To the Editor,
Rapid and accurate measurement of SARS-CoV-2 neutralizing antibodies (nAbs) may aid in understanding the development of immunity against COVID-19 and, therefore, is an important tool in mitigating COVID-19 pandemic. The majority of current point-of-care (POCT) antibody tests developed for SARS-CoV-2 rely on lateral flow assays, but do not offer quantitative information on neutralizing antibodies (Abs) titers. To address this issue, we evaluated the diagnostic performance of a rapid SARS-CoV-2 nAb detection test called “iRapid SARS-CoV-2 Quant Neutralizing Abs” (DIESSE Diagnostica Senese S.p.A, Siena), a semi-quantitative membrane-based rapid immunoassay for the detection of IgG antibodies directed against the receptor binding domain (RBD) of SARS-CoV-2. Briefly, a recombinant SARS-CoV-2 RBD protein is conjugated with colloidal gold nanoparticles; the patient specimen is placed to the pad of the strip (S) and thereafter, two drops of buffer are added. The antibodies against SARS-CoV-2 RBD in the sample will bind to RBD protein coated to colloidal gold present in the conjugate paper. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the monoclonal anti-human IgG in the test line region. The results are shown as coloured lines, the colour intensity being proportional to antibodies concentration, measured in binding antibody units (BAU/mL), calculated with reference to the first international standard WHO 20/136 for anti-SARS-CoV-2. For practical purposes, Neutralizing” Ab titers are classified according to the following cutoffs: <300 BAU/mL (as negative), 450 BAU/mL, 600 BA (/mL), 800 BAU/mL, >1,000 BAU/mL (Figure 1).
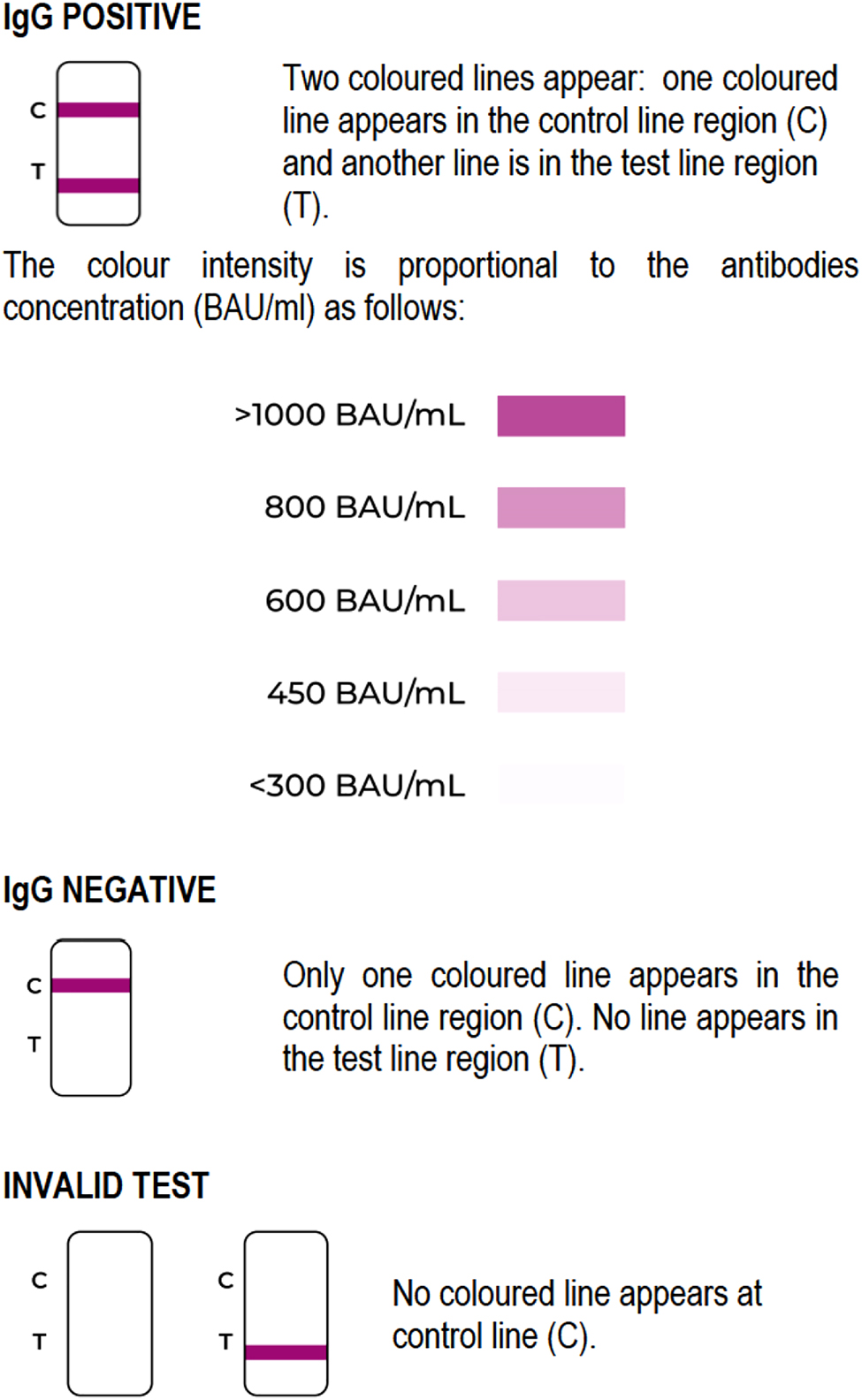
Interpretation of results.
If the specimen contains IgG antibodies against SARS-CoV-2 receptor binding domain at a concentration above limit of detection, a colored line will appear in the test line region. If there are no IgG antibodies against SARS-CoV-2 reception binding domain or to a lower concentration than limit of detection, only the control line will appear. The test will be validated only if the control line appears, otherwise the test is invalid and cannot be interpreted. The control line (C) is composed by anti-RBD antibodies able to bind the RBD protein.
The results have been compared with the “gold standard”, that is the micro‐neutralization assay (MN). This is currently considered the gold‐standard method being the most specific and sensitive serological assay capable of evaluating and detecting functional neutralizing antibodies (nAbs). In this study, a live virus‐based MN assay is presented for the quantification of SARS‐CoV‐2‐specific nAbs in human serum samples by classical method of detection: a read‐out by checking the percentage of cytopathic effect (CPE) in the cell monolayer. Briefly, serum samples were heat-inactivated for 30 min at 56 °C; two-fold serial dilutions, starting from 1:10, were then mixed with an equal volume of viral solution containing 100 TCID50 of SARS-CoV-2 virus. The serum-virus mixture was incubated 1 h at 37 °C in a humidified atmosphere with 5% CO2. After incubation, 100 µL of each dilution mixture was added in duplicate to a cell plate containing a semi-confluent VERO E6 monolayer. The plates were then incubated for three days at 37 °C in a humidified atmosphere with 5% CO2. After three days of incubation, the plates were analyzed with an inverted optical microscope. The highest serum dilution able to protect more than the 50% of cells from CPE was taken as the neutralization threshold. Using MN as a reference, 299 serum specimens collected (period 1–30 July, 2021) from 149 patients vaccinated for SARS-CoV2 without previous COVID-19 (negative for antibodies against SARS-CoV-2 nucleocapsid protein) and 150 subjects non-vaccinated for SARS-CoV2 with confirmed previous COVID-19 by antibodies against SARS-CoV-2 nucleocapsid protein have been investigated. In order to evaluate previously infected individuals with SARS-CoV-2, serial blood samples were tested for IgG antibodies against SARS-CoV-2 nucleocapsid protein using the Abbott ARCHITECT i-system (Abbott, Maidenhead, UK) immunoassay. Antibodies against viral proteins, including nucleocapsid and spike, are produced in response to SARS-CoV-2 infection [1] and probably correlate with immunity against reinfection [2]. There is also growing evidence that previously infected individuals develop greater antibody responses to SARS-CoV-2 vaccination than people who have not been infected [3], [4], [5].
Table 1 shows the diagnostic performances of the iRapid SARS-CoV-2 Quant Neutralizing Abs. Overall, the rapid assay showed a very satisfactory diagnostic performance, with high specificity (>99%) and a very close qualitative and quantitative agreement between nAb values determined by Rapid SARS-CoV-2 Quant Neutralizing Ab and the MN assay gold standard (Weighted Kappa statistic 0.938; 95% confidence interval 0.899–0.978).
iRapid diagnostic performances.
| MN + | MN− | Total | |
|---|---|---|---|
| iRapid + | 122 | 2 | 124 |
| iRapid− | 7 | 168 | 175 |
| Total | 129 | 170 | 299 |
| SE (95% CI) | 0.95 (0.89, 0.98) | ||
| SP (95% CI) | 0.99 (0.96, 1.00) | ||
| PPV (95% CI) | 0.98 (0.94, 1.00) | ||
| NPV (95% CI) | 0.96 (0.92, 0.98) | ||
-
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value.
In summary, we have tested a new platform for SARS-CoV-2 antibody detection, that is faster than current POCT devices and offers a valuable semi-quantitative information. The simplicity and low cost of the assay could enable its widespread use and a range of applications, including testing in low- and middle-income settings, evaluation of the serostatus before vaccination, and post-vaccination surveillance. This test offers the opportunity to quickly assess nAbs levels as a correlate of protection, procrastinating the booster (and saving doses) which could be eventually carried out with vaccines updated on the variants of concern (VOC).
Acknowledgments
We thank Alessandra Brogi, Marinunzia Castria, Giulia Tesi (technical laboratory scientists) for their valuable technical support and Diesse Diagnostica Senese, for kindly supplying reagents without any influence in study design and data analysis.
-
Research funding: None declared.
-
Author contributions: Conception and design: FB and MP; Data collection: FB; Investigations: FB and MP; Data analysis: SF and MC; Writing original: FB; Draft preparation: FB; Revision: FB, MP. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: Not applicable.
References
1. Tut, G, Lancaster, T, Krutikov, M, Sylla, P, Bone, D, Nayakaur, N, et al.. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. Lancet Healthy Longev 2021;2:e544–53. https://doi.org/10.1016/s2666-7568(21)00168-9.Suche in Google Scholar
2. Ebinger, JE, Fert-Bober, J, Printsev, I, Wu, M, Sun, N, Prostko, JC, et al.. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021;27:981–4. https://doi.org/10.1038/s41591-021-01325-6.Suche in Google Scholar PubMed PubMed Central
3. Long, Q-X, Liu, BZ, Deng, HJ, Wu, GH, Deng, K, Chen, YK, et al.. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845–8. https://doi.org/10.1038/s41591-020-0897-1.Suche in Google Scholar PubMed
4. Huang, AT, Garcia-Carreras, B, Hitchings, MDT, Yang, B, Katzelnick, LC, Rattigan, SM, et al.. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 2020;11:4704. https://doi.org/10.1038/s41467-020-18450-4.Suche in Google Scholar PubMed PubMed Central
5. Krammer, F, Srivastava, K, Alshammary, H, Amoalo, AA, Awawda, MH, Beach, KF, et al.. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021;384:1372–4. https://doi.org/10.1056/nejmc2101667.Suche in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Lot-to-lot variation: no longer a neglected issue
- Review
- Health Technology Assessment to assess value of biomarkers in the decision-making process
- Mini Review
- Diagnostic performance of the fully automated Roche Elecsys SARS-CoV-2 antigen electrochemiluminescence immunoassay: a pooled analysis
- Opinion Papers
- Preanalytical quality improvement – an interdisciplinary journey
- Metrological traceability and clinical traceability of laboratory results – the role of commutability in External Quality Assurance
- Lot-to-lot reagent verification: challenges and possible solutions
- EFLM Paper
- An approach for determining allowable between reagent lot variation
- General Clinical Chemistry and Laboratory Medicine
- Introduction of BD Vacutainer® Barricor™ tubes in clinical biobanking and application of amino acid and cytokine quality indicators to Barricor plasma
- Delays during PBMC isolation have a moderate effect on yield, but severly compromise cell viability
- Investigation of the effects of pneumatic tube transport system on routine biochemistry, hematology, and coagulation tests in Ankara City Hospital
- Comparison of three different protocols for obtaining hemolysis
- Report from the HarmoSter study: impact of calibration on comparability of LC-MS/MS measurement of circulating cortisol, 17OH-progesterone and aldosterone
- Point-of-care testing in primary healthcare: a scoring system to determine the frequency of performing internal quality control
- Hematology and Coagulation
- The flagging features of the Sysmex XN-10 analyser for detecting platelet clumps and the impacts of platelet clumps on complete blood count parameters
- Cancer Diagnostics
- Thyroglobulin and thyroglobulin antibodies: assay-dependent management consequences in patients with differentiated thyroid carcinoma
- Infectious Diseases
- Hyris bCUBE SARS-CoV-2 rapid molecular saliva testing: a POCT innovation on its way
- Ultrasensitive assay for saliva-based SARS-CoV-2 antigen detection
- Identification of contagious SARS-CoV-2 infected individuals by Roche’s Rapid Antigen Test
- Monocyte distribution width (MDW) as a screening tool for early detecting sepsis: a systematic review and meta-analysis
- Perinatal asphyxia partly affects presepsin urine levels in non-infected term infants
- Letters to the Editors
- Spurious results for total and free prostate-specific antigen (PSA); sometimes really “a riddle wrapped in a mystery inside an enigma”
- Reply to: Spurious results for total and free prostate-specific antigen (PSA); sometimes really “a riddle wrapped in a mystery inside an enigma”
- Spike vs. nucleocapsid serum antigens for COVID-19 diagnosis and severity assessment
- A rapid semi-quantitative test for determination of SARS-CoV-2 antibody levels
- Diagnostic potential of leukocyte differential and cell population data in prediction of COVID-19 among related viral and bacterial infections at Emergency Department
- Added value of drug-laboratory test interaction alerts in test result authorisation
- Pre-analytical considerations for the analysis of uracil and 5,6-dihydrouracil in heparin plasma
- Rare unstable and low oxygen affinity haemoglobin variant, Hb Hazebrouck, detected on Sysmex XN-9000
- Multicancer early detection
Artikel in diesem Heft
- Frontmatter
- Editorial
- Lot-to-lot variation: no longer a neglected issue
- Review
- Health Technology Assessment to assess value of biomarkers in the decision-making process
- Mini Review
- Diagnostic performance of the fully automated Roche Elecsys SARS-CoV-2 antigen electrochemiluminescence immunoassay: a pooled analysis
- Opinion Papers
- Preanalytical quality improvement – an interdisciplinary journey
- Metrological traceability and clinical traceability of laboratory results – the role of commutability in External Quality Assurance
- Lot-to-lot reagent verification: challenges and possible solutions
- EFLM Paper
- An approach for determining allowable between reagent lot variation
- General Clinical Chemistry and Laboratory Medicine
- Introduction of BD Vacutainer® Barricor™ tubes in clinical biobanking and application of amino acid and cytokine quality indicators to Barricor plasma
- Delays during PBMC isolation have a moderate effect on yield, but severly compromise cell viability
- Investigation of the effects of pneumatic tube transport system on routine biochemistry, hematology, and coagulation tests in Ankara City Hospital
- Comparison of three different protocols for obtaining hemolysis
- Report from the HarmoSter study: impact of calibration on comparability of LC-MS/MS measurement of circulating cortisol, 17OH-progesterone and aldosterone
- Point-of-care testing in primary healthcare: a scoring system to determine the frequency of performing internal quality control
- Hematology and Coagulation
- The flagging features of the Sysmex XN-10 analyser for detecting platelet clumps and the impacts of platelet clumps on complete blood count parameters
- Cancer Diagnostics
- Thyroglobulin and thyroglobulin antibodies: assay-dependent management consequences in patients with differentiated thyroid carcinoma
- Infectious Diseases
- Hyris bCUBE SARS-CoV-2 rapid molecular saliva testing: a POCT innovation on its way
- Ultrasensitive assay for saliva-based SARS-CoV-2 antigen detection
- Identification of contagious SARS-CoV-2 infected individuals by Roche’s Rapid Antigen Test
- Monocyte distribution width (MDW) as a screening tool for early detecting sepsis: a systematic review and meta-analysis
- Perinatal asphyxia partly affects presepsin urine levels in non-infected term infants
- Letters to the Editors
- Spurious results for total and free prostate-specific antigen (PSA); sometimes really “a riddle wrapped in a mystery inside an enigma”
- Reply to: Spurious results for total and free prostate-specific antigen (PSA); sometimes really “a riddle wrapped in a mystery inside an enigma”
- Spike vs. nucleocapsid serum antigens for COVID-19 diagnosis and severity assessment
- A rapid semi-quantitative test for determination of SARS-CoV-2 antibody levels
- Diagnostic potential of leukocyte differential and cell population data in prediction of COVID-19 among related viral and bacterial infections at Emergency Department
- Added value of drug-laboratory test interaction alerts in test result authorisation
- Pre-analytical considerations for the analysis of uracil and 5,6-dihydrouracil in heparin plasma
- Rare unstable and low oxygen affinity haemoglobin variant, Hb Hazebrouck, detected on Sysmex XN-9000
- Multicancer early detection

