Abstract
Gas boriding was used to produce the borided layer containing a mixture of chromium and nickel borides on the Inconel®600-alloy. The borided sample was characterized by a higher corrosion potential (−0.953 V) than the non-borided sample (−1.005 V). The corrosion current density was significantly lower for the borided sample. The oxidation at 1000 °C for 24 h caused the formation of different oxides on the surface of the borided sample. Simultaneously, the presence of nickel and chromium borides was confirmed by XRD analysis after the oxidation test. It was concluded, that the gas boriding could be an effective barrier against corrosion and oxidation of Inconel®600-alloy.
1 Introduction
Boriding is a well-known surface treatment that can be applied for different metals and alloys to improve hardness and wear resistance. The following materials were most often used as the substrate for boriding: steels (Campos-Silva et al. 2013; Hegewaldt et al. 1984; Kayali and Taktak 2015), titanium alloys (Ataibis and Taktak 2015; Duan et al. 2018, 2020; Liu et al. 2020; Makuch et al. 2017a) and nickel alloys (Aytekin and Akcin 2013; Kulka et al. 2013, 2014a,b; Lou et al. 2006, 2009; Makuch 2020; Makuch and Kulka 2014, 2016; Makuch et al. 2015, 2017b,c, 2019; Mu et al. 2009; Sista et al. 2013; Ueda et al. 2000). In the case of nickel-based alloys, various methods of boriding were studied e.g., powder pack (Aytekin and Akcin 2013; Lou et al. 2006; Mu et al. 2009; Ueda et al. 2000), paste (Lou et al. 2009), electrochemical (Sista et al. 2013), plasma (Makuch 2020; Makuch et al. 2019), laser (Kulka et al. 2013, 2014a), and gaseous (Kulka et al. 2014b; Makuch and Kulka 2014, 2016; Makuch et al. 2015, 2017b,c). Depending on the chemical composition of Ni-based alloys, different types of borides were formed as a result of boriding: nickel borides, chromium borides, iron borides, or substitutive complex borides e.g., (Cr, Co)2B or (Ni, Co)2B. In general, the boriding of Ni-based alloys caused an increase in hardness and wear resistance. The gas boriding process was an appropriate method of producing thick, hard, resistant to wear layers on the surface of different Ni-based alloys: Nisil (Kulka et al. 2014b), Nimonic®80A (Makuch and Kulka 2016; Makuch et al. 2015, 2017b,c), Inconel®600 (Makuch and Kulka 2014). The measured depths of gas-borided layers were significantly higher than those obtained for Ni-based alloys by the pack-boriding or paste boriding methods under comparable process conditions (temperature and time). The microstructure, phase composition, and properties of the borided layers produced on nickel alloys strongly depended on the following factors: chemical composition of the substrate material, method of boriding, process conditions (temperature, time, and boron activity).
Generally, Ni-based superalloys are characterized by high corrosion resistance and heat resistance. Therefore, it is very important to keep these properties after gas-boriding of these materials. The influence of gas boriding on the corrosion resistance of Inconel®600 alloy was described in the paper (Dziarski et al. 2017). The gas-borided and non-borided samples were subjected to immersion corrosion tests in the solution of H2O, H2SO4, and Fe2(SO4)3. Based on the corrosion resistance tests, it was found that a strong intergranular attack was observed in the case of the non-borided sample. In contrast, the corrosive behavior of gas-borided Inconel® 600-alloy was more complicated and resulted from the surface condition. The obtained results indicated that the gas boriding in N2–H2–BCl3 atmosphere could provide suitable corrosion protection if the entire surface was covered with a boride layer. The advantageous influence of gas boriding on corrosion resistance in an acidic environment has been proved. However, the influence of gas boriding on heat resistance has not yet been recognized. Therefore, the present work is important in the respect of new information on the protective properties of the borided layers produced on nickel alloys. Especially, in terms of describing the impact of the effects of high-temperature annealing without a protective atmosphere on the durability of the borided layer produced on Inconel®600-alloy.
In this study, the gaseous boriding process was used to produce hard ceramic phases (nickel and chromium borides) on Inconel®600-alloy. The use of N2–H2–BCl3 atmosphere ensured the obtaining of a thick layer in a short time of the process. In the present study, the potentiodynamic polarization test was used to evaluate the corrosion resistance of gas-borided and non-borided Inconel®600-alloy in a salt environment. The behavior of the gas-borided sample under oxidation conditions was also investigated. The microstructure, chemical, and phase composition of the borided samples before and after high-temperature oxidation were compared.
2 Materials and methods
2.1 Materials
The substrate material used in this study was Inconel®600-alloy. This Ni–Cr–Fe alloy contained chromium (15.72 wt. %) and iron (8.63 wt. %) as the main alloying elements. The detailed chemical composition of the Inconel®600-alloy was presented in Table 1. The shape of the samples was adapted to the corrosion test conditions, therefore, flat discs were used for the tests. The dimensions of the samples were as follows: diameter of 20 mm, height of 6 mm.
Chemical composition of Inconel®600-alloy.
| Cr | Fe | Mn | Cu | Si | C | Al | Ni | |
|---|---|---|---|---|---|---|---|---|
| wt. % | 15.72 | 8.63 | 0.16 | 0.04 | 0.18 | 0.078 | 0.06 | Balance |
2.2 Gas boriding in N2–H2–BCl3 atmosphere
Gas boriding in N2–H2–BCl3 atmosphere was selected as the method for production of layer on the Inconel®600-alloy because of its high efficiency. This method results in the formation of thick layers at a lower temperature and shorter process duration in comparison to the pack boriding method. In the present study, gas boriding was carried out as a continuous process. The following process parameters were used: temperature of 920 °C (1193 K), duration of 2 h, average concentration of boron trichloride of 5.23 vol. % (in relation to hydrogen). The devices used for gas boriding, and a detailed description of the process, were presented in the previous work (Makuch et al. 2015).
2.3 Surface roughness
A white light 3D interference microscope (WYKO NT−1100) was used to evaluate the 3D surface profiles of non-borided and borided samples. During the measurements, the interferometric objective moved vertically to scan the surface at changeable heights. The following parameters were determined: Ra, Rq, Sa, and Sq. Amplitude parameters are the most important parameters characterizing the surface topography in respect of the surface deviations. The arithmetic average height Ra is defined as the average absolute deviation of the roughness irregularities from the mean line. The extension of the Ra parameter to the surface is Sa. The root mean square roughness Rq represents the standard deviation of the distribution of surface heights in the linear profile whereas Sq represents the root mean square value in relation to the surface.
The 3D surface topography and roughness parameters of the non-borided and borided samples were compared in Figure 1 and Table 2. The surface topography of non-borided (Figure 1a) and borided (Figure 1b) was uniform, without craters or cracks. Only the effects of mechanical machining were visible. The non-borided sample was characterized by a more uniform profile with a lower roughness (Ra = 1.38 µm, Sa = 1.96 µm) compared to the borided sample (Ra = 1.45 µm, Sa = 2.46 µm).
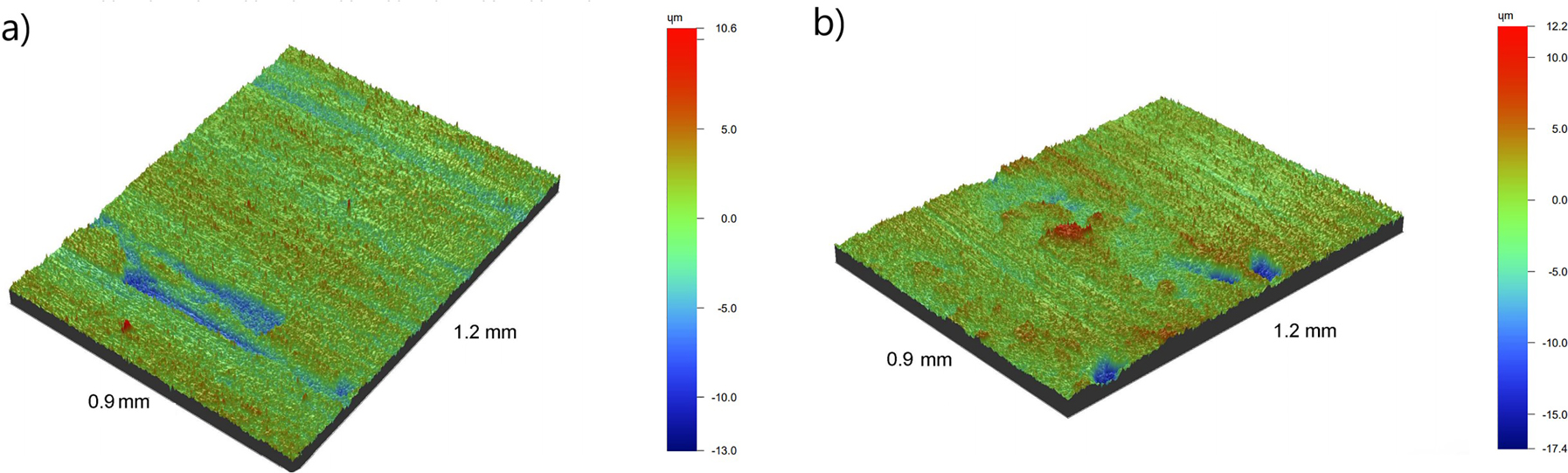
3D images of surface topography of non-borided (a) and borided (b) samples.
Roughness parameters of non-borided and borided samples.
| Line roughness | Area roughness | |||
|---|---|---|---|---|
| R a (µm) | R q (µm) | S a (µm) | S q (µm) | |
| Non-borided sample | 1.38 | 1.86 | 1.96 | 2.16 |
| Borided sample | 1.45 | 2.12 | 2.46 | 2.98 |
2.4 Microstructure characterization
First, the specimens for microstructure characterization were cut out. Standard metallographic preparation included the following steps: mounting in conductive resin, grinding, polishing, etching with the use of a Marble’s reagent.
The microstructure of the boride layer was observed with the use of a Keyence VHX-6000 digital microscope. The porosity of the boride layer was determined on the cross-section of the layer with the use of a digital microscope Keyence VHX-6000. A Mira3 Tescan scanning electron microscope (SEM) equipped with an Energy Dispersive Spectrometer was used for X-ray microanalysis.
2.5 Cohesion evaluation
The bond strength between the gas-borided layer and the substrate material was determined based on the destructive method in accordance with the VDI 3198 norm (Verein Deutscher Ingenieure Normen 1991). The measurements consisted of the generation of a Rockwell indentation mark under a total load of 1470 N (scale C). During the measurements, the cone-shaped indenter caused the destruction of the layer in the form of microcracks, delamination, or flacking (Figure 2). After the Rockwell C test, the generated indentation marks with visible failures were observed with the use of an optical microscope. It is a comparative method in which the indentation craters and generated failure marks were compared with those presented in the norm as quality maps HF1–HF6 (Figure 2).
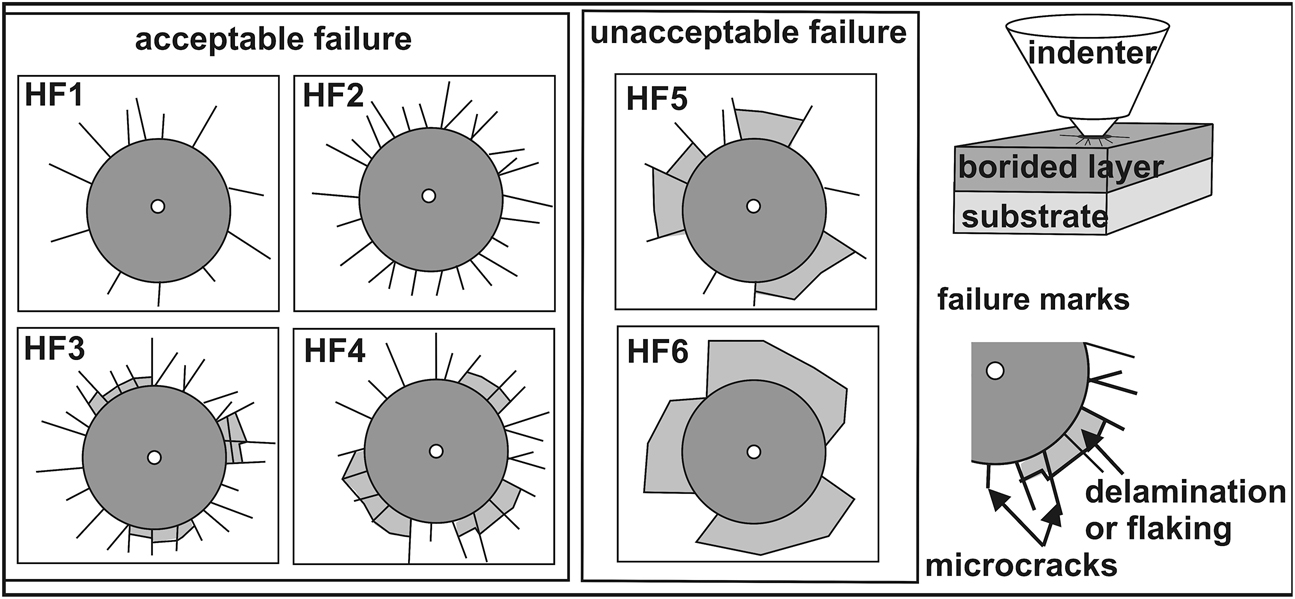
The principle of the VDI 3198 cohesion test with HF1-HF6 quality maps.
2.6 Corrosion resistance evaluation
To determine the corrosion resistance of the samples, a potentiodynamic anodic polarization test was used. The investigations were carried out with the use of the Atlas Solich ATLAS 0531 EU&IA device. A three-electrode cell system containing a platinum electrode as a counter electrode, a saturated calomel electrode as a reference electrode, and a sample as a working electrode, was used for the experiments. Measurements were performed on three test areas for each sample. The tested sample (the exposed surface was 50 mm2) was immersed in a 3.5% NaCl solution at a constant temperature of 22 °C. The specimens were polarized in the anode direction from a potential −1.5 V up to 1.5 V, with a potential change rate of 0.5 mV/s. The results were plotted as a polarization curve showing the E − log I dependence. From the obtained polarization curve, the following quantities were determined: corrosion potential ECORR, corrosion current density ICORR, primary passivation potential EP, Flade potential EF, transpassive potential ETP, secondary passivity potential ESP, oxygen evolution potential EOE, and passivation current density.
2.7 Oxidation test
Oxidation tests were conducted in a resistance furnace at a temperature of 1000 °C (1273 K) for a period of 24 h. First, the cleaned sample was put into the furnace. Then the heating process was started. A heating rate of 10 °C/min was applied to minimize the oxidation during heating. After the furnace reached a temperature of 1000 °C (1273 K), the oxidation test in the open-air atmosphere was started. After 24 h of oxidation, the gas-borided sample was slowly cooled in a furnace to minimize the risk of thermal shock. The oxidized sample was examined by scanning electron microscopy (Mira3 Tescan Vega). The reaction products formed on the surface during oxidation were identified by X-ray diffraction (XRD) and energy dispersive spectroscopy (EDS).
3 Results and discussion
3.1 Microstructure analysis
Gas boriding resulted in the formation of a compact boride layer which was characterized by a high average thickness of 86 μm. The XRD pattern of gas-borided layers formed on the Inconel®600-alloy was analyzed in the previous study (Makuch et al. 2015). The compact boride zone included nickel borides (NiB, Ni2B, Ni3B, and Ni4B3) and chromium borides (CrB and Cr2B). This type of multiphase microstructure did not show zonal phase distribution. It was found that nickel borides, and chromium borides, appeared in the cross-section of the layer only as a mixture. Moreover, the distribution of nickel borides and chromium borides was independent of the distance from the surface.
The high amount of porosity could be the reason for diminished mechanical properties and high brittleness of the borided layer. Therefore, the percentage of pores in the borided layer produced on the Inconel®600-alloy was measured. The OM microstructure (Figure 3a) and the binary image of a layer with marked porosity (Figure 3b) were analyzed. The investigated area was characterized by a small amount of porosity equal to 2.912%. However, the pores were mainly detected near the top-surface region. This specific pore distribution could be the reason for the reduced cohesion of the layer with the substrate material, and the increased brittleness of the layer. In the case of the gas-borided Inconel®600-alloy, the presence of porosity was caused by the high concentration of iron in the base material (8.63 wt. %).
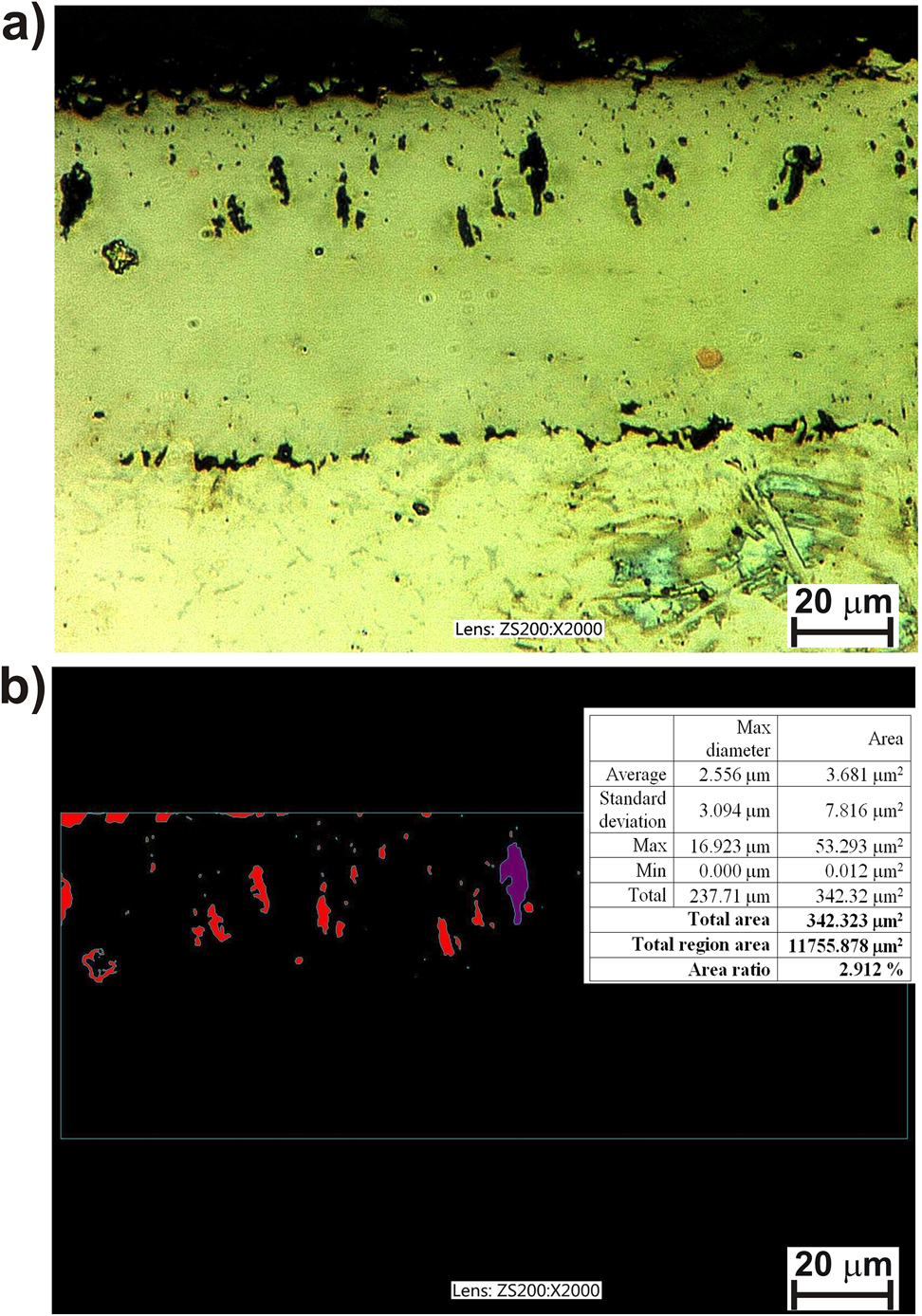
The OM microstructure (a) and binary image (b) of porosity of the gas-boride layer produced on Inconel®600-alloy.
The presence of iron in the base material enabled the formation of ferrous and ferric chlorides (FeCl2 and FeCl3) during gas boriding in N2–H2–BCl3 atmosphere. As a consequence of the reaction of boron trichloride with iron and reducer (in this process – hydrogen), the following products were formed: HCl, BHCl2, FeCl2, and FeCl3. During the process, hydrochloric acid reacted with iron and caused the formation of ferrous and ferric chlorides as a consequence of the reactions (Hegewaldt et al. 1984):
According to the paper (Hegewaldt et al. 1984), the porosity appeared as a result of corrosion caused by the presence of FeCl2.
3.2 Cohesion
Figure 4 shows an OM image of a Rockwell-C indentation crater with visible failure marks. The following failure marks were visible: long radial cracks (Figure 4d), flaking, and delamination (Figure 4b and c). These observed failure marks can be classified as acceptable failures, which indicate sufficient cohesion represented by the HF3 quality map (Figure 4e). The high hardness gradient between the borided layer and the base material could be the cause of delamination. The second important factor that strongly influenced the results of the cohesion test was the presence of porosity below the top surface and the brittleness of the boride layer.
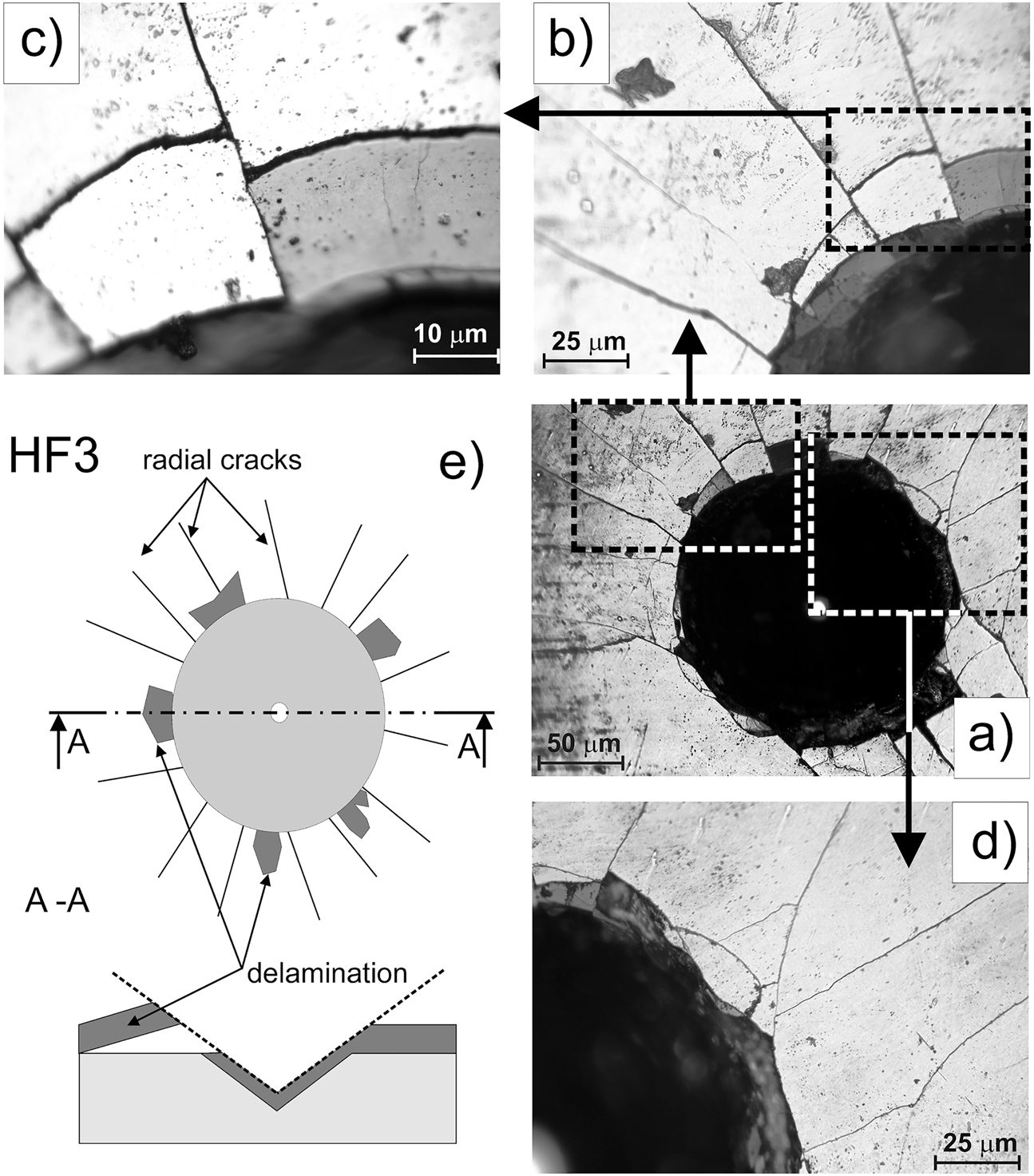
OM images of gas-borided layers produced on Inconel®600-alloy after Rockwell C indentation test (a–d) and scheme of HF3 standard with failure marks (e).
3.3 Corrosion resistance
The corrosion resistance was determined based on the obtained polarization curves. Three curves were plotted for each borided and non-borided sample. Owing to the high similarity of the obtained curves, only one representative polarization curve was presented and described. Simultaneously, the electrochemical parameters derived from the polarization curves were presented in Table 3. The values of ECORR and ICORR evaluated from three measurements for each sample were shown. A representative corrosion potentiodynamic curve recorded in a 3.5% NaCl solution for gas-borided Inconel®600-alloy is shown in Figure 5a. To determine the influence of boriding on the corrosion behavior, the results obtained for the non-borided Inconel®600-alloy were presented (Figure 5b). The width of the corrosion resistance region (1) was higher for the gas-borided sample as compared to the non-borided sample. For this reason, the value of the corrosion potential ECORR was also higher for the borided sample. Both samples show resistant-active–passive-transpassive behavior in the corrosive environment used. However, the passive region differed when the borided and non-borided samples were compared. First, in the case of the borided sample, the passivation current density was 2·10−5 A/cm2. Whereas, in the case of the non-borided Inconel®600-alloy, the passivation current density was 5·10−5 A/cm2.
Electrochemical parameters determined based on potentiodynamic curves.
| Material | Measurement | I CORR (A/cm2) | E CORR (V) |
|---|---|---|---|
| Borided Inconel 600 | 1 | 3.1·10−6 | −0.953 |
| 2 | 2.2·10−6 | −0.948 | |
| 3 | 1.9·10−6 | −0.956 | |
| Non-borided Inconel 600 | 1 | 1.9·10−5 | −1.005 |
| 2 | 2.1·10−5 | −1.004 | |
| 3 | 2.3·10−5 | −1.009 |
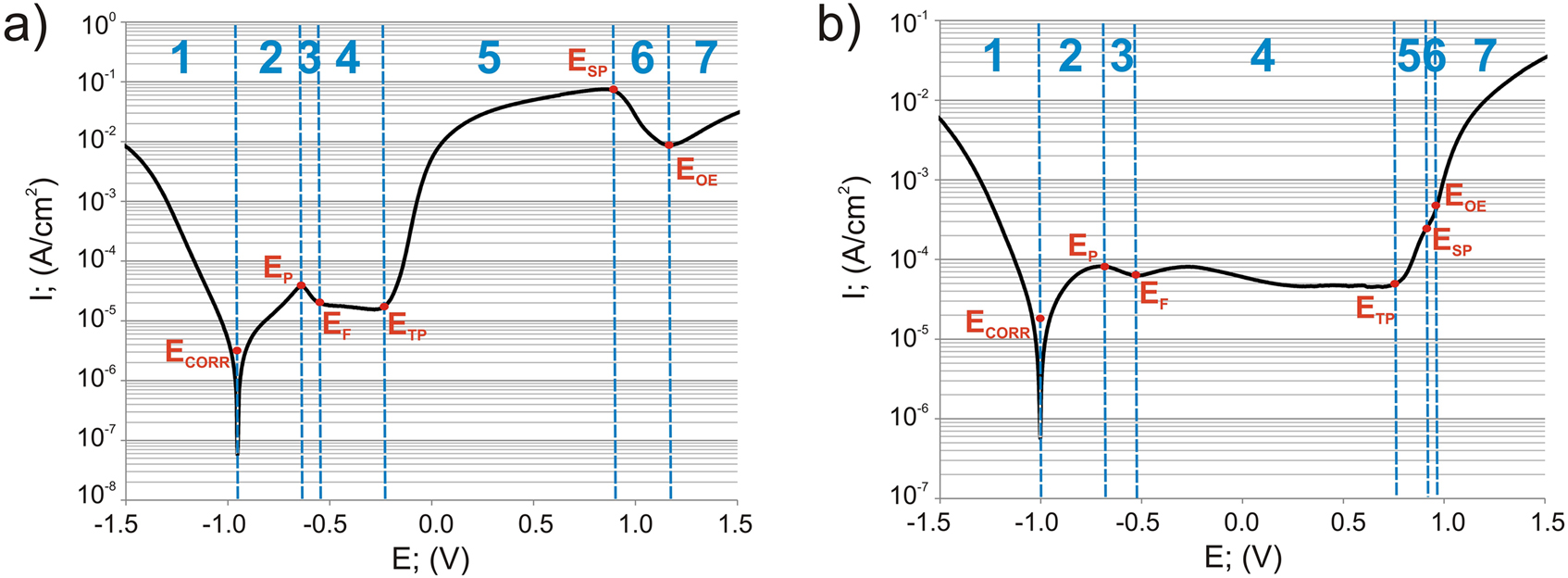
Corrosion potentiodynamic curves recorded in a 3.5% NaCl solution for gas-borided Inconel®600-alloy (a) and non-borided Inconel®600-alloy (b); ECORR-corrosion potential; ICORR-corrosion current density; EP-the primary passivation potential; EF-the Flade potential; ETP-the transpassive potential; ESP-the secondary passive potential; EOE-oxygen evolution potential.
1, corrosion resistance region; 2, active region; 3, primary passivation region; 4, passive region; 5, transpassive region; 6, secondary passivity region; 7, oxygen evolution region.
These results indicated that the presence of a borided layer on the surface of Inconel®600-alloy resulted in reduced corrosion. Comparing the range of the passive region, the non-borided Inconel®600-alloy was found to be passive over a wide potential range (from −0.51 to 0.77 V) before transpassive dissolution occurred at 0.77 V. The borided sample was characterized by the narrower range of the passive state, ranging from −0.52 to −0.22 V. These results indicated a higher susceptibility to passivation in a 3.5% NaCl solution. Moreover, the non-borided sample spontaneously passivated without exhibiting the distinct active to passive transition peak.
The electrochemical parameters ICORR and ECORR determined based on the polarization curves are presented in Table 3. The higher value of the corrosion potential (average ECORR = −0.952 V) characterized the gas-borided Inconel®600-alloy. The non-borided sample demonstrates a slightly lower average ECORR value of −1.006 V. The lower corrosion current density ICORR (average 2.4·10−6 A/cm2) was measured for the gas-borided Inconel®600-alloy. The non-borided sample was characterized by a higher ICORR value (2.1·10−5 A/cm2). Therefore, it can be concluded that the gas-borided sample shows a higher corrosion resistance in a 3.5% NaCl solution.
However, the multicomponent character of the gas-borided layer could be the cause of the formation of corrosive micro-cells. The privileged areas of micro-cells formation were those with different types of borides. In the gas-borided layer produced on Inconel®600-alloy, nickel borides and chromium borides were formed. These borides differed in physical and chemical properties. As a result of these differences, the microstructure of the gas-borided layer produced on the Inconel®600-alloy was divided into the anode region and cathode regions. Therefore, during the potentiodynamic corrosion test, micro-cells were formed between different types of borides, as shown in Figure 6. In the gas-borided layer produced on the Inconel®600-alloy, micro-cells were created between the different types of borides designated MeIxBy and MeIIxBy. Borides marked as MeIxBy donated valence electrons and passed into solution in the form of MeI+ ions. The electrons in the metal migrated to the cathode area (MeIIxBy). Then, they combined with the depolarizer (D), i.e., an ion with the ability to add electrons (reduction).
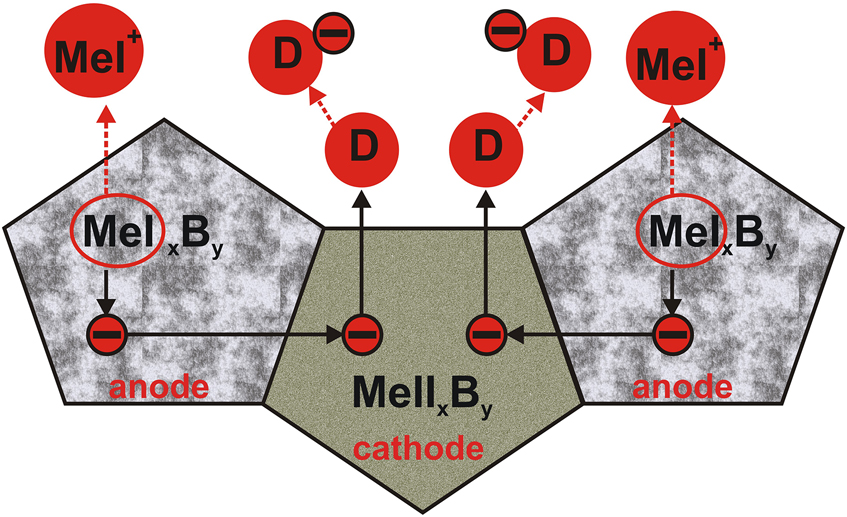
Scheme of work of corrosive micro-cells for a multiphase gas-borided layer.
3.4 Oxidation behavior at 1000 °C
SEM images of the cross-section of the non-oxidized and oxidized sample were presented in Figure 7. The appearance of the gas-borided layer was significantly different in comparison to the borided sample before the oxidation test (Figure 7a). In some regions, layer losses were observed (Figure 7b and d), and delamination of the layer from the substrate material (Figure 7c). This indicated a change in the phase and chemical composition of the borided layer. The oxidation test was carried out in an open-air atmosphere, which allowed for easy diffusion of oxygen into the sample. To confirm the phase composition of the gas-borided layer after the oxidation test, the XRD pattern was analyzed (Figure 8). It was found that the oxidation at 1000 °C for 24 h resulted in the formation of various oxides: Cr2O3, Cr3O4, NiO, B2O3, Fe3O4. However, the phases characteristic of the gas-borided layer have also been identified. Many of the peaks corresponded to nickel borides (NiB, Ni2B, Ni3B, and Ni4B3) and chromium boride (CrB).
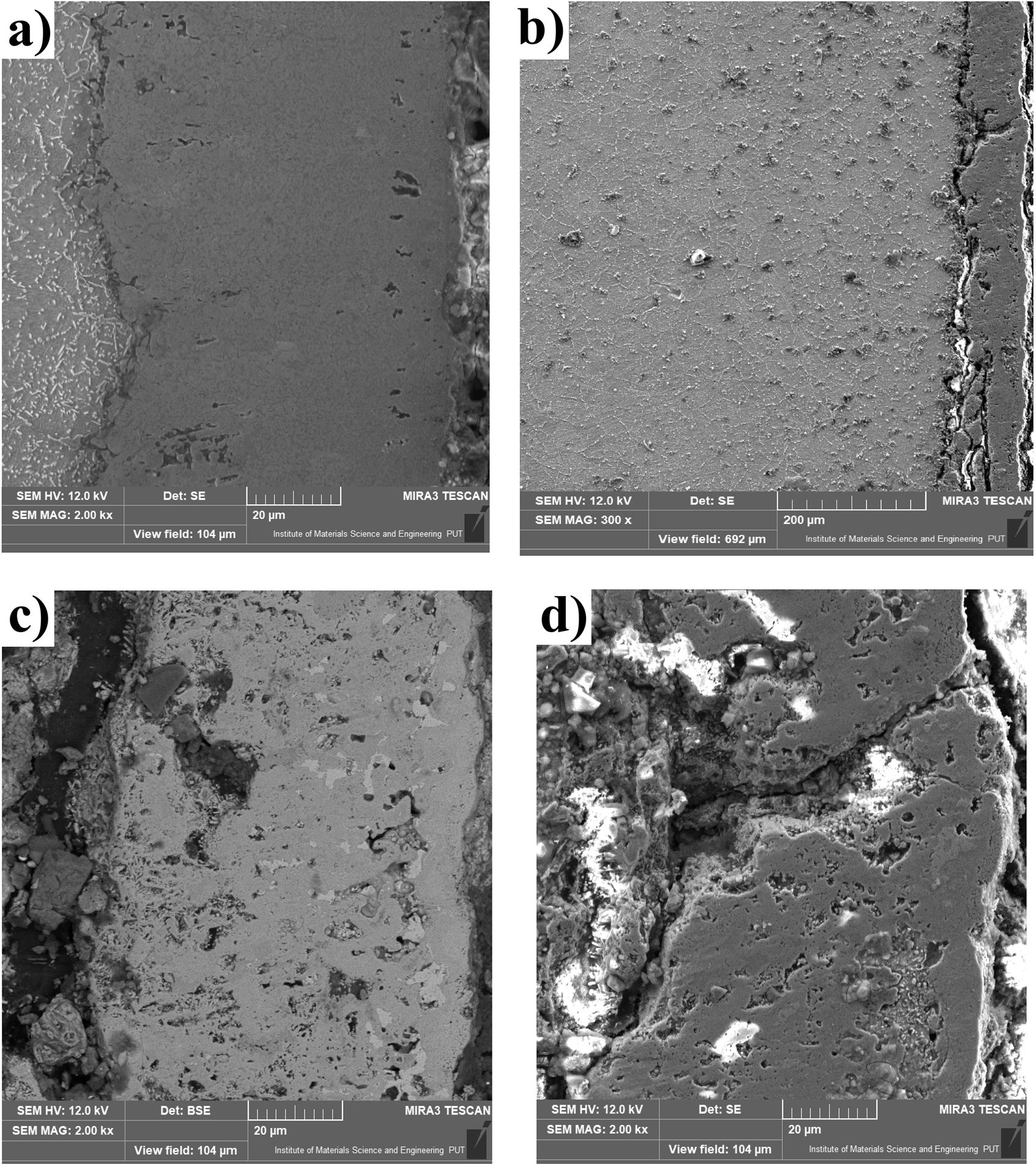
The SEM microstructure of the gas-boride layer produced on Inconel®600-alloy: before oxidation test (a), after oxidation at 1000 °C for 24 h (b), visible delamination of borided layer after oxidation test (c), layer damage after oxidation test (d).
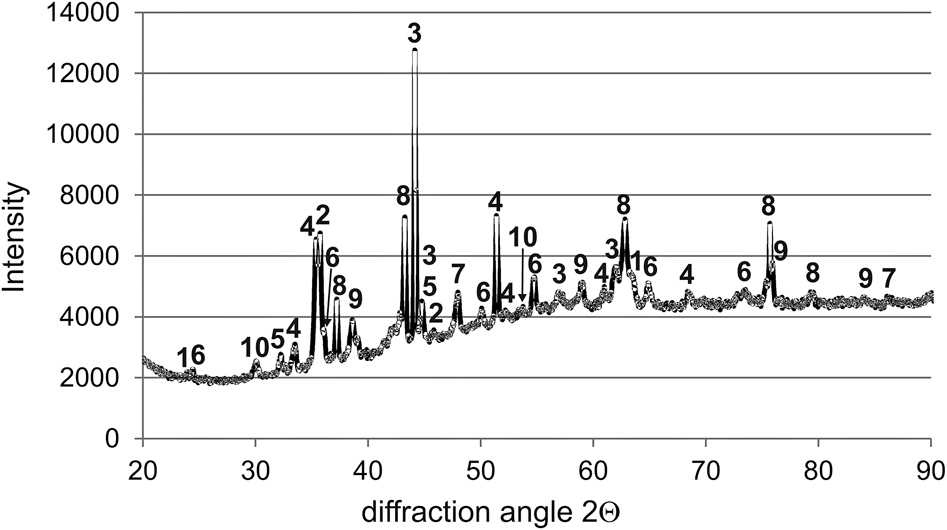
XRD pattern of the gas-borided sample after oxidation test at 1000 °C for 24 h. 1, NiB; 2, Ni2B; 3, Ni3B; 4, Ni4B3; 5, CrB; 6, Cr2O3; 7, Cr3O4; 8, NiO; 9, B2O3; 10, Fe3O4.
It was found that after oxidation, the layer could be classified as a mixed boride-oxide layer. However, it was difficult to identify the phase distribution in the cross-section of the borided layer. Therefore, the linear EDS analysis was carried out in the cross-section of the specimen as shown in Figure 9. The concentration of boron and oxygen was higher in the area corresponding to the mixed boride-oxide layer. However, in some areas, the content of oxygen was significantly lower compared to the boron content. This may indicate the presence of chromium and nickel borides in these regions. Simultaneously, low boron concentration was detected in some areas, especially near the top surface. Chromium oxides were probably the dominant phases in this area. The presence of a compact, dense layer of boron oxide B2O3 after oxidation is important to prevent oxygen penetration. This layer, formed on the top surface of the boride layer, could be a barrier to further diffusion of oxygen through the substrate material (Kartal Sireli et al. 2017). In the present study, the presence of boron oxide was confirmed by XRD analysis, however, this phase was not formed as a compact layer on the top surface.
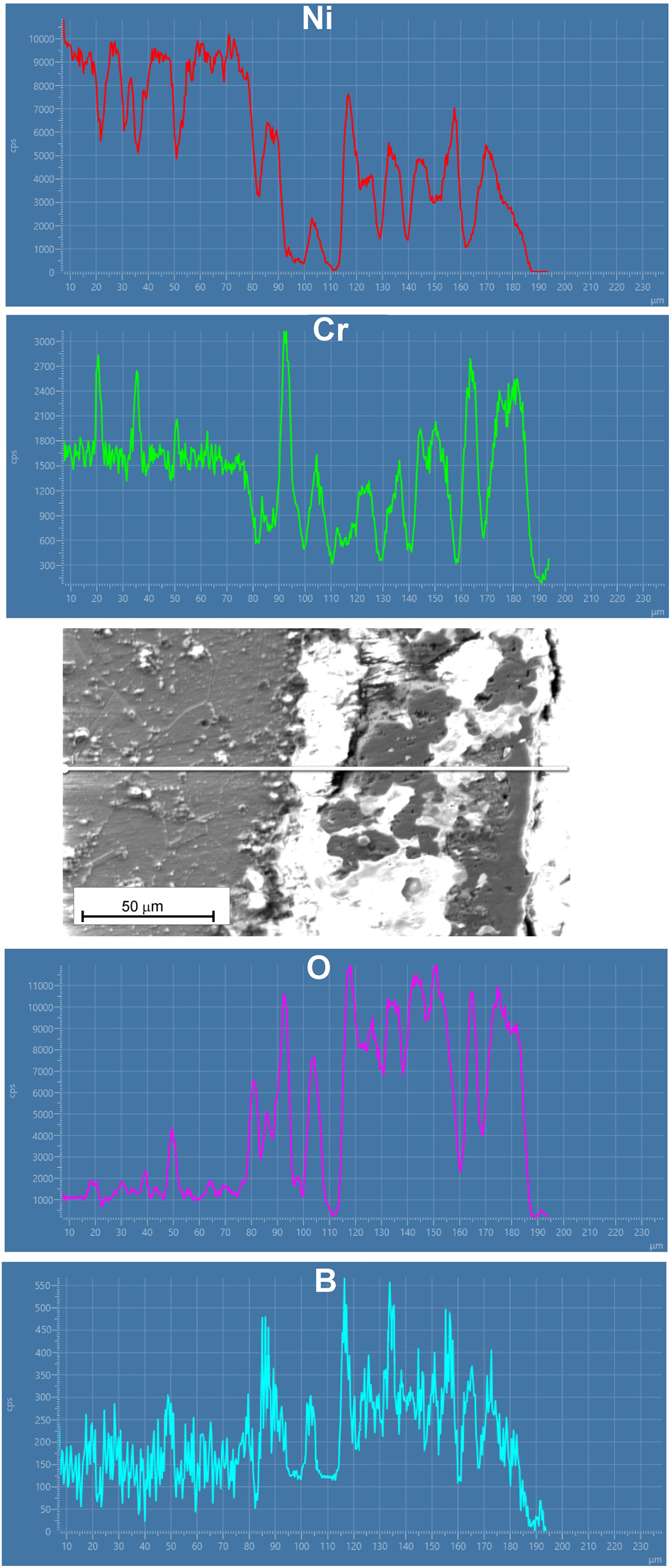
SEM image of the oxidized sample with results of EDS line analysis.
4 Summary and conclusions
Continuous gas-boriding in N2–H2–BCl3 atmosphere was used to produce the multiphase layer on Inconel®600-alloy. Despite the low process temperature (920 °C) and the short duration (2 h), a thick layer (86 µm) was produced. The characteristic compact, fine-grained microstructure consisted of nickel borides (NiB, Ni2B, Ni3B, and Ni4B3,) and chromium borides (CrB and Cr2B). The gas-borided layer produced on Inconel®600-alloy was characterized by sufficient cohesion corresponding to the HF3 standard. The presence of porosity near the top-surface region, and the high hardness gradient between the layer and the substrate material, could be the reason for diminished cohesion. The borided layer produced on the surface of Inconel®600-alloy can provide an effective protective barrier against corrosion in a 3.5% NaCl solution. The electrochemical parameters ICORR and ECORR, derived from the polarization curves, indicated a higher corrosion resistance of the borided sample compared to the non-borided sample. Oxidation at 1000 °C for 24 h caused changes in the phase and chemical composition of the borided layer. As a result of the strong oxidation, various oxides (Cr2O3, Cr3O4, NiO, B2O3, and Fe3O4) were formed. However, the phases characteristic of the gas-borided layer (NiB, Ni2B, Ni3B, and Ni4B3) have also been identified. It was found that after oxidation, the layer could be classified as a mixed boride-oxide layer. This layer is an effective barrier against oxidation of the substrate material. However, a more detailed investigation should be performed to determine the kinetics of the oxidation process.
Funding source: Ministerstwo Nauki i Szkolnictwa Wyższego
Award Identifier / Grant number: 0513/SBAD
Acknowledgments
The authors wish to thank Ph.D. A. Piasecki from the Institute of Materials Science and Engineering for help and cooperation during the realization of this work.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work has been financially supported by the Ministry of Science and Higher Education in Poland as a part of the 0513/SBAD project.
-
Conflicts of interest: The authors declare no conflicts of interest regarding this article.
-
Data availability: Data will be made available on request.
References
Ataibis, V. and Taktak, S. (2015). Characteristics and growth kinetics of plasma paste borided Cp–Ti and Ti6Al4V alloy. Surf. Coating. Technol. 279: 65–71, https://doi.org/10.1016/j.surfcoat.2015.08.023.Suche in Google Scholar
Aytekin, H. and Akcin, Y. (2013). Characterization of borided Incoloy 825 alloy. Mater. Des. 50: 515–521, https://doi.org/10.1016/j.matdes.2013.03.015.Suche in Google Scholar
Campos-Silva, I., Flores-Jiménez, M., Rodríguez-Castro, G., Hernández-Sánchez, E., Martínez-Trinidad, J., and Tadeo-Rosas, R. (2013). Improved fracture toughness of boride coating developed with a diffusion annealing process. Surf. Coating. Technol. 237: 429–439, https://doi.org/10.1016/j.surfcoat.2013.05.050.Suche in Google Scholar
Duan, Y., Li, P., Chen, Z., Shi, J., and Ma, L. (2018). Surface evolution and growth kinetics of Ti6Al4V alloy in pack boriding. J. Alloys Compd. 742: 690–701, https://doi.org/10.1016/j.jallcom.2018.01.383.Suche in Google Scholar
Duan, Y., Wang, X., Liu, D., Bao, W., Li, P., and Peng, M. (2020). Characteristics, wear and corrosion properties of borided pure titanium by pack boriding near α → β phase transition temperature. Ceram. Int. 46: 16380–16387, https://doi.org/10.1016/j.ceramint.2020.03.197.Suche in Google Scholar
Dziarski, P., Makuch, N., and Kulka, M. (2017). Influence of gas boriding on corrosion resistance of Inconel 600-alloy. Arch. Mater. Sci. Eng. 84/1: 23–33, https://doi.org/10.5604/01.3001.0010.3028.Suche in Google Scholar
Hegewaldt, F., Singheiser, L., and Tuerk, M. (1984). Gasborieren. Härt.-Tech. Mittl. 39: 7–15.10.1515/htm-1984-390102Suche in Google Scholar
Kartal Sireli, G., Ozkalafat, P., and Timur, S. (2017). Surface modification of chromium-silicon martensitic steel by forming hard borides. Surf. Coating. Technol. 326: 18–27, https://doi.org/10.1016/j.surfcoat.2017.07.033.Suche in Google Scholar
Kayali, Y. and Taktak, S. (2015). Characterization and Rockwell-C adhesion properties of chromium-based borided steels. J. Adhes. Sci. Technol. 29: 2065–2075, https://doi.org/10.1080/01694243.2015.1052617.Suche in Google Scholar
Kulka, M., Dziarski, P., Makuch, N., Piasecki, A., and Miklaszewski, A. (2013). Microstructure and properties of laser-borided Inconel®600-alloy. Appl. Surf. Sci. 284: 757–771, https://doi.org/10.1016/j.apsusc.2013.07.167.Suche in Google Scholar
Kulka, M., Makuch, N., Dziarski, P., and Piasecki, A. (2014a). A study of nanoindentation for mechanical characterization of chromium and nickel borides’ mixtures formed by laser boriding. Ceram. Int. 40: 6083–6094, https://doi.org/10.1016/j.ceramint.2013.11.059.Suche in Google Scholar
Kulka, M., Makuch, N., and Popławski, M. (2014b). Two-stage gas boriding of Nisil in N2–H2–BCl3 atmosphere. Surf. Coating. Technol. 244: 78–86, https://doi.org/10.1016/j.surfcoat.2014.01.057.Suche in Google Scholar
Liu, D., Duan, Y., Bao, W., and Peng, M. (2020). Characterization and growth kinetics of boride layers on Ti-5Mo-5V-8Cr-3Al alloy by pack boriding with CeO2. Mater. Char. 164: 110362, https://doi.org/10.1016/j.matchar.2020.110362.Suche in Google Scholar
Lou, D.C., Akselsen, O.M., Solberg, J.K., Onsoien, M.I., Berget, J., and Dahl, N. (2006). Silicon-boronising of Nimonic 90 superalloy. Surf. Coating. Technol. 200: 3582–3589, https://doi.org/10.1016/j.surfcoat.2005.03.030.Suche in Google Scholar
Lou, D.C., Solberg, J.K., Akselsen, O.M., and Dahl, N. (2009). Microstructure and property investigation of paste boronized pure nickel and Nimonic 90 superalloy. Mater. Chem. Phys. 115: 239–244, https://doi.org/10.1016/j.matchemphys.2008.11.055.Suche in Google Scholar
Makuch, N. (2020). The importance of phase composition for corrosion resistance of borided layers produced on nickel alloys. Materials 13: 5131, https://doi.org/10.3390/ma13225131.Suche in Google Scholar PubMed PubMed Central
Makuch, N. and Kulka, M. (2014). Microstructural characterization and some mechanical properties of gas-borided Inconel®600-alloy. Appl. Surf. Sci. 314: 1007–1018, https://doi.org/10.1016/j.apsusc.2014.06.109.Suche in Google Scholar
Makuch, N. and Kulka, M. (2016). Fracture toughness of hard ceramic phases produced on Nimonic 80A-alloy by gas boriding. Ceram. Int. 42: 3275–3289, https://doi.org/10.1016/j.ceramint.2015.10.119.Suche in Google Scholar
Makuch, N., Kulka, M., Keddam, M., Taktak, S., Ataibis, V., and Dziarski, P. (2017a). Growth kinetics and some mechanical properties of two-phase boride layers produced on commercially pure titanium during plasma paste boriding. Thin Solid Films 626: 25–37, https://doi.org/10.1016/j.tsf.2017.02.033.Suche in Google Scholar
Makuch, N., Kulka, M., and Paczkowska, M. (2017b). Nanomechanical properties of gas-borided layer produced on Nimonic 80A alloy. Ceram. Int. 43: 8255–8261, https://doi.org/10.1016/j.ceramint.2017.03.156.Suche in Google Scholar
Makuch, N., Kulka, M., and Mikołajczak, D. (2017c). Corrosion behavior of hard boride layer produced on Nimonic 80A-alloy by gas boriding. Trans. Indian Inst. Met. 70: 2509–2527, https://doi.org/10.1007/s12666-017-1113-y.Suche in Google Scholar
Makuch, N., Kulka, M., Dziarski, P., and Taktak, S. (2019). The influence of chemical composition of Ni-based alloys on microstructure and mechanical properties of plasma paste borided layers. Surf. Coating. Technol. 367: 187–202, https://doi.org/10.1016/j.surfcoat.2019.03.042.Suche in Google Scholar
Makuch, N., Kulka, M., and Piasecki, A. (2015). The effects of chemical composition of Nimonic 80A-alloy on the microstructure and properties of gas-borided layer. Surf. Coating. Technol. 276: 440–455, https://doi.org/10.1016/j.surfcoat.2015.06.031.Suche in Google Scholar
Mu, D., Shen, B., Yang, C., and Zhao, X. (2009). Microstructure analysis of boronized pure nickel using boronizing powders with SiC as diluents. Vacuum 83: 1481–1484, https://doi.org/10.1016/j.vacuum.2009.06.048.Suche in Google Scholar
Sista, V., Kahvecioglu, O., Kartal, G., Zeng, Q.Z., Kim, J.H., Eryilmaz, O.L., and Erdemir, A. (2013). Evaluation of electrochemical boriding of Inconel®600-alloy. Surf. Coating. Technol. 215: 452–459, https://doi.org/10.1016/j.surfcoat.2012.08.083.Suche in Google Scholar
Ueda, N., Mizukoshi, T., Demizu, K., Sone, T., Ikenaga, A., and Kawamoto, M. (2000). Boriding of nickel by the powder-pack method. Surf. Coating. Technol. 126: 25–30, https://doi.org/10.1016/s0257-8972(00)00517-x.Suche in Google Scholar
Verein Deutscher Ingenieure Normen (1991). VDI 3198. VDI Verlag, Dusseldorf.Suche in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this issue
- Reviews
- Investigations of the intrinsic corrosion and hydrogen susceptibility of metals and alloys using density functional theory
- Integration of green nanotechnology with silica for corrosion inhibition
- Analysis of internal corrosion of supercritical CO2 pipeline
- A review of thermal spray coatings for protection of steels from degradation in coal fired power plants
- Original articles
- The importance of phase composition for corrosion resistance and thermal oxidation behavior of gas-borided layer produced on nickel alloy
- Tribological behavior of stainless steel in sulfuric acid in the presence of Thymus zygis subsp. gracilis essential oil: experimental and quantum chemical studies
Artikel in diesem Heft
- Frontmatter
- In this issue
- Reviews
- Investigations of the intrinsic corrosion and hydrogen susceptibility of metals and alloys using density functional theory
- Integration of green nanotechnology with silica for corrosion inhibition
- Analysis of internal corrosion of supercritical CO2 pipeline
- A review of thermal spray coatings for protection of steels from degradation in coal fired power plants
- Original articles
- The importance of phase composition for corrosion resistance and thermal oxidation behavior of gas-borided layer produced on nickel alloy
- Tribological behavior of stainless steel in sulfuric acid in the presence of Thymus zygis subsp. gracilis essential oil: experimental and quantum chemical studies

