Abstract
In recent years, magnesium (Mg) alloys have attracted great attention due to superior biocompatibility, biodegradability, and other characteristics important for use in biodegradable implants. However, the development of Mg alloys for clinical application continues to be hindered by high corrosion rates and localized corrosion modes, both of which are detrimental to the mechanical integrity of a load-bearing temporary implant. To overcome these challenges, technologies have been developed to improve the corrosion resistance of Mg alloys, among which surface treatment is the most common way to enhance not only the corrosion resistance, but also the bioactivity of biodegradable Mg alloys. Nevertheless, surface treatments are unable to fundamentally solve the problems of fast corrosion rate and localized corrosion. Therefore, it is of great importance to alter and improve the intrinsic corrosion behavior of Mg alloys for biomedical applications. To show the significance of the intrinsic corrosion resistance of biodegradable Mg alloys and attract much attention on this issue, this article presents a review of the improvements made to enhance intrinsic corrosion resistance of Mg alloys in recent years through the design and preparation of the Mg alloys, including purifying, alloying, grain refinement, and heat treatment techniques. The influence of long-period stacking-ordered structure on corrosion behavior of the biodegradable Mg alloys is also discussed.
1 Introduction
Mg alloys have recently attracted great attention as “smart” temporary implants compared to traditional implant materials (Chen, Xu, Smith, & Sankar, 2014b). The elasticity moduli of traditional clinical implant materials, including stainless steels (189–205 GPa), Titanium alloys (110–117 GPa), and Cobalt-Chromium alloys (230 GPa), are much higher in comparison to human bone tissue (3–20 GPa) (Staiger, Pietak, Huadmai, & Dias, 2006). Therefore, when implanted, these materials can cause stress shielding and result in reduced stimulation of new bone growth as well as a decrease of implant stability. In addition, they do not degrade in the human body and will lead to long-term complications if not removed (Li et al., 2014c; Pound, 2014a,b). Therefore, a second surgery is usually necessary for patients after the tissue has healed sufficiently (Witte et al., 2008). In contrast, Mg alloys have many advantages over traditional metallic implant materials (Chen et al., 2014b; Li & Zheng, 2013; Manivasagam & Suwas, 2014; Staiger et al., 2006; Song & Atrens, 1999; Virtanen, 2011; Waksman, 2006; Witte & Eliezer, 2012; Wu, Ibrahim, & Chu, 2013; Xin, Hu, & Chu, 2011; Yamamoto, 2008). First, because Mg is a nutritive element and is the fourth most abundant element in human body, it is highly biocompatible. Mg is needed for human metabolism reactions and biological mechanisms, and it is recommended that an adult receive 240–420 mg Mg daily. Mg also has desirable physical and mechanical properties. For example, the density of Mg alloys is 1.75–1.85 g/cm3, which is similar to that of the human bone (1.8–2.1 g/cm3). The elastic modulus of Mg alloys (41–45 GPa) is also close to that of the human bone tissue (3–20 GPa), which could make it able to avoid the stress shielding effect caused by usual implants. Furthermore, the compressive yield strength of Mg alloys is also near to that of the natural bone. Mg alloys are also advantageous due to their biodegradability. Mg alloys have low corrosion potential. They are sensitive to chloride ions (Cl-) and can therefore be degraded in the Cl--containing human body environment. The biodegradable Mg implants are able to do their jobs and disappear when the tissue has been healed, either by being absorbed by the tissue or excreted out of the human body. Mg also possesses good processing properties in that Mg alloys can undergo severe plastic deformation even though they have a hexagonal close-packed crystal structure. These alloys can also be machined similarly to traditional metals. A final advantage is availability, as Mg is one of the most abundant lightweight metals on earth. Such outstanding characteristics make Mg alloys superb potential candidates for temporarily biomedical implants.
Previously, commercial Mg alloys, such as AZ31 (Witte, Kaese, Haferkamp, Switzer, & Meyer-Lindenberg, 2005; Zhang, Huang, Yang, Zhang, & Ai, 2007), AZ91 (Liu, Xin, Tian, & Chu, 2007b; Song, Shan, & Han, 2008), WE43 (Mani, Feldman, Patel, & Agrawal, 2007; Mario et al., 2004), have been widely studied for biomedical applications. However, because these alloys were designed for structural materials and did not take into account biocompatibility as biomaterials several disadvantages were observed. For example, exposure to element Aluminum (Al) is linked to the development of Alzheimer disease, muscle fiber damage, and the decrease of osteoclast viability (Ferreira, Piai, Takayanagui, & Segura-Muñoz, 2008). It is therefore suggested that Mg-Al series alloys be used only as experimental materials to study the enhancement of processing and surface modification technologies for biomedical application but should not be implanted in the human body (Witte et al., 2008). Consequently, the development of some novel biodegradable Mg alloys have been seen in recent years, including Mg-Ca, Mg-Zn, Mg-Sr, and Mg-RE (rare earth) series alloys. These novel Mg alloys have low or even no cytotoxicity, in addition to good mechanical properties, all characteristics desirable for clinical implementation.
Nevertheless, the foremost limitation to the application of novel biodegradable Mg alloys is unaccountable corrosion behavior (fast corrosion rate and/or localized corrosion mode). A fast corrosion rate and localized corrosion mode may destroy the mechanical integrity of Mg alloys and leave them unable to meet the requirements of biodegradable implant materials. Moreover, if the corrosion rate of the Mg alloys is too rapid, the evolved hydrogen will not be able to be absorbed quickly enough and a balloon effect will occur (Hornberger, Virtanen, & Boccaccini, 2012; Virtanen, 2011). In addition, it is impossible to control the corrosion rate if the Mg alloys present a localized corrosion mode (Ghali, Dietzel, & Kainer, 2004). Erinc, Sillekens, Mannens, and Werkhoven (2009) suggested that the corrosion rate of useful biodegradable Mg alloys in simulated body fluid (SBF) should be less than 0.5 mm/year. Regretfully, the corrosion rates of most biodegradable Mg alloys are higher than 0.5 mm/year (Gu, Zheng, Cheng, Zhong, & Xi, 2009; Li, Gu, Lou, & Zheng, 2008; Zhang, He, Xue, Wang, & Wang, 2014b), and therefore unsatisfactory for clinical applications.
Surface modification technologies, including coatings (Rojaee, Fathi, & Raeissi, 2013; Zomorodian et al., 2013), ion implantation (Jamesh, Wu, Zhao, & Chu, 2013; Zhao et al., 2013c), microarc oxidation (MAO) (Fischerauer et al., 2013; Lin et al., 2014; Narayanan, Park, & Lee, 2014), laser surface processing (Taltavull et al., 2014), etc., are widely implemented to enhance the corrosion resistance and biocompatibility of Mg alloys. However, the surface treatments are unable to change the localized corrosion mode, and Mg alloys still exhibit localized corrosion once the protective surface is destroyed. This results in the phenomenon of “small anode and large cathode” and causes further corrosion (Kim, Kim, Lee, & Seok, 2008; Liu, Chen, Bhole, Cao, & Jahazi, 2009a). As shown in Figure 1, the surface-treated AZ31 samples still undergo localized corrosion in vitro similar to the untreated samples (Yang et al., 2008). The MAO-treated ZX50 implants undergo severe localized attack in vivo (Figure 2; Fischerauer et al., 2013). Consequently, despite the development of surface modification technologies, the corrosion behavior of Mg alloys, in essence, is under the control of the substrate itself. Therefore, to control the corrosion behavior of Mg alloys for biomedical applications, it is necessary to develop biodegradable Mg alloys with both low corrosion rates and a uniform corrosion mode.
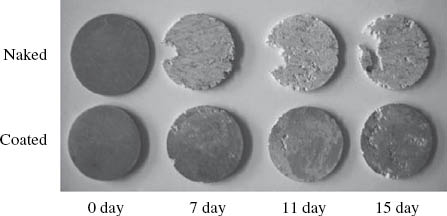
A comparison of the corrosion behavior of untreated versus calcium orthophosphate-coated AZ31 alloy immersion in 3% NaCl solution for an increasing number of days.
Reprinted from Yang et al. (2008), with permission from Elsevier.
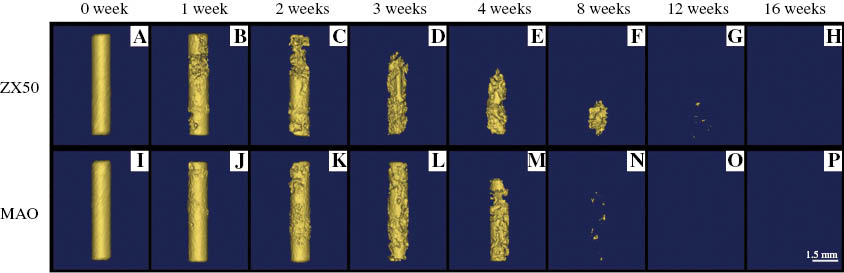
CT images (3-D reconstruction) showing the degradation of untreated (A–H) and MAO-treated (I–P) ZX50 pins after implantation in rats.
Reprinted from Fischerauer et al. (2013), with permission from Elsevier.
Purifying, alloying, grain refinement, and heat treatment are effective methods to improve the corrosion behavior of Mg alloys from the perspective of the components and microstructures of the materials. Additionally, a special arrangement called long-period stacking-ordered (LPSO) structure plays an important role in improving corrosion behavior of Mg alloys. Great developments in biodegradable Mg alloys have been made in recent years, and there are also some reviews on the development of biodegradable Mg alloys (Atrens et al., 2014; Hornberger et al., 2012; Kirkland, 2012; Narayanan et al., 2014; Wang et al., 2012; Wu et al., 2013). However, most of them pay much attention on the surface treatment, but few of them focus on the improvement of intrinsic corrosion of the materials. As we discussed, surface treatments are not able to change the corrosion mode of Mg alloys, and once the protective film is destroyed, the substrate will still suffer rapid corrosion. Therefore, different from the other reviews, the aim of this work is to present an overview of recent improvement of the intrinsic corrosion behavior of the Mg alloys for biomedical applications, and to attract much more attention on the development of intrinsic corrosion resistance of biodegradable Mg alloys.
2 Corrosion mechanism
Mg is a reactive metal. When a Mg alloy is immersed in aqueous solution (for instance, the human body environment), it will easily react with water and produce hydrogen according to Eq. (1) (Mueller, Nascimento, & Mele, 2010). The corrosion reaction can be decoupled into the anodic reaction and the cathodic reaction, as listed in Eqs. (2) and (3), respectively (Atrens, Liu, & Abidin, 2011):
It has been observed that at the beginning of the reaction, a film of Mg(OH)2 formed by Mg2+ and OH- will adsorb on the surface of the Mg alloy. This film is only slightly soluble in water and can restrict corrosion. However, over time, Mg(OH)2 will react with Cl- in aqueous solution to form highly soluble MgCl2 as given by Eq. (4) (Staiger et al., 2006):
The corrosion modes of the Mg alloys can be generally classified into uniform corrosion and localized corrosion (Ghali et al., 2004). Most of the Mg alloys contain a second phase, precipitates, and/or impurities. Due to the presence of these phases, which are cathodic with respect to the α-Mg matrix, the anodic reaction is likely to be accelerated and the Mg(OH)2 will be quickly destroyed. Once the protective film is destroyed, the surrounding solution will continuously penetrate through the porous film and the Mg matrix will suffer from further and accelerated corrosion. If the second phase has a greater corrosion potential and is distributed unhomogeneously, the alloy will tend to corrode in a localized fashion. Rarely, Mg alloy may exhibit uniform corrosion (Song, Atrens, & Dargusch, 1999). It is reported (Kirkland, Lespagnol, Birbilis, & Staiger, 2010b) that 29 out of 31 types of Mg alloys undergo non-uniform corrosion. Wang et al. (2011) reported that the Mg-Mn alloy (M1A) immersed in SBF undergoes non-uniform corrosion, but when immersed in albumin-containing SBF (A-SBF), it exhibits a uniform corrosion mode, as shown in Figure 3. This result indicates that the corrosion mode of the Mg alloys is also dependent on the corrosion media.

Schematic illustration of corrosion behaviors of M1A in SBF and albumin-containing SBF: (A) M1A sample, (B–D) degradation in SBF, (E–G) degradation in albumin-containing SBF.
Reprinted from Wang et al. (2011), with permission from Elsevier.
If used as a biodegradable implant, once localized corrosion occurs, Mg alloys will lose the integrity of their mechanical properties, which will result in the failure of the material (Zheng, Gu, & Witte, 2014). However, uniform corrosion can allow for sustained integrity of the mechanical properties of the Mg alloys as well as slow down the overall corrosion rate to some extent. Therefore, Mg alloys with slow degradation rate and a uniform degradation mode are desirable for clinical applications.
3 Improvements to the intrinsic corrosion resistance of biodegradable Mg alloys
3.1 Purification
The standard reduction potentials of common impurities, such as iron (Fe), nickel (Ni), and copper (Cu), are greater than that of Mg, and hence, result in galvanic corrosion due to the potential difference between the Mg matrix and the impurities. The Mg matrix acts as an anode and is corroded initially and rapidly (Manivasagam & Suwas, 2014; Song & Atrens, 1999). Therefore, impurities have a significant negative effect on the corrosion behavior of the Mg alloys, and it is of great importance to reduce their content. Different elemental impurities exhibit different tolerance limits, so that once the content of these elements exceeds a certain limit, the corrosion will be greatly accelerated (Ren et al., 2005; Song & Atrens, 1999). Therefore, high-purity, even ultra-high-purity, raw materials are favorable for the preparation of biodegradable Mg alloys.
It has been reported (Hofstetter et al., 2015) that ultra-high-purity Mg with an Fe impurity concentration of 2.2 ppm exhibits a much lower degradation rate in vitro (10±3 μm/year) and in vivo (13±3 μm/year) compared to the pure Mg with Fe impurity concentration of 37 ppm. From the Mg-Fe phase diagram, the maximum solubility of Fe in the hexagonal close packed Mg is only ∼10 ppm. Higher Fe concentration will lead to the formation of Fe-rich body-centered cubic (bcc) phase, which would cause high corrosion rates by microgalvanic acceleration of the Mg matrix. The bcc Fe-rich phase can be precipitated by heat treatment, and thus, the Fe tolerance limit is ∼5–10 ppm for heat-treated high-purity Mg (Liu et al., 2009b). Therefore, the impurity of Fe should be restricted using ultra-high-purity raw materials and/or proper processes. Hofstetter et al. (2014) found that the improvement of corrosion resistance of the high-purity Mg-5Zn-0.25Ca alloy is over an order of magnitude greater than that of a sample with standard purity, and the improvement of the ultrahigh impurity alloy is three times than the high-purity alloy. Moreover, some elements (for instance, Manganese (Mn) and Zirconium (Zr)) can increase the tolerance limit of Fe (Atrens et al., 2014; Liu & Song, 2013). Zr added to the Fe-containing molten Mg alloy reacts with Fe and forms FeZrx particles, which settle to the bottom of the melt (Atrens et al., 2014). It has also been demonstrated that rare earth elements can trap impurity elements and form intermetallic compounds (Zhao, Shi, & Xu, 2013a), thereby reducing the negative effects of those impurities on the corrosion resistance.
Further developing the process method to reduce impurities is another way to improve the corrosion properties of the Mg alloys. Peng, Huang, Zhou, Hort, and Kainer (2010) prepared a high-purity Mg-Y binary alloy using a zone solidification method. Their work indicated that any impurities within the sample are mainly distributed in the top or the bottom layers of the specimens, and the corrosion rate for the middle region of the purified alloy is lower than that of the common as-cast alloy. Qiao, Shi, Hort, Abidin, and Atrens (2012) found that permanent mold direct chill casting was able to keep the Fe concentration in the range of 26–48 ppm for high-purity Mg, thereby slowing the corrosion rate. It has also been reported that a lower casting temperature can tolerate a higher Fe without detrimental effects for corrosion resistance at a certain addition of Mn for the AXJ530 Mg alloy (Liu & Song, 2013).
Therefore, adopting high- or even ultra-high-purity raw materials and controlling the impurity concentration by improving the processing techniques serve to significantly enhance corrosion resistance of biodegradable Mg alloys.
3.2 Alloying
Alloying is a common method to improve the corrosion properties of biodegradable Mg alloys. The addition of some elements acts to refine the grain size of the Mg alloys and form a second phase, which surrounds the α-Mg matrix continuously and leads to a decrease in the corrosion rate. While other added elements are able to reduce the precipitation of the second phase at grain boundaries, balancing the potential difference between the α-Mg matrix and the second phase and decreasing the microgalvanic corrosion (Ghali et al., 2004; Manivasagam & Suwas, 2014; Witte et al., 2008). Furthermore, alloying elements can accumulate on the surface of Mg alloys to form a compact oxidation film that protects the Mg matrix, and thus improves the corrosion properties of the biodegradable Mg alloys (Zhao et al., 2013a). As biomaterials, it is important to ensure that alloying elements have no toxicity or low toxicity, in addition to improving the mechanical properties and/or corrosion resistance (Li & Zheng, 2013). Calcium (Ca), zinc (Zn), zirconium (Zr), strontium (Sr), rare earth (RE), manganese (Mn), silver (Ag), etc. are suitable elements to be added in biodegradable Mg alloys.
Ca is the most common alloying element used for biodegradable Mg alloys because Ca is a major component of the human bone and is beneficial for both human metabolism and bone growth (Jeong & Kim, 2014; Jiao et al., 2011). Bornapour, Celikin, Cerruti, and Pekguleryuz (2014) revealed that the corrosion resistance of the Mg-Ca binary alloy is much better than that of pure Mg due to the following factors: (1) the Mg2Ca intermetallic phase is formed both at the grain boundaries and in the grain interior, which decreases the corrosion potential difference between the Mg matrix and the grain boundaries; (2) the formation of calcium phosphate produced by initial corrosion slows down the further corrosion of the alloy. Most available reports (Erdmann et al., 2011; Li et al., 2008; Salahshoor & Guo, 2014; Waizy et al., 2012; Wan et al., 2008) indicate that addition of 0.6–0.8 wt% Ca is the most suitable for Mg-Ca binary alloys because it showed the slowest corrosion rate and good biocompatibility. Once the content of Ca exceeds the solubility limit of 1.34 wt%, the corrosion resistance of the alloy will decrease with the increase of Ca (Kirkland et al., 2010a).
The addition of elemental Zn has an effect on the grain size of the Mg alloys. Below a certain concentration limit, an increase in the Zn content leads to a decrease in grain size. However, at Zn concentration above this limit, the phase change results in the variation of the growth restriction factor of the grains and the grain size increases (Chen, Zhang, Wang, Ma, & Hao, 2014a). The corrosion rates of the Mg-6Zn alloy after 3- and 30-day immersion in SBF are lower than those of pure Mg. However, both pure Mg and the Mg-6Zn alloy suffered from localized corrosion (Zhang et al., 2010). Du, Wei, Liu, and Zhang (2011) indicated that when the content of Zn is 2 wt%, the corrosion resistance of the Mg-3Ca-2Zn Mg alloy is improved due to the presence of Ca2Mg6Zn3 phase, which acts as the cathode and prevents the continuous corrosion of Mg2Ca phases. It was also observed that both Mg-3Ca-2Zn (Du et al., 2011) and Mg-4.0Zn-1.0Ca-0.6Zr alloys (Guan et al., 2012) show localized corrosion.
Zr is usually added as a powerful grain refiner in Mg alloys, and it can react with some impurities such as Fe, Ni, and Co from molten Mg. Therefore, Zr-containing Mg alloys show improved corrosion properties (Li & Zheng, 2013; Li et al., 2012; Zheng et al., 2014). Li, He, Zhang, and Wang (2014a) compared the corrosion behavior of as-cast Mg-1.5Zn-0.6Zr alloy with a single phase to as-cast commercial AZ91D alloy and reported that the Mg-1.5Zn-0.6Zr alloy with a single phase and finer microstructure exhibited a much slower corrosion rate (0.3 mm/year) and a more uniform corrosion mode than the AZ91D alloy with double phase when placed in Hank’s solution. These differences are due to both the elimination of intensive microgalvanic corrosion reactions and the formation of uniform and compact films on the Mg-1.5Zn-0.6Zr alloys refined by the addition of Zr. Li et al. (2012) reported that Zr addition refined the grain size, smoothed the grain boundaries, and improved the corrosion properties of Mg-Zr-Sr alloys. In addition, the Mg-Zr-Sr alloy with the addition of 1 wt% Zr was considered the preferable candidate due to its excellent corrosion resistance during in vitro and in vivo tests.
Sr is a significant component of human bone, and it is beneficial for the growth of osteoblasts and the formation of the bone. In addition, Sr is able to refine the grain sizes and improve the mechanical properties and corrosion resistance of Mg alloys (Bornapour, Muja, Shum-Tim, Cerruti, & Pekguleryuz, 2013; Gu, Xie, Li, Zheng, & Qin, 2012; Li et al., 2014b; Zhang et al., 2014b). For these reasons, it attracted the attention of researchers for use as an alloying element for biodegradable Mg alloys. The corrosion rates, in terms of mass loss for various Mg-Sr-based alloys, are shown in Table 1. Bornapour et al. (2013) suggested that when the content of Sr is less than 1 wt%, the as-cast Mg-Sr binary alloy shows a slower corrosion rate than those with higher Sr content. The in vivo experimental results indicated that the biodegradation rate of Mg-0.5Sr to be around 0.2–0.4 mm/year. Nevertheless, Gu et al. (2012) indicated that the Mg-Sr binary alloy containing 2 wt% Sr shows even better corrosion resistance in vitro and in vivo. The contrary results could most likely be ascribed to the different microstructures formed under different conditions (as-cast versus as-rolled) of the Mg-Sr binary alloy. The corrosion modes of the as-cast Mg-0.5Sr in vitro and the as-rolled Mg-2Sr in vivo are localized and caused by microgalvanic couples due to the presence of the Mg17Sr2 phase, as shown in Figures 4 and 5, respectively. Zhang, He, Xue, Wang and Wang (2014b) found that when the content of Sr is 2 wt%, microgalvanic corrosion plays a dominant role and the corrosion is sharply accelerated. The results of these studies demonstrate the significance of factors such as conditions and the effects of the other alloying elements when developing novel Mg alloys.

The surface appearance of as-cast Mg-0.5Sr removed from SBF after (A) 1-, (B) 2-, and (C) 3-day immersion at 37°C.
Reprinted from Bornapour et al. (2013), with permission from Elsevier.

CT images (3-D reconstruction) showing the in vivo degradation of an intramedullary as-rolled Mg-2Sr alloy implant for different time intervals. (A) Complete outline of the distal femur of a mouse. (B) Complete outline of the Mg-2Sr alloy implant. Bar 1.0 mm.
Reprinted from Gu et al. (2012), with permission from Elsevier.
Corrosion rates of Sr-containing Mg alloys measured by mass loss test.
| Mg alloys | Condition | Corrosion rate (mm/year) | Corrosion medium | References |
|---|---|---|---|---|
| Mg-0.3Sr | As-cast | 2.5 | Hank’s solution | Bornapour et al., 2013 |
| Mg-0.5Sr | As-cast | 1.8 | Hank’ s solution | |
| Mg-0.7Sr | As-cast | 2.8 | Hank’ s solution | |
| Mg-1.0Sr | As-cast | 3.1 | Hank’ s solution | |
| Mg-1.2Sr | As-cast | 3.0 | Hank’ s solution | |
| Mg-1.5Sr | As-cast | 5.1 | Hank’ s solution | |
| Mg-2.0Sr | As-cast | 11.5 | Hank’ s solution | |
| Mg-2.5Sr | As-cast | 23.0 | Hank’ s solution | |
| Mg-1Sr | As-rolled | 0.85 | Hank’ s solution | Gu et al., 2012 |
| Mg-2Sr | As-rolled | 0.37 | Hank’ s solution | |
| Mg-3Sr | As-rolled | 0.75 | Hank’ s solution | |
| Mg-4Sr | As-rolled | 1.65 | Hank’ s solution | |
| Mg-1Zn-0.2Sr | Backward-extruded | 1.8 | SBF | Li et al., 2014b |
| Mg-1Zn-0.5Sr | Backward-extruded | 2.8 | SBF | |
| Mg-1Zn-0.8Sr | Backward-extruded | 3.9 | SBF | |
| Mg-1Zn-1.0Sr | Backward-extruded | 6.29 | SBF | |
| Mg-2.2Nd-0Sr-0.3Zr | As-cast | 0.89 | SBF | Zhang et al., 2014b |
| Mg-2.2Nd-0.4Sr-0.3Zr | As-cast | 0.83 | SBF | |
| Mg-2.2Nd-0.7Sr-0.3Zr | As-cast | 0.77 | SBF | |
| Mg-2.2Nd-2.0Sr-0.3Zr | As-cast | 56.49 | SBF |
Rare earth elements in Mg alloys can act as scavengers to remove impurity elements and thereby strengthen corrosion resistance. The common rare earth elements added in the Mg alloys are yttrium (Y), neodymium (Nd), gadolinium (Gd), dysprosium (Dy), and lanthanum (La). However, Y has been related to hepatotoxicity, and therefore does not meet the requirement of low cell toxicity for biomaterials. Therefore, the addition of Y should not be seriously considered (Feyerabend et al., 2010). The Mg-Y alloys with different Y conditions show that the corrosion rate increases with increasing Y addition in 0.1 m NaCl solution, but decreases when the Y addition is over ∼3.0 wt% in 0.1 m Na2SO4 solution due to a more protective surface film (Liu, Schmutz, Uggowitzer, Song, & Atrens, 2010). It has been reported (Shi, Cao, Song, Liu, & Atrens, 2013; Zhao et al., 2013a) that the corrosion resistance of Mg-RE binary alloys is worse than that of pure Mg mainly because the presence of both the second phase and impurities results in microgalvanic corrosion between them and the α-Mg matrix. While some studies (Hort et al., 2010; Kubásek & Vojtěch, 2013) revealed that proper addition of RE elements is beneficial to corrosion resistance of binary Mg alloys. Zhang, Yuan, Mao, Niu, and Ding (2012d) revealed that the corrosion rate of the as-extruded Mg-Nd-Zn-Zr alloy in SBF is lower and the corrosion morphology of the alloy is more uniform than those of the commercial AZ31 and WE43 alloys, respectively. The corroded surface of the as-extruded Mg-Nd-Zn-Zr alloy is smooth and uniform, while the surface of WE43 suffers from severe pitting corrosion, as shown in Figure 6 (Mao et al., 2012). Zhang, He, Xue, Wang and Wang (2014c) also found that the addition of Gd slows down the corrosion of the Mg-Nd-Sr-Zn-Zr alloy.

Surface (A, B) and cross section (C, D) corrosion morphologies of the as-extruded Mg-Nd-Zn-Zr (A, C) and WE43 (B, D) alloys immersed in artificial plasma for 10 d.
Reprinted from Mao et al. (2012), with permission from Elsevier.
The main effect of the addition of Mn is an increase in the tolerance limit of Fe, making it able to decrease the impact of impurities and hence significantly enhance the corrosion resistance of Mg alloys (Liu & Song, 2013). The grain size of the Mg-Nd-Zn-Ag-Zr alloy is finer and the distribution of the second phase is more continuous with the addition of Ag, leading to improvement of the corrosion resistance. However, the corrosion resistance is lessened when the content of Ag is >0.8 wt% (Zhang et al., 2013a).
In summary, the following factors are recommended to take into account when choosing alloying elements. (1) Special attention must be paid to ensure that each alloying element does not exceed its specific content limit which proves detrimental to the corrosion behavior. (2) The effect of solution types on corrosion resistance of Mg alloys is proposed to be considered. (3) The condition of the Mg alloys and the effects of other elements simultaneously existing in the alloys should also be considered. (4) In vivo degradation must also be taken into account if the in vitro corrosion resistance is shown to meet the requirements of the biomedical application. Only in this way can the biodegradable Mg alloys be recognized as potential candidates for biomaterial.
3.3 Grain refinement
It has been reported that the grain sizes of both the α-Mg matrix and the second phase significantly influence the corrosion resistance of Mg alloys (Ben-Haroush, Ben-Hamu, Eliezer, & Wagner, 2008; Ralston, Birbilis, & Davies, 2010). Most of the literature indicated that grain refinement is good for improving corrosion resistance of Mg alloys. Ralston and Birbilis (2010) explained that grain boundaries have higher energy and provide favorable locations for grain formation, which are then beneficial to the formation of a protective oxide film. With finer grains, there is an improvement in corrosion resistance, which is attributed to better film formation and adhesion due to the increased grain boundary densities. The second phases in Mg alloys always have greater corrosion potential and result in an increase of microgalvanic corrosion and overall accelerate corrosion (Zhao, Teng, Zhou, Leng, & Geng, 2014). Therefore, if grain refinement of the second phase can be achieved to some extent, it is useful to prevent second-phase particles from operating as local cathodes and causing severe localized corrosion.
However, some researchers reported that Mg alloys with finer grains show higher corrosion rate (Kutniy et al., 2009; Song et al., 2011). Song et al. (2011) reported that the corrosion resistance of the ultra-fine grained AZ91D alloy is lower than that of the as-cast AZ91D alloy. It reveals that the strain-induced crystalline defects providing the matrix more corrosion activation and the refined β-phase losing barriers to corrosion propagation in matrix are responsible for the decreased corrosion resistance. In addition, Gollapudi (2012) pointed out that a broader grain size distribution led to increased corrosion resistance in non-passivating environment but decreased corrosion resistance in passivating environment. It has been also concluded that the increased grain boundary densities would likely enhance the corrosion rate of Mg alloy in the absence of oxide film because the increased grain boundary densities would enhance the surface reactivity (Ralston, & Birbilis, 2010). Consequently, some other factors should be considered seriously besides grain refinement.
It has been shown that the grain size of Mg alloys can be refined by hot deformation methods such as extrusion, rolling, double extrusion (DE), backward extrusion (BE), cyclic extrusion and compression (CEC), high-pressure torsion (HPT) process, equal channel angular pressing (ECAP), etc. due to dynamic recrystallization. Furthermore, rapid solidification is another effective way to refine the microstructures of Mg alloys. Here we reviewed the corrosion resistance of Mg alloys improved by grain refinement through hot deformation and rapid solidification.
3.3.1 Hot deformation
Hot plastic deformation is a useful method to refine grains and obtain a homogeneous microstructure. A fine microstructure can often be achieved by common deformation, such as single extrusion (SE) and rolling (Deng, Huang, Zhao, & Wang, 2014; Zakiyuddin, Yun, & Lee, 2014; Zhang, Yuan, & Wang, 2013d). Zhang et al. (2013d) compared the corrosion properties of the as-extruded with the as-cast Mg-Nd-Zn-Zr alloy and found that the as-extruded alloy has better corrosion resistance than the as-cast one, the difference is mainly attributed to a refined and more homogenous microstructure. The corrosion resistance of the as-rolled AZ31 alloy is improved by 39.4% compared to the as-cast alloy due to the refined microstructure (Deng et al., 2014).
However, it has often been observed that common deformation is not able to achieve a totally homogeneous microstructure for Mg alloys (Azeem, Tewari, Mishra, Gollapudi, & Ramamurty, 2010; Xu et al., 2011; Zhang, Wang, Yuan, & Xue, 2012b; Zhang et al., 2013d). Therefore, new deformation methods, such as DE, BE, ECAP, CEC, and HPT processes have been developed to refine the microstructure. As shown in Figure 7, these new plastic deformation methods have a large positive effect on the improvement of the corrosion resistance of biodegradable Mg alloys. Moreover, when Gao et al. (2011) studied the corrosion behavior of the Mg-Zn-Ca alloy in SBF using an HPT process, they found that the second phase has been refined to nano-sized particles and distributed homogeneously in the interior of α-Mg grains. As a result, the alloy shows a uniform corrosion mode, indicating that severe plastic deformation is also useful for improving the corrosion mode of Mg alloys.

Enhanced corrosion rate of Mg alloys following different kinds of hot deformation.
SE, rolling, BE, and HPT compared to the as-cast alloy; DE, ECAP, and CEC are compared to the SE alloy (Deng et al., 2014; Gao et al., 2011; Peng, Li, Ma, Liu, & Zhang, 2012; Wang, Estrin, & Zúberová, 2008; Zakiyuddin et al., 2014; Zhang et al., 2012b; Zhang, Yuan, & Wang, 2012e; Zhang et al., 2013d).
3.3.2 Rapid solidification
Rapid solidification has been shown to refine the microstructure of Mg alloys due to the increased solidification rate (Wang et al., 2010; Willbold et al., 2013). The corrosion resistance is also enhanced by rapid solidification because of grain refinement and fine dispersion of the quasi-crystals and intermetallic compounds in the α-Mg matrix. Liao, Hotta, and Mori (2012) studied the corrosion resistance of the Mg-Al-Mn-Ca alloy (AMX602) by rapid solidification and found that the corrosion rate of the alloy produced by the spinning water atomization process is 2.5–10 times less than that of the hot-extruded and as-cast alloy. Figure 8 indicates the rapid solidification sample exhibits much better corrosion resistance and a more uniform corrosion mode compared with the as-cast and as-extruded alloys prepared by gravity cast. Izumi, Yamasaki, and Kawamura (2009) proposed that increasing the cooling rate can reduce filiform corrosion of the Mg-Zn-Y alloy due to grain refinement and formation of a supersaturated single α-Mg solid solution. In addition, rapid solidification can improve microstructural and electrochemical homogeneities of Mg-Zn-Y alloys as well as enhance the passivity of substrate materials, leading to a reduction of the occurrence of local breakdown of films.
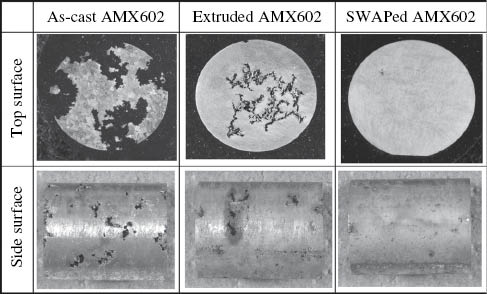
Macro-corrosion appearance of samples after being immersed in 0.1 m NaCl solution for 2 weeks.
Reprinted from Liao et al. (2012), with permission from Elsevier.
3.4 Heat treatment
Available reports indicate that heat treatments have a complex effect on the corrosion rate and corrosion mode of Mg alloys (Liang, Guan, & Tan, 2011; Liu, Xin, Tang, & Chu, 2007a; Peng et al., 2009; Wang, Li, Zeng, Wu, & Ding, 2013). The corrosion resistance of the as-cast Mg-7Gd-3Y-0.4Zr alloy is improved 93.8% and 85.4%, respectively, after solution treatment or peak aged treatment (Liang et al., 2011). Nevertheless, the as-cast Mg-7Al-2Sn alloy exhibits an even lower corrosion rate compared to the solution- and aging-treated Mg-7Al-2Sn alloys due to a barrier of Mg17Al12 phase (Wang et al., 2013).
Generally, the second phase around the Mg matrix can be reduced or removed after solution treatment, a step that is beneficial to decreasing galvanic corrosion and improving the overall corrosion behavior of Mg alloys. Furthermore, aging treatment could allow for the precipitated phase to homogeneously distribute around grain boundaries and the grain interior, which is also good for reducing microgalvanic corrosion and relieving stress.
Proper heat treatment is useful for improving the corrosion resistance and corrosion mode of Mg alloys. It has been reported that solution- and aging-treated Mg-10Gd-3Y-0.4Zr alloys exhibit lower corrosion rates and more uniform corrosion modes than the as-cast alloy (Peng et al., 2009). It has also been found that the corrosion resistance of Mg-Nd-Zn-Zr alloys can be enhanced after solution treatment and aging treatment (Zhang, Yuan, & Wang, 2013c). As shown in Figure 9, the samples immersed in SBF exhibit a favorable uniform corrosion mode. Nevertheless, the corrosion mode was slightly worse after solution treatment and aging treatment, which is illustrated by cyclic polarization scans and shown in Figure 10 (Zhang et al., 2013c). The corrosion potential of the forward scan showed that the polarization behavior of the non-corroded areas is lower than that of the reverse scan associated with the corroded areas. The area with negative potential has been previously corroded owing to the galvanic effect. The corrosion potential difference between the forward scan and reverse scan decreases after T4 and T6 treatment and thus results in a slight worsening of the uniform corrosion mode. Additionally, the corrosion resistance is influenced by the heat-treated parameters (Zhang, Yuan, Fang, Wang, & Zhang, 2013b).

Macro-corrosion morphologies of Mg-3.08Nd-0.27Zn-0.43Zr immersed in SBF before and after removing corrosion products (Zhang et al., 2013c).

Cyclic polarization curves of Mg-Nd-Zn-Zr alloy after immersion in SBF for 1 h (A) F, (B) T4, and (C) T6 (Zhang et al., 2013c).
4 The improvement of corrosion behavior by LPSO structure
The LPSO structure has a high density of plane faults, and the LPSO was found to possess a structure of 6H, 10H, 14H, 18R, or 24R types of close-packed planes of the Mg crystal, all of which are observed in Mg-RE-Zn systems (Kawamura & Yamasaki, 2007; Lu, Ma, Jiang, Yang, & Zhou, 2012; Matsuda, Ii, Kawamura, Ikuhara, & Nishida, 2005; Wu, Lin, Zeng, Peng, & Ding, 2009). The LPSO structure plays a very important role in the corrosion behavior of Mg alloys (Leng et al., 2013; Peng et al., 2014; Zhang, Wu, Xue, Wang, & Yang, 2012c; Zhang et al., 2014a; Zhang et al., 2015).
Zhang et al. (2012c) reported that corrosion rate of the as-extruded Mg-11.3Gd-2.5Zn-0.7Zr alloy with LPSO structure in SBF is only 0.17 mm/year, while that of the as-extruded Mg-10.2Gd-3.3Y-0.6Zr alloy without LPSO structure is 0.55 mm/year. Furthermore, the Mg-11.3Gd-2.5Zn-0.7Zr alloy with LPSO structure shows relatively uniform corrosion even when it has discontinuously distributed second phase (Figure 11A). Meanwhile, the Mg-10.2Gd-3.3Y-0.6Zr alloy without LPSO structure shows pitting corrosion (Figure 11B). This study indicates that the LPSO structure has a positive effect on the corrosion behavior of Mg alloys. Peng et al. (2014) found that the corrosion rate of solution treated Mg-2Dy-0.5Zn with 14H-LPSO is lower than that of the as-cast alloy with 18R-LPSO due to the existence of a homogeneous oxidation film and fast film remediation ability.

Corrosion morphologies of the as-extruded Mg-11.3Gd-2.5Zn-0.7Zr alloy with LPSO structure (A) and Mg-10.2Gd-3.3Y-0.6Zr alloy without LPSO structure (B) after immersion in Hank’s solution for 120 h (Zhang et al., 2012c).
Recently, we have studied the corrosion behavior of the Mg-5Gd-1Zn-0.6Zr (GZ51K) alloy in SBF under as-cast, solution-treated, and aging-treated conditions (Zhang , Ba, Wang, Wu, Wang, & Wang, 2014a). It was observed that the as-cast GZ51K alloy with LPSO structure exhibits better corrosion resistance and a more uniform corrosion mode than aging-treated alloys without LPSO structure. We also found that the corrosion resistance of the GZ51K alloy correlated to the fraction of LPSO structure: a higher fraction of the LPSO structure leads to better corrosion resistance, as shown in Figures 12 and 13, respectively (Zhang et al., 2015). The alloy with the LPSO structure has a uniform corrosion mode, while that without the LPSO structure undergoes localized corrosion, as shown in Figure 14. These results suggest that the corrosion behavior of the GZ51K alloy can be adjusted by controlling the fraction of the LPSO structure. Figure 15 (Zhang et al., 2014a) shows the schematic diagrams of the corrosion process for the GZ51K alloy with and without LPSO structure. In the as-cast GZ51K alloy, the substrate is first corroded and the continuously distributed LPSO structure acts as a barrier, working as a protective structure, and preventing further corrosion. Only when the LPSO structure is destroyed completely can the next substrate be further corroded. In contrast, the substrate and grain boundary of the GZ51K alloy without the LPSO structure is easily corroded and the discontinuously distributed precipitates accelerate the corrosion due to microgalvanic corrosion. Consequently, the alloy with the LPSO structure exhibits good corrosion resistance and a homogeneous corrosion mode.

Microstructure of the GZ51K alloy heat treated at (A) 350°C, (B) 400°C, (C) 450°C, and (D) 500°C (Zhang et al., 2015).

Corrosion rates of GZ51K alloy under different solution temperatures after immersion in SBF for 120 h (Zhang et al., 2015).
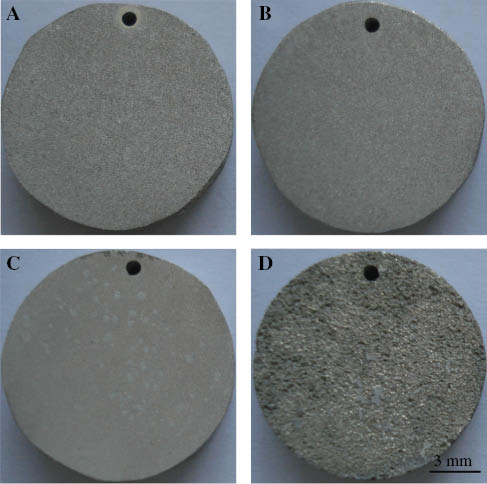
Corrosion morphologies of the GZ51K alloy after immersion in SBF for 120 h heat treated at (A) 350°C, (B) 400°C, (C) 450°C, and (D) 500°C (Zhang et al., 2015).

Schematic diagrams of corrosion process for the as-cast GZ51K alloy with LPSO structure to show the uniform corrosion (A–D) and T6-treated GZ51K without LPSO structure to show localized corrosion (E–H) following prolonged immersion in SBF (Zhang et al., 2014a).
Mg-Y-Zn alloys also possess good corrosion resistance when the fraction of the LPSO structure is increased and forms a network around the Mg matrix (Zhang, Xu, Cheng, Chen, & Kang, 2012a). Furthermore, the nano-spaced basal plane stacking faults (the early stage of the LPSO structure) are helpful in improving corrosion behavior of the Mg-6Ho-1Zn alloy (Zhang et al., 2014d). The alloy with nano-spaced basal plane stacking faults exhibits a uniform corrosion mode and a low corrosion rate of 0.55 mm/year. This low corrosion rate is mainly because of the fact that different orientations of nano-spaced basal plane stacking faults force corrosion to march along their length, thus preventing further corrosion into neighboring grains.
However, the LPSO structure is not always bound to improve the corrosion behavior Mg alloys. The as-cast Mg100-3x(Zn1Y2)x (1≤x≤3) alloys with LPSO structures show a localized corrosion mode, with degradation rates increasing in correlation with an increasing amount of LPSO structure (Zhao, Shi, & Xu, 2013b). Moreover, we have recently studied the corrosion behavior of the Mg-Gd-Cu-Zr alloy with LPSO structure and found that the corrosion rate of the as-cast alloy is around 59.9 mm/year and shows a severely localized corrosion mode. In general, it is possible for the LPSO structure to have a contrary influence on the corrosion behavior of various Mg alloys. It may improve corrosion behavior (including corrosion resistance and corrosion mode) of some Mg alloys and yet may also worsen corrosion behavior of other Mg alloys.
5 Conclusion
Mg alloys have attracted great attention due to their good biocompatibility, biodegradability, excellent physical and mechanical properties, and other characteristics as compared to conventional metallic biomaterials. However, fast corrosion rates and localized corrosion modes remain major obstacles for their use in clinical application, a problem that cannot be solved using only surface-altering technologies. Therefore, more attention should be paid to the improvement of the intrinsic corrosion behavior of the material. The corrosion behavior of Mg alloys is influenced by many factors, such as composition, grain size, the amount and distribution of the other phases. This paper illustrates important methods for improving corrosion behavior of Mg alloys from the perspective of the designing, process, and microstructure. Based on this work, we put forward some suggestions to enhance the intrinsic corrosion behavior of Mg alloys for biomedical application, as follows:
Impurities and alloying elements should be considered when designing Mg alloys for biomedical applications. Adopting high-purity or even ultra-high-purity raw materials, selecting certain alloying elements, and improving the melting process of the Mg alloys are three main ways to reduce the negative effects of impurities. Selecting elements that show good biocompatibility with Mg alloys not only can enhance mechanical properties, but also improve corrosion behavior. It is necessary to systematically research the influence of the category and the amount of an alloying element on biodegradable Mg alloys.
Several effective processes can be used to modify the corrosion behavior during the preparation of Mg alloys, such as rapid solidification, severe plastic deformation, and proper heat treatment.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (51301089), the Natural Science Foundation of Jiangsu Province (BK20130745), the Innovative Foundation Project for Students of Jiangsu Province (201411276005Z) and Nanjing Institute of Technology (N20150206), and the Qing Lan Project of Jiangsu Province.
References
Atrens A, Liu M, Abidin NIZ. Corrosion mechanism applicable to biodegradable magnesium implants. Mater Sci Eng B 2011; 176: 1609–1636.10.1016/j.mseb.2010.12.017Suche in Google Scholar
Atrens A, Song GL, Liu M, Shi ZM, Cao FY, Dargusch MS. Review of recent developments in the field of magnesium corrosion. Adv Eng Mater 2015; 17: 400–453.10.1002/adem.201400434Suche in Google Scholar
Azeem MA, Tewari A, Mishra S, Gollapudi S, Ramamurty U. Development of novel grain morphology during hot extrusion of magnesium AZ21 alloy. Acta Mater 2010; 58: 1495–1502.10.1016/j.actamat.2009.10.056Suche in Google Scholar
Ben-Haroush M, Ben-Hamu G, Eliezer D, Wagner L. The relation between microstructure and corrosion behavior of AZ80 Mg alloy following different extrusion temperatures. Corros Sci 2008; 50: 1766–1778.10.1016/j.corsci.2008.03.003Suche in Google Scholar
Bornapour M, Muja N, Shum-Tim D, Cerruti M, Pekguleryuz M. Biocompatibility and biodegradability of Mg-Sr alloys: the formation of Sr-substituted hydroxyapatite. Acta Biomater 2013; 9: 5319–5330.10.1016/j.actbio.2012.07.045Suche in Google Scholar PubMed
Bornapour M, Celikin M, Cerruti M, Pekguleryuz M. Magnesium implant alloy with low levels of strontium and calcium: the third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Mater Sci Eng C 2014; 35: 267–282.10.1016/j.msec.2013.11.011Suche in Google Scholar PubMed
Chen TJ, Zhang DH, Wang W, Ma Y, Hao Y. Effects of Zn content on microstructures and mechanical properties of Mg-Zn-RE-Sn-Zr-Ca alloys. Mater Sci Eng A 2014a; 607: 17–27.10.1016/j.msea.2014.03.111Suche in Google Scholar
Chen YJ, Xu ZG, Smith C, Sankar J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater 2014b; 10: 4561–4573.10.1016/j.actbio.2014.07.005Suche in Google Scholar PubMed
Deng JF, Huang GS, Zhao YC, Wang B. Electrochemical performance of AZ31 magnesium alloy under different processing conditions. Rare Metal Mat Eng 2014; 43: 316–321.10.1016/S1875-5372(14)60066-7Suche in Google Scholar
Du H, Wei ZJ, Liu XW, Zhang EL. Effects of Zn on the microstructure, mechanical property and bio-corrosion property of Mg-3Ca alloys for biomedical application. Mater Chem Phys 2011; 125: 568–575.10.1016/j.matchemphys.2010.10.015Suche in Google Scholar
Erdmann N, Angrisani N, Reifenrath J, Lucas A, Thorey F, Bormann D, Meyer-Lindenberg A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: a comparative in vivo study in rabbits. Acta Biomater 2011; 7: 1421–1428.10.1016/j.actbio.2010.10.031Suche in Google Scholar PubMed
Erinc M, Sillekens WH, Mannens RGTM, Werkhoven RJ. Applicability of existing magnesium alloys as biomedical implant materials. In: Nyberg EA, Agnew SR, Neelameggham NR, Pekguleryuz MO, editors. Magnesium technology. San Francisco, CA/Warrendale, PA: Minerals, Metals and Materials Society, 2009: 209–214.Suche in Google Scholar
Ferreira PC, Piai KA, Takayanagui AMM, Segura-Muñoz SI. Aluminum as a risk factor for Alzheimer’s disease. Rev Latino-am Enfermagem 2008; 16: 151–157.10.1590/S0104-11692008000100023Suche in Google Scholar
Feyerabend F, Fischer J, Holtz J, Witte F, Willumeit R, Drücker H, Vogt C, Hort N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater 2010; 6: 1834–1842.10.1016/j.actbio.2009.09.024Suche in Google Scholar PubMed
Fischerauer SF, Kraus T, Wu X, Tangl S, Sorantin E, Hänzi AC, Löffler JF, Uggowitzer PJ, Weinberg AM. In vivo degradation performance of micro-arc-oxidized magnesium implants: a micro-CT study in rats. Acta Biomater 2013; 9: 5411–5420.10.1016/j.actbio.2012.09.017Suche in Google Scholar PubMed
Gao JH, Guan SK, Ren ZW, Sun YF, Zhu SJ, Wang B. Homogeneous corrosion of high pressure torsion treated Mg-Zn-Ca alloy in simulated body fluid. Mater Lett 2011; 65: 691–693.10.1016/j.matlet.2010.11.015Suche in Google Scholar
Ghali E, Dietzel W, Kainer KU. General and localized corrosion of magnesium alloys: a critical review. J Mater Eng Perform 2004; 13: 7–23.10.1361/10599490417533Suche in Google Scholar
Gollapudi S. Grain size distribution effects on the corrosion behaviour of materials. Corros Sci 2012; 62: 90–94.10.1016/j.corsci.2012.04.040Suche in Google Scholar
Gu XN, Zheng YF, Cheng Y, Zhong SP, Xi TF. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009; 30: 484–498.10.1016/j.biomaterials.2008.10.021Suche in Google Scholar PubMed
Gu XN, Xie XH, Li N, Zheng YF, Qin L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater 2012; 8: 2360–2374.10.1016/j.actbio.2012.02.018Suche in Google Scholar PubMed
Guan RG, Johnson I, Cui T, Zhao T, Zhao ZY, Li X, Liu HN. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J Biomed Mater Res A 2012; 100: 999–1015.10.1002/jbm.a.34042Suche in Google Scholar PubMed
Hofstetter J, Becker M, Martinelli E, Weinberg AM, Mingler B, Kilian H, Pogatscher S, Uggowitzer PJ, Löffler JF. High-strength low-alloy (HSLA) Mg-Zn-Ca alloys with excellent biodegradation performance. JOM 2014; 66: 566–572.10.1007/s11837-014-0875-5Suche in Google Scholar
Hofstetter J, Martinelli E, Weinberg AM, Becker M, Mingler B, Uggowitzer PJ, Löffler JF. Assessing the degradation performance of ultrahigh-purity magnesium in vitro and in vivo. Corros Sci 2015; 91: 29–36.10.1016/j.corsci.2014.09.008Suche in Google Scholar
Hornberger H, Virtanen S, Boccaccini AR. Biomedical coatings on magnesium alloys – a review. Acta Biomater 2012; 8: 2442–2455.10.1016/j.actbio.2012.04.012Suche in Google Scholar PubMed
Hort N, Huang Y, Fechner D, Störmer M, Blawert C, Witte F, Vogt C, Drücker H, Willumeit R, Kainer KU, Feyerabend F. Magnesium alloys as implant materials – principles of property design for Mg-RE alloys. Acta Biomater 2010; 6: 1714–1725.10.1016/j.actbio.2009.09.010Suche in Google Scholar PubMed
Izumi S, Yamasaki M, Kawamura Y. Relation between corrosion behavior and microstructure of Mg-Zn-Y alloys prepared by rapid solidification at various cooling rates. Corros Sci 2009; 51: 395–402.10.1016/j.corsci.2008.11.003Suche in Google Scholar
Jamesh M, Wu GS, Zhao Y, Chu PK. Effects of silicon plasma ion implantation on electrochemical corrosion behavior of biodegradable Mg-Y-RE Alloy. Corros Sci 2013; 69: 158–163.10.1016/j.corsci.2012.11.037Suche in Google Scholar
Jeong YS, Kim WJ. Enhancement of mechanical properties and corrosion resistance of Mg-Ca alloys through microstructural refinement by indirect extrusion. Corros Sci 2014; 82: 392–403.10.1016/j.corsci.2014.01.041Suche in Google Scholar
Jiao W, Li HF, Zhao K, Bai HY, Wang YB, Zheng YF, Wang WH. Development of CaZn based glassy alloys as potential biodegradable bone graft substitute. J Non-Crystal Solids 2011; 357: 3830–3840.10.1016/j.jnoncrysol.2011.08.003Suche in Google Scholar
Kawamura Y, Yamasaki M. Formation and mechanical properties of Mg97Zn1RE2 alloys with long-period stacking ordered structure. Mater Trans 2007; 48: 2986–2992.10.2320/matertrans.MER2007142Suche in Google Scholar
Kim WC, Kim JG, Lee JY, Seok HK. Influence of Ca on the corrosion properties of magnesium for biomaterials. Mater Lett 2008; 62: 4146–4148.10.1016/j.matlet.2008.06.028Suche in Google Scholar
Kirkland NT. Magnesium biomaterials: past, present and future. Corros Eng Sci Technol 2012; 47: 322–328.10.1179/1743278212Y.0000000034Suche in Google Scholar
Kirkland NT, Birbilis N, Walker J, Woodfield T, Dias GJ, Staiger MP. In-vitro dissolution of magnesium-calcium binary alloys: clarifying the unique role of calcium additions in bioresorbable magnesium implant alloys. J Biomed Mater Res B 2010a; 95: 91–100.10.1002/jbm.b.31687Suche in Google Scholar PubMed
Kirkland NT, Lespagnol J, Birbilis N, Staiger MP. A survey of bio-corrosion rates of magnesium alloys. Corros Sci 2010b; 52: 287–291.10.1016/j.corsci.2009.09.033Suche in Google Scholar
Kubásek J, Vojtěch D. Structural and corrosion characterization of biodegradable Mg-RE (RE=Gd, Y, Nd) alloys. Trans Nonferrous Met Soc China 2013; 23: 1215–1225.10.1016/S1003-6326(13)62586-8Suche in Google Scholar
Kutniy KV, Papirov II, Tikhonovsky MA, Pikalov AI, Sivtzov SV, Pirozhenko LA, Shokurov VS, Shkuropatenko VA. Influence of grain size on mechanical and corrosion properties of magnesium alloy for medical implants. Mat-wiss Werkstofftech 2009; 40: 242–246.10.1002/mawe.200900434Suche in Google Scholar
Leng Z, Zhang JH, Yin TT, Zhang L, Guo XY, Peng QM, Zhang ML, Wu RZ. Influence of biocorrosion on microstructure and mechanical properties of deformed Mg-Y-Er-Zn biomaterial containing 18R-LPSO phase. J Mech Behav Biomed Mater 2013; 28: 332–339.10.1016/j.jmbbm.2013.08.012Suche in Google Scholar PubMed
Li N, Zheng YF. Novel magnesium alloys developed for biomedical application: a review. J Mater Sci Technol 2013; 29: 489–502.10.1016/j.jmst.2013.02.005Suche in Google Scholar
Li ZJ, Gu XN, Lou SQ, Zheng YF. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008; 29: 1329–1344.10.1016/j.biomaterials.2007.12.021Suche in Google Scholar PubMed
Li YC, Wen CE, Mushahary D, Sravanthi R, Harishankar N, Pande G, Hodgson P. Mg-Zr-Sr alloys as biodegradable implant materials. Acta Biomater 2012; 8: 3177–3188.10.1016/j.actbio.2012.04.028Suche in Google Scholar PubMed
Li T, He Y, Zhang HL, Wang XT. Microstructure, mechanical property and in vitro biocorrosion behavior of single-phase biodegradable Mg-1.5Zn-0.6Zr alloy. J Magn Alloy 2014a; 2: 181–189.10.1016/j.jma.2014.05.006Suche in Google Scholar
Li H, Peng QM, Li XJ, Li K, Han ZS, Fang DQ. Microstructures, mechanical and cytocompatibility of degradable Mg-Zn based orthopedic biomaterials. Mater Des 2014b; 58: 43–51.10.1016/j.matdes.2014.01.031Suche in Google Scholar
Li YX, Zhang YY, Jungwirth S, Seely N, Fang YD, Shi XM. Corrosion inhibitors for metals in maintenance equipment: introduction and recent developments. Corros Rev 2014c; 32: 163–181.10.1515/corrrev-2014-0002Suche in Google Scholar
Liang SQ, Guan DK, Tan XP. The relation between heat treatment and corrosion behavior of Mg-Gd-Y-Zr alloy. Mater Des 2011; 32: 1194–1199.10.1016/j.matdes.2010.10.022Suche in Google Scholar
Liao JS, Hotta M, Mori Y. Improved corrosion resistance of a high-strength Mg-Al-Mn-Ca magnesium alloy made by rapid solidification powder metallurgy. Mater Sci Eng A 2012; 544: 10–20.10.1016/j.msea.2012.02.046Suche in Google Scholar
Lin X, Yang XM, Tan LL, Li M, Wang X, Zhang Y, Yang K, Hu ZQ, Qiu JH. In vitro degradation and biocompatibility of a strontium-containing micro-arc oxidation coating on the biodegradable ZK60 magnesium alloy. Appl Surf Sci 2014; 288: 718–726.10.1016/j.apsusc.2013.10.113Suche in Google Scholar
Liu M, Song GL. Impurity control and corrosion resistance of magnesium-aluminum alloy. Corros Sci 2013; 77: 143–150.10.1016/j.corsci.2013.07.037Suche in Google Scholar
Liu CL, Xin YC, Tang GY, Chu PK. Influence of heat treatment on degradation behavior of bio-degradable die-cast AZ63 magnesium alloy in simulated body fluid. Mater Sci Eng A 2007a; 456: 350–357.10.1016/j.msea.2006.12.020Suche in Google Scholar
Liu CL, Xin YC, Tian XB, Chu PK. Corrosion behavior of AZ91 magnesium alloy treated by plasma immersion ion implantation and deposition in artificial physiological fluids. Thin Solid Films 2007b; 516: 422–427.10.1016/j.tsf.2007.05.048Suche in Google Scholar
Liu C, Chen DL, Bhole S, Cao X, Jahazi M. Polishing-assisted galvanic corrosion in the dissimilar friction stir welded joint of AZ31 magnesium alloy to 2024 aluminum alloy. Mater Charact 2009a; 60: 370–376.10.1016/j.matchar.2008.10.009Suche in Google Scholar
Liu M, Uggowitzer PJ, Nagasekhar AV, Schmutz P, Easton M, Song GL, Atrens A. Calculated phase diagrams and the corrosion of die-cast Mg-Al alloys. Corros Sci 2009b; 51: 602–619.10.1016/j.corsci.2008.12.015Suche in Google Scholar
Liu M, Schmutz P, Uggowitzer PJ, Song GL, Atrens A. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys. Corros Sci 2010; 52: 3687–3701.10.1016/j.corsci.2010.07.019Suche in Google Scholar
Lu F, Ma A, Jiang JH, Yang DH, Zhou Q. Review on long-period stacking-ordered structures in Mg-Zn-RE alloys. Rare Metals 2012; 31: 303–310.10.1007/s12598-012-0510-ySuche in Google Scholar
Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials 2007; 28: 1689–1710.10.1016/j.biomaterials.2006.11.042Suche in Google Scholar PubMed
Manivasagam G, Suwas S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater Sci Technol 2014; 30: 515–520.10.1179/1743284713Y.0000000500Suche in Google Scholar
Mao L, Yuan GY, Wang SH, Niu JL, Wu GH, Ding WJ. A novel biodegradable Mg-Nd-Zn-Zr alloy with uniform corrosion behavior in artificial plasma. Mater Lett 2012; 88: 1–4.10.1016/j.matlet.2012.08.012Suche in Google Scholar
Mario CD, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M, Deloose K, Heublein B, Rohde R, Kasese V, Ilsley C, Erbel R. Drug-eluting bioabsorbable magnesium stent. J Interven Cardiol 2004; 17: 391–395.10.1111/j.1540-8183.2004.04081.xSuche in Google Scholar PubMed
Matsuda M, Ii S, Kawamura Y, Ikuhara Y, Nishida M. Variation of long-period stacking order structures in rapidly solidified Mg97Zn1Y2 alloy. Mater Sci Eng A 2005; 393: 269–274.10.1016/j.msea.2004.10.040Suche in Google Scholar
Mueller WD, Nascimento ML, Mele MFL. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater 2010; 6: 1749–1755.10.1016/j.actbio.2009.12.048Suche in Google Scholar PubMed
Narayanan TSNS, Park IS, Lee MH. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: prospects and challenges. Prog Mater Sci 2014; 60: 1–71.10.1016/j.pmatsci.2013.08.002Suche in Google Scholar
Peng LM, Chang JW, Guo XW, Atrens A, Ding WJ, Peng YH. Influence of heat treatment and microstructure on the corrosion of magnesium alloy Mg-10Gd-3Y-0.4Zr. J Appl Electrochem 2009; 39: 913–920.10.1007/s10800-008-9739-4Suche in Google Scholar
Peng QM, Huang YD, Zhou L, Hort N, Kainer KU. Preparation and properties of high purity Mg-Y biomaterials. Biomaterials 2010; 31: 398–403.10.1016/j.biomaterials.2009.09.065Suche in Google Scholar PubMed
Peng QM, Li XJ, Ma N, Liu RP, Zhang HJ. Effects of backward extrusion on mechanical and degradation properties of Mg-Zn biomaterial. J Mech Behav Biomed Mater 2012; 10: 128–137.10.1016/j.jmbbm.2012.02.024Suche in Google Scholar PubMed
Peng QM, Guo JX, Fu H, Cai XC, Wang YN, Liu BZ, Xu ZG. Degradation behavior of Mg-based biomaterials containing different long-period stacking ordered phases. Sci Rep 2014; 4: 3620.10.1038/srep03620Suche in Google Scholar PubMed PubMed Central
Pound BG. Corrosion behavior of metallic materials in biomedical applications. I. Ti and its alloys. Corros Rev 2014a; 32: 1–20.10.1515/corrrev-2014-0007Suche in Google Scholar
Pound BG. Corrosion behavior of metallic materials in biomedical applications. II. Stainless steels and Co-Cr alloys. Corros Rev 2014b; 32: 21–41.10.1515/corrrev-2014-0008Suche in Google Scholar
Qiao ZX, Shi ZM, Hort N, Abidin NIZ, Atrens A. Corrosion behaviour of a nominally high purity Mg ingot produced by permanent mould direct chill casting. Corros Sci 2012; 61: 185–207.10.1016/j.corsci.2012.04.030Suche in Google Scholar
Ralston KD, Birbilis N. Effect of grain size on corrosion: a review. Corrosion 2010; 66: 075005.10.5006/1.3462912Suche in Google Scholar
Ralston KD, Birbilis N, Davies CHJ. Revealing the relationship between grain size and corrosion rate of metals. Scripta Mater 2010; 63: 1201–1204.10.1016/j.scriptamat.2010.08.035Suche in Google Scholar
Ren YB, Huang JJ, Yang K, Zhang BC, Yao ZM, Wang H. Study of bio-corrosion of pure magnesium. Acta Metall Sin 2005; 41: 1228–1232.Suche in Google Scholar
Rojaee R, Fathi M, Raeissi K. Controlling the degradation rate of AZ91 magnesium alloy via sol-gel derived nanostructured hydroxyapatite coating. Mater Sci Eng C 2013; 33: 3817–3825.10.1016/j.msec.2013.05.014Suche in Google Scholar PubMed
Salahshoor M, Guo YB. Biodegradation Control of magnesium-calcium biomaterial via adjusting surface integrity by synergistic cutting-burnishing. Procedia CIRP 2014; 13: 143–149.10.1016/j.procir.2014.04.025Suche in Google Scholar
Shi ZM, Cao FY, Song GL, Liu M, Atrens A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary Mg-RE alloys: RE=Ce, La, Nd, Y, Gd. Corros Sci 2013; 76: 98–118.10.1016/j.corsci.2013.06.032Suche in Google Scholar
Song GL, Atrens A. Corrosion mechanisms of magnesium alloys. Adv Eng Mater 1999; 1: 11–33.10.1002/(SICI)1527-2648(199909)1:1<11::AID-ADEM11>3.3.CO;2-ESuche in Google Scholar
Song GL, Atrens A, Dargusch M. Influence of microstructure on the corrosion of diecast AZ91D. Corros Sci 1999; 41: 249–273.10.1016/S0010-938X(98)00121-8Suche in Google Scholar
Song YW, Shan DY, Han EH. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomedical application. Mater Lett 2008; 62: 3276–3279.10.1016/j.matlet.2008.02.048Suche in Google Scholar
Song D, Ma AB, Jiang JH, Lin PH, Yang DH, Fan JF. Corrosion behaviour of bulk ultra-fine grained AZ91D magnesium alloy fabricated by equal-channel angular pressing. Corros Sci 2011; 53: 362–373.10.1016/j.corsci.2010.09.044Suche in Google Scholar
Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 2006; 27: 1728–1734.10.1016/j.biomaterials.2005.10.003Suche in Google Scholar
Taltavull C, Torres B, Lopez AJ, Rodrigo P, Otero E, Atrens A, Rams J. Corrosion behaviour of laser surface melted magnesium alloy AZ91D. Mater Des 2014; 57: 40–50.10.1016/j.matdes.2013.12.069Suche in Google Scholar
Virtanen S. Biodegradable Mg and Mg alloys: corrosion and biocompatibility. Mater Sci Eng B 2011; 176: 1600–1608.10.1016/j.mseb.2011.05.028Suche in Google Scholar
Waizy H, Weizbauer A, Maibaum M, Witte F, Windhagen H, Lucas A, Denkena B, Meyer-Lindenberg A, Thorey F. Biomechanical characterisation of a degradable magnesium-based (MgCa0.8) screw. J Mater Sci Mater Med 2012; 23: 649–655.10.1007/s10856-011-4544-8Suche in Google Scholar
Waksman R. Biodegradable stents: they do their job and disappear. J Invasive Cardiol 2006; 18: 70–74.Suche in Google Scholar
Wan YZ, Xiong GY, Luo HL, He F, Huang Y, Zhou XS. Preparation and characterization of a new biomedical magnesium-calcium alloy. Mater Des 2008; 29: 2034–2037.10.1016/j.matdes.2008.04.017Suche in Google Scholar
Wang H, Estrin Y, Zúberová Z. Bio-corrosion of a magnesium alloy with different processing histories. Mater Lett 2008; 62: 2476–2479.10.1016/j.matlet.2007.12.052Suche in Google Scholar
Wang J, Wang LG, Guan SK, Zhu SJ, Ren CX, Hou SS. Microstructure and corrosion properties of as sub-rapid body fluid for vascular stent application solidification Mg-Zn-Y-Nd alloy in dynamic simulated body fluid for vascular stent application. J Mater Sci Mater Med 2010; 21: 2001–2008.10.1007/s10856-010-4063-zSuche in Google Scholar PubMed
Wang YS, Lim CS, Lim CV, Yong MS, Teo EK, Moh LN. In vitro degradation behavior of M1A magnesium alloy in protein-containing simulated body fluid. Mater Sci Eng C 2011; 31: 579–587.10.1016/j.msec.2010.11.017Suche in Google Scholar
Wang JL, Tang J, Zhang P, Li YD, Wang J, Lai YX, Qin L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res B 2012; 100: 1691–1701.10.1002/jbm.b.32707Suche in Google Scholar PubMed
Wang ZQ, Li DJ, Zeng XQ, Wu XM, Ding WJ. Effects of heat treatments on corrosion behavior of Mg AT72 alloy. Mater Sci Forum 2013; 747: 230–237.10.4028/www.scientific.net/MSF.747-748.230Suche in Google Scholar
Willbold E, Kalla K, Bartsch I, Bobe K, Brauneis M, Remennik S, Shechtman D, Nellesen J, Tillmann W, Vogt C, Witte F. Biocompatibility of rapidly solidified magnesium alloy RS66 as a temporary biodegradable metal. Acta Biomater 2013; 9: 8509–8517.10.1016/j.actbio.2013.02.015Suche in Google Scholar PubMed
Witte F, Eliezer A. Biodegradable metals. In: Eliaz N, editor. Degradation of implant materials. New York: Springer, 2012: 93–109.10.1007/978-1-4614-3942-4_5Suche in Google Scholar
Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005; 26: 3557–3563.10.1016/j.biomaterials.2004.09.049Suche in Google Scholar PubMed
Witte F, Hort N, Vogt C, Cohen S, Kainer KU, Willumeit R, Feyerabend F. Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci 2008; 12: 63–72.10.1016/j.cossms.2009.04.001Suche in Google Scholar
Wu YJ, Lin DL, Zeng XQ, Peng LM, Ding WJ. Formation of a lamellar 14H-type long period stacking ordered structure in an as-cast Mg-Gd-Zn-Zr alloy. J Mater Sci 2009; 44: 1607–1612.10.1007/s10853-008-3213-xSuche in Google Scholar
Wu GS, Ibrahim JM, Chu PK. Surface design of biodegradable magnesium alloys – a review. Surf Coat Technol 2013; 233: 2–12.10.1016/j.surfcoat.2012.10.009Suche in Google Scholar
Xin Y, Hu T, Chu PK. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: a review. Acta Biomater 2011; 7: 1452–1459.10.1016/j.actbio.2010.12.004Suche in Google Scholar PubMed
Xu SW, Zheng MY, Kamado S, Wu K, Wang GJ, Lv XY. Dynamic microstructural changes during hot extrusion and mechanical properties of a Mg-5.0Zn-0.9Y-0.16Zr (wt.%) alloy. Mater Sci Eng A 2011; 528: 4055–4067.10.1016/j.msea.2011.01.103Suche in Google Scholar
Yamamoto A. Biomedical application of magnesium alloys. J Japan Inst Light Met 2008; 58: 570–576.10.2464/jilm.58.570Suche in Google Scholar
Yang JX, Jiao YP, Cui FZ, Lee IS, Yin QS, Zhang Y. Modification of degradation behavior of magnesium alloy by IBAD coating of calcium phosphate. Surf Coat Technol 2008; 202: 5733–5736.10.1016/j.surfcoat.2008.06.035Suche in Google Scholar
Zakiyuddin A, Yun K, Lee K. Corrosion behavior of as-cast and hot rolled pure magnesium in simulated physiological media. Met Mater Int 2014; 20: 1163–1168.10.1007/s12540-014-6022-6Suche in Google Scholar
Zhang GD, Huang JJ, Yang K, Zhang BC, Ai HJ. Experimental study of in vivo implantation of a magnesium alloy at early stage. Acta Metall Sin 2007; 43: 1186–1190.Suche in Google Scholar
Zhang SX, Zhang XN, Zhao CL, Li JN, Song Y, Xie CY, Tao HR, Zhang Y, He YH, Jiang Y, Bian YJ. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater 2010; 6: 626–640.10.1016/j.actbio.2009.06.028Suche in Google Scholar PubMed
Zhang JS, Xu JD, Cheng WL, Chen CJ, Kang JJ. Corrosion behavior of Mg-Zn-Y alloy with long-period stacking ordered structures. J Mater Sci Technol 2012a; 28: 1157–1162.10.1016/S1005-0302(12)60186-8Suche in Google Scholar
Zhang XB, Wang ZZ, Yuan GY, Xue YJ. Improvement of mechanical properties and corrosion resistance of biodegradable Mg-Nd-Zn-Zr alloys by double extrusion. Mater Sci Eng B 2012b; 177: 1113–1119.10.1016/j.mseb.2012.05.020Suche in Google Scholar
Zhang XB, Wu YJ, Xue YJ, Wang ZZ, Yang L. Biocorrosion behavior and cytotoxicity of a Mg-Gd-Zn-Zr alloy with long period stacking ordered structure. Mater Lett 2012c; 86: 42–45.10.1016/j.matlet.2012.07.030Suche in Google Scholar
Zhang XB, Yuan GY, Mao L, Niu JL, Ding WJ. Biocorrosion properties of as-extruded Mg-Nd-Zn-Zr alloy compared with commercial AZ31 and WE43 alloys. Mater Lett 2012d; 66: 209–211.10.1016/j.matlet.2011.08.079Suche in Google Scholar
Zhang XB, Yuan GY, Wang ZZ. Mechanical properties and biocorrosion resistance of Mg-Nd-Zn-Zr alloy improved by cyclic extrusion and compression. Mater Lett 2012e; 74: 128–131.10.1016/j.matlet.2012.01.086Suche in Google Scholar
Zhang XB, Ba ZX, Wang ZZ, He XC, Shen C, Wang Q. Influence of silver addition on microstructure and corrosion behavior of Mg-Nd-Zn-Zr alloys for biomedical application. Mater Lett 2013a; 100: 188–191.10.1016/j.matlet.2013.03.061Suche in Google Scholar
Zhang XB, Yuan GY, Fang XX, Wang ZZ, Zhang T. Effects of solution treatment on yield ratio and biocorrosion behaviour of as-extruded Mg-2.7Nd-0.2Zn-0.4Zr alloy for cardiovascular stent application. Mater Technol 2013b; 28: 155–158.10.1179/1753555712Y.0000000048Suche in Google Scholar
Zhang XB, Yuan GY, Wang ZZ. Biocorrosion properties of as-cast Mg-Nd-Zn-Zr magnesium alloy. Chin J Nonferrous Met 2013c; 23: 905–911.Suche in Google Scholar
Zhang XB, Yuan GY, Wang ZZ. Effects of extrusion ratio on microstructure, mechanical and corrosion properties of biodegradable Mg-Nd-Zn-Zr alloy. Mater Sci Technol 2013d; 29: 111–116.10.1179/1743284712Y.0000000107Suche in Google Scholar
Zhang XB, Ba ZX, Wang Q, Wu YJ, Wang ZZ, Wang Q. Uniform corrosion behavior of GZ51K alloy with long period stacking ordered structure for biomedical application. Corros Sci 2014a; 88: 1–5.10.1016/j.corsci.2014.07.004Suche in Google Scholar
Zhang XB, He XC, Xue YJ, Wang ZZ, Wang Q. Effects of Sr on microstructure and corrosion resistance in simulated body fluid of as cast Mg-Nd-Zr magnesium alloys. Corros Eng Sci Technol 2014b; 49: 345–351.10.1179/1743278213Y.0000000143Suche in Google Scholar
Zhang XB, He XC, Xue YJ, Wang ZZ, Wang Q. Microstructure and corrosion resistance of as-cast Mg-Nd-Gd-Sr-Zn-Zr alloys for biomedical applications. Mater Technol 2014c; 29: 179–187.10.1179/1753555714Y.0000000129Suche in Google Scholar
Zhang L, Zhang JH, Xu C, Jing YB, Zhuang JP, Wu RZ, Zhang ML. Formation of stacking faults for improving the performance of biodegradable Mg-Ho-Zn alloy. Mater Lett 2014d; 133: 158–162.10.1016/j.matlet.2014.06.171Suche in Google Scholar
Zhang XB, Wang Q, Chen FB, Wu YJ, Wang ZZ, Wang Q. Relation between LPSO structure and biocorrosion behavior of biodegradable GZ51K alloy. Mater Lett 2015; 138: 212–215.10.1016/j.matlet.2014.09.133Suche in Google Scholar
Zhao X, Shi LL, Xu J. A comparison of corrosion behavior in saline environment: rare earth metals (Y, Nd, Gd, Dy) for alloying of biodegradable magnesium alloys. J Mater Sci Technol 2013a; 29: 781–787.10.1016/j.jmst.2013.05.017Suche in Google Scholar
Zhao X, Shi LL, Xu J. Mg-Zn-Y alloys with long-period stacking ordered structure: in vitro assessments of biodegradation behavior. Mater Sci Eng C 2013b; 33: 3627–3637.10.1016/j.msec.2013.04.051Suche in Google Scholar PubMed
Zhao Y, Wu GS, Lu QY, Wu J, Xu RZ, Yeung KWK, Chu PK. Improved surface corrosion resistance of WE43 magnesium alloy by dual titanium and oxygen ion implantation. Thin Solid Films 2013c; 529: 407–411.10.1016/j.tsf.2012.05.046Suche in Google Scholar
Zhao ZW, Teng XY, Zhou GR, Leng JF, Geng JW. Effect of Mg-Zn-Nd quasicrystal addition on corrosion resistance of AZ91 alloys. Rare Metal Mat Eng 2014; 43: 791–795.10.1016/S1875-5372(14)60085-0Suche in Google Scholar
Zheng YF, Gu XN, Witte F. Biodegradable metals. Mater Sci Eng R 2014; 77: 1–34.10.1016/j.mser.2014.01.001Suche in Google Scholar
Zomorodian A, Garcia MP, Silva TM, Fernandes JCS, Fernandes MH, Montemor MF. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater 2013; 9: 8660–8670.10.1016/j.actbio.2013.02.036Suche in Google Scholar PubMed
©2015 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- In this issue
- Reviews
- Improvement of corrosion resistance of magnesium alloys for biomedical applications
- Environment-induced fatigue cracking behavior of aluminum alloys and modification methods
- On high-temperature oxidation and protection of 2:17-type SmCo-based magnets
- Corrosion behaviors of steels under supercritical CO2 conditions
- The natural gas industry: equipment, materials, and corrosion
- Original articles
- Modeling data acquisition during electrochemical noise measurements for corrosion studies
- Corrosion inhibition behavior of two quinoline chalcones: insights from density functional theory
Artikel in diesem Heft
- Frontmatter
- In this issue
- Reviews
- Improvement of corrosion resistance of magnesium alloys for biomedical applications
- Environment-induced fatigue cracking behavior of aluminum alloys and modification methods
- On high-temperature oxidation and protection of 2:17-type SmCo-based magnets
- Corrosion behaviors of steels under supercritical CO2 conditions
- The natural gas industry: equipment, materials, and corrosion
- Original articles
- Modeling data acquisition during electrochemical noise measurements for corrosion studies
- Corrosion inhibition behavior of two quinoline chalcones: insights from density functional theory

