The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
-
Wilson de Melo Cruvinel
, Guilherme Guerra Ferreira
Abstract
The indirect immunofluorescence assay (IFA) on HEp-2 cells is the prevailing method used to screen for autoantibodies in the investigation of systemic autoimmune diseases (SAID). When positive, the titer provides a semi-quantitative assessment of the autoantibody serum concentration whereas the immunofluorescence pattern indicates the possible autoantibody specificities. The Brazilian Consensus on ANA Patterns (BCA) and the International Consensus on ANA Patterns (ICAP) provide recommendations for the harmonization on the pattern nomenclature and test reporting. Nuclear patterns are among the most frequent in the clinical laboratory and some of them are highly relevant in the diagnosis of SAID. Nuclear patterns with stained metaphase plate (MP) indicate autoantibodies against chromatin components or against chromatin-bound antigens. These include the nuclear homogeneous (AC-1), nuclear dense fine speckled (AC-2), Topo 1-like (AC-29), and nuclear fine speckled with stained MP (AC-30) patterns. The Brazilian consensus has also classified the quasi-homogeneous nuclear pattern (QH). The correct identification of these patterns is important because each one is associated with different autoantibody specificities and clinical scenarios. However, the recognition of the nuances in texture of the staining pattern and other specific features that characterize each of them may be challenging for the analyst at the microscope. This review focuses on the morphological characteristics, immunological identities, and clinical relevance of nuclear patterns with stained MP. The aim is to assist laboratory analysts and clinicians in identifying and interpreting these patterns, thus optimizing the use of the HEp-2 IFA test in the investigation of patients under suspicion of SAID.
Introduction
The indirect immunofluorescence assay on HEp-2 cells (HEp-2 IFA) is a laboratory technique in which specific serum autoantibodies are immobilized on a human cellular substrate (HEp-2 cells) and subsequently detected by fluorochrome-labeled secondary antibodies. Although originally characterized as a human laryngeal carcinoma, HEp-2 cells are now known to be contaminated with HeLa cells [1], but regardless of its tissue origin this cell line is a suitable substrate [2]. The HEp-2 IFA is the most frequently used methodology for the detection of autoantibodies in systemic autoimmune rheumatic diseases (SARD) and other systemic autoimmune diseases (SAID), allowing for the screening of numerous clinically relevant autoantibodies [3]. The test provides three crucial pieces of information: the presence or absence of autoantibodies in the sample, the antibody titer, and the immunofluorescence pattern. The titer and immunofluorescence pattern help differentiate positive results obtained in non-autoimmune individuals from those of patients with a probable autoimmune disease. In addition, the HEp-2 IFA pattern provides preliminary indication of the possible targeted autoantigens thereby guiding the investigation of the most likely specific autoantibodies, thus contributing to medical diagnosis and treatment [4], [5], [6], [7].
Given the importance of the HEp-2 IFA test in autoimmunity diagnostics, a group of Brazilian experts established the Brazilian Consensus on Antinuclear Antibodies in HEp-2 cells (BCA) in 2000. Since then, successive workshops were conducted aiming at harmonizing the HEp-2 IFA technique and establishing recommendations for standardization of the test and pattern classification [2], [8], [9], [10], [11]. The BCA presented the first proposal of classification of the most relevant patterns into a hierarchical tree, harmonizing the nomenclature of HEp-2 cell patterns, which was previously heterogeneous across the country. The hierarchical classification tree comprised four major groups addressing the most prominent cell domains, i.e., nucleus, nucleolus, cytoplasm, and mitotic apparatus [2].
In 2014, the Autoantibody Standardization Committee of the International Union of Immunological Societies (IUIS) launched the International Consensus on ANA Patterns (ICAP) initiative [12] that created a HEp-2 IFA pattern classification system built on the Brazilian Consensus experience. Since then, ICAP’s goal has been to promote worldwide harmonization of HEp-2 IFA procedure and interpretation. The guidelines for the interpretation and classification of HEp-2 IFA patterns ultimately aim to help laboratory personnel in the analysis of the test and clinicians in the diagnosis and optimization of medical management of patients under suspicion of SAID [12], 13].
ICAP recognizes 32 different HEp-2 IFA patterns (AC-0 to AC-31) in addition to unusual and non-catalogued patterns, designated as AC-XX [14]. These patterns are categorized into four main groups: negative (n=1), nuclear (n=17), cytoplasmic (n=9), and mitotic (n=5) patterns [14]. The consensus nomenclature is organized into a classification tree displayed on the interactive ICAP website (www.ANApatterns.org). For each pattern, an alphanumeric AC (anti-cell) code is assigned, for example, the homogeneous nuclear pattern is designated as AC-1. The ICAP website provides representative images for each pattern along with other important information such as the recommended designation, previous nomenclature, a description of the main morphological characteristics, potential antigenic specificities, and clinical relevance [13]. The most relevant patterns that are relatively easy to identify are classified as competent-level patterns, i.e., these are expected to be recognized by any HEp-2 IFA analyst. This is in contrast to the group of expert-level patterns, comprised of less common patterns or those that require higher expertise for recognition [12].
Over the years, experts from several countries have made efforts to absorb and harmonize the ICAP recommendations according to their local specific contexts, for instance, in Argentina [15], Uruguay [16], Italy [17], Ukraine [18], and Brazil [10], 11]. In particular, the Brazilian Consensus (BCA) has implemented several modifications towards harmonization with ICAP, including the adoption of the alpha-numeric AC code. However, certain patterns classified in the BCA algorithm are not present in the ICAP classification tree, and, therefore, these received a provisional “BAC” (Brazilian AC) codification [10], 11].
ICAP classifies the HEp-2 IFA patterns into segregated groups according to cell compartments, i.e., nuclear, cytoplasmic, and mitotic apparatus. The nuclear pattern group has the highest level of clinical relevance and has undergone the most updates in recent years. In 2014, the IV Brazilian Consensus incorporated the quasi-homogeneous (QH) nuclear pattern into the nuclear pattern group [9]. The QH pattern has a very fine speckled texture tending to homogenous and is classified as AC-1 in ICAP. However, it has been shown that the frequency of anti-dsDNA and anti-nucleosome antibodies is far lower in samples with the QH pattern in comparison with samples with the bona fide homogeneous pattern [19]. As the QH pattern that has not yet been classified by ICAP, it has received the provisional code BAC-3 [9]. In 2018, ICAP incorporated a compound pattern, previously reported as strongly associated with antibodies against DNA topoisomerase I (Topo I) [20], as the AC-29 pattern [21]. In 2021, there was an update to the ICAP tree, grouping the AC-1, AC-2, and AC-29 patterns to highlight their morphological similarities (www.anapaterns.org). In 2024, additional nuclear patterns were included in the ICAP algorithm, including the nuclear fine speckled with mitotic plate (AC-30) and the nuclear speckled of the myriad discrete type (AC-31). These two patterns were integrated into the group of nuclear patterns with negative plates (AC-4) fine speckled and AC-5, coarse/large speckled [14].
The nuclear patterns are predominant in clinical laboratories and were found to represent more than 70 % of positive samples in one large clinical laboratory [22]. Within this group, there are two main categories, those with no staining of the metaphase chromosome plate and those with distinctive staining of the metaphase plate. These include the nuclear homogeneous (AC-1), nuclear dense fine speckled (AC-2), topo-1-like (AC-29), and nuclear fine speckled with stained metaphase plate (AC-30) patterns. The major segregating factor among these patterns is the staining texture in the interphase nucleus and in the metaphase plate. This difference is subtle and subjective, therefore they are classified at the expert level [14]. Notwithstanding their morphological complexity and potential for misidentification, the distinction of these patterns is important as each one is associated with a specific clinical context. This review aims to examine the spectrum of nuclear patterns with stained metaphase plate, focusing on the morphology and texture of the patterns, autoantibody associations, and clinical relevance.
Nuclear homogeneous pattern (AC-1)
The homogeneous nuclear pattern (AC-1) is characterized by a diffuse, regular, and homogeneous fluorescence throughout the nucleoplasm of the HEp-2 cell. The nucleolar regions are usually stained, but they may also remain unstained depending on the cellular substrate [12]. In interphase cells, higher titer sera may show more intense staining at the nuclear periphery. In mitotic cells, the metaphase chromatin mass is intensely stained in a homogeneous manner, with a distinctly hyaline appearance. The cytoplasm typically appears negative (unstained) in both interphase and mitotic cells [12]. The morphological features of the AC-1 pattern are illustrated in the upper panel of Figure 1A and detailed in Table 1. This pattern is frequently associated with autoantibodies targeting chromatin components such as double-stranded DNA (dsDNA), histones, and nucleosomes [12]. These antigen associations harmonize with the fact that the chromatin consists of the native complex of histones (∼40 %), dsDNA (∼40 %), and non-histone proteins (∼20 %) [34].
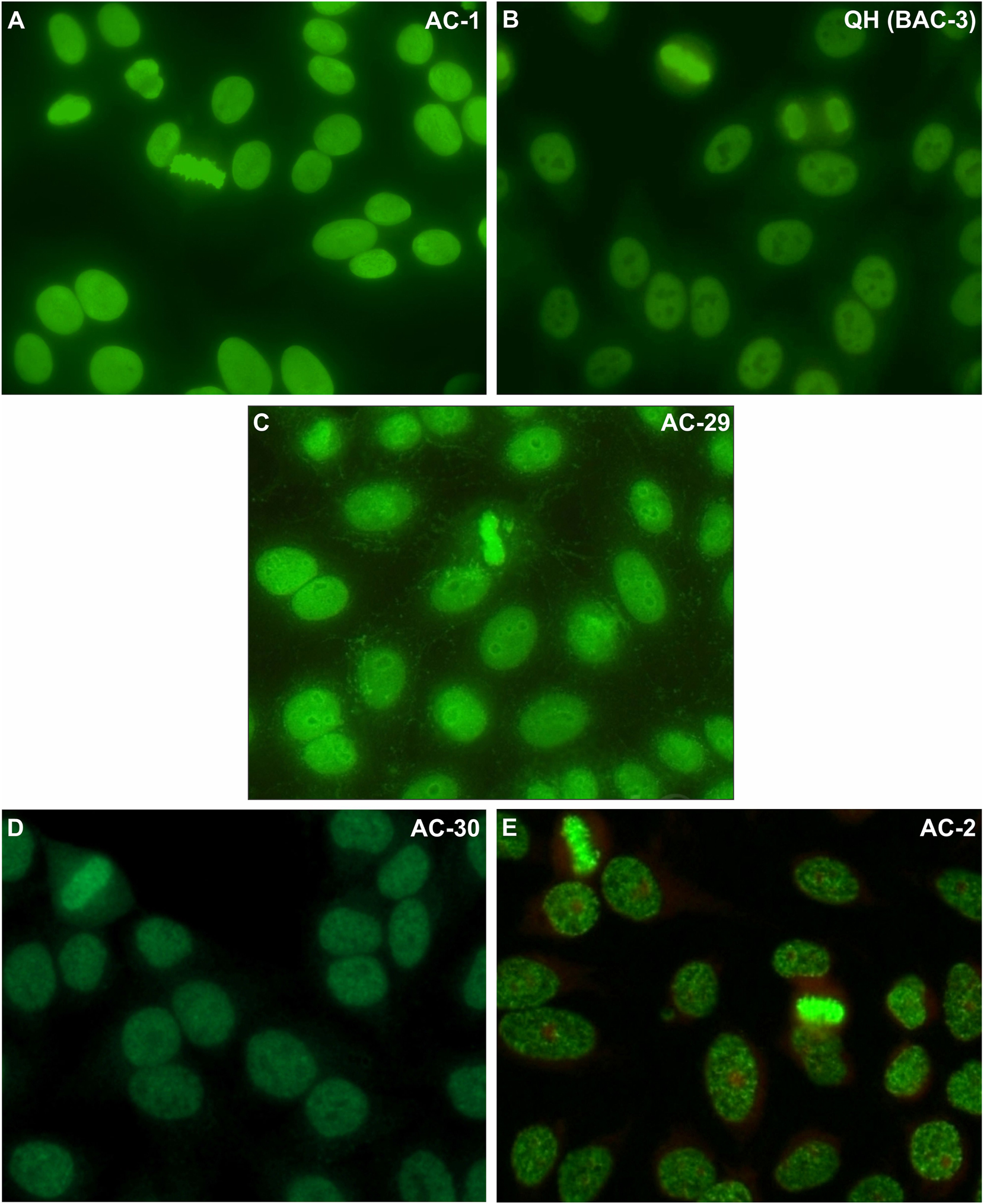
Morphological details of the nuclear patterns that show homogeneous or speckled staining of the metaphase plate: nuclear homogeneous – AC1 (A), nuclear quasi-homogeneous - BAC-3 (B), DNA topoisomerase I/topo I-like – AC-29 (C), nuclear fine speckled with mitotic plate - AC-30 (D), and nuclear dense fine speckled – AC-2 (E). Images from the International Consensus on ANA Patterns, photographs (C) and (E): Werner Klotz & Manfred Herold (Sample dilution: 1:80; microscope magnification: 400×).
Nuclear patterns with stained metaphase chromosome plate: morphological characteristics, antigenic associations, clinical associations, and recommended follow-up testing.
| Code | Morphological characteristics | Antigenic associations | Clinical associations and follow-up testing | References | ||
|---|---|---|---|---|---|---|
| Interphase nucleus | Metaphase plate | |||||
| Nuclear homogeneous | AC-1 | The nucleolar regions are usually stained, but they may also remain unstained depending on the cellular substrate | The metaphase chromatin mass is intensely stained in a homogeneous manner, with a distinctly hyaline appearance | Anti-dsDNA, anti-nucleosome, anti-histone | In patients suspected of SLE with an AC-1 pattern, testing for anti-dsDNA and anti-histone antibodies is recommended. Anti-histone antibodies, though classically associated with SLE and drug-induced lupus, are also found in Sjögren’s disease, inflammatory myositis, autoimmune hepatitis, and rheumatoid arthritis. Anti-nucleosome antibodies, the first described in SLE, are valuable diagnostic biomarkers, especially for lupus nephritis | Rekvig OP [23]; Bizzaro et al. [24]; Krippner et al. [25]; Zirwas et al. [26] |
| Nuclear quasi-homogeneous | QH | Extremely fine speckled diffuse nuclear fluorescence, resembling but not quite homogeneous in texture | Metaphase chromatin mass is intensely stained in a homogeneous texture | Anti-dsDNA, anti-nucleosome, anti-histone, anti-Sm, anti-U1-RNP, anti-SS-A/Ro, anti-SS-B/La (frequently more than one antinuclear antibody) | Although with lower correlation, it can be observed in patients with systemic autoimmune rheumatic diseases. In cases of clinical suspicion, the relevant autoantibodies should be investigated. | França et al. [19] |
| Topo-1-like | AC-29 | Fine, compact, and prominent speckled diffuse nuclear fluorescence in interphase cells with delicate and weak cytoplasmic fluorescence with a web-like appearance radiating from the perinuclear area to the vicinity of the plasma membrane and inconsistent fluorescence of nucleoli with varying aspects. | Consistent and strong fine speckled staining of condensed chromatin in mitotic cells, with the chromatin metaphase plate appearing as homogeneous in high titer samples. Strong staining of the nucleolar organizing regions (NOR) at the metaphase plate | Anti-Scl-70 | In suspected SSc, follow-up testing for anti-topoisomerase I (Scl-70) antibodies is recommended, as they are present in 20–30 % of SSc patients and associated with diffuse cutaneous involvement, interstitial lung fibrosis, and a more aggressive disease course, including higher risks of renal, gastrointestinal, and cardiac complications. | Shero et al. [27]; Ho and Reveille [28]; Gabrielli et al. [29] |
| Nuclear fine speckled with stained metaphase plate | AC-30 | Nuclear speckled fluorescence with uniform distribution of speckles and the nuclear border is regular, | The metaphase plate displays a uniformly distributed fine speckled pattern | Most samples have none of the clinically relevant autoantibodies, but some may have a combination of anti-dsDNA, anti-nucleosome, anti-histone, anti-Sm, anti-U1-RNP, anti-SS-A/Ro, anti-SS-B/La, and anti-Scl-70 | The AC-30 pattern is not associated with anti-DFS70 antibodies and up to 25 % of sera may recognize one or more clinically relevant antinuclear antibodies. So, in cases of clinical suspicion, the disease-related autoantibodies should be investigated. | Landoni et al. [30]; Andrade et al. [14] |
| Nuclear dense fine speckled | AC-2 | Non-uniform speckled fluorescence distributed throughout the interphase nucleus, with characteristic heterogeneity in the size, brightness, and distribution of the speckles. Across the interphase nucleus, some areas exhibit denser or sparser staining. The border of the nucleus is irregular (moth-eaten pattern). | The metaphase plate displays a strong speckled pattern, with some coarse speckles standing out | Anti-DFS70/LEDGF, most frequently as the unique antinuclear antibody in the sample. | If anti-DFS70 autoantibody is confirmed in the absence of relevant autoantibodies against extractable nuclear antigens (including anti-dsDNA), it is rarely associated with systemic autoimmune rheumatic diseases (SARD) [31], [32], [33]. | Muro et al. [33]; Fitch-Rogalsky et al. [31]; Mariz et al. [7] |
Antibodies that bind to double-stranded DNA (dsDNA) or anti-native DNA (nDNA) are the most clinically relevant antibodies against deoxyribonucleic acid antibodies [23]. These include antibodies against a broad spectrum of fine molecular DNA specificities, including native dsDNA and chemically modified dsDNA containing thymidine dimers. These are frequently high affinity autoantibodies that form stable immune complexes that are relevant in the context of systemic lupus erythematosus (SLE) [23], [35], [36], [37]. Anti-dsDNA antibodies can be of different isotypes but IgG-class autoantibodies are predominantly observed in patients with SLE, contributing to the pathogenesis of glomerulonephritis, particularly the high-avidity IgG antibodies [38]. From the pathophysiological point of view, these antibodies are involved in the formation of immune complexes with dsDNA, leading to subsequent deposition in glomeruli. Alternatively, they may cross-react and bind to other autoantigens embedded in the glomerular basement membrane. The immune complexes at the glomerular basement membrane induce an inflammatory process and a variety of lesions that underlie the distinct histopathological classifications and varying degrees of nephritis [39].
A positive HEp-2 IFA test with the AC-1 pattern suggests the presence of anti-dsDNA antibodies, which must be confirmed in antigen-specific immunoassays, such as the Farr immunoprecipitation radioimmunoassay, the indirect immunofluorescence (IIF) with the protozoan Crithidia luciliae (CLIFT), enzyme-linked immunosorbent assays (ELISA), or chemiluminescence immunoassays (CLIA) [40]. Generally, good clinical specificity for SLE is achieved using radioimmunoassay and CLIFT methodologies [41], [42], [43], [44], whereas ELISA and CLIA techniques may detect low avidity and cross-reacting antibodies, thereby providing lower clinical specificity. However, CLIFT shows lower sensitivity compared to immunometric methods, such as fluorescence enzyme immunoassay, CLIA, and multiplex technologies, which offer sensitivities ranging from 67 % to 92 % and specificities between 84 % and 98 % in the analyzed cohorts. The high sensitivity and semiquantitative readout of ELISA and CLIA may render these methods appropriate for monitoring the serum levels of anti-dsDNA antibodies [45]. Considering the limited commutability across platforms, the heterogeneous diagnostic performance of CLIFT and immunometric methods in the evaluation of anti-dsDNA antibodies reinforces the need for harmonization among different immunoassays [46].
Regarding the clinical relevance, anti-dsDNA represents a useful parameter for monitoring SLE disease activity, with elevated levels during disease flares, especially in lupus nephritis, and decreasing levels in response to treatment and quiescence [23]. Therefore, in the presence of an AC-1 pattern in patients suspected of SLE, a confirmatory test for anti-dsDNA antibodies is recommended, as it serves as a powerful parameter for classifying and diagnosing SLE, particularly in patients suspected of having renal involvement [23].
The AC-1 pattern may also be associated with the presence of anti-nucleosome and anti-histone antibodies, the latter representing structural protein subunits that provide a core around which chromatin is wrapped, allowing for its spatial organization within the nucleus. The histone core consists of two H2A-H2B dimers and one H3-H4 tetramer, forming a histone octamer with an external H1 histone, the latter being a conserved subunit that assists in stabilizing the chromatin structure [47]. The histone octamer, coupled with approximately 150 DNA base pairs, is known as the nucleosome and forms the fundamental unit of chromatin [48]. Histones not only provide support for chromatin but also play a significant and complex role in regulating gene expression, being encoded by multiple genes spanning several chromosomes [49].
Anti-nucleosome antibodies consist of a large family of autoantibodies directed against epitopes of histones exposed in chromatin and against conformational epitopes generated by the interaction between dsDNA and core histones [24]. Free nucleosomes are generated during cellular apoptosis by endonuclease-mediated chromatin cleavage [50]. These components may become immunogenic during apoptosis when cellular debris containing chromatin is not properly cleared [51]. There is evidence that, as a result of impaired phagocytic clearance of apoptotic nuclear debris, nucleosomes bound to the cell surface and circulating polynucleosomes stimulate and promote the production of autoantibodies [52].
Various technologies have been used to measure anti-nucleosome antibodies, starting with the now obsolete LE cell test and eventually evolving to immunoprecipitation methodologies, ELISA, and CLIA [47]. Clinically, anti-nucleosome antibodies have proven to be a good diagnostic biomarker for SLE, representing the first autoantibody described in association with the disease, particularly in the development of lupus nephritis [24].
Anti-histone antibodies can recognize total histones or restricted sets of histone subunits, and can bind free histones or histones bound to DNA [25], 53]. In the HEp-2 IFA test, anti-histone antibodies may yield the AC-1 (homogeneous nuclear) pattern, as previously described [54] and can be determined using ELISA or CLIA [55]. The Western blotting technique has been used in the original characterization of autoantibodies against histone subunits. In this case, polyacrylamide gel electrophoresis is used to separate histones into their constituents based on molecular weight [56].
In terms of clinical relevance, anti-histone antibodies have been classically associated with SLE and drug-induced lupus but they are also found in other conditions, including Sjögren’s disease, inflammatory myositis, and rheumatoid arthritis [25], 26].
The AC-1 pattern is one of the common patterns in autoimmune hepatitis but other HEp-2 IFA nuclear patterns may also occur, although the corresponding autoantigens have not yet been fully defined [13], 57].
Nuclear quasi-homogeneous pattern
An interesting and subtle pattern within the group of nuclear patterns with a positive metaphase plate is the quasi-homogeneous speckled nuclear pattern (QH). The QH pattern is characterized by extremely fine speckled diffuse nuclear fluorescence, resembling but not quite homogeneous in texture, and it also includes a similar staining of the metaphase plate. The morphological similarity to the AC-1 pattern may lead to confusion between these two patterns and one needs to exercise the ability to differentiate those two patterns [9].
The immunological characterization of the QH pattern was performed by analyzing 60 samples with this morphological profile on four different HEp-2 cell brands, compared with an equal number of samples with the nuclear homogeneous (AC-1) and nuclear dense fine speckled (AC-2) patterns. Reactivity to double-stranded DNA (dsDNA), nucleosome, and histones was tested in all samples. Autoantibodies against nucleosome, histones, and dsDNA were present in 96.7 %, 60 %, and 36.7 % in AC-1 samples, respectively, compared to 35 %, 16.7 %, and 7 % in the QH samples. None of the AC-2 samples presented any of these autoantibodies [58]. Thus, the QH pattern exhibited an intermediate autoantibody profile between the AC-1 and AC-2 patterns and was seldom observed in patients with systemic autoimmune rheumatic diseases [58].
Considering that the QH pattern does not rule out the possibility of an association with SARD, although with a lower post-test probability than the AC-1 pattern [58]. In a retrospective analysis of 11,478 HEp-2 IFA positive samples in a large Brazilian clinical laboratory, the QH pattern presented a 10 % total prevalence among positive samplesb [22]. The morphological details of the QH pattern can be observed in Figure 1B and detailed in Table 1.
DNA topoisomerase I/topo-I-like pattern (AC-29)
The AC-29 pattern is highly suggestive of reactivity against the enzyme DNA topoisomerase-I (topo-I), a 765-amino acid (91 kDa) protein localized in the cell nucleus due to the presence of a functional nuclear localization signal in the enzyme N-terminal domain. Both topo-I and topo-II are enzymes involved in relaxing the DNA helix as it unwinds for DNA replication and RNA transcription [59], 60]. Several epitopes are recognized by anti-topo I autoantibodies, with the amino acids 489–573 stretch being identified as an immunodominant epitope [61]. Anti-topo I antibodies of the IgG class are most frequently detected compared to IgA and IgM isotypes [62].
In 1979, Eng M. Tan and colleagues described the presence of autoantibodies recognizing a 70 kDa nuclear antigen, termed Scl-70, in patients with SSc [63]. Later studies by van Venrooij et al. and Guldner et al. (1985 and 1986, respectively) demonstrated that this autoantigen corresponded to the 100 kDa Topo I enzyme [64], 65]. Anti-topo-I autoantibodies are highly specific biomarkers for SSc, being present in 20–30 % of SSc patients and are preferentially associated with diffuse cutaneous involvement and interstitial lung fibrosis, suggesting a more aggressive disease course [27], 29] Furthermore, anti-topo-I is associated with a higher likelihood of renal vascular damage, renal crisis, severe gastrointestinal involvement, and cardiac fibrosis [28]. In 2009, Dellavance et al. redefined the fluorescence pattern characteristics associated with this autoantibody, determining that the Topo I-like pattern is a composite pattern characterized by reactivity in five distinct cellular regions of the HEp-2 cell [20]. In 2018, ICAP assigned the AC-29 classification code to the HEp-2 IFA pattern specifically associated with anti-Topo I autoantibodies [21].
The AC-29 pattern has been shown to be strongly associated with autoantibodies to DNA topoisomerase I [20], 21] and is composed of up to five morphological elements: (1) fine, compact, and prominent speckled diffuse nuclear fluorescence in interphase cells; (2) consistent and strong staining of condensed chromatin in mitotic cells, with the chromatin metaphase plate appearing homogeneous in high titer samples; (3) strong staining of the nucleolar organizing regions (NOR) at the metaphase plate, the visualization of which may need adjustment in the fine focus to allow distinction from the bright metaphase plate staining as NOR are not always in the same focal plane as the chromatin; (4) delicate and weak cytoplasmic fluorescence with a web-like appearance radiating from the perinuclear area to the vicinity of the plasma membrane. Frequently, the cytoplasmic staining becomes more prominent during sample titration to higher dilutions; and finally, (5) inconsistent fluorescence of nucleoli with varying aspects [20], 21]. Due to the number of elements and the variation in analysis depending on the brand of the HEp-2 slide, this pattern is considered challenging to identify, and detailed analysis with attention to different focal planes is recommended for proper visualization. For this reason, the pattern is classified at the expert level by ICAP and the Brazilian Consensus on ANA [10], 14]. The morphological details of the AC-29 Topo-1-like pattern can be observed in Figure 1C and revised in Table 1. The five key characteristics of the pattern are highlighted in Figure 2.
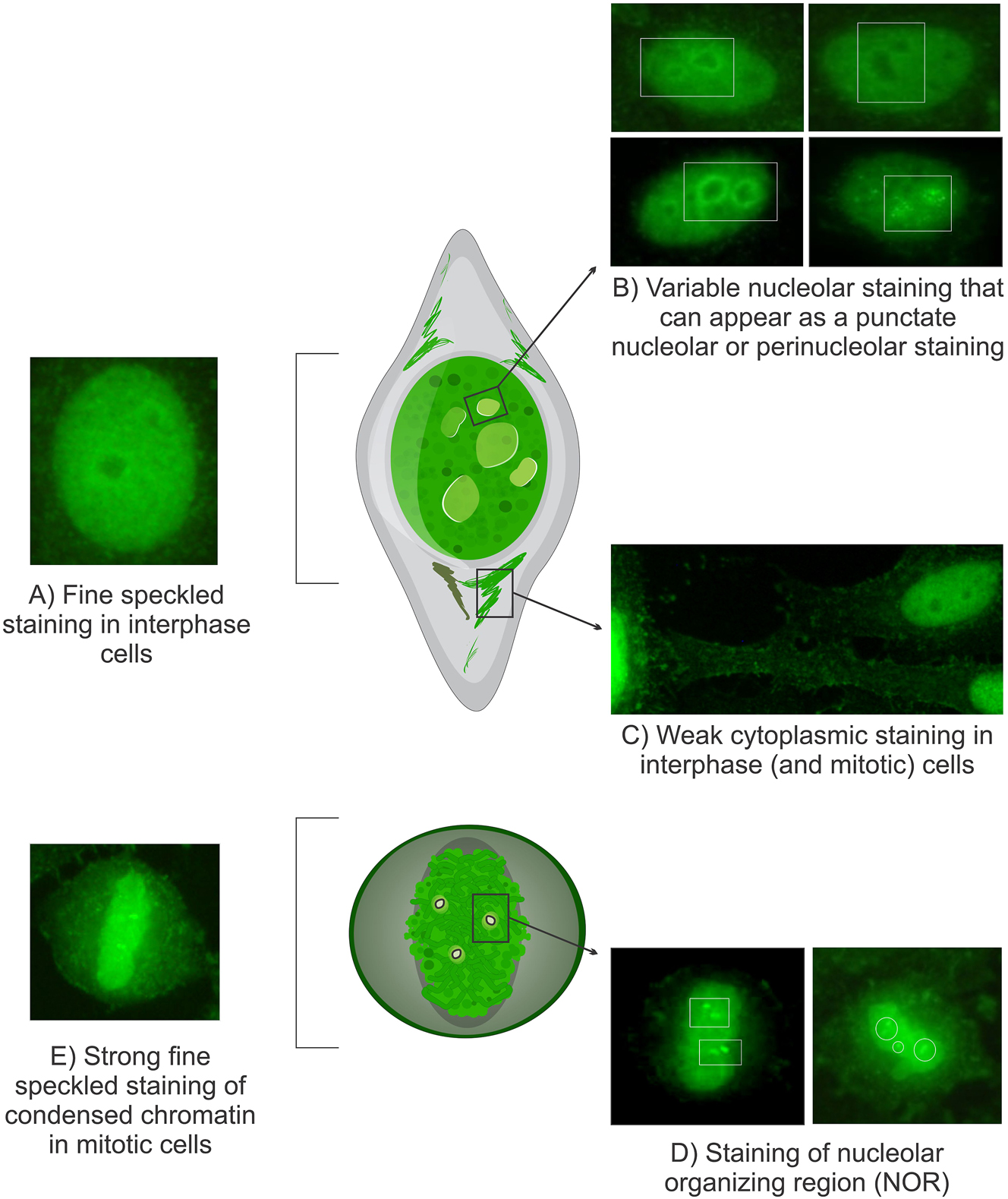
Morphological characteristics of the Topo I-like pattern: The Topo I-like pattern is defined by staining across five distinct subcellular regions. (A) Prominent extremely fine and compact speckled staining in interphase nuclei. (B) Variable nucleolar staining, which may present as punctate nucleolar, homogeneous or perinucleolar staining in interphase cells, although this feature is not consistently observed. (C) Faint cytoplasmic staining in interphase and mitotic cells, forming a delicate network radiating from the perinuclear area to the plasma membrane. (D) Intense staining of NOR over condensed chromosomes in mitotic cells, though this can be obscured by bright chromosomal staining. (E) Strong extremely fine compact speckled staining of condensed chromatin in mitotic cells, which may appear homogeneous with high titer samples. Images sourced from the International Consensus on ANA Patterns (www.ANAPatterns.org), photographs by Werner Klotz & Manfred Herold.
Upon identification of the AC-29 pattern by HEp-2 IFA, the presence of anti-topoisomerase I (anti-topo-I) antibodies should be confirmed using an antigen-specific immunoassay, such as ELISA, double immunodiffusion, or immunoblotting. In high-throughput laboratories, ELISA and CLIA are the preferred methods. Notably, CLIA has demonstrated excellent agreement with the gold standard immunoprecipitation technique for detecting anti-Scl-70 (Topo I) antibodies [66], 67].
Dense fine speckled pattern (AC-2)
The AC-2 pattern is produced by autoantibodies that recognize a 75 kDa chromatin-associated protein designated DFS70, also known as lens epithelium derived growth factor p75 (LEDGF/p75), transcription co-activator p75 (TCP75), and PC4/SRSF1 interacting protein 1 [68], [69], [70]. The DFS pattern was first described in 1994 [71] and the target autoantigen was fully characterized as the Dense Fine Speckled 70 (DFS70) protein in the late 1990s [72]. This designation was based on their distinctive nuclear fluorescence pattern and migration near the 70 kDa region in immunoblotting assays [72]. The entire DFS70 sequence corresponds to the p75 transcriptional coactivator positive cofactor 4 (PC4), which is integral to RNA polymerase II complexes and plays a key role in gene transcription [72], 73]. Contemporarily an independent group reported the same protein as the Lens Epithelium-Derived Growth Factor p75 (LEDGF/p75) with properties of protecting cells from oxidative damage [74]. Acting as a hub for protein-protein interactions, DFS70/LEDGFp75 facilitates the recruitment of transcription factors, chromatin remodelers, and regulatory proteins to RNA polymerase II complexes at active chromatin sites [75].
The dense fine speckled (DFS) pattern is characterized by a non-uniform speckled fluorescence distributed throughout the interphase nucleus, with characteristic heterogeneity in the size, brightness, and distribution of the speckles. Across the interphase nucleus, some areas exhibit denser or sparser staining. The border of the nucleus is irregular (moth-eaten pattern). The metaphase plate displays a strong speckled pattern, with some coarse speckles standing out [12]. It should be emphasized that the AC-2 pattern is clearly recognizable only when anti-DFS70 is the only antinuclear antibody in the sample, as the presence of additional antinuclear antibodies tend to mask the distinctive characteristics of this pattern. Details of the AC-2 pattern can be observed in Figure 1E, in the lower panel, detailed in Table 1.
Anti-DFS70/LEDGF antibodies are primarily of the IgG class, although IgE-class antibodies have been detected in some atopic diseases. Anti-DFS70 antibodies target a highly conserved region in the C-terminal domain of DFS70 and can be detected at moderate to high titers with variable frequency in clinical routines [31], 72], 76].
Initial studies of this autoantibody system primarily relied on the HEp-2 IFA pattern elicited by anti-DFS70 autoantibodies, with just a few initial studies confirming the anti-DFS70 reactivity in antigen-specific immunoassays. Progressively, a multitude of solid-phase assays for detecting these autoantibodies has been developed in recent years, providing excellent platforms for confirming the anti-DFS70 reactivity with high specificity and sensitivity. These assays include serum adsorption against recombinant DFS70/LEDGF protein, IFA with DFS70-knock-out HEp-2 cells, CLIA, ELISA, line immunoassays, dot blot assays and fluorescence enzyme immunoassay (FEIA) [70], 77].
The initial reports suggested that the AC-2 pattern was associated with inflammatory diseases, such as interstitial cystitis, asthma, alopecia areata, atopic dermatitis, and ocular diseases [71], 72], 78], 79] Along the years, it was shown that this pattern and the correspondent anti-DFS70 antibody were frequently found also in apparently healthy individuals [7], 32], 72], 80]. A seminal finding was the demonstration by Mariz et al. that the AC-2 pattern rarely occurs in sera from patients with SARD and, therefore, represent a negative biomarker for systemic autoimmunity [7]. Other studies confirmed that anti-DFS70 autoantibodies in the absence of relevant autoantibodies against extractable nuclear antigens (anti-DNA) are rarely associated with SARD [31], 33], 81]. Therefore, monospecific anti-DFS70 antibodies, strongly speak against the odds of SARD in a given individual [13]. Thus, the accurate identification of the AC-2 pattern produced by autoantibodies to DFS70 is crucial for its clinical use as a potential biomarker to aid in the exclusion of SARD [82].
Nuclear fine speckled with mitotic plate pattern (AC-30)
A further refinement in the classification of nuclear speckled patterns was achieved by the characterization of the nuclear fine speckled pattern with similar staining of the metaphase plate. This was classified by ICAP as the “nuclear fine speckled with mitotic plate” pattern (NFS with plate) and was assigned the AC-30 code [14]. The AC-30 pattern has some similarities with AC-2 as both are fine speckled patterns that stain the interphase nuclei and the metaphase plate. However, in contrast to AC-2, the AC-30 pattern has a uniform distribution of speckles and the nuclear border is regular [81] (Figure 1D, Table 1). The AC-2 pattern, in turn, has a heterogeneous distribution of the nuclear speckles, with areas of dense speckles intermingled with areas of sparse coarse speckles. As a result of such heterogeneity, the border of the nucleus is irregular (moth-eaten pattern). Preliminary evidence indicates that the AC-30 and the AC-2 patterns have distinct immunological associations. While the AC-2 pattern is strongly associated with anti-DFS70 antibodies and the absence of antibodies to most clinically relevant antinuclear antibodies, the AC-30 pattern is not associated with anti-DFS70 antibodies and over 25 % of sera recognize one or more clinically relevant antinuclear antibodies [14]. This could explain the negative results in anti-DFS70-specific tests in AC-30 samples misclassified as AC-2. In a retrospective analysis from the databank of a large clinical laboratory, we explored 2,560 samples concomitantly tested for HEp-2 IFA and autoantibodies to dsDNA, nucleosome, Sm, U1-RNP, Scl-70, SS-A/Ro60, and SS-B/La. The AC-30 and the AC-2 patterns were reported in 609 (23.8 %) and 85 (3.4 %) samples, respectively. The most frequent autoantibodies observed in the AC-30 samples were directed against nucleosomes (17.8 %), SS-A/Ro60 (7.2 %), and dsDNA (6.9 %). In contrast, these autoantibodies were largely not detected in the 87 AC-2 samples: only three (3.4 %) with anti-SS-A/Ro antibodies. Importantly, among 310 samples with confirmed anti-DFS70 reactivity, the HEp-2 IFA tested showed the AC-2 pattern in 285 (92 %) samples, the AC-30 pattern in 21 (6.8 %) samples, AC-4 in seven (1.9 %) samples, and AC-1 in three (1 %) samples [14], 30]. Thus, while the confirmation of an AC-2 pattern with anti-DFS70 suggests the absence of clinically relevant autoantibodies and systemic autoimmune disease, the presence of the AC-30 pattern may indicate a moderate likelihood of association with clinically relevant autoantibodies and autoimmunity [14], 30].
In contrast, the AC-1 is strongly associated with antibodies to chromatin antigens, such as dsDNA, nucleosome and histones. In a comparative study, 29 of 30 samples with the AC-1 pattern had antibodies to dsDNA and/or nucleosome, whereas none of 30 samples with the AC-2 pattern had these autoantibodies. In comparison, the nuclear QH pattern showed an intermediate behavior, with 21 (35 %) of 60 samples presenting anti-nucleosome antibodies. Reactivity to histones was observed in 18 (60 %) of the 30 AC-1 samples, in 10 (16.7 %) of the 60 QH samples, and in only 1 (3.3 %) of the AC-2 samples [19].
Final considerations
HEp-2 IFA patterns are good indicators of the subjacent autoantibodies in the sample under analysis, however, the suspected autoantibodies should always be confirmed according to the clinical context of the patient. For example, the AC-1 pattern suggests reactivity against multiple chromatin-associated antigens, especially dsDNA and nucleosome. Anti-dsDNA and anti-nucleosome antibodies serve as useful biomarkers for monitoring SLE, with elevated levels observed during disease flares, particularly in lupus nephritis, and a decrease in levels following treatment [23]. Therefore, in suspected SLE cases presenting with an AC-1 pattern, confirmatory tests for anti-dsDNA and anti-nucleosome antibodies are recommended.
The QH speckled nuclear pattern has weaker association with clinically relevant autoantibodies and with SARD in comparison with the AC-1 pattern [58]. In addition, the QH pattern is associated with a broader range of antinuclear antibodies than the AC-1 pattern. Therefore, if the patient presents clinical manifestations consistently suggestive of SARD, further investigation for autantibodies to dsDNA, nucleosome, SS-A/Ro, SS-B/La, U1-RNP, and Sm is recommended, according to the most probable autoimmune diseases in consideration [58].
Along the spectrum of nuclear patterns with stained metaphase plate, the AC-30 pattern is characterized by a regular speckled texture. In contrast to the AC-2 pattern, up to 25 % of AC-30 samples may have reactivity to clinically relevant autoantibodies, including those against dsDNA, nucleosomes, Sm, U1-RNP, Scl-70, SS-A/Ro, and SS-B/La, therefore warranting further investigation if the clinical scenario is suggestive of a systemic autoimmune disease. Further down the spectrum of nuclear patterns with stained metaphase plate, when a non-uniform speckled fluorescence is observed throughout the interphase nucleus, characterized by heterogeneity in the size, brightness, and distribution of speckles, the AC-2 pattern is the most likely pattern, which, in most cases, is associated with anti-DFS70 antibodies and is not associated with systemic autoimmune diseases [7], 14], 81].
The AC-29 pattern shows a strong antigenic association with antibodies to DNA topoisomerase I, therefore this pattern is very relevant in cases suspected of SSc, particularly in the diffuse cutaneous SSc and more aggressive forms of the disease. If there is clinical suspicion of systemic sclerosis (SSc), a follow-up test for anti-topoisomerase I antibodies (formerly known as Scl-70) is recommended [13], 21]. Due to the very compact speckled texture of the nuclear staining and also because of staining of the metaphase plate, the AC-29 may be erroneously interpreted as the AC-1 pattern. Therefore, one should carefully look for the presence of the five AC-29 morphological elements in samples provisionally interpreted as AC-1 or QH nuclear pattern. In that sense, the observation of the metaphase under focus fine tuning can be useful in the detection of the NOR, which is not present in the AC-1 and QH patterns but is an integral element of the AC-29 pattern [21]. Figure 3 illustrates the spectrum of morphological characteristics of each of the nuclear patterns with stained metaphase plate, as well as the antigenic identity to be investigated in each context. Figure 4 summarizes the varying likelihoods of autoantibody associations between HEp-2 IFA nuclear patterns and stained metaphase chromosome plates, based on autoantibody specificities. It is important to emphasize that not all cases in the laboratory routine will show images with the distinctive characteristics necessary for a conclusive definition of the pattern. In such cases, the analyst can use the umbrella competent-level classification suggested by ICAP, i.e., AC-1/AC-2/AC-30, whereby a broader spectrum of possibilities of autoantibody associations and clinical relevance are addressed.
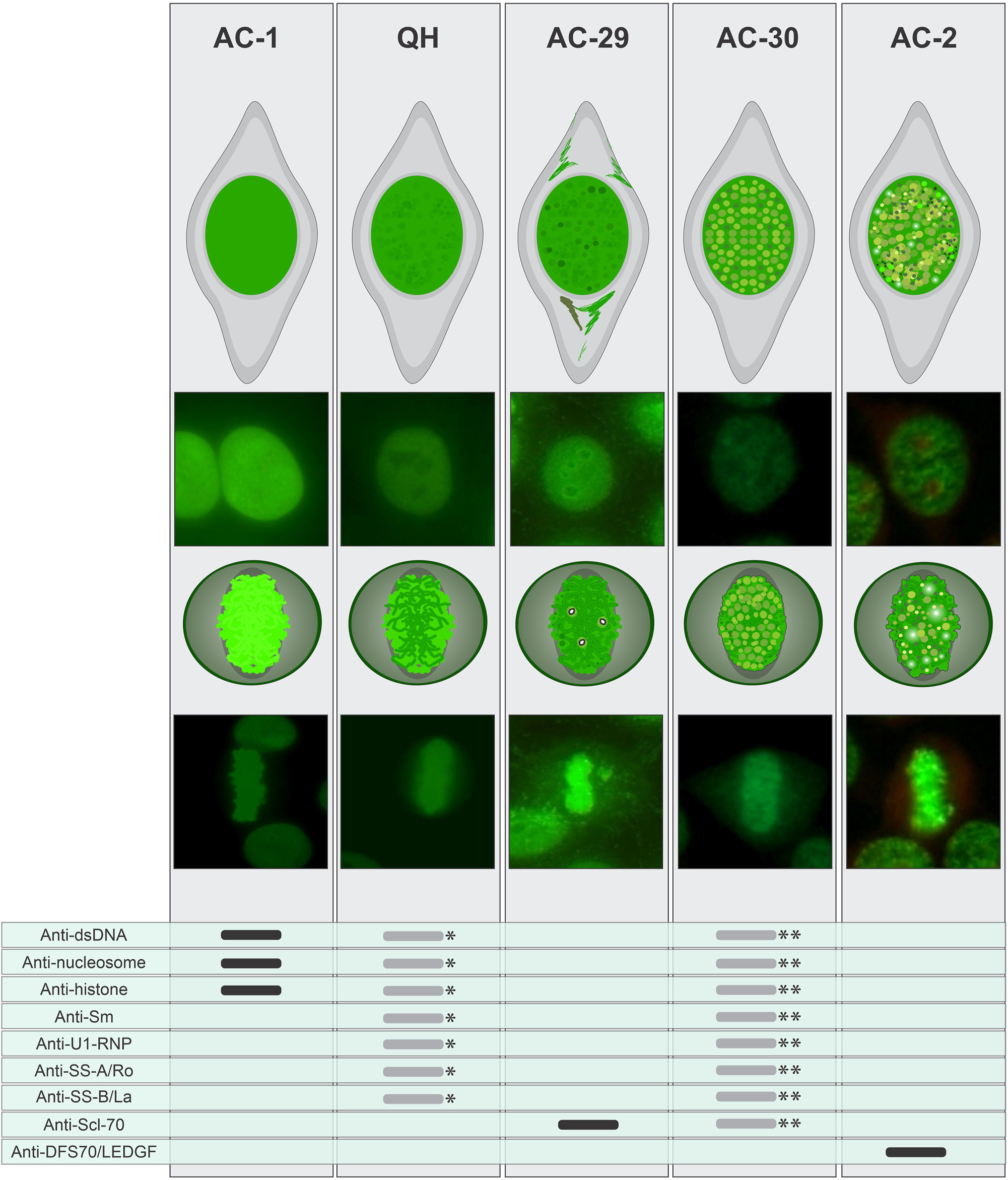
Comparison of morphological characteristics of the AC-1, BAC-3 (QH), AC-29, AC-30, and AC-2 patterns presenting a comparative analysis of the morphological features of the patterns, focusing on the staining in interphase nucleus, metaphase plate, and the reactivity of autoantibodies against immunogenic targets. *If the patient exhibits clinical manifestations suggestive of SARD, further testing for antibodies to dsDNA, nucleosome, SS-A/Ro, SS-B/La, U1-RNP, and Sm is recommended, guided by the most likely autoimmune diseases under consideration.**The AC-30 may exhibit reactivity to dsDNA, nucleosomes, histones, Sm, U1-RNP, Scl-70, SS-A/Ro, and SS-B/La in approximately one-fourth of cases, warranting additional investigation guided by the most likely autoimmune diseases under consideration.
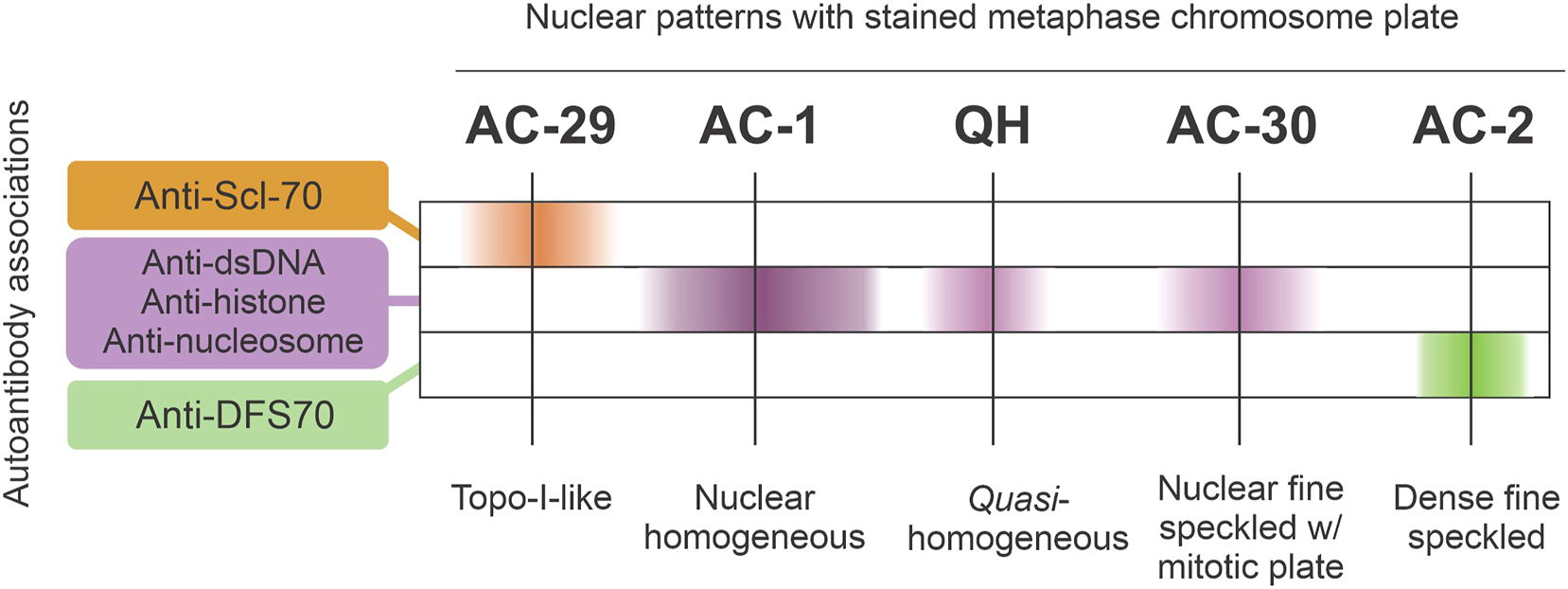
Schematic representation of the likelihood of autoantibody associations (anti-dsDNA, anti-nucleosome, anti-histone, anti-Scl-70, and anti-DFS70) with HEp-2 IFA nuclear patterns that exhibit staining of the metaphase chromosome plate.
Finally, it should be noted that samples with multiple autoantibodies to nuclear antigens may cause a mixed nuclear pattern in which none of the standard nuclear patterns can be clearly recognized. For example, a sample with antibodies to dsDNA, SS-A/Ro, and U1-RNP may produce a mixed nuclear pattern that cannot be classified as AC-1, AC-4 or AC-5. The observed staining texture may vary according to the serum concentration of each of these autoantibodies. In some cases, one may see a blurred speckled in the interphase nucleus and a homogeneous metaphase plate. Such mixed nuclear pattern is not unusual in samples from patients with SLE, a disease characterized by the co-occurrence of multiple antinuclear antibodies.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors’ contributions were as follows: WMC conceived the review and created the figures; GGF, LLS, WCVN, and CMG conducted the literature review and data extraction. WMC and PLCF wrote the original draft, and LECA performed the final revision of the manuscript. The authors have accepted responsibility for the entire content of the manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Kenney, GE. Origin of HEp-2 cells used for culture of chlamydiae. J Clin Microbiol 1993;31:470–1. https://doi.org/10.1128/jcm.31.2.470-471.1993.Search in Google Scholar PubMed PubMed Central
2. Dellavance, A, Gabriel Júnior, A, Nuccitelli, B, Taliberti, BH, von Mühlen, CA, Bichara, CDA, et al.. 3o Consenso Brasileiro para pesquisa de autoanticorpos em células HEp-2 (FAN): recomendações para padronização do ensaio de pesquisa de autoanticorpos em células HEp-2, controle de qualidade e associações clínicas. Rev Bras Reumatol 2009;49:89–98.10.1590/S0482-50042009000200002Search in Google Scholar
3. Meroni, PL, Schur, PH. ANA screening: an old test with new recommendations. Ann Rheum Dis 2010;69:1420–2. https://doi.org/10.1136/ard.2009.127100.Search in Google Scholar PubMed
4. Satoh, M, Chan, EK, Sobel, ES, Kimpel, DL, Yamasaki, Y, Narain, S, et al.. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert Rev Clin Immunol 2007;3:721–38. https://doi.org/10.1586/1744666x.3.5.721.Search in Google Scholar PubMed
5. Sack, U, Conrad, K, Csernok, E, Frank, I, Hiepe, F, Krieger, T, et al.. Autoantibody detection using indirect immunofluorescence on HEp‐2 cells. Ann N Y Acad Sci 2009;1173:166–73. https://doi.org/10.1111/j.1749-6632.2009.04735.x.Search in Google Scholar PubMed
6. Wiik, AS, Høier-Madsen, M, Forslid, J, Charles, P, Meyrowitsch, J. Antinuclear antibodies: a contemporary nomenclature using HEp-2 cells. J Autoimmun 2010;35:276–90. https://doi.org/10.1016/j.jaut.2010.06.019.Search in Google Scholar PubMed
7. Mariz, HA, Sato, EI, Barbosa, SH, Rodrigues, SH, Dellavance, A, Andrade, LEC. Pattern on the antinuclear antibody–HEp‐2 test is a critical parameter for discriminating antinuclear antibody–positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum 2011;63:191–200. https://doi.org/10.1002/art.30084.Search in Google Scholar PubMed
8. Dellavance, A, Junior, AG, Cintra, AFUC, Ximenes, AC, Nuccitelli, B, Taliberti, BH, et al.. II Consenso Brasileiro de Fator Antinuclear em Células HEp-2 (*) II Brazilian Consensus on Antinuclear Antibodies in HEp-2 Cells nucleolus, cytoplasm and mitotic apparatus, as wel as its clinical associations. Rev Bras Reumatol 2003;43:129–40.10.1590/S0482-50042003000300002Search in Google Scholar
9. Francescantonio, PLC, Cruvinel, Wde M, Dellavance, A, Andrade, LEC, Taliberti, BH, von Mühlen, CA, et al.. IV Brazilian guidelines for autoantibodies on HEp-2 cells. Rev Bras Reumatol 2014;54:44–50. https://doi.org/10.1016/j.rbre.2014.02.006.Search in Google Scholar
10. Cruvinel, Wde M, Andrade, LEC, Dellavance, A, Ximenes, AC, Bichara, CDA, Mangueira, CLP, et al.. VI Brazilian consensus guidelines for detection of anti-cell autoantibodies on HEp-2 cells. Adv Rheumatol 2022;62:34. https://doi.org/10.1186/s42358-022-00266-z.Search in Google Scholar PubMed
11. Cruvinel, Wde M, Andrade, LEC, von Mühlen, CA, Dellavance, A, Ximenes, AC, Bichara, CD, et al.. V Brazilian consensus guidelines for detection of anti-cell autoantibodies on hep-2 cells. Adv Rheumatol 2019;59:28. https://doi.org/10.1186/s42358-019-0069-5.Search in Google Scholar PubMed
12. Chan, EKL, Damoiseaux, J, Carballo, OG, Conrad, K, de Melo Cruvinel, W, Francescantonio, PLC, et al.. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014–2015. Front Immunol 2015;6. https://doi.org/10.3389/fimmu.2015.00412.Search in Google Scholar PubMed PubMed Central
13. Damoiseaux, J, Andrade, LEC, Carballo, OG, Conrad, K, Francescantonio, PLC, Fritzler, MJ, et al.. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis 2019;78:879–89. https://doi.org/10.1136/annrheumdis-2018-214436.Search in Google Scholar PubMed PubMed Central
14. Andrade, LEC, Klotz, W, Herold, M, Musset, L, Damoiseaux, J, Infantino, M, et al.. Reflecting on a decade of the international consensus on ANA patterns (ICAP): accomplishments and challenges from the perspective of the 7th ICAP workshop. Autoimmun Rev 2024;23:103608. https://doi.org/10.1016/j.autrev.2024.103608.Search in Google Scholar PubMed
15. Carballo, OG, Ingénito, FB, Ginaca, AA, Carabajal, P, Costa, MA, Balbaryski, J. Primer Consenso Argentino para la Estandarización de la Determinación de Anticuerpos Anti-Nucleares por Inmunof uorescencia Indirecta-HEp-2. Acta Bioquim Clin Latinoam 2012;46:3–13.Search in Google Scholar
16. Rodríguez, N, Buzzi, C, Cairoli, E, Coelho Andrade, LE, Danza, ÁMC, Montenegro, C, et al.. 1er Consenso Uruguayo de Anticuerpos Antinucleares Departamento de Laboratorio Clínico, Unidad de Enfermedades Autoinmunes Sistémicas, Hospital de Clínicas Unidad de Enfermedades Autoinmunes Sistémicas, Hospital Pasteur. Rev Medica Del Uruguay 2019;35.10.29193/RMU.35.4.7Search in Google Scholar
17. Infantino, M, Bizzaro, N, de Melo Cruvinel, W, Chan, EKL, Andrade, LEC. Adopting the International Consensus on ANA Patterns (ICAP) classification for reporting: the experience of Italian clinical laboratories. Clin Chem Lab Med 2024;62:830–4. https://doi.org/10.1515/cclm-2023-0752.Search in Google Scholar PubMed
18. Tarasov, YV, Kurtova, MM, Koltsova, IH, Shevchuk, AY, Gruzevskiy, OA. Ukrainian adaptation of the nomenclature of the international consensus on antinuclear antibody patterns. J V N Karazin Kharkiv Natl Univ Ser “Medicine” 2024:387–413. https://doi.org/10.26565/2313-6693-2024-50-09.Search in Google Scholar
19. França NR de. Caracterização imunológica do padrão Quasi-Homogeneo (QH), observado pela técnica de imunofluorescência indireta em células HEp-2. Universidade Federal de São Paulo; 2011. Available from: http://repositorio.unifesp.br/handle/11600/22520.Search in Google Scholar
20. Dellavance, A, Gallindo, C, Soares, MG, da Silva, NP, Mortara, RA, Andrade, LEC. Redefining the Scl-70 indirect immunofluorescence pattern: autoantibodies to DNA topoisomerase I yield a specific compound immunofluorescence pattern. Rheumatology 2009;48:632–7. https://doi.org/10.1093/rheumatology/kep070.Search in Google Scholar PubMed PubMed Central
21. Andrade, LEC, Klotz, W, Herold, M, Conrad, K, Rönnelid, J, Fritzler, MJ, et al.. International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I. Clin Chem Lab Med 2018;56:1783–8. https://doi.org/10.1515/cclm-2018-0188.Search in Google Scholar PubMed
22. Santos, WFS, Cantuária, APde C, Félix, Dde C, Nardes, LK, de Melo, ICS. The influence of demography and referral medical specialty on the detection of autoantibodies to HEP-2 cells in a large sample of patients. Adv Rheumatol 2022;62:32. https://doi.org/10.1186/s42358-022-00264-1.Search in Google Scholar PubMed
23. Bizzaro, N, Villalta, D, Giavarina, D, Tozzoli, R. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis. Autoimmun Rev 2012;12:97–106. https://doi.org/10.1016/j.autrev.2012.07.002.Search in Google Scholar PubMed
24. Krippner, H, Springer, B, Merle, S, Pirlet, K. Antibodies to histones of the IgG and IgM class in systemic lupus erythematosus. Clin Exp Immunol 1984;58:49–56.Search in Google Scholar
25. Zirwas, MJ, Kress, DW, Deng, J-S. The utility of antihistone antibody screening in the diagnosis of drug-induced lupus erythematosus. Arch Dermatol 2004;140. https://doi.org/10.1001/archderm.140.4.494.Search in Google Scholar PubMed
26. Shero, JH, Bordwell, B, Rothfield, NF, Earnshaw, WC. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science (80-) 1986;231:737–40. https://doi.org/10.1126/science.3003910.Search in Google Scholar PubMed
27. Ho, KT, Reveille, JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther 2003;5:80–93. https://doi.org/10.1186/ar628.Search in Google Scholar PubMed PubMed Central
28. Gabrielli, A, Avvedimento, EV, Krieg, T. Scleroderma. N Engl J Med 2009;360:1989–2003. https://doi.org/10.1056/nejmra0806188.Search in Google Scholar PubMed
29. Landoni, D, Keppke, G, Martins, T, Diaz, C. ALH consistent are the association of He-2 patterns and autoantibody specificities. How consistent are the association of HEp-2 patterns and autoantibody specificities. In: Conrad, K, Andrade, LEC, Chan, EKL, Damoiseaux, J, Fritzler, MJ, Meroni, PL, et al., editors. Achievements and challenges in research, diagnostics and therapy of autoimmune diseases. Dresden: Gesellschaft zur Förderung der Immundiagnostik e.V.; 2023.Search in Google Scholar
30. Fitch-Rogalsky, C, Steber, W, Mahler, M, Lupton, T, Martin, L, Barr, SG, et al.. Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. Kuwana M, editor. PLoS One 2014;9:e93812. https://doi.org/10.1371/journal.pone.0093812.Search in Google Scholar PubMed PubMed Central
31. Mahler, M, Parker, T, Peebles, CL, Andrade, LE, Swart, A, Carbone, Y, et al.. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol 2012;39:2104–10. https://doi.org/10.3899/jrheum.120598.Search in Google Scholar PubMed
32. Muro, Y, Sugiura, K, Morita, Y, Tomita, Y. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody–positive patients with autoimmune rheumatic disease. Lupus 2008;17:171–6. https://doi.org/10.1177/0961203307086311.Search in Google Scholar PubMed
33. Gómez-Puerta, JA, Burlingame, RW, Cervera, R. Anti-chromatin (anti-nucleosome) antibodies: diagnostic and clinical value. Autoimmun Rev 2008;7:606–11. https://doi.org/10.1016/j.autrev.2008.06.005.Search in Google Scholar PubMed
34. Rekvig, OP. The dsDNA, anti-dsDNA antibody, and lupus nephritis: what we agree on, what must be done, and what the best strategy forward could be. Front Immunol 2019;10. https://doi.org/10.3389/fimmu.2019.01104.Search in Google Scholar PubMed PubMed Central
35. Ehrenstein, MR, Katz, DR, Griffiths, MH, Papadaki, L, Winkler, TH, Kalden, JR, et al.. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int 1995;48:705–11. https://doi.org/10.1038/ki.1995.341.Search in Google Scholar PubMed
36. Schiffer, LE, Hussain, N, Wang, X, Huang, W, Sinha, J, Ramanujam, M, et al.. Lowering anti-dsDNA antibodies – what’s new? Lupus 2002;11:885–94. https://doi.org/10.1191/0961203302lu311rr.Search in Google Scholar PubMed
37. Akberova, NI, Zhmurov, AA, Nevzorova, TA, Litvinov, RI. An anti-DNA antibody prefers damaged dsDNA over native. J Biomol Struct Dyn 2017;35:219–32. https://doi.org/10.1080/07391102.2015.1128979.Search in Google Scholar PubMed
38. Villalta, D, Bizzaro, N, Bassi, N, Zen, M, Gatto, M, Ghirardello, A, et al.. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. Rieux-laucat F, editor. PLoS One 2013;8:e71458. https://doi.org/10.1371/journal.pone.0071458.Search in Google Scholar PubMed PubMed Central
39. Yung, S, Chan, TM. Autoantibodies and resident renal cells in the pathogenesis of lupus nephritis: getting to know the unknown. Clin Dev Immunol 2012;2012:1–13. https://doi.org/10.1155/2012/139365.Search in Google Scholar PubMed PubMed Central
40. Pisetsky, DS. Anti-DNA antibodies – quintessential biomarkers of SLE. Nat Rev Rheumatol 2016;12:102–10. https://doi.org/10.1038/nrrheum.2015.151.Search in Google Scholar PubMed
41. Egner, W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol 2000;53:424–32. https://doi.org/10.1136/jcp.53.6.424.Search in Google Scholar PubMed PubMed Central
42. Isenberg, D. Anti-dsDNA antibodies: still a useful criterion for patients with systemic lupus erythematosus? Lupus 2004;13:881–5. https://doi.org/10.1191/0961203304lu2028oa.Search in Google Scholar PubMed
43. Smeenk, RJT. Methodological update detection of antibodies to dsDNA: current insights into its relevance. Clin Exp Rheumatol 2002;20:294–300.Search in Google Scholar
44. Haugbro, K, Nossent, JC, Winkler, T, Figenschau, Y, Rekvig, OP. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann Rheum Dis 2004;63:386–94. https://doi.org/10.1136/ard.2003.016303.Search in Google Scholar PubMed PubMed Central
45. Suh-Lailam, BB, Chiaro, TR, Davis, KW, Wilson, AR, Tebo, AE. Evaluation of a high avidity anti-dsDNA IgG enzyme-linked immunosorbent assay for the diagnosis of systemic lupus erythematosus. Int J Clin Exp Pathol 2011;4:748–54.Search in Google Scholar
46. Infantino, M, Palterer, B, Previtali, G, Alessio, M-G, Villalta, D, Carbone, T, et al.. Comparison of current methods for anti-dsDNA antibody detection and reshaping diagnostic strategies. Scand J Immunol 2022;96:e13220. https://doi.org/10.1111/sji.13220.Search in Google Scholar PubMed
47. Burlingame, RW. The clinical utility of antihistone antibodies. Autoantibodies reactive with chromatin in systemic lupus erythematosus and drug-induced lupus. Clin Lab Med 1997;17:367–78. https://doi.org/10.1016/s0272-2712(18)30201-4.Search in Google Scholar
48. McGinty, RK, Tan, S. Nucleosome structure and function. Chem Rev 2015;115:2255–73. https://doi.org/10.1021/cr500373h.Search in Google Scholar PubMed PubMed Central
49. Prado, F, Jimeno-González, S, Reyes, JC. Histone availability as a strategy to control gene expression. RNA Biol 2017;14:281–6. https://doi.org/10.1080/15476286.2016.1189071.Search in Google Scholar PubMed PubMed Central
50. Flora, PK, Gregory, CD. Recognition of apoptotic cells by human macrophages: inhibition by a monocyte/macrophage‐specific monoclonal antibody. Eur J Immunol 1994;24:2625–32. https://doi.org/10.1002/eji.1830241109.Search in Google Scholar PubMed
51. Düzgün, N, Şahin, M, Genç, Y, Tutkak, H. Antinucleosome antibodies and systemic lupus erythematosus. Ann N Y Acad Sci 2007;1109:421–8. https://doi.org/10.1196/annals.1398.048.Search in Google Scholar PubMed
52. Amoura, Z, Koutouzov, S, Chabre, H, Cacoub, P, Amoura, I, Musset, L, et al.. Presence of antinucleosome autoantibodies in a restricted set of connective tissue diseases: antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis Rheum 2000;43:76–84. https://doi.org/10.1002/1529-0131(200001)43:1<76::aid-anr10>3.0.co;2-i.10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-ISearch in Google Scholar
53. Lee, AY, Ang, EB. A clinical overview of autoantibodies in general practice rheumatology. Br J Gen Pract 2014;64:e599–601. https://doi.org/10.3399/bjgp14x681601.Search in Google Scholar
54. Kubo, M, Ihn, H, Yazawa, N, Kikuchi, K, Tamaki, K, Sato, S. Prevalence and antigen specificity of anti-histone antibodies in patients with polymyositis/dermatomyositis. J Invest Dermatol 1999;112:711–5. https://doi.org/10.1046/j.1523-1747.1999.00580.x.Search in Google Scholar
55. Vordenbäumen, S, Böhmer, P, Brinks, R, Fischer-Betz, R, Richter, J, Bleck, E, et al.. High diagnostic accuracy of histone H4-IgG autoantibodies in systemic lupus erythematosus. Rheumatology 2018;57:533–7. https://doi.org/10.1093/rheumatology/kex462.Search in Google Scholar
56. Suzuki, T, Burlingame, RW, Casiano, CA, Boey, ML, Rubin, RL. Antihistone antibodies in systemic lupus erythematosus: assay dependency and effects of ubiquitination and serum DNA. J Rheumatol 1994;21:1081–91.Search in Google Scholar
57. European Association for the Study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971–1004. https://doi.org/10.1016/j.jhep.2015.06.030.Search in Google Scholar
58. França, NR, Dellavance, A, Rodrigues, SH, Perazzio, SF, Silva, NPAL. Quasi-homogeneous ANA-HEp-2 pattern reflects an autoantibody profile intermediate to the homogeneous and dense fine speckled nuclear patterns. In: Arthritis, R, editor. Arthritis & Rheumatism Abstract supplement 2011 Annual Scientific Meeting. Chicago, Illinois: WILEY-BLACKWELL Arthritis Rheum.; 2011.S901.Search in Google Scholar
59. Mo, Y-Y, Wang, P, Beck, WT. Functional expression of human DNA topoisomerase I and its subcellular localization in HeLa cells. Exp Cell Res 2000;256:480–90. https://doi.org/10.1006/excr.2000.4864.Search in Google Scholar PubMed
60. Champoux, JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 2001;70:369–413. https://doi.org/10.1146/annurev.biochem.70.1.369.Search in Google Scholar PubMed
61. Kuwana, M, Kaburaki, J, Medsger, TA, Wright, TM. An immunodominant epitope on DNA topoisomerase I is conformational in nature: heterogeneity in its recognition by systemic sclerosis sera. Arthritis Rheum 1999;42:1179–88. https://doi.org/10.1002/1529-0131(199906)42:6<1179::aid-anr14>3.0.co;2-e.10.1002/1529-0131(199906)42:6<1179::AID-ANR14>3.0.CO;2-ESearch in Google Scholar
62. Hildebrandt, S, Weiner, E, Senécal, J, Noell, S, Daniels, L, Earnshaw, WC, et al.. The igg, igm, and iga isotypes of anti‐topoisomerase i and anticentromere autoantibodies. Arthritis Rheum 1990;33:724–7. https://doi.org/10.1002/art.1780330515.Search in Google Scholar
63. Douvas, AS, Achten, M, Tan, EM. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem 1979;254:10514–22. https://doi.org/10.1016/s0021-9258(19)86738-8.Search in Google Scholar
64. van Venrooij, WJ, Stapel, SO, Houben, H, Habets, WJ, Kallenberg, CG, Penner, E, et al.. Scl-86, a marker antigen for diffuse scleroderma. J Clin Invest 1985;75:1053–60. https://doi.org/10.1172/jci111767.Search in Google Scholar
65. Guldner, H-H, Szostecki, C, Vosberg, H-P, Lakomek, H-J, Penner, E, Bautz, FA. Scl 70 autoantibodies from scleroderma patients recognize a 95 kDa protein identified as DNA topoisomerase I. Chromosoma 1986;94:132–8. https://doi.org/10.1007/bf00286991.Search in Google Scholar
66. Gelpí, C, Pérez, E, Roldan, C. Efficiency of a solid-phase chemiluminescence immunoassay for detection of antinuclear and cytoplasmic autoantibodies compared with gold standard immunoprecipitation. Auto- Immun highlights 2014;5:47–54. https://doi.org/10.1007/s13317-014-0059-x.Search in Google Scholar
67. de Almeida Brito, F, Maria Elói Santos, S, Aparecida Ferreira, G, Pedrosa, W, Gradisse, J, Cristina Costa, L, et al.. Diagnostic evaluation of ELISA and chemiluminescent assays as alternative screening tests to indirect immunofluorescence for the detection of antibodies to cellular antigens. Am J Clin Pathol 2016;145:323–31. https://doi.org/10.1093/ajcp/aqv083.Search in Google Scholar
68. Ge, H, Si, Y, Roeder, RG. Isolation of cDNAs encoding novel transcription coactivators p52 and p75 reveals an alternate regulatory mechanism of transcriptional activation. EMBO J 1998;17:6723–9. https://doi.org/10.1093/emboj/17.22.6723.Search in Google Scholar
69. Ochs, RL, Mahler, M, Basu, A, Rios-Colon, L, Sanchez, TW, Andrade, LE, et al.. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med 2016;16:273–93. https://doi.org/10.1007/s10238-015-0367-0.Search in Google Scholar PubMed PubMed Central
70. Ortiz-Hernandez, GL, Sanchez-Hernandez, ES, Casiano, CA. Twenty years of research on the DFS70/LEDGF autoantibody-autoantigen system: many lessons learned but still many questions. Autoimmun Highlights 2020;11:3. https://doi.org/10.1186/s13317-020-0126-4.Search in Google Scholar PubMed PubMed Central
71. Ochs, RL, Stein, TW, Peebles, CL, Gittes, RF, Tan, EM. Autoantibodies in interstitial cystitis. J Urol 1994;151:587–92. https://doi.org/10.1016/s0022-5347(17)35023-1.Search in Google Scholar PubMed
72. Ochs, RL, Muro, Y, Si, Y, Ge, H, Chan, EKL, Tan, EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol 2000;105:1211–20. https://doi.org/10.1067/mai.2000.107039.Search in Google Scholar PubMed
73. Ge, H, Si, Y, Wolffe, AP. A novel transcriptional coactivator, p52, functionally interacts with the essential splicing factor ASF/SF2. Mol Cell 1998;2:751–9. https://doi.org/10.1016/s1097-2765(00)80290-7.Search in Google Scholar PubMed
74. Singh, DP, Ohguro, N, Chylack, LT, Shinohara, T. Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci 1999;40:1444–51.Search in Google Scholar
75. Tesina, P, Čermáková, K, Hořejší, M, Procházková, K, Fábry, M, Sharma, S, et al.. Multiple cellular proteins interact with LEDGF/p75 through a conserved unstructured consensus motif. Nat Commun 2015;6:7968. https://doi.org/10.1038/ncomms8968.Search in Google Scholar PubMed
76. Carbone, T, Pafundi, V, Tramontano, G, Gilio, M, Padula, MC, Padula, AA, et al.. Prevalence and serological profile of anti-DFS70 positive subjects from a routine ANA cohort. Sci Rep 2019;9:2177. https://doi.org/10.1038/s41598-019-38686-5.Search in Google Scholar PubMed PubMed Central
77. Wei, X, Chen, R, Yang, C, Nguyen, K, Wakefield, D. Improved performance of confirmatory assays for detecting dense fine speckled (DFS) 70 antibodies. Pathology (Phila, Pa) 2022;54:904–9. https://doi.org/10.1016/j.pathol.2022.05.010.Search in Google Scholar PubMed
78. Yamada, K, Senju, S, Shinohara, T, Nakatsura, T, Murata, Y, Ishihara, M, et al.. Humoral immune response directed against LEDGF in patients with VKH. Immunol Lett 2001;78:161–8. https://doi.org/10.1016/s0165-2478(01)00243-7.Search in Google Scholar PubMed
79. Okamoto, M, Ogawa, Y, Watanabe, A, Sugiura, K, Shimomura, Y, Aoki, N, et al.. Autoantibodies to DFS70/LEDGF are increased in alopecia areata patients. J Autoimmun 2004;23:257–66. https://doi.org/10.1016/j.jaut.2004.07.004.Search in Google Scholar PubMed
80. Watanabe, A, Kodera, M, Sugiura, K, Usuda, T, Tan, EM, Takasaki, Y, et al.. Anti‐DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum 2004;50:892–900. https://doi.org/10.1002/art.20096.Search in Google Scholar PubMed
81. Infantino, M, Bizzaro, N, Grossi, V, Manfredi, M. The long-awaited ‘pseudo-DFS pattern’. Expert Rev Clin Immunol 2019;15:445. https://doi.org/10.1080/1744666x.2019.1596801.Search in Google Scholar
82. Sanchez-Hernandez, ES, Ortiz-Hernandez, GL, Ochoa, PT, Reeves, M, Bizzaro, N, Andrade, LEC, et al.. The nuclear dense fine speckled (DFS) immunofluorescence pattern: not all roads lead to DFS70/LEDGFp75. Diagnostics 2023;13:222. https://doi.org/10.3390/diagnostics13020222.Search in Google Scholar PubMed PubMed Central
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025
Articles in the same Issue
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025

