False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
-
Angelika Hammerer-Lercher
, Christine K. Kissel
, Luca Bernasconi
, Robert Manka
Abstract
Objectives
Cardiac troponins (cTn) are used to detect and quantify acute cardiomyocyte injury. In patients presenting with symptoms that could indicate myocarditis, elevated cTn concentrations typically mandate cardiac catheterization and heart muscle biopsy or cardiac magnetic resonance imaging (CMR). Accordingly, increased cTn levels due to macrotroponin – a complex between patient anti-troponin autoantibodies and cTn – could lead to unnecessary and potentially harmful interventions. In athletes, ensuring cardiac health after infection like COVID-19 is critical, but the occurrence of false-positive cTn levels post-COVID-19 remains unknown.
Methods
This observational study prospectively included 35 healthy athletes (aged 16–75 years; 19 females, 16 males) who underwent post-COVID check-ups during 2022–2023. Athletes’ cTn levels were measured using four different hs-cTn routine immunoassays. If discrepancies were noted between assays, further testing for macrotroponin was conducted using protein G column, sucrose gradient ultracentrifugation and anti-troponin autoantibody immunoassay.
Results
Seventeen athletes had normal cTn levels across all assays, while 18 (51 %) had elevated cTn, mostly cTnI. Despite elevated cTn levels, no signs of myocarditis or other cardiac conditions were found on electrocardiography, echocardiography, or CMR. Macrotroponin was confirmed among 16 of these 18 athletes. Further, IgG anti-troponin autoantibodies correlated significantly with the levels of the two most-commonly affected assays: hs-cTnI-Siemens Atellica and hs-cTnI-Abbott Alinity.
Conclusions
Post-COVID-19, nearly half of athletes showed elevated cTnI levels due to interference from macrotroponin. Awareness among physicians and laboratorians of this analytical confounder can avoid unnecessary invasive or costly diagnostic tests in athletes with false-positive cTnI levels after COVID-19.
Introduction
Myocarditis is an inflammation of the heart muscle, often caused by viral infections of the upper respiratory tract or the gastrointestinal tract. This can potentially lead to dangerous sequelae such as life-threatening arrhythmia or heart failure, particularly if physical exercise is continued during active inflammation [1]. It is one of the potential causes of sudden cardiac death in athletes. Myocarditis diagnosis hence results in a sports restriction for 3–6 months [2], 3]. Symptoms can be unspecific, making early recognition and diagnosis challenging. Electrocardiograms (ECG) and cardiac troponins (cTn) are easily available tools to help evaluate patients with suspected myocarditis. Initial reports of COVID-19 suggested a high rate of myocardial involvement [4], [5], [6]. Therefore, sports medicine governing bodies issued recommendations for a safe return to sports in professional athletes after COVID-19 [7], 8]. Specific medical check-up criteria within 14 days after Covid-19 and before return to intensive sports were formulated based on the severity and symptoms of illness and laboratory results. Further tests, such as echocardiography, or cardiac magnetic resonance imaging (CMR) were recommended if required [2], 3], 9].
If echocardiography and CMR exclude myocardial inflammation or other cardiac involvement, the question arises whether the inflammation is below the detection-threshold of the imaging modality or whether elevated high-sensitivity (hs) cTn concentrations may be false-positive. The latter suspicion emerged because some laboratories utilized by chance two different cTn tests at the same time (hs-cTnI and hs-cTnT) with discordant results. A possible explanation for these discrepancies and increased values is a test interference with macrotroponin. Macrotroponin is a complex formed by endogenous anti-troponin autoantibodies and cardiac troponins. Beside other triggers, autoantibodies may develop upon cardiotropic viral infections, mostly enteroviruses, that set free tissue antigens and, thus, can stimulate the immune system to produce autoantibodies. This may also be true for the COVID-19 causing virus SARS-CoV-2. At moderately elevated levels, the half-life of cTnI and cTnT is approximately 2 h [10], 11] because of the dominant clearance by the kidneys. This clearance is most probably delayed when cTn are bound by anti-cTn antibodies forming macrotroponin complexes which results in accumulation and persistent elevations of cTn levels, although the cTn-release rate from myocardial tissue turnover remains normal. The prevalence of macrotroponin after COVID-19 is unclear. There are two systematic community studies before the COVID-19 pandemic [12], 13] and only limited case studies after COVID-19 [14], 15] that provide insight.
Our hypothesis was that macrotroponin complexes lead to unexpectedly elevated cTn levels in athletes. The occurrence of macrotroponin after COVID-19 in athletes has not been studied before. Therefore, we performed a prosepective study to investigate the presence of macrotroponin and anti-troponin auto-antibodies in athletes post-COVID-19 and the different impacts of macrotroponin on four widely used hs-cTn assays.
Materials and methods
Study design and population
We planned and conducted this observational study in accordance with the Helsinki Declaration and obtained approval by the Ethics Commission of Northwestern and Central Switzerland (project ID 2022-00507). We prospectively followed-up on 35 athletes aged 16 years or older presenting to the recommended investigation before returning to training, at least seven days after COVID-19 between January 2022 and January 2024. Athletes were instructed to withhold exercise before clearance was given by the sports physician. In case of later blood draws, athletes were advised to refrain from training for 48 h. If athletes presented with symptoms suggestive for cardiac involvement or with abnormal ECG and/or laboratory results, they additionally underwent echocardiography. If suspicion persisted, a CMR was performed. Symptoms at time of infection were recorded in addition to sport discipline and concurrent diseases. Plasma samples were sent from the sports center to its local laboratory, where hs-cTnI was routinely measured with an Alinity i analyzer (Abbott Diagnostics). Aliquots were frozen and transferred on dry ice to the central laboratory (Cantonal Hospital Aarau, Switzerland), where samples were stored at −80° C until further assessment or further transferal on dry ice to additional analysis locations.
Cardiac troponin assays and definition of macrotroponin work-up
The samples were measured with three hs-cTnI assays (Alinity i/Abbott Diagnostics, Atellica Solution/Siemens Healthineers, Access 2/Beckman Coulter) and a hs-cTnT assay (Cobas/Roche Diagnostics). Performance characteristics of assays are summarized in Supplementary Table 1. Athletes having a result above the sex-specific cut-off of a specific assay were considered having a positive result with that assay. Those athletes having a positive result with at least one assay or a positive hs-cTn result despite absence of clinical suspicion of cardiac involvement were selected for further investigation of the presence of macrotroponin with sucrose gradient, protein G spin column and anti-troponin autoantibody immunoassay methods.
The technicians performing the assay measurements were blinded to further clinical or laboratory data. All assays were run according to the manufacturer’s instructions and underwent internal and external quality controls.
Sucrose gradient ultracentrifugation
The sucrose density gradient ultracentrifugation is a reference method to separate proteins of different molecular weights. We used this method as previously described [16] to investigate macrotroponin. The applied molecular weight indicators were ferritin (480 kDa) indicating high molecular weight to mark the region of macrotroponin accumulation, and thyroid stimulating hormone (28 kDa) indicating low molecular weight, where free cTn is expected to accumulate. To be able to determine a possible macrotroponin I, hs-cTnI was measured in each of the seven gradient fractions obtained with the assays that gave increased hs-cTnI in the original blood sample. In one athlete with low hs-cTn values and limited sample volume only four gradient fractions were done. Because of limited volumes in the gradient fractions in general, it was not possible to additionally investigate for macrotroponin T with the hs-cTnT assay. Therefore, if not stated otherwise, the term macrotroponin refers to macrotroponin I in this study.
Protein G spin column
As a second method to identify macrotroponin we used the published protein G spin column method [16] with a slight modification (more details in the supplemental methods). This method depletes all immunoglobulin G (IgG) molecules from the sample including the anti-troponin autoantibody – troponin complexes, the macrotroponin. The previously published cut-off of <40 % recovery after immunoglobulin depletion was used to define macrotroponin [13], 17].
Anti-troponin autoantibody immunoassay
The presence of anti-troponin autoantibodies was determined with a sandwich-type immunoassay as previously described [18] (more details in the supplemental methods). The calculated positive assay signals reflect the titer and affinity of the anti-troponin autoantibodies in the circulation.
Statistical analysis
Data are presented as median and interquartile range. Mann-Whitney-U test was used for group comparison. Chi-Squared test and Spearman’s rank correlation coefficient were calculated for associations between macrotroponin presence and anti-troponin autoantibody. A p-value <0.05 was regarded statistically significant. For power calculation see supplemental file. Statistics were calculated using SPSS 30.0.0.0 (IBM, Armonk, IL, USA) for Windows and figures were drawn in Excel (Microsoft Corp., Redmond, WA, USA) for Microsoft 365 and R 4.4.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Population characteristics
This study included 35 athletes with median age of 24 years (16–75 years), among them 19 (54 %) females, at the first post-COVID-19 visit. If symptoms were present, they consisted mostly of postviral exhaustion, mild infectious symptoms or thoracic pain (Table 1). SARS-CoV-2 infection was defined as a positive antigen or polymerase chain reaction test. C-reactive protein was not increased and most of the chemistry biomarkers and blood count parameters were not significantly different between athletes with and without macrotroponin (Supplementary Table 2). Four athletes had secondary diagnoses unrelated to this investigation (supplemental results). Athletes commonly presented with sinus rhythm and, except for one athlete, with no repolarization abnormalities. Twenty-two athletes showed signs of training-induced left ventricular hypertrophy in the ECG, without correlate on CMR (Table 1 and more detailed ECG/imaging findings in Supplementary Table 3). The three most common sports of the athletes were cycling (43 %, mountain biking or road biking), soccer (16 %) and marathon/tri-/ultra-/gigathlon (14 %); other sports comprised climbing, curling, swimming, floorball, cross-country skiing or rhythmic gymnastics.
Characteristics of all athletes in the study group as well as according to the presence or absence of macrotroponin.
| Overall (n=35) | Without macrotroponin (n=19) | With macrotroponin (n=16) | p-Value (with vs. without macrotroponin) | |
|---|---|---|---|---|
| Age, years | 24 (20; 38) | 26 (22; 48) | 22 (20; 26) | 0.088 |
| Females, % | 54 | 47 | 63 | n.s. |
| BMI | 21 (20; 22) | 21 (20; 23) | 21 (20; 22) | n.s. |
| Symptomsa, n | ||||
| Upper respiratory tract infection | 15 | 12 | 3 | |
| Fever or subfebrile temperature | 7 | 5 | 2 | |
| Headache | 5 | 4 | 1 | |
| Myalgia | 6 | 5 | 1 | |
| Chest pain | 10 | 4 | 6 | |
| Gastrointestinal | 1 | 0 | 1 | |
| No symptoms/exhaustion | 9 | 3 | 6 | |
| ECG | ||||
| Repolarization abnormalities | 1 (n=34) | 0 (n=19) | 1 (n=15) | n.a. |
| Echocardiography | ||||
| LVEF % | 63 (61; 66) (n=21) | 64 (63; 66) (n=8) | 63 (58; 66) (n=13) | n.s. |
| GLS %, GE | −20 (−22; −18) (n=11) | n.a. | −21 (−22; −19) (n=9) | n.a. |
| CMR | ||||
| LVEF % | 58 (53; 65) (n=13) | n.a. | 58 (54; 65) (n=9) | n.a. |
| LVEDVI, mL/m2 | 99 (86; 107) (n=12) | n.a. | 91 (80; 106) (n=9) | n.a. |
| hs-cTn | ||||
| hs-cTnI (Atellica; ng/L) | 19 (3.8; 78) | 4.0 (2.5; 12) | 70 (41; 169) | <0.001 |
| hs-cTnI (Alinity; ng/L) | 10 (2.0; 43) | 4.0 (2.0; 10) | 41 (15; 82) | <0.001 |
| hs-cTnI (Access; ng/L) | 3.3 (1.6; 8.3) | 2.9 (1.5; 5.0) | 6.0 (2.8; 25) | 0.088 |
| hs-cTnT (Elecsys; ng/L) | 6.0 (4.0; 8.0) | 5.0 (4.0; 7.0) | 6.0 (4.3; 10) | n.s. |
-
Data were not consistently available for all athletes; therefore, the number of cases is provided in additional parentheses. The p-values were calculated between the groups with and without macrotroponin. Values as median (25th; 75th percentile); n.a., due to insufficient data (n<5). aSome symptoms were overlapping. n.s., not significant; BMI, body mass index; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; GE, gradient echocardiography; CMR, cardiac magnetic resonance imaging; LVEDVI, left ventricular end diastolic volume index.
Cardiac troponin assay results
Seventeen (17/35) athletes had hs-cTn concentrations within the sex-specific cut-off values in all four assays and did not undergo further investigation for macrotroponin. In the remaining 18 athletes (18/35) hs-cTn was increased in at least one assay which was mostly a hs-cTnI method, and three out of them showed increases in all four assays.
Macrotroponin detection with sucrose gradient ultracentrifugation
We applied the reference method sucrose-gradient ultracentrifugation in the 18 athletes presenting with discrepancies between hs-cTn results or between hs-cTn and clinical presentation at the first post-COVID-19 check-up visit (Figure 1). Of these athletes 16 were found positive for macrotroponin while two were found negative. Of these two macrotroponin negative athletes, one had increased hs-cTn in all four assays but normal hs-cTn concentrations in a control measurement two days later (Figure 1, neg1). The other macrotroponin negative athlete had solely borderline hs-cTnT increase at first visit (male 16 ng/L; URL<16 ng/L). The low hs-cTnT values did not allow investigating for a possible macrotroponin T, but no macrotroponin I was detected (Figure 1, neg2). Therefore, these two athletes without detected macrotroponin I were allocated into the non-macrotroponin group resulting in 16 with and 19 without macrotroponin (Supplementary Figure 1).
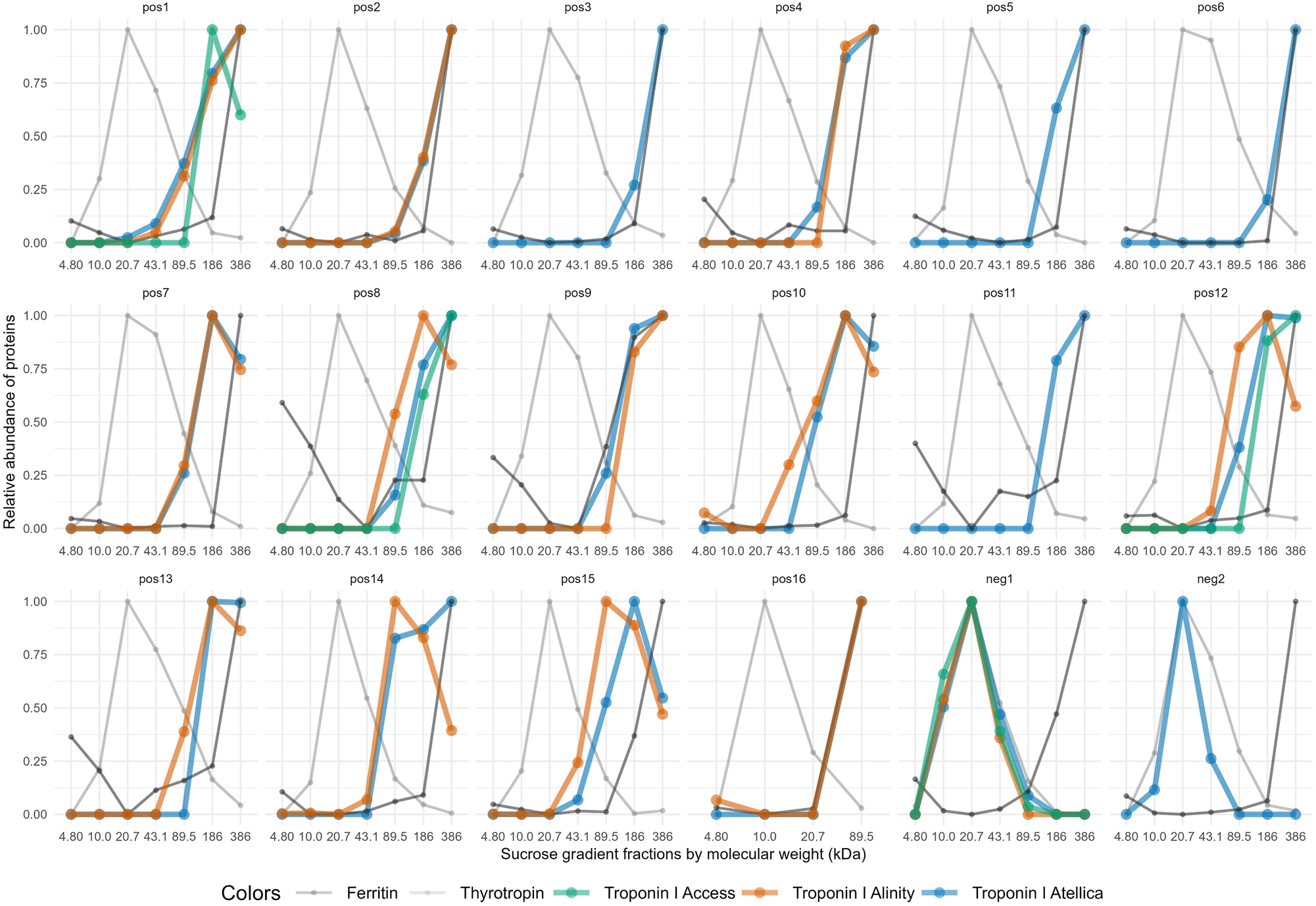
Macrotroponin results by sucrose gradient ultracentrifugation. Athletes with discordant hs-cTn results (n=18) were assessed for the presence of macrotroponin using molecular weight-based fractions of the sucrose gradient ultracentrifugation method. Macrotroponin was assigned to high molecular weight fractions (89.5–386 kDa), which were indicated by the high molecular weight marker ferritin (480 kDa). In contrast, free hs-cTn was associated with lower molecular weight fractions (10.0–43.1 kDa), where thyrotropin (28 kDa) was detected. In general, those hs-cTnI assays that showed increased concentrations in the original sample were applied for this assessment. Among athletes suspected of having macrotroponin, 16 were positive (pos1 – pos16) and two negative for macrotroponion (neg1 – neg2).
The corresponding hs-cTn values of the first check-up visits are outlined in Table 1. Multiples of cut-offs and frequencies of hs-cTn increases above the sex-specific cut-off values in athletes with macrotroponin are shown in Table 2.
Multiple of cut-offs of hs-cTn above the sex-specific upper reference limit (URL) and occurrence of values above URL for each assay in macrotroponin positive athletes (n=16).
| MOC of hs-cTn above URL | Cases with hs-cTn above URL, % | Cases with hs-cTn above URL (n of total 16 cases) | |
|---|---|---|---|
| hs-cTnI (Atellica) | 1.88 (0.95; 4.96) | 93 | 14a |
| hs-cTnI (Alinity) | 2.10 (0.65; 3.68) | 75 | 12 |
| hs-cTnI (Access) | 0.36 (0.23; 1.58) | 31 | 5 |
| hs-cTnT (Roche) | 0.56 (0.40; 0.67) | 31 | 5 |
-
The multiple of cut-offs (MOC) was calculated by normalizing the hs-cTn value according to the sex-specific URL and is given as median (25th; 75th percentile). aDenotes one missing value for Atellica hs-cTnI. n, number.
Macrotroponin detection with protein G spin column method
The additional investigation of macrotroponin by the protein G spin column method in the athletes with hs-cTn increases (n=18) gave congruent results with the reference method achieving 16 macrotroponin positive athletes (median hs-cTnI recovery values: 17 %; min/max recoveries: 2.4 %; 39 %; n=16/18).
The same two athletes (2/18) who were not confirmed to have macrotroponin using the reference method sucrose gradient ultracentrifugation did not show any evidence for macrotroponin by this method either (recoveries: 99 % and 144 % of the original cTn measurement). There was one athlete with sufficiently high hs-cTnT concentration (77 ng/L) and sample volume to search for both, macrotroponin I and T. Despite the presence of macrotroponin I (recovery 12 %), macrotroponin T (recovery 101 %) was not detectable. Two athletes with borderline hs-cTnT increases (male 16 ng/L, female 14 ng/L) that could not be investigated for macrotroponin T by the reference method due to low concentrations, gave uncertain results by the protein G method for macrotroponin T. However, macrotroponin I was detected in one of them by this method as well.
Anti-troponin autoantibodies
The anti-troponin IgG autoantibodies of first-visit samples (n=34, one missing value) were significantly associated with macrotroponin in the crosstable calculation (chi-square test χ2=30.2 at 1 degree of freedom, p<0.001, Phi-coefficient = 0.942, all expected cell frequencies were ≥5; Table 3). One athlete revealed anti-troponin autoantibodies despite normal hs-cTn concentrations. Spearman’s Rho coefficients showed highly significant correlations of anti-troponin autoantibody assay signals with hs-cTnI Siemens Atellica (r=0.601, p<0.001) and hs-cTnI Abbott Alinity (r=0.472, p=0.005), but not with hs-cTnI Beckman Access or hs-cTnT Roche Cobas.
Crosstables for anti-troponin autoantibodies and macrotroponin in athletes at their return to sports check-up first visit.
| Macrotroponin | |||
|---|---|---|---|
| Anti-troponin autoantibody | Negative | Positive | Sum |
| Negative | 18 | 0 | 18 |
| Positive | 1 | 15 | 16 |
| Sum | 19 | 15 | 34 |
-
First-visit samples of athletes were assessed for anti-troponin autoantibodies with a research immunoassay specific for IgG autoantibodies against troponin. Both, autoantibodies and macrotroponins were present in the same sample in all but one case who had anti-troponin autoantibodies despite normal hs-cTn concentrations. There is one missing case because of lack of sample volume at the first post-COVID-19 check-up for anti-troponin autoantibody assessment.
Follow-up of cases
Among all athletes, there were six follow-up cases due to elevated cTn levels including four athletes in the macrotroponin and two in the non-macrotroponin group. The hs-cTn values together with the concomitant results of macrotroponin and anti-troponin autoantibodies at the different timepoints are outlined in Figure 2. Among the macrotroponin positive cases (pos1, 7, 12 and 15), macrotroponin was present at all short- or long-term timepoints in all but one (pos1). However, in this latter case anti-troponin autoantibodies were still detectable at the subsequent visit. In another case (pos7) anti-troponin autoantibodies were absent at follow-up despite evidence of macrotroponin and increased hs-cTn levels in the first three follow-up samples. In the mactrotroponin negative group, one follow-up athlete (neg1) had elevated hs-cTn levels at the initial visit, normalizing within two days. Another athlete (neg3) consistently showed hs-cTn values within the reference range, precluding macrotroponin assessment; but anti-troponin autoantibodies were undetectable either.
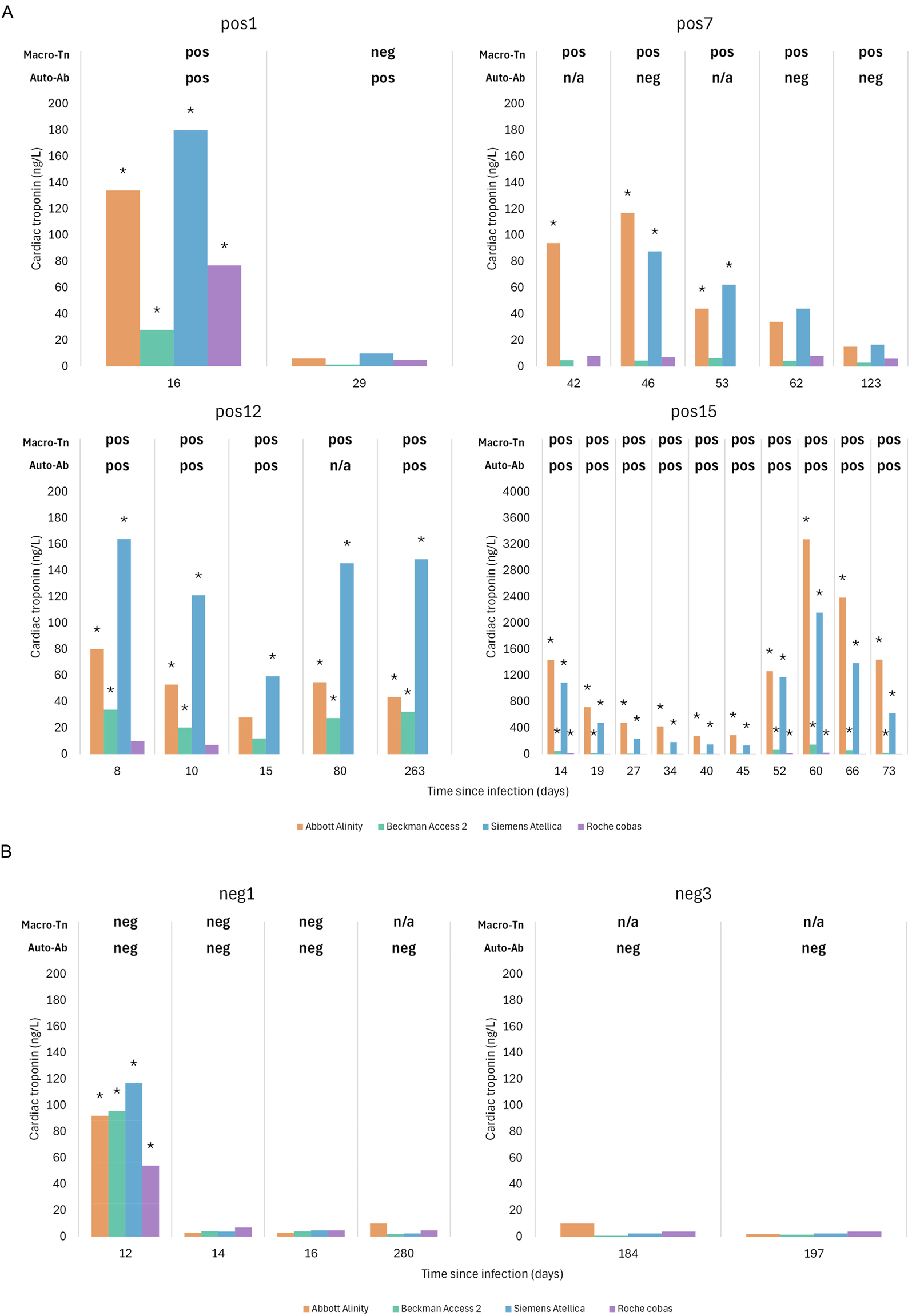
Athletes with follow-up visits. Out of six athletes with available follow-up data four were in the macrotroponin positive group (A) and two in the macrotroponin negative group (B). The graphs summarize the presence of macrotroponin, anti-troponin autoantibodies and the hs-cTn levels. The labelling of athletes corresponds to that of Figure 1 using pos (pos1, pos7, pos12 and pos15) for those suspected of having macrotroponin and neg (neg1, neg3) for those in the macrotroponin negative group. *Significant increase of hs-cTn above the sex-specific upper reference limit; Macro-Tn, macrotroponin; Auto-Ab, anti-troponin autoantibody; n/a, not applicable; neg, negative; pos, positive.
Discussion
Key study findings
To the best of our knowledge, this is the first prospective study investigating false-positive hs-cTn levels due to macrotroponin in athletes after COVID-19. We applied several methods to ascertain our findings in 35 healthy athletes post-COVID-19. First, we showed that macrotroponin was present in most cases with discordant hs-cTnI concentrations. Second, congruent results to the reference method sucrose gradient ultracentrifugation were achieved with the protein G spin column method, offering a less labor-intensive alternative for routine laboratory use. Third, IgG anti-troponin autoantibodies, a prerequisite for the formation of the macrotroponin complex, were detected in almost all cases where macrotroponin was found. Fourth, these antibodies correlated significantly with elevated hs-cTnI levels (Siemens Atellica and Abbott Alinity) drawing connection between the interference, the titer, and affinity of anti-troponin autoantibodies. Fifth, macrotroponin was detectable for long time periods, even at the latest follow-up point of 263 days, and clinicians as well as laboratorians should be aware of this. This is particularly of relevance in otherwise healthy people, such as athletes who may be falsely excluded from sports, potentially causing severe career implications.
Consistency of evidence and novelty
Beside case studies of macrotroponin in pediatric and adult patients, mostly independently of COVID-19 [14], 15], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], there are two systematic studies using leftover samples of laboratory requests before the COVID-19 pandemic [12], 13]. These two studies identified macrotroponin in 5 % of 1,074 and 55 % of 223 leftovers, respectively, using different approaches for the work-up. Warner et al. [12] investigated macrotroponin only in samples with discordant hs-cTnI according to two different methods (Architect hs-cTnI vs. VitrosTnI hs-cTnI (n=56)) and used three detection techniques (polyethylene glycol precipitation, protein A/G/L treatment and sephacryl gel filtration chromatography) for the proof of macrotroponin. On the other hand, Lam et al. [13] evaluated all samples with one detection technique (protein A spin column) to conclude for macrotroponin. They [13] showed that macrotroponin caused increases above URL in all cases with the hs-cTnI Siemens Centaur assay and to a lesser extent with hs-cTnI Abbott Architect (41 %), hs-cTnI Beckman Access (28 %) and hs-cTnT Roche Elecsys (28 %) [13]. In our study, the trigger to further investigate macrotroponin with specific methods was a discordant hs-cTn result among the four assays or a discrepancy between hs-cTn result and clinical presentation. Similarly to the study by Lam et al. [13], we observed increased hs-cTnI concentrations in all but one macrotroponin case with the Atellica assay, the recent generation of the Siemens immunoanalyzers, which has several components of the Centaur method integrated. Concerning the Abbott Alinity cTnI assay [13], we found a higher percentage of increased hs-cTnI values than Lam et al. [13]. However, we had less cases and used sex specific cut-offs to determine increased hs-cTn concentrations, which may partly account for these slightly different findings. Immunoassays differ in their epitope targeting, as manufacturers utilize different assay antibodies binding to distinct regions of the troponin molecule for capture and detection. Anti-troponin autoantibodies may form macrotroponin complexes that mask specific epitopes, thereby interfering with some assays while sparing others. Consequently, susceptibility to interference is assay-dependent.
Similarly to Lam et al. [13], in our study macrotroponin seemed to affect cTnT less frequently and macrotroponin I was detected even in some samples with very low cTn levels. We focused on macrotroponin I using hs-cTnI assays due to limited gradient fraction volumes of the reference method and low concentrations. Above all, hs-cTnT values were less frequently elevated than hs-cTnI by Atellica or Alinity, with isolated hs-cTnT elevations in only two athletes. Macrotroponin T interference appears rare, with Lam et al. [13] reporting only 2 % of cases in their systematic study. In the three athletes, in which we were able to additionally investigate macrotroponin T by the protein G spin column, one was negative and two gave uncertain results. One of these uncertain macrotroponin T results nevertheless turned out with a positive macrotroponin I by the sucrose gradient ultracentrifugation and protein G spin column method, although the first visit hs-cTnI concentrations were within the reference limits. The anti-troponin autoantibody result was also consistently positive, further supporting the presence of macrotroponin. Notably, in this athlete, with limited sample volume and low hs-cTnI concentration, performing only four gradient fractions nonetheless yielded clear results, partly due to higher analyte concentrations compared to the standard seven-fraction protocol used for all others in this study. The standard seven-fractions protocol ensures maximum separation of macro- and non-macrotroponin. Our finding suggests that macrotroponin I may even exist in small amounts below the URL of an hs-cTnI assay. This warrants further investigations, as macrotroponin may impact preventive risk-assessments in asymptomatic individuals, particularly if manufacturers adopt lower cut-offs like those of the Abbott Alinity hs-cTnI Risk Strat assay.
Whether macrotroponin itself has a prognostic impact is not fully elucidated currently. One study showed a favorable prognosis in the presence of macrotroponin in patients with increased hs-cTnI values and demonstrated an adjusted hazard ratio for all-cause mortality of 0.54 and for cardiovascular disease mortality of 0.48 [36]. However, the athletes of this study were healthy or had unremarkable adaptive findings in imaging which are common in elite athletes as also shown by Schneeweis et al. [37].
Beside the proof of macrotroponin complex as the cause of false-positive hs-cTn results, we corroborated the simultaneous presence of anti-troponin autoantibodies themselves, which are mandatory in the complex-formation with troponin. There were only three exceptions: One macrotroponin positive athlete (Figure 2A, pos7) was negative for anti-troponin autoantibodies. This discrepancy may reflect methodological differences. The sucrose gradient detects large molecules, while the protein G column identifies IgG-containing molecules and complexes, which means that both can produce positive results also for crosslinking heterophilic antibodies. On the other hand, the anti-troponin autoantibody assay, that relies on specific cTnI epitopes, may yield false negatives if autoantibodies mask these binding sites. In contrast, anti-troponin autoantibodies were found in one athlete with too low hs-cTn concentrations to generate conclusive macrotroponin results and in another (Figure 2A, pos1) with negativity for macrotroponin in the follow-up. There might have been too few cardiac troponin molecules in the blood to form measurable complexes with autoantibodies, particularly when cTn concentrations were in very low ranges.
In follow-up samples hs-cTn concentrations decreased in all athletes with macrotroponin, except for one, who may have had a symptomless SARS-CoV-2 or other infection. Other cardiotropic viruses could also trigger macrotroponin which warrants investigations in future studies, as this was beyond the scope of the present work. The precise mechanisms driving anti-troponin autoantibody formation remain incompletely understood. We propose that transient post-exercise elevations in circulating cardiac troponins may act as antigenic stimuli as also hypothesized in a recent review [38]. In the setting of COVID-19, such exposures – particularly when recurrent, as in endurance athletes – could facilitate immune recognition and the development of anti-troponin autoantibodies. Notably, not all hs-cTnI assays fell below the reference ranges within the followed period which could have a tremendous impact, particularly on athletes who may have several control visits at their sports physicians because of intensification of training or participation in competitions.
Policy implications
In case hs-cTn is increased, uncertainty about the athlete’s health leads to further costly and timely imaging investigations that could be avoided in case there was knowledge of the presence of macrotroponin. Therefore, awareness of this issue is required, not only among sports physicians but also among other physicians, clinicians and laboratorians. To address this interference, an alternative hs-cTn method such as hs-cTnT should be used when hs-cTnI is elevated. Given that most laboratories lack both assays, inter-laboratory collaboration is advisable. While dilution series and heterophilic antibody blocking reagents are not highly effective for detecting macrotroponin, they are recommended initial steps for suspected interferences in general [39], 40]. For routine macrotroponin detection, the protein G spin column offers a feasible alternative to the reference method and should be established in at least one collaborating laboratory.
Our findings extend and corroborate prior research indicating that macrotroponin and heterophilic antibodies are important analytical confounders for hs-cTnI, but not for hs-cTnT. In contrast, chronic active skeletal muscle disease has been shown to be an important confounder for hs-cTnT, but not hs-cTnI [41]. Accordingly, measuring the alternative analyte is very helpful whenever clinicians are concerned by unexpectedly high hs-cTn concentrations [39], 40].
Strengths and limitations
The first strength is that we used sucrose gradient ultracentrifugation as a labor-intensive reference method to define macrotroponin status, also validating the less demanding protein G column method. Second, we used four widely applied assays, revealing varying susceptibility to macrotroponin. Third, we measured anti-troponin autoantibodies as an indirect proof and prerequisite of macrotroponin.
There are some limitations we want to state. First, it was not possible to study athletes with hs-cTn values below the sex-specific URL with the sucrose gradient as the sensitivity of the method is limited. Second, we had only Caucasian athletes participating in this study. However, we believe that our findings on macrotroponin occurrence following COVID-19 are broadly applicable to other ethnic groups, because the immune system not only triggers antibody production after viral infection in athletes but is a fundamental defending system in humans. Third, we cannot draw epidemiological conclusions because of the observational prospective study setting in a real-life environment. Athletes were not enrolled consecutively and some declined return-to-sports examinations due to concerns about potential sports exclusion.
Conclusions
Post-COVID-19, nearly half of the investigated athletes showed elevated cTnI levels due to interference from macrotroponin. Awareness of this analytical confounder can avoid unnecessary invasive or costly diagnostic tests in athletes with elevated cTnI levels after COVID-19.
Funding source: Universitätsspital Basel
Funding source: LUA/ALF funding at the Sahlgrenska University Hospital
Funding source: Riksförbundet HjärtLung
Funding source: Cancerfonden
Funding source: Universität Basel
Funding source: Forschungsrat des Kantonsspitals Aarau
Award Identifier / Grant number: 1410.000.187
Funding source: Sydäntutkimussäätiö
Acknowledgments
We thank the athletes who participated in this study, Martina Hanke for local study management and performing the routine laboratory tests at the Cantonal Hospital Aarau, Kathrin Meissner for organizing and performing hs-cTnT tests at the University of Basel as well as Franja Dugar, Vanessa Thommen, Rebekka Odermatt for data entry into RedCap at the University of Basel.
-
Research ethics: We planned and conducted this observational study in accordance with the Helsinki Declaration and obtained approval by the Ethics Commission of Northwestern and Central Switzerland (project ID 2022-00507).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: AHL reports speaker honoraria from Abbott Diagnostics, Beckman Diagnostics and Siemens Healthineers. SW declares research funding from Business Finland, research grants from the Turku University Foundation and the Varsinais-Suomi Regional Fund of the Finnish Cultural Foundation, support for travel and meetings from Finnish Society of Clinical Chemistry and International Federation of Clinical Chemistry and Laboratory Medicine. RM reports speaker honoraria from Philips, Siemens Healthineers, Bayer and Bristol Myers Squibb. EK declares speaker honoraria/consulting honoraria from Boehringer Ingelheim and Abbott, outside the submitted work, and all paid to the institution. EK and CM received financial support from the University of Basel and the University Hospital Basel as well as research support from the Swiss National Science Foundation; CM additionally received research support from the Swiss Heart Foundation, Innosuisse, the University Hospital Basel, the University of Basel, Beckman Coulter, Biomerieux, Brahms, Idorsia, Mitsubishi, Novartis, Ortho Clinical, Quidel, Roche, Siemens, Singulex, SpinChip, Sphingotec, and Upstream, as well as speaker honoraria/consulting honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, BMS, Idorsia, Novo-Nordisk, Osler, Novartis, Roche, Siemens, SpinChip, and Singulex, outside the submitted work, and all paid to the institution. OH received financial support from the Swedish Cancer Society, the Swedish Heart and Lung Foundation, and LUA/ALF funding at the Sahlgrenska University Hospital. OH also reports honoraria from Siemens Healthineers and LumiraDX and has stock options from AligendBio.
-
Research funding: This study was supported by research grants from the Research Council at the Cantonal Hospital Aarau/Switzerland (#1410.000.187), the University of Basel, the University Hospital Basel, the Finnish Foundation for Cardiovascular Research, the Swedish Cancer Society, the Swedish Heart and Lung Foundation, and LUA/ALF funding at the Sahlgrenska University Hospital. Abbott Diagnostics, Beckman Coulter and Siemens Healthineers provided reagents free of charge, yet, they had no influence on study design, conduction, interpretation, or writing the manuscript.
-
Data availability: Data are available upon reasonable request from the authors.
References
1. Van Name, J, Wu, K, Xi, L. Myocarditis – a silent killer in athletes: comparative analysis on the evidence before and after COVID-19 pandemic. Sports Med Health Sci 2024;6:232–9. https://doi.org/10.1016/j.smhs.2024.03.003.Search in Google Scholar PubMed PubMed Central
2. Pelliccia, A, Sharma, S, Gati, S, Bäck, M, Börjesson, M, Caselli, S, et al.. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. https://doi.org/10.1093/eurheartj/ehaa605.Search in Google Scholar PubMed
3. Writing, C, Gluckman, TJ, Bhave, NM, Allen, LA, Chung, EH, Spatz, ES, et al.. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–56, https://doi.org/10.1016/j.jacc.2022.02.003.Search in Google Scholar PubMed PubMed Central
4. Puntmann, VO, Carerj, ML, Wieters, I, Fahim, M, Arendt, C, Hoffmann, J, et al.. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–73. https://doi.org/10.1001/jamacardio.2020.3557.Search in Google Scholar PubMed PubMed Central
5. Rajpal, S, Tong, MS, Borchers, J, Zareba, KM, Obarski, TP, Simonetti, OP, et al.. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116–8. https://doi.org/10.1001/jamacardio.2020.4916.Search in Google Scholar PubMed PubMed Central
6. Brito, D, Meester, S, Yanamala, N, Patel, HB, Balcik, BJ, Casaclang-Verzosa, G, et al.. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imag 2021;14:541–55. https://doi.org/10.1016/j.jcmg.2020.10.023.Search in Google Scholar PubMed PubMed Central
7. Bhatia, RT, Marwaha, S, Malhotra, A, Iqbal, Z, Hughes, C, Börjesson, M, et al.. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology and exercise of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2020;27:1242–51. https://doi.org/10.1177/2047487320930596.Search in Google Scholar PubMed PubMed Central
8. Steinacker, JM, Schellenberg, J, Bloch, W, Deibert, P, Friedmann-Bette, B, Grim, C, et al.. Recommendations for return-to-sport after COVID-19: expert consensus. Dtsch Z Sportmed 2022;73:127–36.Search in Google Scholar
9. Löllgen, H, Bachl, N, Papadopoulou, T, Shafik, A, Holloway, G, Vonbank, K, et al.. Recommendations for return to sport during the SARS-CoV-2 pandemic. BMJ Open Sport Exerc Med 2020;6:e000858. https://doi.org/10.1136/bmjsem-2020-000858.Search in Google Scholar PubMed PubMed Central
10. Kristensen, JH, Hasselbalch, RB, Strandkjær, N, Jørgensen, N, Østergaard, M, Møller-Sørensen, PH, et al.. Half-life and clearance of cardiac troponin I and troponin T in humans. Circulation 2024;150:1187–98. https://doi.org/10.1161/circulationaha.123.066565.Search in Google Scholar
11. Starnberg, K, Fridén, V, Muslimovic, A, Ricksten, SE, Nyström, S, Forsgard, N, et al.. A possible mechanism behind faster clearance and higher peak concentrations of cardiac troponin I compared with troponin T in acute myocardial infarction. Clin Chem 2020;66:333–41. https://doi.org/10.1093/clinchem/hvz003.Search in Google Scholar PubMed
12. Warner, JV, Marshall, GA. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med 2016;54:1821–9. https://doi.org/10.1515/cclm-2015-1276.Search in Google Scholar PubMed
13. Lam, L, Aspin, L, Heron, RC, Ha, L, Kyle, C. Discrepancy between cardiac troponin assays due to endogenous antibodies. Clin Chem 2020;66:445–54. https://doi.org/10.1093/clinchem/hvz032.Search in Google Scholar PubMed
14. Kempton, H, Jones, G, McCready, M, Kovacic, J. Macrotroponin in the COVID-19 era: an under-recognised cause of persistent troponin elevation. Heart Lung Circ 2024;33:1147–50. https://doi.org/10.1016/j.hlc.2024.03.007.Search in Google Scholar PubMed
15. Bularga, A, Oskoui, E, Fujisawa, T, Jenks, S, Sutherland, R, Apple, FS, et al.. Macrotroponin complex as a cause for cardiac troponin increase after COVID-19 vaccination and infection. Clin Chem 2022;68:1015–9. https://doi.org/10.1093/clinchem/hvac100.Search in Google Scholar PubMed PubMed Central
16. Hammarsten, O, Becker, C, Engberg, AE. Methods for analyzing positive cardiac troponin assay interference. Clin Biochem 2023;116:24–30. https://doi.org/10.1016/j.clinbiochem.2023.03.004.Search in Google Scholar PubMed
17. Lam, L, Heron, C, Aspin, L, Ha, L, Kyle, CV. Change in troponin concentrations in patients with macrotroponin: an in vitro mixing study. Clin Biochem 2020;85:43–8. https://doi.org/10.1016/j.clinbiochem.2020.08.012.Search in Google Scholar PubMed
18. Savukoski, T, Ilva, T, Lund, J, Porela, P, Ristiniemi, N, Wittfooth, S, et al.. Autoantibody prevalence with an improved immunoassay for detecting cardiac troponin-specific autoantibodies. Clin Chem Lab Med 2014;52:273–9. https://doi.org/10.1515/cclm-2013-0310.Search in Google Scholar PubMed
19. Plebani, M, Mion, M, Altinier, S, Girotto, MA, Baldo, G, Zaninotto, M. False-positive troponin I attributed to a macrocomplex. Clin Chem 2002;48:677–9. https://doi.org/10.1093/clinchem/48.4.677.Search in Google Scholar
20. Salaun, E, Drory, S, Coté, MA, Tremblay, V, Bédard, E, Steinberg, C, et al.. Role of antitroponin antibodies and macrotroponin in the clinical interpretation of cardiac troponin. J Am Heart Assoc 2024;13:e035128. https://doi.org/10.1161/jaha.123.035128.Search in Google Scholar
21. Legendre-Bazydlo, LA, Haverstick, DM, Kennedy, JLW, Dent, JM, Bruns, DE. Persistent increase of cardiac troponin I in plasma without evidence of cardiac injury. Clin Chem 2010;56:702–5. https://doi.org/10.1373/clinchem.2009.138164.Search in Google Scholar PubMed
22. Lucas, R, Roberts, P. Macrotroponin as a cause for an elevated troponin in a 14-year-old boy. J Paediatr Child Health 2020;56:1632–3. https://doi.org/10.1111/jpc.14797.Search in Google Scholar PubMed
23. Akhtar, Z, Dargan, J, Gaze, D, Firoozi, S, Collinson, P, Shanmugam, N. False-positive troponin elevation due to an immunoglobulin-G-cardiac troponin T complex: a case report. Eur Heart J Case Rep 2020;4:1–5. https://doi.org/10.1093/ehjcr/ytaa082.Search in Google Scholar PubMed PubMed Central
24. Lam, L, Ha, L, Heron, C, Chiu, W, Kyle, C. Identification of macrotroponin T: findings from a case report and non-reproducible troponin T results. Clin Chem Lab Med 2021;59:1972–80. https://doi.org/10.1515/cclm-2021-0626.Search in Google Scholar PubMed
25. Kavsak, PA, Hoard, B, Mackett, K, Mukherjee, SD, Bordeleau, L, Ellis, PM, et al.. Detection of macrotroponin in patients receiving treatment for breast cancer. CJC Open 2023;5:658–60. https://doi.org/10.1016/j.cjco.2023.05.010.Search in Google Scholar PubMed PubMed Central
26. Dalle Carbonare, L, Pizzini, M, Micheletti, V, Lo Cascio, C, Bovo, C, Girelli, D, et al.. Interference from immunocomplexes on a high-sensitivity cardiac troponin T immunoassay. Clin Chem Lab Med 2020;58:e225–7. https://doi.org/10.1515/cclm-2020-0028.Search in Google Scholar PubMed
27. Laguë, M, Turgeon, PY, Thériault, S, Steinberg, C. A false-positive troponin assay leading to the misdiagnosis of myopericarditis. CMAJ 2022;194:E456–9. https://doi.org/10.1503/cmaj.211842.Search in Google Scholar PubMed PubMed Central
28. Nichols, M, Silversides, CK, Woo, A, Leung, F, Taher, J, Zhou, Q, et al.. A diagnostic dilemma from a presentation of shortness of breath and chest pain. J Appl Lab Med 2022;7:575–81. https://doi.org/10.1093/jalm/jfab119.Search in Google Scholar PubMed
29. Ghossein, J, Ghossein, J, Booth, RA, Kavsak, P, Chamoun, C. Presence of macrotroponin for over 2 years in a young Woman. CJC Open 2022;4:1012–4. https://doi.org/10.1016/j.cjco.2022.07.016.Search in Google Scholar PubMed PubMed Central
30. Bajwa, O, Hassen, G, Fishbein, J, Uchenna, U, Ammar, H. False elevation of troponin in a case of multiple myeloma. Cureus 2023;15:e34186. https://doi.org/10.7759/cureus.34186.Search in Google Scholar PubMed PubMed Central
31. Harberg, MR, Al-Mousily, MF, Akter, T, Babic, N, Jackson, LB. Persistent elevation of troponin I in a pediatric patient resulting from macrotroponin complex. Pediatrics 2023;151:e2022058374. https://doi.org/10.1542/peds.2022-058374.Search in Google Scholar PubMed
32. van Avezaath, LK, Nijenhuis, HP, Muller Kobold, AC. Unexpected high troponin T and I values in a child with hypertrophic cardiomyopathy and acute chest pain: a case report. Eur Heart J Case Rep 2023;7:ytad375. https://doi.org/10.1093/ehjcr/ytad375.Search in Google Scholar PubMed PubMed Central
33. Bosi, D, Canovi, S, Pennacchioni, A, Demola, P, Corradini, M, Guiducci, V, et al.. “Troponinosis”, the cardiologist’s curse-when clinic-laboratory interaction unveils the mystery: a case report. J Cardiovasc Dev Dis 2023;10:378. https://doi.org/10.3390/jcdd10090378.Search in Google Scholar PubMed PubMed Central
34. Broz, P, Racek, J, Prokop, P, Novak, J, Rajdl, D, Trefil, L. Macrotroponins cause discrepancy in high-sensitivity examination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2024;168:187–90. https://doi.org/10.5507/bp.2023.001.Search in Google Scholar PubMed
35. Kavsak, PA, Ahmed, B, Ivanick, D, Greene, DN, Ranjitkar, P. Patient with macrocomplexes for both creatine kinase and cardiac troponin reveals the importance of immunoassay methods for macrotroponin detection. Clin Chim Acta 2024;562:119885. https://doi.org/10.1016/j.cca.2024.119885.Search in Google Scholar PubMed
36. Lam, L, Ha, L, Gladding, P, Tse, R, Kyle, C. Effect of macrotroponin on the utility of cardiac troponin I as a prognostic biomarker for long term total and cardiovascular disease mortality. Pathology 2021;53:860–6. https://doi.org/10.1016/j.pathol.2021.04.005.Search in Google Scholar PubMed
37. Schneeweis, C, Diebold, K, Schramm, T, Syrek, C, Predel, HG, Manka, R, et al.. Mid- to long-term cardiac magnetic resonance findings in elite athletes recovered from COVID-19: results from an ongoing observational COVID-19 study at a German Olympic medical centre. Swiss Med Wkly 2023;153:3534. https://doi.org/10.57187/s.3534.Search in Google Scholar PubMed
38. Dong, X, Zhao, Y, Zhao, Z, Fang, J, Zhang, X. The association between marathon running and high-sensitivity cardiac troponin: a systematic review and meta-analysis. J Back Musculoskelet Rehabil 2023;36:1023–31. https://doi.org/10.3233/bmr-220352.Search in Google Scholar
39. Hammarsten, O, Warner, JV, Lam, L, Kavsak, P, Lindahl, B, Aakre, KM, et al.. Antibody-mediated interferences affecting cardiac troponin assays: recommendations from the IFCC Committee on Clinical Applications of Cardiac Biomarkers. Clin Chem Lab Med 2023;61:1411–9. https://doi.org/10.1515/cclm-2023-0028.Search in Google Scholar PubMed
40. Mair, J, Giannitsis, E, Mills, NL, Mueller, C, Lindahl, B, Cullen, L, et al.. Study group on biomarkers of the European society of cardiology association for acute CardioVascular care. How to deal with unexpected cardiac troponin results. Eur Heart J Acute Cardiovasc Care 2022;11:e1–3. https://doi.org/10.1093/ehjacc/zuac023.Search in Google Scholar PubMed
41. du Fay de Lavallaz, J, Prepoudis, A, Wendebourg, MJ, Kesenheimer, E, Kyburz, D, Daikeler, T, et al.. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation 2022;145:1764–79. https://doi.org/10.1161/circulationaha.121.058489.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2025-0427).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025
Articles in the same Issue
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025

