Abstract
Objectives
Patient risk management is an essential subject for clinical laboratory which is now central in main international laboratory quality standards (e.g., ISO 15989:2022; ISO 22367:2020 and CLSI EP232nd). Risk analysis is a necessary part of risk management which requires categorizing the severity of patient harm from a laboratory failure. However, this subjective task is not currently the subject of any recommendation and little literature about this topic. To remedy that, we conducted an international survey of medical biology professionals, asking them to rate a panel of 20 analytes the harm potentially induced by an erroneous reported result.
Methods
The survey was published by Bio-Rad® to their customers base and the public with a dedicated webpage. The survey proposes to assign for the submitted analytes the amount of harm among five pre-defined categories of harm: negligible, minor, serious, critical, and catastrophic. Participants were also asked to specify their demographic characteristics.
Results
The questionnaires of 267 respondents coming from 43 countries were analyzed to allocate for each analyte a specific harm category. We highlight that almost all parameters (19/20) were categorized with at least a serious harm category and that none were associated with the negligible category.
Conclusions
This study constitutes the first international attempt to investigate how the laboratory community thinks about patient harm from an erroneous reported result. These results provide support to document the laboratory risk management policy which must now be centered on patient risk.
Introduction
All international quality standards for medical laboratories have updated their recommendations and requirements in recent years, focusing the quality management process on patient risk. ISO/IEC Guide 51:2014 defines risk as the “combination of the probability of occurrence of harm and the severity of that harm” [1]. The general concept of risk can be expressed as the probability of harm=probability of occurrence x the severity of harm [2]. In the clinical laboratory, risk analysis and risk management are focused on the risk of patient harm from erroneous and delayed results. The probability of occurrence is the probability of producing an erroneous or incorrect result. With an erroneous result being defined as a patient sample measurement that contains measurement error in excess of the quality specification for the analyte being measured. Erroneous results can be produced when the test method is “in control” state or “out of control” state of the test method. The probability of producing erroneous results is the sum of the probabilities of producing erroneous results when the test method is “in control” and “out of control” [3]. The severity of harm (SOH) in this context is the consequence to the patient when an erroneous result is produced [4]. As much as the occurrence can be estimated objectively with quantitative data, SOH determination is by nature subjective. Unfortunately, there is actually no guidance to estimate SOH except recommendation for using a qualitative five level scale of harm [4], 5] (Table 1). Some descriptive studies propose to evaluate the SOH caused by lab errors from declarative failure reports. Nevertheless, they do not provide any information on the specific criticality of each examination which can considerably differ from one analysis to another [6], 7]. We propose here, an international survey approach to estimate objectively and specifically the SOH in case of erroneous reported results for a bundle of 20 routine analysis. The aim of this study is to propose some guidance for SOH assignments that can be used by the clinical diagnostic laboratory community to improve compliance with ISO 15189:2022 requirements.
Qualitative description of harm based on scale of severity levels from CLSI EP232nd Edition.
| Descriptive category | Severity level |
|---|---|
| Negligible | Inconvenience or temporary discomfort |
| Minor | Temporary injury or impairment not requiring professional medical intervention |
| Serious | Injury or impairment requiring professional medical intervention |
| Critical | Permanent impairment or life-threatening injury |
| Catastrophic | Patient death |
Materials and methods
Survey design
To determinate the SOH that would be caused to a patient by an incorrect laboratory result only one question was submitted to the responder: “How would you rate the severity of patient harm if a clinician would interpret incorrect reported results from these analytes?”. This question was accompanied by a list of 20 selected blood routine analysis. We surveyed biochemistry analytes (sodium, potassium, troponin, glucose, creatinine, phosphorus, ALT, lipase, protein total, iron, albumin, TSH, CA 19-9 and hCG total), coagulation and hematology parameters (DD-dimer, fibrinogen, prothrombin time, haemoglobin and platelet) and one pharmacology analysis (digoxin). Five pre-defined point scale categories were proposed to the responder: i) negligible ii) minor iii) serious iv) critical v) catastrophic (Table 1). The responder has the possibility to not answer. These categories are those which are given by CLSI EP232nd [4] and ISO 22367:2020 [5] guidelines. A pilot study conducted among the biochemistry’s team of CHU de Rennes revealed some recurrent difficulties to answer this seemingly simple question. Accordingly, a nota bene was added to avoid pitfalls and ensure that responders have the same interpretation of the question. This nota bene was formulated as follows: “as we understand that an erroneous result could generate different severity of harm depending on clinical context and error depth we advise you to consider the most unfavorable situation that can occur in your lab activity”. We also ask for demographic specification to characterize the population of responders: geographic localization, background, principal medical lab activity and the medical structure of the lab. Responses were multi-choice, but also open-ended responses were allowed. The complete survey is available in the Supplementary Material.
Survey deployments and participation
To select appropriate responders “the sample by recommendation” method was used for sampling design. For this purpose, the survey was distributed by Bio-Rad® to both their customer base (via email) and the public (via social media and during events). In august 2021 the final English survey was launched via SurveyMonkey® on a dedicated Bio-Rad® webpage (https://info.bio-rad.com/quality-control-innovation-survey.html). Translated versions were provided on the webpage from November 2021 in French, Italian, Czech and Spanish. A German and a Russian version were also directly shared with interested participants. No compensation was offered to encourage participation in the survey. The data collection process ended in November 2023, but the webpage survey is still available to further integrate new potential data.
Survey analysis
Survey results were extracted then exported into a csv file and loaded into R (version 4.3.2) [8] with RStudio (version 2023.9.1.494) [9] to make calculations, figures and tables. All responses were checked by the authors. Only questionnaires with complete demographic data and from clinical laboratory professionals were retained.
Prevailing Severity of Harm Category attribution
In order to propose some guidance, we decided to assign a unique SOH category for each parameter named the “Prevailing Severity of Harm Category”. By default, this category is the modal category of the survey (the “no answer” category was removed and not considered for calculation). When difference between the modal and the second category is less than 5 %, we choose to assign the highest rank of SOH.
Results
Survey participation
A total of 286 complete questionnaires were collected during the survey process. After review by the authors 19 questionnaires were excluded to be sure that all participants were clinical laboratory professionals. Therefore, a total of 267 (93.4 %) questionnaires were included in the study.
Respondent characteristics
Geographic distribution
The 267 participants originate from five continents and 43 countries. The majority originates from Europe (68.16 %), followed by Africa (15 %), the Middle East (13.1 %) and to a small extent Asia (2.3 %) and North America (1.5 %). Three countries account for more than 10 % of all participants: France (14.6 %), Italy (12 %) and Germany (10.5 %). The first extra-European country is United Arab Emirates (6 %) just followed by Kenya (4.5 %). The complete distribution of all countries is presented in Supplementary Figure S1.
Academic background distribution
Six multi-choices and an open-ended response were proposed to delineate the academic background of the professional laboratory responder. The highest category is represented by the “other” group which corresponds to the open-ended response with a total of 28.1 % of the responders. This group is mostly constituted by lab technicians which have different academic backgrounds according to their country of origin. The “other” category is just followed by the MD background which represents 26.2 % of the total. The complete distribution of the different academic backgrounds is presented in Supplementary Figure S2.
Principal lab activity distribution
Seven multi-choices and an open-ended response were proposed to delineate the principal lab activity of the professional laboratory responder. Two categories are largely over-represented: clinical chemistry and versatile activity which represent respectively 43.5 and 36.7 % for a cumulated frequency of 80.2 %. The six other categories are equal or above 5 % of all participants. The “other” category which represents 5.2 % is principally constituted by immunohematology, toxicology and pre-analytics. The complete distribution of the main lab activity practice by the respondent is presented in Supplementary Figure S3.
Laboratory structure distribution
Three multi-choices and an open-ended response were proposed to delineate the type of laboratory structure of the professional laboratory responder. General hospital laboratories are the most represented type of laboratory with 40.8 % of the total, followed by private laboratories (28.5 %) and university hospital laboratories (24 %). The “other” category which represents 6.7 % is principally constituted by government lab and private hospital Lab. The complete distribution of the main laboratory structure is presented in Supplementary Figure S4.
Severity of harm survey results
Severity of harm level distribution
A total of 267 clinical laboratory professionals categorized for 20 analytes the amount of harm that would be potentially caused if a patient were to be mistreated based on an incorrect laboratory result. We observed a 99.1 % overall response rate for the SOH categorization part of the survey. The most chosen category is serious (33.9 %) followed by critical (30.1 %), catastrophic (16.3 %), minor (16.2 %), and to a much lesser extent the negligible category (2.7 %). The 20 analytes were analyzed individually to establish the SOH category distribution (Table 2). The SOH categorization results are also presented in a Likert plot (Figure 1) which can be used to classify the analytes from the potentially most harmful to the most harmless. We observed that troponin is considered by the responders to be the most harmful analyte; on the opposite blood iron is considered to be the most harmless in case of wrong results.
Distribution of severity of harm category by analyte (in percentage).
| Analyte | Severity of harm category, % | |||||
|---|---|---|---|---|---|---|
| No answer | Negligible | Minor | Serious | Critical | Catastrophic | |
| Albumin | 1.12 | 4.49 | 42.7 | 38.2 | 11.99 | 1.5 |
| ALT | 0.4 | 2.6 | 33.7 | 48.7 | 12 | 2.6 |
| CA 19-9 | 1.5 | 1.87 | 14.23 | 40.82 | 28.09 | 13.48 |
| Creatinine | 0 | 0 | 9 | 43.4 | 41.9 | 5.6 |
| D-dimer | 1.5 | 0.4 | 6.7 | 25.8 | 43.8 | 21.7 |
| Digoxin | 3 | 0.7 | 3 | 19.9 | 40.4 | 33 |
| Fibrinogen | 2.2 | 0.4 | 12.4 | 42.7 | 33.7 | 8.6 |
| Glucose | 0 | 0.4 | 5.6 | 31.8 | 40.4 | 21.7 |
| Haemoglobin | 0.37 | 0.75 | 4.12 | 25.09 | 38.58 | 31.09 |
| hCG total | 1.5 | 3.7 | 11.6 | 35.6 | 32.6 | 15 |
| Iron | 1.12 | 16.48 | 46.82 | 27.34 | 7.87 | 0.37 |
| Lipase | 2.25 | 3 | 18.73 | 45.32 | 25.84 | 4.87 |
| Phosphorus | 1.5 | 5.24 | 37.83 | 37.45 | 17.6 | 0.37 |
| Platelet | 0.7 | 0 | 3.7 | 27 | 42.7 | 25.8 |
| Potassium | 0 | 0 | 1 | 16 | 35 | 48 |
| Protein (total) | 1.9 | 7.5 | 37.1 | 41.2 | 12 | 0.4 |
| Prothrombin time | 1.5 | 0 | 6.4 | 28.5 | 41.6 | 22.1 |
| Sodium | 1.1 | 1.1 | 9.4 | 42.7 | 33.3 | 12.4 |
| Troponin | 0.04 | 0 | 0 | 10 | 32 | 57 |
| TSH | 1.5 | 2.25 | 20.22 | 49.81 | 22.47 | 3.75 |
-
The survey respondent’s category designation is presented in percentage in alphabetical order. The specific matrix of each analyte is serum or plasma for albumin, ALT, CA 19.9, creatinine, digoxin, glucose, hCG, total, iron, lipase, phosphorus, potassium, protein (total), sodium, troponin and TSH; whole blood for haemoglobin and platelet and at last plasma for D-dimer, fibrinogen and prothrombin time.
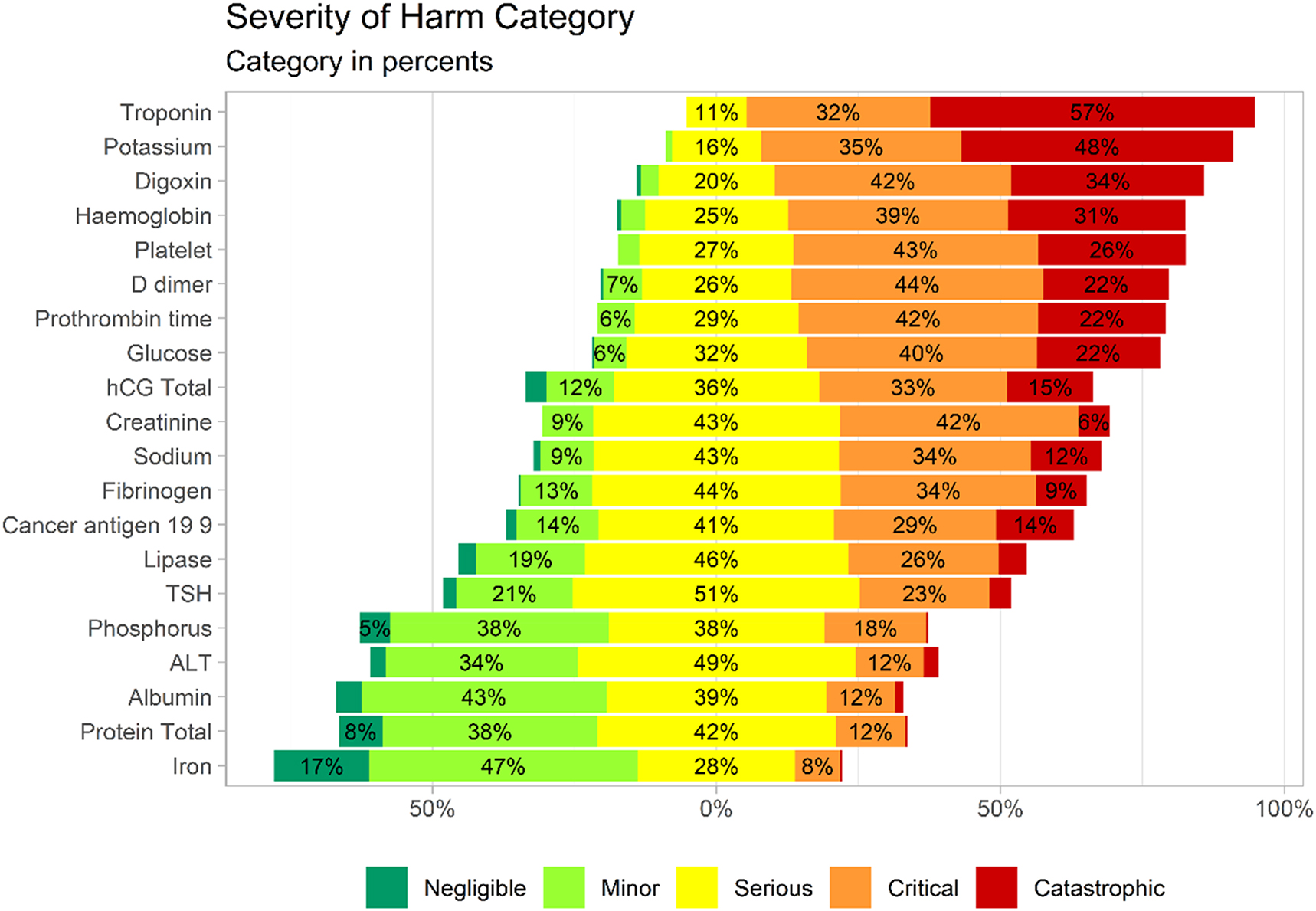
Likert plot representation of survey results of severity of harm categorization. The likert plot centered bars with splits the serious category. Analytes are ordered by the combined critical and catastrophic category. The “no answer” category was removed and not taking into account.
Severity of harm category attribution
To propose guidance for SOH determination, we decided to assign a unique SOH category for each parameter named the “Prevailing Severity of Harm Category”. This main assignment is designed to help clinical laboratory professionals to associate a ranked level of damage with a clinical analysis mistake. By default, the modal category of the survey is assigned to each parameter. In some cases, it could be irrelevant when the modal category is very close to another one. To remedy this lack of significance we choose to assign the highest rank of SOH when the difference between the modal and the second category is less than 5 %. It concerns 5 parameters and four were reclassified in the upper category (Albumin, Creatinine, Phosphorus and Protein Total). After reclassification two parameters were associated with a catastrophic SOH, eight were considered critical, nine as serious, only one as minor and no one was assigned to the negligible category of SOH. The Prevailing SOH Categories are presented for each tested parameter in Table 3 (modal categories are available in the supplementary materials in Supplementary Table S1).
Prevailing severity of harm category for the survey parameters.
| Analyte | Prevailing severity of harm category |
|---|---|
| Potassium (serum/plasma) | Catastrophic |
| Troponin (serum/plasma) | Catastrophic |
| Creatinine (serum/plasma) | Critical |
| D-dimer (plasma) | Critical |
| Digoxin (serum/plasma) | Critical |
| Glucose (serum/plasma) | Critical |
| Haemoglobin (whole blood) | Critical |
| hCG total (serum/plasma) | Critical |
| Platelet (whole blood) | Critical |
| Prothrombin time (plasma) | Critical |
| Albumin (serum/plasma) | Serious |
| ALT (serum/plasma) | Serious |
| CA 19-9 (serum/plasma) | Serious |
| Fibrinogen (plasma) | Serious |
| Lipase (serum/plasma) | Serious |
| Phosphorus (serum/plasma) | Serious |
| Protein (total) (serum/plasma) | Serious |
| Sodium (serum/plasma) | Serious |
| TSH (serum/plasma) | Serious |
| Iron (serum/plasma) | Minor |
Discussion
Risk management in medical laboratories has become a central part of the requirements for quality and competence in the new ISO 15189:2022 [10] which is in alignment with the ISO 22367:2020 specifically devoted to this topic [5]. In ISO 22367:2020 patient harm is specifically addressed in the standard Section 5.7 and states that “Reasonably foreseeable harms that could result from each hazardous situation shall be identified and classified along with the severity of each harm. This process and the identified harms, shall be documented.” [5]. Although this ISO standard was elaborated for medical laboratories, there are actually few literature or consensus guidelines to document patient harm in a risk analysis process. With this study we propose to specifically estimate with an international survey the SOH from erroneous lab results for a panel of 20 analytes.
As recommended in Annex I.4 of ISO 22367:2020 we use an ordinal variable with concise definitions without associated probability elements [5]. Consequently, our five level SOH scale fit with actual requirements and our results can be used in risk assessment process as defined by Janssens et al. [11]. The key point of this study is to define a SOH category which is independent of any clinical context. It cannot be denied that clinical context has major influence on patients’ harm from incorrect lab results. However, the variety of situations encountered for an analyte makes it impossible to consider in a lab risk management system. In addition, the clinical context is not always known by the laboratory. Consequently, in order to provide a usable laboratory tool, we apply CLSI EP232nd recommendations which state that the most harmful scenario had to be assumed for determining SOH when key considerations are unable [4]. As only lab professionals had a complete overview of their activity, we decided to address the survey to laboratory professionals rather than clinicians. This recruitment bias is assumed and makes the proposed SOH classification suitable for medical laboratory use.
A few studies are available to evaluate SOH induced by lab errors in real medical situations. Nevertheless they all indicate that most lab failures do not lead to patient harm which is in contrast to our survey results [6], 7]. Indeed, almost all surveyed parameters (19/20) were associated with at least a serious SOH category which is defined by “injury or impairment requiring professional medical intervention”. This should be explained that for real failure, laboratory errors are detected before they reach the patient. It is supported by the fact that postanalytical events, which are more likely to reach patients, contain the highest ratio of severe harm [7], 12]. It is also supported by O’Kane’s publication who ranked both real and potential patient outcomes for 658 quality failure lab reports. It reveals that despite any significant adverse outcomes, most events (67.9 %) were graded at the maximum rank of damage if the potential worst-case was considered [13]. These findings clarify our results and explain the high rate of serious and critical SOH categories attributed to our 20 evaluated parameters. Our results are also consistent with two recent studies which also used a survey approach with a five level scale to estimate the SOH from laboratory errors [14], 15]. Despite some differences with tested analytes, responder’s demography and numbers, they also found that the majority of asked parameters were associated with at least a serious SOH category (55.4 % [14] and 94.8 % [15]).
The main limitation of the current study is the lack of representativeness of the survey responders. This lack of diversity is particularly important from a geographical point of view. Despite a wide range of represented countries (n=43), four countries are largely over-represented and totalized 46.4 % of the participants (France, Italy, Germany, Spain). We also noted that there were virtually no representatives from Asia (2.3 %), the entire American continent (1.5 %) and no one from Australia. Moreover, the European countries totalized 68.2 % of the responder’s origin. This over-representation of the EU is due to the fact that the survey was launched by the European Bio-Rad® team and therefore was well promoted during congresses or virtual events across Europe. Moving forward, additional efforts in promotion need to take place trying to improve uniformity of the response rate globally.
Hazardous situations occur to patients when an incorrect result is reported to a clinician or when a critical result is delayed [5]. By asking “How would you rate the severity of patient harm if a clinician would interpret incorrect reported results from these analytes?” our SOH evaluation cannot assess harm caused by delayed results. This limitation should be coped in further survey investigations by adding question(s) which specifically deal with this topic. However, our results can document the consequences of laboratory failure for many steps of the total test process and could help to identify the most critical ones. As requested by the ISO 15189:2022 standard Section 7.1 our proposed SOH assignment can provide guidance for laboratories to monitor and evaluate (for a specific test) the identified risk and effectiveness of mitigation process according to the potential harm to the patient [10].
The prevailing SOH category classification can also be used for internal quality control (IQC) establishment strategy by applying the model presented in CLSI document EP232nd [4]. This model proposed to map a SOH category to a corresponding maximum acceptable probability of patient harm from incorrect results. The laboratory can then design a quality control strategy that have a predicted probability of patient harm below this maximum. The use of a such model permits laboratories to satisfy the ISO 15189:2022 subclause 7.3.7.2 which states that IQC shall be performed at a frequency that is based on the stability and robustness of the examination method and the risk of harm to the patient from an erroneous result [10].
Conclusions
To the best of our knowledge, this is the first international attempt to investigate how the laboratory community thinks about patient harm from an erroneous reported result. While severity of harm designations should be specific to the local clinical system, guidance can be provided by collecting the opinion of a wide variety of clinical laboratory professionals. This proposed SOH category evaluation constitutes a valuable tool for laboratories to document their risk management policy and responds to a need prompted by the normative evolution of all international laboratory quality standards. Nevertheless, this approach needs to be extended to a much more exhaustive set of parameters and matrix with more participants to become routinely usable and recognized by the scientific community of clinical diagnostic laboratories. During the last decade this community was deeply involved in elaborating quality measurement tools as Quality Indicators [16] to reduce error rate and enhance patient safety: this study contribute to the global effort and give some guidance to improve risk assessment standardization in laboratory medicine.
Funding source: Bio-Rad Laboratories
Acknowledgments
The authors would like to thank all the participants of the survey for their contributions. Lucas Peltier thanks the biochemistry’s team of CHU de Rennes for their contributions to the pilot study and Charles Brottier for his support with R code.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Bart Peeters (BP), Lucas Peltier (LP) and Sophie Van Aelst (SVA) proposed the survey approach in consultation with John Yundt-Pacheco (JYP). All authors contributed to survey conception and design. Jean-Baptiste Raimbourg (JBR) was responsible for survey deployment, data collection process and administrated the project. LP, BP and SVA did the curation of the data. LP performed computations with R, design figures and tables and took the lead in writing the manuscript. BP, JBR, SVA provided critical feedback and corrected the manuscript. JYP gave input and reviewed the final manuscript. All authors had access to the data from the study and approved the manuscript and had final responsibility for the decision to submit for publication.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Lucas Peltier received from Bio-Rad® consulting fee, payment for writing the draft and for the development of an educational presentation. Sophie Van Aelst & Bart Peeters received from Bio-Rad® consulting fee. Jean-Baptiste Raimbourg & John Yundt-Pacheco are Bio-Rad® employees.
-
Research funding: This study was carried out with funding from Bio-Rad Laboratories.
-
Data availability: The data that support the findings of this study are available from Bio-Rad laboratories but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Bio-Rad laboratories.
References
1. Guidelines for their inclusion in standards, ISO/IEC Guide 51:2014 Safety aspects 2014. [Internet] Available from: https://www.iso.org/standard/53940.html.Search in Google Scholar
2. Signori, C, Ceriotti, F, Sanna, A, Plebani, M, Messeri, G, Ottomano, C, et al.. Process and risk analysis to reduce errors in clinical laboratories. Clin Chem Lab Med 2007;45:742–8. https://doi.org/10.1515/cclm.2007.172.Search in Google Scholar
3. Parvin, CA, Baumann, NA. Assessing quality control strategies for HbA1c measurements from a patient risk perspective. J Diabetes Sci Technol 2018;12:786–91. https://doi.org/10.1177/1932296818758768.Search in Google Scholar PubMed PubMed Central
4. Laboratory quality control based on risk management 2nd ed., CLSI EP23; 2023. [Internet] Available from: https://clsi.org/standards/products/method-evaluation/documents/ep23/.Search in Google Scholar
5. Medical laboratories — application of risk management to medical laboratories 2020, ISO 22367:2020 [Internet]; Available from: https://www.iso.org/standard/71254.html.Search in Google Scholar
6. Snydman, LK, Harubin, B, Kumar, S, Chen, J, Lopez, RE, Salem, DN. Voluntary electronic reporting of laboratory errors: an analysis of 37,532 laboratory event reports from 30 health care organizations. Am J Med Qual Off J Am Coll Med Qual 2012;27:147–53. https://doi.org/10.1177/1062860611413567.Search in Google Scholar PubMed
7. Restelli, V, Taylor, A, Cochrane, D, Noble, MA. Medical laboratory associated errors: the 33-month experience of an on-line volunteer Canadian province wide error reporting system. Diagn Berl Ger 2017;4:79–86. https://doi.org/10.1515/dx-2017-0013.Search in Google Scholar PubMed
8. R Core Team. _R: a language and environment for statistical computing_. Vienna, Austria: R Foundation for Statistical Computing; 2023. https://www.R-project.org/.Search in Google Scholar
9. Posit team. RStudio: integrated development environment for R. Posit software. Boston, MA: PBC; 2023. URL http://www.posit.co/.Search in Google Scholar
10. Medical laboratories — requirements for quality and competence 2022, ISO 15189:2022 [Internet]; Available from: https://www.iso.org/standard/76677.html.Search in Google Scholar
11. Janssens, PM. Practical, transparent prospective risk analysis for the clinical laboratory. Ann Clin Biochem 2014;51:695–704. https://doi.org/10.1177/0004563214521160.Search in Google Scholar PubMed
12. Van Moll, C, Egberts, T, Wagner, C, Zwaan, L, Ten Berg, M. The nature, causes, and clinical impact of errors in the clinical laboratory testing process leading to diagnostic error: a voluntary incident report analysis. J Patient Saf 2023;19:573–9. https://doi.org/10.1097/pts.0000000000001166.Search in Google Scholar
13. O’ Kane, M. The reporting, classification and grading of quality failures in the medical laboratory. Clin Chim Acta 2009;404:28–31. https://doi.org/10.1016/j.cca.2009.03.023.Search in Google Scholar PubMed
14. Ilardo, C, Lamarti, C, Al Muhanna, B, Bastelica, M, Benaily, N. Sentinel testing, analytical sigma metrics and a risk management approach as part of a simplified method verification/validation process. Scand J Clin Lab Invest 2024;84:569–76. https://doi.org/10.1080/00365513.2024.2442512.Search in Google Scholar PubMed
15. Çubukçu, HC, Cihan, M, Alp, HH, Bolat, S, Zengi, O, Uçar, KT, et al.. Measuring the impact: severity of harm from laboratory errors in 195 tests. Am J Clin Pathol 2024:aqae144. https://doi.org/10.1093/ajcp/aqae144.Search in Google Scholar PubMed
16. Plebani, M, Astion, ML, Barth, JH, Chen, W, Galoro, CA, De, O, et al.. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52:951–8. https://doi.org/10.1515/cclm-2014-0142.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2024-1477).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor

