Abstract
Nano- and microgels are promising soft polymer materials for different application fields: stabilizers, sensors, catalysts, selective sorbents, drug delivery carriers etc. They are composed of cross-linked polymer chains swollen with a solvent. The building blocks, synthesis approaches and architecture of nano- and microgels are reviewed. The mechanisms of responsiveness to various stimuli are described, examples of applications are provided. Micro- and nanogels are good objects for learning projects and the ideas for learning projects with microgels are described.
Introduction
According to the IUPAC Goldbook, it is a material that contains individual particles of gel of any shape with an equivalent diameter of approximately 0.1–100 μm. Nanogel contains the particles of gel with the size of 1–100 nm. Microgels can exhibit sizes from 100 nm to 100 μm. Later we will refer both micro- and nanogels as “microgels”.
Nano- and microgels are topical objects in polymer science. The term “microgel” appeared in 1963 (Sieglaff, 1963), “nanogel” – in 1989 (Quellet, Eicke, Gehrke, & Sager, 1989), but the systematic investigations began only in 2000s (Figure 1). Now nano- and microgels are regarded as promising tools for many fields: stabilizers, sensors, catalysts, selective sorbents, drug carriers etc. We know them as reversible glue in Post-It paper stickers, rubber fillers, superabsorber beads in diapers, acne treatment drug Retin-A. Microgels are used in paper industry to modify the printing paper surface for the better adhesion of inks. Their most remarkable feature is the tunability of the properties and responsibility to various stimuli.
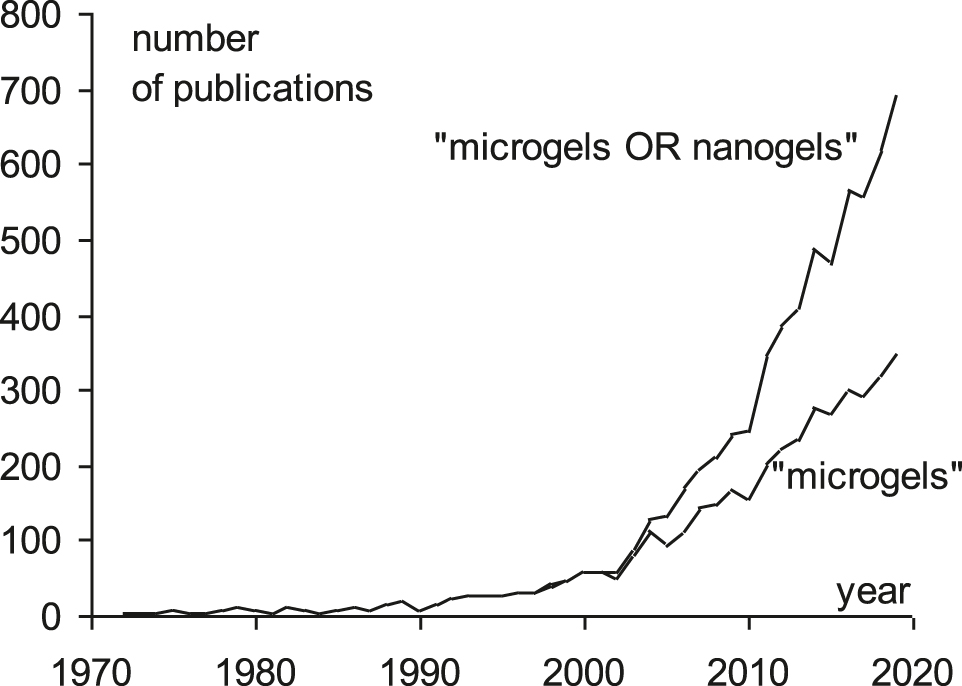
The number of publications by the keywords “microgel” and “microgel OR nanogel”.
Herein we will describe the macroscopic appearance and the structure of microgels at different levels; discuss their properties (first of all – response to stimuli) in relation to their composition and structure; give examples of the applications of microgels and suggest possible topics for learning projects with microgels.
Microgels from macroscopic to molecular level
The household examples of gels are jelly or marmalade (Figure 2). The gels are solid in terms that they hold the shape when they are still, but are easily penetrable and can tremble or slide while shaking. One can imagine a microgel as a jelly cut into stable pieces of micron size.

Jelly.
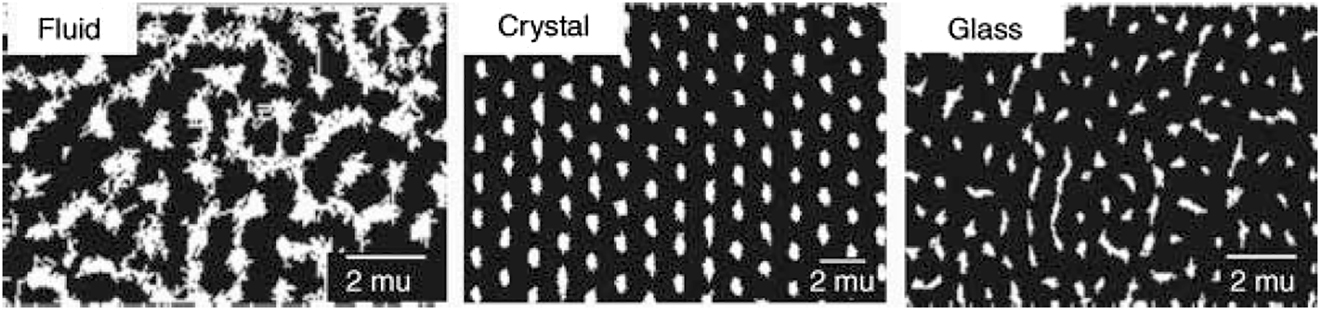
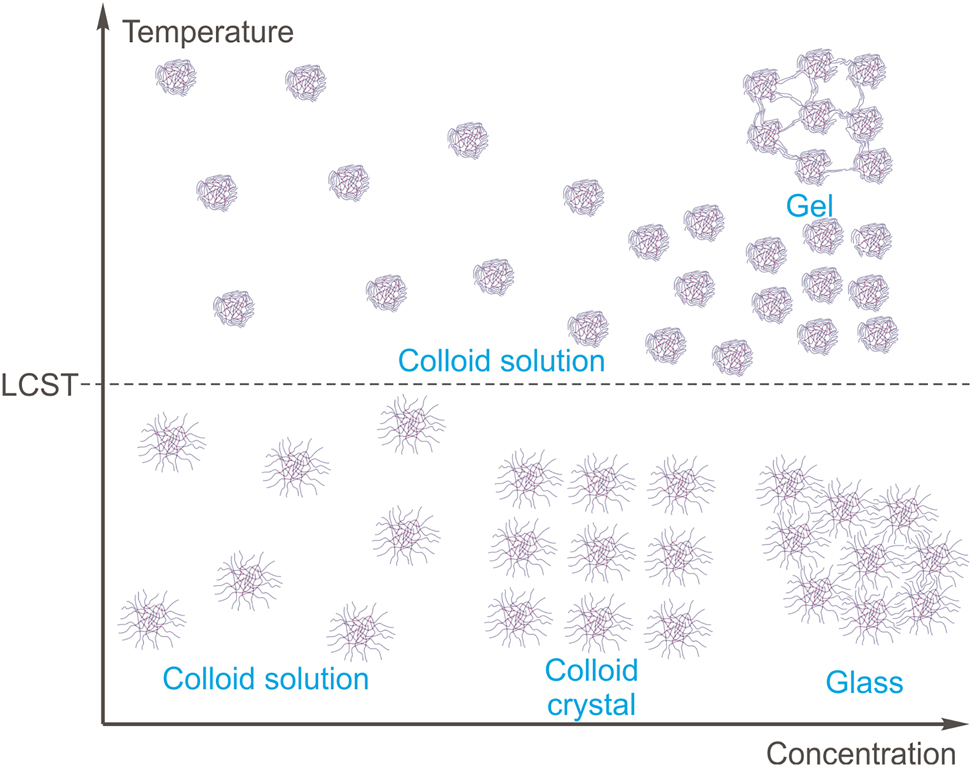
The macroscopic properties of microgels in a solvent depend on the concentration of microgel particles that determines their packing density (Gao & Hu, 2002; Figure 3). When the concentration is low, the colloid suspension is formed. It is a liquid, sometimes viscous, that reveals Tyndall effect, which allows to investigate the size and the density of the particles by light-scattering technique. Dense packing is formed when the concentration of the dry polymer is about 2–3%. If the size of the microgel particles is equal, the microgel forms colloidal crystals (usually – gelly-like substances of opaque appearance, Figure 3). The crystallization is resulted from the interactions of the soft spheres and thus occurs at relatively low concentrations (Lyon et al., 2004). If the concentration is higher, the microgel particles undergo deformation (Matthson et al., 2009) and the glass state is formed. It tends to be transparent and resembles soft glass by mechanical properties.

Color pictures of NIPA microgel dispersions (132 nm) at 21 °C. C/wt.%: A) 0.064, B) 1.47, C) 3.0, D) 3.4, E) 4.2, F) 4.6, G) 5.95, H) 7.92, I) 13.7. Samples A–C are liquid, D–E − crystal, F–I – glass.
Reproduced from Gao & Hu, 2002 with permission from ACS.
Microgels can also adsorb to interfaces and reduce the interfacial tension (Zhang & Pelton, 1999). The efficient reduction of the surface tension at liquid-liquid interfaces is due to the small size and deformability of microgels. Interestingly, the swelling degree of microgels influences strongly the dynamics of the interfacial adsorption and packing density of microgels at interfaces. Microgels in swollen state can reduce more efficiently the surface tension compared to microgels in collapsed state (Wu, Wiese, Balaceanu, Richtering, & Pich, 2014).
From the microscopic point of view a gel is a sparse three-dimensional network of cross-linked polymer chains that are swollen by the molecules of a solvent (Figure 4). If the gel is swollen with water it is called “hydrogel”. The degree of swelling (and, widely, the morphology of microgel particle) is determined by solvent-polymer, solvent-solvent and polymer-polymer interactions. In turn, they depend on many external factors such as temperature, pH, ionic strength etc. On the other hand, the morphology of the microgels determines many properties and processes, such as softness, penetrability, diffusion and so on. This mechanism allows fine tuning of the properties of microgels. Sometimes the properties change abruptly with the change of a stimulus (Figure 5). For example, at a certain temperature the polymer chains coil, the microgel particles collapse and the viscosity of the gel changes. In this case the microgels are called “stimuli-responsive”.
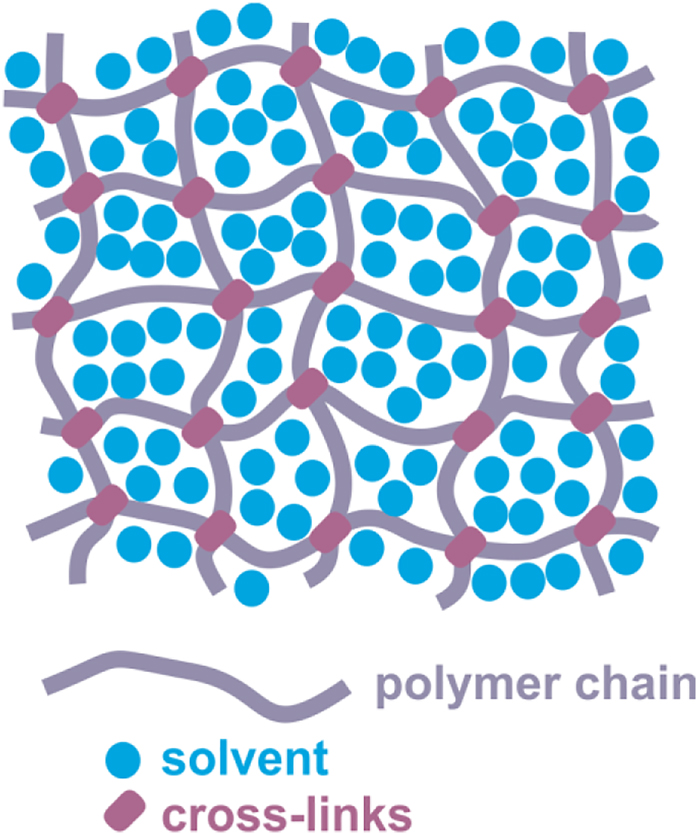
The structure of gel.
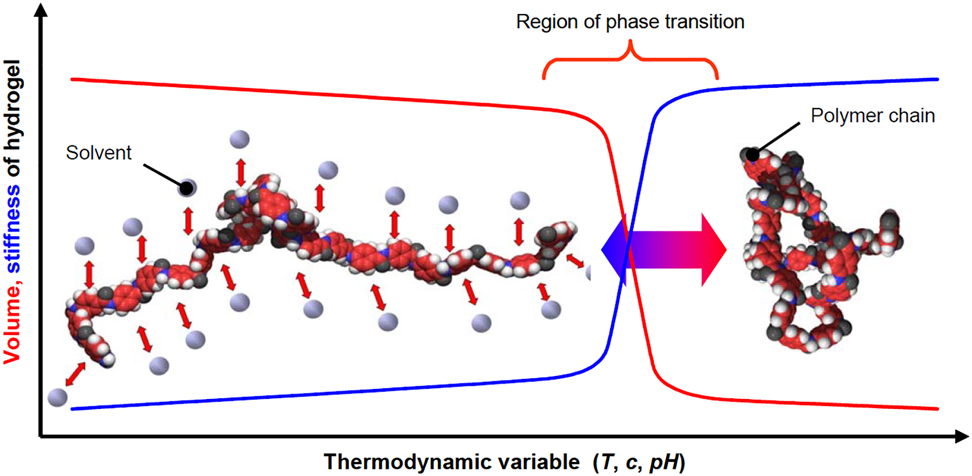
The mechanism of a response to a stimulus (Richter et al., 2008).
The swollen phase of the gel (left) is dominated by polymer-solvent interactions: The solvent is well-distributed among the polymer chains. The collapsed phase of the gel (right) is determined by polymer-polymer-interactions, which remove solution out of the gel. Within the range of phase transition, small alterations of a thermodynamic value result in abrupt changes of the gel properties.
Besides morphology one can talk about network architecture of the microgels (Karg et al., 2019). The simplest architecture is a compartment of a sole polymer cross-linked evenly (Figure 6). Sometimes the polymer chains can be cross-linked in a thick core and dangle in a loose corona. The corona usually reveals bright response to stimuli because it can drastically change its size under change of conditions. A core can be also covered with a shell of another polymer. Such core–shell polymers can either response to different stimuli (the core is responcible for one stimulus and the corona – for another). Several cores in one shell gives pudding-like architecture (Di et al., 2017) and several shells one above another – to the still imaginary onion-like. The microgels also can carry solid nanoparticles (gold, silver, magnetite, quantum dots). These particles provide response of the microgel for either electric or magnetic fields or cause interesting optical properties. The nanoparticles can comprise a core, be trapped in a core or a shell or be distributed in other ways. Farooqi, Khan, Begum, and Ijaz (2016) counted nine types of architecture that includes nanoparticles. All that sophistication provides a great variety of the particles with different properties. That’s why in the past decade, the synthesis of microgels with complex architectures received considerable attention.

The architecture of microgel particles.
If the polymer chains are charged, their morphology is responsive to pH and ionic strength (Bergman, Pedersen, Schurtenberger, & Boon, 2020). In the core-corona or core–shell microgels these stimuli can affect a core and a shell (corona) in different ways. Changing the density of the shell or corona can swell or deswell the core. If the core contains some weakly bound molecules, the molecules can be released. This is the main principle of using microgels for the delivery and controlled release of small molecules, first of all – drugs (Chyzy, Tomczykowa, & Plonska-Brzezinska, 2020).
Building blocks for microgels and how they influence the microgel properties
The microgels contain the following building blocks:
Scaffolding polymer, that forms the chains for the network (obligatory);
Physical or covalent cross-links (obligatory);
Functionalized (“tailored”) blocks within the scaffolding polymer, that modify the chemical properties of the microgel making it responsible to various stimuli (optional, but wide-spread);
Chemically or physically bound small molecules and nanoparticles (optional).
In principle, any cross-linked hydrophilic polymer can be a scaffold for aqueous microgels. However the most widely used scaffold is poly(N-isopropylacrylamide) (pNIPAM) with N,N′-methylenebisacrylamide (BIS) as a cross-linker.
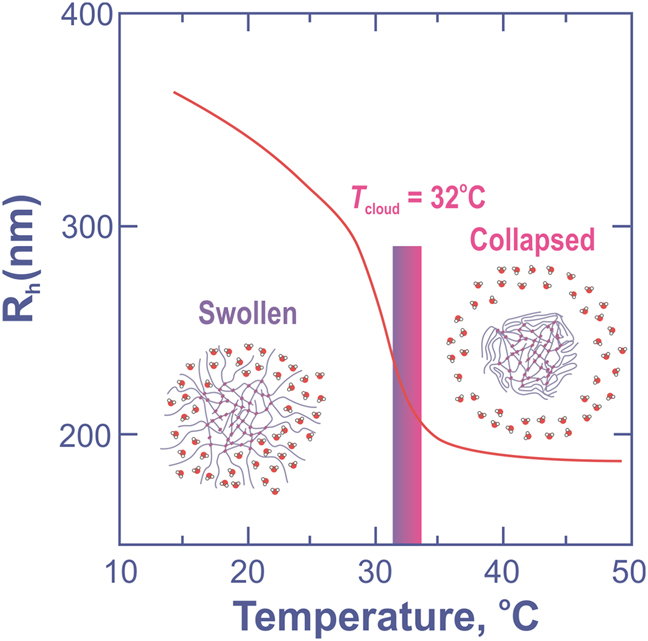
 Why pNIPAM? Because its phase diagram with water (Figure 7) has a remarkable feature: pNIPAM chains are miscible with water only at temperature less than 32 °C – the lower critical solution temperature (LCST aka “cloud point”). Poly(N-vynilcaprolactam) (PVCL) with close LCST = 35 °C (Meeussen, 2000) is also used for synthesis of microgels.
Why pNIPAM? Because its phase diagram with water (Figure 7) has a remarkable feature: pNIPAM chains are miscible with water only at temperature less than 32 °C – the lower critical solution temperature (LCST aka “cloud point”). Poly(N-vynilcaprolactam) (PVCL) with close LCST = 35 °C (Meeussen, 2000) is also used for synthesis of microgels.

Phase diagram for pNIPAM in water basing on Heskins & Guillet, 1968.
The microgels based on the pNIPAM chains exhibit volume phase transition at the temperatures about 32 °C, that is close to the human body. Below this temperature the pNIPAM particles are swollen and form crystals or glasses while packed densely. Above the LCST the microgels dramatically shrink in size changing their volume by more than an order of magnitude (Figure 8). The glass turns into liquid (Figure 9). However, at higher temperatures polymer chains tend to stick together, leading to an interaction between microgel particles. As a result, aggregation or even gelation can occur (Romeo, Fernandez-Nieves, Wyss, Aciernoand, & Weitz, 2010). It means that the phase diagram of pNIPAM microgels with water (Figure 10) is very complicated and sometimes surprising.
Average hydrodynamic radius for a thermoresponsive pNIPAM microgel with a crosslinking density of 5 mol.%.
Adapted from Mohanty, Paloli, Crassous, & Schurtenberger, 2012, with permission from John Wiley & Sons, Inc.
Particle trajectories observed during a period of 10 s.
Reproduced from Mohanty et al., 2012, with permission from John Wiley & Sons, Inc.
The schematic phase diagram for pNIPAM microgels.
However, pNIPAM microgels reveal only thermoresponsive properties. To make them responsive to other stimuli, like pH, light or mechanical stress the pNIPAM chains should be modified accordingly.
Usually the functionalized blocks (Figure 11) are inserted directly into the polymer chain by means of co-polymerization. For example, to add acidic function, the blocks of acrylic acid (AA), metacrylic acid (MA) or fumaric acid (FA) can be integrated into pNIPAM chains. For basic function vynilpyridine (VP) or N-(3-aminopropyl)methecrylamide (APMA) are used. N-tret-butyl acrylamide (NTBAM) is applied to increase hydrophobicity of the polymer chain, monomethyl ether monomethacrylate (PEG-MA) – to decrease hydrophobicity. Chelating monomers, such as N-(4-vinyl)benzyl ethylenediamine (VBEDA) make for metal-binding properties (Kanazawa, 2004). Electochemicaly active moieties such as ferrocene (Mergel et al., 2019) add responsiveness to the redox-potential.
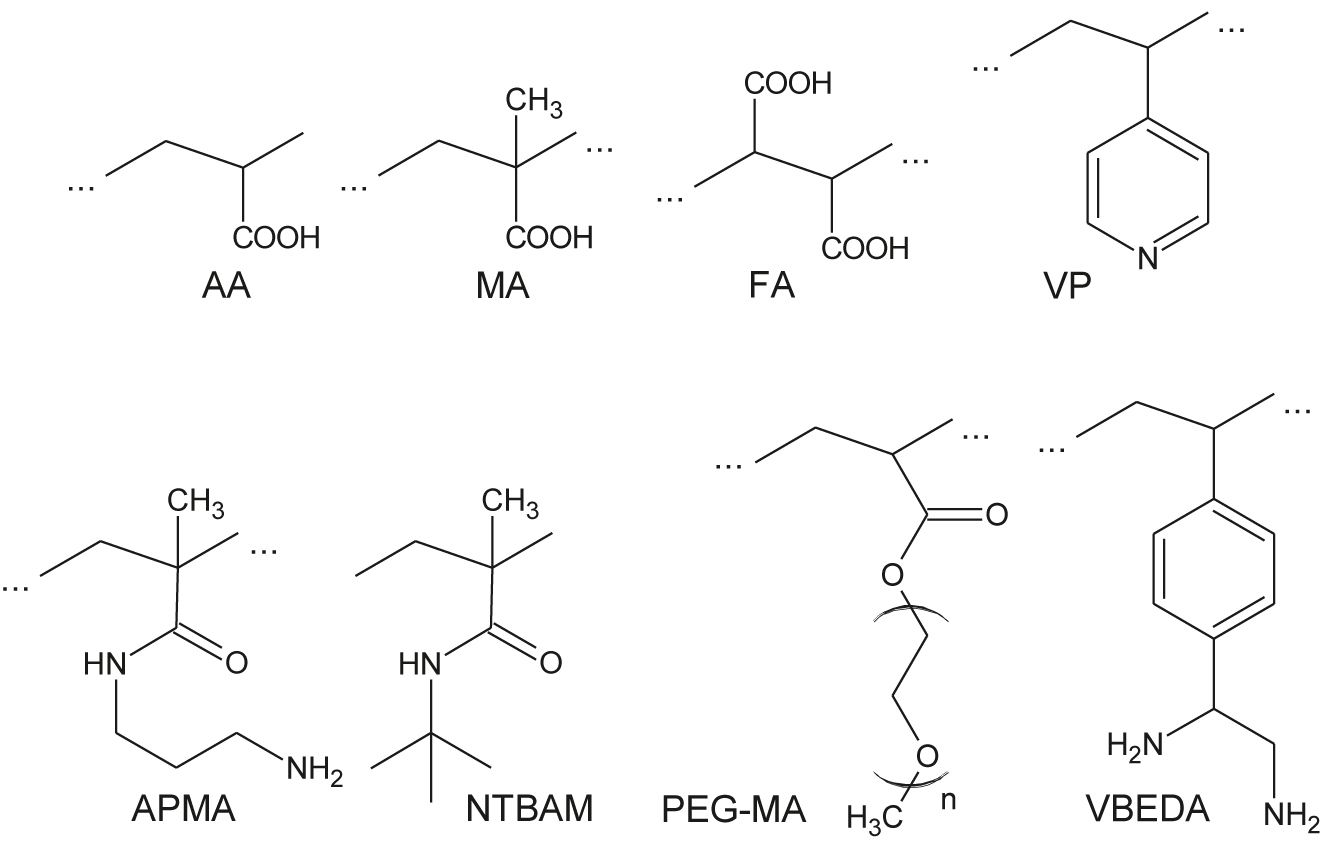
The functionalized blocks for microgels.
Microgels tailored with acidic or basic blocks are pH-sensitive. At a pH value lower than pKa the acidic chain is protonized and thus non-charged. While increasing pH the polymer chain deprotonizes and it gets negative charge. The repulsion of negative charges enlarges the size of the particles (Figure 12) and increases repulsion between the particles.
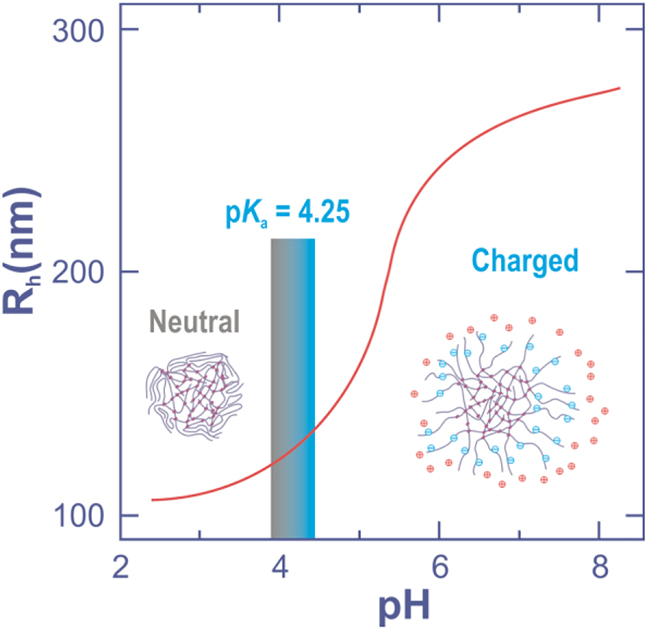
Average hydrodynamic radius for a thermoresponsive pNIPAM microgel tailored with acrylic acid.
Adapted from Mohanty et al., 2012, with permission from John Wiley & Sons, Inc.
Post-modification (polymer analogous reactions) also can be used for tailoring microgel structure. This way is used seldom, but allows to enrich the microgel by very interesting groups such as crown ethers (Wei et al., 2017) or gluccose-binding 3-aminophenylboronic acid (Zhang, Guan, & Zhou, 2006). Responsive surfactants inside the gel (such as light-responsive azobenzene derivatives undergoing cis-trans isomerization, Zakrevskyy et al., 2012) also can form stimuli-responsive microgel.
Synthesis of microgels
The microgels are obtained by polymerization of monomers with simultaneous incorporation of cross-linkers. However, there are challenges in this process:
To synthesize the colloidally stable microgels of a certain size;
To reach high monomer conversions;
To control the distribution of cross-links (either to cross-link all the polymer chains in the core leaving corona with non-cross-linked chains, or, on the contrary, to obtain evenly linked polymer within the whole particle).
To obtain the microgels of the uniform size the polymerization is usually performed in aqueous phase (precipitation polymerization) or in aqueous droplets, emulsified in oil. The precipitation polymerization includes combination of free-radical polymerization and self-assembly/crosslinking of polymer chains to form microgels (Figure 13).
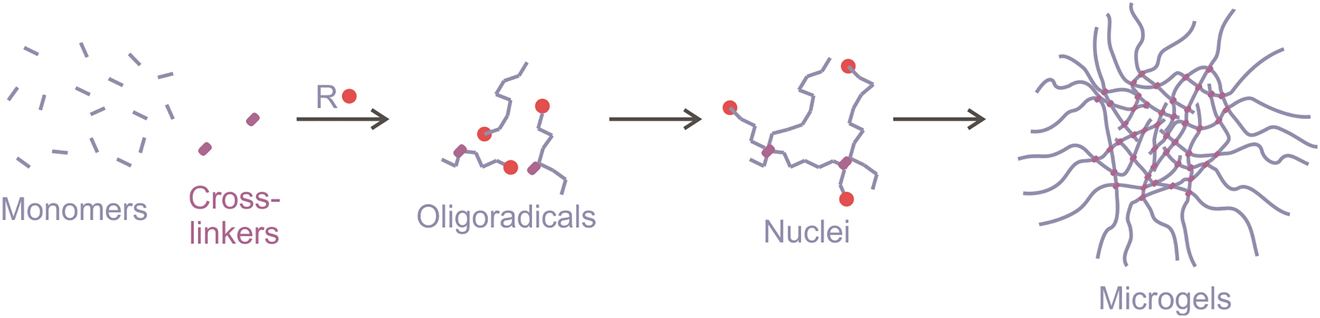
Microgel synthesis by precipitation polymerization.
Organic solvent
The droplets of aqueous emulsion in oil may contain one monomer or a mixture of monomers: scaffolding such as NIPAM, functionalizing such as acrylic acid and cross-linking such as BIS. The uniform size of the gel particles is provided by the uniform size of the emulsion droplets. To stabilize the droplets the surfactants such as sodium dodecylsulfate (SDS) are used (Figure 14).
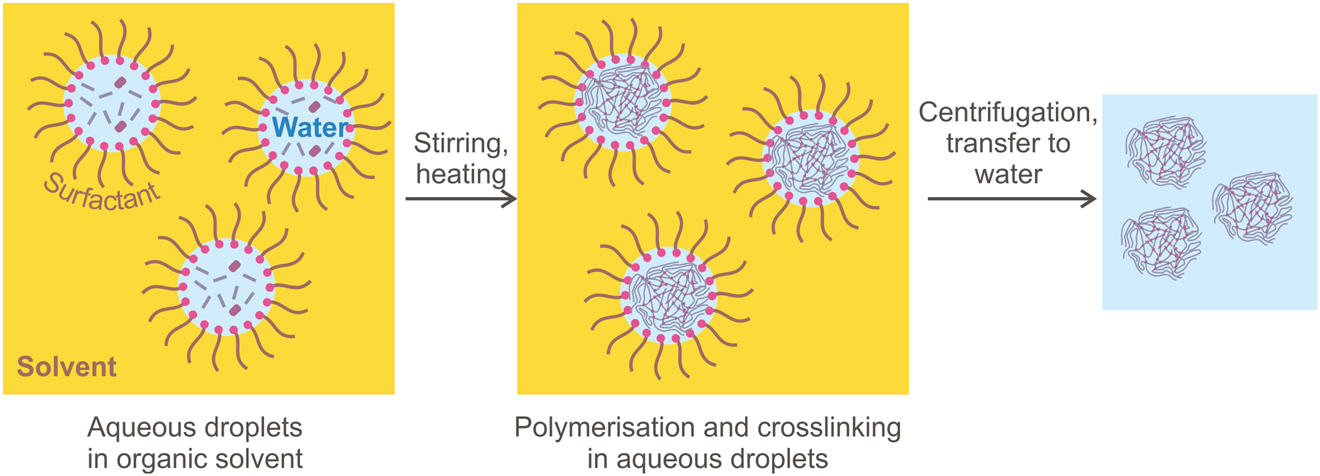
Microgel synthesis by polymerisation/crosslinking in water-in-oil emulsion.
The use of the surfactant in precipitation polymerization is not mandatory because PVCL or pNIPAM microgels exhibit sufficient colloidal stability due to the combination of the electrostatic repulsion forces originating from the ionic initiator fragments and sterical stabilization by amphiphilic dangling chains. However, the use of surfactant may help to regulate the final size of microgels, making it more uniform for example. After dissolving all monomers in the reaction medium, the initiator is added. Here is the extraction of the synthesis technique for precipitation polymerisation (Jones & Lyon, 2000).
NIPAM (1.4 g) and BIS (0.10 g) were dissolved in 150 mL of H2O. Dissolved gas was removed under vacuum. SDS (0.057 g) was then dissolved in the monomer solution. The solution was placed into a 250 mL three-neck, round-bottom flask and heated over period of 1 h with a heating mantle under a constant nitrogen purge and maximum stir rate. After stabilizing the solution for 15 min at 70 °C, polymerization was initiated via addition of ammonium persulfate (0.069 g) dissolved 10 mL of degassed H2O. The reaction was allowed to proceed at a temperature of 70 °C for 6 h.
To graft a shell to a core the core microgel dispersion is mixed with the shell monomers, surfactant and initiator. If the core is charged, it can be also coated with a polymer of the opposite charge and they will stick to each other by Coulomb interactions. In this way Wong, Krishnakumar Gaharwar, Müller-Schulte, Bahadur, and Richtering (2007) coated pNIPAM-pAA core with poly(diallyldimethylammonium chloride).
To upscale the microgel production the jet or continuous polymerization is developed (Figure 15). From the chemical point of view it is the polymerization with initiator, but with a tricky technological arrangement. The reactants (monomer, crosslinker and initiator) are mixed in the static mixer and pumped through the reactor tube of about 1 mm in diameter and 5–10 m length. The microgels are formed while passing the tube reactor and do not coagulate under the laminar conditions into the tube. The size of the particle depends on the time that the substances are in the reactor.
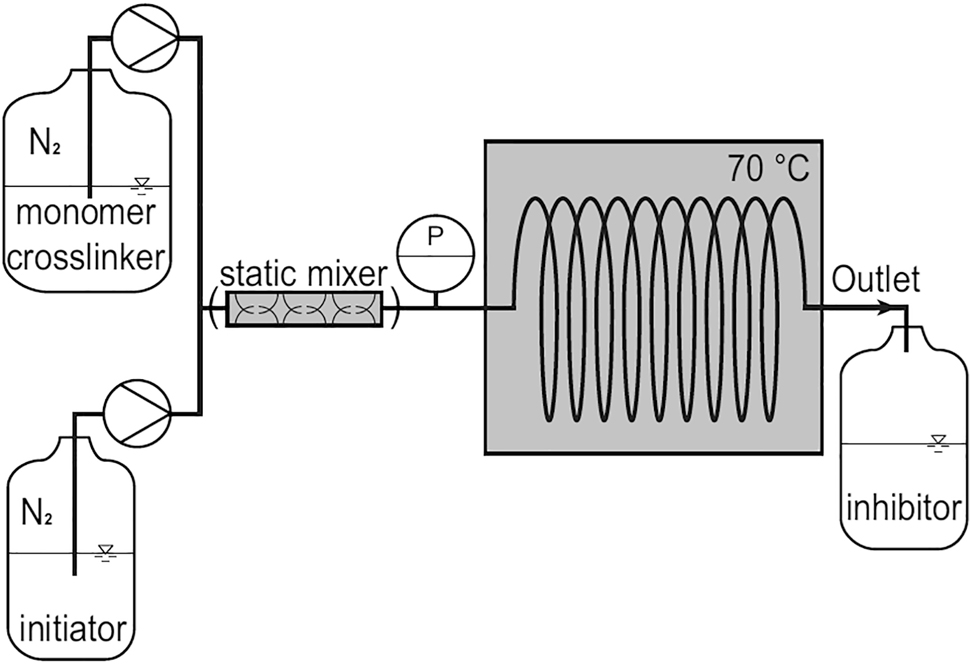
A scheme of continuous polymerization.
Reproduced from Wolff et al. (2018) with permission from ACS.
Applications of microgels: bright examples
Here we will just list some bright examples of microgel applications.
Microgels are used to stabilize “quantum dots” – nanoparticle of CdS and generic compounds that reveal fluorescence. Cai, Du, Gao, Chen, and Fu (2016) used the microgels to tune the fluorescence of CdTe quantum dots (Figure 16). Liu, Shu, Su, Zhang, and Serpe (2018) obtained such quantum dots in pNIPAM-co-AAmicrogels after impregnation the microgel by cadmium salt and adding sodium sulfide. The obtained nanoparticles reveal photocatalytic activity in degradation of dyes – neither isolated CdS nor msole microgel don’t reveal this activity.
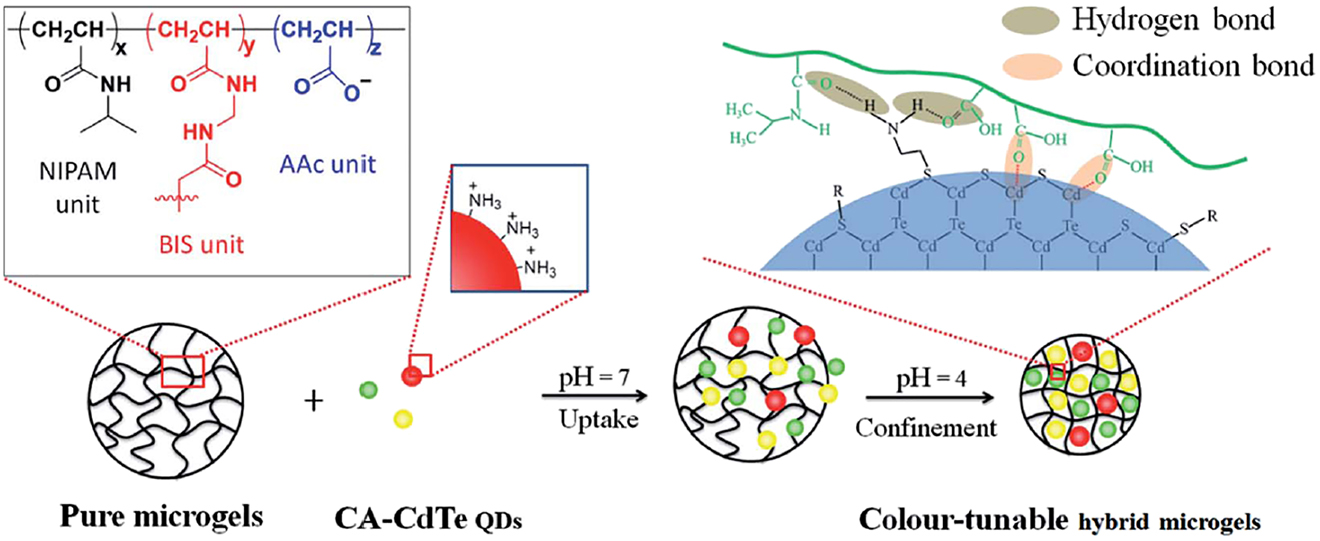
Synthetic scheme for CdTe embedded microgels and illustration of the interactions between cisteamine – CdTe quantum dots.
(Reproduced from Cai et al., 2016 with permission from the Royal Society of Chemistry).
Microgels with hydrophobic hard polysterene core and soft and swollen pNIPAM shell loaded with gold nanoparticles selectively catalyses oxidation of benzil alcohol into benzaldehyde by oxygen at 40 °C with a turnover factor of more than 2000 h−1 (Lu et al., 2009). At 25 °C the turnover factor is more than 10 times less because of the swelling.
Gluccose-sensitive microgels (the radius of the particles increases in the presence of glucose) was obtained by the functionalization of poly(N-isopropylacrylamide-co-acrylic acid) microgels with 3-aminophenylboronic acid (APBA) via carbodiimide coupling (Zhang et al., 2006) (Figure 17). These microgels can be used as a building blocks of the films with tunable permeability. Immobilisation of fluorescent quantum dots at such a microgel allows optical detection of gluccose (Figure 18) (Wu, Zhou, Shen, & Zhou, 2009).
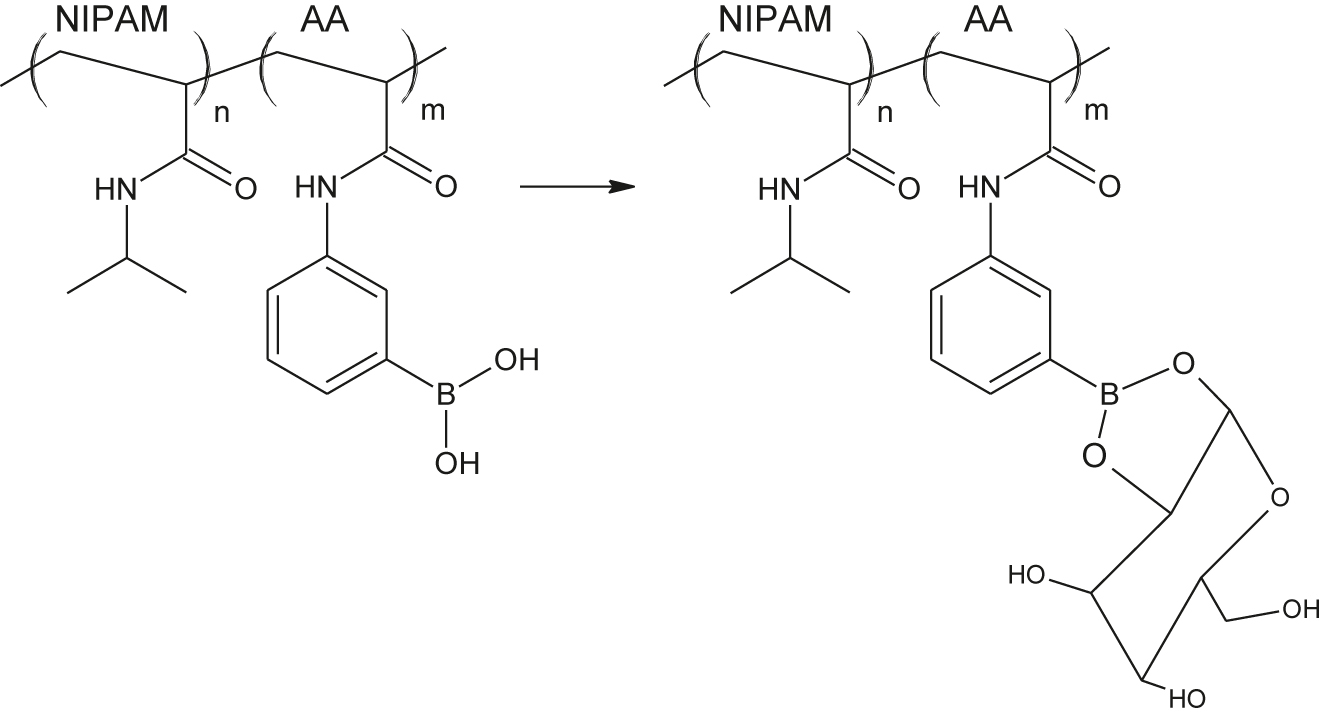
Gluccose-sensitive microgel.
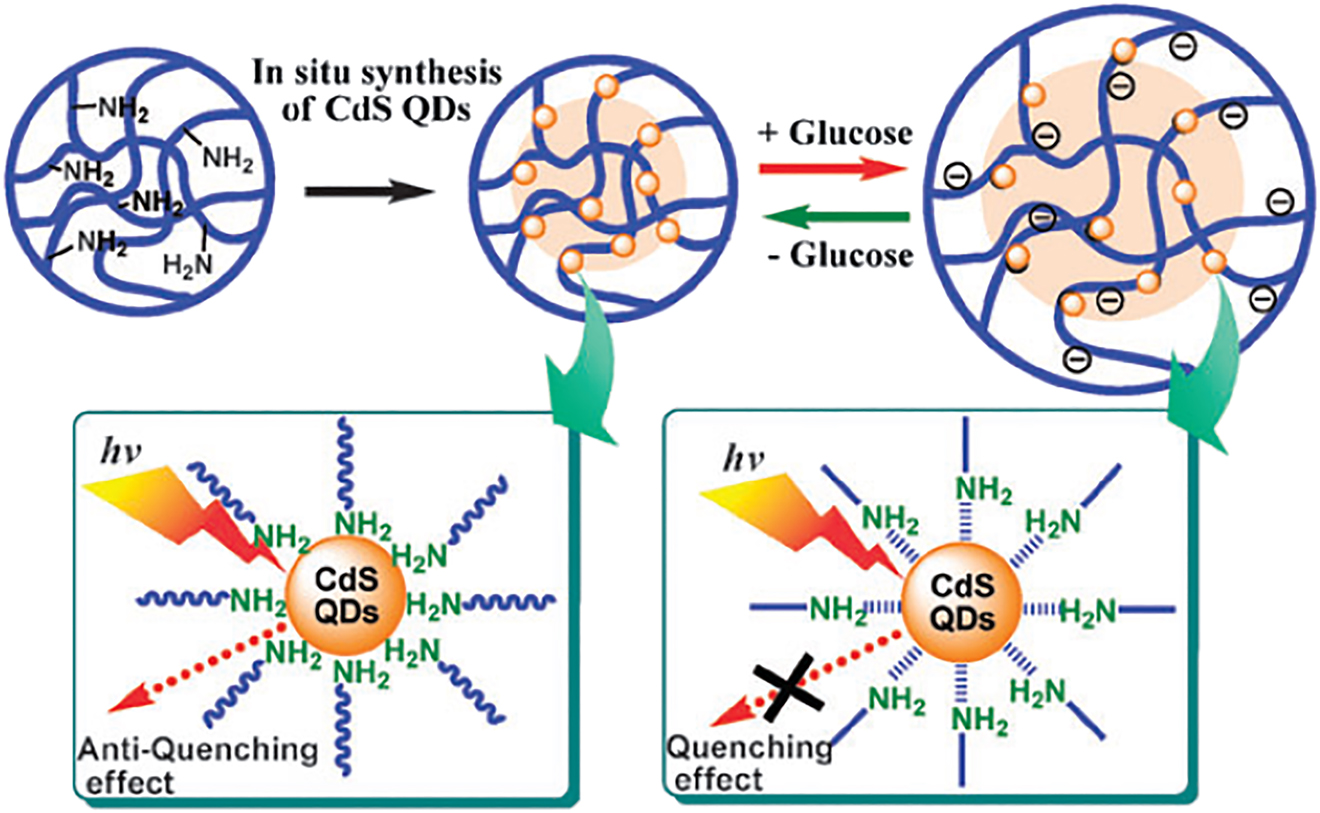
Reversible fluorescence quenching and antiquenching of CdS QDs embedded in the interior of p(NIPAM-AAm-PBA) microgels in response to the change in glucose concentration.
Reproduced from Wu et al., 2009. with permission from the Royal Society of Chemistry.
A photonic device was fabricated by sandwiching the nanoparticles with gold core and pNIPAM shell between two thin layers of gold (Wei & Serpe, 2019). The distance between the layers of gold and hence the capacitance of the system is responsive to temperature and green light. Poly(lactic-co-glycolic acid) nanocapsules loaded with insuline and encapsulated within chitosan microgels release insuilin in vivo under ultrasonic treatment (Di et al., 2017).
Microgels as a topic for learning projects
Microgels are quite accessible objects for learning projects. As one can see from the part “Synthesis of microgels” even the synthesis of PNIPAM microgel is affordable for students if the monomers are accessible. However, there are even more simple techniques that are described below. To assess the resulting microgels their optical, rheological, magnetic and, sometimes, catalytic properties can be estimated. For large (> 100 μm) particles the size changes can be observed via school microscope.
First of all, microgel can be made of common gelation agents (agarose or gelatine) by spraying the small droplets of hot solution into cold water (Brun-Graeppi, 2011). The main challenge is to make a device to spray micron-size droplets – it could be a part of the learning project. The obtained microgels melt irreversibly, so the cross-linking is another challenge. Glutaraldehyde (Huang et al., 2008), formaldehyde or glyoxal (Marquié, 2001) can be used to cross-link gelatine gelatine and genipin – for agarose (Meena, Prasad, & Siddhanta, 2007). Casein microgels cross-linked with genipin (Silva, Saint-Jalmes, De Carvalho, & Gaucheron, 2014) are also suitable.
The most achievable cross-linked microgel for the school lab is calcium (or strontium or barium) alginate. When dropping 0.5–3% of sodium alginate solution into 10–50 mM solution of Ca2+ under vigorous stirring, the microgels form immediately and the drops don’t have time to coagulate (Brun-Graeppi, 2011; Quong, Neufeld, Skjåk-Bræk, & Poncelet, 1998). In contrast, CaCO3 has very low solubility in pure water, allowing its uniform distribution in alginate solution before gelation occurs (Kuo & Ma, 2001). Playing with the salts, concentrations, stirring conditions and admixtures to the reaction mixture allows arranging parallel learning projects. Pravinta et al., 2016 used jet homogenizer to mix the components under highly turbulent conditions. The construction of such a homogenizer also could be a good learning project.
Starch-based microgels (oxidized or carboxymethylated) can be cross-linked by sodium thrimetaphosphate (Li et al., 2009). However, the described method is rough: The macrogel is dried and then grinded. Pre-emulsifying of the starch particles is a good challenge for the learning project.
A “casting” strategy is promising for obtaining gelatine microgels with different nanoparticles. Duan et al. (2019) obtained porous calcium carbonate microparticles by mixing solutions of Na2CO3 and CaCl2, loaded them with nanoparticles of platinum or iron oxide and saturated the pores with aqueous gelatin. Then added cold water, and centrifuged the precipitated particles. The gelatine moleculs were cross-linked with genipine and calcium carbonate was dissolved by EDTA. Loading of CaCO3 with different substances or nanoparticles and playing with cross-linkers allow to arrange many parallel research projets.
The easiest way to add nanoparticles to the microgel is to fabricate them inside the microgel network. For example, silver nanopartilces can be obtained by in-situ reduction of silver compounds (Begum, Naseem, & Farooqi, 2016 and Refs.). To incorporate magnetite nanoparticles the microgel is impregnated with a solution containing FeCl3 and FeCl2 and treated with ammonia solution (Bhattacharya, Eckert, Boyko, & Pich, 2007). Nanopartilces of various metals (including magnetic cobalt) can be incorporated by loading the gel particles by a metal salt solution following by NaBH4 reduction (Ajmal, 2014; Bibi, Ajmal, Naseer, Farooqi, & Siddiq, 2018). All these techniques also could be applied in learning projects.
Conclusions
Microgels are remarkable soft polymer materials with interesting structure and unique properties. These materials show high chemical diversity, tunability of the properties and can be used in various fields. Based on the availability of the molecular building blocks (monomers, pre-polymers or biopolymers) and simple synthesis techniques (free-radical precipitation polymerisation in water, polymerisation/crosslinking in water-in-oil emulsions or gelation processes in aqueous salt solutions) microgels can be easily and safely synthesized in educational laboratories. Microgel properties like deformability, surface-activity or stimuli-responsiveness can be investigated with simple equipment (optical microscope) what makes them suitable and promising objects for project-based learning.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ajmal, M., Siddiq, M., Al-Lohedan, H., & Sahiner, N. (2014). Highly versatile p(MAc)-M (M: Cu, co, ni) microgel composite catalyst for individual and simultaneous catalytic reduction of nitro compounds and dyes. RSC Advances, 4(103), 59562–59570. https://doi.org/10.1039/c4ra11667d.Search in Google Scholar
Begum, R., Naseem, K., & Farooqi, Z. H. (2016). A review of responsive hybrid microgels fabricated with silver nanoparticles: Synthesis, classification, characterization and applications. Journal of Sol-Gel Science and Technology, 77(2), 497–515. https://doi.org/10.1007/s10971-015-3896-9.Search in Google Scholar
Bergman, M. J., Pedersen, J. S., Schurtenberger, P., & Boon, N. (2020). Controlling the morphology of microgels by ionic stimuli. Soft Matter, 16(11), 2786–2794. https://doi.org/10.1039/c9sm02170a.Search in Google Scholar PubMed
Bhattacharya, S., Eckert, F., Boyko, V., & Pich, A. (2007). Temperature-, pH-, and magnetic-field-sensitive hybrid microgels. Small, 3(4), 650–657. https://doi.org/10.1002/smll.200600590.Search in Google Scholar PubMed
Bibi, F., Ajmal, M., Naseer, F., Farooqi, Z. H., & Siddiq, M. (2018). Preparation of magnetic microgels for catalytic reduction of 4-nitrophenol and removal of methylene blue from aqueous medium. International Journal of Environmental Science and Technology, 15(4), 863–874. https://doi.org/10.1007/s13762-017-1446-4.Search in Google Scholar
Brun-Graeppi, A. K. A. S., Richard, C., Bessodes, M., Scherman, D., & Merten, O. (2011). Cell microcarriers and microcapsules of stimuli-responsive polymers. Journal of Controlled Release, 149(3), 209–224. https://doi.org/10.1016/j.jconrel.2010.09.023.Search in Google Scholar PubMed
Cai, Y., Du, G., Gao, G., Chen, J., & Fu, J. (2016). Colour-tunable quantum dots/poly(NIPAM-: Co -AAc) hybrid microgels based on electrostatic interactions. RSC Advances, 6(100), 98147–98152. https://doi.org/10.1039/c6ra19659d.Search in Google Scholar
Chyzy, A., Tomczykowa, M., & Plonska-Brzezinska, M. E. (2020). Hydrogels as potential nano-, micro- and macro-scale systems for controlled drug delivery. Materials, 13, 188. https://doi.org/10.3390/ma13010188.Search in Google Scholar PubMed PubMed Central
Di, J., Yu, J., Wang, Q., Yao, S., Suo, D., Ye, Y., Gu, Z. (2017). Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Research, 10(4), 1393–1402. https://doi.org/10.1007/s12274-017-1500-z.Search in Google Scholar
Duan, L., Qin, C., Wang, A., Wang, S., Li, J., & Bai, S. (2019). Gelatin microgels with various nano-objects fabricated by “casting” strategy and application as a catalytic system. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 580. https://doi.org/10.1016/j.colsurfa.2019.123759.Search in Google Scholar
Farooqi, Z. H., Khan, S. R., Begum, R., & Ijaz, A. (2016). Review on synthesis, properties, characterization, and applications of responsive microgels fabricated with gold nanostructures. Reviews In Chemical Engineering, 32(1), 49–69. https://doi.org/10.1515/revce-2015-0033.Search in Google Scholar
Gao, J., & Hu, Z. (2002). Optical properties of N-isopropylacrylamide microgel spheres in water. Langmuir, 18(4), 1360–1367. https://doi.org/10.1021/la011405f.Search in Google Scholar
Heskins, M., & Guillet, J. E. (1968). Solution properties of poly(N-isopropylacrylamide). Journal of Macromolecular Science Part A - Chemistry, 2(8), 1441–1455. https://doi.org/10.1080/10601326808051910.Search in Google Scholar
Huang, S., Deng, T., Wang, Y., Deng, Z., He, L., Liu, S., Jin, Y. (2008). Multifunctional implantable particles for skin tissue regeneration: Preparation, characterization, in vitro and in vivo studies. Acta Biomaterialia, 4(4), 1057–1066. https://doi.org/10.1016/j.actbio.2008.02.007.Search in Google Scholar PubMed
Jones, C. D., & Lyon, L. A. (2000). Synthesis and characterization of multiresponsive core-shell microgels. Macromolecules, 33(22), 8301–8306. https://doi.org/10.1021/ma001398m.Search in Google Scholar
Kanazawa, R., Mori, K., Tokuyama, H., & Sakohara, S. (2004). Preparation of thermosensitive microgel adsorbent for quick adsorption of heavy metal ions by a temperature change. Journal of Chemical Engineering of Japan, 37(6), 804–807. https://doi.org/10.1252/jcej.37.804.Search in Google Scholar
Karg, M., Pich, A., Hellweg, T., Hoare, T., Lyon, L. A., Crassous, J. J., Richtering, W. (2019). Nanogels and microgels: From model colloids to applications, recent developments, and future trends. Langmuir, 35(19), 6231–6255. https://doi.org/10.1021/acs.langmuir.8b04304.Search in Google Scholar PubMed
Kuo, C. K., & Ma, P. X. (2001). Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials, 22(6), 511–521. https://doi.org/10.1016/S0142-9612(00)00201-5.Search in Google Scholar
Li, Y., De Vries, R., Slaghek, T., Timmermans, J., Cohen Stuart, M. A., & Norde, W. (2009). Preparation and characterization of oxidized starch polymer microgels for encapsulation and controlled release of functional ingredients. Biomacromolecules, 10(7), 1931–1938. https://doi.org/10.1021/bm900337n.Search in Google Scholar PubMed
Liu, J., Shu, T., Su, L., Zhang, X., & Serpe, M. J. (2018). Synthesis of poly (: N -isopropylacrylamide)- co -(acrylic acid) microgel-entrapped CdS quantum dots and their photocatalytic degradation of an organic dye. RSC Advances, 8(30), 16850–16857. https://doi.org/10.1039/c8ra01855c.Search in Google Scholar PubMed PubMed Central
Lu, Y., Proch, S., Schrinner, M., Drechsler, M., Kempe, R., & Ballauff, M. (2009). Thermosensitive core-shell microgel as a "nanoreactor" for catalytic active metal nanoparticles. Journal of Materials Chemistry, 19(23), 3955–3961. https://doi.org/10.1039/b822673n.Search in Google Scholar
Lyon, L. A., Debord, J. D., Debord, S. B., Jones, C. D., McGrath, J. G., & Serpe, M. J. (2004). Microgel colloidal crystals. Journal of Physical Chemistry B, 108(50), 19099–19108. https://doi.org/10.1021/jp048486j.Search in Google Scholar
Marquié, C. (2001). Chemical reactions in cottonseed protein cross-linking by formaldehyde, glutaraldehyde, and glyoxal for the formation of protein films with enhanced mechanical properties. Journal of Agricultural and Food Chemistry, 49(10), 4676–4681. https://doi.org/10.1021/jf0101152.Search in Google Scholar PubMed
Mattsson, J., Wyss, H. M., Fernandez-Nieves, A., Miyazaki, K., Hu, Z., Reichman, D. R., & Weitz, D. A. (2009). Soft colloids make strong glasses. Nature, 462(7269), 83–86. https://doi.org/10.1038/nature08457.Search in Google Scholar PubMed
Meena, R., Prasad, K., & Siddhanta, A. K. (2007). Preparation of genipin-fixed agarose hydrogel. Journal of Applied Polymer Science, 104(1), 290–296. https://doi.org/10.1002/app.25596.Search in Google Scholar
Meeussen, F., Nies, E., Berghmans, H., Verbrugghe, S., Goethals, E., & Du Prez, F. (2000). Phase behaviour of poly(N-vinyl caprolactam) in water. Polymer, 41(24), 8597–8602. https://doi.org/10.1016/S0032-3861(00)00255-X.Search in Google Scholar
Mergel, O., Schneider, S., Tiwari, R., Kühn, P. T., Keskin, D., Stuart, M. C. A., Plamper, F. A. (2019). Cargo shuttling by electrochemical switching of core-shell microgels obtained by a facile one-shot polymerization. Chemical Science, 10(6), 1844–1856. https://doi.org/10.1039/c8sc04369h.Search in Google Scholar PubMed PubMed Central
Mohanty, P., Paloli, D., Crassous, J., & Schurtenberger, P. (Eds.) (2012). Dynamical arrest and crystallization in dense microgel suspensions. Hydrogel Micro and Nanoparticles, (pp. 369–395). https://doi.org/10.1002/9783527646425.ch15.Search in Google Scholar
Pravinata, L., Akhtar, M., Bentley, P. J., Mahatnirunkul, T., & Murray, B. S. (2016). Preparation of alginate microgels in a simple one step process via the Leeds jet homogenizer. Food Hydrocolloids, 61, 77–84. https://doi.org/10.1016/j.foodhyd.2016.04.025.Search in Google Scholar
Quellet, C., Eicke, H., Gehrke, R., & Sager, R. (1989). Evidence of fractal network formation in gelatin-W/O microemulsions. Europhysics Letters, 9(3), 293–298. https://doi.org/10.1209/0295-5075/9/3/018.Search in Google Scholar
Quong, D., Neufeld, R. J., Skjåk-Bræk, G., & Poncelet, D. (1998). External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnology and Bioengineering, 57(4), 438–446. https://doi.org/10.1002/(SICI)1097-0290(19980220)57:4<438::AID-BIT7>3.0.CO;2-N.10.1002/(SICI)1097-0290(19980220)57:4<438::AID-BIT7>3.0.CO;2-NSearch in Google Scholar
Richter, A., Paschew, G., Klatt, S., Lienig, J., Arndt, K., & Adler, H. P. (2008). Review on hydrogel-based pH sensors and microsensors. Sensors, 8(1), 561–581. https://doi.org/10.3390/s8010561.Search in Google Scholar
Romeo, G., Fernandez-Nieves, A., Wyss, H. M., Aciernoand, D., & Weitz, D. A. (2010). Temperature-controlled transitions between glass. liquid, and gel states in dense p-NIPA suspensions. Advanced Materials, 22(31), 3441–3445. https://doi.org/10.1002/adma.200904189.Search in Google Scholar
Sieglaff, C. L. (1963). Viscosity and swelling behaviour of lightly crosslinked microgels. Polymer, 4(C), 281–284. https://doi.org/10.1016/0032-3861(63)90034-X.Search in Google Scholar
Silva, N. F. N., Saint-Jalmes, A., De Carvalho, A. F., & Gaucheron, F. (2014). Development of casein microgels from cross-linking of casein micelles by genipin. Langmuir, 30(34), 10167–10175. https://doi.org/10.1021/la502274b.Search in Google Scholar
Wei, Y., Liu, Z., Luo, F., Zhang, L., Wang, W., Ju, X.-J., Chu, L. (2017). A novel poly(N-isopropylacrylamide-co-acryloylamidobenzo-12-crown-4) microgel with rapid stimuli-responsiveness for molecule-specific adsorption of γ-cyclodextrin. Macromolecular Chemistry and Physics, 218(20). https://doi.org/10.1002/macp.201700216.Search in Google Scholar
Wei, M., & Serpe, M. J. (2019). Temperature–Light dual-responsive Au@PNIPAm core-shell microgel-based optical devices. Particle and Particle Systems Characterization, 36(1). https://doi.org/10.1002/ppsc.201800326.Search in Google Scholar
Wolff, H. J. M., Kather, M., Breisig, H., Richtering, W., Pich, A., & Wessling, M. (2018). From batch to continuous precipitation polymerization of thermoresponsive microgels. ACS Applied Materials and Interfaces, 10(29), 24799–24806. https://doi.org/10.1021/acsami.8b06920.Search in Google Scholar
Wong, J. E., Krishnakumar Gaharwar, A., Müller-Schulte, D., Bahadur, D., & Richtering, W. (2007). Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. Journal of Magnetism and Magnetic Materials, 311(1 SPEC. ISS.), 219–223. https://doi.org/10.1016/j.jmmm.2006.10.1201.Search in Google Scholar
Wu, W., Zhou, T., Shen, J., & Zhou, S. (2009). Optical detection of glucose by CdS quantum dots immobilized in smart microgels. Chemical Communications, 45(29), 4390–4392. doi:https://doi.org/10.1039/b907348e.Search in Google Scholar
Wu, Y., Wiese, S., Balaceanu, A., Richtering, W., & Pich, A. (2014). Behavior of temperature-responsive copolymer microgels at the oil/water interface. Langmuir, 30(26), 7660–7669. https://doi.org/10.1021/la501181k.Search in Google Scholar PubMed
Zakrevskyy, Y., Richter, M., Zakrevska, S., Lomadze, N., Von Klitzing, R., & Santer, S. (2012). Light-controlled reversible manipulation of microgel particle size using azobenzene-containing surfactant. Advanced Functional Materials, 22(23), 5000–5009. https://doi.org/10.1002/adfm.201200617.Search in Google Scholar
Zhang, Y., Guan, Y., & Zhou, S. (2006). Synthesis and volume phase transitions of glucose-sensitive microgels. Biomacromolecules, 7(11), 3196–3201. https://doi.org/10.1021/bm060557s.Search in Google Scholar PubMed
Zhang, J., & Pelton, R. (1999). Poly(N-isopropylacrylamide) microgels at the air-water interface. Langmuir, 15(23), 8032–8036. https://doi.org/10.1021/la990316o.Search in Google Scholar
© 2021 Denis M. Zhilin and Andrij Pich, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Preface
- Special issue of Chemistry Teacher International in Polymer Sciences
- Review Articles
- Fundamentals of reversible addition–fragmentation chain transfer (RAFT)
- Reversible-Deactivation Radical Polymerisation: chain polymerisation made simple
- Ring-opening polymerization
- Other
- Thermal analysis: basic concept of differential scanning calorimetry and thermogravimetry for beginners
- Review Article
- Size-exclusion chromatography as a useful tool for the assessment of polymer quality and determination of macromolecular properties
- Other
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 1: a good practice on estimation of bulk resistance of solid polymer electrolytes
- Special Issue Paper
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 2: dielectric response of (non-) polymer electrolytes
- Good Practice Report
- Chirality analysis of helical polymers
- Other
- Fabrication and function of polymer membranes
- Review Article
- Nano- and microgels: a review for educators
- Other
- Theoretical background on semiconducting polymers and their applications to OSCs and OLEDs
- Good Practice Report
- An understandable approach to the temperature dependence of electric properties of polymer-filler composites using elementary quantum mechanics
- Review Article
- Polymer degradation: a short review
Articles in the same Issue
- Frontmatter
- Preface
- Special issue of Chemistry Teacher International in Polymer Sciences
- Review Articles
- Fundamentals of reversible addition–fragmentation chain transfer (RAFT)
- Reversible-Deactivation Radical Polymerisation: chain polymerisation made simple
- Ring-opening polymerization
- Other
- Thermal analysis: basic concept of differential scanning calorimetry and thermogravimetry for beginners
- Review Article
- Size-exclusion chromatography as a useful tool for the assessment of polymer quality and determination of macromolecular properties
- Other
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 1: a good practice on estimation of bulk resistance of solid polymer electrolytes
- Special Issue Paper
- Basics of teaching electrochemical impedance spectroscopy of electrolytes for ion-rechargeable batteries – part 2: dielectric response of (non-) polymer electrolytes
- Good Practice Report
- Chirality analysis of helical polymers
- Other
- Fabrication and function of polymer membranes
- Review Article
- Nano- and microgels: a review for educators
- Other
- Theoretical background on semiconducting polymers and their applications to OSCs and OLEDs
- Good Practice Report
- An understandable approach to the temperature dependence of electric properties of polymer-filler composites using elementary quantum mechanics
- Review Article
- Polymer degradation: a short review


