Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
-
Ann Helen Kristoffersen
, Martine J. Hollestelle
, Janne Cadamuro
, Andreas Hillarp
, Gro Gidske
, Dagmar Kesseler
Abstract
Objectives
Coagulation test results may be affected by hemolysis, icterus and/or lipemia (HIL). Detailed guidelines for HIL-management are missing, both for manual and automatic HIL-checks. The aim of this survey was to provide an overview of the practical procedures for the detection and handling of HIL-samples used by laboratories in Europe in the context of coagulation testing.
Methods
A SurveyMonkey questionnaire was sent from the Haemostasis Working Group in the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM) to European external quality assurance organizers, who in turn forwarded the link to their participating laboratories. Questions were asked regarding detection and handling of HIL-samples, comment- and reject-levels, and the guidance used by the laboratories.
Results
A written procedure for HIL-management was available in 55–67 % of laboratories, and each sample was checked for HIL in 73–83 % (lowest percentage for icterus, highest for hemolysis). Manual visual inspection as the only method to detect HIL was used by up to 38 % of laboratories, with most relying on personal experience for HIL-level classification. All other laboratories used some type of automated HIL-detection, alone or in combination with visual check. The terms used for classification and the HIL comment- and reject-levels varied widely, even among laboratories using the same manufacturer. Most laboratories state that they use the manufacturer’s guidance.
Conclusions
There is wide heterogeneity in HIL-detection, handling and reporting among European laboratories, which calls for an urgent collaboration among laboratories and manufacturers to harmonize the HIL-management in coagulation testing.
Introduction
Coagulation testing is used for diagnosing thromboembolic and bleeding diseases, assessing risk of thrombosis and bleeding, as well as for monitoring anticoagulant treatment or factor replacement therapy. It is of utmost importance that results of coagulation testing are correct as erroneous results may lead to incorrect diagnosis and treatment, hence increase the risk of bleeding and/or thrombosis. Preanalytical errors are relatively frequent [1] and it is therefore important to detect variables within this part of the total testing process which can interfere with the results of coagulation tests and assess whether these factors may lead to clinically relevant biased results [2], 3].
Interferences on measurements of coagulation tests caused by hemolysis (H), icterus/bilirubinemia (I) and lipemia (L) (HIL) are complex. Interference depends upon the instrument’s clot-detection method (optical or mechanical detection) for clot-based methods (e.g. activated partial thromboplastin time (APTT) and fibrinogen). Immunological and chromogenic methods (which are not clot-based, e.g. D-dimer and antithrombin) are measured using optical detection on all instruments. Optical detection methods (both clot and non-clot methods) are affected by the presence of analytical interference (spectrophotometric), while mechanical clot-detection is not. Notably, some optical methods may reduce analytical HIL-interference by switching to alternative wavelengths and/or use interpretation of coagulation (waveform) curves to draw attention to potential HIL-interference. Biological interference, on the other hand, may affect results both for instruments with optical and mechanical clot-detection methods [4], 5]. Biological interference is caused by coagulation activation when cell contents and phospholipid membranes are released during hemolysis and possibly by lipid particles in lipemia [2], [6], [7], [8], [9], [10]. Lipemia may also cause interference by volume displacement effect or by blocking antigen-antibody binding [7].
There are some general guidelines/recommendations for laboratories on how to detect and handle HIL in coagulation testing [2], [11], [12], [13], [14], as well as some general guidelines in clinical chemistry, which may also be applicable in coagulation [15]. However, these are not detailed enough in most instances to be used in procedures for specific coagulation reagent/instrument combinations. In the package inserts/instrument application manuals, manufacturers often notify about the level/concentration of HIL up to which interference is not influencing the results of their tests, unfortunately, most often without providing information on the study-protocol and acceptability criteria used [16]. Published studies may be difficult to interpret or apply to local settings due to the heterogeneity in the methods used and discrepancies in conclusions regarding magnitude and direction of interference [4], 5], 9], 10], [17], [18], [19], [20], [21], [22], [23], [24]. As there is a lack of detailed guidance, this survey aimed to gain insight into which procedures are used by European laboratories for HIL-detection. Additionally, we investigated how HIL-samples for routine coagulation testing are being handled and finally we aimed to audit which evidence local procedures are based on.
Materials and methods
The survey was led by the Haemostasis Working Group in the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM) [25]. Via the EQALM mailing list, a link to an online questionnaire (SurveyMonkey, Momentive Inc.) on practices regarding handling of HIL-samples in routine coagulation testing, was sent to European external quality assurance organizers, who in turn forwarded to their participating laboratories. The questionnaire was open for responses from June to September 2022. The response rate for each country could not be calculated as the total number of invited laboratories was not fully known. As some EQA organizers have customers outside of Europe, answers from 10 non-European laboratories were included as similar coagulation instruments were used.
The questionnaire covered routine coagulation tests, i.e. activated partial thromboplastin time (APTT), prothrombin time in seconds (PT-sec), international normalized ratio (INR), fibrinogen, D-dimer and antithrombin. Part I and Part II of the questionnaire (Supplemental file Questionnaire) contained questions about the laboratory and their routines, including presence of automatic HIL-check and written procedures for HIL-detection. The middle parts of the questionnaire consisted of similar questions for hemolysis (Part III), icterus (Part IV) and lipemia (Part V), e.g. situations including HIL-checks, method used for HIL-detection and HIL-level and comment- and reject-levels for HIL. Laboratories stating not to check any samples for H, I and/or L, respectively, were directed to the next part of the questionnaire. In the last part (Part VI), the laboratories were asked about HIL-level reports, internal quality controls (IQC) for HIL and participation in external quality assurance (EQA) for HIL in coagulation testing.
Where applicable, the laboratories were grouped according to the manufacturer of their main analytical instrument(s), if using one of the most prevalent instruments: Stago (Asnières-sur-Seine, France), Instrumentation Laboratories (IL), currently Werfen (Bedford, Massachusetts, USA), Sysmex (Kobe, Japan) and Siemens (Erlangen, Germany) (Supplemental Table 1). The results from laboratories using instruments from other manufacturers or manual tilt tube method are included when not grouped into manufacturers.
The laboratories were asked about their comment-level (i.e. the level of HIL (cut-off) where they would report the result with a comment) and their reject-level (i.e. the level of HIL (cut-off) where they would not report the result). These levels were stated either in quantitative or semi-quantitative levels or in concentrations. As most concentrations were given in mg/dL, concentrations in mmol/L (hemoglobin and triglycerides) and µmol/L (total bilirubin) were converted to mg/dL to enable comparison (Supplemental Tables 2–4). When the same level was stated both as comment- and reject-level, it is reported as the reject-level.
Statistics
Descriptive statistics (medians (10- and 90-percentiles) and percentages) were used. Percentages for each question were calculated based on number of laboratories responding to that particular question. Number of missing responses increased gradually throughout the questionnaire (319 (72.5 %) completed the entire survey). All laboratories were not expected to answer all questions, as some questions were shown or hidden, depending on answers to previous questions. SPSS version 22.0 (SPSS Inc.) and Excel (MicroSoft Office 365 v2302) were used for statistics and Figures.
Results
Number and characteristics of the responding laboratories
A total of 440 laboratories responded to the questionnaire, of which 430 (98 %) came from 33 European countries (93 % from 16 of these countries) and 10 from non-European countries (Figure 1).
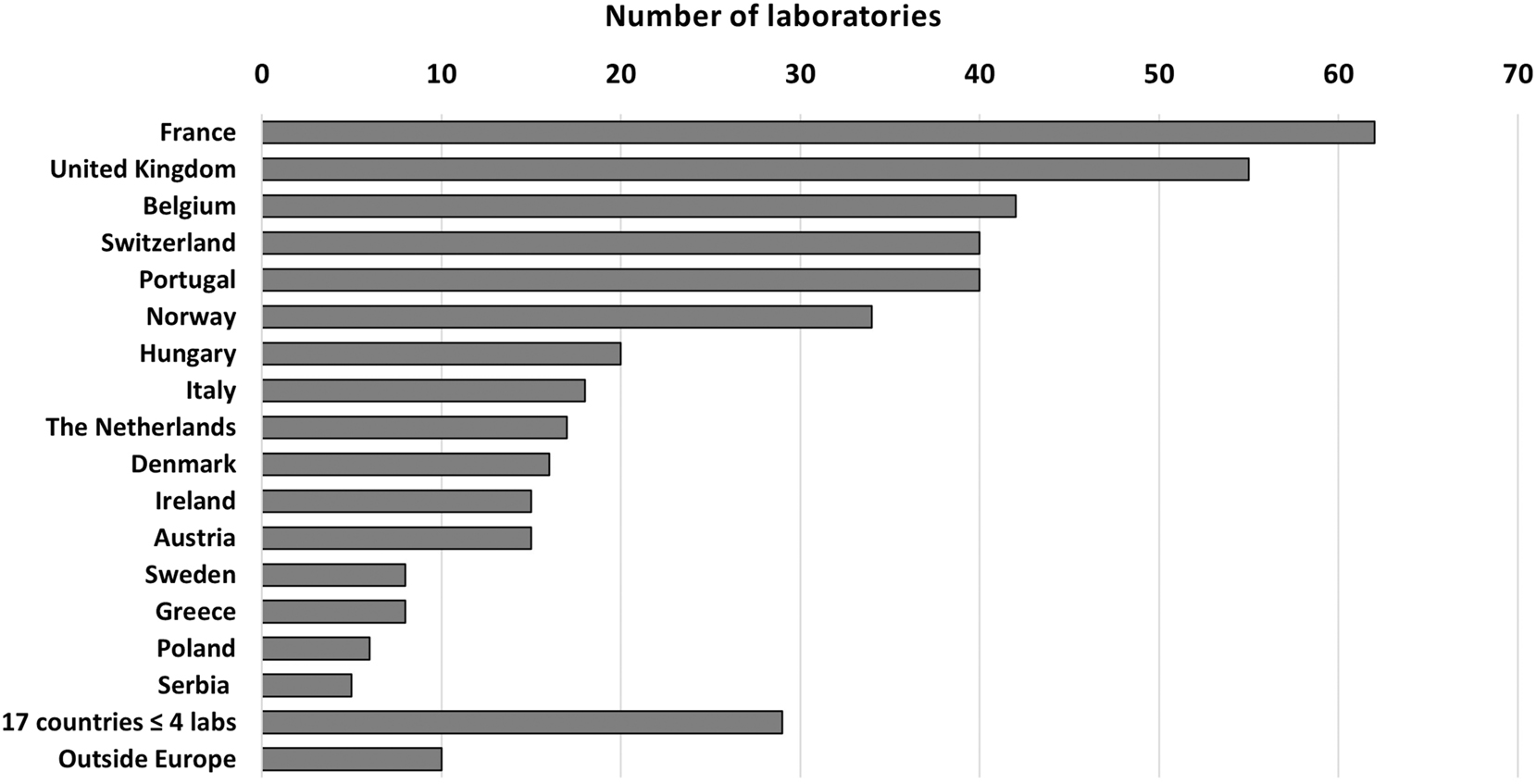
Number of laboratories answering from each country.
The characteristics of the laboratories are shown in Table 1. The majority (95 %) used coagulation instruments from one of the following manufacturers: Stago, IL, Sysmex and Siemens (Figure 2 and Supplemental Table 1). Optical clot-detection for APTT, PT, INR and fibrinogen was most often used among participants (71 %), but only 11 % of these used mechanical clot-detection method or manual tilt tube method as an alternative or back-up method. More than half of the laboratories (54 %) stated using the same instrument both as main and alternative, while the rest stated different other instruments with optical clot-detection, clinical chemistry instruments (D-dimer) or point-of-care devices (INR, D-dimer) as their back-up method (Supplemental Table 1).
Characteristics of the responding laboratories.
| Characteristics of laboratories | Number of laboratories (%) |
|---|---|
| Type of laboratory | |
| Non-private | 331 (75.2) |
| Private | 109 (24.8) |
| Samples received from | |
| Both primary and secondary care | 329 (75) |
| Only secondary care | 71 (16) |
| Only primary care | 40 (9) |
| Pneumatic tube systems (PTS) | 263 (60) |
| Blood collection by non-laboratory staff | 342 (78) |
| Coagulation tests | |
| APTT | 410 (93.2) |
| PT | 365 (83.0) |
| INR | 411 (93.4) |
| Fibrinogen | 379 (86.1) |
| D-dimer | 378 (85.9) |
| Antithrombin | 223 (50.7) |
| Number of coagulation tests in 1 year (2021) | Number of tests Median (10- to 90-percentiles) |
| APTT | 32,406 (3,888–120,777) |
| PT | 48,689 (4,560–138,888) |
| INR | 41,587 (3,960–122,000) |
| Fibrinogen | 7,353 (751–74,000) |
| D-dimer | 7,641 (1,292–24,007) |
| Antithrombin | 500 (75–5,040) |
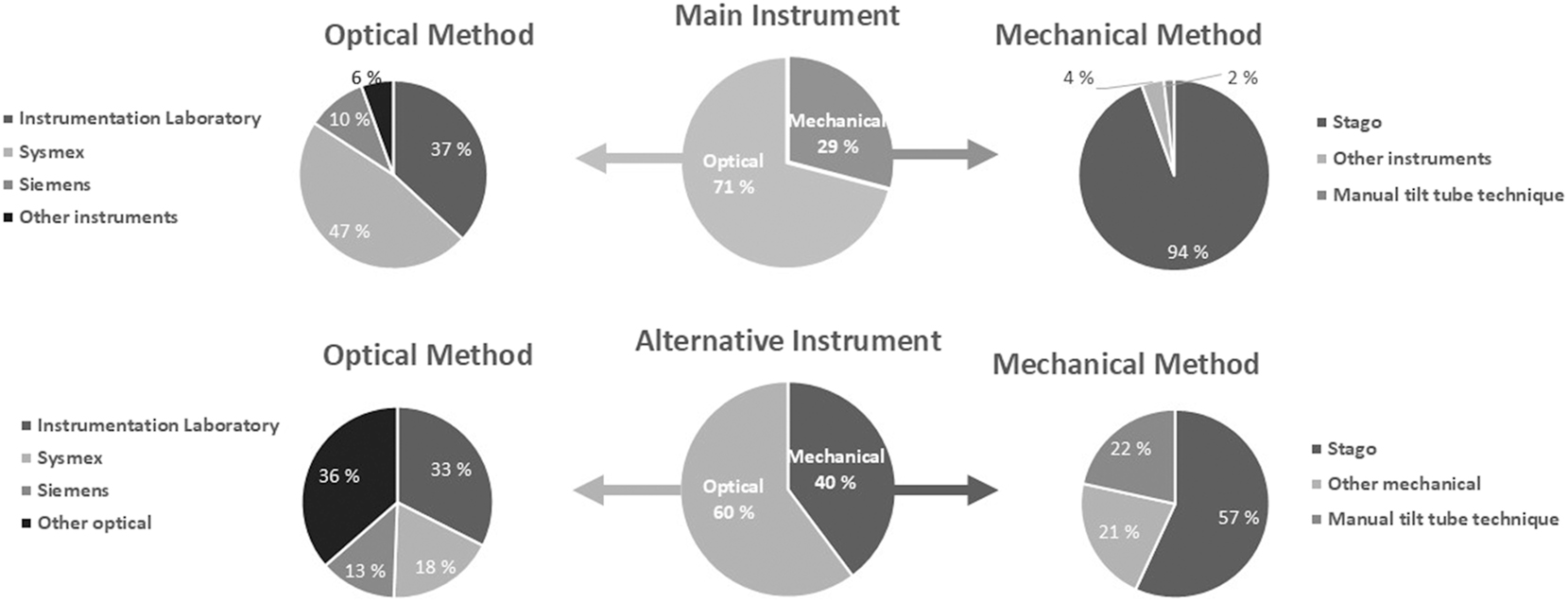
The percentage of laboratories stated using a coagulation instrument with optical or mechanical clot-detection method for the clotting tests, APTT, PT, INR and fibrinogen as the main (middle upper circle) and alternative (lower middle circle) coagulation instrument. Circles to the left show the percentages using different manufacturers for instruments with optical clot-detection and to the right for instruments with mechanical clot-detection for the clotting tests. All instruments use optical methods for D-dimer and antithrombin. See Supplemental Table 1 for more details.
Automated HIL-measurement was available in approximately 2/3 of the main coagulation instruments and 1/4 of the alternative coagulation instruments, with the highest percentage among users of Sysmex and IL instruments (Table 2).
The number and percentage of the laboratories stating to have automatic HIL-detection for their main and alternative coagulation instrument in total; divided into the instrument manufacturer used. n; number of laboratories.
| Automatic HIL detection | Main instrument | Alternative instrument | |||||
|---|---|---|---|---|---|---|---|
| Hemolysis n (%) | Icterus n (%) | Lipemia n (%) | Hemolysis n (%) | Icterus n (%) | Lipemia n (%) | ||
| Total (n=440) | 293 (66.6) | 282 (64.1) | 289 (65.7) | Total (n=128) | 30 (23.3) | 30 (23.3) | 28 (21.7) |
| Stago (n=121) | 67 (55.4) | 66 (54.5) | 67 (55.4) | Stago (n=29) | 8 (27.6) | 7 (24.1) | 7 (24.1) |
| IL (n=115) | 88 (76.5) | 88 (76.5) | 88 (76.5) | IL (n=25) | 8 (32) | 8 (32) | 8 (32) |
| Sysmex (n=148) | 114 (77.0) | 107 (72.3) | 113 (76.4) | Sysmex (n=14) | 6 (42.9) | 6 (42.9) | 6 (42.9) |
| Siemens (n=32) | 17 (53.1) | 16 (50) | 17 (53.1) | Siemens (n=10) | 4 (40) | 4 (40) | 4 (40) |
| Other (n=24) | 7 (29.2) | 5 (20.8) | 4 (16.7) | Other (n=50) | 4 (8) | 5 (10) | 3 (6) |
Frequency of HIL-detection
Participants stated they detect samples with hemolysis more frequently than icterus or lipemia. Detection of hemolysis was positively correlated with the number of processed coagulation samples in the laboratory (Supplemental Figure 1). Information regarding the frequency of detected HIL-samples was mainly based upon personal experience (64 %), with only 21 and 15 % obtaining the data from the laboratory information system (LIS) or from manual registration of HIL-samples, respectively.
Procedures for HIL-detection
A written procedure for detection of H, I and L was available in 67 , 55 and 61 % of the laboratories, respectively (Supplemental Figure 2A). All samples were checked by 83 , 73 and 80 %, for H, I and L, respectively, while 12 , 11 and 11 % checked some samples and 4 , 16 and 9 % none (Supplemental Figure 2B). The most frequent reasons given for not checking any samples or not checking all samples, were that the laboratory had “no written procedure for HIL” and/or “no automatic HIL-detection”. In addition, about 1/3 stated that “results are not affected by HIL” as reason for not checking any samples (Supplemental Tables 5 and 6). The percentage of laboratories not checking any samples, tended to be slightly higher for those using mechanical clot-detection (7 , 16 and 13 % for H, I and L, respectively) compared to optical clot-detection (3 , 12 and 5 %), and the same accounts for laboratories not checking all samples (20 , 8 and 13 % (mechanical) vs. 9 , 10 and 8 % (optical)). However, absolute numbers are small.
HIL was detected by visual inspection only, in 37–38 % of the laboratories, by automatic detection only, in 36–43 %, while the remaining used a combination (Figure 3A). Most laboratories using only visual inspection to detect HIL, used experience to determine HIL-levels or they did not determine HIL-levels (i.e. no levels=HIL positive or negative) (Figure 3B). Very few of these laboratories stated to use a visual chart/scale or to measure HIL-levels by coagulation or clinical chemistry instruments (Figure 3B).
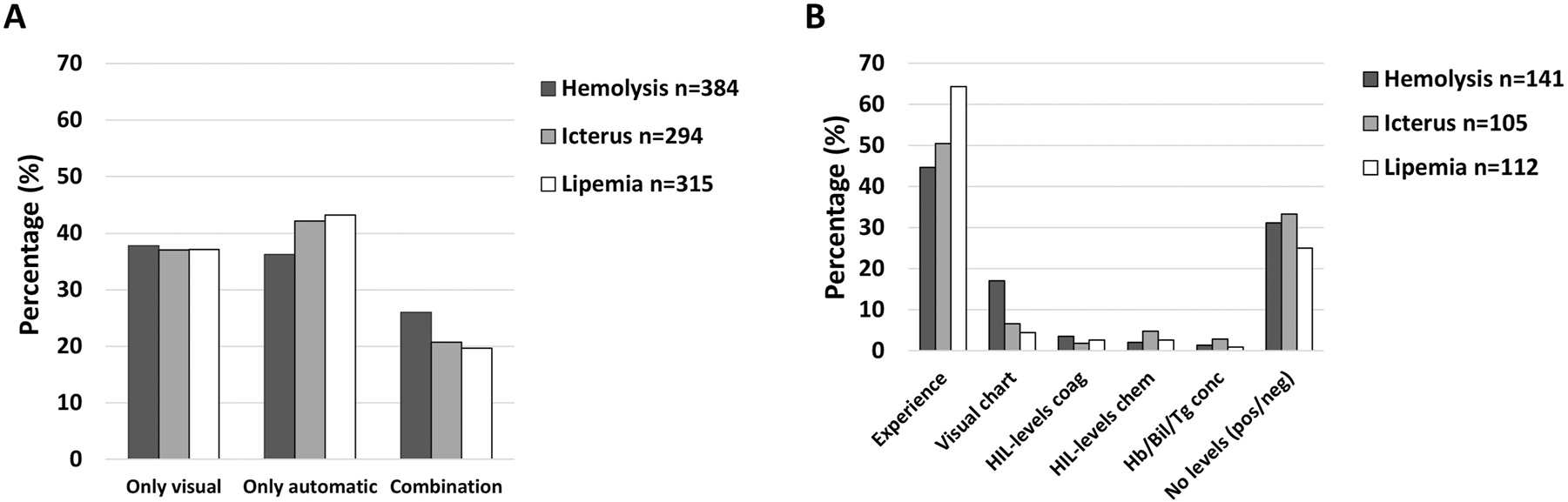
The percentage of laboratories stating to detect HIL by visual inspection only, by automatic (coagulation or chemistry instruments) analyses only or a combination (A), and the methods used to determine HIL-levels by those using only visual inspection to detect HIL (B). HIL-levels coag; measuring by coagulation instrument, HIL-levels chem; measuring by chemistry instrument, Hb/Bil/Tg conc; hemoglobin, bilirubin and/or triglyceride concentration by hematology and/or chemistry instrument, respectively, No levels (pos/neg); only stating HIL presence or not (no classification).
Almost half of the laboratories (40–45 %) did not classify into HIL-levels, but only stated HIL-presence or not (positive or negative for HIL). When determination of HIL-level was performed, most (30 %) classified into qualitative HIL-levels (e.g. 1–5) (Figure 4A). Among laboratories detecting HIL only automatically, the percentage classifying into qualitative HIL-levels (44–54 %) was more frequent than not classifying into levels (29–36 %) (Figure 4C). Among laboratories detecting HIL only visual or by visual and automatic, the percentage not classifying into HIL-levels was higher (Figure 4B) or rather similar (Figure 4D), respectively.
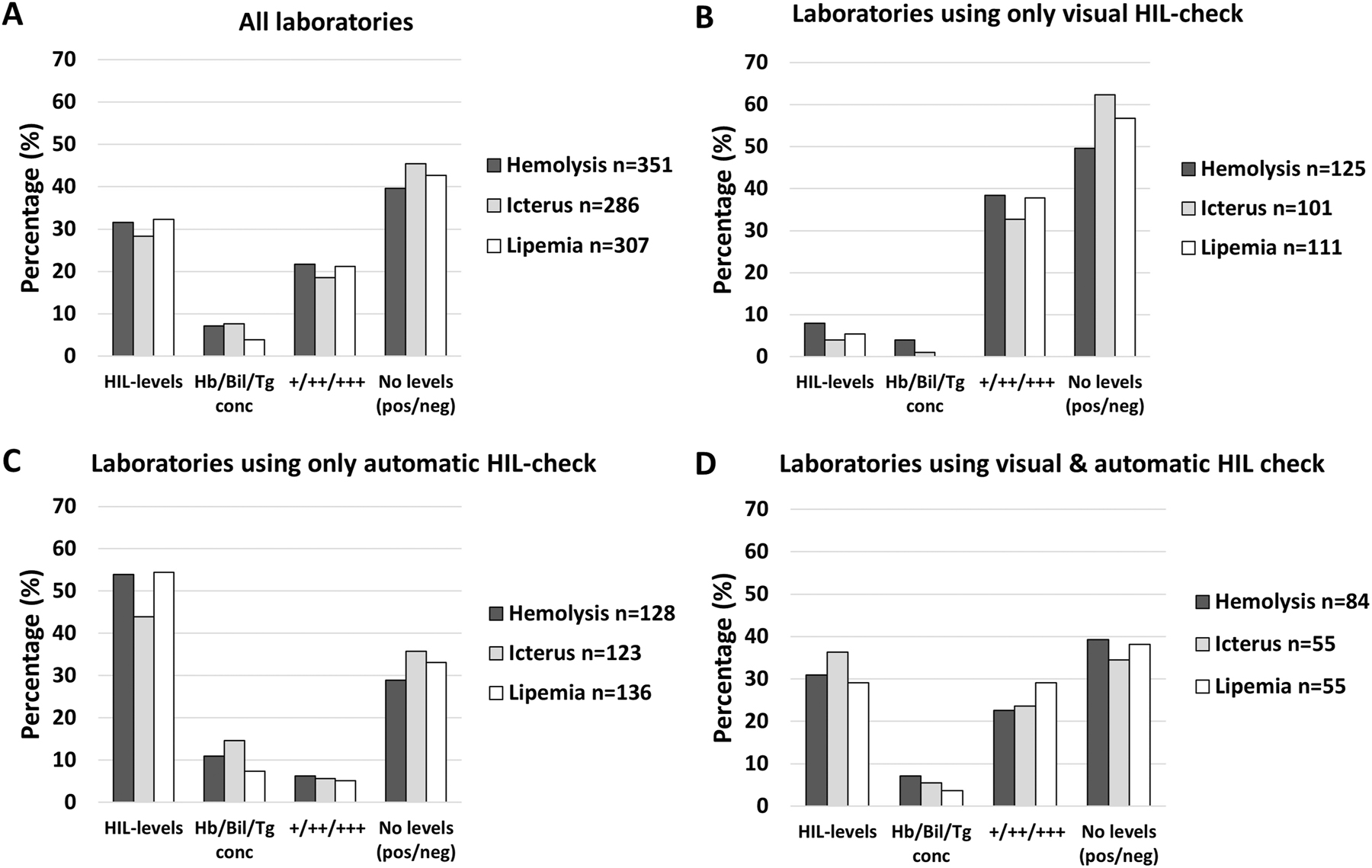
The percentage of laboratories stating to classify into different categories for hemolysis, icterus and lipemia for all laboratories (A), for laboratories using only visual HIL-check (B), for laboratories using only automatic HIL-check (C), and for laboratories using both visual and automatic HIL-check (D). No levels (pos/neg); only stating HIL presence or not (no classification). HIL-levels; semi-quantitative expression of HIL into levels (e.g., 1–5). Hb/Bil/Tg conc; hemoglobin, bilirubin and/or triglyceride concentration by hematology and/or chemistry instrument, respectively. +/++/+++; semi-quantitative expression of HIL into three levels (merged laboratories answering +/++/+++ and slight/moderate/gross).
When looking more closely into which manufacturers were used, Stago and Sysmex users tended to classify into HIL-levels more often than the others, whilst Siemens users tended to more often classify into +/++/+++ (slight/moderate/gross), in particular for hemolysis (Figure 5).
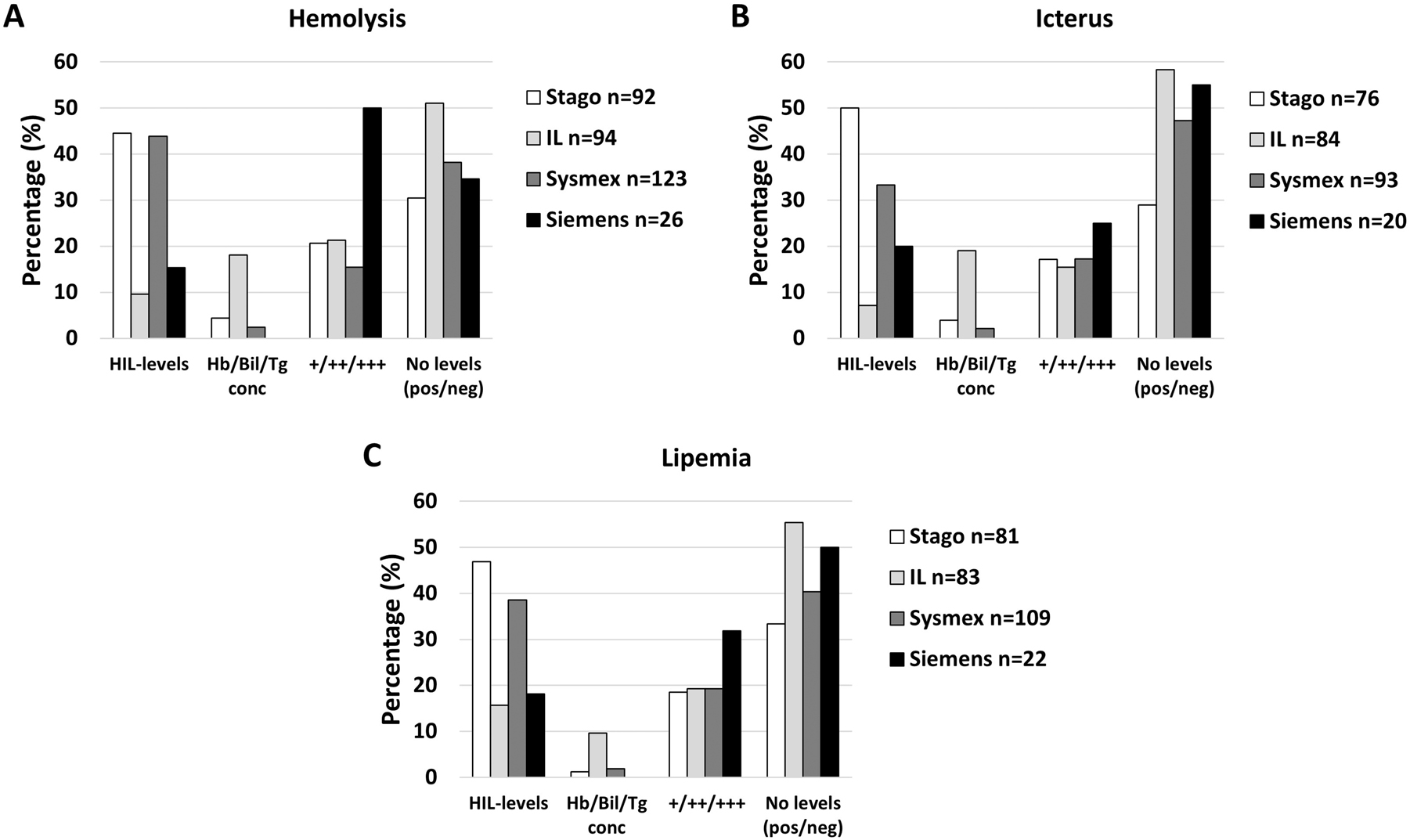
The percentage of laboratories stating they classify into different HIL-categories for hemolysis (A), icterus (B) and lipemia (C) according to instrument manufacturer in use. No levels (pos/neg); only stating HIL-presence or not (no classification). HIL-levels; semi-quantitative levels (e.g., 1–5). Hb/Bil/Tg conc; measurement of hemoglobin, bilirubin and triglycerides concentration by hematology or chemistry instrument, respectively. +/++/+++; semi-quantitative expression of HIL into three levels (merged laboratories answering +/++/+++ and slight/moderate/gross).
Comment- and reject-levels
The HIL-levels at which laboratories stated to comment (report test-result with a comment) or reject (report comment without test-result) were very heterogeneous for the coagulation tests evaluated, even within each manufacturer group (Supplemental Tables 2A–4A). Several laboratories answered this question with text, rather than the required quantitative or semiquantitative HIL-levels at which they comment and/or reject results (Supplemental Tables 2B–4B).
Among laboratories not reporting comment- and/or reject-levels, several stated using “Individual assessment” (45–55 %) or that they evaluated coagulation curves (35–45 %), while some stated switching to other wavelengths or using an alternative instrument (Supplemental Figure 3A–C). “Individual assessment” was stated more often for samples with hemolysis, while “Individual assessment” and “Evaluation of coagulation curves” were stated equally for icterus and lipemia. Minor differences were seen between handling of the different coagulation tests. Stago users tended to state “Individual assessment” more often, while Sysmex and IL users stated “Individual assessment” and/or “Evaluation of coagulation curve”, and the majority of Siemens users stated “Alternative wavelength” (80 % for APTT, PT, INR and fibrinogen and 60 % for D-dimer and antithrombin) (Supplemental Figure 4–6). Some Stago (mechanical clot-detection) users stated that their coagulation tests were not affected by HIL, while only one optical clot-detection user stated this.
When HIL-levels are higher than their own reject-level, 45–60 % of the responding laboratories answered that they would never release the results, with a tendency that results more often were released in cases of icterus and less often for D-dimer and antithrombin (Figure 6). Some laboratories would release results in case of in vivo hemolysis (≈30 %), liver failure (≈30 %) or chronic lipemia (≈20 %), for H, I or L, respectively. When difficult to draw a new blood sample, 25–30 % of the laboratories would release the potentially biased results, approximately 10 % if it was urgent (STAT) and 10 % for results within the reference interval (Figure 6). Multiple answers could be given for this question.
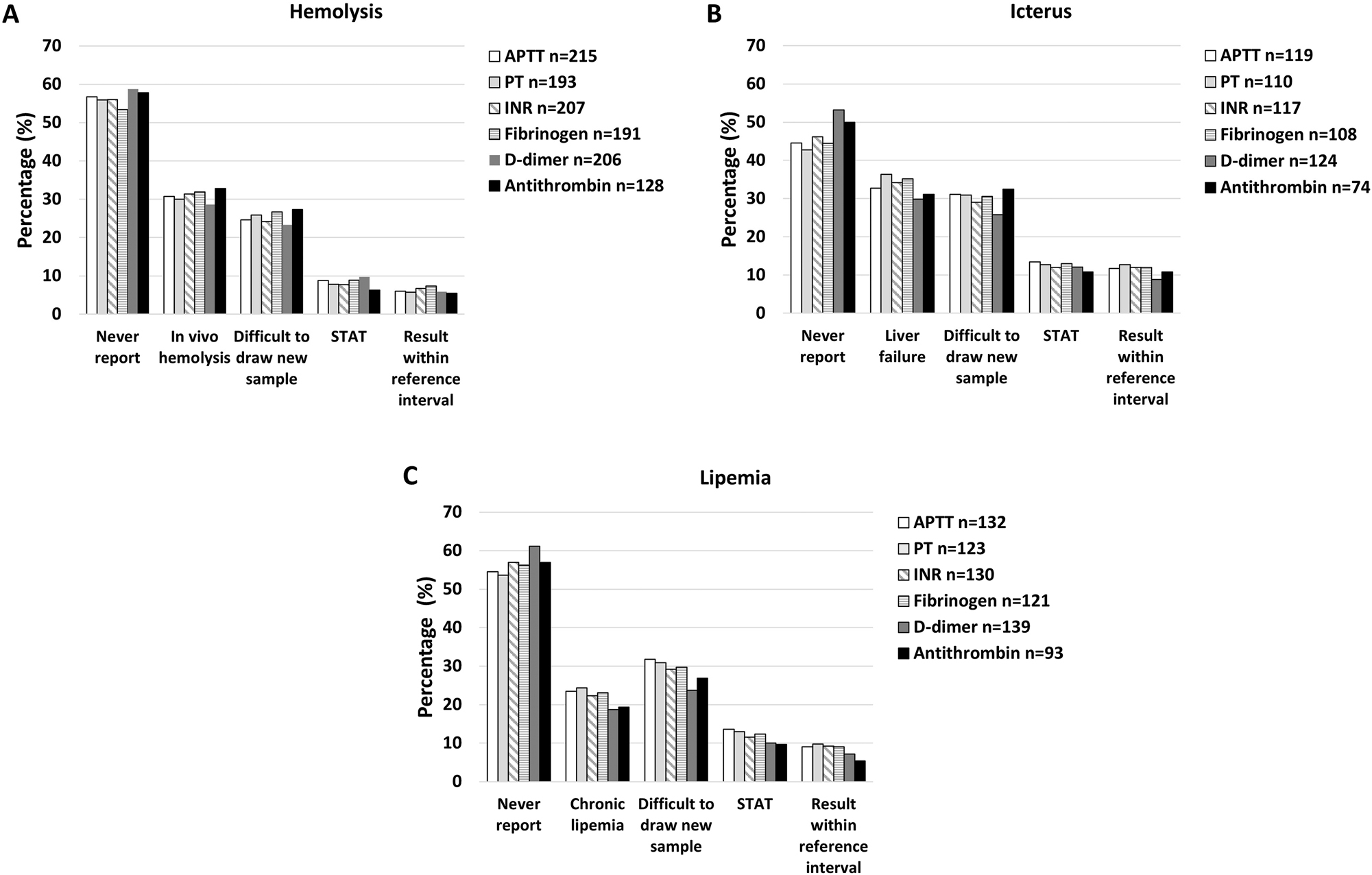
The percentage of laboratories stating never to report the results if the HIL-level is above their own rejection-level (never report) and the percentages reporting in different specific situations for hemolysis (A), icterus (B) and lipemia (C), for each of the different coagulation tests. STAT; urgent sample.
The comment- and reject-levels for HIL for each coagulation test were stated mainly to be derived from the manufacturers (70–80 %), but approximately 35 % reported the laboratories’ own experience as one of the sources. Another 10–20 % claimed to use experts, guidelines, or published studies, but very few relied on studies carried out in their own laboratory (1–6%) (Supplemental Figure 7). Multiple answers could be given for this question.
Type of comments given
Standardized comments for H, I and L were used by 72 , 59 and 64 % laboratories, respectively, while fewer used individualized comments (17 , 19 and 21 %) or did not provide comments (11 , 22 and 15 %). The direction of the potential bias caused by HIL (e.g. increased/decreased) in the sample was included in the comments by less than 10 %, with no agreement on increase/decrease for any coagulation test or interfering substance (Supplemental Figure 8).
Reporting of HIL-levels and internal/external QC
Some laboratories (31–33 %) stated to include results of HIL-checks in the laboratory system available for the laboratory staff, but few (7–8%) included this in reports to the clinicians. Only 1.2–2% measure in-house IQC material and about 5.5 % use commercially available IQC. EQA programs for HIL in coagulation testing were joined by 22 % of the laboratories measuring HIL, while 67 % stated to be interested in participating in EQA for HIL in coagulation testing.
Discussion
The procedures of HIL-detection, handling and reporting in samples for coagulation testing varies largely in European laboratories, and some laboratories state not to have written procedures for HIL-detection.
Frequency of HIL-samples
The laboratories observed hemolysis more frequent than samples with icterus and lipemia, similar to other studies [1], 7], 26]. Hemolysis may originate both in vivo – hemolytic disease or mechanical (e.g. intravascular devices or artificial heart valve) or in vitro – preanalytical issues like e.g. difficult venipuncture, thin needles and vigorous mixing [1], 26], while icterus (e.g. caused by cholestasis or inherited diseases) and lipemia (caused by high fat meals, metabolic diseases, parenteral infusion or propofol anesthesia [7], 27]) – only occur in vivo. Continuous improvement in the quality of sample handling would eventually reduce the number of in vitro hemolytic samples. Some in vivo lipemic samples may be avoided with fasting samples and stopping parenteral infusion, but ultracentrifugation may be an option to clear lipemic samples [17], 28], 29].
Procedures for HIL-detection
The percentage of laboratories checking for HIL-samples in coagulation testing seem to have increased over the last few years [30], probably because of increasing availability of automatic HIL-check on the newer coagulation instruments [18], 19], 31], 32] and increasing use of transport bands [33]. However, it is of great concern that visual checks alone, still are used to a large extent (almost 40 %), as also shown by another survey [34]. Automatic HIL-detection is recommended as visual checks have been shown to be unreliable [9], 15], [35], [36], [37].
The reason for the slightly lower percentage of Stago users performing HIL-checks may be that mechanical clot-detection is not affected by the analytical interference (optical/spectrophotometric). However, biological interference (mainly hemolysis, but also lipemia) may affect results from coagulation tests both for instruments with mechanical (Stago) and optical (IL, Sysmex and Siemens) clot-detection methods [2], 4]. In addition, immunological and chromogenic methods (e.g. D-dimer and antithrombin) use optical detection, independently upon the instrument’s clot-detection method, and lipemia might also affect antibody binding [7]. Consequently, HIL-checks are important regardless of the instrument used.
Comment- and reject-levels
The large heterogeneity in the comment- and reject-levels for HIL and how these are reported, cannot only be explained by using different methods, as a large heterogeneity was also found between laboratories using the same manufacturer. Since most laboratories state to use manufacturers information, part of the heterogeneity could be caused by different interpretation of the information given in the package inserts (e.g. some laboratories stated to report the result with a comment at the same HIL-level/concentration as others would reject). How manufacturers performed their HIL-studies and decided upon the acceptability criteria (allowable bias) as basis for stating not to be affected by HIL up to certain cut-offs, are usually not available [7], 15], 16]. For some methods (mechanical clot-detection) HIL-interference is not always mentioned in the package inserts for the clot-methods. This also makes it more difficult to understand and adhere to manufacturers’ information, which is actually legally binding.
Automatic HIL-detection by coagulation instruments is performed by optical absorbance measurements at different wavelengths. Stago and Sysmex instruments reports this in HIL-levels (e.g. 0–5), each corresponding to an approximate concentration-interval, while IL-instruments transforms optical absorbance measurement into concentration intervals (clouds) for hemolysis and bilirubinemia and milliabsorbances (mAbs) for lipemia/turbidity. Since HIL cut-offs are given in mg/dL or mAbs, respectively, more IL users give comment- and reject-levels in these units, instead of HIL-levels as for other instruments. However, qualitative, quantitative and semi-quantitative cut-offs were seen among all laboratories, regardless of manufacturer.
Few laboratories have the resources to perform in-house HIL-studies, potentially leading to sample-rejection although HIL-levels may be too low to induce clinically significant bias [20] or no sample-rejection even though HIL-levels may cause clinically significant bias [38]. Both the complexity of performing in-house HIL-studies and lack of manufacturers’ transparency may contribute to the large heterogeneity seen in comment- and reject-levels in this survey. Published studies comparing instruments and reagents are needed to make more detailed guidelines, as published studies, with a few exceptions [18], 21], represent one instrument and a few reagents [4], 9], 10], 19], 20], [22], [23], [24]. However, a far easier approach would be if instrument and assay manufacturers would share the raw data on which their recommendations in their package inserts/instrument applications are based upon, a call made by the working group “Preanalytics” (WG-PRE) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) already in 2018 [16]. Harmonizing HIL-levels and reporting in the same unit (preferentially as quantitative concentrations) would also make studies comparing different methods feasible and possibly encourage manufacturers to develop tools to decrease susceptibility to HIL when possible.
In special cases, some laboratories release results even if HIL-levels are higher than the reject-levels. Such decisions may be taken after risk assessment, as laboratories should balance the risk of reporting (sometimes slightly) erroneous results against the risk of withholding clinically important results (causing delayed results, diagnosis and treatment), both potentially affecting patient safety [39]. In situations, in which a re-draw of samples is difficult or in very urgent situations, a biased result may in some instances still be clinically useful, if information on the direction and magnitude of the measurement error are available [39]. Unfortunately, for coagulation tests, the magnitude and direction of changes are not always easy to assess (see below Type of comments given).
Several of the laboratories without comment- and reject-criteria reported to assess HIL-samples individually (“Individual assessments”), which may lead to heterogeneity of handling HIL-samples within the laboratory. Written procedures should be present to harmonize handling of HIL-samples within each laboratory. Flagging of erroneous coagulation curves and/or switch of wavelength may be performed by manufacturers’ instructions for some of the optical instruments, but few published studies are available to evaluate such procedures [40].
Type of comments given
It is understandable that most laboratories do not add the direction of the bias in their comments for HIL-samples. Stating the direction of the biased results for a particular coagulation test may be possible if the interference is only optical (analytical), based on the method of that test, but may be impossible for clot-based assays when both analytical and biological interferences may be present. Manufacturers do not provide information on the direction and performing in-house studies are challenging.
Results from published studies may be difficult to implement in own laboratory as the extent of interference found will depend upon several factors; among others the HIL-level in the sample, the analytical method (i.e. clot-, chromogenic- or immunological method), the coagulation test, reagent and instrument used, the type of clot-detection method (e.g. optical or mechanical) for clot-based assays and the concentration/level of the coagulation parameter measured (normal levels in healthy and/or pathological in disease or anticoagulant treatment) [4], 9], 10], 18], 19], [21], [22], [23], [24]. In addition, different methods for producing HIL-samples [41] (e.g. artificially hemolysis by freeze-thaw cycles or aspiration of blood through a thin needle, or using real-patient paired samples with and without hemolysis [4], 9]), may affect the measurement differently depending on the method used in the preparation of samples. Furthermore, conflicting conclusions may be caused by different methods used to define comment- and reject-levels, with some using an arbitrary ±10 % change in concentration from baseline, and others using analytical change limit and reference change value [7], 15] and sometimes the reflection of the needed setting is not available.
Reporting of HIL-levels and internal/external QC
As recommended for chemistry tests, HIL-levels in samples for coagulation testing should be reported in the laboratory report-system and IQC and EQA should be implemented [7], 15], 42], 43]. Increasing availability of automated HIL-detection in coagulation instruments makes reporting of HIL-levels feasible [33]. However, solutions for automatic transfer of HIL-results and material for IQC and EQA may not yet be widely available. Manufacturers should be encouraged to develop systems for this [16] and EQA organisations should be encouraged to develop EQA-programs for HIL-detection in samples for coagulation testing as very few currently exist [34]. As an added benefit, results from EQA-programs may be used to benchmark between manufacturers, showing potential biases among them, which hopefully will encourage harmonization [16], 44].
Strengths and limitations
One strength of this survey is that multiple European countries were included with as many as 440 responding laboratories in total, and more than 70 % of the participants finishing the survey despite its length (58 questions in total, although some questions were skipped depending upon former answers). It is assumed that laboratories with most interest in HIL and preanalytical issues participated, and the large heterogeneity may therefore also be generalizable.
The survey is limited in that the response rate to the survey could not be calculated because the number of laboratories which received the questionnaire is not fully known. Country specific results could also not be assessed since the percentage of laboratories per country is unknown.
Conclusions
There is a large heterogeneity in HIL-detection, handling and reporting among laboratories, which calls for urgent collaboration between laboratory experts, EQA organisations and instrument/assay manufacturers to improve and harmonize HIL-sample management and reporting in coagulation testing. High-quality studies should be performed on effect of HIL on coagulation tests to make better and more specific recommendations, applicable to local settings.
Acknowledgments
EQALM member EQA organisations are kindly acknowledged for their willingness to forward the questionnaire to their participants, and thanks to the laboratories for their willingness to respond. Thanks to Steve Kitchen, Sverre Sandberg and Gunn BB Kristensen for valuable comments during the project. Thanks also to the Norwegian Western Region Health Authority for post-doctor scholarship for Ann Helen Kristoffersen.
-
Research ethics: The study is a questionnaire to laboratories, no humans or animals or health information. The participating laboratories were informed that data from the questionnaire were going to be published. The data (answers from the questionnaire) were treated anonymously.
-
Informed consent: Not applicable.
-
Author contributions: All authors have contributed to the planning of the questionnaire, data evaluation and writing of the manuscript. MvH performed the SurveyMonkey editing and collection of data. AHK performed the data evaluation and statistics. AHK and JC made the figures. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Data available within the article or its supplementary materials.
References
1. Salvagno, GL, Lippi, G, Bassi, A, Poli, G, Guidi, GC. Prevalence and type of pre-analytical problems for inpatients samples in coagulation laboratory. J Eval Clin Pract 2008;14:351–3. https://doi.org/10.1111/j.1365-2753.2007.00875.x.Suche in Google Scholar PubMed
2. Lippi, G, Plebani, M, Favaloro, EJ. Interference in coagulation testing: focus on spurious hemolysis, icterus, and lipemia. Semin Thromb Hemost 2013;39:258–66. https://doi.org/10.1055/s-0032-1328972.Suche in Google Scholar PubMed
3. Adcock, FDM, Lippi, G, Favaloro, EJ. Quality standards for sample processing, transportation, and storage in hemostasis testing. Semin Thromb Hemost 2012;36:576–85.10.1055/s-0032-1319768Suche in Google Scholar PubMed
4. Woolley, A, Golmard, JL, Kitchen, S. Effects of haemolysis, icterus and lipaemia on coagulation tests as performed on Stago STA-Compact-Max analyser. Int J Lab Hematol 2016;38:375–88. https://doi.org/10.1111/ijlh.12498.Suche in Google Scholar PubMed
5. Laga, AC, Cheves, TA, Sweeney, JD. The effect of specimen hemolysis on coagulation test results. Am J Clin Pathol 2006;126:748–55. https://doi.org/10.1309/03fk-3378-ytra-1frf.Suche in Google Scholar PubMed
6. Favaloro, EJ, Lippi, G, Adcock, DM. Preanalytical and postanalytical variables: the leading causes of diagnostic error in hemostasis? Semin Thromb Hemost 2008;34:612–34. https://doi.org/10.1055/s-0028-1104540.Suche in Google Scholar PubMed
7. Nikolac, N. Lipemia: causes, interference mechanisms, detection and management. Biochem Med 2014;24:57–67. https://doi.org/10.11613/bm.2014.008.Suche in Google Scholar
8. Lippi, G, Montagnana, M, Salvagno, GL, Guidi, GC. Interference of blood cell lysis on routine coagulation testing. Arch Pathol Lab Med 2006;130:181–4. https://doi.org/10.5858/2006-130-181-iobclo.Suche in Google Scholar
9. Novelli, C, Vidali, M, Brando, B, Morelli, B, Andreani, G, Arini, M, et al.. A collaborative study by the Working Group on Hemostasis and Thrombosis of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC) on the interference of haemolysis on five routine blood coagulation tests by evaluation of 269 paired haemolysed/non-haemolysed samples. Biochem Med 2018;28:030711. https://doi.org/10.11613/bm.2018.030711.Suche in Google Scholar
10. Hedeland, Y, Gustafsson, CM, Touza, Z, Ridefelt, P. Hemolysis interference in 10 coagulation assays on an instrument with viscosity-based, chromogenic, and turbidimetric clot detection. Int J Lab Hematol. 2020;42:341–9. https://doi.org/10.1111/ijlh.13188.Suche in Google Scholar PubMed
11. Kitchen, S, Adcock, DM, Dauer, R, Kristoffersen, AH, Lippi, G, Mackie, I, et al.. International Council for Standardization in Haematology (ICSH) recommendations for processing of blood samples for coagulation testing. Int J Lab Hematol. 2021;43:1272–83. https://doi.org/10.1111/ijlh.13702.Suche in Google Scholar PubMed
12. Baker, P, Platton, S, Gibson, C, Gray, E, Jennings, I, Murphy, P, et al.. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Br J Haematol 2020;191:347–62. https://doi.org/10.1111/bjh.16776.Suche in Google Scholar PubMed
13. CLSI. Collection, transport, and processing of blood specimens for testing plasma-based coagulation assays and molecular hemostasis assays; approved guideline - fifth edition. CLSI document H21-A5. Wayne PA: Clinical and Laboratory Standards Institute; 2008.Suche in Google Scholar
14. Gosselin, RC, Marlar, RA. Preanalytical variables in coagulation testing: setting the stage for accurate results. Semin Thromb Hemost 2019;45:433–48. https://doi.org/10.1055/s-0039-1692700.Suche in Google Scholar PubMed
15. Lippi, G, Cadamuro, J, von Meyer, A, Simundic, AM, European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase. Practical recommendations for managing hemolyzed samples in clinical chemistry testing. Clin Chem Lab Med 2018;56:718–27. https://doi.org/10.1515/cclm-2017-1104.Suche in Google Scholar PubMed
16. von Meyer, A, Cadamuro, J, Lippi, G, Simundic, AM. Call for more transparency in manufacturers declarations on serum indices: On behalf of the Working Group for Preanalytical Phase (WG-PRE), European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta 2018;484:328–32. https://doi.org/10.1016/j.cca.2018.03.043.Suche in Google Scholar PubMed
17. Negrini, D, Bernardi, D, Antonelli, G, Plebani, M. Interference of lipemia in samples for routine coagulation testing using a Sysmex CS-5100 coagulometer. Int J Lab Hematol 2019;41:772–7. https://doi.org/10.1111/ijlh.13108.Suche in Google Scholar PubMed
18. Nagant, C, Rozen, L, Demulder, A. HIL interferences on three hemostasis analyzers and contribution of a preanalytical module for routine coagulation assays. Clin Lab 2016;62:1979–87. https://doi.org/10.7754/clin.lab.2016.160313.Suche in Google Scholar
19. Montaruli, B, Guiotto, C, Cosseddu, D. Influence of hemolysis, icterus and lipemia on coagulation tests as performed on Cobas t511 new analyzer. Blood Coagul Fibrinolysis 2020;31:48–54. https://doi.org/10.1097/mbc.0000000000000873.Suche in Google Scholar PubMed
20. Jensen, AK, Christensen, GL, Dalgard, A, Jorgensen, NR. Estimation of lipemia interference with automated HIL-test on d-dimer ACL TOP 50 series analysis - reveals a higher cut-off than manufacturer’s recommendations. Scand J Clin Lab Invest 2020;80:168–71. https://doi.org/10.1080/00365513.2019.1703214.Suche in Google Scholar PubMed
21. Nougier, C, Jousselme, E, Sobas, F, Pousseur, V, Negrier, C. Effects of hemolysis, bilirubin, and lipemia interference on coagulation tests detected by two analytical systems. Int J Lab Hematol. 2020;42:88–94. https://doi.org/10.1111/ijlh.13147.Suche in Google Scholar PubMed
22. Florin, L, Oyaert, M, Van Maerken, T, Devreese, KMJ. Performance of the preanalytical check module of the Stago STA R Max2 mechanical endpoint detection analyzer for assessing the impact of hemolysis, lipemia, and icterus on aPTT and PT. Int J Lab Hematol. 2018;40:e109–2. https://doi.org/10.1111/ijlh.12871.Suche in Google Scholar PubMed
23. Seheult, JN, Dalenberg, D, Sridharan, MR, Stuart, M, Moericke, K, Cardel, L, et al.. Revisiting the effects of spectral interfering substances in optical end-point coagulation assays. Int J Lab Hematol 2021;43:1181–90. https://doi.org/10.1111/ijlh.13465.Suche in Google Scholar PubMed
24. Pan, LL, Lee, CH, Hung, KC, Tsai, IT, Wang, MC, Sun, CK. Differential impacts of hemolysis on coagulation parameters of blood samples: a STROBE-compliant article. Medicine (Baltimore) 2021;100:e25798. https://doi.org/10.1097/md.0000000000025798.Suche in Google Scholar PubMed PubMed Central
25. Stavelin, A, Albe, X, Meijer, P, Sarkany, E, MacKenzie, F. An overview of the European Organization for External Quality Assurance Providers in Laboratory Medicine (EQALM). Biochem Med 2017;27:30–6. https://doi.org/10.11613/bm.2017.005.Suche in Google Scholar
26. Simundic, AM, Baird, G, Cadamuro, J, Costelloe, SJ, Lippi, G. Managing hemolyzed samples in clinical laboratories. Crit Rev Clin Lab Sci 2020;57:1–21. https://doi.org/10.1080/10408363.2019.1664391.Suche in Google Scholar PubMed
27. Negaard, BJ, Hobbs, R, Frye, JR, Merrill, AE. Propofol-induced interference with activated partial thromboplastin time-based monitoring of therapeutic heparin anticoagulation. Am J Health Syst Pharm 2023;80:445–51. https://doi.org/10.1093/ajhp/zxac337.Suche in Google Scholar PubMed
28. Gosselin, RC. Ultracentrifugation for coagulation testing. Methods Mol Biol 2023;2663:63–70. https://doi.org/10.1007/978-1-0716-3175-1_4.Suche in Google Scholar PubMed
29. Gardiner, C, Lane, P, Tailor, H, Mackie, IJ. A practical method for reducing the interference due to lipaemia in coagulation tests. Int J Lab Hematol. 2020;42:140–4. https://doi.org/10.1111/ijlh.13129.Suche in Google Scholar PubMed PubMed Central
30. Cadamuro, J, Lippi, G, von Meyer, A, Ibarz, M, van Dongen, E, Lases, et al.. European survey on preanalytical sample handling - part 2: practices of European laboratories on monitoring and processing haemolytic, icteric and lipemic samples. On behalf of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for the Preanalytical Phase (WG-PRE). Biochem Med 2019;29:020705.10.11613/BM.2019.020705Suche in Google Scholar PubMed PubMed Central
31. Chen, L, Chen, Y. Performance evaluation of the Sysmex CS-5100 automated coagulation analyzer. Clin Lab 2015;61:653–60. https://doi.org/10.7754/clin.lab.2014.141124.Suche in Google Scholar PubMed
32. Lippi, G, Ippolito, L, Favaloro, EJ. Technical evaluation of the novel preanalytical module on instrumentation laboratory ACL TOP: advancing automation in hemostasis testing. J Lab Autom 2013;18:382–90. https://doi.org/10.1177/2211068213491747.Suche in Google Scholar PubMed
33. Lippi, G, Plebani, M, Favaloro, EJ. Technological advances in the hemostasis laboratory. Semin Thromb Hemost 2014;40:178–85. https://doi.org/10.1055/s-0033-1364206.Suche in Google Scholar PubMed
34. Brown, L, Jennings, I, Kitchen, S, Kitchen, DP, Woods, TAL, Walker, ID. Pre-analytical variables in haemostasis: findings from the United Kingdom national external quality assessment scheme for blood coagulation (UK NEQAS BC) haemolysis exercise. Int J Lab Hematol. 2021;43:1198–206. https://doi.org/10.1111/ijlh.13468.Suche in Google Scholar PubMed
35. Simundic, AM, Nikolac, N, Ivankovic, V, Ferenec-Ruzic, D, Magdic, B, Kvaternik, M, et al.. Comparison of visual vs. automated detection of lipemic, icteric and hemolyzed specimens: can we rely on a human eye? Clin Chem Lab Med 2009;47:1361–5. https://doi.org/10.1515/cclm.2009.306.Suche in Google Scholar
36. Hawkins, R. Discrepancy between visual and spectrophotometric assessment of sample haemolysis. Ann Clin Biochem 2002;39:521–2. https://doi.org/10.1258/000456302320314575.Suche in Google Scholar PubMed
37. Glick, MR, Ryder, KW, Glick, SJ, Woods, JR. Unreliable visual estimation of the incidence and amount of turbidity, hemolysis, and icterus in serum from hospitalized patients. Clin Chem 1989;35:837–9. https://doi.org/10.1093/clinchem/35.5.837.Suche in Google Scholar
38. Nikolac, N, Simundic, AM, Miksa, M, Lima-Oliveira, G, Salvagno, GL, Caruso, B, et al.. Heterogeneity of manufacturers’ declarations for lipemia interference--an urgent call for standardization. Clin Chim Acta 2013;426:33–40. https://doi.org/10.1016/j.cca.2013.08.015.Suche in Google Scholar PubMed
39. Cadamuro, J, Simundic, AM, Ajzner, E, Sandberg, S. A pragmatic approach to sample acceptance and rejection. Clin Biochem 2017;50:579–81. https://doi.org/10.1016/j.clinbiochem.2017.02.001.Suche in Google Scholar PubMed
40. Junker, R, Kase, M, Schulte, H, Baumer, R, Langer, C, Nowak-Gottl, U. Interferences in coagulation tests--evaluation of the 570-nm method on the Dade Behring BCS analyser. Clin Chem Lab Med 2005;43:244–52. https://doi.org/10.1515/cclm.2005.041.Suche in Google Scholar PubMed
41. Gidske, G, Solvik, UO, Sandberg, S, Kristensen, GBB. Hemolysis interference studies: freeze method should be used in the preparation of hemolyzed samples. Clin Chem Lab Med 2018;56:e220–2. https://doi.org/10.1515/cclm-2018-0193.Suche in Google Scholar PubMed
42. Lippi, G, Cadamuro, J, von Meyer, A, Simundic, AM, European Federation of Clinical Chemistry; Laboratory Medicine (EFLM) Woring Group, for Preanalytical Phase (WG-PRE). Local quality assurance of serum or plasma (HIL) indices. Clin Biochem 2018;54:112–8. https://doi.org/10.1016/j.clinbiochem.2018.02.018.Suche in Google Scholar PubMed
43. Lippi, G, Cadamuro, J, Danese, E, Gelati, M, Montagnana, M, von Meyer, A, et al.. Internal quality assurance of HIL indices on Roche Cobas c702. PLoS One 2018;13:e0200088. https://doi.org/10.1371/journal.pone.0200088.Suche in Google Scholar PubMed PubMed Central
44. Dolci, A, Panteghini, M. Harmonization of automated hemolysis index assessment and use: is it possible? Clin Chim Acta 2014;432:38–43. https://doi.org/10.1016/j.cca.2013.10.012.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2025-0319).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025
Artikel in diesem Heft
- Frontmatter
- Editorial
- Quality indicators: an evolving target for laboratory medicine
- Reviews
- Regulating the future of laboratory medicine: European regulatory landscape of AI-driven medical device software in laboratory medicine
- The spectrum of nuclear patterns with stained metaphase chromosome plate: morphology nuances, immunological associations, and clinical relevance
- Opinion Papers
- Comprehensive assessment of medical laboratory performance: a 4D model of quality, economics, velocity, and productivity indicators
- Detecting cardiac injury: the next generation of high-sensitivity cardiac troponins improving diagnostic outcomes
- Perspectives
- Can Theranos resurrect from its ashes?
- Guidelines and Recommendations
- Australasian guideline for the performance of sweat chloride testing 3rd edition: to support cystic fibrosis screening, diagnosis and monitoring
- General Clinical Chemistry and Laboratory Medicine
- Recommendations for the integration of standardized quality indicators for glucose point-of-care testing
- A cost-effective assessment for the combination of indirect immunofluorescence and solid-phase assay in ANA-screening
- Assessment of measurement uncertainty of immunoassays and LC-MS/MS methods for serum 25-hydroxyvitamin D
- A novel immunoprecipitation-based targeted liquid chromatography-tandem mass spectrometry analysis for accurate determination for copeptin in human serum
- Histamine metabolite to basal serum tryptase ratios in systemic mastocytosis and hereditary alpha tryptasemia using a validated LC-MS/MS approach
- Machine learning algorithms with body fluid parameters: an interpretable framework for malignant cell screening in cerebrospinal fluid
- Impact of analytical bias on machine learning models for sepsis prediction using laboratory data
- Immunochemical measurement of urinary free light chains and Bence Jones proteinuria
- Serum biomarkers as early indicators of outcomes in spontaneous subarachnoid hemorrhage
- High myoglobin plasma samples risk being reported as falsely low due to antigen excess – follow up after a 2-year period of using a mitigating procedure
- Candidate Reference Measurement Procedures and Materials
- Commutability evaluation of glycated albumin candidate EQA materials
- Reference Values and Biological Variations
- Health-related reference intervals for heavy metals in non-exposed young adults
- Hematology and Coagulation
- Practical handling of hemolytic, icteric and lipemic samples for coagulation testing in European laboratories. A collaborative survey from the European Organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM)
- Cancer Diagnostics
- Assessment of atypical cells in detecting bladder cancer in female patients
- Cardiovascular Diseases
- False-positive cardiac troponin I values due to macrotroponin in healthy athletes after COVID-19
- Diabetes
- A comparison of current methods to measure antibodies in type 1 diabetes
- Letters to the Editor
- The neglected issue of pyridoxal- 5′ phosphate
- Error in prostate-specific antigen levels after prostate cancer treatment with radical prostatectomy
- Arivale is dead ‒ Hooke is alive
- A single dose of 20-mg of ostarine is detectable in hair
- Growing importance of vocabularies in medical laboratories
- Congress Abstracts
- 62nd National Congress of the Hungarian Society of Laboratory Medicine Szeged, Hungary, August 28–30, 2025

