Abstract
The measurement of the active hormone of B-type natriuretic peptide (BNP) system actually has several analytical limitations and difficulties in clinical interpretations compared to that of inactive peptide N-terminal proBNP (NT-proBNP) because of the different biochemical and pathophysiological characteristics of two peptides and quality specifications of commercial immunoassay methods used for their measurement. Because of the better analytical characteristics of NT-proBNP immunoassays and the easier pathophysiological and clinical interpretations of variations of NT-proBNP levels in patients with heart failure (HF), some authors claimed to measure the inactive peptide NT-proBNP instead of the active hormone BNP for management of HF patients. The measurement of the active peptide hormone BNP gives different, but complementary, pathophysiological and clinical information compared to inactive NT-proBNP. In particular, the setup of new more sensitive and specific assays for the biologically active peptide BNP1-32 should give better accurate information on circulating natriuretic activity. In conclusion, at present time, clinicians should accurately consider both the clinical setting of patients and the analytical characteristics of BNP and NT-proBNP immunoassays in order to correctly interpret the variations of natriuretic peptides measured by commercially available laboratory methods, especially in patients treated with the new drug class of angiotensin receptor-neprilysin inhibitors.
Analytical characteristics of BNP and NT-proBNP methods
The measurement of the active hormone of B-type natriuretic peptide (BNP) system actually presents several analytical limitations and difficulties in clinical interpretations compared to that of inactive peptide N-terminal proBNP (NT-proBNP) because of the different biochemical and pathophysiological characteristics of two peptides (Table 1) and the quality specifications of commercial immunoassay methods. Indeed, the inactive peptide NT-proBNP fits better the analytical and clinical characteristics required for an ideal laboratory biomarker than the active peptide BNP. NT-proBNP is more stable in vivo and in vitro: it has a higher molecular mass and a longer biological plasma half-live and a lower intra-individual biological variation (Table 1). Furthermore, data from external quality assessment studies demonstrated that immunoassays methods for NT-proBNP share on average better analytical performances (including better sensitivity and imprecision) than those of BNP [1], [2], [3], [4]. Furthermore, these studies also reported that there are large systematic differences (up to twofolds) between the BNP values measured by the most popular immunoassay methods, whereas the NT-proBNP methods show a between-method variability lower than 20% because they use calibrators and materials from the same manufacturer [3], [4]. Finally, from a clinical point of view, the circulating levels of NT-proBNP show a greater and progressive increment (on average, more than 120-folds compared to healthy subjects) from early to more severe disease in patients with heart failure (HF), as assessed by NYHA functional class, than those of BNP (i.e. on average, only an increment of about 50-folds) (Figure 1 and Table 2), measured by an immunoradiometric assay (IRMA) [5], [6]. Of course, the ratio between NT-proBNP and BNP is greatly method dependent [5], [6], [7], [8]; as a result, the data reported in Figure 1 are only an example. The wider clinical range of NT-proBNP should theoretically allow a better discrimination between the clinical phases of HF than BNP [8]; however, usually there are no differences in the diagnostic use of BNP and NT-proBNP immunoassays [1].
Biochemical and physiological characteristics of BNP, NT-proBNP and proBNP peptides.
| BNP | NT-proBNP | proBNP | |
|---|---|---|---|
| Molecular mass | 3462 Da | 8457 Daa | 11,900 Daa |
| Amino acids | 32 | 76 | 108 |
| Biological function | Active hormone | Inactive | Pro-hormone |
| Half life | 15–20 min | >60 min | >60 min |
| Glycosylation | Non-glycosylated | Highly glycosylated in vivo | Highly glycosylated in vivo |
aThe molecular mass (MM) of NT-proBNP and proBNP depends to the degree of glycosylation of the peptide; the MM of non-glycosylated peptides is reported in the table.
![Figure 1: Progressive increase in BNP and NT-proBNP levels from less to more severe stages of HF, assessed by NYHA functional class.The plasma concentration values of NT-proBNP, measured by the ECLIA method (Roche Diagnostics, Germany), and BNP, measured by the immunoradiometric assay (IRMA) Shionoria (Shionogi &Co, Japan), are expressed as the ratio between the mean biomarker concentration values found in HF groups and that found in healthy control subjects. Original BNP and NT-proBNP values measured in healthy subjects and patients with heart failure are reported in Table 2 and were previously discussed in detail by Prontera et al. [2].](/document/doi/10.1515/cclm-2017-0433/asset/graphic/j_cclm-2017-0433_fig_001.jpg)
Progressive increase in BNP and NT-proBNP levels from less to more severe stages of HF, assessed by NYHA functional class.
The plasma concentration values of NT-proBNP, measured by the ECLIA method (Roche Diagnostics, Germany), and BNP, measured by the immunoradiometric assay (IRMA) Shionoria (Shionogi &Co, Japan), are expressed as the ratio between the mean biomarker concentration values found in HF groups and that found in healthy control subjects. Original BNP and NT-proBNP values measured in healthy subjects and patients with heart failure are reported in Table 2 and were previously discussed in detail by Prontera et al. [2].
BNP and NT-proBNP values (mean±SD) measured in normal subjects and patients with heart failure (HF), divided according to New York Heart Association (NYHA) functional class groups.
| Group | Number | BNP | NT-proBNP | NT-proBNP/BNP molar ratio |
|---|---|---|---|---|
| Healthy subjects | 85 | 12.5±10.7 (3.6±3.1) | 55.9±37.7 (6.60±4.45) | 1.8 |
| All HF patients | 193 | 287.7±350.5 (81.1±101.3) | 3087.4±5439.2 (364.3±641.8) | 4.5 |
| HF NYHA I | 25 | 71.3±103.3 (20.6±29.8) | 588.3±950.8 (69.4±112.2) | 3.4 |
| HF NYHA II | 91 | 185.1±263.0 (53.6±76.0) | 1424.3±1676.3 (168.1±197.8) | 3.1 |
| HF NYHA III | 48 | 412.8±283.7 (119.2±82.0) | 4959.0±6976.2 (585.2±823.2) | 4.9 |
| HF NYHA IV | 29 | 598.0±528.2 (172.8±152.6) | 7362.4±8477.7 (868.8±1000.49) | 5.0 |
These data were used for the preparation of Figure 1. Values are expressed in both conventional units (ng/L) and SI units (pmol/L) within brackets. These data were previously published by Prontera et al. [2]. Plasma NT-proBNP was measured with the ECLIA method (Roche Diagnostics, Germany), and plasma BNP with the immunoradiometric assay (IRMA) Shionoria (Shionogi &Co, Japan) [2].
Two recent studies from the PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial [9], [10] reported conflicting results between BNP and NT-proBNP levels. PARADIGM-HF study evaluated the clinical effects of a new composite drug (denominated LCZ696 or Entresto), first-in-class composite angiotensin receptor-neprilysin inhibitors (ARNi) [11]. The action mechanism of this drug is complex, combining the effect of the angiotensin II receptor blocker and that of the neprilysin inhibitor. The enzyme neprilysin causes degradation not only of natriuretic peptides but also of a variety of components affecting the mechanisms of action for several other circulating biological active peptides, including enkephlins, bradykinins, angiotensins, endorphins, insulin and gastrin [12]. The rationale for the use of a drug containing a neprilysin inhibitor in HF patients is that this proteolytic enzyme can degrade the biologically active natriuretic peptides, especially CNP and ANP and to a lesser extent BNP [11], [12], [13]. For this reason, a drug, containing a substance inhibiting natriuretic peptide degradation, may increase the circulating levels of the biologically active natriuretic hormones (Figure 2) and, by doing so, may improve the clinical conditions of HF patients by increasing diuresis and natriuresis and also by reducing cardiac stress [11]. The results of the PARADIGM-HF study indicated that plasma BNP levels were higher during treatment with LCZ696 than with enalapril, but on the contrary, circulating levels of NT-proBNP and cardiac troponin T (cTnT) were lower during treatment with LCZ696 than with enalapril after the first weeks of treatment [9]. Authors explained these conflicting results obtained in the PARADIGM-HF study by considering the combined action of LCZ696 drug. Indeed, BNP (but not NT-proBNP) is a substrate for neprilysin [11], [13]; as a result, the increase in BNP levels after LCZ696 administration should reflect the inhibiting action of the drug on neprilysin, even if BNP1-32 is degraded with minor efficiency by neprilysin than ANP and CNP [11], [12], [13]. On the contrary, the decrease in NT-proBNP levels should reflect the beneficial effects of the drug on myocardial function and vascular hemodynamics, especially because of the inhibition in renin-angiotensin-aldosterone system activity [11], [13]. Indeed, the reduction of cardiac stress during LCZ696 treatment should reduce the production and secretion of natriuretic peptides from cardiomyocytes, hence producing a fall in circulating levels of NT-proBNP, too [14]. Considering these pathophysiological premises, clinicians should accurately evaluate the clinical setting in order to correctly interpret the variations of natriuretic peptides, measured by commercially available laboratory methods. In particular, clinicians should take into account that an increase in BNP levels in HF patients could be caused by inhibiting effect of LCZ696, but also by deterioration of clinical conditions in patients who do not respond to treatment. On the other hand, an increase in plasma NT-proBNP is usually due to deterioration of clinical conditions of HF patients [1], [14].
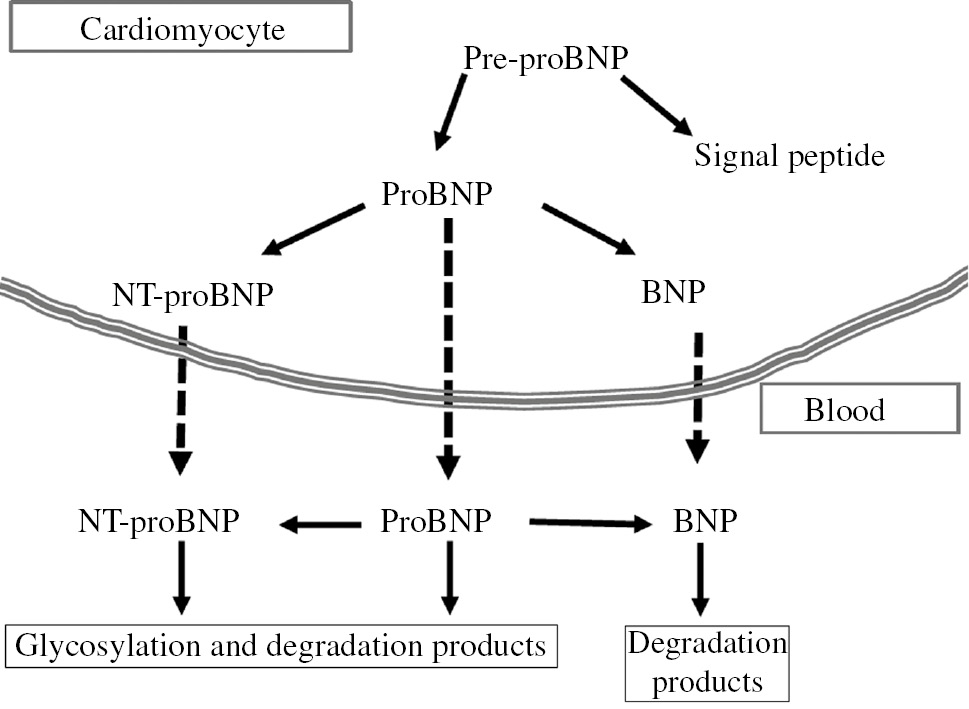
Schematic representation of biosynthesis, secretion and distribution of B-type-related natriuretic peptides.
Human BNP is synthesized as a 134-amino acid precursor protein (pre-proBNP), including a signal peptide of 26 amino acids, and is subsequently processed to form a 108-aa pro-peptide (proBNP). The proBNP can be enzymatically cleaved both in cardiomyocytes and in plasma by some pro-protein convertases to form the 76 amino acids N-terminal peptide (NT-proBNP) and the biologically active 32 amino acid C-terminal peptide (BNP). In plasma, proBNP, NT-proBNP and BNP are further degraded by some proteolytic enzymes.
Because of the better analytical characteristics of NT-proBNP immunoassays and the easier pathophysiological and clinical interpretations of variations of NT-proBNP levels in HF patients, some authors claimed to assay the inactive peptide NT-proBNP instead of the active hormone BNP for management of HF patients under treatment with LCZ696 [15], [16]. On the contrary, we do not think that it is yet the time to celebrate a requiem for the death of BNP assay, and we would like to explain in the present article why BNP assay is still alive, and why the measurement of the active natriuretic hormone may still be useful in both experimental research and clinical practice.
Rationale for BNP measurement
More than 30 years ago, De Bold et al. [17] reported that atrial extracts contain some biological active peptides, which promote a rapid and massive diuresis and natriuresis when injected in rats. After only few years, several endogenous peptide hormones with natriuretic and vasodilator activity have been identified in the human blood and peripheral tissues [1], [14]. Atrial natriuretic peptide (ANP), BNP and their related peptides are predominantly produced by atrial and ventricular cardiomyocytes, constituting the cardiac endocrine function [14]. From a pathophysiological point of view, cardiac endocrine function is an essential component of the integrated systems of the body, and thus, it plays a pivotal role in fluid, electrolyte and hemodynamic homeostasis [14]. A continuous exchange of information flows from the cardiac endocrine system to nervous and immunological systems and to other organs, including kidney, endocrine glands, liver, adipose tissue, immunocompetent cells and vice versa. This close link between cardiac natriuretic peptide system and counter-regulatory systems could explain the increase in circulating levels of BNP/NT-proBNP in some non-cardiac-related clinical conditions, including kidney, liver, pulmonary, metabolic and endocrine disorders [14].
Although even the most recent international guidelines reported only an interlocutory judgment about the natriuretic peptide-guided HF management [18], [19], [20], the monitoring of ambulatory patients with acute (decompensated) or chronic HF is frequently done, especially in specialized HF centers [21], [22]. From a clinical point of view, both BNP and NT-proBNP levels usually monitor the effectiveness of the pharmacological treatment of HF patients: the HF patients, “responder” to treatment, usually show a progressive reduction in circulating levels (more than 30%) and also a better clinical outcomes [22], [23]. However, it is important to note that pathophysiological interpretations of plasma concentration variations of these two peptides (BNP vs. NT-proBNP) should be conceptually different in both healthy subjects and patients with cardiac disease [1], [14].
NT-proBNP is an inactive peptide produced in an equimolar ratio together with BNP by action of some cellular or circulating proteolytic enzymes (such as corin and furin) on the precursor pro-hormone (proBNP) (Figure 2) [1], [14], [24], [25], [26], [27], [28]. As a result, NT-proBNP assay is not a reliable index of the biological (natriuretic) activity of cardiac endocrine system in severe HF because the concentration of less active peptides in patients with severe HF (such as NT-proBNP and proBNP) is greatly higher than that of the active peptide BNP1-32 (Figure 1) [1], [29], [30], [31], [32], [33]. Moreover, a recent study [34] suggested that the commercial immunoassays, commonly used for NT-proBNP assay, employ non-glycosylated calibrator materials and mostly antibodies directed against epitopes with potential O-glycosylation site occupancy. Because the most part of circulating inactive peptides related to NT-proBNP and proBNP are O-glycosylated [7], [18], [35], these immunoassays cannot measure some glycosylated peptides [34], [36]. Moreover, the commercially available immunoassays for NT-proBNP are interfered by the intact peptide proBNP (especially non-glycosylated peptide) [36] and also by some other shorter peptides derived from the proteolytic degradation of this pro-hormone [1] (Figure 2). In conclusion, the commercially available immunoassays for NT-proBNP do not allow an accurate measurement of this peptide.
On the other hand, plasma levels of the active hormone BNP1-32 are directly related to peripheral hormonal response of the cardiac endocrine function throughout the binding to specific natriuretic peptides receptors (NPR-A), which are present in all tissues of the body, including the central nervous system [14]. In particular, considering the natriuretic activity, circulating active peptide BNP1-32 can stimulate the specific natriuretic receptor (i.e. NPR-A) on renal tubular cells, inducing natriuresis. As a result, the specific measurement of the active peptide BNP1-32 (like that of ANP) should be considered a direct index of cardiac endocrine system activity [14]. Another reliable index of natriuresis induced by cardiac nariuretic peptides is the assay of cyclic GMP (cGMP) in the urine: this cyclic nucleoside is the second messenger of the natriuretic hormone system. cGMP is released in tubular renal cells as consequence of ANP and BNP binding to NPR-A sites on cell membranes of tubular renal cells [37].
Quality specifications for an accurate BNP assay
Recent studies revealed that the cardiac B-type natriuretic system is far more complex than we ever envisaged [1], [14], [24]. In addition to the peptide hormone BNP and the inactive peptide NT-proBNP, a huge numbers of other circulating peptides derived from the pro-hormone proBNP can be identified by chromatographic procedures in human plasma, including the intact and glycosylated forms of proBNP itself [29], [30], [31], [32], [33], [38] (Figure 2). Furthermore, the active hormone BNP may be produced even in vivo from the circulating intact precursor proBNP through enzymatic cleavage by some plasma proteases (such as corin) [35], [36], [39] (Figure 2). Indeed, a recent study, using an in vivo experimental rat model, demonstrated that the split of proBNP into BNP and NT-proBNP can actually occur in circulation [40]. From a clinical point of view, the peripheral processing of circulating proBNP could likely be submitted to regulatory mechanisms, which might be impaired in patients with HF, opening new perspectives in the treatment of HF [41].
The active hormone BNP, including 32 amino acids (BNP1-32), can be accurately detected and measured with mass spectrometry methods [1]. In particular, some studies [32], [42], [43], [44] reported that the true plasma BNP1-32 concentration measured with mass spectrometry methods in patients with severe HF is much lower than BNP values usually measured by commercially available immunoassay methods. At present time, the mass spectrometry methodology cannot be used in clinical laboratories for measurement of BNP because of the high cost of instrumentation, the long laboratory turnaround time, the complex analytical procedure, which generally include a preliminary extraction and/or chromatography processing of sample, and the need of highly qualified laboratory staff for analysis.
On the other hand, there are large systematic differences (up to twofolds) between the commercial immunoassay methods so far available for BNP assay [45], [46], [47], [48]. These differences are in part attributable to specific interferences related to the presence in clinical samples of proBNP and its degradation products [1], [36], [49]. According to these evidences [1], [36], [44], [45], [46], [47], [48], [49], the commercially available immunoassay methods, considered specific for the active hormone BNP, present an obvious paradox. As discussed in the previous paragraph, BNP immunoassays should specifically measure only the active peptide hormone because clinicians could be interested in evaluating the “true biologically active status” of the cardiac endocrine function [1], [14]. Unfortunately, none of the commercially available methods is able to accurately provide this important pathophysiological information because all BNP immunoassays are greatly affected by some less active peptides, especially glycosylated and non-glycosylated proBNP, which are the predominant peptides present in blood samples of patients with severe HF [1], [29], [30], [31], [32], [33], [38], [49]. Furthermore, the commercially available immunoassay methods for BNP measure not only the peptide BNP1-32, but also several degradation products with varying biological activities, derived from the active peptide due to action of some proteolyic enzymes (Figure 2) [1], [14], [24]. Several proteolytic enzymes can degrade natriuretic peptides in plasma and tissues, including neprilysin, dipeptidyl peptidase IV, insulin degrading enzyme and peptidyl arginine aldehyde protease, but their action on BNP seems to be lower that observed on ANP and CNP [50], [51], [52].
Fortunately, a very recent study [53] may offer a solution to this clinically relevant analytical problem. For the first time, Lewis et al. [53] were able to set up an ELISA method that measures BNP1-32 in plasma without interference by proBNP. This two-site ELISA uses a specific polyclonal antibody that recognizes the amino-terminal end of BNP1-32 and does not cross-react with proBNP, in conjunction with a C-terminal antibody, which recognizes BNP26-32. Detection by this assay requires both amino-terminus and carboxy-terminus of BNP to be intact. As expected, this new ELISA for BNP1-32 measured much lower BNP values than the commercial BNP method using the fully automated ARCHITECT platform. In 22 healthy subjects, the median (interquartile range) BNP concentration measured by the specific ELISA was 0.29 (0.2–0.6) ng/L, whereas the median value measured by ARCHITECT method was 17.3 (8.7–22.5) ng/L; even larger was the difference in BNP values measured by these two methods in a group of HF patients: 40.7 (2.0–90.1) ng/L (n=42) vs. 1778 (1134–2853) ng/L (n=39), respectively [53]. Interestingly, despite greatly lower values measured, this ELISA for BNP1-32 showed a percent increase in median BNP concentrations from the groups of healthy controls to HF patients slightly higher than that of ARCHITECT method (59% vs. 44%). Of course, the analytical performance and clinical results of this ELISA should be evaluated and confirmed in other studies using larger populations of both healthy controls and HF patients before to definitely state that we have found an immunoassay method able to measure the “true” concentration of the active hormone BNP1-32.
HF therapy with ARNi: Is it the time for the renaissance of BNP assay?
The results reported by Lewis et al. [53] confirmed that on average, the BNP1-32 concentration in healthy subjects is very low (median under 1 ng/L). The setup of a new generation of commercial BNP immunoassay methods with a relevant increment in both analytical sensitivity and specificity is needed in order to detect these very low analyte concentrations. This is a very difficult challenge, but the very recent experience on the development of highly sensitive methods for cardiac troponins suggests that this mission is not impossible [1].
More sensitive and specific immunoassay methods for the active hormone BNP1-32 will allow a more accurate estimation of circulating natriuretic activity in patients with HF, including those under treatment with ARNi. The time courses of plasma BNP and NT-proBNP concentrations of patients enrolled in the PARADIGM-HF study [9] levels tell us the same story, although seen from two different perspectives. BNP, measured with ADVIA Centaur method [54], increased at 4 weeks of LCZ696 treatment with a slightly decrease after 8 months of treatment, whereas NT-proBNP levels, measured by ECLIA Elecsys method [55], were significantly lower after 4 weeks compared to baseline and then they further decreased after 9 months of therapy. The rapid decrease in NT-proBNP levels confirms the clinical evidences, suggesting that the majority of patients under LCZ696 treatment shows a clinical benefit from the drug, whereas the rapid increase followed by a trend to decrease of BNP levels indicates that this improvement in clinical conditions is almost in part due to the increase in natriuretic activity due to neprilysin inhibition on BNP and ANP degradation. Therefore, BNP and NT-proBNP assay allows complementary (not contradictory) information on clinical conditions of patients treated with ARNi. It is important to note that the same complementary information is respectively allowed by cTnT assay (suggesting a reduction in heart stress and damage) and urinary cGMP assay (indicating an increased natriuretic activity under pharmacological treatment) [9].
At this point, it is important to discuss whether a more sensitive and specific BNP assay, as that described by Lewis et al. [53], could add further and/or more accurate information on pathophysiological and clinical conditions of HF patients under LCZ696 treatment. As a matter of fact, proBNP cross-reacts by about 14%–17% with BNP1-32 in the ADVIA BNP assay [36], [56], which is the laboratory method used in the PARADIGM-HF trial for BNP assay [9]. All the other commercially available BNP immunoassays are also (or even more) cross-reacted by proBNP than ADVIA BNP assay (from about 20% to 40%) [36], [56]. From a physiological point of view, proBNP is able to bind the specific natriuretic receptor NPR-A, but it stimulates guanylyl cyclase-A (GC-A) with reduced potency (i.e. 13-fold less) than BNP [37], [57]. Considering that the proBNP and its related peptides are the predominant peptides present in plasma of HF patients [29], [30], [31], [32], [33], [38], the measurement of BNP with the immunoassays at present commercially available gives an inaccurate estimation of biologically active B-type peptides present in patients with severe HF. Therefore, the results of a more sensitive and specific assay for BNP1-32 should theoretically allow a better correlation between immunoreactivity and biological activity (i.e. natriuretic activity) [14]. Furthermore, a more specific BNP1-32 assay should display a better correlation with rapid variations in pathophysiological conditions compared to other less specific immunoassay methods significantly interfered by proBNP because the active peptide has a greatly lower plasma half-life than less active pro-hormone (Table 1). In particular, it is conceivable that a more sensitive and specific method, such as that by Lewis et al. [53], should show a greater decrease in BNP levels compared to the ADVIA Centaur method after 8 months of treatment similarly to that observed with NT-proBNP levels in the PARADIGM-HF trial [9].
New perspectives and conclusions
Because of complex metabolic pathways and pathophysiological mechanisms in which B-type natriuretic peptides are involved, as well as to the differences in quality specifications of immunoassay methods, clinicians should give great care to the pathophysiological interpretation of plasma BNP and NT-proBNP variations in HF patients. Indeed, the measurement of the active peptide hormone BNP should theoretically give different, but complementary, pathophysiological and clinical information than that of the inactive NT-proBNP. Unfortunately, the clinical interpretation of variations of natriuretic peptides in HF is made still more difficult because current BNP methods cross-react with proBNP, and also they measure several BNP fragments instead of the intact hormone; on the other hand, NT-proBNP assays are cross-reacted by proBNP and its degradation products [1], [49].
Therefore, it is needed to set up some more sensitive and specific immunoassays for the active peptide BNP1-32, as that described by Lewis et al. [53]. These new and specific methods should give more accurate information on the circulating natriuretic activity [1]. On the other hand, the measurement of the pro-hormone proBNP with highly specific immunoassays [29], [30], [31], [58] may be useful as an estimate of less active B-type natriuretic peptides produced by cardiomyocytes and circulating as predominant peptides in plasma of patients with severe HF [1], [29], [30], [31], [49].
In conclusion, at present time, clinicians should accurately consider both the clinical setting of patients and the analytical characteristics of BNP and NT-proBNP immunoassays in order to correctly interpret the variations of natriuretic peptides measured by commercially available laboratory methods, especially in patients treated with ARNi.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Clerico A, Passino C, Franzini M, Emdin M. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin Chim Acta 2015;443:17–24.10.1016/j.cca.2014.06.003Search in Google Scholar
2. Prontera C, Emdin E, Zucchelli GC, Ripoli A, Passino C, Clerico A. Analytical performance and diagnostic accuracy of a fully automated electrochemiluminescent assay for the N-terminal fragment of the pro-peptide of brain natriuretic peptide in patients with cardiomyopathy: comparison with immunoradiometric assay methods for brain natriuretic peptide and atrial natriuretic peptide. Clin Chem Lab Med 2004;42:37–44.10.1515/CCLM.2004.008Search in Google Scholar
3. Prontera C, Zaninotto M, Giovannini S, Zucchelli GC, Pilo A, Sciacovelli L, et al. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NT-proBNP) immunoassays: the CardioOrmocheck study. Clin Chem Lab Med 2009;47:762–8.10.1515/CCLM.2009.153Search in Google Scholar
4. Clerico A, Zaninotto M, Prontera C, Giovannini S, Ndreu R, Franzini M, et al. State of the art of BNP and NT-proBNP immunoassays: the CardioOrmoCheck study. Clin Chim Acta 2012;414:112–9.10.1016/j.cca.2012.07.017Search in Google Scholar
5. Storti S, Prontera C, Emdin M, Passino C, Prati P, Fontani G, et al. Analytical performance and clinical results of a fully automated MEIA system for brain natriuretic peptide assay: comparison with a point of care testing method. Clin Chem Lab Med 2004;42:1178–85.10.1515/CCLM.2004.238Search in Google Scholar
6. Prontera C, Stori S, Emdin M, Passino C, Zyw L, Zucchelli GC, et al. Comparison of a fully automated immunoassay with a point-of-care testing method for B-type natriuretic peptide. Clin Chem 2004;51:1274–6.10.1373/clinchem.2005.048496Search in Google Scholar
7. Emdin M, Passino C, Prontera C, Iervasi A, Ripoli A, Masini S, et al. Cardiac natriuretic hormones, neuro-hormones, thyroid hormones and cytokines in normal subjects and patients with heart failure. Clin Chem Lab Med 2004;42:627–36.10.1515/CCLM.2004.108Search in Google Scholar
8. Emdin M, Passino C, Prontera C, Fontana M, Poletti R, Gabutti A, et al. Comparison of brain natriuretic peptide (BNP) and amino-terminal ProBNP for early diagnosis of heart failure. Clin Chem 2007;53:1289–97.10.1373/clinchem.2006.080234Search in Google Scholar
9. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61.10.1161/CIRCULATIONAHA.114.013748Search in Google Scholar
10. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:387–95.10.1016/S0140-6736(12)61227-6Search in Google Scholar
11. McCormack PL. Sacubitril/Valsartan: a review in chronic heart failure with reduced ejection fraction. Drugs 2016;76:387–96.10.1007/s40265-016-0544-9Search in Google Scholar
12. Erdös EG, Skidgel RA. Neutral endopeptidases 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 1989;3:145–51.10.1096/fasebj.3.2.2521610Search in Google Scholar
13. Bayés-Genis A. Neprilysin in heart failure: from oblivion to center stage. JACC Heart Fail 2015;3:637–40.10.1016/j.jchf.2015.03.010Search in Google Scholar
14. Clerico A, Giannoni A, Vittorini S, Passino C. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol 2011;301:H12–20.10.1152/ajpheart.00226.2011Search in Google Scholar
15. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004.10.1056/NEJMoa1409077Search in Google Scholar
16. Vasile VC, Jaffe AS. Natriuretic peptides and analytical barriers. Clin Chem 2017;63:50–8.10.1373/clinchem.2016.254714Search in Google Scholar
17. De Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and important natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci 1981;28:89–94.10.1016/0024-3205(81)90370-2Search in Google Scholar
18. National Clinical Guideline Centre (UK). Acute heart failure: diagnosing and managing acute heart failure in adults. London: National Institute for Health and Care Excellence (UK); 2014.Search in Google Scholar
19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200.10.1093/eurheartj/ehw128Search in Google Scholar PubMed
20. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803.10.1161/CIR.0000000000000509Search in Google Scholar PubMed
21. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017;135:e1054–91.10.1161/CIR.0000000000000490Search in Google Scholar PubMed
22. Troughton R, Michael Felker G, Januzzi JL, Jr. Natriuretic peptide-guided heart failure management. Eur Heart J 2014;35:16–24.10.1093/eurheartj/eht463Search in Google Scholar PubMed
23. Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J 2009;158:422–30.10.1016/j.ahj.2009.06.018Search in Google Scholar PubMed
24. Goetze JP. Biosynthesis of cardiac natriuretic peptides. Results Probl Cell Differ 2010;50:97–120.10.1007/400_2009_25Search in Google Scholar PubMed
25. Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem 2010;56:1166–76.10.1373/clinchem.2010.143883Search in Google Scholar PubMed
26. Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem 2011;286:10066–72.10.1074/jbc.M110.185082Search in Google Scholar PubMed PubMed Central
27. Knappe S, Wu F, Masikat MR, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem 2003;278:52363–70.10.1074/jbc.M309991200Search in Google Scholar PubMed
28. Dong N, Chen S, Yang J, He L, Liu P, Zheng D, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail 2010;3:207–11.10.1161/CIRCHEARTFAILURE.109.903849Search in Google Scholar PubMed PubMed Central
29. Hammerer-Lercher A, Halfinger B, Sarg B, Mair J, Puschendorf B, Griesmacher A, et al. Analysis of circulating forms of proBNP and NT-proBNP in patients with severe heart failure. Clin Chem 2008;54:858–65.10.1373/clinchem.2007.090266Search in Google Scholar PubMed
30. Dries DJ, Ky B, Wu A, Rame JE, Putt M, Cappola T. Simultaneous assessment of unprocessed ProBNP 1-108 in addition to processed BNP32 improves risk stratification in ambulatory patients with systolic heart failure. Circ Heart Fail 2010;3:220–7.10.1161/CIRCHEARTFAILURE.109.903153Search in Google Scholar PubMed PubMed Central
31. Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, et al. Pro-B-type natriuretic peptide 1-108 circulates in the general community: plasma determinants and detection of left ventricular systolic dysfunction. J Am Coll Cardiol 2011;57:1386–95.10.1016/j.jacc.2011.01.005Search in Google Scholar PubMed PubMed Central
32. Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail 2011;4:355–60.10.1161/CIRCHEARTFAILURE.110.960260Search in Google Scholar
33. Shimizu H, Masuta K, Asada H, Sugita K, Sairenji T. Characterization of molecular forms of probrain natriuretic peptide in human plasma. Clin Chim Acta 2003;334:233–9.10.1016/S0009-8981(03)00240-7Search in Google Scholar
34. Halfinger B, Hammerer-Lercher A, Amplatz B, Sarg B, Kremser L, Lindner HH. Unraleveling the molecular complexity of O-glycosylated endogenous (N-Terminal) pro_B-type natriuretic peptide forms in blood plasma of patients with seveere heart failure. Clin Chem 2017;63:359–68.10.1373/clinchem.2016.265397Search in Google Scholar PubMed
35. Seferian KR, Tamm NN, Semenov AG, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem 2008;54:866–73.10.1373/clinchem.2007.100040Search in Google Scholar PubMed
36. Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem 2008;54:619–21.10.1373/clinchem.2007.097998Search in Google Scholar PubMed
37. Potter LR. Guanyl cyclase structure, function and regulation. Cell Signal 2011;23:1921–6.10.1016/j.cellsig.2011.09.001Search in Google Scholar PubMed PubMed Central
38. Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol 2007;49:1071–8.10.1016/j.jacc.2006.10.063Search in Google Scholar PubMed
39. Semenov AG, Postnikov AB, Tamm NN, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem 2009;55:489–98.10.1373/clinchem.2008.113373Search in Google Scholar PubMed
40. Semenov AG, Seferian KR, Tamm NN, Artem’eva MM, Postnikov AB, Bereznikova AV, et al. Human pro-B-type natriuretic peptide is processed in the circulation in a rat model. Clin Chem 2011;57:883–90.10.1373/clinchem.2010.161125Search in Google Scholar PubMed
41. Del Ry S, Cabiati M, Clerico A. Recent advances on natriuretic peptide system: new promising therapeutic targets for the treatment of heart failure. Pharmacol Res 2013;76:190–8.10.1016/j.phrs.2013.08.006Search in Google Scholar PubMed
42. Niederkofler EE, Kiernan UA, O’Rear J, Menon S, Saghir S, Protter AA, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail 2008;1:258–64.10.1161/CIRCHEARTFAILURE.108.790774Search in Google Scholar PubMed
43. Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Ichiki T. ProBNP1-108 processing and degradation in human heart failure. Circ Heart Fail 2015;8:89–97.10.1161/CIRCHEARTFAILURE.114.001174Search in Google Scholar PubMed PubMed Central
44. Hawkridge AM, Heublein DM, Bergen HR 3rd, Cataliotti A, Burnett JC Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA 2005;102:17442–7.10.1073/pnas.0508782102Search in Google Scholar PubMed PubMed Central
45. Rawlins ML, Owen WE, Roberts WL. Performance characteristics of four automated natriuretic peptide assays. Am J Clin Pathol 2005;123:439–45.10.1309/PDJ2RMM80FVRDH7WSearch in Google Scholar
46. Prontera C, Zaninotto M, Giovannini S, Zucchelli GC, Pilo A, Sciacovelli L, et al. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NT-proBNP) immunoassays: the CardioOrmoCheck study. Clin Chem Lab Med 2009;47:762–8.10.1515/CCLM.2009.153Search in Google Scholar PubMed
47. Yeo KT, Dumont KE, Brough T. Elecsys NT-ProBNP and BNP assays: are there analytically and clinically relevant differences? J Card Fail 2005;11(5 Suppl):S84–8.10.1016/j.cardfail.2005.04.017Search in Google Scholar PubMed
48. Franzini M, Masotti S, Prontera C, Ripoli A, Passino C, Giovannini S, et al. Systematic differences between BNP immunoassays: comparison of methods using standard protocols and quality control materials. Clin Chim Acta 2013;424:287–91.10.1016/j.cca.2013.07.001Search in Google Scholar PubMed
49. Clerico A, Franzini M, Masotti S, Prontera C, Passino C. State of the art of immunoassay methods for B-type natriuretic peptides: an update. Crit Rev Clin Lab Sci 2015;52:56–69.10.3109/10408363.2014.987720Search in Google Scholar PubMed
50. Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J 2001;278:1808–11.10.1111/j.1742-4658.2011.08082.xSearch in Google Scholar PubMed PubMed Central
51. Vanderheyden M, Bartunek J, Goethals M, Verstreken S, Lambeir AM, De Meester I, et al. Dipeptidyl-peptidase IV and B-type natriuretic peptide. From bench to bedside. Clin Chem Lab Med 2009;47:248–52.10.1515/CCLM.2009.065Search in Google Scholar PubMed
52. Ralat LA, Guo Q, Ren M, Ren M, Funke T, Dickey DM, et al. Insulin degrading enzyme modulates the natriuretic peptide-mediated signaling response. J Biol Chem 2011;286:4670–9.10.1074/jbc.M110.173252Search in Google Scholar PubMed PubMed Central
53. Lewis LK, Raudsepp SD, Yandle TG, Prickett TC, Richards AM. Development of a BNP1-32 immunoassay that does not cross-react with proBNP. Clin Chem 2017;63:110–7.10.1373/clinchem.2016.269712Search in Google Scholar PubMed
54. Prontera C, Fortunato A, Storti S, Mercuri A, Longombardo G, Zucchelli GC, et al. Evaluation of analytical performance of the Siemens ADVIA TnI ultra immunoassay. Clin Chem 2007;53:1722–3.10.1373/clinchem.2007.089995Search in Google Scholar PubMed
55. Prontera C, Zucchelli CG, Vittorini S, Storti S, Emdin M, Clerico A. Comparison between analytical performances of polyclonal and monoclonal electrochemiluminescence immunoassays for NT-proBNP. Clin Chim Acta 2009;400:70–3.10.1016/j.cca.2008.10.011Search in Google Scholar PubMed
56. Saenger AK, Rodriguez-Fraga O, Ler R, Ordonez-Llanos J, Jaffe AS, Goetze JP, et al. Specificity of B-type natriuretic peptide assays: cross-reactivity with different BNP, NT-proBNP, and proBNP peptides. Clin Chem 2017;63:351–8.10.1373/clinchem.2016.263749Search in Google Scholar PubMed
57. Dickey DM, Potter LR. ProBNP1–108 is resistant to degradation and activates guanylyl cyclase-A with reduced potency. Clin Chem 2011;9:1272–8.10.1373/clinchem.2011.169151Search in Google Scholar PubMed PubMed Central
58. Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, et al. Assay for measurement of intact B-type natriuretic peptide rohormone in blood. Clin Chem 2006;52:1054–61.10.1373/clinchem.2005.061770Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Method comparison – a practical approach based on error identification
- Review
- Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease
- Mini Reviews
- α-Defensin point-of-care test for diagnosis of prosthetic joint infections: neglected role of laboratory and clinical pathologists
- The diagnostic accuracy of biomarkers for diagnosis of primary biliary cholangitis (PBC) in anti-mitochondrial antibody (AMA)-negative PBC patients: a review of literature
- Opinion Paper
- New issues on measurement of B-type natriuretic peptides
- Genetics and Molecular Diagnostics
- The SEeMORE strategy: single-tube electrophoresis analysis-based genotyping to detect monogenic diseases rapidly and effectively from conception until birth
- General Clinical Chemistry and Laboratory Medicine
- Determination of serum calcium levels by 42Ca isotope dilution inductively coupled plasma mass spectrometry
- The effects of dry ice exposure on plasma pH and coagulation analyses
- Placental protein-13 (PP13) in combination with PAPP-A and free leptin index (fLI) in first trimester maternal serum screening for severe and early preeclampsia
- Circulating CD89-IgA complex does not predict deterioration of kidney function in Korean patients with IgA nephropathy
- Performance analysis of automated evaluation of Crithidia luciliae-based indirect immunofluorescence tests in a routine setting – strengths and weaknesses
- Performance of automated digital cell imaging analyzer Sysmex DI-60
- Reference Values and Biological Variations
- Determination of reference intervals for urinary steroid profiling using a newly validated GC-MS/MS method
- Reference intervals and longitudinal changes in copeptin and MR-proADM concentrations during pregnancy
- Definition of the upper reference limit of glycated albumin in blood donors from Italy
- Reference values of fecal calgranulin C (S100A12) in school aged children and adolescents
- Processing-independent proANP measurement for low concentrations in plasma: reference intervals and effect of body mass index and plasma glucose
- Cancer Diagnostics
- Cancer sniffer dogs: how can we translate this peculiarity in laboratory medicine? Results of a pilot study on gastrointestinal cancers
- Cardiovascular Diseases
- NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis
- Analytical evaluation of the new Beckman Coulter Access high sensitivity cardiac troponin I immunoassay
- Infectious Diseases
- Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor
- Effects of procalcitonin testing on antibiotic use and clinical outcomes in patients with upper respiratory tract infections. An individual patient data meta-analysis
- Acknowledgment
- Letters to the Editor
- Handling the altered test results of hemolyzed samples. Recommendations of the Quality, Management, Safety and Evidence Committee (CCGSE) of the Spanish Association of Medical Biopathology and Laboratory Medicine (AEBM-ML)
- Reply to: Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor
- Excessive hypercortisolemia due to ectopic Cushing’s syndrome requiring extending the reportable range for plasma cortisol for management
- Heavy chain disease: our experience
- An abnormal elevation of serum CA72-4 due to taking colchicine
- Rivaroxaban non-responders: do plasma measurements have a place?
- Next generation sequencing and immuno-histochemistry profiling identify numerous biomarkers for personalized therapy of endometrioid endometrial carcinoma
- A multicenter effort to improve comparability of vitamin B6 assays in whole blood
- PR3-anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis
Articles in the same Issue
- Frontmatter
- Editorial
- Method comparison – a practical approach based on error identification
- Review
- Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease
- Mini Reviews
- α-Defensin point-of-care test for diagnosis of prosthetic joint infections: neglected role of laboratory and clinical pathologists
- The diagnostic accuracy of biomarkers for diagnosis of primary biliary cholangitis (PBC) in anti-mitochondrial antibody (AMA)-negative PBC patients: a review of literature
- Opinion Paper
- New issues on measurement of B-type natriuretic peptides
- Genetics and Molecular Diagnostics
- The SEeMORE strategy: single-tube electrophoresis analysis-based genotyping to detect monogenic diseases rapidly and effectively from conception until birth
- General Clinical Chemistry and Laboratory Medicine
- Determination of serum calcium levels by 42Ca isotope dilution inductively coupled plasma mass spectrometry
- The effects of dry ice exposure on plasma pH and coagulation analyses
- Placental protein-13 (PP13) in combination with PAPP-A and free leptin index (fLI) in first trimester maternal serum screening for severe and early preeclampsia
- Circulating CD89-IgA complex does not predict deterioration of kidney function in Korean patients with IgA nephropathy
- Performance analysis of automated evaluation of Crithidia luciliae-based indirect immunofluorescence tests in a routine setting – strengths and weaknesses
- Performance of automated digital cell imaging analyzer Sysmex DI-60
- Reference Values and Biological Variations
- Determination of reference intervals for urinary steroid profiling using a newly validated GC-MS/MS method
- Reference intervals and longitudinal changes in copeptin and MR-proADM concentrations during pregnancy
- Definition of the upper reference limit of glycated albumin in blood donors from Italy
- Reference values of fecal calgranulin C (S100A12) in school aged children and adolescents
- Processing-independent proANP measurement for low concentrations in plasma: reference intervals and effect of body mass index and plasma glucose
- Cancer Diagnostics
- Cancer sniffer dogs: how can we translate this peculiarity in laboratory medicine? Results of a pilot study on gastrointestinal cancers
- Cardiovascular Diseases
- NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis
- Analytical evaluation of the new Beckman Coulter Access high sensitivity cardiac troponin I immunoassay
- Infectious Diseases
- Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor
- Effects of procalcitonin testing on antibiotic use and clinical outcomes in patients with upper respiratory tract infections. An individual patient data meta-analysis
- Acknowledgment
- Letters to the Editor
- Handling the altered test results of hemolyzed samples. Recommendations of the Quality, Management, Safety and Evidence Committee (CCGSE) of the Spanish Association of Medical Biopathology and Laboratory Medicine (AEBM-ML)
- Reply to: Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor
- Excessive hypercortisolemia due to ectopic Cushing’s syndrome requiring extending the reportable range for plasma cortisol for management
- Heavy chain disease: our experience
- An abnormal elevation of serum CA72-4 due to taking colchicine
- Rivaroxaban non-responders: do plasma measurements have a place?
- Next generation sequencing and immuno-histochemistry profiling identify numerous biomarkers for personalized therapy of endometrioid endometrial carcinoma
- A multicenter effort to improve comparability of vitamin B6 assays in whole blood
- PR3-anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis

