Abstract
A series of pyrazolines 4a–h has been synthesized by Michael addition of chalcones 3a–h with hydrazine hydrate in the presence of sodium acetate under conventional heating or microwave irradiation. Structures of the newly synthesized pyrazolines 4a–h have been established on the basis of IR, 1H, 13C NMR and mass spectral data.
Introduction
The recent literature is enriched with progressive findings about the synthesis and pharmacological properties of pyrazolines [1–9]. Pyrazolines have been found to possess antimicrobial [1], antibacterial [2], antiamoebic [3, 4], antidepressant [5], anticonvulsant [6], anti-inflammatory [7, 8] and antitumor [9] activities.
Quinoline derivatives are also known to exhibit antiallergic [10], anticonvulsant [11], antimicrobial [12] and antimalarial [13] activities. Many chalcone derivatives have been reported to show antimalarial [14] and anticancer [15] activities. Synthesis of quinolinyl chalcones is scarcely reported in the literature, whereas chalcones derived from tetrazolo[1,5-a]quinoline-4-carbaldehyde have not been reported so far. Fusion of tetrazole, which is considered a planar acidic heterocyclic analog of carboxylic function, has the ability to increase potency and bioavailability of quinolinyl chalcones. Several substituted tetrazoles have been shown to possess anticonvulsant [16], anti-inflammatory [17], central nervous system (CNS) dispersant [18], anti-HIV [19] and antifertility [20, 21] properties. The aim of the present study is to prepare the title compounds.
Results and discussion
In recent years, reports on microwave-assisted synthesis revealed that it is a safe, rapid, economic and environmentally friendly method. Owing to increased regulatory pressure in research and industry, tremendous efforts have been made to reduce the amount of pollutants produced, including organic solvents in chemical synthesis. To enforce such practices, the discovery and invention of new synthetic methods are required. Microwave-assisted synthesis leads to significantly reduced reaction times, enhanced conversions and it is an environmentally friendly [22, 23] method.
The synthesis of new derivatives of pyrazolines was carried out as outlined in Scheme 1. The chalcones were prepared by reacting substituted 2-hydroxyacetophenones 1a–h with tetrazolo[1,5-a]quinoline-4-carbaldehyde 2 [23] in the presence of KOH by conventional as well as microwave irradiation methods by using Claisen-Schmidt condensation. The reaction of (2E)-1-(2-hydroxy-substituted phenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl) prop-2-en-1-ones 3a–h with hydrazine hydrate in dimethylformamide (DMF) was carried out by either heating conventionally or by microwave irradiation in the presence of sodium acetate to give 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols 4a–h.
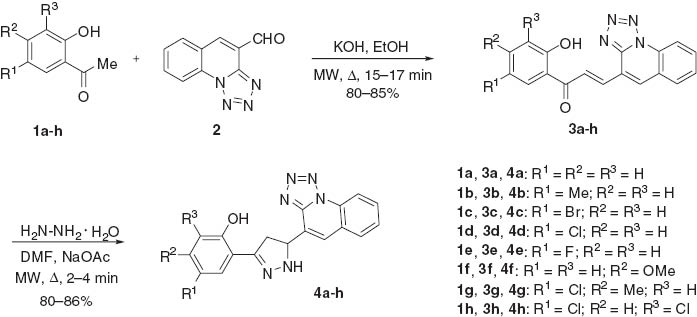
It was found that the synthesis of pyrazolines 4a–h by the conventional method took a longer time (2–3 h) and gave lower yields when compared to the microwave irradiation technique, in which the reaction proceeded smoothly with excellent yields and within a few minutes (2–3 min).
The 1H NMR spectrum of pyrazoline 4a displays three characteristic signals due to the diastereotopic protons, HA, HB and HX [24, 25]. The HA proton, which is cis to HX, resonates upfield at δ 3.38 as doublet of doublet (dd), whereas the HB proton, which is trans to HX, resonates downfield at δ 3.88 (dd). The HX proton, which is vicinal to two methylene protons (HA and HB), is also observed as dd at δ 5.43.
The cyclization of chalcones into pyrazolines was further supported by the 13C NMR of 4a, in which the C-4 and C-5 carbons resonate at δ 40.3 and 57.8, respectively. These values are in close agreement with the reported values for pyrazolines carbons C-4 and C-5 [26, 27]. The combination of 1H NMR and 13C NMR provides strong evidence in support of structures assigned to the pyrazoline derivatives. The mass spectrum of 4a shows a peak at m/z = 331 for [M+H]+.
Conclusion
High yielding, convenient methods for the synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols 4a–h from (2E)-1-(2-hydroxy substituted phenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-ones 3a–h under both conventional heating and microwave irradiation conditions are reported. The microwave irradiation process is a simple, environmentally friendly technique.
Experimental
Melting points were determined by the open capillary method using the electrical melting point apparatus and are uncorrected. Microwave reactions were carried out in a multi-SYNTH series microwave system (Milestone). The IR spectra were recorded as KBr pellets on a Shimadzu FT-IR-8400s spectrophotometer. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker DPX 400 spectrophotometer using tetramethylsilane (TMS) as internal standard and DMSO-d6 as solvent. Mass spectra were recorded on a GCMS-QP 1000 EX mass spectrometer and thin layer chromatography (TLC) was performed to check the purity of the compounds, the spot being located under UV light and iodine vapors.
General procedure for the synthesis of compounds 3a–h
Conventional method A
A solution of a 2-hydroxyacetophenone 1a–h (0.01 mol) and tetrazolo[1,5-a]quinoline-4-carbaldehyde 2 (0.01 mol) in ethanol (30 mL) was treated with KOH and the mixture was stirred overnight at room temperature. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was poured into crushed ice and neutralized with diluted hydrochloric acid. The yellow solid thus obtained was filtered, washed with water and dried. Crystallization from methanol afforded pure chalcone 3a–h.
Microwave method B
A solution of a 2-hydroxyacetophenone 1a–h (0.01 mol) and tetrazolo[1,5-a]quinoline-4-carbaldehyde 2 (0.01 mol) in ethanol (10 mL) in a 30-mL glass vial equipped with a cap was treated with KOH and the mixture was irradiated for 15–17 min at 125°C, using an irradiation power of 180 W. The progress of the reaction was monitored by TLC. After completion of the reaction, the vial was cooled, diluted with crushed ice and neutralized with diluted hydrochloric acid. The yellow solid thus obtained was filtered, washed with water and dried. Crystallization from methanol afforded pure chalcone 3a–h.
(2E)-1-(2-Hydroxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl) prop-2-en-1-one (3a)
A yellow solid; yield 68% (method A) and 80% (method B); mp 238–240°C (dec); IR: 3423 (OH), 1642 (C=O), 1613 (C=N), 1588 (C=C), 1267 cm-1 (Ar-O); 1H NMR: δ 11.98 (s, 1H, OH), 8.90 (d, 1H, Hβ), 8.81 (s, 1H, Ar-H), 8.67 (d, 1H, Ar-H), 8.26 (d, 1H, Ar-H), 8.06 (m, 3H, Ar-H and Hα), 7.59 (m, 2H, Ar-H), 7.08 (m, 2H, Ar-H); 13C NMR: δ 190.9 (C=O), 161.1 (Ar-O), 136.8, 136.6, 133.0, 132.2, 131.3, 130.6, 129.0, 128.0, 126.9, 124.3, 124.0, 122.4, 121.3, 118.4, 116.8, 116.6; MS: m/z 317 [M+H]+ (30%). Anal. Calcd for C18H12N4O2: C, 68.41; H, 3.82; N, 17.73. Found: C, 68.39; H, 3.76; N, 17.79.
(2E)-1-(2-Hydroxy-5-methylphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3b)
A yellow solid; yield 66% (method A) and 85% (method B); mp 245–247°C (dec); IR: 3423 (OH), 1646 (C=O), 1614 (C=N), 1582 (C=C), 1254 cm-1(Ar-O); 1H NMR: δ 12.61 (s, 1H, OH), 9.18 (d, 1H, Hβ), 8.76 (d, 1H, Ar-H), 8.16 (s, 1H, Ar-H), 8.07 (m, 2H, Ar-H and Hα), 7.97 (m, 2H, Ar-H), 7.79 (m, 1H, Ar-H), 7.39 (m, 1H, Ar-H), 6.98 (d, 1H, Ar-H), 2.44 (s, 3H, CH3); 13C NMR: δ 189.9 (C=O), 159.1 (Ar-O), 147.8, 145.6, 144.0, 136.2, 134.3, 131.6, 130.0, 129.0, 128.9, 128.3, 128.0, 127.4, 122.3, 120.4, 118.8, 116.4, 23.3 (CH3); MS: m/z 331 [M+H]+ (100%). Anal. Calcd for C19H14N4O2: C, 69.15; H, 4.27; N, 16.97. Found: C, 69.11; H, 4.28; N, 17.05.
(2E)-1-(5-Bromo-2-hydroxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3c)
A yellow solid; yield 58% (method A) and 80% (method B); mp 242–244°C (dec); IR: 3421 (OH), 1644 (C=O), 1613 (C=N), 1578 (C=C), 1252 cm-1 (Ar-O); 1H NMR: δ 12.71 (s, 1H, OH), 9.07 (d, 1H, Hβ), 8.77 (d, 1H, Ar-H), 8.26 (s, 1H, Ar-H), 8.16 (m, 2H, Ar-H and Hα), 8.09 (m, 1H, Ar-H), 7.98 (m, 1H, Ar-H), 7.80 (m, 1H, Ar-H), 7.64 (m, 1H, Ar-H), 6.99 (d, 1H, Ar-H); 13C NMR: δ 190.6 (C=O), 160.6 (Ar-O), 146.8, 145.2, 144.3, 138.2, 135.4, 133.6, 131.1, 129.4, 127.9, 125.2, 124.0, 122.1, 121.2, 117.3, 116.8, 116.4; MS: m/z 394 [M]+ (100%), 396 [M+2]+ (97%). Anal. Calcd for C18H11N4O2Br: C, 54.73; H, 2.81; N, 14.18. Found: C, 54.70; H, 2.88; N, 14.17.
(2E)-1-(5-Chloro-2-hydroxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3d)
A yellow solid; yield 65% (method A) and 82% (method B); mp 246–248°C (dec); IR: 3424 (OH), 1645 (C=O), 1615 (C=N), 1580 (C=C), 1251 cm-1 (Ar-O); 1H NMR: δ 12.62 (s, 1H, OH), 9.04 (d, 1H, Hβ), 8.75 (d, 1H, Ar-H), 8.30 (s, 1H, Ar-H), 8.16 (m, 2H, Ar-H and Hα), 8.12 (m, 1H, Ar-H), 8.00 (m, 1H, Ar-H), 7.83 (m, 1H, Ar-H), 7.54 (d, 1H, Ar-H); 13C NMR: δ 189.8 (C=O), 161.2 (Ar-O), 145.7, 145.6, 144.1, 141.3, 137.5, 136.4, 134.5, 131.2, 130.8, 129.2, 128.5, 128.3, 127.5, 121.4, 117.8, 116.5; MS: m/z 350 [M]+ (100%), 352 [M+2]+ (30%). Anal. Calcd for C18H11ClN4O2: C, 61.77; H, 3.17; N, 16.01. Found: C, 61.68; H, 3.19; N, 16.12.
(2E)-1-(5-Fluoro-2-hydroxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3e)
A yellow solid; yield 68% (method A) and 80% (method B); mp 239–241°C (dec); IR: 3421 (OH), 1644 (C=O), 1614 (C=N), 1583 (C=C), 1257 cm-1 (Ar-O); 1H NMR: δ 12.51 (s, 1H, OH), 8.82 (d, 1H, Hβ), 8.66 (d, 1H, Ar-H), 8.25 (s, 1H, Ar-H), 8.18 (m, 2H, Ar-H and Hα), 8.05 (m, 1H, Ar-H), 8.02 (m, 1H, Ar-H), 7.87 (m, 1H, Ar-H), 7.63 (m, 1H, Ar-H), 7.02 (d, 1H, Ar-H); 13C NMR: δ 189.9 (C=O), 158.3 (Ar-O), 156.8, 148.5, 147.5, 146.1, 144.1, 138.4, 138.1, 136.5, 135.5, 132.3, 131.2, 130.5, 128.3, 127.1, 124.6, 116.3; MS: m/z 335 [M+H]+ (100%). Anal. Calcd for C18H11FN4O2: C, 64.73; H, 3.32; N, 16.77. Found: C, 64.69; H, 3.29; N, 16.78.
(2E)-1-(2-Hydroxy-4-methoxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3f)
A yellow solid; yield 56% (method A) and 81% (method B); mp 244–246°C (dec); IR: 3423(OH), 1641 (C=O), 1616 (C=N), 1577 (C=C), 1277 cm-1 (Ar-O); 1H NMR: δ 12.62 (s, 1H, OH), 9.02 (d, 1H, Hβ), 8.70 (d, 1H, Ar-H), 8.28 (s, 1H, Ar-H), 8.15 (m, 2H, Ar-H and Hα), 8.03 (m, 1H, Ar-H), 8.00 (m, 1H, Ar-H), 7.90 (m, 1H, Ar-H), 7.58 (m, 1H, Ar-H), 6.99 (d, 1H, Ar-H), 3.68 (s, 3H, CH3); 13C NMR: δ 191.5 (C=O), 168.5, 163.8 (Ar-O), 148.5, 146.2, 145.0, 141.7, 140.1, 138.5, 136.3, 134.0, 128.6, 127.1, 125.4, 124.6, 116.5, 108.6, 103.3, 56.9 (CH3); MS: m/z 347 [M+H]+ (100%). Anal. Calcd for C19H14N4O3: C, 65.96; H, 4.08; N, 16.19. Found: C, 65.84; H, 4.19; N, 16.25.
(2E)-1-(5-Chloro-2-hydroxy-4-methylphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3g)
A yellow solid; yield 59% (method A) and 80% (method B); mp 252–254°C (dec); IR: 3425 (OH), 1645 (C=O), 1616 (C=N), 1578 (C=C), 1258 cm-1 (Ar-O); 1H NMR: δ 12.64 (s, 1H, OH), 9.04 (d, 1H, Hβ), 8.74 (d, 1H, Ar-H), 8.15 (s, 1H, Ar-H), 8.06 (m, 2H, Ar-H and Hα), 7.95 (m, 1H, Ar-H), 7.77 (m, 2H, Ar-H), 6.94 (d, 1H, Ar-H), 2.42 (s, 3H, CH3); 13C NMR: δ 188.5 (C=O), 158.5 (Ar-O), 145.7, 144.8, 143.2, 142.5, 138.1, 137.7, 136.1, 135.8, 131.1, 128.5, 127.4, 126.1, 124.3, 117.9, 116.8, 116.0, 16.0 (CH3); MS: m/z 364 [M]+ (100%), 366 [M+2]+ (30%). Anal. Calcd for C19H13ClN4O2: C, 62.69; H, 3.60; N, 15.39. Found: C, 62.60; H, 3.63; N, 15.41.
(2E)-1-(3,5-Dichloro-2-hydroxyphenyl)-3-(tetrazolo[1,5-a]quinoline-4-yl)prop-2-en-1-one (3h)
A yellow solid; yield 66% (method A) and 82% (method B); mp 247–249°C (dec); IR: 3422 (OH), 1645 (C=O), 1616 (C=N), 1578 (C=C), 1258 cm-1 (Ar-O); 1H NMR: δ 13.28 (s, 1H, OH), 9.07 (d, 1H, Hβ), 8.76 (d, 1H, Ar-H), 8.18 (s, 1H, Ar-H), 8.07 (m, 3H, Ar-H and Hα), 7.97 (m, 1H, Ar-H), 7.79 (m, 1H, Ar-H), 7.65 (d, 1H, Ar-H); 13C NMR: δ 190.9 (C=O), 161.1 (Ar-O), 136.8, 136.6, 133.0, 132.2, 131.3, 130.6, 129.0, 128.0, 126.9, 124.3, 124.0, 122.4, 121.3, 118.4, 116.8, 116.6; MS: m/z 384 [M]+ (100%), 386 [M+2]+ (62%), 388 [M+4]+ (30%). Anal. Calcd for C18H10Cl2N4O2: C, 56.30; H, 2.62; N, 14.59. Found: C, 56.26; H, 2.59; N, 14.61.
General procedure for the synthesis of compounds 4a–h
Conventional method C
To a solution of chalcone 3a–h (0.01 mol) in DMF (5 mL) containing sodium acetate (0.01 mol), hydrazine hydrate (0.01 mol) was added and the reaction mixture was heated at 80–90°C for 2–3 h. The progress of the reaction was monitored by TLC. After the completion of the reaction, ice water was added. A solid product separated was filtered, washed with water and dried. Crystallization from MeOH/CHCl3(1:1) afforded pure product.
Microwave method D
To a solution of chalcone 3a–h (0.01 mol) in DMF (5 mL) containing sodium acetate (0.01 mol) in a 10 mL glass vial equipped with a cap, hydrazine hydrate (0.01 mol) was added and the mixture was irradiated for 2–4 min at 130°C using an irradiation power of 180 W. The progress of the reaction was monitored by TLC for each of the 30-s time interval. After completion of the reaction, ice water was added. A solid product separated was filtered, washed with water and dried. Recrystallization from MeOH/CHCl3 (1:1) afforded pure product.
2-(4,5-Dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)phenol (4a)
A white solid; yield 68% (method C) and 80% (method D); mp 208–210°C (dec); IR: 3424 (OH), 1621 cm-1 (C=N); 1H NMR: δ 11.13 (s, 1H, OH), 8.63 (d, 1H, Ar-H), 8.27 (t, 2H, Ar-H and N-H), 8.03 (d, 1H, Ar-H), 7.96 (m, 1H, Ar-H), 7.82 (m, 1H, Ar-H), 7.27 (m, 2H, Ar-H), 6.90 (m, 2H, Ar-H), 5.43 (dd, 1H, HX, J = 3.6, 11.6 Hz), 3.89 (dd, 1H, HB, J = 17.2, 11.6 Hz), 3.39 (dd, 1H, HA, J = 3.6, 17.2 Hz); 13C NMR: δ 157.2, 153.6, 147.1, 131.5, 130.4, 129.9, 129.8, 129.5, 128.7, 128.4, 127.4, 124.3, 119.6, 117.0, 116.5, 116.2, 57.8 (CH), 40.3 (CH2); MS: m/z 331 [M+H]+ (100%). Anal. Calcd for C18H14N6O: C, 65.52; H, 4.27; N, 25.46. Found: C, 65.57; H, 4.20; N, 25.40.
2-(4,5-Dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)-4-methylphenol (4b)
A white solid; yield 60% (method C) and 84% (method D); mp 200–202°C (dec); IR: 3425 (OH), 1614 cm-1 (C=N); 1H NMR: δ 10.69 (s, 1H, OH), 8.70 (d, 1H, Ar-H), 8.11 (s, 1H, Ar-H), 7.99 (d, 1H, Ar-H), 7.89 (m, 1H, Ar-H), 7.73 (m, 1H, Ar-H), 7.07 (d, 1H, Ar-H), 6.99 (s, 1H, Ar-H), 6.91 (m, 1H, Ar-H), 6.34 (s, 1H, N-H), 5.60 (dd, 1H, HX, J = 4.2, 10.6 Hz), 4.04 (dd, 1H, HB, J = 10.6, 16.4 Hz), 3.22 (dd, 1H, HA, J = 4.2, 16.4 Hz), 2.26 (s, 3H, CH3); 13C NMR: δ 155.5, 153.3, 146.9, 131.6, 131.2, 131.0, 130.1, 129.1, 128.4, 128.2, 128.0, 126.8, 123.9, 116.7, 116.4, 115.6, 57.6 (CH), 40.0 (CH2), 20.4 (CH3); MS: m/z 345 [M+H]+ (25%). Anal. Calcd for C19H16N6O: C, 66.34; H, 4.68; N, 24.43. Found: C, 66.32; H, 4.60; N, 24.49.
4-Bromo-2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)phenol (4c)
A white solid; yield 62% (method C) and 85% (method D); mp 218–220°C (dec); IR: 3425 (OH), 1615 cm-1 (C=N); 1H NMR: δ 10.87 (s, 1H, OH), 8.71 (d, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.00 (d, 1H, Ar-H), 7.89 (m, 1H, Ar-H), 7.74 (m, 1H, Ar-H), 7.33 (m, 2H, Ar-H), 6.91 (d, 1H, Ar-H), 6.44 (s, 1H, N-H), 5.63 (dd, 1H, HX, J = 4.0, 10.8 Hz), 3.99 (dd, 1H, HB, J = 10.8, 16.6 Hz), 3.26 (dd, 1H, HA, J = 4.0, 16.6 Hz); 13C NMR: δ 155.9, 152.2, 146.1, 132.1, 131.0, 130.4, 129.3, 129.2, 128.6, 128.4, 127.7, 125.8, 123.3, 117.6, 115.9, 110.0, 57.3 (CH), 40.2 (CH2); MS: m/z 408 [M]+ (100%), 410 [M+2]+ (97%). Anal. Calcd for C18H13N6OBr: C, 52.86; H, 3.20; N, 20.54. Found: C, 52.80; H, 3.27; N, 20.54.
4-Chloro-2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)phenol (4d)
A white solid; yield 69% (method C) and 80% (method D); mp 212–214°C (dec); IR: 3426 (OH), 1617 cm-1 (C=N); 1H NMR: δ 10.84 (s, 1H, OH), 8.69 (d, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 8.00 (d, 1H, Ar-H), 7.89 (t, 1H, Ar-H), 7.74 (t, 1H, Ar-H), 7.19 (m, 2H, Ar-H), 6.95 (d, 1H, Ar-H), 6.44 (s, 1H, N-H), 5.63 (dd, 1H, HX, J = 7.2, 10.6 Hz), 3.98 (dd, 1H, HB, J = 10.6, 16.6 Hz), 3.25 (dd, 1H, HA, J = 7.2, 16.6 Hz); 13C NMR: δ 155.8, 152.1, 147.0, 131.6, 129.9, 129.8, 129.7, 129.5, 128.7, 127.5, 127.2, 124.3, 123.2, 118.7, 118.0, 116.5, 58.0 (CH), 40.5 (CH2); MS: m/z 364 [M]+ (100%), 366 [M+2]+ (33%). Anal. Calcd for C18H13Cl N6: C, 59.39; H, 3.60; N, 23.08. Found: C, 59.33; H, 3.68; N, 23.13.
4-Fluoro-2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)phenol (4e)
A white solid; yield 65% (method C) and 86% (method D); mp 206–208°C (dec); IR: 3421 (OH), 1614 cm-1 (C=N); 1H NMR: δ 10.84 (s, 1H, OH), 8.63 (d, 1H, Ar-H), 8.26 (s, 1H, Ar-H), 8.14 (d, 1H, Ar-H), 7.97 (m, 1H, Ar-H), 7.82 (m, 1H, Ar-H), 7.12 (m, 2H, Ar-H), 6.91 (d, 1H, Ar-H), 6.51 (s, 1H, N-H), 5.46 (dd, 1H, HX, J = 2.8, 10.8 Hz), 3.87 (dd, 1H, HB, J = 10.8, 17.0 Hz), 3.38 (dd, 1H, HA, J = 2.8, 17.0 Hz); 13C NMR: δ 157.2, 153.6, 147.1, 131.5, 130.4, 129.9, 129.8, 129.5, 128.7, 128.4, 127.4, 124.3, 119.6, 117.0, 116.5, 116.2, 57.8 (CH), 40.1 (CH2); MS: m/z 349 [M+H]+ (100%). Anal. Calcd for C18H13N6OF: C, 62.12; H, 3.76; N, 24.15. Found: C, 62.09; H, 3.72; N, 24.19.
2-(4,5-Dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)-5-methoxyphenol (4f)
A white solid; yield 64% (method C) and 83% (method D); mp 230–232°C (dec); IR: 3425 (OH), 1620 cm-1 (C=N); 1H NMR: δ 11.08 (s, 1H, OH), 8.70 (d, 1H, Ar-H), 8.13 (s, 1H, Ar-H), 7.99 (d, 1H, Ar-H), 7.87 (t, 1H, Ar-H), 7.72 (t, 1H, Ar-H), 7.10 (d, 1H, Ar-H), 6.56 (d, 1H, Ar-H), 6.45 (m, 1H, Ar-H) 6.22 (s, 1H, N-H), 5.57 (dd, 1H, HX, J = 6.4, 10.6 Hz), 4.00 (dd, 1H, HB, J = 10.6, 16.4 Hz), 3.81 (s, 3H, CH3), 3.18 (dd, 1H, HA, J = 6.4, 16.4 Hz); 13C NMR: δ 161.4, 158.9, 154.0, 147.1, 131.5, 130.4, 129.9, 129.8, 129.5, 128.7, 127.6, 124.3, 116.5, 110.4, 106.2, 101.4, 57.5 (CH), 55.7 (CH3), 40.3 (CH2); MS: m/z 361 [M+H]+ (25%). Anal. Calcd for C19H16N6O2: C, 63.39; H, 4.48; N, 23.34. Found: C, 63.35; H, 4.44; N, 23.37.
4-Chloro-2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)-5-methylphenol (4g)
A white solid; yield 62% (method C) and 82% (method D); mp 234–236°C (dec); IR: 3424 (OH), 1615 cm-1 (C=N); 1H NMR: δ 10.72 (s, 1H, OH), 8.69 (d, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 7.99 (d, 1H, Ar-H), 7.89 (t, 1H, Ar-H), 7.73 (t, 1H, Ar-H), 7.14 (s, 1H, Ar-H), 6.88 (s, 1H, Ar-H), 6.39 (s, 1H, N-H), 5.58 (dd, 1H, HX, J = 3.4, 10.4 Hz), 3.97 (dd, 1H, HB, J = 10.4, 16.2 Hz), 3.22 (dd, 1H, HA, J = 3.4, 16.2 Hz), 2.34 (s, 3H, CH3); 13C NMR: δ 157.2, 153.6, 147.1, 131.5, 130.4, 129.9, 129.8, 129.5, 128.7, 128.4, 127.4, 124.3, 119.6, 117.0, 116.5, 116.2, 57.8 (CH), 40.0 (CH2), 16.8 (CH3); MS: m/z 378 [M]+ (100%), 380 [M+2]+ (30%). Anal. Calcd for C19H15N6Cl: C, 60.37; H, 3.99; N, 22.23. Found: C, 60.25; H, 4.00; N, 22.19.
2,4-Dichloro-6-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazo-3-yl)phenol (4h)
A white solid; yield 60% (method C) and 85% (method D); mp 242–244°C (dec); IR: 3425 (OH), 1614 cm-1 (C=N); 1H NMR: δ 11.49 (s, 1H, OH), 8.71 (d, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.00 (d, 1H, Ar-H), 7.90 (t, 1H, Ar-H), 7.75 (t, 1H, Ar-H), 7.35 (d, 1H, Ar-H), 7.09 (d, 1H, Ar-H), 6.50 (s, 1H, N-H), 5.66 (dd, 1H, HX, J = 4.0, 10.6 Hz), 4.00 (dd, 1H, HB, J = 10.6, 16.8 Hz), 3.25 (dd, 1H, HA, J = 4.0, 16.8 Hz); 13C NMR: δ 157.2, 153.6, 147.1, 131.5, 130.4, 129.9, 129.8, 129.5, 128.7, 128.4, 127.4, 124.3, 119.6, 117.0, 116.5, 116.2, 57.8 (CH), 40.0 (CH2); MS: m/z 398 [M]+ (100%), 400 [M+2]+ (62%), 402 [M+4]+ (30%). Anal. Calcd for C18H12Cl2N6: C, 54.32; H, 3.04; N, 21.12. Found: C, 54.27; H, 3.09; N, 21.14.
The authors are grateful to The Head, Department of Chemistry, Osmania University, Hyderabad for providing laboratory facilities. V.H.R. is grateful to CSIR, New Delhi, for the award of a Junior Research Fellowship.
References
[1] Abdel-Wahab, B. F.; Abdel-Aziz, H. A.; Ahmed, E. M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635.Search in Google Scholar
[2] Nauduri, D.; Reddy, G. B. Antibacterials and antimycotics: part 1: synthesis and activity of 2-pyrazoline derivatives. Chem. Pharm. Bull. (Tokyo)1998, 46, 1254–1260.10.1248/cpb.46.1254Search in Google Scholar PubMed
[3] Abid, M.; Azam, A. Synthesis, characterization and antiamoebic activity of 1-(thiazolo[4,5-b] quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 2812–2816.Search in Google Scholar
[4] Abid, M.; Bhat, A. R.; Athar, F.; Azam, A. Synthesis, spectral studies and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines. Eur. J. Med. Chem. 2009, 44, 417–425.Search in Google Scholar
[5] Prasad, Y. R.; Rao, A. L.; Prasoona, L.; Murali, K.; Kumar, P. R. Synthesis and antidepressant activity of some 1,3,5- triphenyl-2-pyrazolines and 3-(2”-hydroxy naphthalen-1”-yl)-1,5-diphenyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034.Search in Google Scholar
[6] Ozdemir, Z.; Kandilci, H. B.; Gumusel, B.; Calıs, U.; Bilgin, A. A. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 373–379.Search in Google Scholar
[7] Rathish, I. G.; Javed, K.; Ahmad, S.; Bano, S.; Alam, M. S.; Pillai, K. K.; Singh, S.; Bagchi, V. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg. Med. Chem. Lett. 2009, 19, 255–258.Search in Google Scholar
[8] Gökhan-Kelekçi, N.; Yabanoğlu, S.; Küpeli, E.; Salgın, U.; Ozgen, O.; Ucar, G.; Yeşilada, E.; Kendi, E.; Yeşilada, A.; Bilgin, A. A. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786.Search in Google Scholar
[9] Taylor, E. C.; Patel, H. H, Synthesis of pyrazolo[3,4,-d]pyrimidine analogues of the potent antitumor agent N-{4-[2-(2-amino-4(3H)-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-l-glutamic acid (LY231514). Tetrahedron1992, 48, 8089–8100.Search in Google Scholar
[10] Althuis, T. S.; Moore, P. F.; Hess, J. H. Development of ethyl 3,4-dihydro-4-oxopyrimido[4,5-b]quinoline-2-carboxylate, a new prototype with oral antiallergy activity. J. Med. Chem. 1979, 22, 44–48.Search in Google Scholar
[11] Rowley, M.; Leeson, P. D.; Stevenson, G. I.; Moseley, A. M.; Stansfield, I.; Sanderson, I.; Robinson, L.; Baker, R.; Kemp, J. A.; Marshall, G. R. 3-Acyl-4-hydroxyquinolin-2(1H)-ones. Systemically active anticonvulsants acting by antagonism at the glycine site of the N-methyl-d-aspartate receptor complex. J. Med. Chem. 1993, 36, 3386–3396.Search in Google Scholar
[12] Abdel-Moty, S. G.; Abdel-Rahman, M. H.; Elsherief, H. A.; Kafafy, A. H. N. Synthesis of some quinoline thiosemicarbazone derivatives of potential antimicrobial activity. Bull. Pharm. Sci. 2005, 28, 79–93.Search in Google Scholar
[13] Vlahov, R.; Parushev, R.; St. Valvov, J.; Nickel, P.; Snatzke, G. Synthesis of some new quinoline derivatives – potential antimalarial drugs. Pure Appl. Chem. 1990, 62, 1303–1306.Search in Google Scholar
[14] Dominguez, J.; Basante, W.; Charris, J.; Riggione, F. Synthesis and activity of some quinolone derivatives against Plasmodium falciparum in vitro. Farmaco1996, 51, 407–412.Search in Google Scholar
[15] Liu, M.; Wilairat, P.; Croft, S. L.; Tan, A. L.; Go, M. L. Structure-activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem. Lett. 2003, 11, 2729–2738.Search in Google Scholar
[16] Shekarchi, M.; Marvasti, M. B.; Sharifzadeh, M.; Shafiee, A. Anticonvulsant activities of 7-phenyl-5H-thiazolo[5,4-e][1,2,3,4] tetrazolo[5,1-c]pyrrolo[1,2-a][1,4]diazepine and 7-phenyl-5H-thiazolo[5,4-e][1,3,4]triazolo[5,1-c] pyrrolo[1,2-a][1,4]diazepines. Iran. J. Pharm. Res. 2005, 1, 33–36.Search in Google Scholar
[17] Kumar, P.; Knaus, E. E. Synthesis and antiinflammatory activity of 5-(1,6-dihydropyridyl)-tetrazol-2-acetic acids, esters and amides. Drug Des. Discov. 1994, 11, 15–22.Search in Google Scholar
[18] Shukla, J. S.; Saxena, S. Studies on substituted tetrazoles as CNS depressant, anticonvulsant and monoamine oxidase inhibitory agents. Indian Drugs1980, 18, 15–18.Search in Google Scholar
[19] Dereu, N.; Evers, M.; Poujade, C.; Soler, F. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. WO 94267251994 [Chem. Abstr. 1995, 122, 214297p].Search in Google Scholar
[20] Singh, H.; Bhutani, K. K.; Malhotra, R. K.; Paul, D. 7a-Aza-B-homo[7a,7-d]tetrazole analogues of progesterone and testosterone. Experientia1978, 34, 557–558.Search in Google Scholar
[21] Singh, H.; Bhutani, K. K.; Malhotra, R. K.; Paul, D. Steroids and related studies. Part 49. 7a-Aza-B-homo[7a,7-d]tetrazole analogues of progesterone and testosterone. J. Chem. Soc. Perkin Trans. 1. 1979, 3166–3170.10.1039/p19790003166Search in Google Scholar
[22] Ahluwalia, V. K.; Kidwai, M. New Trends in Green Chemistry, 2nd Edition; Anamaya Publishers: New Delhi, 2006.Search in Google Scholar
[23] Kappe, C. O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005.Search in Google Scholar
[24] Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinolines and related fused pyridines, part 5. The synthesis of 2-chloroquinoline-3-carboxaldehyde. J. Chem. Soc. Perkin Trans. 1. 1981, 1520–1530.10.1039/p19810001520Search in Google Scholar
[25] Amir, M.; Kumar, S. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of 3,5-dimethyl pyrazoles, 3-methyl pyrazol-5-ones and 3,5-disubstituted pyrazolines. Indian J. Chem. 2005, 44B, 2532–2537.Search in Google Scholar
[26] Aggarwal, R.; Kumar, V.; Singh, S. P. Synthesis of some new 1-(6-fluorobenzothiazol-2-yl)-3-(4-fluoro-phenyl)-5-arylpyrazolines and their iodine (III) mediated oxidation to corresponding pyrazoles. Indian J. Chem. 2007, 46B, 1332–1336.Search in Google Scholar
[27] Ozdemir, A.; Turan-Zitouni, G.; Kaplancikh, Z. A.; Revial, G.; Guven, K. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-5-aryl-2-pyrazoline derivatives. Eur. J. Med. Chem. 2008, 42, 403–409.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes

