Abstract
Von Willebrand factor (VWF), a blood multimeric protein with a very high molecular weight, plays a crucial role in the primary haemostasis, the physiological process characterized by the adhesion of blood platelets to the injured vessel wall. Hydrodynamic forces are responsible for extensive conformational transitions in the VWF multimers that change their structure from a globular form to a stretched linear conformation. This feature makes this protein particularly prone to be investigated by mechanochemistry, the branch of the biophysical chemistry devoted to investigating the effects of shear forces on protein conformation. This review describes the structural elements of the VWF molecule involved in the biochemical response to shear forces. The stretched VWF conformation favors the interaction with the platelet GpIb and at the same time with ADAMTS-13, the zinc-protease that cleaves VWF in the A2 domain, limiting its prothrombotic capacity. The shear-induced conformational transitions favor also a process of self-aggregation, responsible for the formation of a spider-web like network, particularly efficient in the trapping process of flowing platelets. The investigation of the biophysical effects of shear forces on VWF conformation contributes to unraveling the molecular mechanisms of many types of thrombotic and haemorrhagic syndromes.
Introduction
Vascular damage is rapidly followed by adhesion and firm attachment of blood platelets at the site of endothelial injury. This process is mediated by von Willebrand factor (VWF), a multimeric protein of variable molecular weight ranging from about 0.5 to about 20 megadalton, which acts as a bridge between the subendothelial collagen and platelets, a process referred to as primary haemostasis [1, 2]. VWF multimers link, in fact, the platelet receptor GpIb/IX/V to collagen fibrils of the extracellular matrix [2, 3]. VWF immobilized on subendothelial collagen forms a reactive surface, which can capture platelets from flowing blood [4]. The latter in the circulatory tree isare subjected to forces generated by flow itself. Shear stress is a force that is mathematically expressed as a vector, having both a magnitude and direction. The magnitude of the shear stress vector is directly proportional to blood flow and fluid viscosity and indirectly proportional to the vessel radius. Consequently, blood vessels with high flow and small lumens are exposed to high shear stress, while vessels with low flow and large diameters are exposed to low shear stress. Hence, macromolecules in blood flow experience the effect of high shear in the microcirculation (arterioles and capillaries), where the values of forces are >20-30 dyn/cm2 (corresponding to 2-3 pascal), whereas the shear effect is minimal in the venous circulation. Notably, at shear stress> 20 dyn/cm2, VWF undergoes micro- and macro-conformational changes from a globular state to a stretched chain conformation, where especially the A2 domain undergoes relevant structural changes [5, 6, 7]. VWF derives from a huge gene (180 kb, 52 exons), and is synthesized as pre-pro-VWF, including a 22-residue signal peptide and a 741-residue propeptide. VWF undergoes extensive posttranslational processing, consisting of glycosylation, sulfation, and assembly in the endoplasmic reticulum, Golgi, and post-Golgi organelles. The ~250 kDa monomeric subunits form disulfide-linked multimers of 500 to 20,000 kDa. Each mature VWF monomer contains 2050 amino acids made up of conserved domains arranged in the order D1-D2-D’-D3-A1-A2-A3-D4-B1-B2-B3-C1-C6-CK [1]. In Figure 1 a cartoon shows the scheme of the various VWF domains with their main relative functions, which

Schematic representation of the VWF monomer. VWF is synthesized as a pre-pro-VWF that comprises 763 residue propeptide, and the 2050-residue mature subunit. After initial removal of the signal peptide, the pro-VWF monomers associate in the endoplasmic reticulum in “tail-to-tail” dimers by the formation of disulfide bonds engaging the carboxyl-terminal CK domains (formation of the “dimeric bouquet”). Thereafter, dimers further multimerize by forming “head-to-head” disulfide bonds between the amino-terminus (D3 domain) in the Golgi apparatus. Some binding sites that are important for the hemostatic function of VWF are also indicated together with the ADAMTS13 cleavage site in the A2 domain. Relevant regions in which mutations have been found to be associated with VWD types 1 and 2 are also shown. The symbol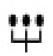 indicates the glycosylation sites.
indicates the glycosylation sites.
are variably inhibited by inherited mutations present in the different subtypes of von Willebrand disease. Binding of the platelet GpIbα receptor to the A1 domain of immobilized VWF results in an initial adhesion, characterized by a continuous surface translocation of the platelets [8, 9]. This process ultimately leads to a stable platelet adhesion through interaction with the platelet collagen receptors GpVI and α2β1 integrin (GpIa/IIa) [10, 11, 12], activation of the platelet GpIIb/IIIa receptor complex and, finally, to platelet aggregation. During the process of primary haemostasis, the interaction between VWF and GpIbα does not occur under static conditions but it needs a pre-activation of soluble VWF. This process consists of a conformational change of VWF molecules, which expose within the A1 domain the binding site for GpIbα. This mechanism is triggered either by mechanical forces, such as a high shear rate (>5000 s-1), found in the microcirculation or by chemical potentials generated by the interaction with biochemical effectors [5, 6, 13, 14, 15]. However, even natural mutations causing type 2B von Willebrand disease (VWD 2B), such as the Arg1306Trp or Arg1341Trp or Arg1306Trp [16, 17] in the A1 domain, are also able to stabilize a conformational state favoring the interaction with the platelet receptor even under very low shear conditions [18]. The stretched conformation of VWF not only permits the interaction with the platelet GpIb receptor, but also the proteolytic attack by the zincprotease ADAMTS-13 (A Disintegrin And Metalloproteinase with a Thrombospondin type 1 motif, member 13) [7, 19, 20, 21, 22], which, cleaving the Tyr1605-Met1606 in the A2 domain of VWF, fragments the protein and limits the prohaemostatic and prothrombotic activity of the ultra-large VWF multimers [7].
Besides conferring the ability to VWF multimer to interact with the platelet receptor and at the same time to be fragmented by ADAMTS-13, high shear stress can favor a VWF self-association, a process by which multimeric VWF binds to or aggregates with additional VWF multimers. This process is responsible for the formation of VWF fibers, that are organized as spiderweb like structures [5, 23]. This network constitutes an ideal anchor to recruit flowing platelets in the circulation during the process of primary haemostasis. All these VWF properties stem from the unique capacity of this multimeric protein to be very sensitive to external hydrodynamic forces [5]. Many biological processes are controlled and variably modulated by external physical forces, such as cell adhesion, cytoskeleton organization, cell division, bacteria invasion, and the activity of ion channels [24, 25, 26, 27, 28, 29, 30].
The high sensitivity of VWF to hydrodynamic forces raises the question concerning the molecular mechanisms by which VWF multimers undergo major conformational changes responsible for the acquisition of their full pro-haemostatic and prothrombotic activities. Besides the shear-dependent conformational changes of VWF multimers, also the binding of VWF A1 domain with the platelet receptor GpIb/IX/V and subendothelial collagen have been extensively studied from a biophysical point of view. To characterize the acting biophysical mechanisms, different types of bonds were identified, such as the catch-slip bond [31, 32, 33] or flex bond [34] (see below). Notably, the flex bond was demonstrated to occur in the binding of the VWF A1 domain to GpIb [34]. In elegant experiments using the ReaLiSM method (repeated measurements of the binding and unbinding of a receptor and ligand in a single-molecule), Kim et al. demonstrated two states of the receptor-ligand bond, that is, a flex-bond [34]. One state was observed at low hydrodynamic force (<5pN), whereas a second state begins to engage at about 10 pN with a ∼20-fold longer lifetime and greater force resistance. The second state can resist to flowing forces that could disaggregate the platelet plug. Nonetheless, although plenty of experimental evidence was provided by different Authors, no definitive conclusion has been reached on what type of bond stabilizes and/or controls VWF interaction with platelets or the vessel wall. Thus, before analyzing what is known about the molecular mechanisms adopted by VWF in responding to hydrodynamic forces, we will describe the main aspects of the scientific theory developed to analyze and unravel the mechanisms by which many natural proteins sense the action of external physical forces, that is the scientific branch referred to as mechanochemistry.
Principles of mechanochemistry
Between 1978 and 1988 different authors formulated and developed the theory of mechanochemistry, based on the assumption that in the interaction between two macromolecules an external force exponentially accelerates dissociation between interacting particles [35, 36]. The basic principle for the application of mechanochemistry in biochemical systems relies on the fact that macromolecules in living systems do not often interact each other freely in solution, according to classical kinetics and thermodynamics principles, but very often interact tethered to other subcellular structures and in a highly structured mode. The difference between the mechanochemical approach to manipulating chemical reactivity is that mechanical force, unlike temperature, pressure, or solution composition, is a vector, whose direction, which can be changed in the attachment points, can heavily modify the observed kinetic rate of the reaction. The study by Dembo et al. [36] proposed a classification of three types of molecular bonds according to how the off-rate depends on that force. The behavior described earlier by the Bell model [35, 36] was called slip bonds, as force shortens the bond lifetime. This model is very intuitive, as it predicts that the external force increases the rate of dissociation. This model was compatible with results obtained with atomic force microscopy, which were theoretically validated by Shapiro & Qian in 1997 in the framework of the Kramers’ rate of molecular dissociation theory [37, 38]. This model predicts that in any experimental measurement of the interaction between two molecules, the bond may break down at any time and space point over a range of forces, as the Brownian motion of the molecules must be considered [37, 38]. Force (f) exponentially increases the rate at which bonds are broken, according to the relation:
where k(f) is the rate at which the bond breaks at a certain force, f, and fC is the characteristic force for dissociation. The force (f) in eq. 1 can be related to an interaction distance (Dx = kBT / fC ), where kB is the Boltzmann constant and T is the absolute temperature. Based on this relation, the force reduces the magnitude of the energy barrier by the amount f ·Dx.
The second type of bond was defined catch bond: it is characterized by a counter-intuitive behavior, as force prolongs bond lifetime. Catch bonds show different characteristics from the slip bonds, in any kind of experimental methodology employed. The occurrence of the apparently counterintuitive catch bonds was demonstrated in several biochemical systems, comprising the interaction between VWF and the platelet receptor GpIb/IX/V [34]. However, the simple catch bond model can also change into a more complex system. If in atomic force experiments on single-molecule a constant force and velocity of stretching is used, catch bonds show simple mono-exponential dissociation kinetics. A typical example is given by the interaction between P-selectin and its receptor PSLG-1 [39]. However, in the case of catch bonds, another scenario could be depicted: the energy landscape of a catch-slip bond has two bound states and dissociates along two pathways according to a sliding-rebinding mechanism. Under the catch regime, the binding affinity of one molecule (ligand, “L”) for the interacting molecule (receptor, “R”) progressively grows with force owing to mechanical interface remodeling and formation of additional binding contacts, which results in bond strengthening. This trend continues until the size and conformation of the binding pocket of R becomes comparable with the molecular dimension and conformation of L at a critical force f=fc, at which point the slip regime sets in. Under the slip regime, the binding affinity of L gradually decreases with the increasing force f>fc due to the disruption of binding contacts, which weakens the bond. Different biophysical mechanisms have been proposed to drive this complex behavior: 1) single or dual bound states with two dissociation pathways [40, 41, 42], allostery [43, 44, 45, 46], combined entropic controlled dissociation and energetic-controlled break [47], and global deformation of the molecule [48, 49]. The third class is represented by ideal bonds, which indicate the case in which bond lifetime is independent of an externally applied force. A theoretical plot of the dependence the lifetime of all the above bond types as a function of the applied external force is shown in Fig. 1. Based on these theoretical concepts, several experimental methods of dynamic force spectroscopy on single-molecule were developed and applied to investigate some biological systems concerning protein-protein interactions and cell-protein interactions [50, 51, 52]. These methods, as reported below, have been widely applied also to the study of the mechanical force-dependence of the VWF conformation in relation to its interaction with GpIb and ADAMTS-13.
The effect of shear forces on von Willebrand Factor
The ability of VWF to interact with platelets and sub-endothelial collagen contributes to the arrest of bleeding by sequestering platelets from vascular blood flow, thus mediating their adhesion to the exposed subendothelial matrix at sites of vascular damage. Under arterial flow, VWF drives this process [1, 2], as it can change its conformation as a function of shear stress, generating a conformer suitable to the above interactions. Once secreted from the Weibel-Palade bodies into the plasma milieu at physiological pH, VWF assumes a more globular and compact conformation. In most part of the circulatory tree, VWF remains in this conformational state, but can rapidly change its conformational state in the microcirculation flow. Although most of the interactions that VWF can make with many macromolecular ligands are possible only if VWF assumes a stretched conformation under high shear forces, the interaction with subendothelial collagen can take place even by globular VWF conformer, as collagen was demonstrated to be rather insensitive to hydrodynamic forces [53, 54, 55]. Collagen III is believed to be essential for the interaction with the A3 domain, whereas collagen VI is believed to be essential for the interaction with the A1 domain. Experiments in vitro showed that the interaction between the A3 domain and type VI collagen has only an ancillary role that can be useful in the case of natural mutations that impair the A1 interaction with type III collagen. The latter appears to be far more abundant in artery walls and skin than collagen type VI, which makes it the prevalent binding partner for VWF [53]. Recent studies showed that platelets would strongly adhere to high molecular weight VWF multimers (HMW-VWF), present in the circulation after their release from the endothelial storage granules, prior to their deposition onto collagen at a damaged vascular site [53]. As anticipated above, the interaction between the VWF A1 domain and the GpIb/IX/V platelet receptor can occur only in the microcirculation, where a shear rate>2000 sec-1 is generated. From the mechanochemistry standpoint, the interaction between VWF and collagen can be thus characterized by an “ideal bond”, thus independent of externally applied forces. This finding agrees with the possibility to measure the interaction between HMW-VWF with collagen in ELISA tests even under static conditions in the common diagnostic practice of von Willebrand disease (VWD) [56]. Once immobilized on subendothelial collagen, VWF forms a reactive surface capable of capturing platelets from flowing blood [57, 58]. Furthermore, when VWF multimers are bound to collagen, their rotational entropy, present when the protein is free in solution, is drastically reduced and the elongational force becomes predominant. This force acts on the multimers so that the latter form an elongated strain on the endothelial surface that exposes multiple binding sites for ligands and receptor. Of interest, this biophysical process is induced, besides hydrodynamic forces, also by specific interactions with pharmacological and biochemical agents, such as the antibiotic glycopeptide ristocetin, the snake venom botrocetin and polyphosphate [15, 59, 60, 61, 62]. Finally, even gain-of-function natural mutations causing type 2B von Willebrand diseases (2B VWD), such as the p.Arg1306Trp or p.Arg1341Trp, are able to stabilize a conformational state that is prone to the interaction with the platelet receptor GpIb even under very low shear conditions, thus causing platelet clumping in the systemic circulation [17, 63].
What is the structural basis of such conformational transition induced by mechanical forces under flow? Elegant biophysical and biochemical studies together with findings on natural VWD variants in the A1 and A2 VWF domains have largely, although not completely, clarified this issue. Conformational flexibility of VWF was previously limited to the context of polymer physics, whereby a shear stress level>30 dyn cm2 would cause the unfurling of VWF multimeric strings. This stretching phenomenon can expose the A1 domains that bind to platelet GpIb. However, the studies by Auton et al. demonstrated that under certain shear stress conditions the above macro-conformational transition is accompanied by a certain degree of molecular flexibility that allows the VWF A1 residues engaged in the binding to GpIb to fit into the interacting cleft of the receptor [64]. Natural mutations causing type 2M von Willebrand disease, such as p.G1324A and p.G1324S, generate in fact a more rigid sequence due to the substitution of the simple side chain of glycine (hydrogen atom) with a larger side chain (methyl group and hydroxyl group of alanine and serine, respectively) that strongly limits the conformational flexibility of that part of the molecule contained in the β-turn between β-strands 2 and 3 within the native state of VWF [64]. Substitution of a bulky side chain-containing amino acid sterically hinders, in fact, the ϕ-ψ conformational space of the peptide, making this region of the structure more rigid. Furthermore, the hydroxyl group of Ser1324 provides additional structural stability as it forms an additional hydrogen bond with histidine 1322 (see Fig. 2). Hence, not even external hydrodynamic forces are able to counteract the rigidity of this segment of the protein, which has become much more rigid due to the type 2M mutations. By contrast, in the wild type VWF hydrodynamic forces induce conformational transitions that, occurring mostly in the A2 domain, propagate thereafter through the A1 and other domains of the monomer, causing ultimately a stretching of the multimer.
![Figure 2 Different types of bonds as a function of applied external force, according to mechanochemistry principles. The figure illustrates the mains types of bond lifetime according to the mechanochemistry theory proposed by Dembo et al. [36]: the external forces enhance the bond lifetime (catch bond), reduce the bond lifetime (slip bond), did not change the bond’s lifetime (ideal bond) or have a mixed effect (catch and slip bond).](/document/doi/10.1515/bmc-2019-0022/asset/graphic/j_bmc-2019-0022_fig_002.jpg)
Different types of bonds as a function of applied external force, according to mechanochemistry principles. The figure illustrates the mains types of bond lifetime according to the mechanochemistry theory proposed by Dembo et al. [36]: the external forces enhance the bond lifetime (catch bond), reduce the bond lifetime (slip bond), did not change the bond’s lifetime (ideal bond) or have a mixed effect (catch and slip bond).
The effects of hydrodynamic forces on VWF: the A2 domain as a mechanosensitive switch
Shear forces act along the entire contour of a macromolecule, thus the long chains of high molecular weight multimers of VWF are more easily stretched in shear flow. However, the VWF monomer contains a domain that acts as a mechanosensitive machine, that is the A2 domain. The X-ray crystal structure of the A2 domain [6] showed, in fact, that this part resembles the overall conformation of the so-called VWA domain, present in a protein superfamily comprising integrins on cell surfaces, complement components and DNA repair proteins [65]. However, the A2 domain shows a unique structure if compared to other VWA domains. In fact, the A2 domain has a sequence of α-helices and β-strands that alternate in sequence (see Fig. 3). The β-sheet core is highly hydrophobic and contains β-strands with the order β3-β2-β1-β4-β5-β6, with β3 antiparallel to the others. The domains belonging to the VWA superfamily show six amphipathic α-helices around the β-sheet. However, the A2 domain of VWF lacks an α4-helix (Fig. 3A) having a long loop replacing the α4-helix running from the C terminus of the β4-strand to the N terminus of the β5-strand. This loop has a higher B-factor compared to the α4-helix of A1 or A3 domain and shows an apolar interaction between the side chain of Leu1619 end the core β strand. Overall, this loop is thus characterized by high conformational flexibility and has contact with the core β4 strand that contains the Tyr1605-Met1606 peptide bond cleaved by ADAMTS-13. Another aspect renders the A2 domain unique from a structural standpoint: the presence of the vicinal disulfide Cys1669–Cys1670 peptide backbone, which forms an 8-membered ring (Fig. 3B) The ring is nonplanar and thus strained (mean ω angle »152°), confers rigidity to this protein segment, thus acting as a barrier to external hydrodynamic forces, and interacts with some apolar side chains of amino acids in the β4-strand, such as Leu1603 and Tyr1605 (Fig. 3B) Furthermore, the vicinal disulfide gets in contact with the α1-β2 loop residue Met1528, β2-strand residue Ile1535, and N- terminal residue Met1495 [6]. The structural peculiarity of the vicinal disulfide Cys1669-Cys1670 plays a central
![Figure 3 Structure of the type 2M p.Gly1324Ser VWF A1 domain (PDB accession code 5BV8) [64]. The dashed line shows the hydrogen bond between the hydroxyl group of Ser1324 and the imidazole of His1322. This hydrogen bond contributes to rigidify this part of the domain, rendering it much less sensitive to external shear forces. The figure is generated by the program Pymol (v. 2.3)](/document/doi/10.1515/bmc-2019-0022/asset/graphic/j_bmc-2019-0022_fig_003.jpg)
Structure of the type 2M p.Gly1324Ser VWF A1 domain (PDB accession code 5BV8) [64]. The dashed line shows the hydrogen bond between the hydroxyl group of Ser1324 and the imidazole of His1322. This hydrogen bond contributes to rigidify this part of the domain, rendering it much less sensitive to external shear forces. The figure is generated by the program Pymol (v. 2.3)
role in the mechanosensitive properties of the entire A2 domain, whose genetic mutations are largely responsible for many forms of Group II type 2A von Willebrand disease (p.R1597W, p.G1505E, p.I1628T, and p.E1638K) that shows a loss of high molecular weight multimer because of an increased proteolysis by ADAMTS-13 in the bloodstream. The supernormal proteolysis by ADAMTS-13 is linked to the tendency of these VWF mutants to be in a more “open”, unfolded conformations that render the Tyr1605-Met1605 in the hydrophobic core of the β4 strand susceptible to the proteolytic attack by the metalloprotease. The hydrodynamic shear forces, due to the structural aspects described above, act mostly on the A2 domain, as the unfolding will proceed from the C-terminal end rather than the N-terminal one of the A2 domain, because the C-terminal structural elements can be more easily removed one at a time from the end of the domain, while the β1 strand at the N-terminal is surrounded by counterforts constituted by β2 and β4 strands in the core of the domain (see Fig. 4). Based on this structural arrangement, any external hydrodynamic force acting on the C-terminus of the A2 domain will be simultaneously sensed by the 8-membered Cys1670-Cys1669 disulfide ring, which acts a high energy barrier to the external force. If this energetic barrier is overcome, the external force will be transmitted to the hydrophobic core containing the Tyr1605-Met1606, because of the apolar interactions with Leu1603 and Tyr1605. At this point, the poor packing of the β4 strand and the high conformational flexibility of the long loop replacing the α4 strand give rise to an easy unfolding of the hydrophobic core. The unfolding of the inner hydrophobic core of the A2 domain is accompanied by solvation of the region and the Tyr1605-Met1606 becomes available to the proteolysis by ADAMTS-13 (see Fig. 5).

A) Crystal structure of calcium-bound human VWF A2 domain (PDB accession code 3ZQK), shown in cartoon representation. The central β-sheets are encircled by α-helices. The blue arrow indicates the α4-less loop.. B) Structure of human A2 domain of VWF (PDB accession code 3GXB, shown in the same prospective of panel A). The figure shows the interactions between the vicinal Cys1669–Cys1670 peptide bond (yellow) interacting with some apolar side chains of amino acids in the β4-strand, such as Leu1603 (orange) and Tyr1605-Met1606 (magenta). The Ile1619 (orange) in the α4-less loop is also shown. C), D and E) panels show the cartoon representation of the A1 (PDB 1AUQ) , A2 (PDB 3GXB), and A3 (PDB 1AO3) domain of VWF, respectively. Note the similar global structures of the three domains containing a central region composed of β-sheets encircled by α-helices. The major structural difference resides in the presence of the absence of the α4-loop, substituted by a loop (red arrow). NH2- and COOH- represent the N- and C- terminal ends of the domains. The figure is generated by the program Pymol (v. 2.3)

Effect of shear forces on the A2 domain of VWF. The A2 domain is shown in cartoon representation (PDB accession code 3gxb). As explained in the text, shear forces act on this domain and particularly on the 8-membered Cys1670-Cys1669 disulfide ring. The external hydrodynamic force acts primarily on this Cys1670-Cys1669 disulfide ring that represents a high energy barrier to the mechanical force. If the external force overcomes this energetic barrier, it will be transmitted to the hydrophobic core containing the Tyr1605-Met1606, via the apolar interactions of the vicinal Cys1670-Cys1669 ring with Leu1603 and Tyr1605. By virtue of this mechanical energy transfer, both the poor packing of the β4 strand and the high conformational flexibility of the long a4-less loop destabilize the hydrophobic core, which is solvated so that the Tyr1605-Met1606 peptide bond becomes available to proteolytic attack by ADAMTS-13. The conformational transition eliminates also the shielding effect of the A2 domain on the A1 domain that can bind to the platelet GpIb.
We should consider that the above biochemical pathway, unraveled by the X-ray diffraction studies by Zhang et al [6], induces a shift of the reversible reaction F↔U, where F and U are the folded and unfolded conformers of the A2 domain, toward the unfolded form. It must be outlined that in the flowing blood VWF multimers are exposed to high hydrodynamic forces for a limited time and, due to the reversibility of the unfolding reaction, ADAMTS-13 could exert its proteolytic activity for a very short time [21, 22]. However, the nature has provided VWF with a biochemical mechanism that allows conversion from a cis- to a trans Trp1644-Pro1645 peptide bond [66]. This structural transition is capable to greatly delay the process of refolding of the A2 domain, thus allowing a more prolonged activity by ADAMTS-13. It also to be remarked that, when VWF multimers bound to collagen at sites of vascular injury, the rotational flow vanishes and the elongational flow is sufficiently high to unfold the molecule and allow at the same time to interact with platelet GpIb and be proteolyzed by ADAMTS-13 during vessel repair.
The effect of the mechanosensitive properties of the A2 domain on the other domains of VWF
Once the unfolding of the A2 domain takes place, conformational transitions involve also the vicinal A1 and A3 domains, so that the binding sites for other ligands become available for interactions. Hence, the conformational instability of the A2 domain under enough shear forces can affect also the interaction between the A1 and A2 domains. These two domains are adjacent to each other and connected by a linker of 30 amino acids. Previous studies showed that under low shear conditions the A1 domain, and particularly the GpIb binding site, is shielded by a specific inhibitory interaction with the vicinal A2 domain [54]. Thus, once enough shear force overcomes the resistance of the A2 domain to unfold, the conformational transition in the A2 domain gives rise to two different effects: 1) the exposition of the Tyr1605-Met1606 to solvent, rendering it available to the proteolytic attack by ADAMTS-13; 2) the elimination of the shielding effect of the A2 on the A1 domain with exposition of the GpIb binding site in the A1 domain. Furthermore, the shear force-dependent stretching of the whole VWF multimer creates many binding sites available for interactions with cell receptors and other protein ligands.
What is, from a biophysical standpoint, the relationship between the magnitude of the shear force and the molecular size of VWF that makes possible these functional effects described above? The study by Zhang et al. predicted that the A2 domain unfolds around 11 pN of tensile force [6]. However, when it circulates in the blood, VWF achieves this unfolding force in an A2 domain as a function of several factors, such as macromolecular conformation, location along the multimer contour, and the corresponding distribution of local internal tensile force. With experimental and computational methods, it was shown that that unfolding of A2 domains, and thus proteolysis of VWF multimers by ADAMTS-13, occurs preferentially near the center of untangled multimers. Experimentally we know that ultra-large VWF multimers undergo rapid scission upon secretion into the vasculature because of their length [67]. Zhang et al. [7] analyzed the distribution of internal tensile force in a computational analysis for a multimer composed of 25 dimers along the multimer contour, each dimer modeled as a dumbbell with two spheres of radius a = 13 nm separated by a rigid tether of length d = 94 nm, obtaining an interesting result. In fact, the tensile force F(j) to the inside of any sphere pair j in a chain with N dimers, is the sum of the force on all the outer dimer pairs. Hence, the total tensile force is given by [7, 68]
Where f(i) is the normal force between two spheres that are a certain distance (x) apart. From eq. 2, the normal force on a monomer in the center of a multimer is approximately proportional to N2 (i.e., when j=1), whereas the force on a monomer at the end of a multimer is proportional to N (i.e., when j = N).
This quadratic dependence markedly separates longer and shorter multimers, if referred to the capacity of external shear force to stretch the molecule. Hence, in a simulation, Zhang et al. calculated that 11 pN can unfold an upper length limit for VWF of 200 monomers, as observed in atomic force experiments [7]. The relation between the tensile force and the length of the VWF multimers expressed in eq. 2, affects the behavior of VWF multimers that undergo shear forces in the circulation. To perform their physiological pro-haemostatic functions, VWF multimers must undergo a correct transition of stretching, which, however, is not sufficient to fully activate adhesiveness to platelets through binding to GpIb. However, although not sufficient, the stretching is a necessary step, since weak interactions between distal monomers of the VWF multimer tethered on endothelium must be broken to re-direct tension and enable it to build up along the central part of the multimer chain. When tension is >20 pN, it mechanically induces a second transition of the A1 domain to a high-affinity conformation suitable to binding to GpIb. Thus, the length of VWF multimers is essential for the VWF’s haemostatic function: short multimers are not able to reach sufficient forces to induce the conformational transitions described above. Another advantage of having sufficiently long multimers resides in their ability to expose more interaction sites than shorter chains. The occurrence of these biophysical mechanisms is observed also in pathological conditions responsible for opposite effects. Inherited VWF diseases characterized by severe deficiency of high molecular weight VWF multimers (e.g. type 2A VWD) cause haemorrhages, whereas in the acquired disease referred to as thrombotic thrombocytopenic purpura, the accumulation of ultra-large VWF multimers, due to deficiency of ADAMTS-13, causes thrombotic complications in the microcirculation, where high shear stress is generated [69, 70, 71]. Moreover, natural mutations of the VWF A1 domain may differently affect the conformational transitions of the whole VWF multimer linked to external hydrodynamic forces, as noticed above for the type 2M von Willebrand mutations p.G1324A and p.G1324S. This effect is associated with resistance to unfolding induced by denaturing agents, reduced sensitivity to hydrodynamic force and decrease of affinity for GpIb under shear flow and resistance to limited proteolysis [64]. By virtue of these mutations, the physiological equilibrium between a more compact and a more disordered conformation of the A1 domain of VWF is shifted toward the former and this structural effect results in a decrease of both the interaction with GpIb and proteolysis by ADAMTS-13 [64]. By contrast, the natural mutation R1306W in the A1 domain, causing a type 2B von Willebrand disease, switches the equilibrium toward an unfolded conformation of VWF multimers, thus showing a higher sensitivity to shear stress [17], which facilitates exposure of GPIb binding sites. The mean hydrodynamic diameter of resting p.R1306W VWF multimers was indeed significantly greater than that of wild type VWF multimers (210±60 nm vs. 87±22 nm, respectively) [64]. At shear forces <14 dyn cm2, the p.R1306W multimers rapidly populates stretched conformers, acquiring the capacity to interact with GpIb which, instead, was induced for WT VWF by shear forces >30 dyn cm2 only.
Another relevant VWF activity linked to the acquisition of a stretched conformation under shear flow is the propensity to self-aggregate [5]. The stretched VWF conformation favors, in fact, a process of self-aggregation, responsible for the formation of a spider web-like network, particularly efficient in the mechanical trapping of flowing platelets [5]. Thus, the effect of shear stress on conformational changes in VWF shows a close correlation with the platelet adhesion and thrombus formation in the arterial microcirculation, where high shear stress is present. Recent studies have remarked that the sensitivity of the A2 domain to shear forces plays a major role in inducing a process of self-aggregation of VWF [23]. Thus, when the unfolding prevails under the effect of shear forces, VWF multimers act as promoters of thrombotic mechanisms but at the same time are prone to the proteolysis by ADAMTS-13 that limits the pro-hemostatic and prothrombotic function of VWF. When this proteolytic process cannot take place, due to the formation of auto-antibodies against ADAMTS-13 or for genetic deficiency of the metalloprotease, thrombotic microangiopathy occurs, characterized by an accumulation of ultra-large VWF multimers that have a high prothrombotic activity causing a multi-organ ischemic failure [70, 72]. Prothrombotic effects of VWF multimers may be also generated when, once the “open” conformation of the VWF multimer is generated by shear, the side chain of Met1606 may be more easily oxidized by chemical radicals produced by activated polymorphonuclear cells at sites of inflamed endothelium. Under these conditions, oxidizing species, such as ROS and HOCl, can modify the Met1606 residue to sulphonyl-methionine, rendering VWF resistant to ADAMT-13 proteolysis [73, 74]. Furthermore, molecular dynamics simulation showed that oxidation of Met1606 reduces the force necessary to initiate unfolding [75]. Altogether, these phenomena promote an accumulation of ultra-large VWF multimers that, being more sensitive to shear stress, favors platelet adhesion and aggregation, and contributes to thrombotic complications in several clinical settings [76, 77]. As anticipated in a previous section, the same transition from a folded to a stretched VWF conformer can be attained even under static conditions upon the interaction of some molecules with the A1 domain of VWF. These molecules, essentially represented by ristocetin, botrocetin and polyphosphate, bind to different sites in the A1 domain and trigger the conformational transition of VWF multimers [15, 59, 60]. Ristocetin, an old antibiotic with a glycopeptide structure, withdrawn from the market as it was associated with the development of thrombocytopenia, binds in the A1 domain to a specific region comprising Lys534 (VWF sequence: 1297), Arg 571 (VWF sequence: 1334), Lys572 (VWF sequence: 1335), Glu596 (VWF sequence: 1359), Glu613 (VWF sequence: 1376), Arg616 (VWF sequence: 1379), Glu626 (VWF sequence: 1389), and Lys642 (VWF sequence: 1405) [15]. Botrocetin, a venom from Bothrops jararaca snake, promotes platelet-VWF agglutination in several mammalian species, interacting with a cluster of two amino acids, constituted of Arg636 (VWF sequence: 1399) and Lys667 (VWF sequence:1430) [15]. Thus, both physical (shear forces) and chemical (ligand binding) potentials can generate the free energy change needed to sustain the conformational transitions in VWF multimers. In both cases, the induced conformational changes disrupt the multiple inter-domain interactions present in the native VWF structure [67]. Upon stabilization of the stretched conformation, the swapped domains of a VWF multimer can interact with the same domain but belonging to a different multimer. Hence, the non-covalent intra-monomer interactions between domains are broken and restored between different multimer chains: this aggregation mechanism may be an example of the biophysical phenomenon referred to as 3D domain swapping [78, 79]. The latter is a mechanism that allows protein molecules to form dimers or polymers by exchanging an identical structural element, referred to as “domain”. More properly, we should consider the process of VWF multimerization and subsequent molecular aggregation as a 3D domain swapping mechanism. The dimerization process of the VWF mature monomers takes place at acidic pH in the endoplasmic reticulum and occurs through the formation of disulfide bonds at the C-terminal cystine knot (CK) domain, as detailed in the next section. The stable dimer represents the fundamental structure that allows a progressive polymerization process that culminates in the formation of very high molecular weight VWF multimers in the endothelial Weibel-Palade bodies (WPB), through the further formation of disulfide bonds. However, these ultra-large VWF multimers, once secreted in the circulation, under the action of shear forces exploit a 3D swapping mechanism for the creation of extended VWF fibril networks, where flowing platelets can be easily trapped through the interaction between the GpIb and VWF A1 domain. This scenario was confirmed by experimental studies, as described in the next paragraph.
The intracellular dimerization and multimerization processes of VWF affect the sensitivity to shear of mature VWF multimers
VWF glycoprotein is biosynthesized as a preproprotein: the signal peptide sequence is removed during translocation into the endoplasmic reticulum (ER), while cleavage by furin takes place later to generate the pro-piece and mature fragments. Most disulfide bonds are formed in the endoplasmic reticulum, and proVWF dimerizes after the formation of intersubunit disulfide bonds at the C-terminal cystine knot (CK) domain. ProVWF dimers undergo a drastic pH change through the trans-Golgi network and the so-called Weibel-Palade bodies, where pH»6. In these granules, VWF dimers are further polymerized by the formation of additional disulfide bonds and are assembled into helical structures that form well-organized, parallel tubule-like structures [80, 81, 82]. In vitro studies showed that the non-covalent dimerization of the N-terminal ends of proVWF dimers are favored by homotypic interactions between the D1 and D2 domains [81]. Assembly of these dimers onto the end of a growing helical tubule puts the D’D3 domains within a dimer 13 nm apart from one another, and places one D’D3 domain adjacent to a different D’D3 domain from another dimer. This is a first mechanism compatible with a 3D domain swapping, which induces a correct disposition of two monomers for the formation of a physiological form of a dimer (Fig. 6A) Helical arrangement constitutes, in fact, a useful template for disulfide bond formation during N-terminal multimerization, preventing N-terminal disulfide bonds between monomers within the same dimer [83]. At the same time, this mechanism can ensure that covalent interaction between VWF monomers is co-linear with assembly into helical tubules, as electron microscopy studies have demonstrated [81]. Notably, this geometric arrangement of VWF multimers in the Weibel-Palade bodies of endothelial cells can compact mature VWF molecules »50-fold compared with stretched VWF after secretion in the blood stream [81]. Under the effect of external shear forces that induce the conformational changes and stretching of the ultra-large VWF multimers, the molecules can interact each other, forming spiderweb like structures (see cartoon in Fig. 6B) where flowing platelets can easily be trapped in the process of primary haemostasis [5]. This is the molecular process that should be considered bona fide a 3D domain swapping, as it engages multimeric proteins as basic elements functional to the process.
![Figure 6 Schematic cartoon showing the assembly of proVWF dimers in helical tubules according to the data reported in [83, 88]. A) Assembly templates disulfide bond formation between D’D3 domains of adjacent proVWF dimers is favored by the non-covalent dimerization of the N-terminal ends of proVWF dimers through homotypic interactions between the D1 and D2 domains. Note the partial shielding of the A2 on the A1 domain in the dimer in the absence of shear forces. B) Under the effect of mechanical forces, the multimers are unfolded and stretched, with the A1 domain available to interact with the platelet receptor GpIb. At the same time, through the 3D swapping mechanism, the unfolded dimers can interact each other to form a spider web-like lattice.](/document/doi/10.1515/bmc-2019-0022/asset/graphic/j_bmc-2019-0022_fig_006.jpg)
Schematic cartoon showing the assembly of proVWF dimers in helical tubules according to the data reported in [83, 88]. A) Assembly templates disulfide bond formation between D’D3 domains of adjacent proVWF dimers is favored by the non-covalent dimerization of the N-terminal ends of proVWF dimers through homotypic interactions between the D1 and D2 domains. Note the partial shielding of the A2 on the A1 domain in the dimer in the absence of shear forces. B) Under the effect of mechanical forces, the multimers are unfolded and stretched, with the A1 domain available to interact with the platelet receptor GpIb. At the same time, through the 3D swapping mechanism, the unfolded dimers can interact each other to form a spider web-like lattice.
Experimental evidences show that the early dimerization process could affect the final conformation (and thus function) of VWF multimers. For instance, the presence of the von Willebrand factor (VWF) variant c.2771G>A, causing the p.R924Q mutation in the D’D3 domain in combination with another mutation, the rare G allele of c.5843-8C>G in the intron 34 region, was reported to be associated with a reduced level of VWF multimers and coagulation FVIII [18]. Very recently, a compound heterozygous mutation in cis position in the D’D3 and D4 domains (p.R924Q and p.A2178S, respectively) was found by our group in a 55-year-old Italian man characterized by mucocutaneous bleedings with inconstant thrombocytopenia. This subject showed a low level VWF:Ag and even lower VWF:act levels, with a functional and electrophoretic pattern resembling that of a canonical form of type 2B VWD with enhanced interaction with GpIb and ADAMTS-13. (Lancellotti S et al., submitted for publication). It is likely that the contemporary mutations in the D’D3 and D4 domain in cis position can perturb the non-covalent interactions between the monomers, reducing the shielding effect of the A2 on the A1 domain. This phenomenon could explain the above findings and the behavior as a 2B-like form of VWD, although the canonical forms of type 2B VWD are so classified when generated by mutations in the A1 domain [84]. Notably, previous results showed that the 764–1035 region comprising the D’D3 domains of mature VWF plays an inhibitory role in the multimer molecule for GpIb interaction. In the absence of external shear forces, the A1 domain and the D’D3 region are in proximity, possibly mediated by the positively charged segment centered on Lys968 [85]. If the interaction between the A1 and D’D3 domain is disrupted by physical forces, then VWF undergoes a conformational transition leading to the exposition of the A1 domain that can bind to its ligands. A similar effect was observed in fact with an anti-VWF monoclonal antibody, 1C1E7, which binds to the 764-1035 region of VWF. This MoAb, in fact, when bound to the D’D3 domain, enhanced the VWF/GPIb binding, confirming that the elimination of the interaction between the D’D3 and A1 domains allows the latter to be exposed and interact with GpIb [86, 87].
Conclusions
In this review, we have described how the high sensitivity of VWF multimers to mechanical forces, which is centered in the A2 domain, plays a pivotal role in the pathophysiology of this haemostatic protein. From a pure mechanochemistry standpoint, VWF sensitivity to shear forces derives from most types of bonds. The multimer interaction with subendothelial collagen is regulated in fact by ideal bonds, as the binding is not affected by shear forces. On the contrary, the subsequent steps, including the transition from a folded to an unfolded conformation, are driven by catch bonds, as elevated shear stress induces a conformational transition that, starting in the A2 domain of the protein, is subsequently transmitted to the A1 domain and the other domains. The shear-induced conformational transitions of VWF play also a fundamental role in haemorrhagic and thrombotic diseases. It was shown indeed how the phenotypes of some forms of type 2 von Willebrand disease stem from altered structural responses of VWF to external shear forces. In some types of 2M VWD (G1324A and G1324S) the rigidity conferred to the A1 domain of the protein hampers the fitting process into the binding site of platelet GpIb that even high shear stress is not capable to overcome. At variance with this scenario, some type 2B VWF forms are mostly populated by stretched VWF multimer, which is more sensitive to lower shear forces, causing increased proteolysis by ADAMTS-13 and interaction with GpIb. Hence, we should always keep in mind that shear forces finely regulate the properties of VWF both in physiological and pathological conditions. Even acquired cardiovascular diseases, such as severe stenosis of the cardiac aortic valve, could affect the shear stress-induced conformational transitions of VWF multimers. Aortic stenosis is in fact associated locally with high shear stress, which favors the proteolytic interaction with ADAMTS-13, leading to an enhanced degradation or clearance of von Willebrand factor. Mechanochemistry principles and their experimental application in biochemical studies are a powerful tool to elucidate the functional properties of VWF and will further provide in the next years insights that will thoroughly change our understanding of the biophysical mechanisms responsible for the activation of VWF multimers under physiological and pathological conditions. The application of experimental strategies exploiting the mechanochemistry principles will help us to understand also how variably modulating with pharmacological tools the functional behavior of VWF multimers under flow conditions in haemorrhagic and thrombotic diseases.
Acknowledgements
RDC gratefully acknowledges financial support from the Catholic University (“Linea D1-AA 2017)
Conflict of interest: Authors state no conflict of interest
References
1 Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 1998;67:395-424.10.1146/annurev.biochem.67.1.395Search in Google Scholar PubMed
2 Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res 2007;100:1673-85.10.1161/01.RES.0000267878.97021.abSearch in Google Scholar PubMed
3 Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost 2012;10:2428-37.10.1111/jth.12008Search in Google Scholar PubMed
4 Huck V, Schneider MF, Gorzelanny C, Schneider SW. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb Haemost 2014;111:598-609.10.1160/TH13-09-0800Search in Google Scholar
5 Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci US A 2007;104:7899-903.10.1073/pnas.0608422104Search in Google Scholar
6 Zhang Q, Zhou YF, Zhang CZ, Zhang X, Lu C, Springer TA. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc Natl Acad Sci US A 2009;106:9226-31.10.1073/pnas.0903679106Search in Google Scholar
7 Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 2009;324:1330-4.10.1126/science.1170905Search in Google Scholar PubMed
8 Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996;84:289-97.10.1016/S0092-8674(00)80983-6Search in Google Scholar PubMed
9 Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006;108:1903-10.10.1182/blood-2006-04-011551Search in Google Scholar PubMed PubMed Central
10 Nurden AT. Clinical significance of altered collagen-receptor functioning in platelets with emphasis on glycoprotein VI. Blood Rev 2019: 100592.10.1016/j.blre.2019.100592Search in Google Scholar PubMed
11 Nissinen L, Koivunen J, Kapyla J, Salmela M, Nieminen J, Jokinen J, et al. Novel alpha2beta1 integrin inhibitors reveal that integrin binding to collagen under shear stress conditions does not require receptor preactivation. J Biol Chem 2012;287:44694-702.10.1074/jbc.M111.309450Search in Google Scholar PubMed PubMed Central
12 Cruz MA, Yuan H, Lee JR, Wise RJ, Handin RI. Interaction of the von Willebrand factor (vWF) with collagen. Localization of the primary collagen-binding site by analysis of recombinant vWF a domain polypeptides. J Biol Chem 1995;270:10822-7.10.1074/jbc.270.18.10822Search in Google Scholar PubMed
13 Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin Thromb Hemost 2003;29:479-88.10.1055/s-2003-44556Search in Google Scholar PubMed
14 Matsui T, Hamako J. Structure and function of snake venom toxins interacting with human von Willebrand factor. Toxicon 2005;45:1075-87.10.1016/j.toxicon.2005.02.023Search in Google Scholar PubMed
15 Matsushita T, Meyer D, Sadler JE. Localization of von Willebrand factor-binding sites for platelet glycoprotein Ib and botrocetin by charged-to-alanine scanning mutagenesis. J Biol Chem 2000;275:11044-9.10.1074/jbc.275.15.11044Search in Google Scholar PubMed
16 Auton M, Sedlak E, Marek J, Wu T, Zhu C, Cruz MA. Changes in thermodynamic stability of von Willebrand factor differentially affect the force-dependent binding to platelet GPIbalpha. Biophys J 2009;97:618-27.10.1016/j.bpj.2009.05.009Search in Google Scholar PubMed PubMed Central
17 Scaglione GL, Lancellotti S, Papi M, De Spirito M, Maiorana A, Baronciani L, et al. The type 2B p.R1306W natural mutation of von Willebrand factor dramatically enhances the multimer sensitivity to shear stress. J Thromb Haemost 2013;11:1688-98.10.1111/jth.12346Search in Google Scholar PubMed
18 Hickson N, Hampshire D, Winship P, Goudemand J, Schneppenheim R, Budde U, et al. von Willebrand factor variant p.Arg924Gln marks an allele associated with reduced von Willebrand factor and factor VIII levels. J Thromb Haemost 2010;8:1986-93.10.1111/j.1538-7836.2010.03927.xSearch in Google Scholar PubMed PubMed Central
19 South K, Lane DA. ADAMTS-13 and von Willebrand factor: a dynamic duo. J Thromb Haemost 2018;16:6-18.10.1111/jth.13898Search in Google Scholar PubMed PubMed Central
20 De Ceunynck K, Rocha S, Feys HB, De Meyer SF, Uji-i H, Deckmyn H, et al. Local elongation of endothelial cell-anchored von Willebrand factor strings precedes ADAMTS13 protein-mediated proteolysis. J Biol Chem 2011;286:36361-7.10.1074/jbc.M111.271890Search in Google Scholar PubMed PubMed Central
21 Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci US A 2006;103:19099-104.10.1073/pnas.0607264104Search in Google Scholar PubMed PubMed Central
22 Di Stasio E, Lancellotti S, Peyvandi F, Palla R, Mannucci PM, De Cristofaro R. Mechanistic studies on ADAMTS13 catalysis. Biophys J 2008;95:2450-61.10.1529/biophysj.108.131532Search in Google Scholar PubMed PubMed Central
23 Zhang C, Kelkar A, Neelamegham S. von Willebrand factor self-association is regulated by the shear-dependent unfolding of the A2 domain. Blood Adv 2019;3:957-68.10.1182/bloodadvances.2018030122Search in Google Scholar PubMed PubMed Central
24 Rakshit S, Sivasankar S. Biomechanics of cell adhesion: how force regulates the lifetime of adhesive bonds at the single molecule level. Phys Chem Chem Phys 2014;16:2211-23.10.1039/c3cp53963fSearch in Google Scholar PubMed
25 Thomas WE, Vogel V, Sokurenko E. Biophysics of catch bonds. Annu Rev Biophys 2008;37:399-416.10.1146/annurev.biophys.37.032807.125804Search in Google Scholar PubMed
26 McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 2015;107:331-9.10.1093/cvr/cvv154Search in Google Scholar PubMed PubMed Central
27 Manakova K, Yan H, Lowengrub J, Allard J. Cell Surface Mechanochemistry and the Determinants of Bleb Formation, Healing, and Travel Velocity. Biophys J 2016;110:1636-47.10.1016/j.bpj.2016.03.008Search in Google Scholar PubMed PubMed Central
28 Strakova K, Assies L, Goujon A, Piazzolla F, Humeniuk HV, Matile S. Dithienothiophenes at Work: Access to Mechanosensitive Fluorescent Probes, Chalcogen-Bonding Catalysis, and Beyond. Chem Rev 2019.10.1021/acs.chemrev.9b00279Search in Google Scholar PubMed
29 Liu Z, Yago T, Zhang N, Panicker SR, Wang Y, Yao L, Mehta-D’souza P, et al. L-selectin mechanochemistry restricts neutrophil priming in vivo. Nat Commun 2017;8:15196.10.1038/ncomms15196Search in Google Scholar PubMed PubMed Central
30 Brooks DE, Trust TJ. Enhancement of bacterial adhesion by shear forces: characterization of the haemagglutination induced by Aeromonas salmonicida strain 438. J Gen Microbiol 1983;129:3661-9.10.1099/00221287-129-12-3661Search in Google Scholar PubMed
31 Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest 2008;118:3195-207.10.1172/JCI35754Search in Google Scholar PubMed
32 Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol 2013;33:105-13.10.1161/ATVBAHA.112.300522Search in Google Scholar PubMed
33 Ju L, Dong JF, Cruz MA, Zhu C. The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibalpha. J Biol Chem 2013;288:32289-301.10.1074/jbc.M113.504001Search in Google Scholar PubMed
34 Kim J, Zhang CZ, Zhang X, Springer TA. A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 2010;466:992-5.10.1038/nature09295Search in Google Scholar PubMed
35 Bell GI. Models for the specific adhesion of cells to cells. Science 1978;200:618-27.10.1126/science.347575Search in Google Scholar PubMed
36 Dembo M, Torney DC, Saxman K, Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci 1988;234:55-83.10.1098/rspb.1988.0038Search in Google Scholar PubMed
37 Shapiro BE, Qian H. A quantitative analysis of single proteinligand complex separation with the atomic force microscope. Biophys Chem 1997;67:211-9.10.1016/S0301-4622(97)00045-8Search in Google Scholar
38 Kramers HA. Brownian motion in a field of force and the diffusion model of chemical reaction. Physica 1940;7:284-304.10.1016/S0031-8914(40)90098-2Search in Google Scholar
39 Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol 2006;7:883-9.10.1038/ni1366Search in Google Scholar PubMed PubMed Central
40 Pereverzev YV, Prezhdo OV, Forero M, Sokurenko EV, Thomas WE. The two-pathway model for the catch-slip transition in biological adhesion. Biophys J 2005;89:1446-54.10.1529/biophysj.105.062158Search in Google Scholar PubMed PubMed Central
41 Barsegov V, Thirumalai D. Dynamics of unbinding of cell adhesion molecules: transition from catch to slip bonds. Proc Natl Acad Sci US A 2005;102:1835-9.10.1073/pnas.0406938102Search in Google Scholar PubMed PubMed Central
42 Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc Natl Acad Sci US A 2004;101:11281-6.10.1073/pnas.0401870101Search in Google Scholar PubMed PubMed Central
43 Pereverzev YV, Prezhdo OV, Sokurenko EV. Regulation of catch binding by allosteric transitions. J Phys Chem B 2010;114:11866-74.10.1021/jp1031459Search in Google Scholar PubMed PubMed Central
44 Springer TA. Structural basis for selectin mechanochemistry. Proc Natl Acad Sci US A 2009;106:91-6.10.1073/pnas.0810784105Search in Google Scholar PubMed PubMed Central
45 Tchesnokova V, Aprikian P, Yakovenko O, Larock C, Kidd B, Vogel V, et al. Integrin-like allosteric properties of the catch bond-forming FimH adhesin of Escherichia coli. J Biol Chem 2008;283:7823-33.10.1074/jbc.M707804200Search in Google Scholar PubMed
46 Thomas W, Forero M, Yakovenko O, Nilsson L, Vicini P, Sokurenko E, et al. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys J 2006;90:753-64.10.1529/biophysj.105.066548Search in Google Scholar PubMed PubMed Central
47 Wei Y. Entropic-elasticity-controlled dissociation and energetic-elasticity-controlled rupture induce catch-to-slip bonds in cell-adhesion molecules. Phys Rev E Stat Nonlin Soft Matter Phys 2008;77:031910.10.1103/PhysRevE.77.031910Search in Google Scholar PubMed
48 Pereverzev YV, Prezhdo OV. Force-induced deformations and stability of biological bonds. Phys Rev E Stat Nonlin Soft Matter Phys 2006;73:050902.10.1103/PhysRevE.73.050902Search in Google Scholar PubMed
49 Pereverzev YV, Prezhdo OV, Sokurenko EV. Allosteric role of the large-scale domain opening in biological catch-binding. Phys Rev E Stat Nonlin Soft Matter Phys 2009;79:051913.10.1103/PhysRevE.79.051913Search in Google Scholar PubMed
50 Rief M, Oesterhelt F, Heymann B, Gaub HE. Single Molecule Force Spectroscopy on Polysaccharides by Atomic Force Microscopy. Science 1997;275:1295-7.10.1126/science.275.5304.1295Search in Google Scholar PubMed
51 Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 1999;397:50-3.10.1038/16219Search in Google Scholar PubMed
52 Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods 2008;5:491-505.10.1038/nmeth.1218Search in Google Scholar PubMed PubMed Central
53 Machha VR, Tischer A, Moon-Tasson L, Auton M. The Von Willebrand Factor A1-Collagen III Interaction Is Independent of Conformation and Type 2 Von Willebrand Disease Phenotype. J Mol Biol 2017;429:32-47.10.1016/j.jmb.2016.11.014Search in Google Scholar PubMed PubMed Central
54 Aponte-Santamaria C, Huck V, Posch S, Bronowska AK, Grassle S, Brehm MA, et al. Force-sensitive autoinhibition of the von Willebrand factor is mediated by interdomain interactions. Biophys J 2015;108:2312-21.10.1016/j.bpj.2015.03.041Search in Google Scholar PubMed PubMed Central
55 Fuchs B, Budde U, Schulz A, Kessler CM, Fisseau C, Kannicht C. Flow-based measurements of von Willebrand factor (VWF) function: binding to collagen and platelet adhesion under physiological shear rate. Thromb Res 2010;125:239-45.10.1016/j.thromres.2009.08.020Search in Google Scholar PubMed
56 Favaloro EJ. Diagnosing von Willebrand disease: a short history of laboratory milestones and innovations, plus current status, challenges, and solutions. Semin Thromb Hemost 2014;40:551-70.10.1055/s-0034-1383546Search in Google Scholar PubMed
57 Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 2006;107:3537-45.10.1182/blood-2005-02-0618Search in Google Scholar PubMed PubMed Central
58 Bryckaert M, Rosa JP, Denis CV, Lenting PJ. Of von Willebrand factor and platelets. Cell Mol Life Sci 2015;72:307-26.10.1007/s00018-014-1743-8Search in Google Scholar PubMed PubMed Central
59 Di Stasio E, Romitelli F, Lancellotti S, Arcovito A, Giardina B, De Cristofaro R. Kinetic study of von Willebrand factor self-aggregation induced by ristocetin. Biophys Chem 2009;144:101-7.10.1016/j.bpc.2009.07.002Search in Google Scholar PubMed
60 Papi M, Maulucci G, De Spirito M, Missori M, Arcovito G, Lancellotti S, et al. Ristocetin-induced Self-Aggregation of Von Willebrand Factor. European biophysics Journal 2010;39:159760310.1007/s00249-010-0617-8Search in Google Scholar PubMed
61 Di Stasio E, De Cristofaro R. The effect of shear stress on protein conformation: Physical forces operating on biochemical systems: The case of von Willebrand factor. Biophys Chem 2010;153:1-8.10.1016/j.bpc.2010.07.002Search in Google Scholar PubMed
62 Montilla M, Hernandez-Ruiz L, Garcia-Cozar FJ, Alvarez-Laderas I, Rodriguez-Martorell J, Ruiz FA. Polyphosphate binds to human von Willebrand factor in vivo and modulates its interaction with glycoprotein Ib. J Thromb Haemost 2012;10:2315-23.10.1111/jth.12004Search in Google Scholar PubMed
63 Kruse-Jarres R, Johnsen JM. How I treat type 2B von Willebrand disease. Blood 2018;131:1292-300.10.1182/blood-2017-06-742692Search in Google Scholar
64 Tischer A, Campbell JC, Machha VR, Moon-Tasson L, Benson LM, Sankaran B, et al. Mutational Constraints on Local Unfolding Inhibit the Rheological Adaptation of von Willebrand Factor. J Biol Chem 2016;291:3848-59.10.1074/jbc.M115.703850Search in Google Scholar PubMed
65 Springer TA. Complement and the multifaceted functions of VWA and integrin I domains. Structure 2006;14:1611-6.10.1016/j.str.2006.10.001Search in Google Scholar PubMed
66 Valiaev A, Lim DW, Oas TG, Chilkoti A, Zauscher S. Force-induced prolyl cis-trans isomerization in elastin-like polypeptides. J Am Chem Soc 2007;129:6491-7.10.1021/ja070147rSearch in Google Scholar PubMed
67 Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014;124:1412-25.10.1182/blood-2014-05-378638Search in Google Scholar PubMed
68 Shankaran H, Neelamegham S. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys J 2004;86:576-88.10.1016/S0006-3495(04)74136-3Search in Google Scholar PubMed
69 Day MA. The no-slip condition of fluid dynamics Springer Netherlands, 2004.Search in Google Scholar
70 George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:1847-8.10.1056/NEJMra1312353Search in Google Scholar PubMed
71 Fu H, Jiang Y, Yang D, Scheiflinger F, Wong WP, Springer TA. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat Commun 2017;8:324.10.1038/s41467-017-00230-2Search in Google Scholar PubMed PubMed Central
72 Saha M, McDaniel JK, Zheng XL. Thrombotic thrombocytopenic purpura: pathogenesis, diagnosis and potential novel therapeutics. J Thromb Haemost 2017;15:1889-900.10.1111/jth.13764Search in Google Scholar PubMed
73 Fu X, Chen J, Gallagher R, Zheng Y, Chung DW, Lopez JA. Shear stress-induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood 2011;118:5283-91.10.1182/blood-2011-01-331074Search in Google Scholar PubMed
74 Lancellotti S, De Filippis V, Pozzi N, Peyvandi F, Palla R, Rocca B, et al. Formation of methionine sulfoxide by peroxynitrite at position 1606 of von Willebrand factor inhibits its cleavage by ADAMTS-13: A new prothrombotic mechanism in diseases associated with oxidative stress. Free Radic Biol Med 2010;48:446-56.10.1016/j.freeradbiomed.2009.11.020Search in Google Scholar PubMed
75 Interlandi G. Destabilization of the von Willebrand factor A2 domain under oxidizing conditions investigated by molecular dynamics simulations. PLoS One 2018;13:e0203675.10.1371/journal.pone.0203675Search in Google Scholar PubMed
76 De Filippis V, Lancellotti S, Maset F, Spolaore B, Pozzi N, Gambaro G, et al. Oxidation of Met1606 in von Willebrand factor is a risk factor for thrombotic and septic complications in chronic renal failure. Biochem J 2012;442:423-32.10.1042/BJ20111798Search in Google Scholar PubMed
77 Oggianu L, Lancellotti S, Pitocco D, Zaccardi F, Rizzo P, Martini F, et al. The oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PLoS One 2013;8:e55396.10.1371/journal.pone.0055396Search in Google Scholar PubMed
78 Bennett MJ, Schlunegger MP, Eisenberg D. 3D domain swapping: a mechanism for oligomer assembly. Protein Sci 1995;4:2455-68.10.1002/pro.5560041202Search in Google Scholar PubMed
79 Liu Y, Eisenberg D. 3D domain swapping: as domains continue to swap. Protein Sci 2002;11:1285-99.10.1110/ps.0201402Search in Google Scholar PubMed
80 Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, et al. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell 1991;64:403-13.10.1016/0092-8674(91)90648-ISearch in Google Scholar PubMed
81 Huang RH, Wang Y, Roth R, Yu X, Purvis AR, Heuser JE, et al. Assembly of Weibel-Palade body-like tubules from N-terminal domains of von Willebrand factor. Proc Natl Acad Sci US A 2008;105:482-7.10.1073/pnas.0710079105Search in Google Scholar PubMed PubMed Central
82 Berriman JA, Li S, Hewlett LJ, Wasilewski S, Kiskin FN, Carter T, et al. Structural organization of Weibel-Palade bodies revealed by cryo-EM of vitrified endothelial cells. Proc Natl Acad Sci US A 2009;106:17407-12.10.1073/pnas.0902977106Search in Google Scholar PubMed PubMed Central
83 Zhou YF, Eng ET, Nishida N, Lu C, Walz T, Springer TA. A pH-regulated dimeric bouquet in the structure of von Willebrand factor. Embo J 2011;30:4098-111.10.1038/emboj.2011.297Search in Google Scholar PubMed PubMed Central
84 Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14.10.1111/j.1538-7836.2006.02146.xSearch in Google Scholar PubMed
85 Ulrichts H, Udvardy M, Lenting PJ, Pareyn I, Vandeputte N, Vanhoorelbeke K, et al. Shielding of the A1 domain by the D’D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J Biol Chem 2006;281:4699-707.10.1074/jbc.M513314200Search in Google Scholar PubMed
86 Tornai I, Arnout J, Deckmyn H, Peerlinck K, Vermylen J. A monoclonal antibody recognizes a von Willebrand factor domain within the amino-terminal portion of the subunit that modulates the function of the glycoprotein IB- and IIB/IIIA-binding domains. J Clin Invest 1993;91:273-82.10.1172/JCI116181Search in Google Scholar PubMed PubMed Central
87 Ulrichts H, Harsfalvi J, Bene L, Matko J, Vermylen J, Ajzenberg N, et al. A monoclonal antibody directed against human von Willebrand factor induces type 2B-like alterations. J Thromb Haemost 2004;2:1622-8.10.1111/j.1538-7836.2004.00865.xSearch in Google Scholar PubMed
88 Huang J, Roth R, Heuser JE, Sadler JE. Integrin alpha(v)beta(3) on human endothelial cells binds von Willebrand factor strings under fluid shear stress. Blood 2009;113:1589-97.10.1182/blood-2008-05-158584Search in Google Scholar PubMed PubMed Central
© 2019 Stefano Lancellotti et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Article
- ATP Synthase: Structure, Function and Inhibition
- Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics
- Mini review
- The disordered boundary of the cell: emerging properties of membrane-bound intrinsically disordered proteins
- Research Article
- Expression of OX40 Gene and its Serum Levels in Neuromyelitis Optica Patients
- Advances in Molecular biomarker for early diagnosis of Osteoarthritis
- Identification of BRCA1/2 p.Ser1613Gly, p.Pro871Leu, p.Lys1183Arg, p.Glu1038Gly, p.Ser1140Gly, p.Ala2466Val, p.His2440Arg variants in women under 45 years old with breast nodules suspected of having breast cancer in Burkina Faso
- Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation
- Integral membrane protein expression of human CD25 on the cell surface of HEK293 cell line: the available cellular model of CD25 positive to facilitate in vitro developing assays
- Review Article
- Role of Nanomedicine in Redox Mediated Healing at Molecular Level
- Research Article
- Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) variants and breast cancer risk in Burkina Faso
- Incidence of viruses infecting pepper in Thailand
- Mechanochemistry of von Willebrand factor
- Schizophrenia phenomenology comprises a bifactorial general severity and a single-group factor, which are differently associated with neurotoxic immune and immune-regulatory pathways
- Role of Killer cell immunoglobulin-like receptors (KIR) genes in stages of HIV-1 infection among patients from Burkina Faso
- The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis
- Special Issue: Recent Advances in Basic and Clinical Medicine
- Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in Streptozotocin-induced diabetic rats
- Comparison of the effect of vitamin D on osteoporosis and osteoporotic patients with healthy individuals referred to the Bone Density Measurement Center
- Zinc enhances the expression of morphine-induced conditioned place preference through dopaminergic and serotonergic systems
- Pre-operative laparoscopic staging of gastric cancer in patients who are candidates for neo-adjuvant chemotherapy: A Cross Sectional Study
- Neurotoxic Effects of Stanozolol on Male Rats‘ Hippocampi: Does Stanozolol cause apoptosis?
- Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer
- Evaluation of Quality of Life in Terms of Sinonasal Symptoms in Children with Cystic Fibrosis
- The Effect of Fine needle aspiration on Detecting Malignancy in Thyroid Nodule
- Diagnosis of Primary Hydatid Cyst of Thyroid Gland: A Case Report
- Ultrasonography in the diagnosis of lung adhesion before surgery
Articles in the same Issue
- Research Article
- ATP Synthase: Structure, Function and Inhibition
- Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics
- Mini review
- The disordered boundary of the cell: emerging properties of membrane-bound intrinsically disordered proteins
- Research Article
- Expression of OX40 Gene and its Serum Levels in Neuromyelitis Optica Patients
- Advances in Molecular biomarker for early diagnosis of Osteoarthritis
- Identification of BRCA1/2 p.Ser1613Gly, p.Pro871Leu, p.Lys1183Arg, p.Glu1038Gly, p.Ser1140Gly, p.Ala2466Val, p.His2440Arg variants in women under 45 years old with breast nodules suspected of having breast cancer in Burkina Faso
- Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation
- Integral membrane protein expression of human CD25 on the cell surface of HEK293 cell line: the available cellular model of CD25 positive to facilitate in vitro developing assays
- Review Article
- Role of Nanomedicine in Redox Mediated Healing at Molecular Level
- Research Article
- Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) variants and breast cancer risk in Burkina Faso
- Incidence of viruses infecting pepper in Thailand
- Mechanochemistry of von Willebrand factor
- Schizophrenia phenomenology comprises a bifactorial general severity and a single-group factor, which are differently associated with neurotoxic immune and immune-regulatory pathways
- Role of Killer cell immunoglobulin-like receptors (KIR) genes in stages of HIV-1 infection among patients from Burkina Faso
- The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis
- Special Issue: Recent Advances in Basic and Clinical Medicine
- Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in Streptozotocin-induced diabetic rats
- Comparison of the effect of vitamin D on osteoporosis and osteoporotic patients with healthy individuals referred to the Bone Density Measurement Center
- Zinc enhances the expression of morphine-induced conditioned place preference through dopaminergic and serotonergic systems
- Pre-operative laparoscopic staging of gastric cancer in patients who are candidates for neo-adjuvant chemotherapy: A Cross Sectional Study
- Neurotoxic Effects of Stanozolol on Male Rats‘ Hippocampi: Does Stanozolol cause apoptosis?
- Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer
- Evaluation of Quality of Life in Terms of Sinonasal Symptoms in Children with Cystic Fibrosis
- The Effect of Fine needle aspiration on Detecting Malignancy in Thyroid Nodule
- Diagnosis of Primary Hydatid Cyst of Thyroid Gland: A Case Report
- Ultrasonography in the diagnosis of lung adhesion before surgery

