Abstract
The paper gives an introduction to membrane science and technology, an area of research of high significance for the development of a sustainable life for human beings. It is therefore intended to be a guide for teachers in the areas of chemistry, physics, or biology, who can incorporate the presented materials in their respective courses. The paper gives some insights into the different types of membranes, their functions, production and use in some selected areas.
Introduction
Separation of components from mixtures in a given system can occur in many ways and always leads to an increase of order within this system. In order to increase the order some energy is required. For example, the water cycle is based on evaporation of clean water mainly from the oceans, which are mainly mixtures of water and salts. However, evaporation costs energy. The necessary energy for evaporation comes from the sun. The sun heats up the ocean and evaporates the water. The water vapor will then condense to rain and feeds the springs and wet the land, thereby dissolving again minerals and other components. In a technical process the separation of water or other liquids from other components can be done by evaporation (which requires large surfaces between liquid and air and thus is not suitable in most cases), or by thermal distillation where the water or another liquid is heated to the respective boiling temperature (for water at ambient pressure this is above 100 °C in saline water). Distillation is a well-established procedure both in laboratory and industry. However, it costs a lot of energy to distill a liquid like water (imagine the huge amount of drinking water required for Earth’s population!) and therefore less energy intensive processes are preferable. Membrane based separations require less energy, as no phase transition energy is required. In comparison to thermal separation processes, which follow a thermodynamic equilibrium, membrane based separations happen either by size separation, which allows only particles or solutes below a critical size limit to pass a porous membrane (these are so called filtrations) or the separation is due to the different kinetics of different components when they diffuse through a dense membrane. In comparison to thermal separations, membrane based separations can occur at much lower temperatures compared to the boiling temperature, and often no heating is required. For example, fresh water production using a membrane to separate pollutants can be performed at the temperature of the liquid water and there is no need to boil the water. Rather than investing the evaporation energy required in a distillation (ca. 2500 kJ/L), only the osmotic pressure of the solution must be overcome, which relates to much less energy demand (ca. 15 kJ/L for sea water, the exact value depends mostly on the salt concentration). That is why many desalination plants for the fresh water production take advantage of membranes since approximately 50 years ago, when suitable membranes for such application were developed for the first time. Also in chemical and food industry membranes are of increasing interest because of the reduction in energy costs when separating or purifying chemicals or products from liquids or gas mixtures. Besides saving energy also the thermal stability of components can be an important issue, as sometimes a component in a system, which needs to be purified, cannot withstand high temperature. This is the case in living cells, where highly functional membranes can do selective separation of different solutions outside and inside the cell at the temperature of the organism (for a human being: ca. 37 °C). Above 45 °C sensitive proteins in our cells would denature and lose their functionality and lead to death. Well-known is the application of membranes for hemodialysis, and there is no alternative separation way to purify blood.
In the following some fundamentals about membranes will be given, their structures and the separation mechanisms, so this important topic can be integrated into courses of chemistry, biology, or physics, depending on where the question of “separating mixtures” may come up. In physics this may be in the context of phase transitions, such as the liquid gas transition at the boiling point, requiring the evaporation energy (enthalpy, if processes at constant pressure are discussed), in chemistry the topic may be integrated when separations by filtering or distillations are discussed (for example when removing a precipitate from a solution or a liquid from a mixture). Finally, in a biology course, membranes can be discussed in more general when the structure of cells is discussed. Even in some overarching subjects as the discussion of climate change membranes might mentioned since they provide a means for separating CO2 from point sources as industrial flue gases.
Membranes for selective transport
Membranes separate two volumes, which can be both in a similar phase state, such as two gases or two liquids. However, they can also separate a liquid from a gas. Membranes separate molecules or very little particles up to the size of colloids, this means on a length scale from 0.1 nm up to a few 100 nm. When we consider the separation of sand from a mixture of sand with stones (this is a solid mixture), this happens by filters separating much larger particles, which we do not consider here, although the principle of size selectivity is also used in some membrane separations. Figure 1 shows schematically a membrane in a separation process and Figure 2 shows different size regimes of molecules or particles, which are considered in membrane science and technology.
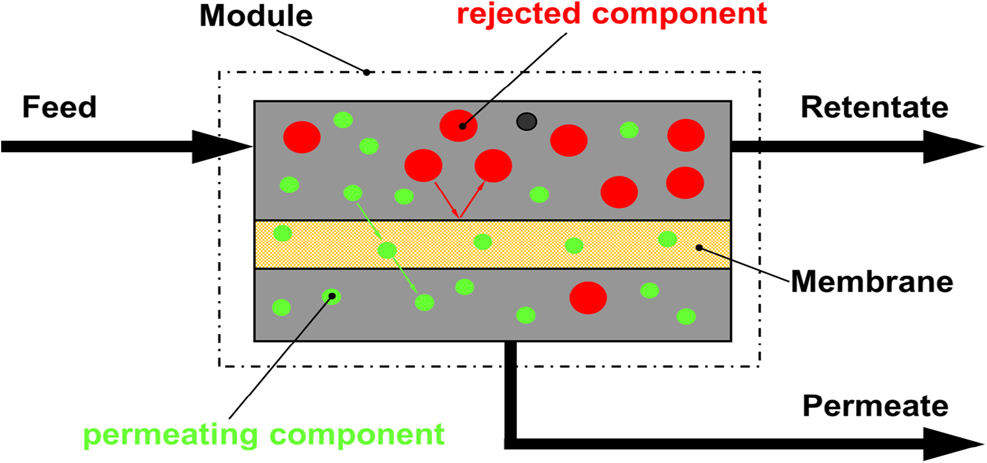
A mixed feed (gas or liquid) enters a module which houses the membrane. The feed moves along the membrane layer and components, which can pass through the membrane, will permeate through the membrane to the permeate side. The permeate is thus separated from the feed. The feed loses permeating components and changes its composition. It leaves as a retentate from the module. This is the typical way for a continuous process, in contrast to a discontinuous, so-called dead-end filtration, where a feed is pressed through a pressure vessel equipped with a membrane filter at its outlet and the matter not passing the membrane accumulating on the membrane surface. Not shown are additional features in a membrane based separation process, such as pumps, heat exchangers, condensers, etc., which are required depending on the components to be separated and the level of purification.
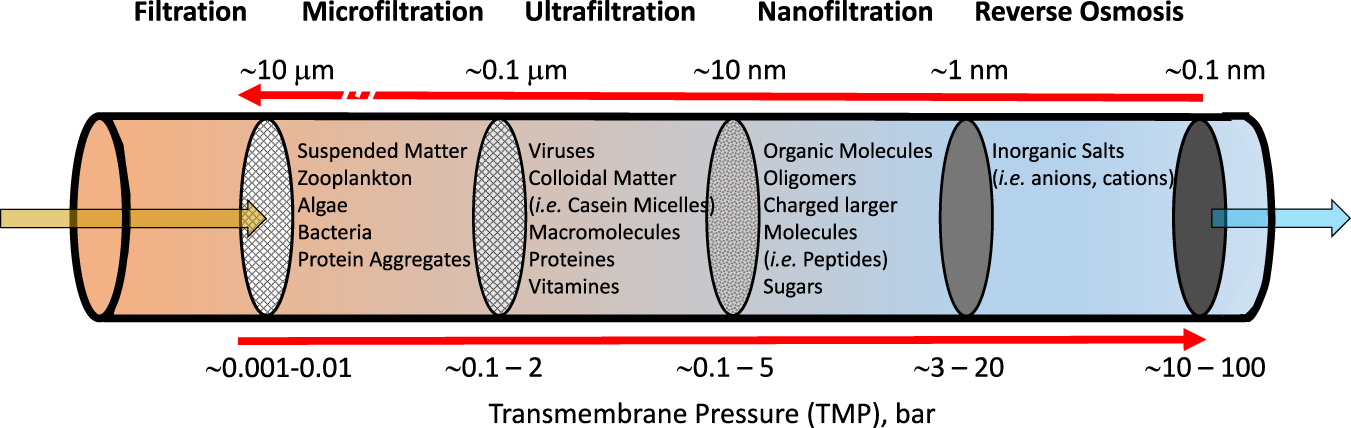
Different regimes of membrane separations for liquids are shown schematically in a dead-end configuration, from 100 nm (microfiltration) down to 0.1 nm (reverse osmosis). The brown arrow indicates a liquid (like water), carrying particles and dissolved molecules and salts. The Figure displays the different species typically retained by the respective membrane right next to it. As an example, viruses may pass a microfiltration membrane, but they will be blocked by an ultrafiltration membrane. Note that besides electrically neutral particles or molecules, also electrically charged molecules or ions can be separated, which is important for electrically driven membrane processes as electro dialysis. In the ideal case the blue arrow indicates a pure liquid, like water.
To understand the function of a membrane in some more detail, we first look at the structure of a membrane. This accounts for both flat sheet or hollow fiber membranes, which we will not distinguish from one another for the remainder of this article.
Membranes can be made of many solid materials and also liquids. Membranes of living cells are liquid in nature. They are composed of bilayers of phospholipids, which are amphiphilic with hydrophilic head groups pointing towards the inside and the outside of the cell, while their lipophilic parts are constituting the inner part of the double layer. Transport of molecules and ions occur through specific proteins embedded in the bilayer. Figure 3 shows schematically such a biological membrane. We note here that these membranes are much more functional than any artificial membrane developed so far.
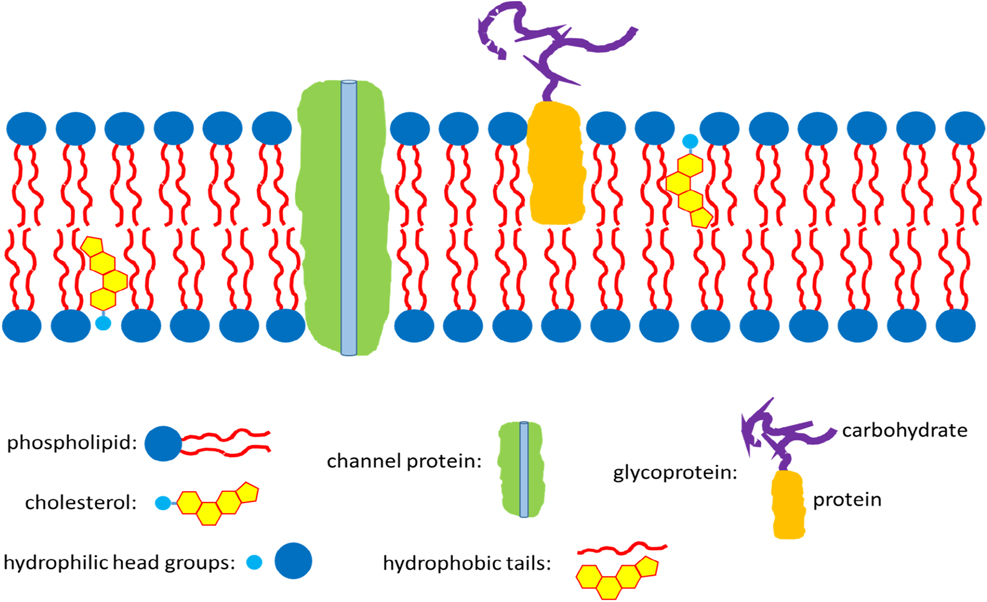
Cell membrane composed of phospholipid bilayer, stabilizing cholesterol, different proteins for selective transports, glycolipids and glycoproteins (only the latter are shown in the schema) serving with their carbohydrates for cell communication and mechanical properties (they are oriented to the outside of the cell only).
The vast majority of synthetic membranes are solid in nature and can be composed of ceramics, metals, polymers, or combinations thereof. Especially polymer membranes are of increasing technological interest as there are many different polymers available, they are cheap in production and easy in mechanical handling (i.e., less brittle than ceramic membranes) (Abetz et al., 2006). However, when it comes to extreme conditions for membrane based separation process (such as harsh oxidizing agents or high temperatures), inorganic membranes may be the only choice. Also there are sometimes specific properties required, such as an absolute selective transport of one type of molecules, where polymer membranes will fail. Such examples are absolutely selective transport of hydrogen, which can be achieved by thin layers of palladium, or the selective transport of oxygen in perovskites. However, in many cases 100% purity of a product is not required and so polymer membranes can do the separation job. Moreover, instead of only one step in the separation process also a process can be designed where several membrane modules are arranged sequentially and by this a higher purity of the product can be achieved. Depending on the specific separation task, suitable membranes can vary in their structure. These different structures are schematized in Figure 4.
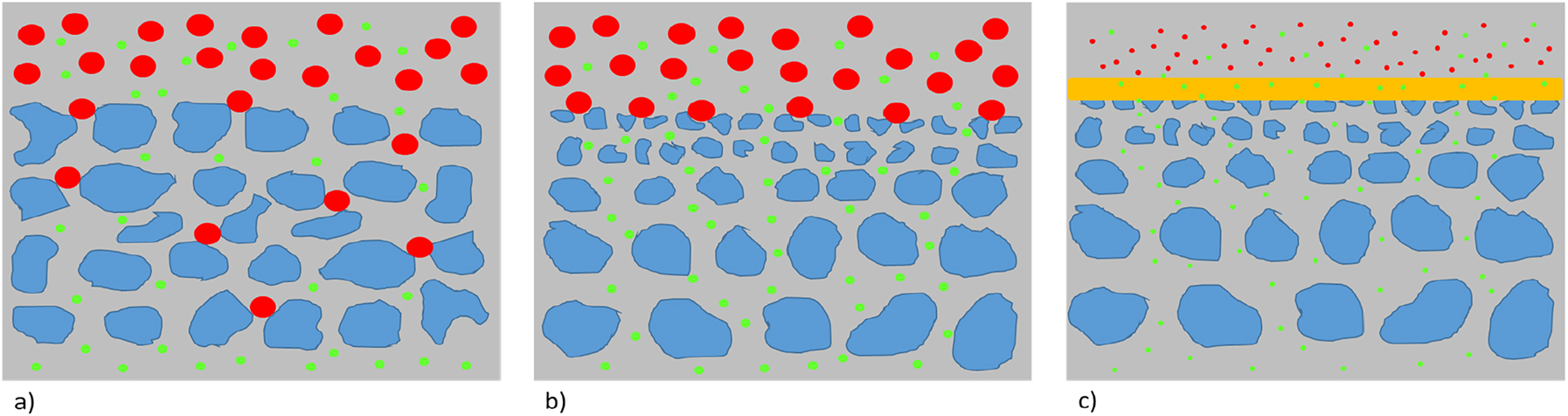
Cross sections of different membranes. (a) Symmetric porous, (b) Asymmetric porous, and (c) Thin film composite membrane.
All membranes are selective for the green particles. In (a) and (b) the green particles permeate because of their size. In (a) the red particles are blocked by in depth filtration, i.e., the particles may be anywhere blocked within the membrane due to an overall homogeneous size distribution. In (b) the red particles are blocked at the surface of the membrane, what is called a surface or screen filtration. In (c) the green molecules diffuse across the yellow dense selective layer after have been adsorbed by the polymer, while the equally sized (or even smaller or bigger) red molecules are incompatible with the dense layer and cannot pass. How good the selectivity is in reality depends on the perfection of the membrane structure. Defects, for example, may lead to decrease of selectivity. Also, in (c), the yellow layer may let also pass some red molecules, it may just be less compatible with them compared to the green ones.
There are dense and porous membranes. They can be formed as symmetric membranes, which show the same structure all over the cross section. They can also exist as asymmetric membranes, where a thin selective layer (dense or porous) is supported by a large-porous substructure. These membranes have the advantage to have a thin selective layer, which lowers the resistance for the molecules which can pass the membrane. Hence, they can pass at an increased rate. The large-porous substructures serve for mechanical robustness of the membrane. Such asymmetric structures may be produced in different ways, depending also on the type of material (here we focus on polymers, but note that similar structures can also be produced from metals or ceramics following different ways). In so-called thin film composite membranes, the selective layer is coated on top of a porous support layer, such as an integral asymmetric membrane shown in Figure 4c. The coating may be obtained by casting a polymer solution on the support followed by evaporation of the solvent. The polymer solution can also contain additives or very small filler particles, such as, e.g. titania, silica, carbon black, or carbon nanotubes, which improve certain properties of the membrane, like mechanical stability (Majeed, Fierro, et al., 2012) or electrical conductivity (Majeed, Filiz, et al., 2012), but also may affect the permeation properties (Mushardt, Müller, Shishatskiy, Wind, & Brinkmann, 2016) (Sun et al., 2018). Such membranes are called thin film composite (TFC) membranes. Another way to introduce a thin selective layer is to carry out an interfacial polymerization. This latter procedure is often used to prepare polyamide membranes for desalination. Here two solutions of the comonomers (on one side the diamines and on the other side of the support membrane the diacid monomers and triacid crosslinkers) are brought together at one surface of the support membrane, where polymerization occurs instantaneously and a crosslinked thin polyamide layer is formed, as the yellow dense layer in Figure 4c.
Another way is to produce the whole asymmetric structure from one polymer in one step. For this, typically a polymer solution is cast on a large-porous substrate and then after a short time, during which part of the solvent evaporates, the whole film or fiber (if it is a hollow fiber from a spinning device) is immersed into a coagulation bath filled with a non-solvent for the polymer. Precipitation occurs and the solvent and non-solvent (these two are miscible with each other), exchange and result in a so-called integral-asymmetric membrane, where a rather dense top layer (this occurs where the non-solvent first touched the polymer solution) transforms gradually into a structure with larger and larger pores from the top towards the bottom of the membrane. This structure is shown in Figure 4b. If cast on a metal film, such membrane can be lifted off after it solidifies completely. In other cases, it may stay on a porous support structure giving more robustness to it. Although this is the most common way to integral asymmetric membranes, it is not the only one. Besides non-solvent induced phase inversion also other procedures are known, such as evaporation induced phase separation, crystallization induced phase separation, etc. For more details the reader is referred to the literature (Strathmann, 2011). A special case of polymer membranes obtained from non-solvent induced phase separation are membranes from amphiphilic block copolymers with a short polar and a long nonpolar block. These block copolymers tend to microphase separate as the polar and nonpolar segments dislike each other (similar as the polar head groups and the lipophilic tail of a soap molecule or phospholipid). When cast from a suitable solution, this microphase separation can be combined with the non-solvent induced phase separation and a so-called isoporous integral asymmetric membrane is obtained, where the selective top layer is composed of rather uniform pores. Figure 5 shows an electron micrograph of such a membrane, which still did not find its way to large scale application so far, but may have a great potential for size selective and even charge selective separations (Abetz, 2015; Radjabian & Abetz, 2020; Wang, Rahman, Abetz, & Abetz, 2020; Zhang et al., 2020).
What drives separation across a membrane?
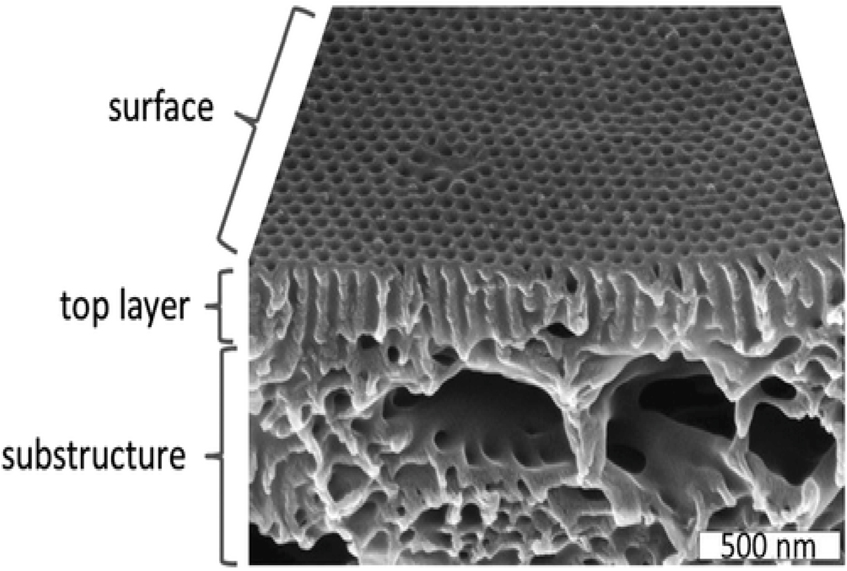
Scanning electron micrograph of a so-called isoporous integral asymmetric membrane formed by a polystyrene-block-poly(2-vinylpyridine) diblock copolymer from the non-solvent induced phase separation. Reproduced with permission (Abetz, 2015) Copyright 2015, Wiley‐VCH GmbH.
After we saw different possible membrane structures we still must address the question, what are the driving forces leading to a separation of different components across a membrane. From daily life there is an observation probably most of us have already made: a fruit juice, a cup of tea or any other drink may be too concentrated for our taste. Addition of water will dilute the drink and the taste will be homogeneously adjusted to all of the volume of the liquid. This comes from a basic tendency in nature, which is to achieve a similar concentration everywhere within one phase (maximizing entropy).
Imagine now that the same experiment is done in a slightly different way (Figure 6): our drink is in a cup with a sealed hole in the side wall, which is put in a water bath in such a way that the levels of the liquids are at the same height (Figure 6b). No exchange of liquids between cup and water bath is possible. Now we open the hole and the liquids can exchange freely. As a result they mix homogeneously, which is illustrated in Figure 6a. Figure 6c shows the case, where the hole is covered by a membrane which lets only water pass through. After a while we observe that the level of the drink becomes higher than the one of the water bath. The level of the drink increases to a finite level and we call this an equilibrated state between the hydrostatic pressure built up by the amount of liquid which is above the level of the surrounding water bath and the osmotic pressure caused by the difference of concentration of our solutes in the drink and in the water bath (where no solutes are present, as the membrane is selective for water). In principle, one could also do the opposite experiment, when a more concentrated drink is aimed for. In that case we have to apply a pressure on the solution (our drink) and overcome the osmotic pressure to squeeze water from the concentrated solution into the water bath. The membrane will keep the delicious ingredients in our cup, of course. This is the same way how a reverse osmosis process works for desalination of sea water (although both the sea water and the brine after removing some water by this process are not tasty at all …). In more general terms, separation across a membrane occurs in such a way that a similarity of the concentrations of those components, which can pass the membrane, is approached on both sides. Or, by applying an external force, a dissimilarity of concentrations can be generated and increased in such a way, that the more concentrated side of the membrane is exposed to an external pressure which overcomes the osmotic pressure, for example. Membranes of living cells can also transport ions or molecules from a more diluted to a more concentrated side, against the driving force of the concentration gradient. However, instead of an external pressure (which is hardly available …) the necessary energy for transportation is generated within the membrane. These are complex metabolic mechanisms leading to so called active membrane transport. Technical membranes are far from applying such mechanisms and require the necessary energy input from outside, such as a difference of pressure across the membrane or an electrochemical potential. Instead of concentrations, in even more general terms, the chemical potentials of the components, which can pass through the membrane, have to be considered. It is the difference in the chemical potentials, which leads to a corresponding flux across the membrane. The relationship between a flux ji of a component i across a membrane with a thickness d and the driving force ΔXi can be expressed in the following equation (Strathmann, 2011):
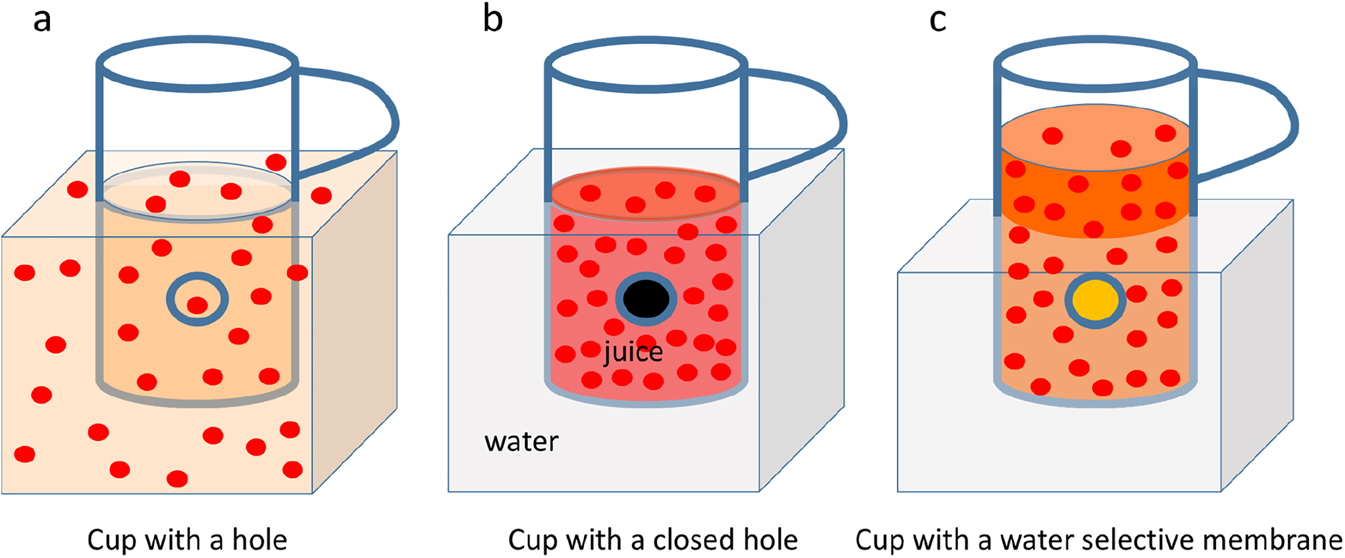
A cup with some red juice is placed in a water bath. (a) The cup has a hole and juice and surrounding water mix; (b) The cup has a sealed hole and juice and water stay separated; (c) The hole of the cup is covered by a membrane, which lets only water pass through. The level of juice increases with respect to the water bath (can be seen by comparing the lower part of the handle and the liquid level in (b) and (c).
The phenomenon of solvent flux through a membrane can be used to determine the molecular weight of a solute. A more detailed analysis of the thermodynamic relationships tells us that the measured osmotic pressure is directly proportional to the number of dissolved molecules. Therefore, if we know the mass of the dissolved molecules, the obtained osmotic pressure will allow the determination of the mass of the individual dissolved molecules. The same mass of large molecules leads to a smaller osmotic pressure compared to the same mass of small molecules. This observation was first reported by van’t Hoff (Hiemenz & Lodge, 2007; Rubinstein & Colby, 2003).
How does transport across a membrane occur?
As shown before, there are different types of membranes, porous and dense ones. Porous membranes act to a certain extent like sieves, which separate by size: if a solute or a particle is too big, it cannot pass through the pores. If we consider monodisperse particles like proteins in a buffer solution, the separation of proteins kept by the membrane from the low molecular liquid is expressed by a selectivity (or separation factor), αi,j, which is the ratio of the sieving coefficients of the low molecule liquid Sci and of the solute particle Scj (Kanani et al., 2010):
As the retention of the solvent molecules (buffer) is negligible, the corresponding sieving coefficient is 1. The flux across the membrane can be described by hydrodynamics. The performance of an ultrafiltration membrane depends also on the pore geometry. Thus, the selectivity depends on the permeability of the solvent (low molecular compounds, which pass through the membrane) in different ways, depending on the pore geometry (Kanani et al., 2010). If the permeability (as given in Eq. (1)) is scaled by the tortuosity (i.e., the factor expressing the increase of pore length compared to a pore perpendicular to the membrane surface) of the pores τ and viscosity of the solution μ, then a comparison of the separation of the solvent from a well-defined particle for different ultrafiltration membranes having cylindrical pores and slit pores can be compiled into one plot, as displayed schematically in Figure 7.
![Figure 7: Selectivity (or separation factor) as a function of the scaled permeability of the solvent i. j indicates the non-permeating, retained solute. Here monodisperse pores are considered without any specific interactions with the permeant. Slit-shaped pores show a higher selectivity compared to cylindrical pores. For membranes with large pore size distributions, however, the differences between slit- and cylindrical-shaped pores become less pronounced [Adapted from (Kanani et al., 2010)].](/document/doi/10.1515/cti-2020-0023/asset/graphic/j_cti-2020-0023_fig_007.jpg)
Selectivity (or separation factor) as a function of the scaled permeability of the solvent i. j indicates the non-permeating, retained solute. Here monodisperse pores are considered without any specific interactions with the permeant. Slit-shaped pores show a higher selectivity compared to cylindrical pores. For membranes with large pore size distributions, however, the differences between slit- and cylindrical-shaped pores become less pronounced [Adapted from (Kanani et al., 2010)].
However, when we consider rather small pores (see Figure 2), also the nature of the pores can be important. There may be adhesion of matter on the pore walls and they are blocked after some time of membrane operation. This is called fouling. They can also carry electrical surface charges or other physical properties, leading to some interactions with the solutes passing through the pores. As a result, a combination of different properties besides the size of a particle or molecule can influence its permittivity across a given porous membrane.
Also gases can be separated by porous membranes, if the pores are in the range of a nanometer or below. Here kinetic effects can play a role, like the probability of gas molecules to collide with one another or rather with the pore wall, which depends on a quantity called the mean free path length. The mean free path length is the average distance a gas molecule can travel between two collisions with other gas particles. If the pores are large, collisions between the particles happen more often than between particles and pore wall. So there is no separation and just a viscous hydrodynamic flow of the gas mixture is observed, without any separation. If the pore is narrow enough, i.e., below the mean path length, then the particles collide more often with the pore wall than with each other. Then the transport of the light gas molecules becomes faster than of the heavier gas molecules. One can show that the selectivity αi,j defined by the permeabilities Pi and Pj of the components i, j
depends on the mass of the gas molecules in the following way (Strathmann, 2011):
This phenomenon is called Knudsen-diffusion can be used to enrich uranium isotopes (235UF6 vs 238UF6). However, the selectivity is very low for this pair of gases (α235UF6,238UF6 ≅ 1.0043), so the procedure needs to be repeated several times, if a significant enrichment is to be achieved. Also other phenomena in such small pores can occur leading to separation of different gases. Gases can selectively adsorb on the pore wall, and the inner part the pore is than too small to let the non-adsorbing gas pass through, while the adsorbed gas can diffuse along the pore wall. This is called surface diffusion. If the pores are narrow enough, condensation can occur in the pores and they are blocked. If no adsorption on the pore walls happen and the pores are small enough, they can become size selective towards the gas molecules and this is called molecular sieving. The different types of porous membranes for gas separation are schematically shown in Figure 8.
![Figure 8: Different gas permeation mechanisms in porous membranes. (a) Knudsen diffusion and (b) Molecular sieving for non-condensable gases. (c) Knudsen diffusion (green gas) and surface diffusion of condensable red gas, (d) capillary condensation of red gas blocking the permeation of the non-condensable green gas [Adapted from (Strathmann, 2011)].](/document/doi/10.1515/cti-2020-0023/asset/graphic/j_cti-2020-0023_fig_008.jpg)
Different gas permeation mechanisms in porous membranes. (a) Knudsen diffusion and (b) Molecular sieving for non-condensable gases. (c) Knudsen diffusion (green gas) and surface diffusion of condensable red gas, (d) capillary condensation of red gas blocking the permeation of the non-condensable green gas [Adapted from (Strathmann, 2011)].
Finally, we consider dense membranes which can be found in gas separation and reverse osmosis (like the yellow layer in Figure 4c). In dense membranes the transport of a component i depends on three steps: adsorption on the membrane’s feed side, Fickian diffusion across the membrane where the diffusing component i is dissolved in the dense membrane material, and desorption from the membrane’s surface on the permeate side. The permeability Pi is determined by the solubility Si of the component i in the membrane and its diffusivity Di within the membrane. Hence, this model is also called the solution-diffusion model (Strathmann, 2011):
In this case the size of a molecule mainly affects the diffusivity, while the solubility also depends on the interactions (i.e., van der Waals interactions based on the polarizability) between the solute or gas and the membrane material. These two factors also depend on the physical state of the polymer the membrane is made from, i.e., if it is a glassy membrane or a soft rubbery membrane. In glassy membranes the so-called free volume is essential for the transport or the permeating molecule. The free volume is the empty space between atoms of the membrane material, due to its glassy nature, which means that there is not optimal dense packing of the polymer segments (repeating units) like in a crystal. In rubbery membranes, the polymer segments have some conformational freedom and therefore there is a dynamics of local density in the membrane, which allows the permeant to move across the membrane. Therefore, the transport and separation mechanism in here differs from the one in porous membranes.
If the selectivity as defined in Eq. (3) of a given pair of gases is plotted as a function of the permeability of the faster permeating component for a huge number of different membrane materials, a linear line can be recognized which seems to limit the selectivity for a given permeability in a double logarithmic plot. This observation was first reported by Robeson (Robeson, 1991) and is commonly referred to as the “Robeson upper bound” (Figure 9).
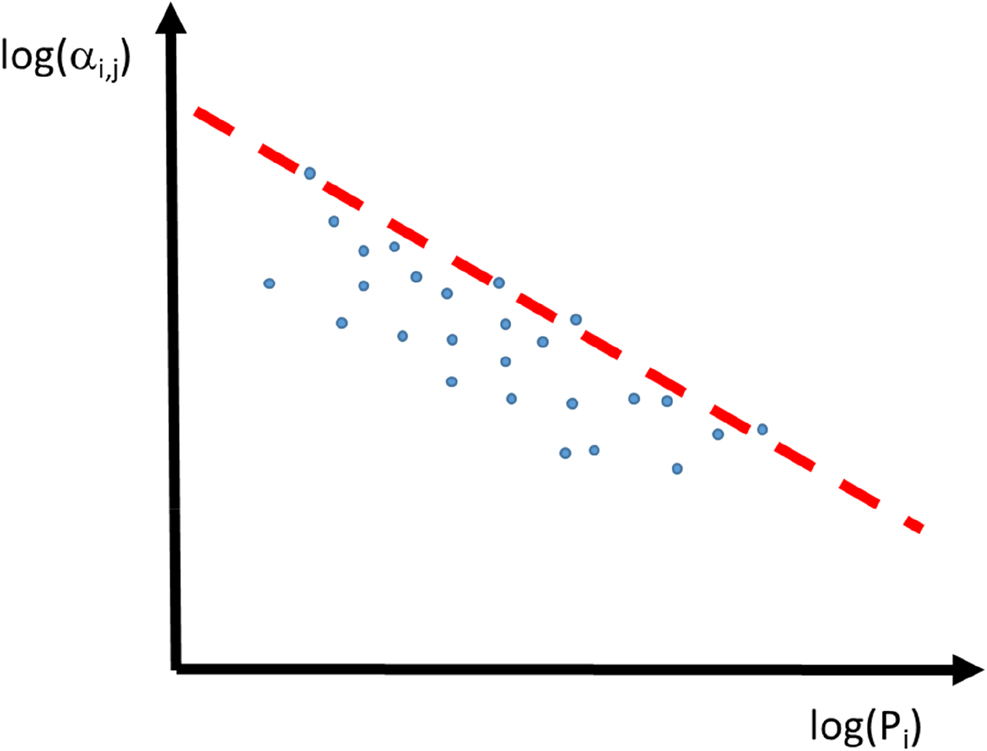
Schematic representation of the selectivity of a gas pair as a function of permeability of the fast permeating species. Experimental data points of different polymer membranes and the red “upper bound” line for the given gas pair i and j.
For more details of the transport phenomena in gas separation membranes the reader is referred to the literature (Freeman, 1999; Robeson, 2008; Robeson, Liu, Freeman, & Paul, 2015; Strathmann, 2011).
Practical application aspects
Membranes can be used in different geometries. There are so called flat sheet membranes and there are cylindrical configurations, which are named hollow fiber membranes when there diameter is below 300 μm (for example in desalination), capillary membranes when their diameter up to ca. 300–1000 μm (typically used in hemodialysis, i.e., serving as an artificial kidney) and tubular membranes when they are having diameters in the range of a few cm. Tubular membranes are preferable if the feed contains a lot of solid particles like in ultrafiltration, which would block a hollow cylinder membrane geometry with a smaller diameter (typically the feed is on the inside of the membrane). They are also common for ceramic as opposed to polymeric membranes.
In a technical process the aim is to put as much membrane area as possible into a given volume, in order to save space. That is why hollow fiber membranes are often preferred compared to flat sheet membranes, which are often spiral wound in order to be space efficient. In the following we give some insight into technical or industry scale membrane fabrication and typical module designs.
Imagine a smooth, non-woven and very permeable fabric made from polymer fibers – it is strong but offers hardly any resistance to mass transfer. On top of this, a thin film (50–100 µm) of polymer solution is applied. The thickness is adjusted by a blade, i.e., the casting knife. Once the polymer film enters a coagulation bath (typically water) the organic solvent of the polymer dissolves in the bath whilst the polymer itself does not, i.e., precipitation occurs. The process depends on operating parameters such as velocity of the casting process, composition and viscosity of the solution or temperature of the coagulation bath. The result is an asymmetric flat sheet membrane as shown in Figures 4b or 5. Figure 10 shows the schematic drawing of a membrane casting machine.
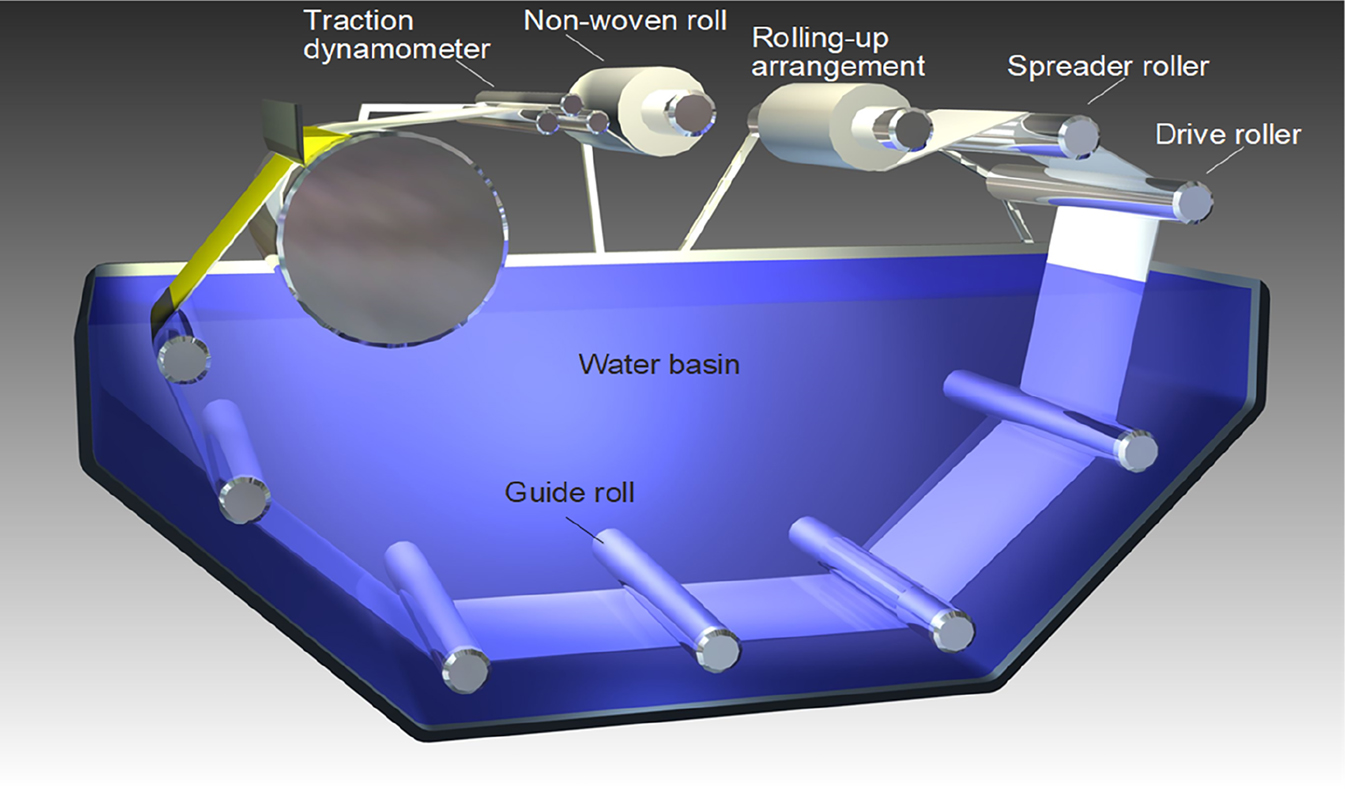
Schematic representation of a membrane casting machine for the production of flat sheet membranes.
In case a tubular geometry is desired, no non-woven is required. Instead, the polymer solution is pumped through a purpose designed nozzle, the so-called spinneret. The resulting tube made from polymer solution starts to solidify in an air gap before entering the coagulation bath where precipitation is induced as previously described. In case the solvent for the polymer consists of a mixture of a light boiling and a high boiling component, the light boiling component starts to evaporate in the air gap prior to the polymer solution film or tube entering the coagulation bath: a dense top layer will form resulting in an integrally asymmetric membrane where porous support structure and dense separation layer are made from one and the same material. Alternatively, a membrane as shown in Figure 4c can be formed by using the porous support structure as shown in Figure 4b as substrate for being coated with thin polymer films made from dilute polymer solutions of rather low viscosity, resulting in a thin film composite membrane. This can be done by either dragging the support across the surface of a basin filled with the solution (dip coating) or by transporting it by means of a roller mechanism past a roller wetted with solution which applies the thin film due to surface forces (Figure 11). The final step is the evaporation of the solvent and an optional thermally induced crosslinking of the polymer. Using these procedures membrane areas of several 100 m2 per batch or even continuous production can be achieved in an industrial set-up.

Roller coating of flat sheet membrane for the production of thin film composite membranes.
The membranes are mounted into a housing, i.e., the membrane module. It is purpose designed to allow for the installation of large membrane areas in as low a volume as possible whilst adhering to optimized fluid dynamics. The most common module types are shown in Figure 12.
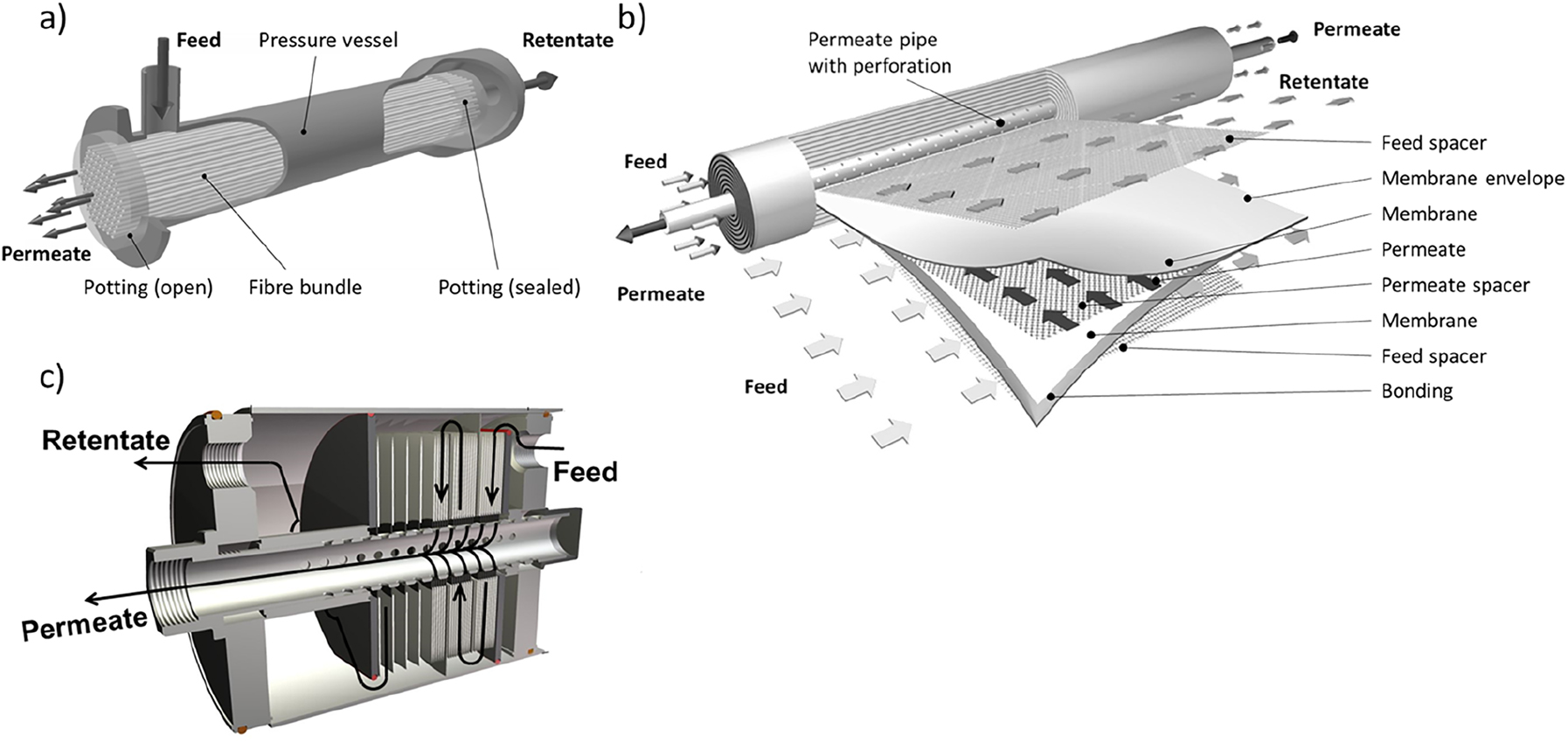
Common membrane module types: (a) Hollow fiber module, (b) Spiral wound module, and (c) Envelope type module.
The hollow fiber module houses bundles of hollow fibers. The feed can be supplied from the outside (as shown in Figure 12a) or from the inside of the fibers. These modules allow for large amounts of membrane area installed into a small volume, i.e., up to 10,000 m2/m3. However, they suffer of rather poor fluid dynamics and hence are employed in gas separation for “slow” membranes. Therefore, modern membranes for the separation of CO2 from flue or industrial waste gases are of the flat sheet geometry. They possess high permeances (i.e., the amount of material passing through the membrane per unit of time, unit of membrane area and unit of partial pressure or fugacity driving force) (Brinkmann et al, 2017). These membranes are mounted into spiral wound (Figure 12b) or, less often, envelope type modules (Figure 12c). For spiral wound modules, envelopes are formed from the membrane material with the selective layer facing to the outside. In between the membrane sheets, a webbing, a so called spacer, is placed to facilitate the withdrawal of the permeate through a channel perpendicular in flow direction to the feed flow. The envelope is glued tight on three sides whilst the fourth side is attached to a perforated tube around which the membrane envelope together with layers of feed spacer is wrapped, resulting in the spiral wound geometry (Baker, 2012). The envelopes in envelope type modules consist of circular membrane sheets where segments are cut off at opposing ends. Inside of the membrane envelope spacer material is placed as before. However, the entire circumference is sealed in this case. In the center, a hole is punched and the envelopes are stacked on a perforated tube, separated by spacer material, gaskets and baffle plates for controlling the feed velocity (Figure 12c) (Brinkmann, Pohlmann, Withalm, Wind, & Wolff, 2013).
One of the most interesting applications for gas separation is the removal of CO2 from flue gas aiming at the mitigation of climate change. Figure 13 shows a pilot plant for the treatment of coal fired power plant flue gas. The unit was operated in the scope of the project MemKoR funded by the German Federal Ministry of Economic Affairs and Energy. Using the described flat sheet thin film composite membranes with a poly(ethylene oxide) containing separation layer of high CO2 selectivity in a two stage configuration, the CO2 content could be increased from 12 Vol.-% in the feed to >94 Vol.-% in the permeate of the second stage.
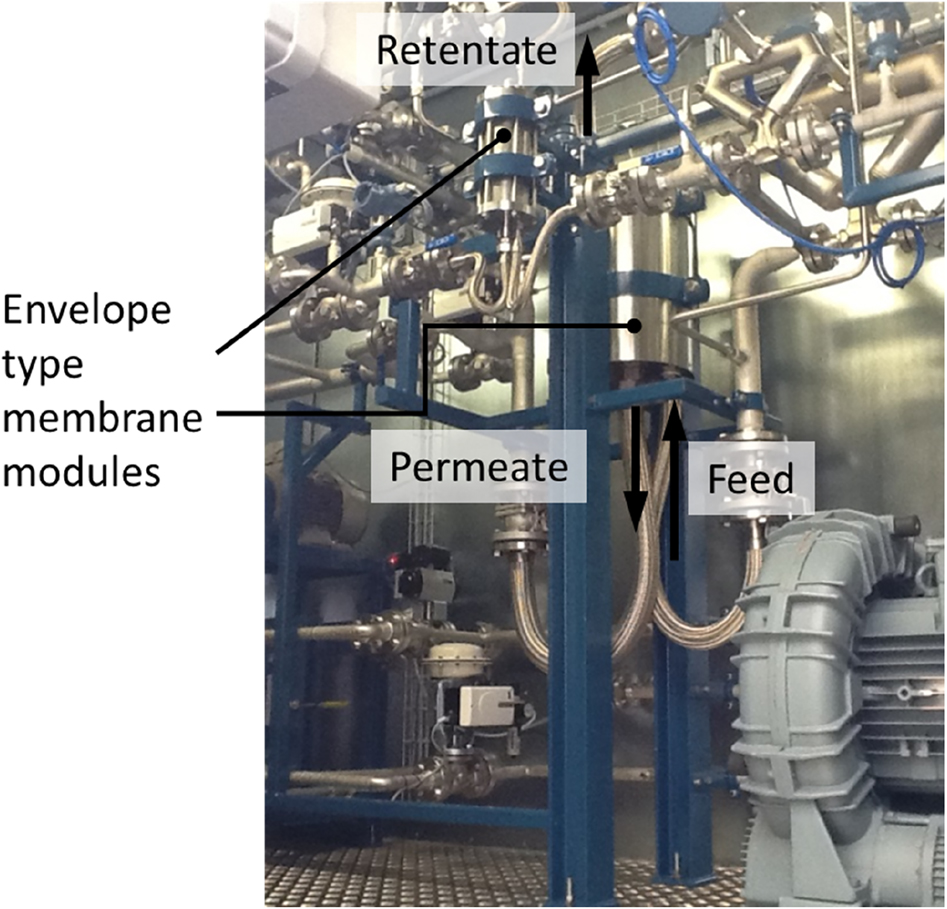
Gas separation pilot plant for CO2 removal from flu gas.
Final remarks
In this short article we tried to give some basic insights into the world of polymer membranes, which play an increasingly important role when we face big challenges in our world. Examples are the need for drinking water and energy to enable an acceptable quality of life for all of us. Membranes cannot only help to clean exhaust gases (as we have shown here) but we emphasize that there are many more existing and also upcoming membrane applications. Examples are membrane technologies for desalination of sea water and brines or detoxification of polluted water. Membranes find use in batteries and fuel cells. They may be used to supply sufficiently clean chemicals for the chemical industry at rather low cost, and they can be used for purification of biofuels (such as ethanol, methanol, methane) and hydrogen. They find also many applications in specific areas in pharmaceuticals, medicine and biotechnology or food processing. However, there is a need for better membranes in many applications, as they often suffer from corrosive environments or they get blocked by scaling or fouling. That is why there is always the aim to improve selectivity and permeability at the same time, which is a big challenge as usually permeability can be increased when lower selectivity is accepted. In order to increase selectivity and keep the permeability constant for a specific component, the structure and/or the composition of a membrane needs to be changed. Not discussed, but also important are polymer membranes containing inorganic filler particles, which can contribute to the membrane separation performance and also its mechanical and electrical properties. For example, introducing carbon nanotubes or carbon black can introduce electrical conductivity across a membrane and increase operational safety when electrical discharges must be avoided in the separation of explosive mixtures. Stimuli-responsive membranes with switchable permeation and selectivity are another example for future developments. Such new membrane developments also will require the necessary best fitting module design and membrane process design. Moreover, in a holistic approach membrane technology can be combined with other separation technologies, such as thermal separation or adsorption. In many cases a combination of these different technologies may result in an optimized separation. Besides experimental approaches also computer simulations will become more important in the future for the development of membranes, modules and the whole membrane-based processes. As shown in the paper, many disciplines contribute to membrane science, especially chemistry, physics and engineering, but also biology and computer sciences. Thus there is still a lot of interesting and highly interdisciplinary work left for future generations!
Funding source: German Federal Ministry of Economic Affairs and Energy
Award Identifier / Grant number: 03ET7064
Funding source: FP7 EU NMP
Award Identifier / Grant number: NMP3-LA-2008-213277NMP3-SL-2009-228652
Acknowledgments
This article benefitted from some collaborative projects. We acknowledge FP7 EU-project HARCANA (High Aspect Ratio Carbon-based Nanocomposites, NMP3-LA-2008-213277) and FP7 EU-project SELFMEM (Self-assembled Polymer Membranes, NMP3-SL-2009-228652) funded within the European Community’s 7th Framework Programme for Research and Technological Development and the project MemKoR (Membranverfahren für die Abtrennung von Kohlendioxid aus Kraftwerksrauchgasen, Membrane processes for the separation of carbon dioxide from power plant flue gases, 03ET7064) funded by the German Federal Ministry of Economic Affairs and Energy. We would also like to thank Thorsten Wolff for the preparation of the images shown in Figures 10 and 12.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This article was supported by FP7 EU-project HARCANA (High Aspect Ratio Carbon-based Nanocomposites, NMP3-LA-2008-213277) and FP7 EU-project SELFMEM (Self-assembled Polymer Membranes, NMP3-SL-2009-228652) and MemKoR funded by the German Federal Ministry of Economic Affairs and Energy under grant no. 03ET7064.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Abetz, V. (2015). Isoporous block copolymer membranes. Macromolecular Rapid Communications, 36(1), 10–22. https://doi.org/10.1002/marc.201400556.Search in Google Scholar
Abetz, V., Brinkmann, T., Dijkstra, M., Ebert, K., Fritsch, D., Ohlrogge, K., & Schossig, M. (2006). Developments in membrane research: From material via process design to industrial application. Advanced Engineering Materials, 8, 328–358. https://doi.org/10.1002/adem.200600032.Search in Google Scholar
Baker, R. W. (2012). Membrane technology and applications (3rd ed.). Chichester, United Kingdom: John Wiley & Sons, Ltd.10.1002/9781118359686Search in Google Scholar
Brinkmann, T., Pohlmann, J., Withalm, U., Wind, J., & Wolff, T. (2013). Theoretical and experimental investigations of flat sheet membrane module types for high capacity gas separation applications. Chemie Ingenieur Technik, 85(8), 1210–1220. https://doi.org/10.1002/cite.201200238.Search in Google Scholar
Brinkmann, T., Lillepärg, J., Notzke, H., Pohlmann, J., Shishatskiy, S., Wind, J., & Wolff, T. (2017). Development of CO2 selective poly(ethylene oxide)-based membranes: From laboratory to pilot scale. Engineering, 3, 485–493. https://doi.org/10.1016/j.eng.2017.04.004.Search in Google Scholar
Freeman, B. D. (1999). Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules, 32(2), 375–380. https://doi.org/10.1021/ma9814548.Search in Google Scholar
Hiemenz, P. C., & Lodge, T. P. (2007). Polymer Chemistry. Boca Raton, Florida, United States of America: CRC Press.10.1201/9781420018271Search in Google Scholar
Kanani, D. M., Fissell, W. H., Roy, S., Dubnisheva, A., Fleischman, A., & Zydney, A. L. (2010). Permeability–selectivity analysis for ultrafiltration: Effect of pore geometry. Journal of Membrane Science, 349(1), 405–410. https://doi.org/10.1016/j.memsci.2009.12.003.Search in Google Scholar
Majeed, S., Fierro, D., Buhr, K., Wind, J., Du, B., Boschetti-de-Fierro, A., & Abetz, V. (2012). Multi-walled carbon nanotubes (MWCNTs) mixed polyacrylonitrile (PAN) ultrafiltration membranes. Journal of Membrane Science, 403–404, 101–109. https://doi.org/10.1016/j.memsci.2012.02.029.Search in Google Scholar
Majeed, S., Filiz, V., Shishatskiy, S., Wind, J., Abetz, C., & Abetz, V. (2012). Pyrene-POSS nanohybrid as a dispersant for carbon nanotubes in solvents of various polarities: Its synthesis and application in the preparation of a composite membrane. Nanoscale Research Letters, 7, 296. https://doi.org/10.1186/1556-276X-7-296.Search in Google Scholar
Mushardt, H., Müller, M., Shishatskiy, S., Wind, J., & Brinkmann, T. (2016). Detailed investigation of separation performance of a MMM for removal of higher hydrocarbons under varying operating conditions. Membranes, 6(1), 16. https://doi.org/10.3390/membranes6010016.Search in Google Scholar
Radjabian, M., & Abetz, V. (2020). Advanced porous polymer membranes from self-assembling block copolymers. Progress in Polymer Science, 102, 101219. https://doi.org/10.1016/j.progpolymsci.2020.101219.Search in Google Scholar
Robeson, L. M. (1991). Correlation of separation factor versus permeability for polymeric membranes. Journal of Membrane Science, 62(2), 165–185. https://doi.org/10.1016/0376-7388(91)80060-j.Search in Google Scholar
Robeson, L. M. (2008). The upper bound revisited. Journal of Membrane Science, 320(1), 390–400. https://doi.org/10.1016/j.memsci.2008.04.030.Search in Google Scholar
Robeson, L. M., Liu, Q., Freeman, B. D., & Paul, D. R. (2015). Comparison of transport properties of rubbery and glassy polymers and the relevance to the upper bound relationship. Journal of Membrane Science, 476, 421–431. https://doi.org/10.1016/j.memsci.2014.11.058.Search in Google Scholar
Rubinstein, M., & Colby, R. H. (2003). Polymer Physics. Oxford, United Kingdom: Oxford University Press.10.1093/oso/9780198520597.001.0001Search in Google Scholar
Strathmann, H. (2011). Introduction to Membrane Science and Technology. Weinheim, Germany: Wiley-VCH Verlag & Co. KGaA.Search in Google Scholar
Sun, W., Shi, J., Cheng, C., Li, N., Xu, Z., Li, J., & Zhao, L. (2018). A review on organic–inorganic hybrid nanocomposite membranes: A versatile tool to overcome the barriers of forward osmosis. RSC Advances, 8, 11040–10056. https://doi.org/10.1039/C7RA12835E.Search in Google Scholar
Wang, J., Rahman, M. M., Abetz, C., & Abetz, V. (2020). Tuning the size selectivity of isoporous membranes for protein fractionation via two scalable post treatment approaches. Journal of Membrane Science, 614, 118535. https://doi.org/10.1016/j.memsci.2020.118535.Search in Google Scholar
Zhang, Z., Rahman, M. M., Abetz, C., Höhme, A. L., Sperling, E., Abetz, V. (2020). Chemically tailored multifunctional asymmetric isoporous triblock terpolymer membranes for selective transport. Advanced Materials, 32, 1907014. https://doi.org/10.1002/adma.201907014.Search in Google Scholar
© 2020 Volker Abetz et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Preface
- Special issue of Chemistry Teacher International in Polymer Sciences
- Review Articles
- Fundamentals of reversible addition–fragmentation chain transfer (RAFT)
- Reversible-Deactivation Radical Polymerisation: chain polymerisation made simple
- Ring-opening polymerization
- Other
- Thermal analysis: basic concept of differential scanning calorimetry and thermogravimetry for beginners
- Review Article
- Size-exclusion chromatography as a useful tool for the assessment of polymer quality and determination of macromolecular properties
- Other
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 1: a good practice on estimation of bulk resistance of solid polymer electrolytes
- Special Issue Paper
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 2: dielectric response of (non-) polymer electrolytes
- Good Practice Report
- Chirality analysis of helical polymers
- Other
- Fabrication and function of polymer membranes
- Review Article
- Nano- and microgels: a review for educators
- Other
- Theoretical background on semiconducting polymers and their applications to OSCs and OLEDs
- Good Practice Report
- An understandable approach to the temperature dependence of electric properties of polymer-filler composites using elementary quantum mechanics
- Review Article
- Polymer degradation: a short review
Articles in the same Issue
- Frontmatter
- Preface
- Special issue of Chemistry Teacher International in Polymer Sciences
- Review Articles
- Fundamentals of reversible addition–fragmentation chain transfer (RAFT)
- Reversible-Deactivation Radical Polymerisation: chain polymerisation made simple
- Ring-opening polymerization
- Other
- Thermal analysis: basic concept of differential scanning calorimetry and thermogravimetry for beginners
- Review Article
- Size-exclusion chromatography as a useful tool for the assessment of polymer quality and determination of macromolecular properties
- Other
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 1: a good practice on estimation of bulk resistance of solid polymer electrolytes
- Special Issue Paper
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 2: dielectric response of (non-) polymer electrolytes
- Good Practice Report
- Chirality analysis of helical polymers
- Other
- Fabrication and function of polymer membranes
- Review Article
- Nano- and microgels: a review for educators
- Other
- Theoretical background on semiconducting polymers and their applications to OSCs and OLEDs
- Good Practice Report
- An understandable approach to the temperature dependence of electric properties of polymer-filler composites using elementary quantum mechanics
- Review Article
- Polymer degradation: a short review

