Abstract
The allylic monomer N-allyl-N-butylbenzimidazolium bromide (ABBB) was synthesized by reacting allyl bromide with benzimidazole and subsequent quaternization with 1-bromobutane. Copolymers of ABBB with butyl acrylate (BA) were prepared in dimethyl formamide solution at 70°C using a free-radical polymerization technique with different monomer feed ratios. Monomer reactivity ratios for the ABBB-BA pairs were evaluated by the Fineman-Ross (r1=0.40; r2=1.49) and Kelen-Tüdõs (r1=0.36; r2=1.46) linearization methods. Thermal behavior of the copolymers of different compositions was evaluated by thermogravimetric analysis. The copolymers were tested for antimicrobial properties against selected strains of bacteria and fungi and were found to be active against the strains. The growth-inhibitory effect of the copolymers varied according to the composition of the polymer: the effect increased with increasing content of the substituted benzimidazolium group in the polymer chain.
1 Introduction
Functional polymers offer the additional advantages of having small molecules with same functional groups, aside from their exceptional properties due to their large size. Frequently, new polymeric materials are designed to meet the challenges in all fields of life. There has been a growing global concern about the emerging infectious diseases over the past few years. New methods, chemicals and drugs, and modes of application have been developed to fight the microbes responsible for these diseases (1). Polymers can act as a matrix for hosting antimicrobial agents. However, because functionalized polymers exhibit antimicrobial activity, their use can pose the problem of diffusion of low molecular weight antimicrobial compounds into the environment. Development of polymers with quaternary ammonium group in the polymer backbone is a significant development in this regard. Quaternary ammonium compounds (QACs) containing at least one alkyl group have the ability to kill microorganisms, such as bacteria and fungi. It is generally accepted that QACs kill microorganisms by destroying their cell walls (2). A number of polymeric antimicrobial agents have been prepared using synthetic polymers, including poly(vinyl alcohol)s (3–7), poly(vinyl pyridine)s (8–10), polyguanidines (11–13), and acrylates (14, 15). Functional polymers with pendant antimicrobial groups have the advantage of non-leaching biocide effects without compromising their antimicrobial properties. Imidazolium and benzimidazolium compounds with N-substituted alkyl groups have demonstrated good antimicrobial activities (16, 17). It has been reported that such polymers, prepared from imidazole derivatives, offer the advantage of good biocompatibility and bioactivity (18).
Acrylic polymers are a class of polymers that find applications in adhesives, bindings, films, fibers, coatings, and pharmacological drugs as polymer support. In the present study, we exploited the idea to incorporate the biocide activity of benzimidazolium compounds in acrylate polymer backbones. We prepared novel acrylate copolymers with benzimidazolium moiety in its backbone as a functional antimicrobial agent. A screening for bioactivity of these acrylate polymers revealed their high potential as antimicrobial agents.
2 Experimental
2.1 Materials
All chemicals and solvents were purchased from Sigma-Aldrich (Germany). Solvents were fractionally distilled before use in the reaction. Synthesis of N-allyl-N-butylbenzimidazolium bromide (ABBB) (Scheme 1) was conducted in air, whereas copolymerization reactions were performed in an oxygen-free environment.

Synthesis of N-allyl-N-butylbenizimidazolium brodmide (ABBB).
2.2 Scheme 1: Synthesis of ABBB
ABBB was prepared in a two-step reaction. In the first step, N-allylbenzimidazole was prepared and subsequently alkylated with 1-bromobutane to yield dialkylatedbenzimidazole.
Benzimidazole (2 mmol, 236 mg) was dissolved in DMF (10 ml). KOH (300 mg) was added and the reaction mixture was stirred for 5 min at room temperature. Allyl bromide (2 mmol, 242 mg) was added drop wise, and stirring was continued for 4 h at 60°C. After the completion of the reaction (as indicated by TLC), 50 ml distilled water was added. Organic layer was extracted with chloroform (4×25 ml). After the solvent was evaporated, N-allylbenzimidazole was obtained as transparent colorless oil.
ABBB was prepared by subsequent N-alkylation of N-allylbenzimidazole using 1-bromobutane as the alkylating agent (Scheme 1). N-Allylbenzimidazole (2 mmol, 264 mg) was dissolved in 10 ml THF. Excess of 1-bromobutane (3 mmol, 274 mg) was added, and the reaction mixture was allowed to stir at room temperature for 72 h. The crude product was filtered and thoroughly washed with diethyl ether to remove the excess 1-bromobutane. The product was recrystallized from THF as colourless crystals with an overall yield of 79%. The product was confirmed by 1H NMR spectroscopy: NMR 1H (CDCl3) δ: 11.3 (s, HN-CH-N); 7.6, 7.6 (m, Har); 6.1 (m, 1Hallyl), 5.4 (d, 2Hallyl); 5.3 (d, 2Hallyl); 4.6 (t, 2Hbutyl); 2.0 (m, 2Hbutyl); 1.4 (m, 2Hbutyl); 0.9 (t, 3Hbutyl).
2.3 Copolymerization
Copolymers of ABBB with butyl acrylate (BA) of different compositions were prepared by free-radical polymerization using DMF as solvent and azobisisobutyronitrile (AIBN) as free-radical initiator. The respective composition of feeds is given in Table 1. Suitable quantities of monomer, co-monomer, DMF (10 ml), and AIBN (0.8% based on total monomers) were refluxed at 70°C under an oxygen-free nitrogen environment for 6 h. The polymer solution was then cooled to room temperature. The polymer was precipitated out by slowly pouring in large volume of methanol with stirring. It was filtered and washed with methanol and further purified by repeated re-precipitation by methanol and finally dried.
Composition, Fineman-Ross and Kelen-Tüdõs parameters for the copolymers of ABBB with BA.
| Sample code no. | ABBB (M1) | BA (M2) | Conversion (w) | Composition of ABBB in the copolymer (m1) | x | y | F | G | ξ | η |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.5 | 8.98 | 0.36 | 1.00 | 0.56 | 1.78 | -0.78 | 0.77 | -0.34 |
| 2 | 0.4 | 0.6 | 8.59 | 0.28 | 0.67 | 0.39 | 1.14 | -1.05 | 0.68 | -0.62 |
| 3 | 0.3 | 0.7 | 8.51 | 0.21 | 0.43 | 0.27 | 0.69 | -1.18 | 0.56 | -0.96 |
| 4 | 0.2 | 0.8 | 8.72 | 0.13 | 0.25 | 0.15 | 0.42 | -1.42 | 0.44 | -1.49 |
| 5 | 0.1 | 0.9 | 8.65 | 0.07 | 0.11 | 0.08 | 0.16 | -1.37 | 0.23 | -1.94 |
where x=M1/M2; y=m1/(1-m1); F=x2/y; G=x[(y-1)/y];
2.4 Antimicrobial Activity
The copolymer prepared was tested for its antimicrobial activity against bacterial (i.e., Escherichia coli and Bacillus subtilis) and fungal strains (i.e., Aspergillus niger and Trichoderma lignorum). These microbial strains were chosen because these are among the most common pathogens. The bacterial strains were grown on nutrient broth, whereas the fungal strains were grown in Sabourand’s dextrose broth. A bacterial culture (5%, v/v) was used to inoculate a 100 ml solution of nutrient broth. For antibacterial evaluations, test media were prepared by adding 50 mg of a polymer sample in a 100 ml nutrient broth solution. Control and test media were incubated at room temperature. After 24 h, 0.5 ml samples were withdrawn and diluted with suitable quantities of DNS reagent and distilled water. Optical density was measured at 660 nm, and growth was recorded as optical density per millilitre. As cell number increases, an increase in optical density is observed. However, the optical method cannot be used to monitor filamentous fungal growth. Thus, gravimetric analysis was performed to measure dry cell mass. Suspensions containing the fungal cultures were harvested after 48 h, and the weight of the dry cell mass was recorded.
The inhibition percentage (I) was calculated from the relation:
In the case of antibacterial screening, X is the optical density of bacterial solution in the control and Y is that in the test polymers. In the case of antifungal screening, X is the weight of the dry fungal cell mass in control and Y is that in the test media. Details of the experimental procedure have already been reported elsewhere (19).
3 Results and discussion
ABBB was prepared by N-alkylation of benzimidazole with allyl bromide and 1-bromobutane in two steps (Scheme 1). The 1H NMR spectrum is shown in Figure 1. Five copolymers of ABBB with BA were synthesized by taking from the monomer different mole fractions ranging from 0.2 to 0.8. A free-radical solution polymerization technique was employed in this work. Polymerization reaction is depicted in Scheme 2. The copolymers were found to be soluble in DMF and DMSO but insoluble in water, methanol, ethanol, and n-hexane. The copolymers were characterized by FTIR spectral data. The bands shown in IR spectrum of the copolymer (Figure 2) at 2997–2843 cm-1 can be assigned to stretching of aromatic, methyl and methylene groups. The band at 1730 cm-1 is attributed to >C=O stretching vibrations of ester group, whereas the bands at 1240 and 1145 cm-1 can be attributed to C-O stretching vibrations of ester group.

1H NMR spectrum of ABBB.
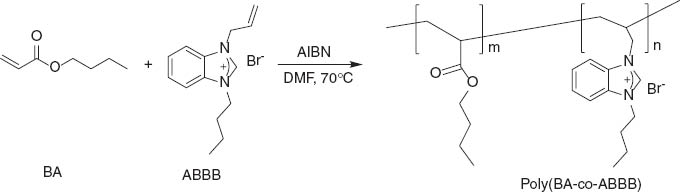
Free-radical copolymerization of ABBB and butyl acrylate (BA).

FTIR spectrum of poly(ABBB-co-BA)(1).
The average composition of the copolymers of BA and ABBB was determined from elemental analysis data. The reactivity ratios of BA and ABBB were determined from the monomer feed ratios and the copolymer composition. Conventional linearization methods such as the Fineman-Ross (F-R) (20) and Kelen-Tüdõs (K-T) (21) methods were employed to obtain the reactivity ratio, and these ratios are shown in Tables 1 and 2. Reaction time was optimized during trials to achieve conversion <10% to satisfy the differential copolymerization equation. The F-R method uses the following equation:
Monomer reactivity ratio obtained by different linearization methods.
| Method | r1 | r2 | r1·r2 |
|---|---|---|---|
| Fineman-Ross | 0.40 | 1.49 | 0.59 |
| Kelen-Tüdõs | 0.36 | 1.46 | 0.52 |
The K-T method employs the following equation:
The F-R and K-T plots obtained by linear regression analysis are presented in Figures 3 and 4, respectively. The reactivity ratio values indicate that the BA radical favours its own monomer rather than ABBB. BA was found to have greater reactivity. The product of r1·r2 is <1, which indicates that the system follows a random distribution of monomer units. The steric effects and overall polarity of the molecules play a role in the overall reactivity of the monomers.

P(ABBB-co-BA) system of the Finemann-Ross plot.

P(ABBB-co-BA) system of the Kelen-Tüdõs plot.
The number and weight average molecular weight of poly(ABBB-co-BA) were determined by gel permeation chromatography and are presented in Table 3. The values of number average and weight average molecular weight ranged from19,460 to 24,590 and 29,370 to 38,640, respectively. The polydispersity indices of copolymers ranged from 1.51 to 1.58, whereas intrinsic viscosity (η) ranged from 0.172 to 0.206 dl.g-1. These data do not clearly suggest that with the decrease in ABBB content in copolymers, molecular weights, polydispersity and viscosity also decrease.
Average molecular weights obtained by gel permeation chromatography for the copolymers of ABBB with BA.
| Sample code number | Mn | Mw | Polydispersity (Mw/Mn) | Intrinsic viscosity [η] (dLg-1) |
|---|---|---|---|---|
| 1 | 24,590 | 38,640 | 1.57 | 0.205 |
| 2 | 23,750 | 37,130 | 1.56 | 0.195 |
| 3 | 23,670 | 38,860 | 1.64 | 0.206 |
| 4 | 20,370 | 32,180 | 1.58 | 0.180 |
| 5 | 19,460 | 29,370 | 1.51 | 0.172 |
Thermogravimetric analysis was performed to investigate the thermal stability of the copolymer, the results of which are presented in Figure 5. The curves indicate that the copolymers undergo a two-step decomposition process within the range of 270–320°C and 370–450°C in relation to ester and benzimidazole fragments, respectively. Positions and intensities of the peaks depended on the ABBB unit content in the copolymer. It can be observed that high temperature degradation peaks grew at the expense of low temperature shoulders.

Thermogravimetric analysis curves of the ABBB-BA copolymers.
The antimicrobial activity of benzimidazolium compounds is well established (22). To the best of our knowledge, the polymers in this study have not been reported in the literature, and this is the first report to evaluate antimicrobial activities of this class of polymers. The results obtained are presented in Figure 6. Copolymer 1 showed maximum inhibitory effect. It is observed that the presence of benzimidazolium moiety is of prime importance. As the proportion of benzimidazolium moiety increased in the copolymer, microbial inhibition characteristic of the copolymer also increased.

Effect of ABBB-co-BA copolymers on the growth (%) of bacteria and fungi.
4 Conclusion
New acrylate copolymers were synthesized by a free-radical polymerization technique. Different copolymers were prepared using different feed ratios of the monomers. The reactivity ratios indicated that the monomers were distributed in polymer chain in a random fashion. The results of thermogravimetric analysis revealed that the copolymers undergo a three-step degradation process. With regard to the screening for antimicrobial activity, the copolymers with benzimidazolium moiety exhibited very good antimicrobial properties. The work is being extended to prepare acrylate antimicrobial finishes for textile materials in the future.
Funding: Higher Education Commission Pakistan via grant number PM-IPFP/HRD/HEC/2011/346
References
1. Saif MJ, Anwar J, Munawar MA. A novel application of quaternary ammonium compounds as antibacterial hybrid coating on glass surfaces. Langmuir 2008;25(1):377–9.10.1021/la802878pSuche in Google Scholar PubMed
2. Kenawy E-R, Abdel-Hay FI, El-Shanshoury AE-RR, El-Newehy MH. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J Polym Sci A1. 2002;40(14):2384–93.10.1002/pola.10325Suche in Google Scholar
3. Kenawy E-R, El-Newehy MH, Abdel-Hay FI, El-Shanshoury AE-RR. Synthesis and biocidal activity of modified poly(vinyl alcohol). Arab J Chem. 2014;7(3):355–61.10.1016/j.arabjc.2013.04.005Suche in Google Scholar
4. Poverenov E, Shemesh M, Gulino A, Cristaldi DA, Zakin V, Yefremov T, Granit R. Durable contact active antimicrobial materials formed by a one-step covalent modification of polyvinyl alcohol, cellulose and glass surfaces. Colloid Surf B 2013;112:356–61.10.1016/j.colsurfb.2013.07.032Suche in Google Scholar PubMed
5. Bonilla J, Fortunati E, Atarés L, Chiralt A, Kenny JM. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocolloid. 2014;35:463–70.10.1016/j.foodhyd.2013.07.002Suche in Google Scholar
6. Liu S, He J, Xue J, Ding W. Efficient fabrication of transparent antimicrobial poly(vinyl alcohol) thin films. J Nanopart Res. 2009;11:553–60.10.1007/s11051-007-9321-8Suche in Google Scholar
7. Abdelgawad AM, Hudson SM, Rojas OJ. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohyd Polym. 2014;100:166–78.10.1016/j.carbpol.2012.12.043Suche in Google Scholar PubMed
8. Qiu J-H, Zhang Y-W, Zhang Y-T, Zhang H-Q, Liu J-D. Synthesis and antibacterial activity of copper-immobilized membrane comprising grafted poly(4-vinylpyridine) chains. J Colloid Interf Sci. 2011;354(1):152–9.10.1016/j.jcis.2010.09.090Suche in Google Scholar PubMed
9. Sahiner N, Yasar AO. The generation of desired functional groups on poly(4-vinyl pyridine) particles by post-modification technique for antimicrobial and environmental applications. J Colloid Interf Sci. 2013;402:327–33.10.1016/j.jcis.2013.03.032Suche in Google Scholar PubMed
10. Siedenbiedel F, Fuchs A, Moll T, Weide M, Breves R, Tiller JC. Star-shaped poly(styrene)-block-poly(4-vinyl-N-methylpyridiniumiodide) for semipermanent antimicrobial coatings. Macromol Biosci. 2013;13(10):1447–55.10.1002/mabi.201300219Suche in Google Scholar PubMed
11. Wang H, Synatschke CV, Raup A, Jerome V, Freitag R, Agarwal S. Oligomeric dual functional antibacterial polycaprolactone. Polym Chem. 2014;5(7):2453–60.10.1039/c3py01467cSuche in Google Scholar
12. Mattheis C, Wang H, Meister C, Agarwal S. Effect of guanidinylation on the properties of poly(2-aminoethylmethacrylate)-based antibacterial materials. Macromol Biosci. 2013;13(2):242–55.10.1002/mabi.201200217Suche in Google Scholar PubMed
13. Mattheis C, Zhang Y, Agarwal S. Thermo-switchable antibacterial activity. Macromol Biosci. 2012;12(10):1401–12.10.1002/mabi.201200207Suche in Google Scholar PubMed
14. Siedenbiedel F, Tiller JC. Antimicrobial polymers in solution and on surfaces: overview and functional principles. Polymers 2012;4(1):46–71.10.3390/polym4010046Suche in Google Scholar
15. Sellenet PH, Allison B, Applegate BM, Youngblood JP. Synergistic activity of hydrophilic modification in antibiotic polymers. Biomacromolecules 2006;8(1):19–23.10.1021/bm0605513Suche in Google Scholar PubMed
16. Özkay Y, Tunalı Y, Karaca H, Işıkdağ İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur J Med Chem. 2010;45(8):3293–8.10.1016/j.ejmech.2010.04.012Suche in Google Scholar PubMed
17. Özkay Y, Tunalı Y, Karaca H, Işıkdağ İ. Antimicrobial activity of a new series of benzimidazole derivatives. Arch Pharm Res. 2011;34(9):1427–35.10.1007/s12272-011-0903-8Suche in Google Scholar PubMed
18. Anderson EB, Long TE. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010;51(12):2447–54.10.1016/j.polymer.2010.02.006Suche in Google Scholar
19. Patel JN, Patel MV, Patel RM. Copolymers of 2,4-dichlorophenyl methacrylate with styrene: synthesis, thermal properties, and antimicrobial activity. J Macromol Sci A 2005;42(1):71–83.10.1081/MA-200040970Suche in Google Scholar
20. Fineman M, Ross SD. Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci. 1950;5(2):259–62.10.1002/pol.1950.120050210Suche in Google Scholar
21. Kelen T, Tüds F. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J Macromol Sci A 1975;9(1):1–27.10.1080/00222337508068644Suche in Google Scholar
22. Pernak J, Rogoża J, Mirska I. Synthesis and antimicrobial activities of new pyridinium and benzimidazolium chlorides. Eur J Med Chem. 2001;36(4):313–20.10.1016/S0223-5234(01)01226-0Suche in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Editorial November 2014
- Full length articles
- Controlled ring-opening polymerization of 1,2,6-tricyclic orthoesters of mannose toward size–controlled α-d-mannopyranan
- Acrylate copolymers containing benzimidazolium moieties: synthesis and antimicrobial applications
- Structure, mechanism and application of vinyl alcohol oligomers grafted onto poly(3-hydroxybutyrate): a proposal
- A study on the relationship between polycarbonate microstructure and performance as determined by a combined experimental and molecular dynamics simulation method
- Highly compact co-poly(amide-imide)s from polycondensation of an imide-modified derivative of l-aspartic acid
- UV-curable electromagnetic shielding composite films produced through waterborne polyurethane-acrylate bonded graphene oxide: preparation and effect of different diluents on the properties
- Characterization of auxetic polyurethanes foam for biomedical implants
- Notice of retraction
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Editorial November 2014
- Full length articles
- Controlled ring-opening polymerization of 1,2,6-tricyclic orthoesters of mannose toward size–controlled α-d-mannopyranan
- Acrylate copolymers containing benzimidazolium moieties: synthesis and antimicrobial applications
- Structure, mechanism and application of vinyl alcohol oligomers grafted onto poly(3-hydroxybutyrate): a proposal
- A study on the relationship between polycarbonate microstructure and performance as determined by a combined experimental and molecular dynamics simulation method
- Highly compact co-poly(amide-imide)s from polycondensation of an imide-modified derivative of l-aspartic acid
- UV-curable electromagnetic shielding composite films produced through waterborne polyurethane-acrylate bonded graphene oxide: preparation and effect of different diluents on the properties
- Characterization of auxetic polyurethanes foam for biomedical implants
- Notice of retraction

