Abstract
The presence of high levels of neutrophil associated immunoglobulins (NAIG) in the serum of newborns with neutropenia and their mothers is usually associated with the diagnosis of allo-immune neonatal neutropenia (AINN). We describe a set of otherwise healthy late preterm monozygotic twins who presented with an isolated severe neonatal neutropenia on the first day of life. Flow cytometry for neutrophil antibody screen for both twins detected elevated levels of NAIG with normal serum levels of allo anti-neutrophil antibody (allo-NAB). Maternal serum did not contain either NAIG or allo-NAB. Also, the NAIG immunoglobulin M (IgM) levels were markedly increased in both twins if compared to the increase in the NAIG immunoglobulin G (IgG). Both twins showed very good response to a short course treatment with granulocyte colony stimulating factor (G-CSF), they remained clinically well until 12 months of age. We suggest that this case may be an early presentation of autoimmune neutropenia of infancy. This case study is the earliest report of autoimmune neutropenia of infancy in preterm monozygotic twins.
Introduction
Neutropenia in neonates is a common finding in the neonatal intensive care unit (NICU). However, the vulnerability of developing sepsis in neonates with neutropenia is generally high and this risk is correlated to a large extent to the absolute neutrophil count (ANC). With a persistent ANC of less than 500/μL, the liability to hospital-acquired infections is higher than it is in neonates with ANC of more than 1000/μL. The risk is further elevated in the presence of other comorbidities such as respiratory distress or requirement of invasive respiratory support. The risk of infection also increases tremendously with prematurity and low birth weight neonates if compared to the risk in good sized full-term neonates [1], [2].
Immune mediated neutropenia should be considered in healthy looking neonates with persistently low ANC. Destruction of peripheral neutrophils is usually observed in the presence of circulating antibodies in maternal and infant serum in allo immune neonatal neutropenia (AINN) and neonatal autoimmune neutropenia. Autoimmune neutropenia of infancy usually presents between 5 and 15 months of age (average 8 months). In autoimmune neutropenia of infancy, destruction of neutrophils is caused by the infant’s own antibodies and the maternal serum lacks any circulating antibodies [1]. Other causes of neutropenia in preterm infants include neonatal sepsis, maternal hypertension, pre-eclampsia or placental insufficiency with growth restriction, an infectious illness during pregnancy, twin-twin transfusion and hemolytic disease [1], [3].
Case reports
We are reporting the first described congenital autoimmune neutropenia in late preterm monozygotic female twins who were born at 34 + 4 weeks’ gestation to a gravida 2 para 1, 27-year-old mother. The pre-pregnancy maternal medical history was negative for any chronic illnesses, with no history suggestive of autoimmune disorder. Conception was non-assisted. The mother was diagnosed with hypothyroidism in the first trimester and treated with levothyroxine. Pregnancy was not complicated with pregnancy-induced hypertension (PIH) or gestational diabetes. The mother’s blood type was O positive. Her routine antenatal infectious screening was negative for HIV, HBV, HCV, syphilis, chlamydia and gonorrhea. She was immune to rubella and varicella. Her family history was unremarkable. The parents were not consanguineous. The parents’ first child was a healthy 2-year-old boy who was born by uncomplicated spontaneous vaginal delivery. He did not need NICU admission. Antenatal ultrasound examinations were normal until 19 weeks’ gestation. Later examinations showed growth restriction of twin B with evidence of cerebral redistribution by Doppler, however, no evidence of twin -to-twin transfusion syndrome (TTTS) was detected. A primary cesarean section was arranged due to the detection of growth restriction and cerebral redistribution in twin B. The mother received intrapartum cefazolin antibiotic prophylaxis. Her temperature was normal, and there was no clinical suspicion of chorioamnionitis.
At birth both twins were vigorous (Table 1). They did not require any resuscitative intervention. They were started on dextrose 10% water through a peripheral intravenous line in addition to enteral feeds as per the unit’s protocol with good tolerance and maintained blood glucose levels. A complete blood count (CBC) with differential was done at 6 h of age as a screening for hematological abnormalities that are commonly associated with growth restriction and placental insufficiency (Table 2). With the confirmation of severe neutropenia, our initial management plan included clinical monitoring with a high index of suspicion for any signs of infection, checking daily CBC and differential counts. A pediatric hematology consultation recommended testing for cord blood TORCH infection, urine cytomegalovirus nucleic acid testing (CMV NAT) and testing for neutrophil antibodies by flow cytometry for the twins. On the third day of life (DOL), both twins started to develop frequent episodes of apnea and shallow breathing, however, twin B was more affected. A thorough clinical examination was normal and vital signs were stable, no specific focus of infection was found in either of the twins. Blood cultures were collected followed by starting ampicillin and gentamicin.
Clinical characteristics of the twins at birth.
| Twin A | Twin B | |
|---|---|---|
| Amniotic fluid | Clear | Clear |
| Cord clamping | Immediate | Immediate |
| Resuscitative measures | No intervention | No intervention |
| APGAR score at 1 and 5 min | 9 and 9 | 9 and 9 |
| Anthropometry | ||
| Weight, g (percentile) | 1855 (16%) | 1430 (2%) |
| HC, cm (percentile) | 30 (22%) | 29 (8%) |
| Length, cm (percentile) | 41.5 (11%) | 40 (4%) |
| Vital signs on admission | Temp: 36.3, RR: 46, HR: 132, SpO2 96% in RA, BP: 51/36 (38), CRT < 3 s | Temp: 36.0, RR: 48, HR: 140, SpO2 97% in RA, BP: 65/37 (48), CRT < 3 s |
| Examination | Unremarkable | Unremarkable |
-
HC = Head circumference; Temp = temperature; RR = respiratory rate; HR = heart rate; RA = room air; BP = blood pressure; CRT = capillary refill time.
Initial CBC at 6 h of age for our cases.
| CBC | Twin A | Twin B |
|---|---|---|
| Hemoglobin (145–225, g/L) | 184 | 189 |
| Hematocrit (0.45–0.67, L/L) | 0.54 | 0.55 |
| RBC (4.0– 6.6 10E12/L) | 5.0 | 5.2 |
| MCV (95–121 Fl) | 107 | 108 |
| MCHC (310–350, g/L) | 343 | 341 |
| RDW (11.0–16.0%) | 16.1 | 16.2 |
| Platelet count (150–400 10E9/L) | 256 | 251 |
| WBC (9.4–34.0 10E9/L) | 5.0 | 5.5 |
| Neutrophils (5.0–21.0 10E9/L) | 0.0 | 0.1 |
| Lymphocytes (2.0–11.5 10E9/L) | 3.6 | 4.5 |
| Monocytes (0.5–1.7 10E9/L) | 1.1 | 0.8 |
| Eosinophils (0.2–0.7 10E9/L) | 0.2 | 0.1 |
| Basophils (0.0–0.3 10E9/L) | 0.0 | 0.0 |
| Myelocytes (0.0–0.0 10E9/L)–myelocytes confirmation | 0.0 – 1% | 0.0 |
| Nucleated RBC/100 WBC (/100 WBC) | 6 | 11 |
| Polychromasia | Present | Present |
Twin B was given a loading dose of caffeine and a maintenance dose was continued on fourth DOL. Treatment with granulocyte colony stimulating factor (G-CSF) was started with an initial proposed 3-day course at a daily dose of 5 μg/kg given intravenously. After the second dose of G-CSF, white blood cell (WBC) count and ANC started to increase to the normal reference range for their gestational age (Figure 1). Clinically, both twins remained well without any further apneic episodes or feeding intolerance. Other laboratory tests were negative for bacterial infection and urine CMV NAT. TORCH testing could not be done as there was not enough cord blood and both twins responded well. Antibiotics and G-CSF were discontinued for both twins and caffeine was discontinued for twin B on DOL 6. Flow cytometry for neutrophil-associated antibodies for both twins showed increased levels of NAIG immunoglobulin G (IgG) and immunoglobulin M (IgM), to almost 4 times and more than 20 times the reference range, respectively (Table 3).
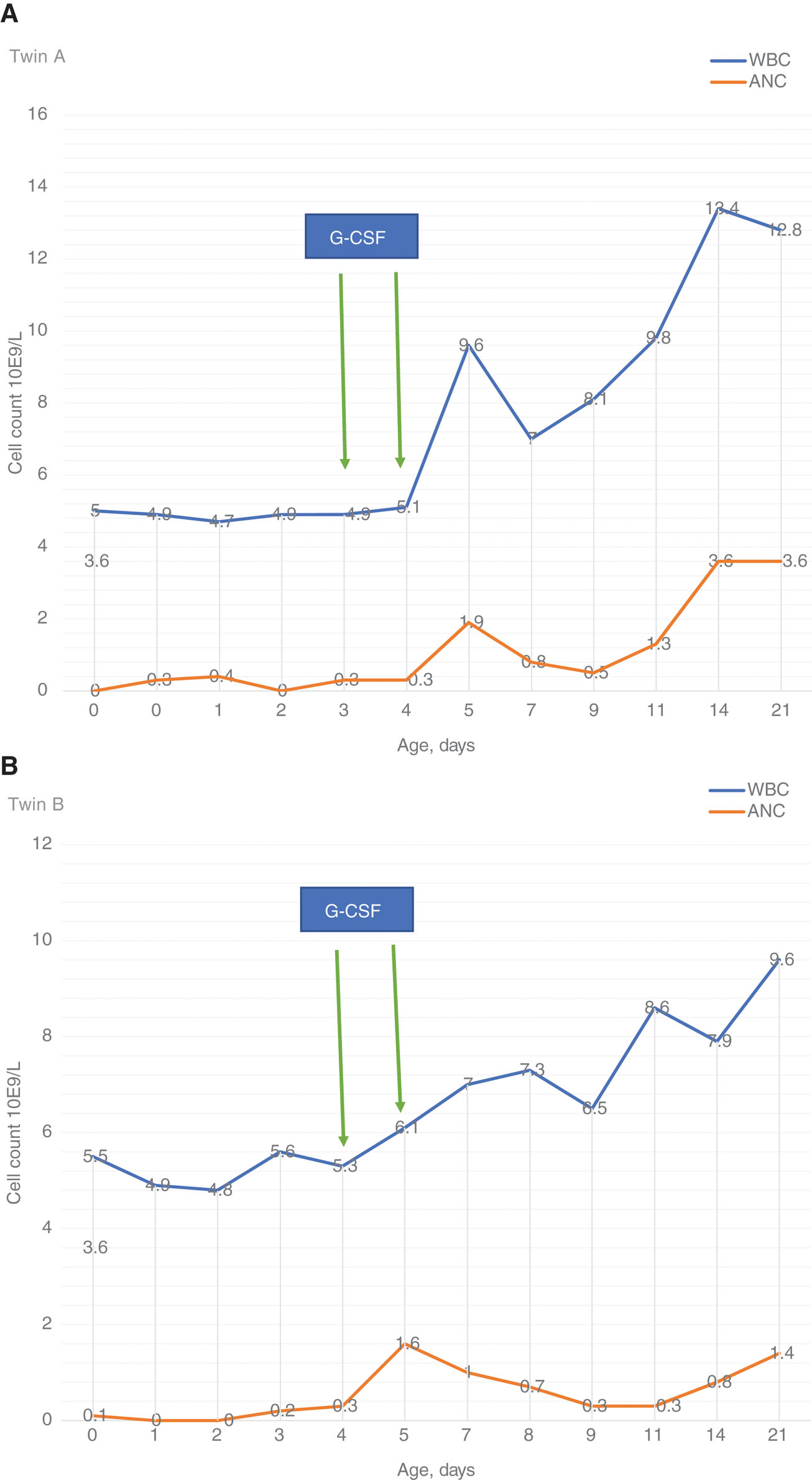
(A) WBC and ANC Response to G-CSF in twin A. (B) WBC and ANC Response to G-CSF in twin B.
G-CSF = Granulocyte colony stimulating factor, WBC = white blood cell, ANC = absolute neutrophil count.
Flow cytometry neutrophil antibody screen.
| Parameter (reference range) | Twin A | Twin B |
|---|---|---|
| Result (comment) | Result (comment) | |
| Neutrophil associated immunoglobulin (NAIG) | ||
| IgG (0–14.11) | 54.74 (Detected) | 46.56 (Detected) |
| IgA (0–2.83) | 1.13 (Not detected) | 1.22 (Not detected) |
| IgM (0–6.08) | 164.74 (Detected) | 137 (Detected) |
| Allo anti-neutrophil antibody (allo-NAB) | ||
| Target cells | Normal group O neutrophils | Normal group O neutrophils |
| Serum IgG (0–10) | 9 (Not detected) | 5 (Not detected) |
| Serum IgM (0–10) | 0 (Not detected) | 2 (Not detected) |
-
IgG = Iimmunoglobulin G; IgA = immunoglobulin A; IgM = immunoglobulin M.
Postnatal maternal investigations confirmed the normal WBC and ANC. Placental histopathology showed no evidence of placental disease. Maternal flow cytometry for neutrophil-associated antibodies showed normal levels of both of neutrophil associated immunoglobulins (NAIG) NAIG and allo anti-neutrophil antibody (allo-NAB).
The rest of hospital course for the twins was uneventful with appropriate growth rate for gestational age. Repeated CBC done on a weekly basis showed normal WBC and ANC. They were discharged home on regular vitamins. At 2 months of age, twin B developed a brief febrile illness on the same day of vaccination. The fever responded well to antipyretics and no further investigations were done at that time. Follow-up CBC done 4 weeks later for twin A and twin B showed persistently normal WBC (10.9 and 10.2, respectively) and normal ANC (1.1 and 2.3, respectively).
Discussion
Neonatal neutropenia in preterm infants is a common finding. A clinical approach to neonatal neutropenia includes reviewing maternal history for risk factors, clinical assessment and laboratory investigations. Sepsis must be ruled out especially in critically-ill newborns. Associated anemia and or thrombocytopenia in neonates with neonatal neutropenia may be a sign of generalized bone marrow failure [1]. Bone marrow biopsy is generally not indicated except for prolonged persistent neutropenia that does not respond to treatment with G-CSF [1].
Multiple gestation with disparity between twins and TTTS may be the cause of neonatal neutropenia in the donor and smaller twin [3]. Also, intra uterine growth restriction caused by PIH, preeclampsia or placental insufficiency are common causes of benign and self-limiting neonatal neutropenia [3] with earlier reports suggesting a correlation between the severity of neutropenia and the degree of placental insufficiency [4]. In our cases, it is difficult to relate the neutropenia to any of these factors in the absence of maternal risk factors, evidence of TTTS or placental pathology.
Sepsis induced neutropenia is usually transient and associated with maternal risk factors such as chorioamnionitis or prolonged rupture of membranes [2]. Our cases were clinically well with no evidence of maternal perinatal infections. They presented with severe neutropenia in the first DOL with ANC of <200/mm3 before developing any clinical signs of infection, that were obvious by the third DOL. This lag between the onset of illness and the detection of neutropenia disproves sepsis to be the cause of neutropenia in our cases.
Immune-related neutropenia is commonly reported in newborns. Trans-placental transmission of maternal antibodies causes peripheral destruction of the circulating neutrophils in the newborn [5]. In AINN, maternal sensitization to the paternal antigens expressed on the fetal neutrophils produces antineutrophil antibodies [1]. Also, the presence of pre-existing maternal antineutrophil autoantibodies as in mothers with autoimmune diseases [6] similarly causes neutropenia as in neonatal autoimmune neutropenia [1]. In both conditions, maternal serum has circulating antineutrophil antibodies [6]. In our cases, we assumed that maternal serum possibly should have circulating antineutrophil antibodies similar to the other study [6]. However, the absence of NAIG and allo-NAB from the maternal serum in our cases refutes this theory to be the cause of neutropenia in the twins.
In 2001, Calhoun et al. [5], reported two preterm cases who presented with congenital autoimmune neutropenia which was considered as a neonatal presentation of autoimmune neutropenia of infancy. Prior to the Calhoun et al. [5] report, the youngest age of onset of the autoimmune neutropenia of infancy was 33 days with an average age of presentation of 8 months [7]. In contrast to the other immune-related neonatal neutropenia, autoimmune neutropenia of infancy is characterized by the presence of antineutrophil autoantibodies in the infant’s serum that are absent from the maternal serum [7].
In our case report, the serum of the twins had markedly elevated levels of NAIG IgM and to some extent NAIG IgG. Increased levels of NAIG IgM, with concomitant neutropenia has been noted in autoimmune neutropenia of infancy [8], [9]. The detection of neutropenia with high levels of NAIG in the serum of our cases on the first DOL with the absence of the NAIG in the maternal serum is consistent with Calhoun et al.’s [5] report that suggests the prenatal or congenital onset of the autoimmune neutropenia of infancy.
Hwang et al. [10] found that NAIG IgM levels detected by flow cytometry were significantly increased in patients with severe neutropenia. The autoantibodies can mediate the peripheral destruction of the neutrophils and/or neutrophil precursors and lead to a more severe neutropenia [6]. In our twins, despite the severe neutropenia, bone marrow precursors were absent in the peripheral blood during the neutropenic phase indicating failure of bone marrow to respond to the decrease in the neutrophil peripheral pool.
Calhoun et al. [5] suggested that congenital autoimmune neutropenia is not strictly inherited due to the absence of neutropenia in the other monozygotic twin in their second case. However, both our identical twins were affected which suggests that a hereditary background should still be considered as a contributing factor.
Neutropenia in our cases was transient with no other clinical presentations until 12 months of age. This is different from Calhoun et al.’s [5] report that suggested that congenital autoimmune neutropenia is the early presentation of the more severe autoimmune neutropenia of infancy and that the later condition is likely present at birth to variable extents. It is difficult to predict if our cases may develop neutropenia in the future. Also, the brief course in our cases has been limiting in terms of performing further investigations to confirm the diagnosis of autoimmune neutropenia as detecting the neutrophil specific antibodies or examining the bone marrow.
Treatment of neutropenia in neonates in challenging and decisions must be individualized depending on the cause and severity [1]. Recombinant G-CSF and granulocyte-macrophage-colony stimulating factor have been used in many cases with a good success rate and very few reported side effects [1]. However, their effect in the prevention and/or treatment neonatal sepsis is controversial. The mechanism of action is through both increasing bone marrow production of neutrophils and decreasing neutrophil apoptosis. Also by reversing some of anti-neutrophil antibodies it induced functional impairments in neutrophils [1].
Calhoun et al. [5] used G-CSF in their cases based on the severe and prolonged neutropenia. In our cases, the rationale for the use of G-CSF and antibiotics was based on developing early signs of infection, severity of neutropenia and the absence of myeloid precursors in the peripheral blood suggesting bone marrow involvement. Both twins showed very good clinical and laboratory response to the treatment after only two doses. Our report suggests that in the absence of confirmed bacterial infection, prolonged courses of treatment with antibiotics and or G-CSF in cases of neutropenia are not necessary.
Recommendations
Diagnosis of congenital autoimmune neutropenia should be considered in otherwise healthy neonates with severe neutropenia not secondary to neonatal sepsis in the absence of maternal circulating antibodies.
The absence of neutrophil precursors in neonates with severe neutropenia is not consistently due to bone marrow failure. Autoimmune mediated neutropenia is usually severe as the immunoglobulins are directed against bone marrow precursors as well as peripherally circulating neutrophils.
Prophylactic antibiotic use is not generally indicated in cases of neonatal neutropenia.
However, a high index of suspicion of infection is mandatory to prevent development of fulminant sepsis.
Congenital autoimmune neutropenia may respond well to treatment with G-CSF as most cases of neonatal neutropenia. Side effects and long-term complications for the use of G-CSF in neonates still need further multicenter studies to be determined.
Educational gap
Autoimmune neutropenia of infancy is usually diagnosed between 5 and15 months of age; however, few reports suggest an earlier onset. Neutropenia caused by autoimmune antibodies is not well recognized in neonates.
-
Financial Disclosures: The authors have no financial relationships relevant to this article to disclose.
-
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
[1] Maheshwari A. Neutropenia in the newborn. Curr Opin Hematol. 2014;21:43–9.10.1097/MOH.0000000000000010Search in Google Scholar
[2] Funke A, Berner R, Traichel B, Schmeisser D, Leititis JU, Niemeyer CM. Frequency, natural course, and outcome of neonatal neutropenia. Pediatrics. 2000;106(Pt 1):45–51.10.1542/peds.106.1.45Search in Google Scholar
[3] Koenig JM, Christensen RD. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Engl J Med. 1989;321:557–62.10.1056/NEJM198908313210901Search in Google Scholar
[4] Kush ML, Gortner L, Harman CR, Baschat AA. Sustained hematological consequences in the first week of neonatal life secondary to placental dysfunction. Early Hum Dev. 2006;82:67–72.10.1016/j.earlhumdev.2005.06.009Search in Google Scholar
[5] Calhoun DA, Rimsza LM, Burchfield DJ, Millsaps M, Christensen RD, Budania J, et al. Congenital autoimmune neutropenia in two premature neonates. Pediatrics. 2001;108:181–4.10.1542/peds.108.1.181Search in Google Scholar
[6] Lakshman R, Finn A. Neutrophil disorders and their management. J Clin Pathol. 2001;54:7–19.10.1136/jcp.54.1.7Search in Google Scholar
[7] Bux, J, Behrens G, Jaeger G, Welte K. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood. 1998;91:181–6.10.1182/blood.V91.1.181Search in Google Scholar
[8] Silliman CC, Cusack NA, Swanson NJ, Ghaffarifar S, Ambruso DR. Platelets and neutrophils from healthy term neonates exhibit increased levels of immunoglobulins. Pediatr Res. 1995;38:993–7.10.1203/00006450-199512000-00027Search in Google Scholar
[9] Lalezari P, Khorshidi M, Petrosova M. Autoimmune neutropenia of infancy. J Pediatr. 1986;109:764–9.10.1016/S0022-3476(86)80690-4Search in Google Scholar
[10] Hwang K, Park CJ, Huh HJ, Han SH, Jang S, Chi HS. Flow cytometric detection of neutrophil-associated immunoglobulin in patients with or without neutropenia and establishment of the reference interval. Ann Clin Lab Sci. 2011;41:144–9.Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Case Reports – Obstetrics
- Trisomy 9 presenting in the first trimester as a fetal lateral neck cyst and increased nuchal translucency
- A case of intrauterine closure of the ductus arteriosus and non-immune hydrops
- Pregnancy luteoma: a rare presentation and expectant management
- A pregnant woman with an operated bladder extrophy and a pregnancy complicated by placenta previa and preterm labor
- Consecutive successful pregnancies of a patient with nail-patella syndrome
- A multidisciplinary management approach for patients with Klippel-Trenaunay syndrome and multifetal gestation with successful outcomes
- A uterus didelphys with a spontaneous labor at term of pregnancy: a rare case and a review of the literature
- Case Reports – Fetus
- Prenatal diagnosis of ring chromosome 13: a rare chromosomal aberration
- Case Reports – Newborn
- Late-onset pubic-phallic idiopathic edema in premature recovering infants
- An unusual cause of neonatal shock: a case report
- Early ultrasonographic follow up in neonatal pneumatocele. Two case reports
- Nonsyndromic extremely premature eruption of teeth in preterm neonates – a report of three cases and a review of the literature
- Successful outcome of a preterm infant with severe oligohydramnios and suspected pulmonary hypoplasia following premature rupture of membranes (PPROM) at 18 weeks’ gestation
- Onset of Kawasaki disease immediately after birth
- Short rib-polydactyly syndrome (Saldino-Noonan type) undetected by standard prenatal genetic testing
- Severe congenital autoimmune neutropenia in preterm monozygotic twins: case series and literature review
- Verona integron-encoded metallo-β-lactamase-producing Klebsiella pneumoniae sepsis in an extremely premature infant
Articles in the same Issue
- Case Reports – Obstetrics
- Trisomy 9 presenting in the first trimester as a fetal lateral neck cyst and increased nuchal translucency
- A case of intrauterine closure of the ductus arteriosus and non-immune hydrops
- Pregnancy luteoma: a rare presentation and expectant management
- A pregnant woman with an operated bladder extrophy and a pregnancy complicated by placenta previa and preterm labor
- Consecutive successful pregnancies of a patient with nail-patella syndrome
- A multidisciplinary management approach for patients with Klippel-Trenaunay syndrome and multifetal gestation with successful outcomes
- A uterus didelphys with a spontaneous labor at term of pregnancy: a rare case and a review of the literature
- Case Reports – Fetus
- Prenatal diagnosis of ring chromosome 13: a rare chromosomal aberration
- Case Reports – Newborn
- Late-onset pubic-phallic idiopathic edema in premature recovering infants
- An unusual cause of neonatal shock: a case report
- Early ultrasonographic follow up in neonatal pneumatocele. Two case reports
- Nonsyndromic extremely premature eruption of teeth in preterm neonates – a report of three cases and a review of the literature
- Successful outcome of a preterm infant with severe oligohydramnios and suspected pulmonary hypoplasia following premature rupture of membranes (PPROM) at 18 weeks’ gestation
- Onset of Kawasaki disease immediately after birth
- Short rib-polydactyly syndrome (Saldino-Noonan type) undetected by standard prenatal genetic testing
- Severe congenital autoimmune neutropenia in preterm monozygotic twins: case series and literature review
- Verona integron-encoded metallo-β-lactamase-producing Klebsiella pneumoniae sepsis in an extremely premature infant

