Abstract
After a long history of medical and biochemical investigations of vitamin B1, N-heterocyclic carbenes (NHCs) have recently been used as organocatalysts for a variety of synthetic reactions. This review article highlights the application of NHC-catalyzed reactions including enantioselective reactions to the heterocyclic synthesis. NHC-catalyzed benzoin condensation and Stetter reaction of ether- and amine-linked aldehydes give four- to six-membered oxygen and nitrogen heterocycles. NHC-catalyzed conjugate additions of enals to ketones and imines afford γ-lactones and γ-lactams. When the NHC-catalyzed reaction intermediates of enals are protonated, the reaction of the resulting enols with enones and ene-imines furnish formal hetero-Diels-Alder products. Acyl fluoride and esters can be activated by NHCs, and subsequent aldol and Michael reactions give β-lactones. Oxidation of intermediates derived from enals or the intermediates from ynals provide unsaturated acyl azolium which are transformed into cyclic products via subsequent nucleophilic reactions. NHCs also catalyze [2+2] cycloadditions of ketenes and other heterocumulenes and ring expansion of cyclic aldehydes.
Introduction
Five-membered azolium ylides such as 1,3-thiazolium, imidazolium, and 1,2,4-triazolium ylides are now commonly called N-heterocyclic carbenes (NHCs). These species have intensively been investigated after the success of ligands for transition metal complexes such as Grubbs catalysts of olefin metathesis reactions [1]. NHCs are highly stabilized singlet carbenes with the vacant p-orbital delocalized to the five-membered azolium aromatic ring, and the non-bonding electrons on the sp2 carbon atom interacting with the neighboring iminium nitrogen (Scheme 1). Thus, NHCs have no carbene-like reactivity such as cyclopropanation with olefins, but behave as highly stable carbanions. They can be formed by deprotonation of the precursor azolium salts with base, and the higher stability enables reversible nucleophilic attack on aldehydes, ketones, and acyl derivatives. This versatile reactivity can be utilized to catalytic reactions. From the late 1980s, various organocatalytic reactions using NHCs have been reported [2, 3], and they have also been applied to the synthesis of nitrogen- and oxygen-heterocyclic compounds. This review is focused on the recent advances in heterocyclic synthesis using NHCs and provides a concise overview of a long history of the research of the biological catalysis of an NHC, vitamin B1 [4].

Thiamin (1,3-thiazolylidene)
Although NHC-catalyzed synthetic reactions seem to be newer in organic synthesis, biochemically, the importance was established during the research of east Asian local disease ‘beriberi’ or ‘kakke’, the deficiency of vitamin B1 (1: thiamin). Thiamin is a 1,3-thiazolium salt found in rice brans, and its diphosphate functions as a coenzyme for decarboxylation of pyruvate in glycolysis to afford an acetyl anion equivalent 3 (Scheme 2); its deficiency results in disorders of the nervous system. After many efforts devoted to conquer the disease, thiamin 1 has been found to be as an essential nutrient. During the research of the action mechanism of thiamin [5–9], its catalytic property of benzoin condensation was unveiled [10–12].

In 1958, Breslow analyzed this thiamin-catalyzed benzoin condensation and proposed the nucleophilic enol-enamine intermediate (7: Breslow intermediate) formed by proton migration of the first addition product 6, that is, the addition product of an NHC species 5 to the aldehyde [13] (Scheme 3). This intermediate 7 is involved in exchange of the reactivity of the aldehyde carbon atom to nucleophilic from electrophilic (umpolung). Thus, NHC-catalyzed reaction of aldehydes can be used as the acyl anion equivalent 3. In 1976, Stetter reported the NHC-catalyzed conjugate addition of aldehydes to α,β-unsaturated carbonyl compounds to give 1,4-dicarbonyl compounds 12 [14] (Stetter reaction: Scheme 4).


N-Heterocyclic carbenes for heterocyclic synthesis
In the early examples, thiazolylidene-catalyzed benzoin condensation related to thiamin-based reaction was first investigated, and Breslow had already reported high C-H acidity of benzimidazolium [13]. In recent years, both monocyclic and poly-fused cyclic imidazolylidenes 13, 1,2,4-triazolylidenes 14 and 15, and particularly dimesitylimidazolylidene 16, have been employed as the catalyst. These NHCs are produced in situ from the parent azolium salts in the presence of usual strong bases such as DBU, DMAP, Cs2CO3, KOtBu, NaH, and KN(TMS)2. Even weaker Et3N and DIPEA are also applicable. A new method for the generation of NHCs is cathodic reduction of imidazolium salts which have recently become commercially available as ionic liquid materials. Sterically hindered NHCs can effectively change the steric environment of the transition state. The use of chiral 1,2,4-triazolylidene NHCs such as 17–19 results in high enantioselectivity and diastereoselectivity. A chiral catalyst 20 and its enantiomer are frequently employed in many asymmetric reactions.
Breslow intermediates 21 react as nucleophiles at the formyl carbon atom. However, oxidation of 21 with a weak oxidant affords electrophilic acyl azoliums 22 which react as acylating species (Scheme 5). Reactions of α,β-unsaturated aldehydes 23 are somewhat complicated; they give vinylogous Breslow intermediates 24 which react as nucleophiles at the β-position, whereas protonation from the conjugate acid of the base used for deprotonation of the starting azolium gives an enol-azoliums 25 which also react as nucleophiles at the α-position (Scheme 6). Ketene 26 or other oxocumulenes react with NHC to give enolates 27 which, in turn, can react as acyl enolates (Scheme 7).



Intra- and intermolecular benzoin condensation and related reactions
Intramolecular benzoin condensation of oxoalkoxybenzoic aldehyde 28 catalyzed by chiral NHCs gives chiral hydroxychromanone 30 [15, 16] (Scheme 8). The acyl anion intermediate can be a carbon nucleophile for the nucleophilic substitution, and the reaction of tosylates 31 yields annulated chromanone 32 and dihydrobenzofuranone 33 through an unclear reaction mechanism [17] (Scheme 9).


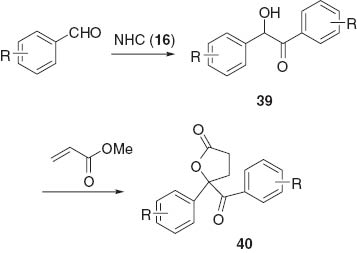
Intermolecular benzoin condensation followed by oxidation gives 1,2-diketones such as benzil, which can be utilized to obtain heterocycles such as quinoxaline 34 [18] and imidazole 35 [19] in one-pot reactions (Scheme 10). NHC-catalyzed reaction of imidoyl chloride 36 and aldehyde directly furnishes iminoketone 37 which can be cyclized to quinoxaline 38 [20] (Scheme 11). Benzoin 39 obtained by NHC-catalyzed benzoin condensation undergoes reaction with methyl acrylate to give lactone 40 [21] (Scheme 12). The NHC-catalyzed sequential benzoin condensation of phthalaldehyde 41 gives spirocyclic lactone 42 or tetrahydropentalene 43 depending on the used NHC catalyst [22] (Scheme 13). The NHC-catalyzed reaction proceeds under the basic conditions, and the reaction of α-aminosulfone 44 yields aldimine, which is a precursor to N-acylaminoketone 45. Imidazole 46 has been obtained by the reaction of 45 with primary amines in a one-pot manner [23] (Scheme 14).




An electrolytically generated NHC is a catalyst in the reaction of aldimine 47 and carbonyl chloride to give β-lactam 49. In this transformation, the initially formed zwitterionic intermediate 48 undergoes a reaction with carbonyl chloride without proton migration to the enamine intermediate, similarly to the Breslow intermediate [24] (Scheme 15). The Breslow intermediate of ketoaldehyde 50 can undergo a reaction with a nitroso group to form furan-, thiophene-, and pyridine-fused oxazinones 51 [25] (Scheme 16).


Intra- and intermolecular Stetter reaction
In 1995, Ciganek reported an intramolecular Stetter reaction of o-alkenyloxy benzaldehyde 52 to chromanone 53 [26] (Scheme 17). In the following year, Enders reported the asymmetric intramolecular Stetter reaction [27]. Thereafter, this reaction became a standard to measure enantioselectivity of the various chiral NHC catalysts [28–31] including peptidic thiazolylidene [32] (Scheme 18). Pd(II)-catalyzed substitution of 54 with allyl acetate 55 followed by NHC-catalyzed cyclization furnishes quinolone product 56 [33] (Scheme 17). Butyrolactone 60 has been obtained by the enantioselective intramolecular Stetter reaction [34] (Scheme 18).


Another enantioselective 5-exo-trigonal intramolecular Stetter reaction yields pyrrolidinone and sulfone 62 and the benzene-fused products [35, 36] (Scheme 19). Desymmetrization of cyclohexadienones 63 and 65 has been achieved by asymmetric intramolecular Stetter reaction [37, 38] (Scheme 20).


Because the Stetter reaction is related to Michael addition, the acceptor double bond needs activation by an electron-withdrawing group. However, the intramolecular Stetter reaction of allyl ethers 67 and 69 need not necessarily be activated by the electron-withdrawing group [39, 40] (Scheme 21). When aerial oxygen has been incorporated with the NHC-catalyzed cyclization of ynal 71, unsaturated 5-exo-digonal and 6-endo-digonal cyclization products 72 and 73 have been obtained [41] (Scheme 22). The Stetter reaction of propargyl ether 74 yields chromanone 76, which can be transformed under basic conditions into the ring contracted product 79 through intermediaries of 77 and 78 [42, 43] (Scheme 23).



Silyl ketone 80 can serve as the equivalent of aldehyde function, which is exemplified in Scheme 24 by intermolecular Stetter reaction with α,β-unsaturated ketone to give 1,4-diketone 82. This product can be converted into furan 83 and pyrrole 84 in one-pot reactions [44] (Scheme 24).

Conjugate addition of enals under NHC catalysis
As shown in Scheme 25, NHC reacts with the enal 85 at the aldehyde group to form the vinylogous Breslow intermediate 86. Then, the reaction course takes two pathways. Intramolecular nucleophilic addition of the β-carbon-centered anion yields pyrrolidinone 87, which undergoes lactonization to 88. Another pathway involves protonation of 86 to an enol azolium intermediate 89, which is then transformed into 92 through the intermediaries of 90 and 91 [45]. NHC-catalyzed reactions of enals with aldehydes [46–49] and ketones 93 [50], 1,2-dicarbonyl compounds [51], and sulfonylimines 97 [52] affords the respective stereoselectively γ-lactones 94, spirolactones 95, 96, and γ-lactams 98 in a stereoselective manner (Scheme 26). Enantioselective syntheses of γ-lactones and γ-lactams using various chiral NHC catalysts have been reported [53–57] (Scheme 27). Because the reaction of an ester carbonyl group is faster than the reaction with an imidate C-N double bond, the imidate 109 undergoes a chemoselective reaction with enal to give piperidinedione 110 after cyclization of intermediate amide anion [58] (Scheme 28).




Lewis acid-catalyzed reaction of enal 111 generates 112 which then undergoes intramolecular aldol condensation. The resultant β-lactone 113 is decarboxylated to cyclopentene 114. The related reaction of enal and sulfonylimine 115 gives β-lactam 117 through an intermediate 116 [59].
However, for this reaction, the intermediate 116 can also be considered to be generated through the oxy-Cope rearrangement of the initially formed intermediate of benzoin condensation [60] (Scheme 29).

After conjugate addition of enal to enone alcohol 118, the successive lactonization varies depending on the NHC catalyst. When imidazolylidene catalyst is used, cyclization occurs at the oxygen atom of the hydroxyl group to give β-lactone 119. By contrast, when triazolylidene catalyst is used, cyclization occurs at the oxide anion generated by the addition of enal to afford γ-lactone 120 [61] (Scheme 30). NHC-catalyzed conjugate addition of enal to tropone 121 followed by 1,3-hydrogen shift furnishes δ-lactone 122 [62] (Scheme 31).


Protonation of the vinylogous Breslow intermediate 123 generates cationic enol 124, which is nucleophilic enough to undergo conjugate addition to enone yielding δ-lactone 126 through an open-chain intermediate 125 [63, 64] (Scheme 32). The product can be considered as a formal hetero-Diels-Alder reaction product of a heterodiene and a ketene dienophile.

The use of chiral NHCs provides enantioselective routes to dihydropyranone 128 [65] and dihydropyridone 130 [66] (Scheme 33). Because α-chloroaldehyde 131 [67, 68] and β-chloro-α-hydroxysulfonate 136 [69] are equivalent species of the corresponding enals, their hetero-Diels-Alder reactions are stereoselective (Scheme 34). A simple reaction of an ester 139 with an NHC gives acyl azolium 140, which can be deprotonated to the ketene dienophile intermediate 141, which is the precursor to the hetero-Diels-Alder product 142 [70] (Scheme 34).


Reaction of cyclopropanecarbaldehyde 143 with NHC gives ring-opened enolate 144, which is equilibrated with the enolate 145. The reaction of this ketene dienophile with enone furnishes dihydropyranone 146 [71] (Scheme 35).

The NHC-catalyzed hetero-Diels-Alder reaction of nitroalkene 147 with cyanoenone is related to the reaction of enals; synthesis of dihydropyran 149 has been reported [72] (Scheme 36).

Reaction of phenoxyaldehyde 150 with NHC causes cleavage of the C-O bond to give phenoxide 151. Michael addition of the enol azolium with 151 followed by cyclization of the phenoxide anion yields lactone 152 instead of the expected hetero-Diels-Alder product [73] (Scheme 37).

Acyl azolium as an acylating reagent
Nucleophilic substitution of acyl fluoride 153 with NHC generates acyl azolium 154. The resulting fluoride anion activates silyl enol ether 155 to form enolate 156, which undergoes a reaction with acyl azolium 154 to give Michael adduct 157. Successive aldol reaction followed by β-lactonization yields 158, which is finally decarboxylated to cyclohexadiene 159 [74] (Scheme 38). A similar reaction of silyl enol ether 160 affords β-lactone 161, which can be converted to the corresponding diol 162 by LiAlH4 reduction [75]. Silyloxycyclopropane 163 can also be activated to enolate 164 by fluoride generated in the reaction of acyl fluoride. Claisen rearrangement of the intermediate product 165–166 followed by lactonization yields β-lactone 167 [76] (Scheme 39).


The Breslow intermediate 168 can be oxidized with a weak oxidant 169 to give acyl azolium 170; the macrolide formation gives 171 [77] (Scheme 40). Acyl azolium intermediates derived from enals in the presence of oxidant 169 are Michael acceptors. Thus, acyl azolium 172 undergoes a reaction with stabilized enamine, which followed by cyclization produces dihydropyridone 173 [78]. Tandem Michael addition of enal 174 and 1,3-diketone 175 affords tricyclic lactone 176 [79] (Scheme 41). The reaction of enol ester 177 affords a pair of enolate 178 and unsaturated acyl azolium 179. Michael addition of the pair followed by lactonization gives 181 [80] (Scheme 42).



Protonation to the Breslow intermediate of ynal 182 by oxindole 183 also generates unsaturated acyl azolium 184 and enolate 185. Michael addition of 185 to 184 and successive lactonization similarly furnish spiro-lactone 187 [81] (Scheme 43). In a similar way, cyano-1,4-diketone 188 has been reacted with ynal to yield 189 after successive cyclization [82]. Another precursor of the unsaturated acyl azolium is α-bromo enal. For example, the treatment of enal 190 with stabilized enamine 191 in the presence of NHC produces hydroquinolinone 192 [83] (Scheme 44).


Activation of the leaving group by an NHC has been observed in the transformation of α-bromo ketone 193 to epoxide 194. The latter compound has been reacted with o-phenylenediamine under air oxidation conditions to give quinoxaline 195 [84] (Scheme 45).

NHC-catalyzed [2+2] cycloaddition of heterocumulene and related reactions
Reaction of ketenes 196 and NHCs gives enol intermediates 197, and the subsequent formal [2+2] cyclization of enols with ketones [85, 86] (Scheme 46), activated imines 201 and 203 [87, 88] (Scheme 47) yields β-lactones 198 and 200, and β-lactams 202 and 204, respectively. The reactions of ketene and azodicarboxylate 205 [89, 90] or iminosulfane oxide 207 [91] also enantioselectively afford [2+2] cycloadducts, diazetidinone 206 or thiazetidinone oxide 208 (Scheme 48).



Although the treatment of isothiocyanate 209 with nitroalkene 147 gives [2+2] cycloadduct azatidinethione 211 [92] through the enethiol intermediate 210, the use of isocyanate 212 furnishes [2+2+2] cycloadduct 213 [93] (Scheme 49). The reaction of an aryl ketene with substrate 214 or 216 in the presence of NHC furnishes the respective adduct 215 or 217 [94, 95] (Scheme 50).


NHC-catalyzed reaction of carbon dioxide with tosylaziridine 218 followed by desulfonylation with samarium diiodide in a one-pot manner gives the insertion product 219 [96] (Scheme 51).

Ring expansion of cyclic aldehydes
Because the saturated C-N and C-O bond migrate to the acyl anion intermediate generated by the reaction of cyclic aldehyde and NHC, the treatment of pyrrolidine, azetidine, oxetane, oxolane, and hydropyran 220–222 furnish the corresponding ring-expanded lactams and lactones 223–225 [97–99] (Scheme 52). Successive enantioselective Michael reaction and ring expansion of aldehyde 226 yields chromanone 228 [100] (Scheme 53). Optically active product 230 has been obtained by treatment of azetidinone 229 with chiral NHC in a process of kinetic enantioselective ring expansion followed by NaBH4 reduction of the unreacted aldehyde to alcohol 231 [101] (Scheme 54).



Conclusions
Starting from analysis of the biochemical behavior of vitamin B1, NHCs including thiazolylidene, imidazolylidene, and 1,2,4-triazolylidene carbenes can be applied to umpolung of aldehydes to form acyl anion equivalents. NHC-catalyzed Stetter reaction with α,β-unsaturated compounds extends the ability of the NHC catalysts to be employed in various heterocyclic syntheses. Recently, NHC-catalyzed conjugate addition of α,β-unsaturated aldehydes has established the ability to provide new strategies for the synthesis of heterocyclic compounds. Treatment with NHCs not only activates aldehydes but also acyl groups and heterocumulenes. For asymmetric reactions, NHC-catalyzed reactions are usually highly enantioselective as the steric environment can flexibly be controlled by the choice of chiral substituents. Because NHCs are relatively new catalysts, more novel reaction patterns will be developed.
I am grateful to Emeritus Biochemistry Professor Makoto Matsuda of The Jikei University School of Medicine who taught me the history of medical and biochemical research of vitamin B1 and stimulated my interest to consider the correlation between biochemistry and biomimetic organic synthesis.
References
[1] Vougioukalakis, G. C.; Grubbs, R. H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010, 110, 1746–1787.Suche in Google Scholar
[2] Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655.Suche in Google Scholar
[3] Vora, H. U.; Rovis, T. Asymmetric N-heterocyclic carbene (NHC) catalyzed acyl anion reactions. Aldrichim. Acta 2011, 44, 3–11.Suche in Google Scholar
[4] Kluger, R.; Tittmann, K. Thiamin diphosphate catalysis: enzymic and nonenzymic covalent intermediates. Chem. Rev. 2008, 108, 1797–1833.Suche in Google Scholar
[5] Takaki, K. Three lectures on the preservation of health amongst the personnel of the Japanese Navy and Army. Lancet 1906, 167, 1369–1374.10.1016/S0140-6736(00)68249-1Suche in Google Scholar
[6] Eijkman, C. Ein Versuch zur Bekampfung der Beri-beri. Virchow Archiv. 1897, 149, 187–194.Suche in Google Scholar
[7] Makino, K.; Imai, T. Bemerkung uber die Chemie des antineuritische Vitamins. Z. Physiol. Chem. 1936, 239, 1–2.Suche in Google Scholar
[8] Williams, R. R.; Cline, J. K. Synthesis of vitamin B1. J. Am. Chem. Soc. 1936, 58, 1504–1505.10.1021/ja01299a505Suche in Google Scholar
[9] Lohmann, K.; Schuster, P. Untersuchungen uber die Cocarboxylase. Biochem. Z. 1937, 294, 188–214.Suche in Google Scholar
[10] Ukai, T.; Tanaka, R.; Dokawa, S. On the new catalysts of acyloin condensations. 1. Effect of thiazolium salts and their ring-opened forms. J. Pharm. Soc. Jpn. 1943, 63, 296–300.Suche in Google Scholar
[11] Mizuhara, S. Action mechanism of vitamin B1. J. Jpn. Biochem. 1950, 22, 102–106.Suche in Google Scholar
[12] Mizuhara, S.; Handler, P. Mechanism of thiamine-catalyzed reactions. J. Am. Chem. Soc. 1954, 76, 571–573.Suche in Google Scholar
[13] Breslow, R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J. Am. Chem. Soc. 1958, 80, 3719–3720.Suche in Google Scholar
[14] Stetter, H. Catalyzed addition of aldehydes to activated double bonds – a new synthetic approach. Angew. Chem. Int. Ed. 1976, 15, 639–647.Suche in Google Scholar
[15] Takikawa, H.; Hachisu, Y.; Bode, J. W.; Suzuki, K. Catalytic enantioselective crossed aldehyde–ketone benzoin cyclization. Angew. Chem. Int. Ed. 2006, 45, 3492–3494.Suche in Google Scholar
[16] Enders, D.; Niemeier, O.; Raabe, G. Asymmetric synthesis of chromanones via N-heterocyclic carbene catalyzed intramolecular crossed-benzoin reactions. Synlett 2006, 2431–2334.10.1055/s-2006-950403Suche in Google Scholar
[17] He, J.; Zheng, J.; Liu, J.; She, X.; Pan, X. N-Heterocyclic carbene catalyzed nucleophilic substitution reaction for construction of benzopyrones and benzofuranones Org. Lett. 2006, 8, 4637–4640.Suche in Google Scholar
[18] Lin, Y.; Lei, X.; Yang, Q.; Yuan, J.; Ding, Q.; Xua, J.; Peng, Y. N-Heterocyclic carbene catalyzed one-pot synthesis of 2,3-diarylquinoxalines. Synthesis 2012, 44, 2699–2706.Suche in Google Scholar
[19] Wu, L.; Jing, X.; Zhu, H.; Liu, Y.; Yan, C. One-pot synthesis of polysubstituted imidazoles from arylaldehydes in water catalyzed by NHC using microwave irradiation. J. Chilean Chem. Soc. 2012, 57, 1204–1207.Suche in Google Scholar
[20] Suzuki, Y.; Murofushi, M.; Kei Manabe, K. One-pot synthesis of unsymmetrical benzils and N-heteroarenes through nucleophilic aroylation catalyzed by N-heterocyclic carbene. Tetrahedron 2013, 69, 470–473.Suche in Google Scholar
[21] Ye, W.; Cai, G.; Zhuang, Z.; Jia, X.; Zhai, H. One-step assembly of functionalized γ-butyrolactones from benzoins or benzaldehydes via an N-heterocyclic carbene-mediated tandem reaction. Org. Lett. 2005, 7, 3769–3771.Suche in Google Scholar
[22] Cheng, Y.; Peng, J.-H.; Li, Y.-J.; Shi, X.-Y.; Tang, M.-S.; Tan, T.-Y. N-Heterocyclic carbene catalyzed reaction of phthalaldehydes: controllable stereoselective synthesis of polyhydroxylated spiro- and fused indenones dictated by the structure of NHC catalysts. J. Org. Chem. 2011, 76, 1844–1851.Suche in Google Scholar
[23] Frantz, D. E.; Morency, L.; Soheili, A.; Murry, J. A.; Grabowski, E. J. J.; Tillyer, R. D. Synthesis of substituted imidazoles via organocatalysis. Org. Lett. 2004, 6, 843–846.Suche in Google Scholar
[24] Feroci, M. Investigation of the role of electrogenerated N-heterocyclic carbene in the Staudinger synthesis in ionic liquid. Int. J. Org. Chem. 2011, 1, 191–201.Suche in Google Scholar
[25] Qu, J.; Cheng, Y. A versatile method for the synthesis of heterocyclic ring-fused 1,2-oxazinones from the NHC-catalyzed reactions of 2-aroylvinylarylaldehydes with nitrosoarenes. Tetrahedron 2013, 69, 888–894.10.1016/j.tet.2012.10.089Suche in Google Scholar
[26] Ciganek, E. Esters of 2,3-dihydro-3-oxobenzofuran-2-acetic acid and 3,4-dihydro-4-oxo-2H-1-benzopyran-3-acetic acid by intramolecular Stetter reactions. Synthesis 1995, 1311–1314.10.1055/s-1995-4100Suche in Google Scholar
[27] Enders, D.; Breuer, K.; Runsink, J.; Teles, J. H. The first asymmetric intramolecular Stetter reaction. Preliminary communication. Helv. Chim. Acta 1996, 79, 1899–1902.Suche in Google Scholar
[28] Kerr, M. S.; de Alaniz, J. R.; Rovis, T. A highly enantioselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 2002, 124, 10298–10299.Suche in Google Scholar
[29] Kerr, M. S.; Rovis, T. Effect of the Michael acceptor in the asymmetric intramolecular Stetter reaction. Synlett 2003, 1934–1936.10.1055/s-2003-41458Suche in Google Scholar
[30] Nakamura, T.; Hara, O.; Tamura, T.; Makino, K.; Hamada, Y. A facile synthesis of chroman-4-ones and 2,3-dihydroquinolin-4-ones with quaternary carbon using intramolecular Stetter reaction catalyzed by thiazolium salt. Synlett 2005, 155–157.10.1055/s-2004-835666Suche in Google Scholar
[31] Rong, Z.-Q.; Li, Y.; Yang, G.-Q.; You, S.-L. D-Camphor-derived triazolium salts for enantioselective intramolecular Stetter reactions. Synlett 2011, 1033–1037.10.1055/s-0030-1259732Suche in Google Scholar
[32] Mennen, S. M.; Blank, J. T. B.; Tran-Dube, M. B. Imbriglio, J. E.; Miller, S. J. A. Peptide-catalyzed asymmetric Stetter reaction. Chem. Commun. 2005, 195–197.10.1039/B414574GSuche in Google Scholar PubMed
[33] Nemoto, T.; Fukuda, T.; Hamada, Y. Efficient synthesis of 3-substituted 2,3-dihydroquinolin-4-ones using a one-pot sequential multi-catalytic process: Pd-catalyzed allylic amination-thiazolium salt-catalyzed Stetter reaction cascade. Tetrahedron Lett. 2006, 47, 4365–4368.Suche in Google Scholar
[34] de Alaniz, J. R.; Rovis, T. A highly enantio- and diastereoselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 2005, 127, 6284–6289.Suche in Google Scholar
[35] Kerr, M. S.; Rovis, T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J. Am. Chem. Soc. 2004, 126, 8876–8877.Suche in Google Scholar
[36] Moore, J. L.; Kerr, M. S.; Rovis, T. Enantioselective formation of quaternary stereocenters using the catalytic intramolecular Stetter reaction. Tetrahedron 2006, 62, 11477–11482.Suche in Google Scholar
[37] Liu, Q.; Rovis, T. Asymmetric synthesis of hydrobenzofuranones via desymmetrization of cyclohexadienones using the intramolecular Stetter reaction. J. Am. Chem. Soc. 2006, 128, 2552–2553.Suche in Google Scholar
[38] Jia, M.-Q.; Liu, C.; You, S.-L. Diastereoselective and enantioselective desymmetrization of α-substituted cyclohexadienones via intramolecular Stetter reaction. J. Org. Chem. 2012, 77, 10996–11001.Suche in Google Scholar
[39] Hirano, K.; Biju, A. T.; Piel, I.; Glorius, F. N-Heterocyclic carbene-catalyzed hydroacylation of unactivated double bonds. J. Am. Chem. Soc. 2009, 131, 14190–14191.Suche in Google Scholar
[40] Piel, I.; Steinmetz, M.; Hirano, K.; Froehlich, R. Grimme, R. S.; Glorius, F. Highly asymmetric NHC-catalyzed hydroacylation of unactivated alkenes. Angew. Chem. Int. Ed. 2011, 50, 4983–4987.Suche in Google Scholar
[41] Park, J. H.; Bhilare, S. V.; Youn, S. W. NHC-catalyzed oxidative cyclization reactions of 2-alkynylbenzaldehydes under aerobic conditions: synthesis of O-heterocycles. Org. Lett. 2011, 13, 2228–2231.Suche in Google Scholar
[42] Franz, J. F.; Fuchs, P. J. W.; Zeitler, K. A versatile combined N-heterocyclic carbene and base-catalyzed multiple cascade approach for the synthesis of functionalized benzofuran-3-(2H)-ones. Tetrahedron Lett. 2011, 52, 6952–6956.Suche in Google Scholar
[43] Padmanaban, M.; Biju, A. T.; Glorius, F. Efficient synthesis of benzofuranones: N-heterocyclic carbene (NHC)/base-catalyzed hydroacylation-Stetter-rearrangement cascade. Org. Lett. 2011, 13, 5624–5627.Suche in Google Scholar
[44] Mattson, A. E.; Bharadwaj, A. R.; Zuhl, A. M.; Scheidt, K. A. Thiazolium-catalyzed additions of acylsilanes: a general strategy for acyl anion addition reactions. J. Org. Chem. 2006, 71, 5715–5724.Suche in Google Scholar
[45] Struble, J. R.; Bode, J. W. Formal synthesis of salinosporamide A via NHC-catalyzed intramolecular lactonization. Tetrahedron 2009, 65, 4957–4967.Suche in Google Scholar
[46] Burstein, C.; Glorius, F. Organocatalyzed conjugate umpolung of α,β-unsaturated aldehydes for the synthesis of γ-butyrolactones. Angew. Chem. Int. Ed. 2004, 43, 6205–6208.Suche in Google Scholar
[47] Sohn, S. S.; Rosen, E. L.; Bode, J. W. N-Heterocyclic carbene-catalyzed generation of homoenolates: γ-butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc. 2004, 126, 14370–14371.Suche in Google Scholar
[48] Feroci, M.; Chiarotto, I.; Orsini, M.; Pelagalli, M.; Inesi, A. Umpolung reactions in an ionic liquid catalyzed by electrogenerated N-heterocyclic carbenes. Synthesis of saturated esters from activated α,β-unsaturated aldehydes. Chem. Commun. 2012, 48, 5361–5363.Suche in Google Scholar
[49] Dunn, M. H.; Cole, M. L.; Harper, J. B. Effects of an ionic liquid solvent on the synthesis of γ-butyrolactones by conjugate addition using NHC organocatalysts. RSC Adv. 2012, 2, 10160–10162.Suche in Google Scholar
[50] Burstein, C.; Tschan, S.; Xie, X.; Glorius, F. N-Heterocyclic carbene-catalyzed conjugate umpolung for the synthesis of γ-butyrolactones. Synthesis 2006, 2418–2439.10.1055/s-2006-942447Suche in Google Scholar
[51] Nair, V.; Vellalath, S.; Poonoth, M.; Mohan, R.; Suresh, E. N-Heterocyclic carbene catalyzed reaction of enals and 1,2-dicarbonyl compounds: stereoselective synthesis of spiro γ-butyrolactones. Org. Lett. 2006, 8, 507–509.Suche in Google Scholar
[52] He, M.; Bode, J. W. Catalytic synthesis of γ-lactams via direct annulations of enals and N-sulfonylimines. Org. Lett. 2005, 7, 3131–3134.Suche in Google Scholar
[53] Zhao, X.; DiRocco, D. A.; Rovis, T. N-Heterocyclic carbene and Brønsted acid cooperative catalysis: asymmetric synthesis of trans-γ-lactams. J. Am. Chem. Soc. 2011, 133, 12466–12469.Suche in Google Scholar
[54] Rommel, M.; Fukuzumi, T.; Bode, J. W. Cyclic ketimines as superior electrophiles for NHC-catalyzed homoenolate additions with broad scope and low catalyst loadings. J. Am. Chem. Soc. 2008, 130, 17266–17267.Suche in Google Scholar
[55] Raup, D. E. A.; Cardinal-David, B.; Holte, D.; Scheidt, K. A. Cooperative catalysis by carbenes and Lewis acids in a highly stereoselective route to γ-lactams. Nature Chem. 2010, 2, 766–771.Suche in Google Scholar
[56] Lv, H.; Tiwari, B.; Mo, J.; Xing, C.; Chi, Y. R. Highly enantioselective addition of enals to isatin-derived ketimines catalyzed by N-heterocyclic carbenes: synthesis of spirocyclic γ-lactams. Org. Lett. 2012, 14, 5412–5415.Suche in Google Scholar
[57] Sun, L.-H.; Shen, L.-T.; Ye, S. Highly diastereo- and enantioselective NHC-catalyzed [3+2] annulation of enals and isatins. Chem. Commun. 2011, 47, 10136–10138.Suche in Google Scholar
[58] Singh, A. K.; Chawla, R.; Rai, A.; Yadav, L. D. S. NHC-catalysed diastereoselective synthesis of multifunctionalised piperidines via cascade reaction of enals with azalactones. Chem. Commun. 2012, 48, 3766–3768.Suche in Google Scholar
[59] Cardinal-David, B.; Raup, D. E. A.; Scheidt, K. A. Cooperative N-heterocyclic carbene/Lewis acid catalysis for highly stereoselective annulation reactions with homoenolates. J. Am. Chem. Soc. 2010, 132, 5345–5347.Suche in Google Scholar
[60] He, M.; Bode, J. W. Enantioselective, NHC-catalyzed bicyclo-β-lactam formation via direct annulations of enals and unsaturated N-sulfonyl ketimines. J. Am. Chem. Soc. 2008, 130, 418–419.Suche in Google Scholar
[61] Kaeobamrung, J.; Bode, J. W. Stereodivergency of triazolium and imidazolium-derived NHCs for catalytic, enantioselective cyclopentane synthesis. Org. Lett. 2009, 11, 677–680.Suche in Google Scholar
[62] Nair, V.; Poonoth, M.; Vellalath, S.; Suresh, E.; Thirumalai, R. An N-heterocyclic carbene-catalyzed [8+3] annulation of tropone and enals via homoenolate. J. Org. Chem 2006, 71, 8964–8965.Suche in Google Scholar
[63] Nair, V.; Paul, R. R.; Lakshmi, K. C. S.; Menon, R. S.; Jose, A.; Sinu, C. R. N-Heterocyclic carbene (NHC) catalyzed annulation of enals and vinyl ketones: a novel synthesis of [2H]-pyranones. Tetrahedron Lett. 2011, 52, 5992–5994.Suche in Google Scholar
[64] Takaki, K.; Shiraishi, K.; Okinaga, K.; Takahashi, S.; Komeyama, K. NHC-catalyzed reaction of enals with unactivated enones: substituent effect on the formation of 1:1 and 1:2 adducts. RSC Adv. 2011, 1, 1799–1807.Suche in Google Scholar
[65] Fang, X.; Chen, X.; Chi, Y. R. Enantioselective Diels-Alder reactions of enals and alkylidene diketones catalyzed by N-heterocyclic carbenes. Org. Lett. 2011, 13, 4708–4711.Suche in Google Scholar
[66] He, M.; Struble, J. R.; Bode, J. W. Highly enantioselective azadiene Diels-Alder reactions catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2006, 128, 8418–8420.Suche in Google Scholar
[67] He, M.; Uc, G. J.; Bode, J. W. Chiral N-heterocyclic carbene catalyzed, enantioselective oxodiene Diels-Alder reactions with low catalyst loadings. J. Am Chem. Soc. 2006, 128, 15088–15089.Suche in Google Scholar
[68] Yang, L.; Wang, F.; Chua, P. J.; Lv, Y.; Zhong, L.-J.; Zhong, G. N-Heterocyclic carbene (NHC)-catalyzed highly diastereo- and enantioselective oxo-Diels-Alder reactions for synthesis of fused pyrano[2,3-b]indoles. Org. Lett. 2012, 14, 2894–2897.Suche in Google Scholar
[69] He, M.; Beahm, B. J.; Bode, J. W. Chiral NHC-catalyzed oxodiene Diels-Alder reactions with α-chloroaldehyde bisulfite salts. Org. Lett. 2008, 10, 3817–3820.Suche in Google Scholar
[70] Hao, L.; Du, Y.; Lv, H.; Chen, X.; Jiang, H.; Shao, Y.; Chi, Y. R. Enantioselective activation of stable carboxylate esters as enolate equivalents via N-heterocyclic carbene catalysts. Org. Lett. 2012, 14, 2154–2157.Suche in Google Scholar
[71] Lv, H.; Mo, J.; Fang, X.; Chi Y. R. Formal Diels-Alder reactions of chalcones and formylcyclopropanes catalyzed by chiral N-heterocyclic carbenes. Org. Lett. 2011, 13, 5366–5369.Suche in Google Scholar
[72] Chen, X.-Y.; Sun, L.-H.; Ye, S. N-Heterocyclic carbene catalyzed [4+2] cycloaddition of nitroalkenes with oxodienes. Chem. Eur. J. 2013, 19, 4441–4445.Suche in Google Scholar
[73] Phillips, E. M.; Wadamoto, M.; Roth, H. S.; Ott, A. W.; Scheidt, K. A. NHC-catalyzed reactions of aryloxyacetaldehydes: a domino elimination/conjugate addition/acylation process for the synthesis of substituted coumarins. Org. Lett. 2009, 11, 105–108.Suche in Google Scholar
[74] Ryan, S. J.; Candish, L.; Lupton, D. W. N-Heterocyclic carbene-catalyzed (4+2) cycloaddition/decarboxylation of silyl dienol ethers with α,β-unsaturated acid fluorides. J. Am. Chem. Soc. 2011, 133, 4694–4697.Suche in Google Scholar
[75] Ryan, S. J.; Stasch, A.; Paddon-Row, M. N.; Lupton, D. W. Synthetic and quantum mechanical studies into the N-heterocyclic carbene catalyzed (4+2) cycloaddition. J. Org. Chem. 2012, 77, 1113–1124.Suche in Google Scholar
[76] Candish, L.; Lupton, D. W. N-Heterocyclic carbene-catalyzed Ireland-Coates Claisen rearrangement: synthesis of functionalized β-lactones. J. Am. Chem. Soc. 2013, 135, 58–61.Suche in Google Scholar
[77] Lee, K.; Kim, H.; Hong, J. N-Heterocyclic carbene catalyzed oxidative macrolactonization: total synthesis of (+)-dactylolide. Angew. Chem. Int. Ed. 2012, 51, 5735–5738.Suche in Google Scholar
[78] Wanner, B.; Mahatthananchai, J.; Bode, J. W. Enantioselective synthesis of dihydropyridinones via NHC-catalyzed aza-Claisen reaction. Org. Lett. 2011, 13, 5378–5381.Suche in Google Scholar
[79] Biswas, A.; De Sarkar, S.; Fröhlich, R.; Studer, A. Highly stereoselective synthesis of 1,2,3-trisubstituted indanes via oxidative N-heterocyclic carbene-catalyzed cascades. Org. Lett. 2011, 13, 4966–4969.Suche in Google Scholar
[80] Ryan, S. J.; Candish, L.; Lupton, D. W. N-Heterocyclic carbene-catalyzed generation of α,β-unsaturated acyl imidazoliums: synthesis of dihydropyranones by their reaction with enolates. J. Am. Chem. Soc. 2009, 131, 14176–14177.Suche in Google Scholar
[81] Du, D.; Hu, Z.; Jin, J.; Lu, Y.; Tang, W.; Wang, B.; Lu, T. N-Heterocyclic carbene-catalyzed three-component domino reaction of alkynyl aldehydes with oxindoles. Org. Lett. 2012, 14, 1274–1277.Suche in Google Scholar
[82] Romanov-Michailidis, F.; Besnard, C.; Alexakis, A. N-Heterocyclic carbene-catalyzed annulation of α-cyano-1,4-diketones with ynals. Org. Lett. 2012, 14, 4906–4909.Suche in Google Scholar
[83] Yao, C.; Jiao, W.; Xiao, Z.; Liu, R.; Li, T.; Yu, C. NHC-catalyzed cascade synthesis of 1-substituted 4-aryl-tetrahydroquinoline-2,5-diones. Tetrahedron 2013, 69, 1133–1137.Suche in Google Scholar
[84] Singh, S.; Mishra, P.; Srivastava, M.; Singh, S. B.; Singh, J.; Tiwari, K. P. N-Heterocyclic carbene (thiamine) promoted eco-friendly synthesis of quinoxalines under mild reaction conditions. Green Chem. Lett. Rev. 2012, 5, 587–593.Suche in Google Scholar
[85] Wang, X.-N.; Shao, P.-L.; Lv, H.; Ye, S. Enantioselective synthesis of β-trifluoromethyl-β-lactones via NHC-catalyzed ketene-ketone cycloaddition reactions Org. Lett. 2009, 11, 4029–4031.Suche in Google Scholar
[86] Wang, X.-N.; Zhang, Y.-Y.; Ye, S. Enantioselective synthesis of spirocyclic oxindole-β-lactones via N-heterocyclic carbene-catalyzed cycloaddition of ketenes and isatins. Adv. Synth. Catal. 2010, 352, 1892–1895.Suche in Google Scholar
[87] Duguet, N.; Donaldson, A.; Leckie, S. M.; Douglas, J.; Shapland, P.; Brown, T. B.; Churchill, G.; Slawin, A. M. Z.; Smith, A. D. Chiral relay in NHC-mediated asymmetric β-lactam synthesis I; substituent effects in NHCs derived from (1R,2R)-cyclohexane-1,2-diamine. Tetrahedron Asym. 2010, 21, 582–600.Suche in Google Scholar
[88] Zhang, Y.-R.; He, L.; Wu, X.; Shao, P.-L.; Ye, S. Chiral N-heterocyclic carbene catalyzed Staudinger reaction of ketenes with imines: highly enantioselective synthesis of N-Boc β-lactams. Org. Lett. 2008, 10, 277–280.Suche in Google Scholar
[89] Huang, X.-L.; Chen, X.-Y.; Ye, S. Enantioselective synthesis of aza-β-lactams via NHC-catalyzed [2+2] cycloaddition of ketenes with diazenedicarboxylates. J. Org. Chem. 2009, 74, 7585–7587.Suche in Google Scholar
[90] Wei, D.; Zhu, Y.; Zhang, C.; Sun, D.; Zhang, W.; Tang, M. A DFT study on enantioselective synthesis of aza-β-lactams via NHC-catalyzed [2+2] cycloaddition of ketenes with diazenedicarboxylates. J. Mol. Cat. A Chem. 2011, 334, 108–115.Suche in Google Scholar
[91] Jian, T.-Y.; He, L.; Tang, C.; Ye, S. N-Heterocyclic carbene catalysis: enantioselective formal [2+2] cycloaddition of ketenes and N-sulfinylanilines. Angew. Chem. Int. Ed. 2011, 50, 9104–9107.Suche in Google Scholar
[92] Awasthi, C.; Yadav, L. D. S. N-Heterocyclic carbene catalyzed [2+2] cycloaddition of aryl isothiocyanates and nitroolefins: an efficient synthesis of β-thiolactams. Synlett 2010, 1783–1788.10.1055/s-0030-1258103Suche in Google Scholar
[93] Duong, H. A.; Cross, M. J.; Louie, J. N-Heterocyclic carbenes as highly efficient catalysts for the cyclotrimerization of isocyanates. Org. Lett. 2004, 6, 4679–4681.Suche in Google Scholar
[94] Lv, H.; Chen, X.-Y.; Sun, L.; Ye, S. Enantioselective synthesis of indole-fused dihydropyranones via catalytic cycloaddition of ketenes and 3-alkylenyloxindoles. J. Org. Chem. 2010, 75, 6973–6976.Suche in Google Scholar
[95] Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. [4+2] Cycloaddition of ketenes with N-benzoyldiazenes catalyzed by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2009, 48, 192–195.Suche in Google Scholar
[96] Seayad, J.; Seayad, A. M.; Ng, J. K. P.; Chai, C. L. L. N-Heterocyclic carbene (NHC) catalyzed cycloaddition of CO2 to N-tosyl aziridines: regio and stereoselective synthesis of oxazolidin-2-ones. ChemCatChem 2012, 4, 774–777.Suche in Google Scholar
[97] Thai, K.; Wang, L.; Dudding, T.; Bilodeau, F.; Gravel, M. NHC-catalyzed intramolecular redox amidation for the synthesis of functionalized lactams. Org. Lett. 2010, 12, 5708–5711.Suche in Google Scholar
[98] Li, G.-Q.; Li, Y.; Dai, L.-X.; You, S.-L. N-Heterocyclic carbene catalyzed ring expansion of 4-formyl-β-lactams: synthesis of succinimide derivatives. Org. Lett. 2007, 9, 3519–3521.Suche in Google Scholar
[99] Wang, L.; Thai, K.; Gravel, M. NHC-catalyzed ring expansion of oxacycloalkane-2-carboxaldehydes: a versatile synthesis of lactones. Org. Lett. 2009, 11, 891–893.Suche in Google Scholar
[100] Jacobsen, C. B.; Albrecht, Ł.; Udmark, J.; Jørgensen, K. A. Enantioselective formation of substituted 3,4-dihydrocoumarins by a multicatalytic one-pot process. Org. Lett. 2012, 14, 5526–5529.Suche in Google Scholar
[101] Li, G.-Q.; Li, Y.; Dai, L.-X.; Youa, S.-L. Enantioselective synthesis of cis-4-formyl-β-lactams via chiral N-heterocyclic carbene-catalyzed kinetic resolution. Adv. Synth. Catal. 2008, 350, 1258–1262.Suche in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes
Artikel in diesem Heft
- Masthead
- Masthead
- Reviews
- Methods for the synthesis of xanthine-derived polycyclic fused systems
- Heterocyclic synthesis via catalysis of N-heterocyclic carbenes: very classical and very modern chemical species
- Preliminary Communication
- Efficient synthesis of substituted imidazo[4,5-b]pyridines
- Research Articles
- Synthesis of N-(2-pyridyl)imidazolidin-2-ones and 1-(2-pyridyl)-2,3,7,8-tetrahydro-1H-imidazo[2,1-b][1,3,5]triazepin-5(6H)-ones with potential biological activities
- Design, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivatives
- Synthesis, characterization, and antimicrobial screening of s-triazines linked with piperazine or aniline scaffolds
- Synthesis of 9-(Cn-1F2n-1)-substituted acridine by the reaction of 2-(CnF2n+1)-substituted aniline with ortho-methyl-substituted aromatic Grignard reagent
- Microwave-assisted synthesis of 2-(4,5-dihydro-5-(tetrazolo[1,5-a]quinoline-4-yl)-1H-pyrazol-3-yl)-substituted phenols
- A computational study on azaazulenes

