The seaweed resources of Peru
-
José Avila-Peltroche
and Jaraj Padilla-Vallejos
Caulerpa filiformis . She is currently a research member at “Vera Alleman Haeghebaert” Natural History Museum, Lima, Peru.
Abstract
The coast of Peru (3.5–18°S) is one of the most productive marine areas in the world. The updated seaweed checklist reports a total of 260 specific/intraspecific taxa, of which the Rhodophyta show the highest number (185 species), followed by Chlorophyta (41 species) and Phaeophyceae (34 species). Since 2012, an increase in molecular studies has been reported mostly in Rhodophyta, while Chlorophyta and Phaeophyceae have lagged far behind. Utilization of seaweed resources has been based on artisanal collection from natural beds. These are mainly consumed fresh or salt-dried (Chondracanthus chamissoi and Porphyra/Pyropia species), or exported for polysaccharide extraction (Lessonia nigrescens, Lessonia trabeculata, Macrocystis pyrifera). Although some regulations for brown seaweed extraction exist, overexploitation is a constant threat that must be addressed. Biomass from seaweed farms accounts, at most, for only 4% of the total annual landings, and there is a decreasing trend on seaweed aquaculture production since 2012. However, some cultivation projects for C. chamissoi and Porphyra/Pyropia species are currently being undertaken. In addition, recent studies have started to explore new uses of commercial and non-commercial seaweeds. Uncovering the Peruvian seaweed diversity and establishing well sustained culture and management projects are essential for utilizing and preserving the seaweed resources of Peru.
1 Introduction
Peru borders the Pacific Ocean to the east. Its coastline stretches from 3°23′S to 18°21′S on the western side of South America. It has a length of 3080 km, with a continental shelf with a variable width: from 150 km off northern and central Peru to less than 10 km off parts of southern Peru (Bruland et al. 2005; Hooker et al. 2013). Around 77 islands (94.36 km2) can be found in the Peruvian Sea, 22 of them belonging to the “Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras” (RNSIIPG), a marine protected network (Hooker et al. 2013; MINAM 2019). Diverse ecosystems are presented on the coast, such as bays, cliffs, kelp and macroalgal beds, rocky shores and sandy beaches. Coastal wetlands, which include the southernmost limit to the tropical Pacific mangrove ecosystem, are also important components of the Peruvian coast (Fernandez-Baca et al. 2007; Tarazona et al. 2003).
The ocean circulation on the coast of Peru is dominated by two systems: the northward flowing cold Peruvian Current (PC), coming from the southern tip of Chile; and the southward flowing warm South Equatorial Current (SEC) (Kämpf and Chapman 2016; Spalding et al. 2007). The coastal upwelling ecosystem supported by the PC makes the marine ecosystem of Peru one of the most productive in the world, earning the title of the “heavyweight champion” in terms of producing fish biomass (Bakun and Weeks 2008). This ecosystem is largely affected by inter-annual variations during El Niño Southern Oscillation (ENSO) events, where warm equatorial and oceanic waters are brought to the coast by the intrusion of Kelvin waves (Arntz et al. 2006; Fahrbach et al. 1991).
In this review, three biogeographical units are considered for the Peruvian coast (Figure 1): the Panamic Province (PP; 3.5–4.5°S), a Transitional Zone (TZ; between 5 and 5.5°S), and the Humboldt (Peruvian) Province (HP; 6–13.5°S). Ibanez-Erquiaga et al. (2018) proposed this zonation based on rocky intertidal communities along the coast of Peru, increasing the precision of the previous biogeographical zonations. In a regional perspective, three out of 27 marine Eco regions described for South America are within (totally or partially) the Exclusive Economic Zone (EEZ) of Peru (Chatwin 2007). The Guayaquil region includes PP, TZ and the northern part of HP, while the Central Peru and Humboldt regions encompass the rest of HP. The diversity of marine environments (shaped by the PC and SEC, continental shelf, islands and the Peru trench) allows a diversity of the marine macroalgae in these zones (Figure 2) that, in turn, supports diverse ecosystems (e.g., kelp forests in central and southern Peru; IMARPE 2012) and provides important resources that need to be regulated (Alemañ et al. 2019).
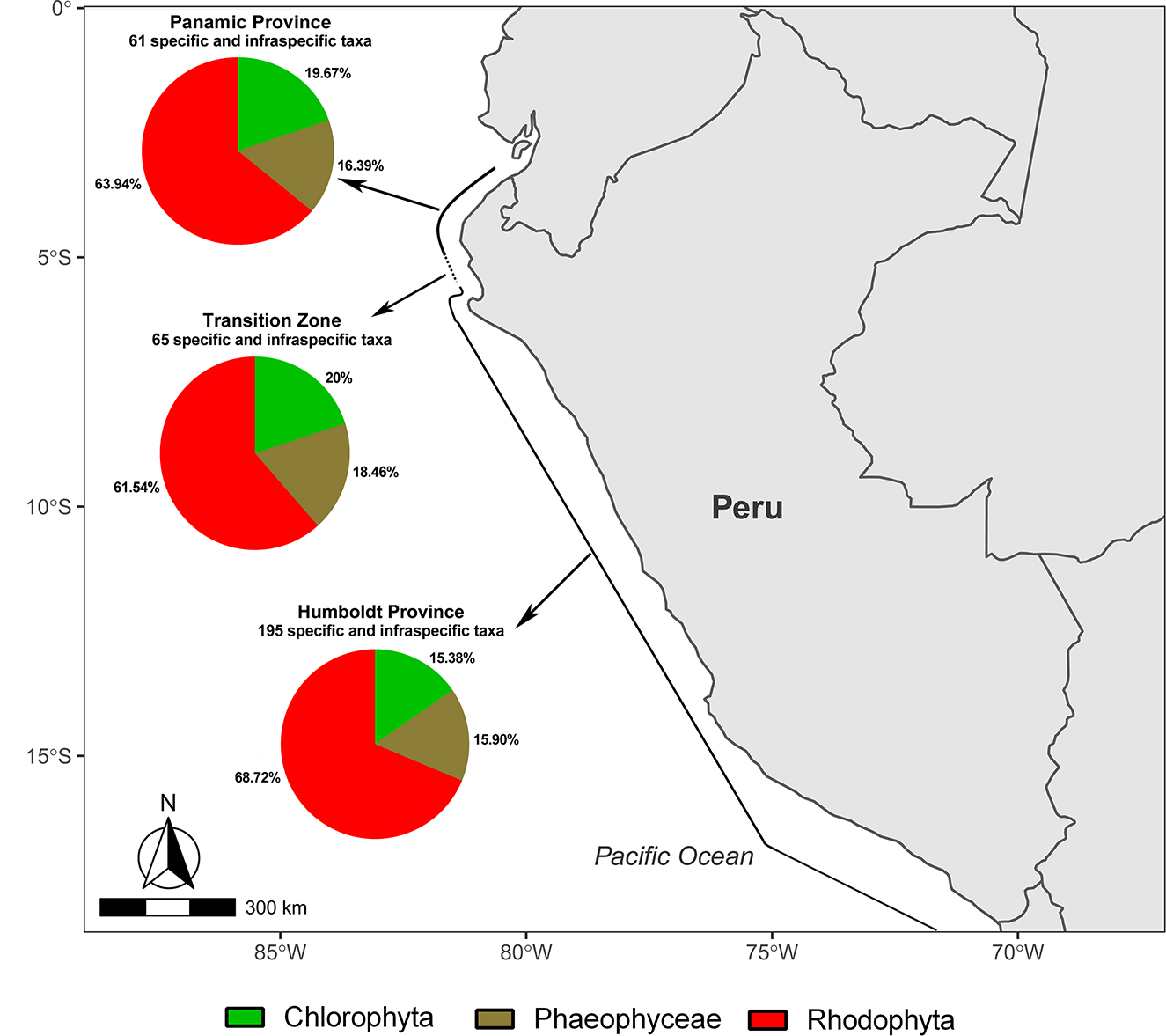
Map of Peru showing the proportions of specific and intraspecific. taxa, belonging to Chlorophyta, Phaeophyceae and Rhodophyta.

Some characteristic seaweed communities from the Peruvian coast. (A) Kelp forests seen from the cliffs of Marcona (Humboldt Province). (B) Algal diversity on the intertidal shores of Marcona (Humboldt Province). (C) Intertidal Petalonia fascia and Ulva spp. from Paracas (Humboldt Province). (D) A patch of Caulerpa filiformis in Cabo Blanco (Panamic Province).
In this context, the present work aims: 1) to compile all the information regarding Peruvian seaweed diversity into a comprehensive list accessible to the world scientific community; 2) to assess its current biodiversity status; 3) to report uses and potential applications of seaweeds in Peru; and 4) to summarize the past and present of seaweed cultivation in the country. We discuss our perspectives for seaweed research in Peru, with emphasis on taxonomy and culture.
2 Seaweed flora of Peru
2.1 A brief history of seaweed biodiversity studies
The history of seaweed exploration in Peru dates back to 1802, when Humboldt and Bonpland collected the first specimens from the Peruvian coast, in places such as Huanchaco, in the north coast, and Callao, in the central coast (Humboldt and Bonpland 1814). These collections were later studied by Humboldt (1815–1825) and C. A. Agardh (1822), thus giving the starting point of seaweed biodiversity studies in the country. During the 19th century, several researchers such as Bory de Saint Vincent (1826–1828); Montagne (1839, 1846); Suhr (1840); Kützing (1843, 1849, 1851–1869); J. G. Agardh (1841, 1851–1863) and Piccone (1886), increased the list of Peruvian macroalgal species. De Toni’s Sylloge Algarum (1900-1924) closed the century, listing about 65 species attributable to Peru.
The 20th century began with a landmark document for Peruvian phycology: The Marine Algae of Peru, by M. A. Howe (1914), the first comprehensive account of marine algal biodiversity in the country. This work was based on the materials collected by R. Coker (1906–1908), the specimens from various European herbaria, and a few collections of the U.S. Exploring Expedition (Wilkes Expedition) of 1839. Howe reported 115 species (excluding Cyanobacteria), 26 of which were new species and 29 were new records for Peru. A few years later, Collins (1915) added five more species to the Peruvian algal list based on a small collection made at Chincha Islands, while Kylin and Skottesberg (1919) reported for the first time the red alga Callophyllis variegata. Subsequent studies did not mention Peruvian algae until 1941, when Dawson revised several species of Rhodymenia (Dawson 1941). During the 1940s and 1950s, Taylor (1947) contributed to the description of several new Peruvian species, whereas Juhl-Noodt (1958) provided a brief annotated list of seaweeds collected during a survey for the Compañía Administradora del Guano in 1956. Later, Dawson et al. (1964) published The Seaweeds of Peru, a taxonomic review that reported 156 species, seven of which were new species and 29 were new records. This review constitutes an important landmark for seaweed taxonomy and diversity in the country during the second half of the 20th century.
During the 1970s and 1980s, Acleto (1972, 1973, 1980, 1981, 1984, 1988), and Acleto and Endo (1977) contributed greatly to the knowledge of the macroalgal flora along the coast of Peru. Acosta (1977a) provided the first detailed algal list of Pisco, one of the most productive marine areas in the country. By the beginning of the 1990s, Ramírez and Santelices (1991) published a catalogue of the benthic marine algae from the southeastern Pacific Ocean, which compiled all the reported taxa, including their bibliography and distribution, from Peru and Chile. This work registered 226 species on the Peruvian coast (39 Chlorophyta, 31 Phaeophyceae and 156 Rhodophyta), and it represents the last checklist of the marine macroalgae of Peru to date.
The 21st century brought the first molecular studies that included Peruvian samples, such as the model organism Ectocarpus siliculosus (Peters et al. 2004) and specimens of the genus Lessonia (Cho et al. 2006). However, it was not until 2012 that the molecular taxonomy of seaweeds started to increase, with works in Rhodomelaceae (Bustamante et al. 2012a,b, 2016, 2019b), Halymeniaceae (Calderon et al. 2014a,b), Gracilariaceae (Arakaki et al. 2015), Phyllophoraceae (Calderon and Boo 2016a,b, 2017) and Bangiaceae (Márquez-Corigliano et al. 2019). Additional regional lists have contributed to a better understanding of seaweed distribution in the country (Gil-Kodaka et al. 2002; Ramírez et al. 2015; Rodríguez-Rodríguez et al. 2018; Roque-Sánchez et al. 2017). Also, three catalogues of seaweeds from the central coast of Peru (Arakaki et al. 2018a,b; Carbajal et al. 2018, 2019) have been released as a result of a DNA barcoding project for marine macroalgae. Finally, the first transcriptomic and genomic analyses of Peruvian seaweeds have been recently published (Bustamante et al. 2019a; Salavarría et al. 2018).
2.2 Diversity of marine macroalgae in Peru
As a result of compiling all earlier species records in both international and Peruvian publications, the updated checklist of seaweeds from Peru consists of a total of 260 specific/intraspecific taxa (41 Chlorophyta, 34 Phaeophyceae and 185 Rhodophyta; Supplementary Tables S1–S3). A summary of the seaweed flora of Peru can be found in Table 1. This work represents the most inclusive list of Peruvian flora to date, including 34 species more than in the list of Ramírez and Santelices (1991). We expect this number to increase as there are several generic records that might represent new species or new reports for the country (Table S4). Although the number of species reported for Peru in AlgaeBase (Guiry and Guiry 2019) is slightly higher (277), 39.35% of the records are synonyms. Also, 14 species have been incorrectly attributed to Peru in AlgaeBase (Table S5). One red algal species, Archeolithothamniun chilense, did not have matching records, either in AlgaeBase or in the INA Database (Silva 2019), and possibly represents an invalid name (Table S6). The best represented genera in Peru are Ulva with 10 species, Desmarestia with four species and two subspecies, Gelidium with nine species, Gracilaria with six species and one subspecies, and Cryptonemia and Rhodymenia with seven species each. Key references for seaweed diversity in Peru are presented in Table 2. The entire literature used for the checklist can be found in the reference list for the Supplementary Tables.
Summary of the seaweed flora of Peru.
| Phylum/Class | Numbers | ||||||
|---|---|---|---|---|---|---|---|
| Classes | Orders | Families | Genera | Species | Sub-species | Varieties | |
| Chlorophyta | 1 | 4 | 11 | 14 | 39 | 2 | |
| Phaeophyceae | 1 | 7 | 12 | 22 | 32 | 2 | |
| Rhodophyta | 4 | 21 | 35 | 90 | 182 | 3 | |
| Total | 6 | 32 | 58 | 126 | 253 | 2 | 5 |
Main studies on Peruvian seaweed diversity.
| Reference | Title | Publication |
|---|---|---|
| Howe (1914) | The marine algae of Perua | Memoirs of the Torrey Botanical Club 15:1–185 |
| Dawson et al. (1964) | The seaweeds of Peru | Beihefte zur Nova Hedwigia 13:1–111 |
| Acleto (1973) | The marine algae of Peru (in Spanish) | Boletín de la Sociedad Peruana de Botánica 6:1–164 |
| Acosta (1977) | The marine algae of Pisco, Ica, Peru (in Spanish)b | Publicaciones del Museo de Historia Natural Javier Prado Serie B 28:1–34 |
| Acleto (1980) | Notes on marine algae from Peru I. New records (in Spanish)b | Publicaciones del Museo de Historia Natural Javier Prado Serie B 30:1–33 |
| Ramirez and Santelices (1991) | Catalogue of the benthic marine algae from the temperate coast of Pacific South America (in Spanish)a | Monografías Biológicas 5:1–437 |
| Alvítez and Rodríguez (2005) | Diversity, taxonomy and ecology of Phaeophyceae from the Peruvian coast (in Spanish)c | Rebiol 25:15–30 |
| Arakaki et al. (2018b) | Macroalgae from central coast of Peru I-Rhodophyta (in Spanish)d | Universidad Nacional Agraria La Molina, Perú |
| Carbajal et al. (2018) | Macroalgae from central coast of Peru II-Chlorophyta & Phaeophyceae (in Spanish)d | Universidad Nacional Agraria La Molina, Perú |
aAvailable online in Algaebase (http://www.algaebase.org).
bAvailable online in the website of the Natural History Museum “Javier Prado” (https://museohn.unmsm.edu.pe/pub_bot.html).
cAvailable online in https://es.slideshare.net/egc1981/rebiol-vol-25?fbclid=IwAR09D0gqI5PvycNQy9GecDEFTiFNnQhB9RgNoOC8xdAwy6VSQ-WDvpFSSrw.
dAvailable online in the website of the National Agrarian University La Molina (http://www.lamolina.edu.pe/eventos/pesqueria/2019/MACROALGAS_DE_LA_COSTA_CENTRAL_DEL_PERU_CATALOGO.pdf).
The bulk of new species and new records of seaweeds from Peru were published in the following publications: Howe (1914), Taylor (1947), Dawson et al. (1964), and Acleto (1973, 1980). From 1984 to 2009, the discovery rate of new species/records diminished (less than one per year). However, from 2011 onward, there has been an increase in this number (more than two species/records per year) together with an increase in the number of algal publications (Figure 3). When divided into algal groups, most of the new species/records relate to red algae (Figure 4). In fact, molecular phylogeny has helped to describe nine new species and to establish five new genera based on Peruvian samples in recent years (Bustamante et al. 2012a,b, 2019b, Calderon and Boo 2016a,b, 2017, Calderon et al. 2014a,b). On the other hand, no new records have been reported for green and brown algae since 1980 and 2006, respectively. Also, no new species in these two groups have been described from Peru for over 50 years (Dawson et al. 1964; Taylor 1947). From this, it is clear that taxonomic and biodiversity studies on Peruvian seaweeds have begun to increase significantly with the help of molecular techniques. However, less attention has been paid to the taxonomy of green and brown seaweeds compared to red ones.
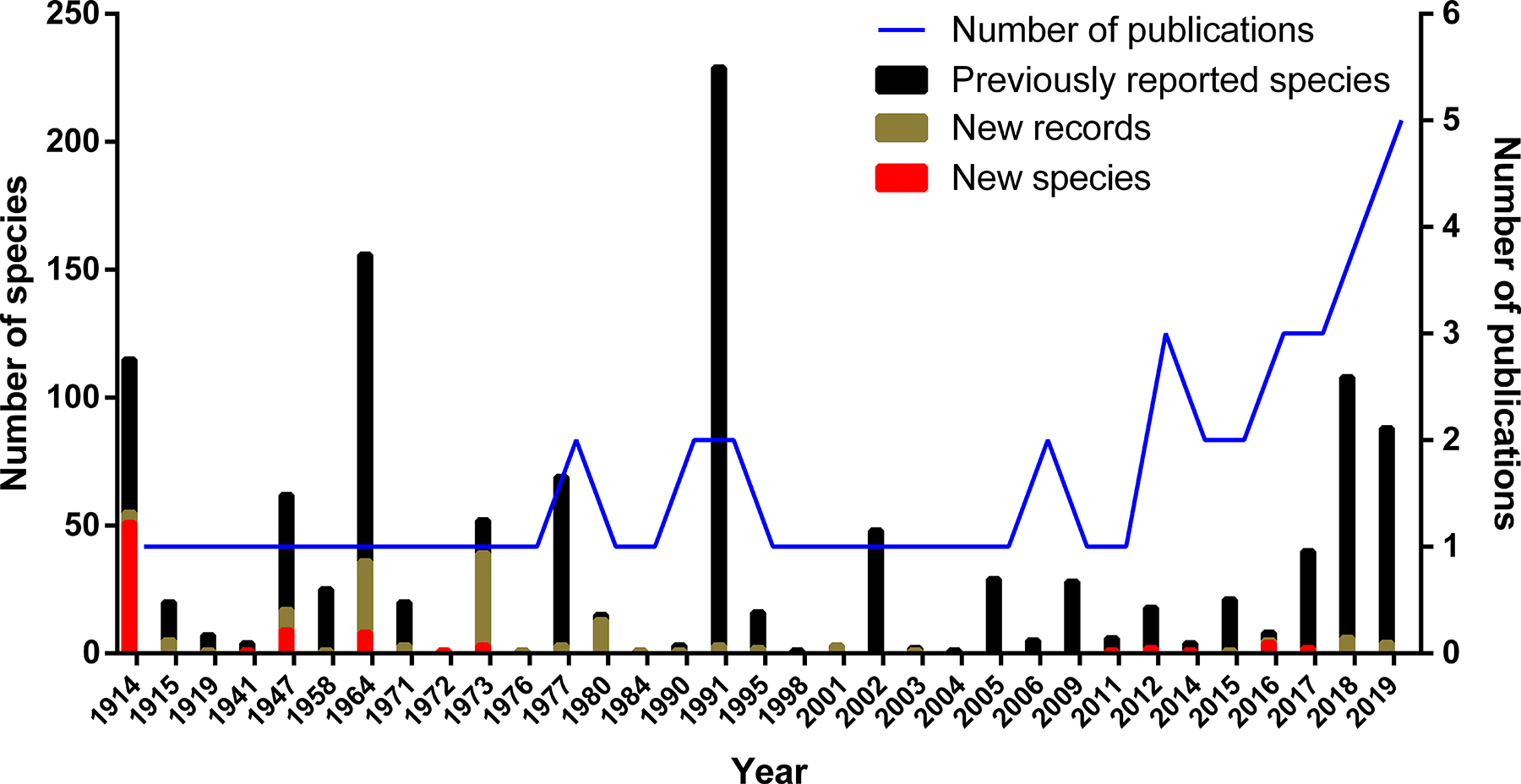
Number of publications on Peruvian seaweeds (blue line, right axis) and numbers of previously reported species (black bar chart, left axis), new species (red bar chart, left axis) and new algal records (brown bar chart, left axis) by year between 1914 and 2019.
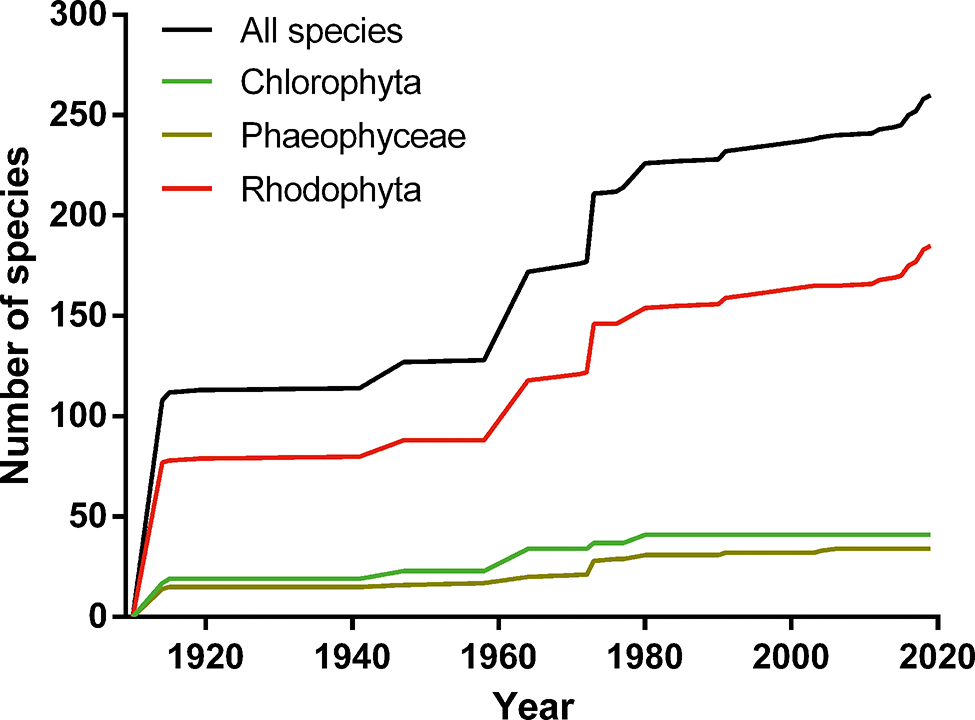
Cumulative curve of new seaweed species and new records (pooled) from Peru between 1914 and 2019.
2.3 Distribution of seaweeds along the Peruvian coast
Locality data enabled us to analyse, to some extent, biogeographic and richness patterns. Among the three biogeographical units considered in this review, the highest number of seaweed species is found in the HP (195), followed by the TZ (65) and the PP (61), from which 71.28, 24.62 and 44.26%, respectively, are exclusive to each region. More than 60% of the reports for each region correspond to Rhodophyta (Figure 1). Most of the species have been found in intertidal habitats. Some species or genera are representative of these areas, such as Bryopsis plumosa, Lessonia and Rhodymenia species for the HP; Ulva compressa, Gracilaria textorii and Laurencia paitensis for the TZ; and Caulerpa racemosa, Bachelotia antillarum and Caloglossa species for the PP. The differences in species diversity among the provinces are most likely influenced by differences in research effort and their geographical extent. For example, HP presents the highest seaweed diversity among the three regions, probably because that it covers most of the Peruvian coast and more research has been conducted there. It is worth noticing that some species might not be present any longer, in areas where they had been reported decades ago. For example, Macrocystis pyrifera is currently reported to have its northernmost limit in the central coast (Lima; Carbajal and Gamarra 2018); however, Juhl-Noodt (1958) found this species on the northern coast (Mancora) more than 60 years ago.
In relation to endemism, five green algae, three brown algae and 27 red algae have been described as endemic so far. These numbers are likely to change in the next years as molecular analyses can help to clarify their endemic status (e.g., Bustamante et al. 2019a). Regarding potential invasive species, there are studies only on the rapid expansion of the green alga Caulerpa filiformis along the Peruvian coast (MINAM 2014; Ramsar 2015); and on the possibly fortuitous introduction and spread of the green alga, Codium fragile in Cherrepe Bay, northern Peru (Castilla and Neill 2009).
2.4 Diversity of marine macroalgae in Peru compared to neighbouring countries
The diversity of the Peruvian seaweeds was compared with that of a number of neighbouring areas (Chile, Galapagos Archipelago and Continental Ecuador). Chile appears to be the most diverse country with a total of 633 species. Peru reports 260 species, which is comparable to the 321 species reported for the Galapagos Archipelago (Table 3). We used the Sørensen similarity index to compare seaweed diversity with neighbouring countries (Nguyen et al. 2013; Phang et al. 2019). The values obtained were all low (Cs < 0.5; Table 3), which is counterintuitive, especially in the case of Chile. This country, together with Peru, makes up the Humboldt Current Large region. One might expect that under similar climatic zones and without any major dispersal barriers, the similarity index would have been higher (Spalding et al. 2007). Although the subtropical influence in the HP could partially explain the low similarity with Chile, we are of the opinion that this value is primarily an artifact resulting from taxonomic inconsistencies and unequal research effort. The limited knowledge of seaweeds from Ecuadorian coastal waters can also explain the extremely low Sørensen similarity index for continental Ecuador (Miloslavich et al. 2011).
Comparison of Peruvian seaweed flora with neighbouring countries.
| Neighbouring country | Peru (Na = 260) | ||
|---|---|---|---|
| Nb | Na+b | Cs | |
| Chile | 633 | 129 | 0.289 |
| Ecuador (Continental) | 49 | 6 | 0.039 |
| Ecuador (Galapagos) | 321 | 51 | 0.176 |
The floristic data of the neighbouring countries were derived from Algaebase (Guiry and Guiry 2020). Nb, number of individuals in the neighbouring country; Na+b, the number of taxa shared with Peru; Sørensen similarity index: Cs = (2Na+b)/(Na + Nb).
3 Economic seaweeds of Peru
3.1 Uses and potential applications
Peru is one of the few places outside of Asia with a long tradition of seaweed consumption (Mouritsen et al. 2018). According to archeological evidence along the coast, seaweeds have been found in places that date back as far as 2500 B.C. (Patterson and Moseley 1968). Marine macroalgae have also been registered in mummies and represented in pottery from pre-Columbian Peruvian cultures (Kiple and Ornelas 2000; Yacovleff and Herrera 1934; Yacovleff and Muelle 1934). Apart from human consumption, seaweeds could have been used as means of exchange (Rostworowski 1981). Today, seaweeds are mostly used for human food or exported for the extraction of phycocolloids. Table 4 shows the uses and potential applications of Peruvian seaweeds. For a detailed review of the uses of marine macroalgae in Peru, we recommend the works of Acosta (1977b), Acleto (1986), and Noriega (2011).
Commercial and non-commercial seaweeds from Peru with their common names and uses or potential uses in the country.
| Phylum/Class | Species | Common name | Use/Potential use | Reference |
|---|---|---|---|---|
| Chlorophyta | Bryopsis plumosa | None | Antibacterial | Magallanes et al. (2003) |
| Caulerpa filiformis | None | Photoprotective, antioxidant, spermicide | Egg et al. (2015), Hernández-Gómez et al. (2015), and Mamani et al. (2020) | |
| Ulva sp. | Lechuga de mar | Human food, animal feed, compost | Cruz (2019), Noriega (2011), Sumarriva-Bustinza et al. (2019), and Wosnitza and Barrantes (2005) | |
| Phaeophyceae | Eisenia cokeri | None | Antioxidant | Rodríguez and Castro (2018) |
| Lessonia nigrescens | Aracanto negro, aracanto | Agricultural growth promoter, alginates, heavy metal removal | Noriega (2011), Reyes et al. (2009), and PSW (2020) | |
| Lessonia trabeculata | Aracanto palo, calatillo | Agricultural growth promoter, alginates, heavy metal removal | Rivera et al. (2004), Noriega (2011), Valiente and Mogollón (2013), and PSW (2020) | |
| Macrocystis pyrifera | Aracanto huiro, huiro | Agricultural growth promoter, alginates, dermocosmetic, heavy metal removal | Reyes et al. (2009), Noriega (2011), Valiente and Mogollón (2013), Castro et al. (2014), and PSW (2020) | |
| Petalonia fascia | None | Antibacterial | Magallanes et al. (2003) | |
| Rhodophyta | Chondracanthus chamissoi, Gigartina paitensis | Yuyo, mococho | Carrageenans, human food | Salas et al. (2009) and Noriega (2011) |
| Gracilariopsis lemaneiformis | Pelillo, pelo de mujer, pelo de indio | Forage | Noriega (2011) | |
| Neorubra decipiens | Piscuchaqui | Human food | Noriega (2011) | |
| Porphyra/Pyropia spp. | Cochayuyoa | Human food | Noriega (2011), Aguilar-Velasquez (2015), and Rosado-Alejos (2017) | |
| Rhodymenia howeana | None | Food supplement | Rojas-Vega et al. (2018) |
aOther species like Grateloupia doryphora, Mazzaella denticulata and Rhodymenia corallina are also commercialized under this name in Peru.
Among red seaweeds, Porphyra/Pyropia species, locally known as “cochayuyo” (Márquez-Corigliano et al. 2019), and Chondracanthus chamissoi, locally known as “yuyo” or “mococho”, are commonly consumed (Figure 5A and B). Acleto (1986) indicated that “cochayuyo” mostly include Py. columbina and Py. pseudolanceolata. These ones are eaten salt-dried in salads or stews after being washed and passed through hot water. Due to problems distinguishing species based on morphology and misapplied names, Porphyra/Pyropia species are currently being taxonomically re-assessed using molecular analysis (Guillemin et al. 2016; Márquez-Corigliano et al. 2019). C. chamissoi is consumed fresh in salads and in the traditional Peruvian ceviche, or added at the end of the preparation of fish stews. Some health food stores sell dried C. chamissoi as a food supplement or weight loss food (Noriega 2011). C. chamissoi is also an important source of carrageenan. Salas et al. (2009) showed that the highest yield of λ-carrageenan from this species was obtained from the tetrasporophytic phase (45.3%), while κ-carrageenan was predominant in the gametophytic phase (35.4–36.2%). They reported the use of λ-carrageenan as thickener in chocolate milk, and the inclusion of κ-carrageenan as a stabilizer in dairy products. Gracilariopsis lemaneiformis (Figure 5C), an important agarophyte worldwide, is used as forage in only small amounts (Noriega 2011). Recent studies have shown that Porphyra/Pyropia possess antioxidant and hypolipidemic properties (Aguilar-Velasquez 2015). Also, they can be used as flour in the preparation of cookies (Rosado-Alejos 2017).
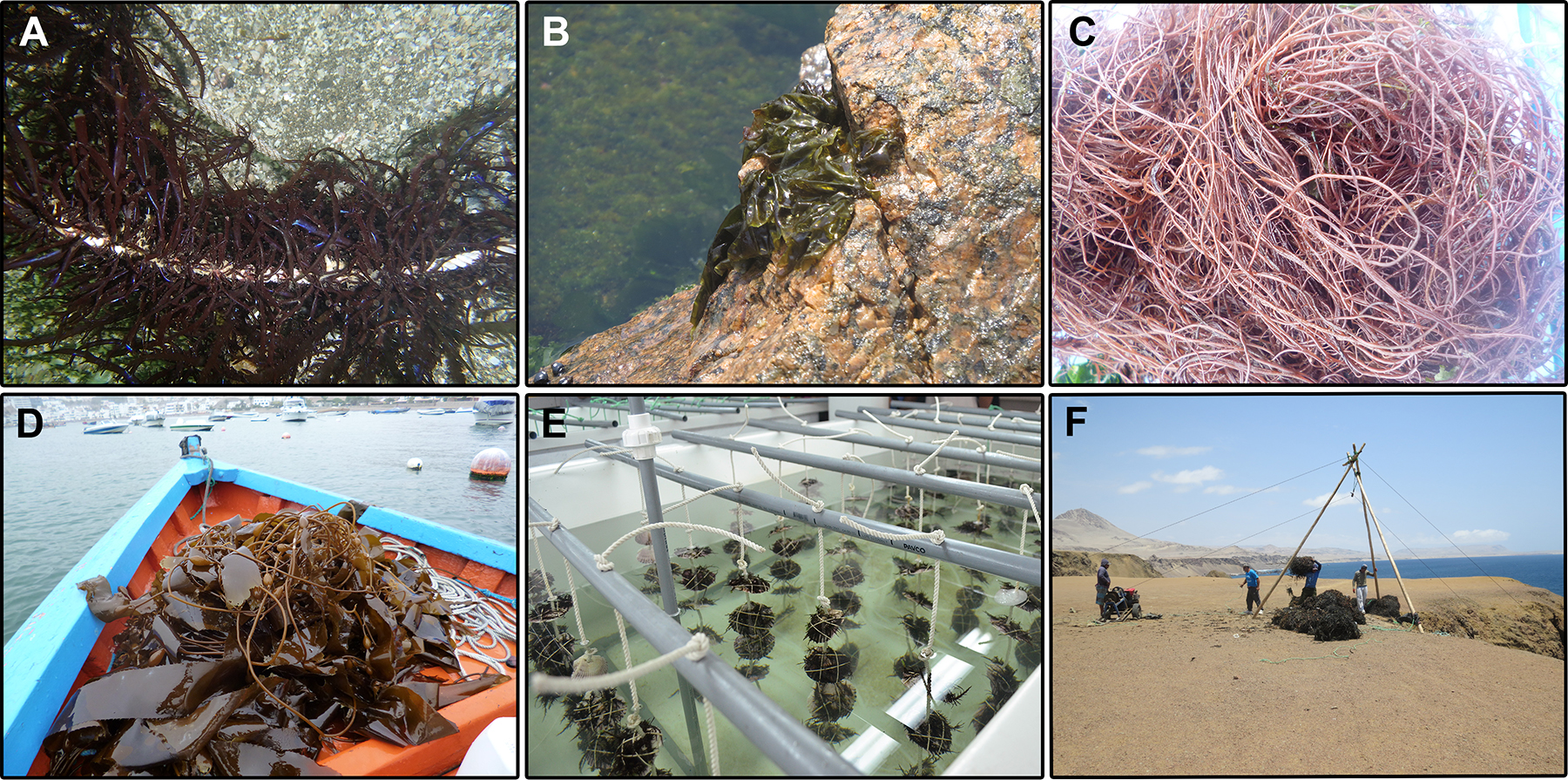
Economic seaweeds from Peru. (A) Chondracanthus chamissoi, locally known as “yuyo” or “mococho”, growing on ropes from a farm in Pisco (photo credit: Paul Baltazar). (B) A Porphyra/Pyropia specimen, locally known as “cochayuyo”, from the intertidal of Marcona. (C) A freshly collected Gracilariopsis lemaneiformis, locally known as “pelillo”, from the bay of Ancon. (D) Macrocystis pyrifera, locally known as “aracanto”, from the bay of Pucusana. (E) Vegetative propagation of C. chamissoi through secondary attachment discs in scallop shells at indoor cultures in Pisco (photo credit: Paul Baltazar). (F) Dried brown seaweeds (mostly Macrocystis pyrifera) being transported to the cliffs from a hard-to-access beach in Marcona. A pulley system is usually used by the fishermen, who later stack the seaweeds for air-drying and selling.
Brown seaweeds are not usually part of Peruvian cuisine. Species like M. pyrifera, Lessonia nigrescens and Lessonia trabeculata (Figure 5D and F) are mainly exported for alginate production due to its gelling, thickening, emulsifying and stabilizing properties. Valiente and Mogollón (2013) showed that alginic acid was predominant in L. nigrescens (37.15%), followed by L. trabeculata (35.20%) and M. pyrifera (31.36%). Fishermen also harvest M. pyrifera for the extraction of flying fish ovas, although this represents a small percentage (less than 3.5%) of the harvested biomass in a year (PRODUCE 2015; Vásquez-Castro 2009). Recently, Castro et al. (2014) included an extract of M. pyrifera in a dermocosmetic formulation due to its antioxidant and antiaging properties. Currently, one company (PSW S.A. Peruvian Seaweeds) includes in its product portfolio agricultural growth promoters based on extracts from these three species (PSW 2020).
Green seaweeds are the less exploited algal group. They are not usually consumed in Peru, although Ulva species can be used instead of lettuce in some food applications (Noriega 2011). The nutritional properties of Ulva lactuca make it ideal for human consumption (Sumarriva-Bustinza et al. 2019) and animal feeds (Cruz 2019). In the last few years, C. filiformis has caught the attention of researchers due to its large unexploited biomass in places such as Paracas Bay (central coast of Peru). Biological and pharmacological properties of its extracts have been analysed with promising results (Egg et al. 2015; Hernández-Gómez et al. 2015; Mamani et al. 2020).
3.2 Traditional harvesting and aquaculture
Seaweed exploitation in Peru largely relies on harvesting from natural beds. In Latin America, Peru contributes 4% of the total seaweed harvest in the region. However, with respect to seaweed cultivation, Peru only accounts for 0.01% of the biomass in Latin America (FAO 2018). According to annual landing data provided by the Ministry of Production (PRODUCE 2015, 2017), seaweed exploitation in Peru has increased since 2013 (Figure 6A), while the biomass from seaweed farms has been decreasing since 2012 (Figure 6B). A decrease in seaweed production from 2008 to 2012 can be attributed to legislation that is discussed later in Section 3.2. At its peak, only 4.1% of Peruvian seaweed production came from aquaculture. It is clear from this that cultivation of marine macroalgae in Peru is still in its infancy; however, some progress has been achieved with species such as C. chamissoi. In this regard, previous reviews have dealt with the situation of seaweed aquaculture of Peru in a regional perspective (Alemañ et al. 2019; Hayashi et al. 2013; Rebours et al. 2014). Therefore, Section 3.2 will attempt to summarize the main findings of these authors and complement them with recent Peruvian publications and projects focused on seaweed culture.
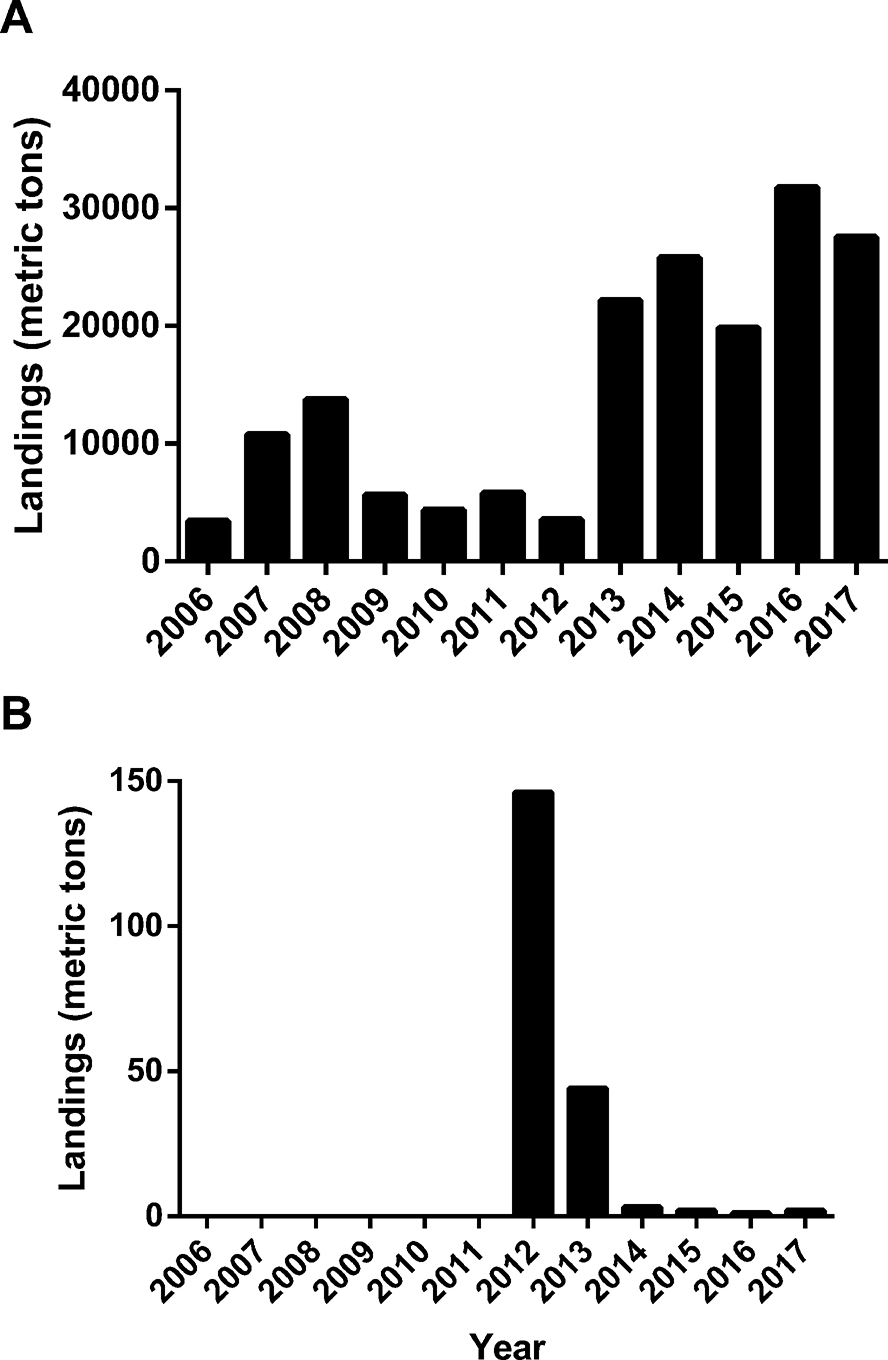
Landings of harvested seaweed in Peru from 2006 to 2017. (A) Total landings. (B) Aquaculture landings. From PRODUCE (2015, 2017).
As previously mentioned, the most exploited red algae in Peru are C. chamissoi and Porphyra/Pyropia. Most of their biomass comes from natural beds. In the case of C. chamissoi, overexploitation has led to a decrease in the populations together with size reduction and lower gel quality. Scallop cultivation in the north of Peru has also affected the populations of this carrageenophyte (Hayashi et al. 2013). Given this threat, cultivation of C. chamissoi was explored during the early 2000s and from 2012 to 2014 (Rebours et al. 2014). This last attempt at cultivation ceased in 2015 according to the FAO (2020). However, recent studies have started to examine the factors affecting carpospore cultures of C. chamissoi (Arbaiza et al. 2019; Castañeda et al. 2018), and a project is currently being implemented for its vegetative propagation (Figure 5E) in Paracas Bay with fishermen (Paul Baltazar, Universidad Científica del Sur, 2020, pers. comm.). As suggested by Alemañ et al. (2019), the investment for C. chamissoi cultivation must be focused on human consumption due to the Peruvian gastronomic boom. Prices of C. chamissoi have experienced an increase of around 400% due to the few existing beds, the short time allowed for plant regeneration, and the effort involved in small-scale harvesting (Hayashi et al. 2013). Fresh C. chamissoi is sold at US$ 2.99–3.59 kg−1, while C. chamissoi flour packages (200 g) cost US$ 4.19 (Noriega 2011). Also, the current legislation does not regulate the harvesting of this or other red seaweeds in Peru.
Cultivation of Porphyra/Pyropia has just started, with studies of the effects of photoperiod and a liquid commercial foliar fertilizer (Bayfolan®) on its biomass under semi-controlled conditions (Arbaiza et al. 2018). This is part of a larger project involving the establishment of a culture system in Pisco (central Peru) together with fishermen associations. The resulting biomass is planned to be used as flour in the bakery industry (Toribio-Chahua 2017). As previously mentioned, Porphyra/Pyropia is consumed salt-dried. It is sold as sheets (250 g) for US$ 0.45 by fishermen, and the same amount costs US$ 2.69 (almost six times the original price) in the capital (Noriega 2011).
Attempts at cultivating the agarophyte G. lemaneiformis were performed with governmental support in the late 1990s. Despite the initial success of the cultures in the Bay of San Nicolas (central Peru), seaweeds were negatively impacted by the “El Niño” phenomena of 1997–1998. Increased temperature, grazers, and epiphytism caused high rates of mortality on the farms. Since then, no more attempts at G. lemaneiformis culture have been reported (Hayashi et al. 2013).
Important populations of L. nigrescens, L. trabeculata and M. pyrifera can be found along the southern coast of Peru. Their importance relies on the ecosystem services they provide, such as elevated secondary production, coastal erosion defence, direct applications, and biodiversity repositories, among others (Carbajal and Gamarra 2018; Smale et al. 2013). Drift brown seaweeds, but also plants harvested directly from natural beds, are collected and air-dried for at least two days in order to get final moisture content of 15–20%. A higher content usually reduces their price. Dried seaweeds are then sold to processing companies that export them (Noriega 2011). Rebours et al. (2014) reported four companies that purchase seaweeds from the local communities: two Peruvian companies, one Chilean, and one Chinese-owned company. During years where “El Niño” does not affect brown seaweed populations, about 3000 tonnes of dry L. nigrescens and dry L. trabeculata is exported to Asia. Due to overexploitation, the prices of dried seaweeds have been increased from US$60 to US$400 t−1 between 2006 and 2008 (Rebours et al. 2014). In 2008, Peru banned the direct harvesting of these species to protect their natural populations along the Peruvian coast (RM N° 839-2008-PRODUCE). The next year, the government allowed the collection of only beach-cast material (RM N° 264–2009–PRODUCE; IMARPE 2020). Nevertheless, illegal harvesting is still reported and a scientific-based management plan for these resources is urgently needed (Noriega 2011). Apart from the cultivation of M. pyrifera in San Nicolas Bay by the company PSW (Murias 2010), no other attempts at brown seaweed aquaculture have been reported (Alemañ et al. 2019).
Green seaweeds have not been subjected to major cultivation studies in Peru. This might be explained by the scarce use they have in the country and their high natural biomass, as explained in Section 2. Efforts to implement new cultivation technologies for Ulva spp. and Codium spp. have been reported (PSW 2020), but the results are not currently available.
4 Conclusions
Studies on Peruvian seaweed diversity started more than 200 years ago. With some exceptions, these works have not been updated and sustained over the years and have lagged behind the neighbouring countries like Chile and Brazil. However, during the last eight years, there has been a “re-emergence” of seaweed taxonomic research assisted by the development of molecular approaches. Despite the increase in algal publications during this period, little is still known about the taxonomy and diversity of Chlorophyta and Phaeophyceae, as these groups have not been extensively studied compared to red algal families such as Halymeniaceae, Phyllophoracea and Rhodomelaceae. Also, the seaweed diversity of subtidal habitats remains largely unexplored. Molecular systematic, genomic and transcriptomic studies with Peruvian seaweeds are currently available, mostly for Rhodophyta. These works will not only help to uncover the real seaweed diversity of Peru and understand basic aspects of these organisms, but also provide essential information for their culture, management, application and preservation (Huete-Pérez and Quezada 2013). While basic research on seaweeds (e.g., growth and developmental studies) must be encouraged, the information must also be linked with culture studies and new applications for seaweed biomass (Charrier et al. 2017). Peru needs to stop relying only on natural beds, as this can lead to overexploitation of the seaweed resources (which is already known for some species) with profound consequences for Peruvian fisheries and the Peruvian seaweed industry. Although the government has established some regulations for brown seaweed collection, these have been ineffective and a science-based management system is needed for the sustainable exploitation of marine macroalgae, involving scientists, policy makers and coastal communities. Seaweed aquaculture can become an alternative source of income for coastal communities, and it can provide several ecosystem services. Peru is starting to prioritize cultivation projects on its coast, bringing together universities, companies and fishermen, in an effort to diversify its production. These projects (and future ones) must consider “phyconomic” issues (Hurtado et al. 2019) in order to assure short and long-term success. Diversification of seaweed species for culture and utilization has just started to be explored with studies on non-commercial species such as C. filiformis, U. lactuca and Rhodymenia howeana. Also, species from the PP, which have received little attention, represent a big opportunity for Peruvian basic and applied phycology.
Finally, Peruvian scientific and non-scientific communities need to work together for a better understanding and adequate management of seaweed resources. The challenge now is to sustain this “re-emergence” of phycological studies and improve its accessibility to the international scientific community as well as to the whole of society. Through this manner, the seaweed resources of Peru can be preserved for the benefit of the country and the world.
About the authors

José Avila-Peltroche is a fifth year PhD student in Chosun University, South Korea. He obtained his BSc in Biological Sciences at Ricardo Palma University (URP), Peru. His research interests include taxonomy, phylogeny, culture and cryopreservation of macroalgae, focussing on protoplast isolation and culture of Phaeophyceae.
Jaraj Padilla-Vallejos, obtained her BSc in Biology at Ricardo Palma University (URP), Peru. Her research interests are marine invasive species and seaweed ecology, focussing on the invasive green alga Caulerpa filiformis. She is currently a research member at “Vera Alleman Haeghebaert” Natural History Museum, Lima, Peru.
Acknowledgements
The authors would like to express here their sincere thanks to Alan T. Critchley who gave us the opportunity to write this review. We kindly appreciate Paul Martín Baltazar Guerrero (Universidad Científica del Sur) for comments, photos and unpublished data on seaweed aquaculture in Peru. We would also like to thank María Luisa Eche Villa (Certificaciones del Perú), Lenin Chumbe Nolasco (Museo de Historia Natural UNMSM), Antony Otinga Oteng’o (Chosun University) and Karthikeyan Vijayakumar (Chosun University) for their suggestions and English review. Special thanks to Elaine Alison Enriquez Marin (Red de Ficología, Universidad Nacional Federico Villareal) for providing us with key literature about Peruvian seaweed taxonomy. The authors thank the reviewers for their contribution to our work. This review is dedicated to Dr. Cesar Acleto, for his significant contributions to the marine flora of Peru, and to all those who constantly work on seaweed research in Peru.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Acleto, C. (1972). Structure and reproduction of Schimmelmannia dawsonii sp. nov. (Rhodophyceae, Cryptonemiales). Phycologia 11: 5–9. https://doi.org/10.2216/i0031-8884-11-1-5.1.Search in Google Scholar
Acleto, C. (1973). Las algas marinas del Perú. Bol. Soc. Per. Bot. 6: 1–164.Search in Google Scholar
Acleto, C. (1980). Notas sobre las algas marinas del Perú I. Nuevos registros. Publ. Mus. Hist. Nat. ‘Javier Prado’ Ser. B Bot. 30: 1–33.Search in Google Scholar
Acleto, C. (1981). Estado de nuestro conocimiento acerca de la flora marina del Perú. Phycol. Latinoam. 1: 26–30.Search in Google Scholar
Acleto, C. (1984). Las especies peruanas de Porphyra (Rhodophyta, Bangiales), ll. Porphyra crispata Kjellman, un nuevo registro para nuestra flora. Publ. Mus. Hist. Nat. ‘Javier Prado’ Ser. B Bot. 31: 1–8.Search in Google Scholar
Acleto, C. (1986). Algas marinas del Perú de importancia económica. Serie de Div. Museo de Historia Natural “Javier Prado”. Dep. de Botánica 5: 1–107.Search in Google Scholar
Acleto, C. (1988). Aspectos fitogeográficos y taxonómicos de las algas marinas del Perú. Gayana. Bot. 45: 143–146.Search in Google Scholar
Acleto, C. and Endo, J. (1977). Las especies peruanas de Porphyra (Rhodophyta, Bangiales), l. Taxonomía y distribución geográfica. Publ. Mus. Hist. Nat. ‘Javier Prado’ Ser. B Bot. 29: 1–19.Search in Google Scholar
Acosta, J. (1977a). Las Algas Marinas de la Provincia de Pisco, Departamento de Ica, Perú. Publ. Mus. Hist. Nat. ‘Javier Prado’ Ser. B Bot. 28: 1–34.Search in Google Scholar
Acosta, J. (1977b). Nombres vulgares y usos de las algas en el Perú. Serie de Div. Museo de Historia Natural “Javier Prado”. Dep. de Botánica 7: 1–9.Search in Google Scholar
Agardh, C.A. (1822). Algae. In: Kunth, C.S. (Ed.). Synopsis plantarum, quas, in itinere ad plagam aequinoctialem orbis novi, collegerunt Al. de Humboldt et Am. Bonpland. Volume Primus, Paris, pp. 1–6.Search in Google Scholar
Agardh, J.G. (1841). In historiam algarum symbolae. Linnaea 15: 1–50, 443–457.Search in Google Scholar
Agardh, J.G. (1851–1863). Species genera et ordines algarum, vol. 2. Algas florideas compiectens, Lund, p. 1291 (1851 = part. 1, pp. i-xii + 1-351; 1852 = part. 2, pp. 337 (bis) – 351 (bis), 352–720; part. 3, pp. 701 (bis) – 720 (bis), 721–786; 1863 = part. 3, pp. 787–1291 (1139–1158 omitted).Search in Google Scholar
Aguilar-Velasquez, B.Y. (2015). Elucidación estructural del polisacárido del alga roja Porphyra columbina y determinación in vivo de la capacidad antioxidante e hipolipemiante, Dissertation. Universidad Nacional Mayor de San Marcos. p. 137.Search in Google Scholar
Alvítez, E. and Rodríguez, E. (2005). Diversidad, taxonomía y ecología de las Phaeophyceae del litoral peruano. Rebiol 25: 15–30.Search in Google Scholar
Alemañ, A.E., Robledo, D., and Hayashi, L. (2019). Development of seaweed cultivation in Latin America: current trends and future prospects. Phycologia 58: 462–471. https://doi.org/10.1080/00318884.2019.1640996.Search in Google Scholar
Arakaki, N., Carbajal, P., Gamarra, A., Gil-Kodaka, P., and Ramírez, M.E. (2018a). Macroalgas de Pucusana. Guía de campo. UNALM, Lima, Perú, p. 56.Search in Google Scholar
Arakaki, N., Gil-Kodaka, P., Carbajal, P., Gamarra, A., and Ramírez, M.E. (2018b). I- rhodophyta. In: Macroalgas de la Costa Central del Perú. UNALM, Lima, Perú, p. 126.Search in Google Scholar
Arakaki, N., Schmidt, W., Carbajal, P., and Frederiq, S. (2015). First occurrence of Gracilaria chilensis, and distribution of Gracilariopsis lemaneiformis (Gracilariaceae, Gracilariales) in Peru on the basis of rbcL sequence analysis. Phytotaxa 208:175–181. https://doi.org/10.11646/phytotaxa.208.2.7.Search in Google Scholar
Arbaiza, S., Castañeda, M., Gerónimo, G., Munayco, P., Reynaga, R., and Advíncula, O. (2018). Efecto del fotoperiodo y nutriente foliar comercial en el crecimiento (biomasa) de cochayuyo Porphyra spp. bajo condiciones semicontroladas de cultivo [abstract]. In: Proceedings of the Annual Meeting of the Latin American & Caribbean Aquaculture Societies; 2018, p. 25, Available at: https://wasblobstorage.blob.core.windows.net/meeting-abstracts/LacQua18AbstractBook.pdf (Accessed 14 April 2020).Search in Google Scholar
Arbaiza, S., Gil-Kodaka, P., Arakaki, N., and Alveal, K. (2019). Primeros estadios de cultivo a partir de carpósporas de Chondracanthus chamissoi de tres localidades de la costa peruana. Rev. Biol. Mar. Oceanogr. 54: 198–207. https://doi.org/10.22370/rbmo.2019.54.2.1901.Search in Google Scholar
Arntz, W.E., Gallardo, V.A., Gutierrez, D., Isla, E., Levin, L.A., Mendo, J., Neira, C., Rowe, G.T., Tarazona, J., and Wolff, M. (2006). El Niño and similar perturbation effects on the benthos of the Humboldt, California, and Benguela current upwelling ecosystems. Adv. Geosci. 6: 243–265.10.5194/adgeo-6-243-2006Search in Google Scholar
Bakun, A. and Weeks, S.J. (2008. The marine ecosystem off Peru: What are the secrets of its fishery productivity and what might its future hold? Prog. Oceanogr. 79: 290–299, https://doi.org/10.1016/j.pocean.2008.10.027.Search in Google Scholar
Bory de Saint Vincent, J.B. (1826-1828). Cryptogamie. In: Duperrey, L.I. (Ed.). Voyage autour du monde sur “La Coquille”, pendant 1822, 1823, 1824 et 1825. Botanique, Paris, p. 301.Search in Google Scholar
Bruland, K.W., Ruea, E.L., Smith, G.J., and DiTullio, G.R. (2005). Iron, macronutrients and diatom blooms in the Peru upwelling regime: brown and blue waters of Peru. Mar. Chem. 93: 81–103, https://doi.org/10.1016/j.marchem.2004.06.011.Search in Google Scholar
Bustamante, D.E., Calderon, M.S., and Hughey, J.R. (2019a). Conspecificity of the Peruvian Corallina ferreyrae with C. caespitosa (Corallinaceae, Rhodophyta) inferred from genomic analysis of the type specimen. Mitochondrial DNA B 4: 1285–1286, https://doi.org/10.1080/23802359.2019.1591203.Search in Google Scholar
Bustamante, D.E., Won, B.Y., and Cho, T.O. (2012a). Neosiphonia ramirezii sp. nov. (Rhodomelaceae, Rhodophyta) from Peru. ALGAE Int. J. Algal Res. 27: 79–82. https://doi.org/10.4490/algae.2013.28.1.073.Search in Google Scholar
Bustamante, D.E., Won, B.Y., and Cho, T.O. (2016). The conspecificity of Pterosiphonia spinifera and P. arenosa (Rhodomelaceae, Ceramiales) inferred from morphological and molecular analyses. ALGAE Int. J. Algal Res. 31: 105–115, https://doi.org/10.4490/algae.2016.31.5.13.Search in Google Scholar
Bustamante, D.E., Won, B.Y., Lindstrom, S.C., and Cho, T.O. (2019b). The new genus Symphyocladiella gen. nov. (Ceramiales, Rhodophyta) based on S. bartlingiana comb. nov. from the Pacific Ocean. Phycologia 58: 9–17, https://doi.org/10.1080/00318884.2018.1517240.Search in Google Scholar
Bustamante, D.E., Won, B.Y., Ramírez, M.E., and Cho, T.O. (2012b). Neosiphonia peruviensis sp. nov. (Rhodomelacea, Rhodophyta) from the Pacific coast of South America. Bot. Mar. 55: 359–366, https://doi.org/10.1515/bot-2012-0146.Search in Google Scholar
Calderon, M.S. and Boo, S.M. (2016a). A new genus Phyllophorella gen. nov. (Phyllophoraceae, Rhodophyta) from central Peru, including Phyllophorella peruviana comb. nov. Phyllophorella humboldtiana sp. nov., and Phyllophorella limaensis sp. nov. Bot. Mar. 59: 339–352, https://doi.org/10.1515/bot-2016-0070.Search in Google Scholar
Calderon, M.S. and Boo, S.M. (2016b). Phylogeny of Phyllophoraceae (Rhodophyta, Gigartinales) reveals Asterfilopsis gen. nov. from the Southern Hemisphere. Phycologia 55: 543–554, https://doi.org/10.2216/16-9.1.Search in Google Scholar
Calderon, M.S. and Boo, S.M. (2017). The Phyllophoraceae (Gigartinales, Rhodophyta) from Peru with descriptions of Acletoa tarazonae gen. & sp. nov. and Gymnogongrus caespitosus sp. nov. Phycologia 56: 686–696, https://doi.org/10.2216/16-126.1.Search in Google Scholar
Calderon, M.S., Boo, G.H. and Boo, S.M. (2014a). Morphology and phylogeny of Ramirezia osornoensis gen. & sp. nov. and Phyllymenia acletoi sp. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 53(1): 23–36, https://doi.org/10.2216/13-158.1.Search in Google Scholar
Calderon, M.S., Boo, G.H. and Boo, S.M. (2014b). Neorubra decipiens gen. & comb. nov. and Phyllymenia lancifolia comb. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 53: 409–422, https://doi.org/10.2216/14-027.1.Search in Google Scholar
Carbajal, P., Arakaki, N., Gil-Kodaka, P., Gamarra, A., and Ramírez, M.E. (2018). II- chlorophyta & phaeophyceae. In: Macroalgas de la Costa Central del Perú. UNALM, Lima, Perú, p. 126.Search in Google Scholar
Carbajal, P. and Gamarra, A. (2018). Guía para la recolección y reconocimiento de macroalgas pardas comerciales del Perú. Inf. Inst. Mar. Perú 45: 169–181.Search in Google Scholar
Carbajal, P., Gamarra, A., Arakaki, N., Gil-Kodaka, P., and Ramírez, M.E. (2019). Guía para el reconocimiento en campo de las macroalgas del Callao. Instituto del Mar del Perú, Callao, Perú, p. 58.Search in Google Scholar
Castañeda, M., Arbaiza, S., Diaz, F., Castillo, Y., Baltazar, P., and Advíncula, O. (2018). Evaluación del fotoperiodo en el asentamiento de tetraesporas de Chondracanthus chamissoi sobre cuerdas de polipropileno en condiciones semi-controladas de laboratorio. An. Cient. 79: 459–465. http://dx.doi.org/10.21704/ac.v79i2.1256.10.21704/ac.v79i2.1256Search in Google Scholar
Castilla, J.C. and Neill, P.E. (2009). Marine bioinvasions in the Southeastern Pacific: Status, ecology, economic impacts, conservation and management. In: Rilov, G. and Crooks, J.A. (Eds.), Biological Invasions in Marine Ecosystems. Springer, Berlin, pp. 439–457.10.1007/978-3-540-79236-9_26Search in Google Scholar
Castro, A.J., Juárez, J.R., Suárez, S., Alcarraz, M., Ramos, N.J., Hinostroza, L., Ráez, E., Ponce, J.J., Santa María, O., Gutiérrez, P., et al. (2014). Efecto antioxidante y antifotoenvejecimiento de extractos de la macroalga del litoral peruano de Macrocystis integrifolia Bory y elaboración de una forma dermocosmética. Cienc. Invest. 17: 80–87.10.15381/ci.v17i2.13594Search in Google Scholar
Charrier, B., Abreu, M.H., Araujo, R., Bruhn, A., Coates, J.C., De Clerck, O., Katsaros, C., Robaina, R.R., and Wichard, T. (2017). Furthering knowledge on seaweed growth and development to facilitate sustainable aquaculture. New Phytol. 216: 967–975, https://doi.org/10.1111/nph.14728.Search in Google Scholar PubMed
Chatwin, A. (Ed.) 2007). Priorities for coastal and marine conservation in South America. The Nature Conservancy, Arlington, USA, p. 63.Search in Google Scholar
Cho, G.Y., Klochkova, N.G., Krupnova, T.N., and Boo, S.M. (2006). The reclassification of Lessonia laminarioides (Laminariales, Phaeophyceae): Pseudolessonia gen. nov. J. Phycol. 42: 1289–1299, https://doi.org/10.1111/j.1529-8817.2006.00280.x.Search in Google Scholar
Collins, F.S. (1915). Algae from the Chincha islands. Rhodora 17: 89–96.Search in Google Scholar
Cruz, C.A. (2019). Una dieta a base de harina de Ulva lactuca mejora el crecimiento de alevines de bauncos Girella laevifrons (Pisces: Kyphosidae). Sci. Agropecu. 10: 191–197. http://dx.doi.org/10.17268/sci.agropecu.2019.02.04.10.17268/sci.agropecu.2019.02.04Search in Google Scholar
Dawson, E.Y. (1941). A review of the genus Rhodymenia with descriptions of new species. Allan Hancock Pacific Exped. 3: 123–181.Search in Google Scholar
Dawson, E.Y., Acleto, O.C., and Foldvik, N. (1964). The seaweeds of Peru. Beih. Nova Hedwigia 13: 1–111.Search in Google Scholar
De Toni, G.B. (1900–1924). Silloge Algarum omnium hucusque cognitarum. Vol. IV, Florideae, p. 1525.Search in Google Scholar
Egg, K, Avia, S., Villalobos, L., Wong, Y., and Gonzales, H. (2015). Efecto in vitro del extracto etanólico de Caulerpa filiformis en parámetros seminales humanos. Rev. Ciencias 11: 7–19.10.31381/revista_ciencias.v11i0.564Search in Google Scholar
Fahrbach, E., Trillmich, F., and Arntz, W. (1991). The time sequence and magnitude of physical effects of El Niño in the Eastern Pacific. In: Trillmich, F. and Ono, K.A. (Eds.). Pinnipeds and El Niño. Springer, Berlin, pp. 8–21.10.1007/978-3-642-76398-4_2Search in Google Scholar
FAO. (2018). The global status of seaweed production, trade and utilization, vol. 124. Globefish Research Programme, Rome, Italy, p. 120.Search in Google Scholar
FAO. (2020). FishStat. Global aquaculture production 1950–2017. Available at: https://www.fao.org/fishery/statistics/global-aquaculture-production/query/en (Accessed 20 April 2020).Search in Google Scholar
Fernandez-Baca, J., Miethke, S., Reichle, S., Armijo, E., Ferdaña, E.Z., Sotomayor, L., and Chatwin, A. (2007). Coastal and marine conservation priorities in Peru. In: Chatwin, A. (Ed.). Priorities for coastal and marine conservation in South America. The Nature Conservancy, Arlington, USA, pp 44–47.Search in Google Scholar
Gil-Kodaka, P., Mendo, J., and Fernández, E. (2002). Diversidad de macroalgas del submareal en la Reserva Nacional de Paracas y notas sobre su uso potencial. In: Mendo, J. and Wolff, M. (Eds.). Memoria I Jornada Científica Bases ecológicas para el manejo de los recursos vivos de la Reserva Nacional de Paracas, pp. 154–163.Search in Google Scholar
Guillemin, M.L., Contreras-Porcia, L., Ramírez, M.E., Macaya, E.C., Contador, C.B., Woods, H., Wyatt, C., and Brodie, J. (2016). The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: molecular species delimitation reveals extensive diversity. Mol. Phylogenet. Evol. 94: 814-826, https://doi.org/10.1016/j.ympev.2015.09.027.Search in Google Scholar PubMed
Guiry, M.D. and Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway, Available at: http://www.AlgaeBase.org (Accessed 19 September 2019).Search in Google Scholar
Guiry, M.D. and Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway, Available at: http://www.AlgaeBase.org (Accessed 10 March 2020).Search in Google Scholar
Hayashi, L., Bulboa, C., Kradolfer, P., Soriano, G., and Robledo, D. (2013). Cultivation of red seaweeds: a Latin American perspective. J. Appl. Phycol. 26: 719–727, https://doi.org/10.1007/s10811-013-0143-z.Search in Google Scholar
Hernández-Gómez, P., Huamaní-Sayritupac, L.A., and Mirano-Casafranca, M.A. (2015). Efecto fotoprotector y calidad del gel cosmético a base del extracto del alga marina Caulerpa filiformis (Subr) Hering recolectada en la Provincia de Pisco-Ica, Dissertation, Universidad Nacional San Luis Gonzaga, p. 93.Search in Google Scholar
Hooker, Y., Prieto-Rios, E., and Solís-Marín, F.A. (2013). Echinoderms of Peru. In: Alvarado, J. and Solis-Marin, F. (Eds.). Echinoderm research and diversity in Latin America. Springer, Berlin, Heidelberg, pp. 277–297.10.1007/978-3-642-20051-9_8Search in Google Scholar
Howe, M.A. (1914). The marine algae of Peru. Mem. Torrey Bot. Club 15: 1–185, https://doi.org/10.5962/bhl.title.97549.Search in Google Scholar
Huete-Pérez, J.A. and Quezada, F. (2013). Genomic approaches in marine biodiversity and aquaculture. Biol. Res. 46: 353–361, https://doi.org/10.4067/s0716-97602013000400007.Search in Google Scholar
Humboldt, A. (1815–1825). Nova genera et species plantarum, Vol. 7. Paris. 714 pls.Search in Google Scholar
Humboldt, A. and Bonpland, A. (1814). Voyage aux regions équinoxiales du nouveau continent fait en 1799-1800-1801-1803-1804, Vol. 2. Paris. 140 pls.Search in Google Scholar
Hurtado, A.Q., Neish, I.C., and Critchley, A.T. (2019). Phyconomy: the extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia 58: 472–483, https://doi.org/10.1080/00318884.2019.1625632.Search in Google Scholar
Ibanez-Erquiaga, B., Pacheco, A.S., Rivadeneira, M.M., and Tejada, C.L. (2018). Biogeographical zonation of rocky intertidal communities along the coast of Peru (3.5–13.5°S Southeast Pacific). PloS One 13(11): e0208244, https://doi.org/10.1371/journal.pone.0208244.Search in Google Scholar PubMed PubMed Central
IMARPE. (2012). Estudios sobre macroalgas pardas en el sur del Perú. 2011–2015. IMARPE, Callao, Peru, p. 200, Available at: http://www.imarpe.pe/imarpe/archivos/macro_algas/estud_macroalg.pdf (Accessed 14 April 2020).Search in Google Scholar
Juhl-Noodt, H. (1958). Beitrage zur kenntnis der peruanischen Meeresalgen 1, Vol. 14. Kieler Meeresforsch, Institut Meereskuste Universitet Kiel, pp. 167–174.Search in Google Scholar
Kämpf, J. and Chapman, P. (2016). The Peruvian-Chilean coastal upwelling system. In: Upwelling systems of the world. Springer, Switzerland, pp. 161–201.10.1007/978-3-319-42524-5_5Search in Google Scholar
Kiple, K.F. and Ornelas, K.C. (Eds.) (2000). The Cambridge world history of food. Cambridge University Press, Cambridge, p. 2153.10.1017/CHOL9780521402149Search in Google Scholar
Kützing, F.T. (1843). Phycologia Generalis. Leipzig, p. 458.Search in Google Scholar
Kützing, F.T. (1849). Species Algarum. Leipzig, p. 922.Search in Google Scholar
Kützing, F.T. (1851–1869). Tabulae phycologicae, Vol. 1–19. Nordhausen. 100 pls.Search in Google Scholar
Kylin, H. and Skottsberg, C. (1919). Zur Kenntnis der subantarktischen und antarktischen Meeresalgen. II. Rhodophyceen. In: Nordenskjöld, O. (Ed.). Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903, Vol. 4:2. Litographisches Institut des Generalstabs, Stockholm, pp. 1–88.Search in Google Scholar
Magallanes, C., Córdova, C., and Orozco, R. (2003). Actividad antibacteriana de extractos etanólicos de macroalgas marinas de la costa central del Perú. Rev. Peru. Biol. 10: 125–132.10.15381/rpb.v10i2.2494Search in Google Scholar
Mamani, J., Chávez, J., Apumayta, E., and Gil-Kodaka, P. (2020). Antioxidant activity and total phenolic content in Caulerpa filiformis (Chlorophyta) from Sechura Bay and Paracas Bay, Peru. In: I Congreso Internacional de Biotecnología e innovación (ICBi). Rev. Peru. Biol. Número especial 27: 61–66. http://dx.doi.org/10.15381/rpb.v27i1.17596.10.15381/rpb.v27i1.17596Search in Google Scholar
Márquez-Corigliano, D., Arakaki, N., Gil-Kodaka, P., and Tellier, F. (2019). Diversidad de especies de Porphyra y Pyropia (Bangiaceae, Rhodophyta) de Marcona (Ica, Perú) bajo la evidencia molecular. Arnaldoa 26: 623–642. http://doi.org/10.22497/arnaldoa.261.26207.Search in Google Scholar
Miloslavich, P., Klein, E., Díaz, J., Hernández, C., Bigatti, G., Campos, L., Artigas, F., Castillo, J., Penchaszadeh, P., Neill, P., et al. (2011). Marine biodiversity in the Atlantic and Pacific coasts of South America: knowledge and gaps. PLoS One 6: e14631, https://doi.org/10.1371/journal.pone.0014631.Search in Google Scholar PubMed PubMed Central
MINAM. (2014). Quinto informe nacional ante el convenio sobre la diversidad biológica: Perú (2010–2013). Viceministerio de Desarrollo Estratégico de los Recursos Naturales. Dirección General de Diversidad Biológica. Proyecto PNUD-GEF (Programas de las Naciones Unidas para el Desarrollo-Global Environment Facility), p. 198.Search in Google Scholar
MINAM. (2019). Sexto informe nacional sobre diversidad biológica: la biodiversidad en cifras. Ministerio del Ambiente, Lima, p. 51.Search in Google Scholar
Montagne, C. (1839). Cryptogamie. Voyage dans l’Amerique Meridionale par M. Alcide D’Orbigny. Botanique, Sertum Patagonicum et Flora Boliviensis. Vol. 7, p. 110.Search in Google Scholar
Montagne, C. (1846). Cryptogames cellulaires. Algues, lichens, hépatiques et mousses. In: Gaudichaud, C. (Ed.). Botanique. Voyage autour du monde exécuté sur la corvette La Bonite. Vol. 1, p. 355.Search in Google Scholar
Mouritsen, O.G., Rhatigan, P., and Pérez-Lloréns, J.L. (2018). World cuisine of seaweeds: science meets gastronomy. Int. J. Gastron. Food Sci. 14: 55–65, https://doi.org/10.1016/j.ijgfs.2018.09.002.Search in Google Scholar
Murias, A. (2010). Algas pardas, un aglutinante orgánico con gran potencial. Fish Information and Services, Available at: https://www.fis.com/fis/techno/newtechno.asp?l=s&id=36301&ndb=1 (Accessed 14 April 2020).Search in Google Scholar
Nguyen, T.V., Le, N.H., Lin, S.M., Steen, F., and De Clerck, O. (2013). Checklist of the marine macroalgae of Vietnam. Bot. Mar. 56: 207–227, https://doi.org/10.1515/bot-2013-0010.Search in Google Scholar
Noriega, C. (2011). Algas comestibles del Perú. Pan del Futuro. Universidad de San Martín de Porres, Lima, p. 175.Search in Google Scholar
Patterson, T.C. and Moseley, M.E. (1968). Late preceramic and early ceramic cultures of the central coast of Peru. Ñawpa Pach 6: 115–133, https://doi.org/10.1179/naw.1968.6.1.006.Search in Google Scholar
Peters, A.F., Marie, D., Scornet, D., Kloareg, B., and Cock, J.M. (2004). Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J. Phycol. 40: 1079–1088, https://doi.org/10.1111/j.1529-8817.2004.04058.x.Search in Google Scholar
Phang, S.M., Yeong, H.Y., and Lim, P.E. (2019). The seaweed resources of Malaysia. Bot. Mar. 62: 265–273. https://doi.org/10.1515/bot-2018-0067.Search in Google Scholar
Piccone, A. (1886). Alghe del viaggio di circumnavegazione della Vettor Pisani. R. Istituto Sordo‐muti, Genova, p. 97.10.5962/bhl.title.64275Search in Google Scholar
PRODUCE. (2015). Anuario Estadístico Pesquero y Acuícola 2015. Ministerio de la Producción, Lima, p. 193.Search in Google Scholar
PRODUCE. (2017). Anuario Estadístico Pesquero y Acuícola 2017. Ministerio de la Producción, Lima, p. 200.Search in Google Scholar
PSW (2020), Available at: http://www.pswsa.com/ (Accessed 27 March 2020).Search in Google Scholar
Ramírez, P., De la Cruz, J., and Torres, D. (2015). Biodiversidad en la isla Lobos de Tierra, región Lambayeque, Setiembre 2015. Inf. Inst. Mar. Perú 46: 341–359.Search in Google Scholar
Ramírez, M.E. and Santelices, B. (1991). Catálogo de las algas marinas bentónicas de la costa temperada del Pacífico de Sudamérica. Monogr. Biol. 5: 1–437.Search in Google Scholar
Ramsar. (2015). Informe Nacional sobre la aplicación de la Convención de Ramsar sobre los humedales. COP12 National Reports, Perú, p. 39.Search in Google Scholar
Rebours, C., Marinho-Soriano, E., Zertuche-González, J.A.M., Hayashi, L., Vásquez, J.A., Kradolfer, P., Soriano, G., Ugarte, R., Abreu, M.H., Bay-Larsen, I., et al. (2014). Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 26: 1939–1951, https://doi.org/10.1007/s10811-014-0304-8.Search in Google Scholar PubMed PubMed Central
Reyes, U.F., Navarro, A.B., and Llanos, B.P. (2009). Algas marinas del litoral peruano como biosorbentes potenciales de ion Cu(II) en tratamiento de efluentes industriales. Rev. Soc. Quím. Perú 75: 353–361.Search in Google Scholar
Rivera, J., Tapia, N., Caja, V., Yarango, A., Reyes, I., Borja, A., and Figueroa, A. (2004). Biosorción del cobre (II) por el alga marina pretratada Lessonia trabeculata. Rev. Per. Quím. Ing. Quím. 7:35–39.Search in Google Scholar
Rodríguez, J. and Castro, A. (2018). Evaluación del contenido de polifenoles y actividad antioxidante del extracto hidroalcohólico de Eisenia cokeri M.A. Howe. Cienc. Invest. 21: 11–17.10.15381/ci.v21i1.15737Search in Google Scholar
Rodríguez-Rodríguez, E.F., Fernández-Honores, M.A., Alvítez-Izquierdo, E., Pollack-Velásquez, L.E., Luján-Bulnes, L.A., Geldres-Cruz, C.W., and Paredes-Pizarro, Y. (2018). Algas marinas del litoral de la región La Libertad, Perú. Sci. Agropecu. 9: 71–81. http://dx.doi.org/10.17268/sci.agropecu.2018.01.08.10.17268/sci.agropecu.2018.01.08Search in Google Scholar
Rojas-Vega, N.C., Valdivieso-Izquierdo, R., and Arnao-Salas, I. (2018). Composición nutricional de la alga roja Rhodymenia howeana de la bahía de Ancón, Perú. Rev. Soc. Quím. Perú 84: 488–498.10.37761/rsqp.v84i4.68Search in Google Scholar
Roque-Sánchez, M., Oscco-Oscco, R., and Ríos-Suarez, J. (2017). Aportación al conocimiento de las macroalgas del litoral costero de la Reserva Nacional San Fernando (Nasca, Perú). AquaTIC 47: 1–9.Search in Google Scholar
Rosado-Alejos, M.A.S. (2017). Elaboración de galletas proteinizadas a base de harina de alga cochayuyo (Porphyra columbina), Dissertation, Universidad Nacional Jorge Basadre Grohmann, p. 79.Search in Google Scholar
Rostworowski de Diez Canseco, M. (1981). Recursos naturales renovables y pesca, siglos XVI y XVII. Instituto de Estudios Peruanos, Lima, p. 180.Search in Google Scholar
Salas, N., Córdova-Castañeda, C., Lengua-Calle, R., and Anaya-Meléndez, F. (2009). Cuantificación de κ y λ-carragenanos a partir de la macroalga Chondracanthus chamissoi. Rev. Soc. Quím. Perú 75: 414–421.Search in Google Scholar
Salavarría, E., Paul, S., Gil-Kodaka, P., and Villena, G.K. (2018). First global transcriptome analysis of brown algae Macrocystis integrifolia (Phaeophyceae) under marine intertidal conditions. 3 Biotech. 8: 185, https://doi.org/10.1007/s13205-018-1204-4.Search in Google Scholar PubMed PubMed Central
Silva, P.C. (2019). Index Nominum Algarum, University Herbarium. University of California, Berkeley, Available at: http://ucjeps.berkeley.edu/INA.html (Accessed 19 September 2019).Search in Google Scholar
Smale, D.A., Burrows, M.T., Moore, P., O’Conner, N., and Hawkings, S.J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3: 4016–4038, https://doi.org/10.1002/ece3.774.Search in Google Scholar PubMed PubMed Central
Spalding, M., Fox, H., Allen, G., Davidson, N., Ferdaña, Z., Finlayson, M., Halpern, B., Jorge, M., Lombana, A., Lourie, S., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57: 573–583, https://doi.org/10.1641/b570707.Search in Google Scholar
Suhr, J.N. von (1840). Beiträge zur Algenkunde. Nr. 3. Flora 22: 65–75.Search in Google Scholar
Sumarriva-Bustinza, L., Castro-Luna, A., Sotelo-Méndez, A., and Chávez-Sumarriva, N. (2019). Evaluación biológica de proteínas, vitaminas, minerales y aminoácidos del alga comestible Ulva lactuca “lechuga de mar” del litoral peruano. Rev. Soc. Quím. Perú 85: 31–42.10.37761/rsqp.v85i1.234Search in Google Scholar
Tarazona, J., Gutierrez, D., Paredes, C., and Indacochea, A. (2003). Overview and challenges of marine biodiversity research in Peru. Gayana 67: 206–231, https://doi.org/10.4067/s0717-65382003000200009.Search in Google Scholar
Taylor, W.R. (1947). Algae collected by the “Hassler”, “Albatross” and Schmitt expeditions. III. Marine algae from Peru and Chile. Pap. Mich. Acad. Sci. Arts Lett. 31: 57–90.Search in Google Scholar
Toribio-Chahua, H. (2017). Uso del cochayuyo en la panificación. Gaceta Molinera. Universidad Nacional Agraria La Molina, Available at: http://www.lamolina.edu.pe/Gaceta/edicion2017/notas/nota105.htm (Accessed 14 April 2020).Search in Google Scholar
Valiente, O. and Mogollón, E. (2013). Contenido de ácido algínico, manitol y laminarano en algas pardas de importancia económica. Bol. invest. Inst. tecnol. Prod. Perú 11: 91–98.Search in Google Scholar
Vásquez-Castro, J.A. (2009). Estudio de investigación de poblaciones y de las condiciones de viabilidad ecológica de las actividades extractivas de algas pardas e invertebrados en la zona costera sur, en apoyo a la investigación y desarrollo del Instituto del Mar del Perú (IMARPE). Informe Final. Proyecto UE – Perú/ PENX. Ala 2004/016 -913. Código Ref. Mincetur POG 3.3.2.1. Sector Pesca y Acuicultura, Lima, p. 90.Search in Google Scholar
Wosnitza, T.M.A. and Barrantes, J.G. (2005). Utilization of seaweed Ulva sp. in Paracas Bay (Peru): experimenting with compost. J. Appl. Phycol. 18: 27–31, https://doi.org/10.1007/s10811-005-9010-x.Search in Google Scholar
Yacovleff, E. and Herrera, F.L. (1934). El mundo vegetal de los antiguos peruanos. Rev. Museo Nac. Museo Nac. 3: 241–322.Search in Google Scholar
Yacovleff, E. and Muelle, J.C. (1934). Un fardo funerario de Paracas. Rev. Museo Nac. Museo Nac. 3: 63–153.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/bot-2020-0026).
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- Seaweed resources of the world: a 2020 vision. Part 4
- Applications
- Seaweed utilisation in New Zealand
- Trends in seaweed resource use and aquaculture in South Africa and Namibia over the last 30 years
- Challenges for marine macroalgal biomass production in Indian coastal waters
- Technical challenges for offshore cultivation of kelp species: lessons learned and future directions
- Using macroalgae as biofuel: current opportunities and challenges
- Biogeography
- Seaweed resources of Tanzania: status, potential species, challenges and development potentials
- The seaweed resources of Peru
- Seaweed resources of Korea
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- Seaweed resources of the world: a 2020 vision. Part 4
- Applications
- Seaweed utilisation in New Zealand
- Trends in seaweed resource use and aquaculture in South Africa and Namibia over the last 30 years
- Challenges for marine macroalgal biomass production in Indian coastal waters
- Technical challenges for offshore cultivation of kelp species: lessons learned and future directions
- Using macroalgae as biofuel: current opportunities and challenges
- Biogeography
- Seaweed resources of Tanzania: status, potential species, challenges and development potentials
- The seaweed resources of Peru
- Seaweed resources of Korea

