = 0.0368, wR2 = 0.0985 (R1 = 0.0509, wR2 = 0.1014 for all 2958 data). CCDC 778724.
1-(6-Butoxy-2-pyridyl)imidazolidin-2-one (3s)
Compound 3s was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 9%; mp 146–148°C; IR: ν 3246 (NH), 1732, 1695 (CO), 1592, 1456, 1270 cm-1; 1H NMR (DMSO-d6): δ 0.92 (t, 3H, CH3), 1.34–1.45 (m, 2H, CH2), 1.61–1.75 (m, 2H, CH2), 3.38 (t, 2H, CH2), 3.98 (t, 2H, CH2), 4.21 (t, 2H, OCH2), 6.32 (d, J= 7.7 Hz, 1H, Ar-H), 7.15 (s, 1H, NH), 7.52–7.69 (m, 2H, Ar-H); 13C NMR (CDCl3): δ 14.4, 19.8, 31.6, 37.9, 44.4, 66.1, 103.4, 104.7, 140.6, 150.9, 159.7, 162.9. Anal. Calcd for C12H17N3O2: C, 61.26; H, 7.28; N, 17.86. Found: C, 61.02; H, 7.09; N, 17.89.
1-(4-Butoxy-2-pyridyl)imidazolidin-2-one (3t)
Compound 3t was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 3:2, v/v); yield 6%; mp 101–103°C; IR: ν 3237 (NH), 1698 (CO), 1602, 1590, 1560, 1264 cm-1; 1H NMR (DMSO-d6): δ 0.94 (t, 3H, CH3); 1.44 (m, 2H, CH2); 1.73 (m, 2H, CH2); 3.32 (t, 2H, CH2); 4.06 (m, 4H, CH2+OCH2); 6.71 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H); 7.82 (d, J = 2 Hz, 1H, Ar-H); 8.16 (d, J = 6 Hz, 1H, Ar-H); 13C NMR (DMSO-d6): δ 13.9, 18.9, 30.7, 36.6, 44.0, 67.4, 97.0, 105.7, 148.6, 154.4, 158.6, 165.6. Anal. Calcd for C12H17N3O2: C, 61.26; H, 7.28; N, 17.86. Found: C, 60.97; H, 7.11; N, 17.76.
1-(4-Isobutoxy-2-pyridyl)imidazolidin-2-one (3u)
Compound 3u was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone, 2:1:1, v/v/v); yield 5%; mp 170–171°C; IR: ν 3226 (NH), 1701 (CO), 1605, 1590, 1440, 1259; 1H NMR (500 MHz, CDCl3): δ 1.04 (d, J = 7 Hz, 6H, 2×CH3), 2.12 (q, 1H, CH), 3.59 (t, 2H, CH2), 3.82 (d, J = 6 Hz, 2H, OCH2), 4.21 (t, 2H, CH2), 5.16 (br s, 1H, NH), 6.54 (dd, J1 = 2 Hz, J2 = 5.5 Hz, 1H, Ar-H), 7.87 (d, J = 2 Hz, 1H, Ar-H), 8.10 (d, J = 5.5 Hz, 1H, Ar-H); 13C NMR (125 MHz, CDCl3): δ 19.4 (two overlapping signals), 28.3, 37.5, 44.6, 74.5, 97.7, 107.3, 147.7, 154.1, 159.3, 166.8. Anal. Calcd for C12H17N3O2: C, 61.26; H, 7.28; N, 17.86. Found: C, 61.12; H, 7.21; N, 18.01.
1,3-Bis(4-methyl-2-pyridyl)imidazolidin-2-one (4b)
Compound 4b was purified by crystallization from methanol; yield 11%; mp 188–190°C ([35], mp 189–191°C); IR and NMR data are virtually identical to those described in [35].
1,3-Bis(4-ethyl-2-pyridyl)imidazolidin-2-one (4c)
Compound 4c was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 4%; mp 155–157°C; IR: ν 1720 (CO), 1603, 1557, 1478, 1416 cm-1; 1H NMR (CDCl3): δ 1.28 (t, 6H, 2×CH3), 2.68 (q, 4H, 2×CH2), 4.19 (s, 4H, 2×CH2), 6.87 (d, J = 5.5 Hz, 2H, 2×Ar-H), 8.23 (m, 4H, 2×Ar-H). Anal. Calcd for C17H20N4O: C, 68.89; H, 6.80; N, 18.90. Found: C, 68.76; H, 6.74; N, 18.76.
1,3-Bis(4-phenyl-2-pyridyl)imidazolidin-2-one (4f)
Compound 4f was purified by use of chromatotron (eluent: chloroform/ethyl acetate, 2:1, v/v); yield 4%; mp 224–225°C; IR: ν 1724 (CO), 1593, 1546, 1387, 1245; 1H NMR (CDCl3): δ 4.27 (s, 4H, CH2), 7.25 (dd, J1 = 1.5 Hz, J2 = 5 Hz, 2H, 2×Ar-H), 7.48 (m, 6H, 2×Ar-H), 7.73 (m, 4H, 2×Ar-H), 8.40 (d, J = 5 Hz, 2H, 2×Ar-H), 8.64 (s, 2H, 2×Ar-H). Anal. Calcd for C25H20N4O: C, 76.51; H, 5.14; N, 14.28. Found: C, 76.42; H, 5.01; N, 14.40.
1,3-Bis(4-methoxy-2-pyridyl)imidazolidin-2-one (4i)
Compound 4i was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 5%; mp 198–199°C; IR: ν 1717 (CO), 1595, 1567, 1483, 1393, 1240 cm-1; 1H NMR (DMSO-d6): δ 3.84 (s, 6H, 2×OCH3), 4.05 (s, 4H, 2×CH2), 6.72 (dd, J1 = 2.0 Hz, J2 = 6 Hz, 2H, 2×Ar-H), 7.83 (d, J= 2 Hz, 2H, 2×Ar-H), 8.17 (d, J = 6 Hz, 2H, 2×Ar-H). Anal. Calcd for C15H16N4O3: C, 59.99; H, 5.37; N, 18.66. Found: C, 59.78; H, 5.34; N, 18.55.
1,3-Bis(4-ethoxy-2-pyridyl)imidazolidin-2-one (4k)
Compound 4k was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/triethylamine, 12:7:1, v/v/v); yield 4%; mp 141–143°C; IR: ν 1708 (CO), 1600, 1570, 1426, 1243 cm-1; 1H NMR (DMSO-d6): δ 1.35 (t, 6H, 2×CH3), 4.04 (s, 4H, 2×CH2), 4.11 (q, 4H, 2×OCH2), 6.69 (dd, J1 = 2 Hz, J2 = 6 Hz, 2H, 2×Ar-H), 7.82 (d, J = 2 Hz, 2H, 2×Ar-H), 8.16 (d, J = 6 Hz, 2H, 2×Ar-H). Anal. Calcd for C17H20N4O3: C, 62.18; H, 6.14; N, 17.06. Found: C, 61.98; H, 6.06; N, 16.88.
1,3-Bis(4-benzyloxy-2-pyridyl)imidazolidin-2-one (4l)
Compound 4l was purified by use of flash column chromatography (eluent: chloroform/ethyl acetate, 5:4, v/v); yield 5%; mp 219–221°C; IR: ν 1719 (CO), 1590, 1476, 1394, 1237; 1H NMR (CDCl3): δ 4.23 (s, 4H, 2×CH2), 5.19 (s, 4H, 2×OCH2), 6.68 (dd, J1 = 2 Hz, J2 = 6 Hz, 2H, 2×Ar-H), 7.44 (m, 10H, 2×Ar-H), 8.09 (d, J = 2 Hz, 2H, 2×Ar-H), 8.18 (d, J = 6 Hz, 2H, 2×Ar-H). Anal. Calcd for C27H24N4O3: C, 71.67; H, 5.35; N, 12.38. Found: C, 71.59; H, 5.19; N, 12.53.
Crystal data for 4l: C27H24N4O3, monoclinic, space group P21/c, a = 23.1330(3), b = 5.4593(1), c = 17.9505(2) Å, β = 100.195(1)°, V = 2231.18(6) Å3, Z = 4, T = 293 K, dx = 1.347 g cm-3, μ(Cu Kα) = 0.726 mm-1, 23 091 data were collected up to θmax = 76.62° for a crystal with dimensions 0.50×0.05×0.05 mm3 (Rint = 0.0255, Rσ = 0.0149). Final R indices for 3688 reflections with I>2σ(I) and 308 refined parameters are: R1 = 0.0351, wR2 = 0.1032 (R1 = 0.0445, wR2 = 0.1075 for all 4646 data). CCDC 808498.
1,3-Bis(4-isopropoxy-2-pyridyl)imidazolidin-2-one (4q)
Compound 4q was purified by use of chromatotron (eluent: chloroform/ethyl acetate/acetone, 2:1:1, v/v/v); yield 4%; mp 171–173°C; IR: ν 1720 (CO), 1598, 1477, 1395, 1238 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 1.32 (d, J = 6 Hz, 12H, 4×CH3), 4.05 (s, 4H, 2×CH2), 4.70–4.75 (m, 2H, 2×CH), 6.52 (dd, J1 = 2 Hz, J2 = 6 Hz, 2H, 2×Ar-H), 7.80 (s, 2H, 2×Ar-H), 8.16 (d, J = 6 Hz, 2H, 2×Ar-H). Anal. Calcd for C19H24N4O3: C, 64.03; H, 6.79; N, 15.72. Found: C, 63.89; H, 6.65; N, 15.52.
1,3-Bis(4-isobutoxy-2-pyridyl)imidazolidin-2-one (4u)
Compound 4u was purified by use of chromatotron (eluent: chloroform/ethyl acetate/acetone, 2:1:1, v/v/v); yield 4%; mp 201–203°C; IR: ν 1718 (CO), 1595, 1567, 1396, 1238 cm-1; 1H NMR (500 MHz, CDCl3): δ 1.06 (d, J = 6.5 Hz, 12H, 4×CH3), 2.13 (q, 2H, 2×CH), 3.85 (d, J = 6.5 Hz, 4H, 2×OCH2), 4.20 (s, 4H, 2×CH2), 6.59 (d, J = 6 Hz, 2H, 2×Ar-H), 7.92 (s, 2H, 2×Ar-H), 8.14 (d, J = 6 Hz, 2H, 2×Ar-H); 13C NMR (125 MHz, CDCl3): δ 19.4 (four overlapping signals), 28.4 (two overlapping signals), 41.3 (two overlapping signals), 74.6 (two overlapping signals), 98.5 (two overlapping signals), 107.6 (two overlapping signals), 148.1 (two overlapping signals), 153.7 (two overlapping signals), 154.6, 166.9 (two overlapping signals). Anal. Calcd for C21H28N4O3: C, 65.60; H, 7.34; N, 14.57. Found: C, 65.52; H, 7.26; N, 14.49.
1-(4-Ethyl-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]- triazepin-5(6H)-one (5c)
Compound 5c was purified by use of chromatotron (eluent: ethyl acetate/dichloromethane/triethylamine, 5:4:1, v/v/v); yield 6%; mp 205–207°C; IR: ν 3374, 3339, 3309, 3218 (NH), 1705 (CO), 1662, 1600, 1554, 1417, 1238 cm-1; 1H NMR (CDCl3): δ 1.25 (t, 3H, CH3), 2.65 (q, 2H, CH2), 3.41 (m, 2H, CH2), 3.74 (m, 2H, CH2), 4.00 (m, 4H, 2×CH2), 6.38 (br s, 1H, NH), 6.77 (d, J = 5 Hz, 1H, Ar-H), 8.18 (d, J = 5 Hz, 1H, Ar-H), 8.22 (s, 1H, Ar-H); 13C NMR (CDCl3): δ 15.0, 29.1, 43.0, 43.2, 43.6, 49.1, 114.2, 118.1, 147.5 (two overlapping signals), 153.7, 154.5, 157.0. Anal. Calcd for C13H17N5O: C, 60.21; H, 6.61; N, 27.01. Found: C, 60.01; H, 6.48; N, 27.14.
1-(4,5-Dimethyl-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5d)
Compound 5d was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/methanol/triethylamine, 12:6:1:1, v/v/v/v); yield 5%; mp 239–241°C; IR: ν 3308, 3215 (NH), 1698 (CO), 1664, 1461, 1214 cm-1; 1H NMR (DMSO-d6): δ 2.13 (s, 3H, CH3), 2.20 (s, 3H, CH3), 3.18 (m, 2H, CH2), 3.53 (m, 2H, CH2), 3.74 (m, 2H, CH2), 3.86 (m, 2H, CH2), 7.68 (t, 1H, NH), 8.00 (s, 1H, Ar-H), 8.20 (s, 1H, Ar-H). Anal. Calcd for C13H17N5O: C, 60.21; H, 6.61; N, 27.01. Found: C, 59.99; H, 6.55; N, 26.86.
1-[4-(3-Phenylpropyl)-2-pyridyl]-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-one (5g)
Compound 5g was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/methanol, 13:6:1, v/v/v); yield 4%; mp 166–168°C; IR: ν 3307, 3217 (NH), 1700 (CO), 1661, 1557, 1470, 1239 cm-1; 1H NMR (DMSO-d6): δ 1.86 (m, 2H, CH2); 2.59 (m, 4H, 2×CH2); 3.20 (m, 2H, CH2); 3.54 (m, 2H, CH2); 3.76 (m, 2H, CH2); 3.89 (m, 2H, CH2); 6.83 (d, J = 5 Hz, 1H, Ar-H); 7.25 (m, 5H, Ar-H); 7.68 (t, 1H, NH); 8.17 (d, J = 5 Hz, 1H, Ar-H); 8.35 (s, 1H, Ar-H). Anal. Calcd for C20H23N5O: C, 68.74; H, 6.63; N, 20.04. Found: C, 68.61; H, 6.59; N, 19.81.
1-(4-Methoxy-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5i)
Compound 5i was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/triethylamine, 12:7:1, v/v/v); yield 5%; mp 239–241°C; IR: ν 3305, 3213 (NH), 1707 (CO), 1604, 1447, 1384, 1221 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 3.19 (m, 2H, CH2), 3.53 (m, 2H, CH2), 3.74 (t, 2H, CH2), 3.77 (s, 3H, OCH3), 3.90 (t, 2H, CH2), 6.60 (dd, J1 = 1.9 Hz, J2 = 5.9 Hz, 1H, Ar-H), 7.70 (br s, 1H, NH), 8.10 (d, J = 5.9 Hz, 1H, Ar-H), 8.18 (s, 1H, Ar-H); 13C NMR (50 MHz, DMSO-d6): δ 41.7, 42.3, 42.7, 49.0, 55.3, 99.0, 104.7, 142.3, 148.4, 154.7, 155.7, 166.0. Anal. Calcd for C12H15N5O2: C, 55.16; H, 5.79; N, 26.80. Found: C, 55.02; H, 5.68; N, 26.48.
1-(4-Ethoxy-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5k)
Compound 5k was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/triethylamine, 12:7:1, v/v/v); yield 12%; mp 221–223°C; IR: ν 3302, 3220 (NH), 1707 (CO), 1661, 1597, 1569, 1470, 1223 cm-1; 1H NMR (DMSO-d6): δ 1.33 (t, 3H, CH3); 3.19 (m, 2H, CH2); 3.54 (m, 2H, CH2); 3.74–3.00 (m, 2H, CH2); 3.88 (m, 2H, CH2); 4.05 (q, 2H, OCH2); 6.57 (dd, J1 = 2.5 Hz, J2 = 5.5 Hz, 1H, Ar-H); 7.07 (t, 1H, NH); 8.08 (d, J = 5.5 Hz, 1H, Ar-H); 8.15 (d, J = 2.5 Hz, 1H, Ar-H); 13C NMR (DMSO-d6): δ 14.6, 41.7, 42.3, 42.7, 49.0, 63.4, 99.5, 105.0, 142.3, 148.4, 154.7, 155.7, 165.2. Anal. Calcd for C13H17N5O2: C, 56.71; H, 6.22; N, 25.44. Found: C, 56.56; H, 6.15; N, 25.63.
1-(4-Benzyloxy-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5l)
Compound 5l was purified by use of the flash column chromatography (chloroform/ethyl acetate, 5:4, v/v); yield 10%; mp 211–212°C; IR: ν 3307, 3218 (NH), 1701 (CO), 1660, 1560, 1463, 1353 cm-1; 1H NMR (DMSO-d6): δ 3.23 (m, 2H, CH2), 3.54 (m, 2H, CH2), 3.73 (t, 2H, CH2), 3.89 (t, 2H, CH2), 5.14 (s, 2H, CH2), 6.67 (dd, J1 = 1.5 Hz, J2 = 5.5 Hz, 1H, Ar-H), 7.38 (m, 5H, Ar-H), 7.69 (t, 1H, NH), 8.09 (d, J = 5.5 Hz, 1H, Ar-H), 8.27 (d, J = 1.5 Hz, 1H, Ar-H); 13C NMR (DMSO-d6): δ 41.7, 42.3, 42.6, 49.0, 69.4, 99.7, 105.4, 128.2 (two overlapping signals), 128.3, 128.7 (two overlapping signals), 136.6, 142.2, 148.4, 154.7, 155.7, 165.0. Anal. Calcd for C18H19N5O2: C, 64.08; H, 5.68; N, 20.76. Found: C, 63.92; H, 5.56; N, 20.56.
1-(4-Methoxy-6-methyl-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-one (5m)
Compound 5m was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone/methanol/triethylamine, 14:2:2:1:1, v/v/v/v/v); yield 6%; mp 219–222°C; IR: ν 3306 (NH), 1685 (CO), 1664, 1592, 1234 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 2.34 (s, 3H, CH3), 3.18 (m, 2H, CH2), 3.34 (s, 3H, OCH3), 3.55 (m, 2H, CH2), 3.74 (m, 2H, CH2), 3.90 (m, 2H, CH2), 6.48 (d, J = 2 Hz, 1H, Ar-H), 7.69 (t, 1H, NH), 8.01 (d, J = 2 Hz, 1H, Ar-H). Anal. Calcd for C13H17N5O2: C, 56.71; H, 6.22; N, 25.44. Found: C, 56.59; H, 5.98; N, 25.74.
Crystal data for 5m: C13H17N5O2, triclinic, space group P-1, a = 8.1785(5), b = 8.8308(5), c = 9.7563(5) Å, α = 81.049(5), β = 74.533(5), γ = 73.360(5)°, V = 648.27(6) Å3, Z = 2, T = 293 K, dx = 1.410 g cm-3, μ(Cu Kα) = 0.818 mm-1, 6677 data were collected up to θmax = 75.45° for a crystal with dimensions 0.3×0.3×0.2 mm3 (Rint = 0.0132, Rσ = 0.0124). Final R indices for 2342 reflections with I>2σ(I) and 199 refined parameters are: R1 = 0.0366, wR2 = 0.0981 (R1 = 0.0389, wR2 = 0.0998 for all 2542 data). The iminoethylene fragment of the seven-membered ring shows a minor disorder. CCDC 778725.
1-(4-Isopropoxy-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5q)
Compound 5q was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone/methanol/triethylamine, 14:2:2:1:1, v/v/v/v/v); yield 4%; mp 218–220°C; IR: ν 3302, 3214 (NH), 1708 (CO), 1664, 1596, 1385, 1224 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 1.28 (d, J = 6.0 Hz, 6H, 2×CH3); 3.19–3.30 (m, 2H, CH2), 3.53–3.56 (m, 2H, CH2), 3.74 (m, 2H, CH2), 3.89 (m, 2H, CH2), 4.63 (m, 1H, OCH), 6.56 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H), 7.67 (t, 1H, NH), 8.07 (d, J = 6 Hz, 1H, Ar-H), 8.12 (d, J = 2 Hz, 1H, Ar-H). Anal. Calcd for C14H19N5O2: C, 58.12; H, 6.62; N, 24.21. Found: C, 57.94; H, 6.51; N, 23.99.
1-(4-Isobutoxy-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5u)
Compound 5u was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate/acetone/methanol/triethylamine, 14:2:2:1:1, v/v/v/v/v); yield 9%; mp 195–196°C; IR: ν 3311, 3232 (NH), 1707 (CO), 1591, 1416, 1385, 1222 cm-1; 1H NMR (500 MHz, CDCl3): δ 0.98 (d, J = 6.8 Hz, 6H, 2×CH3); 2.04 (m, 1H, CH); 3.19 (m, 2H, CH2); 3.56 (m, 2H, CH2); 3.77 (m, 4H, CH2+OCH2); 3.91 (t, 2H, CH2); 6.59 (dd, J1 = 2 Hz, J2 = 6 Hz, 1H, Ar-H); 7.69 (t, 1H, NH); 8.09 (d, J = 6 Hz, 1H, Ar-H); 8.17 (s, 1H, Ar-H); 13C NMR (125 MHz, CDCl3): δ 19.7 (two overlapping signals), 28.5, 43.0, 43.3, 43.5, 49.2, 74.6, 100.1, 106.2, 143.0, 148.5, 155.1, 157.0, 166.5. Anal. Calcd for C15H21N5O2: C, 59.39; H, 6.98; N, 23.09. Found: C, 59.23; H, 6.92; N, 22.91.
1-(4-Chloro-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5v)
Compound 5v was purified by crystallization from isopropanol; yield 4%; mp 265–269°C; IR: ν 3305, 3211 (NH), 1697 (CO), 1664, 1578, 1245 cm-1; 1H NMR (DMSO-d6): δ 3.20 (m, 2H, CH2), 3.57 (m, 2H, CH2), 3.76 (m, 2H, CH2), 3.93 (m, 2H, CH2), 7.07 (dd, J1 = 2 Hz, J2 = 5.5 Hz, 1H, Ar-H), 7.75 (t, 1H, NH), 8.27 (d, J = 5.5 Hz, 1H, Ar-H), 8.67 (d, J = 2 Hz, 1H, Ar-H). Anal. Calcd for C11H12ClN5O: C, 49.72; H, 4.55; N, 26.36. Found: C, 49.54; H, 4.36; N, 25.99.
1-(4-Bromo-2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b]- [1,3,5]triazepin-5(6H)-one (5w)
Compound 5w was purified by crystallization from ethanol; yield 11%; mp 263–266°C; IR: ν 3307, 3209 (NH), 1698 (CO), 1664, 1572, 1385, 1243 cm-1; 1H NMR (DMSO-d6): δ 3.20 (m, 2H, CH2), 3.57 (m, 2H, CH2), 3.76 (m, 2H, CH2), 3.90 (m, 2H, CH2), 7.20 (dd, J1 = 2 Hz, J2 = 5.5 Hz, 1H, Ar-H); 7.73 (t, 1H, NH), 8.19 (d, J = 5.5 Hz, 1H, Ar-H); 8.82 (d, J = 2 Hz, 1H, Ar-H). Anal. Calcd for C11H12BrN5O: C, 42.60; H, 3.90; N, 22.58. Found: C, 42.48; H, 3.78; N, 22.36.
1-(2-Quinolyl)imidazolidin-2-one (7a)
Compound 7a was prepared by reacting quinoline N-oxide (6a) with 2-chloro-4,5-dihydroimidazole (2), as described previously [34], and purified by crystallization from ethanol; yield 36%, mp 245–247°C ([34], mp 244–246°C).
Crystal data for 7a: C12H11N3O, monoclinic, space group P21/c, a = 19.7129(19), b = 6.6524(7), c = 7.7984(8) Å, β = 98.710(9)°, V = 1010.87(18) Å3, Z = 4, T = 293 K, dx = 1.401 g cm-3, μ(Mo Kα) = 0.093 mm-1, 6373 data were collected up to θmax = 25.02° for a crystal with dimensions 0.5×0.5×0.3 mm3 (Rint = 0.0231, Rσ = 0.0219). Final R indices for 1234 reflections with I>2σ(I) and 150 refined parameters are: R1 = 0.0387, wR2 = 0.0997 (R1 = 0.0657, wR2 = 0.1233 for all 1775 data). CCDC 780760.
1-(6-Methoxy-2-quinolyl)imidazolidin-2-one (7b)
Compound 7b was purified by crystallization from N,N-dimethylformamide/methanol (1:1, v/v); yield 65%; mp 254–257°C; IR: ν 3252 (NH), 1694 (CO), 1605, 1500, 1421, 1365, 1268, 1228 cm-1; 1H NMR (DMSO-d6): δ 3.44 (t, 2H, CH2), 3.86 (s, 3H, OCH3), 4.11 (t, 2H, CH2), 7.30 (m, 3H, Ar-H+NH), 7.68 (m, 1H, Ar-H), 8.13 (d, J = 9 Hz, 1H, Ar-H), 8.42 (d, J = 9 Hz, 1H, Ar-H). Anal. Calcd for C13H13N3O2: C, 64.19; H, 5.39; N, 17.27. Found: C, 64.02; H, 5.18; N, 17.60.
References
[1] Xuan, W.; Ding, W.; Hui, H.; Zhang, S. Synthesis and cytotoxic activity of diaryl urea derivatives with a 4-methylpiperazinylcarbonyl moiety. Med. Chem. Res. 2013, 22, 3857–3862.Suche in Google Scholar
[2] Smith, R. A.; Dumas, J.; Adnane, L.; Wilhelm, S. M. Recent advances in the research and development of RAF kinase inhibitors. Curr. Top. Med. Chem. 2006, 6, 1071–1089.Suche in Google Scholar
[3] Montagut, C.; Settleman, J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009, 283, 125–134.Suche in Google Scholar
[4] Kamal, A.; Suresh, P.; Ramaiah, M. J.; Reddy, T. S.; Kapavarapu, R. K.; Rao, B. N.; Imthiajali, S.; Reddy, T. L.; Pushpavalli, S. N. C. V. L. 4β-[4′-(1-(Aryl)ureido)benzamide]-podophyllotoxins as DNA topoisomerase I and IIα inhibitors and apoptosis inducing agents. Bioorg. Med. Chem. 2013, 21, 5198–5208.Suche in Google Scholar
[5] Albaugh, P.; Fan, Y.; Mi, Y.; Sun, F.; Adrian, F.; Li, N.; Jia, Y.; Sarkisova, Y.; Kreusch, A.; Hood, T.; et al. Discovery of GNF-5837, a selective TRK inhibitor with efficacy in rodent cancer tumor models. ACS Med. Chem. Lett. 2012, 3, 140–145.Suche in Google Scholar
[6] Iwata, H.; Oki, H.; Okada, K.; Takagi, T.; Tawada, M.; Miyazaki, Y.; Imamura, S.; Hori, A.; Lawson, J. D.; Hixon, M. S.; et al. A back-to-front fragment-based drug design search strategy targeting the DFG-out pocket protein tyrosine kinases. ACS Med. Chem. Lett. 2012, 3, 342–346.Suche in Google Scholar
[7] Solinas, A.; Faure, H.; Roudaut, H.; Traiffort, E.; Schoenfelder, A.; Mann, A.; Manetti, F.; Taddei, M.; Ruat, M. Acylthiourea, acylurea, and acylguanidine derivatives with potent hedgehog inhibiting activity. J. Med. Chem. 2012, 55, 1559–1571.Suche in Google Scholar
[8] Anderson, J. W.; Sarantakis, D.; Terpinski, J.; Kumar, T. R. S.; Tsai, H.-C.; Kuo, M.; Ager, A. L.; Jacobs, W. R.; Schiehser, G. A.; Ekins, S.; et al. Novel diaryl ureas with efficacy in a mouse model of malaria. Bioorg. Med. Chem. Lett. 2013, 23, 1022–1025.Suche in Google Scholar
[9] Robert, J.-M. H.; Sabourin, C.; Alvarez, N.; Robert-Piessard, S.; Le Baut, G.; Le Pape, P. Synthesis and antileishmanil activity of new imidazolidin-2-one derivatives. Eur. J. Med. Chem. 2003, 38, 711–718.Suche in Google Scholar
[10] Heidebrecht, R. W.; Mulrooney, C.; Austin, C. P.; Barker, R. H.; Beaudoin, J. A.; Cheng, K. C.-C.; Comer, E.; Dandapani, S.; Dick, J.; Duvall, J. R.; et al. Diversity-oriented synthesis yields a novel lead for the treatment of malaria. ACS Med. Chem. Lett. 2012, 3, 112–117.Suche in Google Scholar
[11] Shia, K.-S.; Li, W.-T.; Chang, C.-M.; Hsu, M.-C.; Chern, J.-H.; Leong, M. K.; Tseng, S.-N.; Lee, C.-C.; Lee, Y.-C.; Chen, S.-J.; et al. Design, synthesis, and structure-activity relationship of pyridyl imidazolidinones: a novel class of potent and selective human enterovirus 71 inhibitors. J. Med. Chem. 2002, 45, 1644–1655.Suche in Google Scholar
[12] Miguet, L.; Zervosen, A.; Gerards, T.; Pasha, F. A.; Luxen, A.; Disteche-Nguyen, M.; Thomas, A. Discovery of new inhibitors of resistant Streptococcus pneumoniae penicillin binding protein (PBP) 2× by structure-based virtual screening. J. Med. Chem. 2009, 52, 5926–5936.Suche in Google Scholar
[13] Lopez, L. C.; Dos-Reis, S.; Espargaro, A.; Carrodeguas, J. A.; Maddelin, M.-L.; Ventura, S.; Sancho, J. Discovery of novel inhibitors of amyloid β-peptide 1–42 aggregation. J. Med. Chem. 2012, 55, 9521–9530.Suche in Google Scholar
[14] Jansen, M.; Potschka, H.; Brandt, C.; Loscher, W.; Dannhardt, G. Hydantoin-substituted 4,6-dichloroindole-2-carboxylic acids as ligands with high affinity for the glycine binding site of the NMDA receptor. J. Med. Chem. 2003, 46, 64–73.Suche in Google Scholar
[15] Zhang, M.; Yang, X.-Y.; Tang, W.; Groeneveld, T. W. L.; He, P.-L.; Zhu, F.-H.; Li, J.; Lu, W.; Blom, A. M.; Zuo, J.-P.; et al. Discovery and structural modification of 1-phenyl-3-(1-phenylethyl)urea derivatives as inhibitors of complement. ACS Med. Chem. Lett. 2012, 3, 317–321.Suche in Google Scholar
[16] Yonova, P.; Stoilkova, G. Synthesis and plant-growth-regulating activity of pyridyl-imidazolidinone compounds. Dokl. Bulgar. Akad. Nauk. 2005, 58, 595–600.Suche in Google Scholar
[17] Schulz, M. N.; Hubbard, R. E. Recent progress in fragment-based lead discovery. Curr. Opin. Pharmacol. 2009, 9, 615–621.Suche in Google Scholar
[18] Erlanson, D. A.; Hansen, S. T. Making drugs on proteins: site-directed ligand discovery for fragment-based lead assembly. Curr. Opin. Chem. Biol. 2004, 8, 399–406.Suche in Google Scholar
[19] Erlanson, D. A.; McDowell, R. S.; O’Brien, T. Fragment-based drug discovery. J. Med. Chem. 2004, 47, 3463–3482.Suche in Google Scholar
[20] Congreve, M.; Carr, R.; Murray, C.; Jhoti, H. A ‘rule of three’ for fragment-based lead discovery. Drug. Disc. Today 2003, 8, 876–877.Suche in Google Scholar
[21] Abdala, H.; Robert, J.-M.; Le Pape, P.; Wielgosz, G.; Robert-Piessard, S.; Le Baut, G. Synthesis and antileishmanial activity of new 1-(pyridin-2-yl)imidazolidin-2-ones derived from 2-amino-4,6-dimethylpyridine. Arzneim. Forsch. Drug Res. 2000, 50, 479–484.Suche in Google Scholar
[22] Wolin, R. L.; Bembenek, S. D.; Wei, J.; Crawford, S.; Lundeen, K.; Brunmark, A.; Karlsson, L.; Edwards, J. P.; Blevitt, J. M. Dual binding site inhibitors of B-RAF kinase. Bioorg. Med. Chem. Lett. 2008, 18, 2825–2829.Suche in Google Scholar
[23] Janardhan, B.; Laxmi, S. V.; Rajitha, B. One-pot synthesis of fused 3,4-dihydropyrimidin-2(1H)-ones and thiones using a novel ionic liquid as an efficient and reusable catalyst: improved protocol conditions for the Biginelli-like scaffolds. Heterocycl. Commun. 2012, 18, 93–97.Suche in Google Scholar
[24] Takeda, M.; Inage, M.; Wada, H.; Tamaki, H.; Ochiai, T. Imidazolidinones as brain activators; US Patent 4,886,817, 1989.Suche in Google Scholar
[25] Wang, L.; Zhang, B.; Ji, J.; Li, B.; Yan, J.; Zhang, W.; Wu, Y.; Wang, X. Synthesis and evaluation of structurally constrained imidazolidin derivatives as potent dipeptidyl peptidase IV inhibitors. Eur. J. Med. Chem. 2009, 44, 3318–3322.Suche in Google Scholar
[26] Lundgren, R. J.; Stradiotto, M. Recent advances in the Buchwald-Hartwig amination reaction enabled by the application of sterically demanding phosphine ancillary ligands. Aldrichim. Acta 2012, 45, 59–65.Suche in Google Scholar
[27] Yin, J.; Buchwald, S. L. Palladium-catalyzed intermolecular coupling of aryl halides and amides. Org. Lett. 2000, 2, 1101–1104.Suche in Google Scholar
[28] Lee, C.-C.; Wang, P.-S.; Viswanath, M. B.; Leung, M. Synthesis of symmetrical and unsymmetrical N-aryl-substituted cyclic ureas through copper(I) iodide catalyzed Goldberg-Buchwald-Nandakumar C-N coupling reactions. Synthesis 2008, 9, 1359–1366.Suche in Google Scholar
[29] Stabile, P.; Lamonica, A.; Ribecai, A.; Castoldi, D.; Guercio, G.; Curcuruto, O. Mild, convenient and versatile Cu-mediated synthesis of N-aryl-2-imidazolidinones. Tetrahedron Lett. 2010, 51, 3232–3235.Suche in Google Scholar
[30] Hosseinzadeh, R.; Sarrafi, Y.; Mohadjerani, M.; Mohammadpourmir, F. Copper-catalyzed arylation of phenylurea using KF/Al2O3. Tetrahedron Lett. 2008, 49, 840–843.Suche in Google Scholar
[31] Manley, P. J.; Bilodeau, M. T. A mild method for formation and in situ reaction of imidoyl chlorides: conversion of pyridine-1-oxides to 2-aminopyridine amides. Org. Lett. 2002, 4, 3127–3129.Suche in Google Scholar
[32] Nandakumar, M. V. Copper catalyzed arylation of urea. Tetrahedron Lett. 2004, 45, 1989–1990.Suche in Google Scholar
[33] Wengryniuk, S. E.; Weickgenannt, A.; Reiher, C.; Strotman, N. A.; Chen, K.; Eastgate, M. D.; Baran, P. S. Regioselective bromination of fused heterocyclic N-oxides. Org. Lett. 2013, 15, 792–795.Suche in Google Scholar
[34] Saczewski, F. 2-Chloro-4,5-dihydroimidazole. 1. Reactions with some hetroaromatic N-oxides, cyclic nitrones and aldoximes. Synthesis 1984, 2, 170–172.10.1055/s-1984-35466Suche in Google Scholar
[35] Saczewski, F.; Bulakowska, A.; Gdaniec, M. 2-Chloro-4,5-dihydroimidazole, part X. Revisiting route to N-heteroaryl-2-imidazolidinones. J. Heterocycl. Chem. 2002, 39, 911–915.Suche in Google Scholar
[36] Wolińska, E. Sequential amination of heteroaromatic halides with aminopyridine 1-oxides and their N-protected derivatives based on novel aza-Smiles rearrangement. Heterocycl. Commun. 2012, 18, 227–232.Suche in Google Scholar
[37] Abramovitch, R. A.; Singer, G. M. A direct alkyl and aryl amination of heteroaromatic nitrogen compounds. J. Am. Chem. Soc. 1969, 91, 5672–5673.Suche in Google Scholar
[38] Abramovitch, R. A.; Rogers, R. B. Direct acylamination of 3-substituted pyridine N-oxides. Directive effect of the substituent. J. Org. Chem. 1974, 39, 1802–1807.Suche in Google Scholar
[39] Ochiai, E. Recent Japanese work on the chemistry of pyridine 1-oxide and related compounds. J. Org. Chem. 1952, 18, 534–551.Suche in Google Scholar
[40] Katritzky, A. R. The preparation of some substituted pyridine 1-oxides. J. Chem. Soc. 1956, 2404–2408.10.1039/jr9560002404Suche in Google Scholar
[41] Brown, E. V. Preparation and reactions of 2-nitropyridine-1-oxides. J. Am. Chem. Soc. 1957, 79, 3565–3566.Suche in Google Scholar
[42] Shaw, E. Pyridine N-oxides. In The Chemistry of Heterocyclic Compounds. Pyridine and its Derivatives, Part Two; Chapter IV; Klingsberg, E., Ed. Interscience Publishers, Inc.: New York, 1961, 97–148.10.1002/9780470186657.ch2Suche in Google Scholar
[43] CrysAlis Pro Software, Ver. 1.171.33; Oxford Diffraction Ltd.: Abingdon, Oxfordshire, 2008.Suche in Google Scholar
[44] Sheldrick, G. M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122.Suche in Google Scholar
[45] Bracht, K.; Boubakari; Grunert, R.; Bednarski, P. J. Correlations between the activities of 19 anti-tumor agents and the intracellular glutathione concentrations in a panel of 14 human cancer cell lines: comparisons with the National Cancer Institute data. Anticancer Drugs 2006, 17, 41–51.Suche in Google Scholar
[46] Trani, A.; Bellasio, E. Synthesis of 2-chloro-2-imidazoline and its reactivity with aromatic amines, phenols, and thiophenols. J. Heterocycl. Chem. 1974, 11, 257–261.Suche in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes

