Abstract
Introduction
To analyze the available evidence about the correlation between the presence of detectable amounts of clostebol metabolites in urine and the transdermal or transmucosal contact of clostebol.
Content
A systematic review was performed. A systematic search was conducted in PubMed/MEDLINE, Scopus, Web of Science and the Cochrane library databases. Criteria for including studies were clinical studies reporting: (i) adult subjects; (ii) detection of urine clostebol metabolites derived from transdermal or transmucosal contact of clostebol.
Summary
Seven papers pertinent to our questions were found: 3 case reports, one experimental study and 3 case reports with an experimental section for a total of 32 subjects. The median concentration of urine clostebol’s metabolite 4-chloro-androst-4-en-3α-ol-17-one, M1 was 0.5 ng/mL (range 0.086–4.000 ng/mL; 25%–75 % IQ: 0.5–0.9 ng/mL) and 8.1 ng/mL (range 1.0–36.6 ng/mL; 25%–75 % IQ: 2.8–22.0 ng/mL), in subjects with indirect and direct exposure of clostebol, respectively (p=0.005).
Outlook
We found consistent data that the detection of M1 in urine can be reconcilable with a transdermal or transmucosal contact of clostebol. In the cases of indirect exposure, the urine concentrations of M1 seem to be far lower than the concentrations found in case of direct exposure.
Introduction
Clostebol (4-chloro-testosterone; 4-chloro-4-androsten-17β-ol-3-one) is the 4-chloro derivative of testosterone. It is a synthetic androgenic steroid with anabolic effect (AAS). The approved medical use of clostebol in humans is limited to topical administration, primarily clostebol acetate in dermatological drugs (Veterabol® or Trofodermin® cream or spray containing 0.5 % clostebol acetate, equivalent to 5 mg of clostebol acetate each application), as well as ophthalmological and gynecological preparations. These pharmaceutical formulations are available in several countries such as Italy, where it can be obtained in the pharmacy without a medical prescription, Brazil, Peru, Central America, and Pakistan. Clostebol, as other AAS or anabolic agents, if taken for long periods, can boost physical performance, for example increase strength, support muscle growth and accelerate recovery [1]. Clostebol is listed as a prohibited substance by the World Anti-Doping Agency (WADA). An increased number of adverse analytical findings (AAFs) involving clostebol were reported in the last years. Some cases where athletes claimed an inadvertent uptake were famous. Before the Rio de Janeiro 2016 Summer Olympic Games, some athletes tested positive to clostebol. The case of the skier Therese Johaug and a very recent case involved a top tennis athlete were subjects of controversial discussions. Several metabolites have been detected in the urine of subjects exposed to clostebol, including glucurono- and sulforo-conjugated metabolites [2] using a gas chromatography–mass spectrometry (GC-MS) or liquid chromatography–mass spectrometry (LC-MS) [3], 4]. It is not clear if the detection of clostebol metabolites in urine, and in particular the main clostebol metabolite (4-chloro-androst-4-en-3α-ol-17-one, M1) generally used at the screening level, can be reconcilable with a transdermal or transmucosal direct or indirect transfer, nor at which concentration, even in the case of a single intake of clostebol, whether or not intentional or inadvertent [5], 6]. Therefore, we performed a systematic review (SR) regarding the correlation between the presence of detectable amounts of clostebol metabolites in urine and the transdermal or transmucosal contact of clostebol. Moreover, we want to analyze if the urine concentration of clostebol metabolites derived from transdermal or transmucosal contact with clostebol is lower than therapeutic or recreative concentration (for example to enhance muscular performance).
Methods
Protocol and registration
We registered this study on PROSPERO (registration number CRD42020137629; a protocol was not prepared). Regarding the SR, search strategy, study selection as well as data extraction and analysis were performed and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Institutional review board approval was not required.
Eligibility criteria
We included clinical studies (clinical trials, cohort observational studies, case series and case reports) reporting: (i) adult subjects; (ii) detection of urine clostebol metabolites derived from transdermal or transmucosal contact of clostebol. Criteria for excluding studies were in vitro studies or animal studies.
Information sources
A systematic search was conducted on clostebol in PubMed/MEDLINE, Scopus, Web of Science and the Cochrane library databases up to the end of December 2024, through a comprehensive search strategy without language restriction, combining MeSH terms and free terms (Supplemental Table S1). Reference lists of all pertinent retrieved clinical studies were also analyzed through a manual search, in order to identify additional relevant papers. Moreover, the grey literature, such as conference abstracts, was searched in the Scopus database and screened for pertinence.
Study selection and data extraction
Three blinded investigators (V.G.M., G.R. and A.F.) independently screened titles and abstracts to identify potentially relevant articles. Duplicate publications were actively searched and excluded. Full-texts of potentially pertinent articles were obtained and analyzed by three independent investigators and data were extracted in a pre-designed structured form. Any disagreement about a paper’s inclusion or data extraction was resolved by discussion with a fourth independent reviewer (G.P.). The quality of the included studies was not formally assessed.
In detail, data from each study were extracted as follows: first author name and year of publication; study design; subjects’ characteristics, gender, athlete or volunteer; type of contact, direct defined as trans-dermal or trans-mucosal contact with the drug containing clostebol or indirect defined as contact of the skin or mucosa of the subject with the skin or mucosa (or fur) of another person (or animal) that had direct exposure with the drug containing clostebol; time of exposure to clostebol: we distinguished fast contact (<5 s), intermediate contact (between 6 s and 5 min) and prolonged (>6 min in a single contact or as the sum of the durations of multiple contacts); time from the last exposure to clostebol and the urine exam; the type of urine exam, GC-MS and/or LC-MS; the type of clostebol urine metabolite analyzed and its concentration. Missing information or data were requested from corresponding authors, contacting them by e-mail.
Statistical analysis
Data were expressed as median (range and interquartile range) unless otherwise stated. Comparisons were made using Mann–Whitney U test or chi-square test as appropriate. A significance level of 0.05 was used for the statistical tests. We analyzed our data descriptively with SPSS Statistics, version 23.0 (New York, USA).
Results
The extensive search of the literature followed by a careful manual screening of retrieved articles led to the identification of 7 papers pertinent to our questions. The search algorithm is detailed in Figure 1. According to our aims, all retrieved studies have been included for data extraction regardless of their methodological quality. In detail, we found three case reports [7], [8], [9] one experimental study [10] and three case reports with an experimental section [11], [12], [13]. Overall, 32 cases of determination of urine clostebol metabolite after TD or TM exposure to the drug were found (Table 1): 7 athletes (2 males, 2 females and 3 unspecified) and 25 healthy volunteers (19 males, 5 females and 1 unspecified). In 19 cases the exposure of clostebol was direct: 8 subjects self-applied cream (5 TM and 3 TD), 4 subjects applied cream to another person (TD) and 7 subjects received application of cream (TD). In 13 cases the exposure of clostebol was indirect: 3 subjects had sexual intercourse (TM), 9 subjects had contact with the hand of a volunteer self-applying cream (TD) and in one case the subject touched her dog, which she applied spray to. The dose of clostebol to which the subjects was exposed varied from 0.5 to 200 mg, whilst it was not specified in the case reports. The time of exposure varied in the different studies, but only in the study by de la Torre et al. in 8 subjects (24 %) it could be defined as a fast contact (<1 min). In 26 cases (79 %) GC-MS was performed within 24 h from the exposure to clostebol. The median concentration of M1 in the overall population was 2.6 ng/mL (range 0.086–36.6 ng/mL; 25 %–75 % IQ: 0.805–10.625 ng/mL). The median concentration of M1 was 0.5 ng/mL (range 0.086–4.000 ng/mL; 25 %–75 % IQ: 0.5–0.9 ng/mL) and 8.1 ng/mL (range 1.0–36.6 ng/mL; 25 %–75 % IQ: 2.8–22.0 ng/mL), in subjects with indirect and direct exposure of clostebol, respectively (Table 2). These values were significantly different (p=0.005). The overall rate of M1 concentration<2 ng/mL was 50 %: 85 % (11/13) and 26 % (5/19) in subjects indirectly exposed to clostebol and in those directly exposed to clostebol, respectively (p=0.004). Moreover, M1 was<5 ng/mL in all subjects indirectly exposed to clostebol.
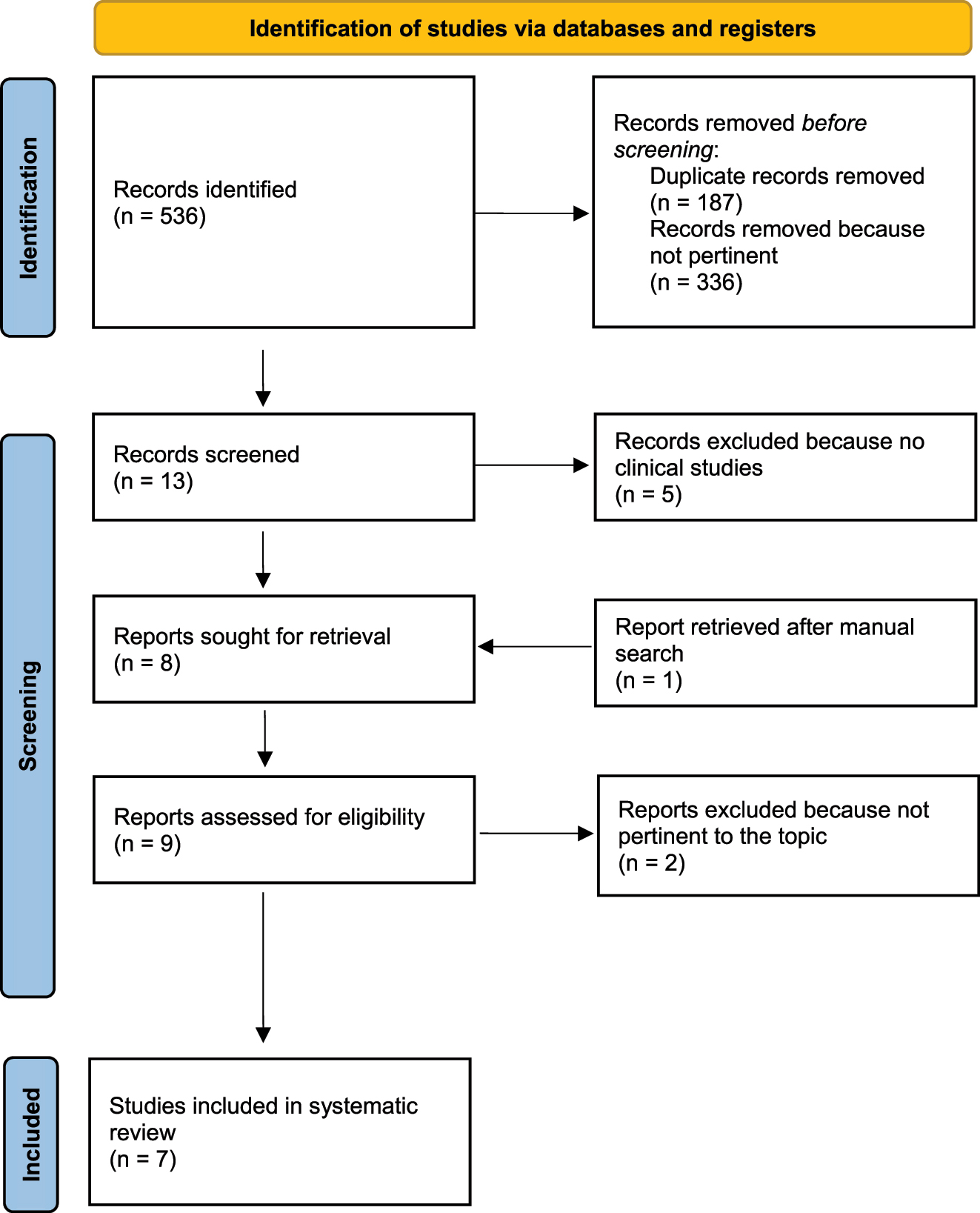
Flow diagram of the study selection process.
Table of evidence of the retrieved studies.
| Article | Study design | Type of contact | Subject | Dose of clostebol | Type of exposurea | Time of exposure | Time exposure-exam, hours | Time exposure-exam < 24 h | Urine exam MS | Urine M1 concentration, ng/mL | Time exposure-exam (2), hours | Urine M1 concentration (2), ng/mL | Details of the case |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pereira 2004 [11] | Case report | TM | Athlete | nd | Indirect | nd | nd | No | GC | 0.9 | nd | nd | Subject had sexual intercourse with a woman taking gynecological cream |
| Pereira 2004 [11] | Experimental | TM | M Volunteer | 5 g | Indirect | nd | 16 | Yes | GC | 0.9 | nd | nd | Subject had sexual intercourse with a woman taking gynecological cream |
| Pereira 2004 [11] | Experimental | TM | M Volunteer | 5 g | Indirect | Prolonged | 16 | Yes | GC | 3.5 | nd | nd | Subject had sexual intercourse with a woman taking gynecological cream |
| Pereira 2004 [11] | Experimental | TM | F Volunteer | 5 g | Direct | Prolonged | 23 | Yes | GC | 35 | nd | nd | Subject self-applied cream to her vagina |
| Pereira 2004 [11] | Experimental | TM | F Volunteer | 5 g | Direct | Prolonged | 23 | Yes | GC | 35 | nd | nd | Subject self-applied cream to her vagina |
| Pereira 2004 [11] | Experimental | TM | M Volunteer | 200 mg | Direct | Intermediate | 3.5 | Yes | GC | 22 | 15 | detectableb | Subject self-applied cream to his penis |
| Pereira 2004 [11] | Experimental | TM | M Volunteer | 200 mg | Direct | Intermediate | 3.5 | Yes | GC | 22 | 15 | detectableb | Subject self-applied cream to his penis |
| Salomone 2019 [7] | Case report | TD | M Athlete | nd | Direct | Prolonged | 168 | No | GC | 1 | nd | nd | Subject applied cream to his wife |
| de la Torre 2020 [12] | Case report | TD | Athlete | nd | Direct | nd | nd | No | GC | 1 | nd | nd | Subject applied cream to a teammate |
| de la Torre 2020 [12] | Case report | TD | Athlete | nd | Direct | nd | nd | No | GC | 1 | nd | nd | Subject applied cream to a teammate |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 15 mg | Direct | Prolonged | 48 | No | GC | 32 | 263 | 0.8 | Subject received three applications of cream (5 mg of clostebol in each one) |
| de la Torre 2020 [12] | Experimental | TD | F Volunteer | 15 mg | Direct | Prolonged | 15 | Yes | GC | 20 | 146 | 0.4 | Subject applied cream to another volunteer |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Direct | Intermediate | 11 | Yes | GC | 8 | 165 | 1.3 | Subject self-applied cream |
| de la Torre 2020 [12] | Experimental | TD | F Volunteer | 5 mg | Indirect | Fast | 5 | Yes | GC | 4 | 48 | 0.5 | Subject had close contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 4 | Yes | GC | 0.5 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 4 | Yes | GC | 0.5 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 4 | Yes | GC | 0.1 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 7 | Yes | GC | 0.5 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 7 | Yes | GC | 0.5 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| de la Torre 2020 [12] | Experimental | TD | M Volunteer | 5 mg | Indirect | Fast | 4 | Yes | GC | 0.5 | nd | nd | Subject had fast contact with the hand of the volunteer self-applying cream |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 8.1 | 288 | tracesb | Subject received application of cream at the back of his neckd |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 7.4 | 264 | tracesb | Subject received application of cream at the back of his neckd |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 1.7 | 360 | tracesb | Subject received application of cream on top of his handd |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 36.6 | 120 | tracesb | Subject received application of cream on top of his handd |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 3.9 | 96 | tracesb | Subject received application of cream on top of his lower armd |
| Gessner 2024 [13] | Experimental | TD | M Volunteer | 10 mgc | Direct | Prolonged | 6 | Yes | GC | 6.4 | 312 | tracesb | Subject received application of cream on top of his lower armd |
| Gessner 2024 [13] | Case report | TM | F Athlete | 5 mg | Direct | Prolonged | 23 | Yes | GC | 13 | 144 | 13 | Subject self-applied cream (lip-balm) to her lips |
| Kintz 2024 [10] | Experimental | TD | F Volunteer | 5 mg | Direct | Intermediate | 8 | Yes | LC and GC | 1.02 | 15 | nd | Subject self-applied cream on top of her hand |
| Kintz 2024 [10] | Experimental | TD | M Volunteer | 50 mg | Direct | Intermediate | 8 | Yes | LC and GC | 9.83 | 15 | nd | Subject self-applied cream on top of her hand |
| Kintz 2024 [10] | Experimental | TD | Volunteer | 0.5 mge | Indirect | Prolonged | 21 | Yes | LC and GC | 0.52 | nd | nd | Subject received 5 massages (ankles and foots) from the volunteer self-applying spray (one time) |
| Kintz 2024 [8] | Case report | TD | M Athlete | nd | Indirect | Prolonged | 12 | Yes | LC and GC | 0.086 | 192 | 0.076 | Subject received massages on ankles and foots from a masseur self-applying spray |
| Pokrywka 2024 [9] | Case report | TD/TM | F Athlete | 3.5 mge | Indirect | Prolonged | 48 | No | LC | 1.7 | nd | nd | Subject applied spray (14 times) to her dog and after that she had close contact with it |
-
MS, mass spectrometry; TM, trans mucosal; TD, trans dermal; nd, no data; M, male; F, female; GC, gas chromatography (mass spectrometry); LC, liquid chromatography (mass spectrometry). Time exposure-exam (2): time between exposure and the last detection of urine M1 during control urine exams. Urine M1 concentration (2): concentration of urine M1 at the last detection during control urine exams. aDirect exposure: drug/skin or drug/mucosa; indirect exposure: skin/skin or mucosa/mucosa, but also fur/skin. bformally 0.1 ng/mL; c10 mg of each AAS: oxandrolone, metandienone (17β-hydroxy-17α-methylandrosta-1,4-dien-3-one), clostebol acetate and DHCMT (dehydrochloro-methyltestosterone) dissolved in 100 μ of dimethyl sulfoxide (DMSO) 99.8 %. dMaximum value of the following ranges: 0.2–8.1 ng/mL; 0.2–7.4 ng/mL; 0.2–1.7 ng/mL; 0.1–36.6 ng/mL; 0.4–3.9 ng/mL; 0.1–6.4 ng/mL. eSpray 5 mg/mL: 1 sprinkle of 0.1 mL.
Characteristics of the study population.
| Type of exposure | Subject | Dose of clostebol | Time of exposure | Urine M1, ng/mL |
|---|---|---|---|---|
| Indirect | ||||
|
|
||||
| #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 #12 #13 |
Athlete M Volunteer M Volunteer F Volunteer M Volunteer M Volunteer M Volunteer M Volunteer M Volunteer M Volunteer volunteer M Athlete F Athlete |
nd 5 g 5 g 5 mg 5 mg 5 mg 5 mg 5 mg 5 mg 5 mg 0.5 mg nd 3.5 mg |
Missing Intermediate Prolonged Fast Fast Fast Fast Fast Fast Fast Prolonged Prolonged Prolonged |
0.9 0.9 3.5 4 0.5 0.5 0.1 0.5 0.5 0.5 0.52 0.09 1,7 |
|
|
||||
| Direct | ||||
|
|
||||
| #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 #12 #13 #14 #15 #16 #17 #18 #19 |
F Volunteer F Volunteer M Volunteer M Volunteer M Athlete athlete Athlete M Volunteer F Volunteer M Volunteer M Volunteer M Volunteer M Volunteer M Volunteer M Volunteer M Volunteer F Athlete F Volunteer M Volunteer |
5 g 5 g 200 mg 200 mg nd nd nd 15 mg 15 mg 5 mg 10 mg 10 mg 10 mg 10 mg 10 mg 10 mg nd 5 mg 50 mg |
Prolonged Prolonged Intermediate Intermediate Prolonged Missing Missing Prolonged Prolonged Intermediate Prolonged Prolonged Prolonged Prolonged Prolonged Prolonged Missing Intermediate Intermediate |
35 35 22 22 1 1 1 32 20 8 8.1 7.4 1.7 36.6 3.9 6.4 13 1.02 9.83 |
Only in 14 cases (43 %) a control value of M1 concentration after 15–288 h was available (Table 2).
Discussion
To the best of our knowledge, this is the first-ever SR including adults with detection of clostebol urine metabolites after transdermal or transmucosal contact.
As expected, we did not find observational studies, but only case reports of athletes and results of experiments conducted on healthy volunteers. We decided to manage data from all subjects together because the biological characteristics of the athletes cited in the case reports can be considered very similar to those of the volunteers. Moreover, the same urine metabolite M1 was reported, and GC-MS was used in all the cases, although in the works of Gessner [13] and Kintz [8], 10] LC-MS was also performed. Both GC-MS and LC-MS are the most reported systems in literature [3], 4] and, interestingly, in the last decade laboratories have developed methods lowering the limit of detection, which is now established at 0.1 ng/mL [12].
We found that the detection of clostebol metabolites in urine can be reconcilable with a transdermal or transmucosal direct or indirect exposure to clostebol, even in case of a single or fast contact. The concentration of urine M1 was significantly higher in the case of direct exposure (median 33.5 ng/mL) than in the case of indirect exposure (median 0.5 ng/mL). The dose of clostebol to which the subjects were directly or indirectly exposed was similar in all the studies, but two: it was higher (200 mg) in two of the volunteers in the study by Pereira [11] and in one (50 mg) of the volunteers in the study by Kintz [10]. In these three cases the concentration of M1 was higher than in the others, confirming a direct correlation between administered doses and urinary metabolites’ levels. We found that time of direct or indirect exposure to clostebol and time from exposure to urine exam correlated with M1, too. The values of M1 were higher after intermediate/prolonged exposure and if the urine exams were performed more than 24 h after the exposure (only in 5/31 cases, 16 %). In our opinion, it would be very interesting to observe at what time and at what concentration urine clostebol metabolites remain detectable, but only a few studies reported a control value of urine M1, which was lower than the first one and almost always<0.1 ng/mL [8], 12], 13].
Other factors influencing the detection of clostebol urine metabolites are individual metabolism, such as genetic and ethnical variations [14], creatinine concentration, but the latter was studied only by Gessner et al. [13], confirming the correlation between dilution of urine samples and the detectability of substances’ metabolites. In the case of transdermal contact, skin thickness could be a key factor influencing the detection of clostebol urine metabolites. Moreover, hand washing could increase removal of the substance from the palm of the hands. Therefore, interpretations of the data must be considered carefully.
Indeed, the case described by Pokrywka [9] appeared unprecedented because it involves the use of clostebol in spray form and its absorption both trough hand-to-dog’s paw contact, and via product inhalation.
Finally, a low urine concentration can also be interpreted as the residue of a drug voluntarily used to enhance physical performance. Illegal preparations, such as clostebol caproate or propionate tablets or injectable clostebol esters can be found on the black market [15], and their use seems to correlate to clostebol urine metabolites exceeding 2–5 ng/mL. This finding seems to be confirmed in our SR. In fact, we found that the urine concentrations of M1 derived from transdermal or transmucosal indirect contact of clostebol were slower than 5 ng/mv in all the cases and also lower than 2 ng/mL in 86 % of cases. On the other hand, after direct exposure to clostebol only one in five subjects had M1 concentration lower than 5 (and also<2) ng/mL.
Finally, the identification of clostebol in hair has been seldom reported [7], [8], [9]. Although WADA does not recognize the use of hair as routine doping control matrix, it can support athletes by demonstrating the contamination scenario.
Limitations
The main limitations of our SR are the relative heterogeneity of the included studies in terms of the study design, different modality of clostebol exposure, for example as a mixture containing other AAS, and the relative high prevalence of missing data, which was only marginally corrected by contacting corresponding authors. Moreover, the available studies are case reports or very small-sized. Unfortunately, it was impossible to perform a multiple imputation and only a qualitative analysis was done. The low methodological quality prevents any attempt to verify the consistency of the results and to generate reliable summary measures of efficacy. Thus, the pooled response rate shown should be interpreted as the theoretical average values perceived by readers of literature about use of clostebol metabolites after transdermal/transmucosal direct or indirect contact, rather than the true effect. In addition, we found some difficulty in finding and extracting data about the use of clostebol. For example, de la Torre [12] cited 47 positive cases of adverse analytical findings involving clostebol, with concentrations<2 ng/mL in 77 % of the cases from 2003 to 2018 and Kintz [10] reported other 50 positives cases, starting from 2018. However, we were unable to find scientific reports regarding these cases, therefore it is not known if some of these cases were due to transdermal or transmucosal exposure to clostebol. Finally, there were few data about the effective urine concentration that correlates with the use of clostebol to enhance physical performance, thus the value we proposed could be questionable.
Conclusions
In conclusion, our SR identified some flaws in the available literature. However, we found consistent data that the detection of M1 in urine can be reconcilable with a transdermal or transmucosal direct or indirect transfer of clostebol. In the cases of indirect exposure to clostebol, the urine concentrations of clostebol metabolites seem to be far lower than the concentrations found in case of therapeutic or recreative use of clostebol.
Acknowledgments
In memory of Dr. Lucia Manfredi.
-
Research ethics: Because the study is a systematic review, Institutional Review Board approval was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). None.
-
Informed consent: Not applicable.
-
Author contributions: V.G.M. and G.P. conceived this study. M.C. and A.P. conducted the systematic search. V.G.M., G.R. and A.F. conducted data collection. G.R. was responsible for data management. V.G.M. and A.F. analyzed all data. V.G.M. drafted the article, and all authors contributed substantially to its revision. V.G.M. takes responsibility for the paper as a whole. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
-
Clinical trial regiatration: We registered this study on PROSPERO (registration number CRD42020137629).
References
1. Geyer, H, Schänzer, W, Thevis, M. Anabolic agents: recent strategies for their detection and protection from inadvertent doping. Br J Sports Med 2014;48:820–6.10.1136/bjsports-2014-093526Search in Google Scholar PubMed PubMed Central
2. Lu, J, Fernández-Álvarez, M, Yang, S, He, G, Xu, Y, Aguilera, R. New clostebol metabolites in human urine by liquid chromatography time-of-flight tandem mass spectrometry and their application for doping control. J Mass Spectrom 2015;50:191–7.10.1002/jms.3517Search in Google Scholar PubMed
3. Balcells, G, Pozo, OJ, Garrostas, L, Esquivel, A, Matabosch, X, Kotronoulas, A, et al.. Detection and characterization of clostebol sulfate metabolites in Caucasian population. J Chromatogr B 2016;1022:54–63. https://doi.org/10.1016/j.jchromb.2016.03.028.Search in Google Scholar PubMed
4. Iannone, M, Botrè, F, Martinez-Brito, D, Matteucci, R, de la Torre, X. Development and application of analytical procedures for the GC–MS/MS analysis of the sulfates metabolites of anabolic androgenic steroids: the pivotal role of chemical hydrolysis. J Chromatogr B 2020;1155:122280. https://doi.org/10.1016/j.jchromb.2020.122280.Search in Google Scholar PubMed
5. Thieme, D, Anielski, P, Grosse, J, Sachs, H, Mueller, RK. Identification of anabolic steroids in serum, urine, sweat and hair: comparison of metabolic patterns. Anal Chim Acta 2003;483:299–306. https://doi.org/10.1016/s0003-2670(02)01604-5.Search in Google Scholar
6. Magnusson, BM, Cross, SE, Winckle, G, Roberts, MS. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacol Physiol 2006;19:336–42.10.1159/000095254Search in Google Scholar PubMed
7. Salomone, A, Gerace, E, Di Corcia, D, Alladio, E, Vincenti, M, Kintz, P. Hair analysis can provide additional information in doping and forensic cases involving clostebol. Drug Test Anal 2019;11:95–101.10.1002/dta.2469Search in Google Scholar PubMed
8. Kintz, P, Gheddar, L, Pichini, S, Plebani, M, Salomone, A. Clostebol and sport: about controversies involving contamination vs. doping offence. Clin Chem Lab Med 2025;63:258–61.10.1515/cclm-2024-1165Search in Google Scholar PubMed
9. Pokrywka, A, Sitkowski, D, Surała, O, Gheddar, L, Kintz, P. Case Report: a case study of positive doping control by animal-to-human drug transfer after an athlete administered medicine in spray format, containing clostebol acetate, to a pet dog. Front Sports Act Living 2024;6. https://doi.org/10.3389/fspor.2024.1480373.Search in Google Scholar PubMed PubMed Central
10. Kintz, P, Gheddar, L. Acétate de Costolo (Trofodermin®): vérification du passage transdermique d’un anabolisant souvent impliqué dans les affaires de dopage. Toxicol Anal Clin 2024;36:317–22. https://doi.org/10.1016/j.toxac.2024.10.001.Search in Google Scholar
11. Pereira, HMG, Marques, MAS, Talhas, IB, Neto, FRA. Incidental clostebol contamination in athletes after sexual intercourse. Clin Chem 2004;50:456–7, https://doi.org/10.1373/clinchem.2003.022210.Search in Google Scholar PubMed
12. de la Torre, X, Colamonici, C, Iannone, M, Jardines, D, Molaioni, F, Botrè, F. Detection of clostebol in sports: accidental doping? Drug Test Anal 2020;12:1561–9.10.1002/dta.2951Search in Google Scholar PubMed
13. Gessner, L, Thevis, M, Rothschild, MA, Jübner, M. Detectability of oxandrolone, metandienone, clostebol and dehydrochloromethyltestosterone in urine after transdermal application. Drug Test Anal 2022;14:1744–61. https://doi.org/10.1002/dta.3355.Search in Google Scholar PubMed
14. Dooley, TP, Haldeman-Cahill, R, Joiner, J, Wilborn, TW. Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultured cells. Biochem Biophys Res Commun 2000;277:236–45.10.1006/bbrc.2000.3643Search in Google Scholar PubMed
15. Kim, ED, Rahnema, CD, Crosnoe, LE, Kim, ED. Designer steroids-over-the-counter supplements and their androgenic component: review of an increasing problem. 2015. Available from: https://onlinelibrary.wiley.com/doi/10.1111/andr.307.10.1111/andr.307Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2025-0331).
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor

