Harnessing microalgae: from biology to innovation in sustainable solutions
-
Simon Greulich
, Nam Trung Tran
Abstract
Microalgae, small single or multicellular photosynthetic active organisms, could be a component to solve our urgent global challenges. This review provides a concise introduction to biology and applications of microalgae. On an example from our own scientific studies, we illustrate how these organisms could replace respectively optimize carbon producing processes. We will also describe the potential of microalgae for sustainable production towards atmospheric CO2 sequestration. Development of machine learning techniques forecast a paradigm shift regarding scientific methods. It concerns synthetic biology as well as engineering of metabolism in microalgae.
Zusammenfassung
Mikroalgen, kleine einzelne oder mehrzellige photosynthetisch aktive Organismen, könnten eine Komponente sein, um unsere dringenden globalen Herausforderungen zu lösen. Diese Übersicht bietet eine kurze Einführung in die Biologie und Anwendungen von Mikroalgen. In einem Beispiel aus unseren eigenen wissenschaftlichen Studien veranschaulichen wir, wie diese Organismen die Kohlenstoffproduktionsprozesse ersetzen können. Wir werden auch das Potenzial von Mikroalgen für eine nachhaltige Produktion in Bezug auf atmosphärische CO2 -Sequestrierung beschreiben. Die Entwicklung von Techniken für maschinelles Lernen prognostiziert eine Paradigmenverschiebung in Bezug auf wissenschaftliche Methoden. Es betrifft die synthetische Biologie sowie das Engineering des Stoffwechsels in Mikroalgen.
1 Introduction
To successfully address urgent challenges such as the escalating impacts of climate change, the demographic shift towards larger and older population, and the limitations imposed by finite resources, it is imperative to adopt a comprehensive and forward-thinking approach [1], [2]. To sustain our quality of life and prevent the imminent threat of energy shortages, we need to find innovative scientific strategies which integrate cutting-edge production chains, and energy generation methods that liberate us from dependence on fossil fuels [3]. One promising way opens by establishing green production lines that employ photosynthetic microorganisms, in particular microalgae, as versatile production platforms – a solution with potential transformative impact [1], [2]. Their ability to propagate in simple growth media, their high contents of vitamins, nutrients, and trace elements, coupled with their capability to synthesize a diverse array of high-value products and their unique capacity to bind atmospheric carbon dioxide (CO2) together with oxygen-production, speaks for them as a promising tool for the management of current and future tasks concerning environmental challenges as well as human food and medicine supply (Figure 1). These goals can only be achieved by interdisciplinary cooperation of engineering and biological science. Only if the expertise from both sites are combined, the joint effort can provide systems that are effective enough to be of commercial interest and at the same time energy efficient as well as environment friendly.
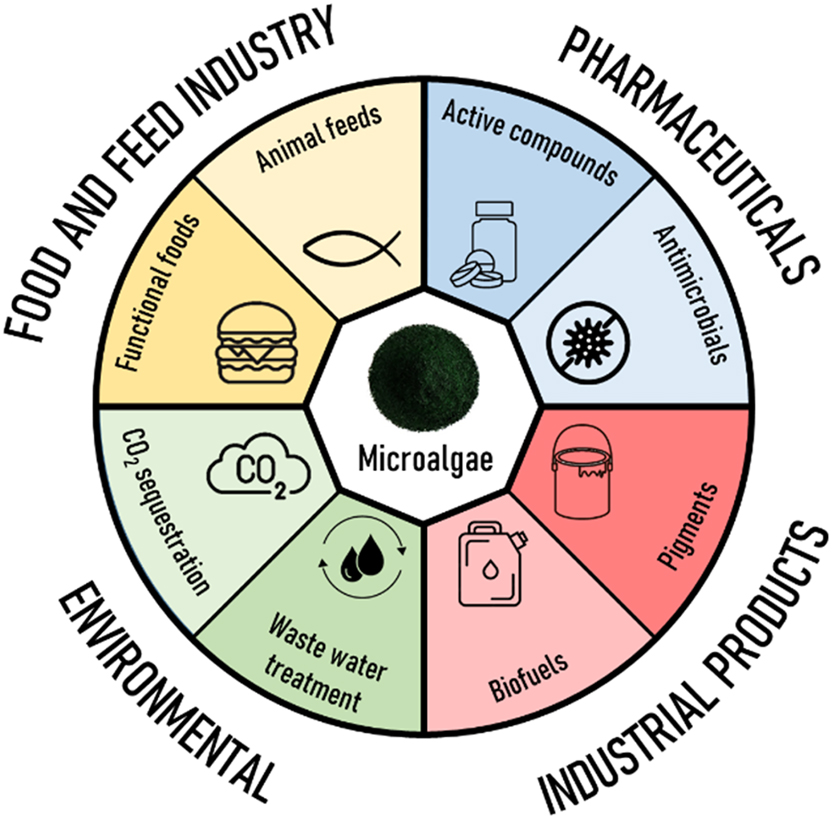
Main applications of microalgae.
2 Microalgae: biology, applications and challenges
Microalgae, photosynthetic uni- or multi-cellular organisms, comprise between 45,000 and 100,000 species thriving across a wide range of habitats worldwide. These include marine environments and freshwater and unexpected niches such as forests, stone walls or ice surfaces [4]–[6]. Often, microalgae play pivotal roles in ecosystems or influence conditions even in worldwide extend. Marine microalgae, for instance, contribute significantly to nearly half of the Earth’s oxygen production [7] which is substantially more than trees for example. Moreover, these microorganisms serve as a fundamental food source for aquatic organisms, permitting the complexities of marine life. On the other hand, algal blooms, a phenomenon characterized by rapid and excessive proliferation of microalgae, frequently is caused by over-fertilization in agriculture and poses significant risks to the environment. Under these conditions, in some microalgae species release of toxic substances is induced [8].
Humans use the potential of microalgae for industrial applications [9]. Microalgae cultivation primarily focussed on bulk biomass production for food or feed industry. In recent years a shift towards more value-generating processes can be observed. Here, microalgae are employed in bio-based production of different chemical compounds [10]–[12]. These encompass products with applications in chemistry, medicine, cosmetics, or food industry, e.g., as food supplement [13]. For cosmetics and pharmaceutical industry, microalgae can produce high-value active compounds. The biosynthetic pathways are occurring naturally or were artificially added through metabolic engineering. Currently, there is intensive research exploring the potential of engineered microalgae to produce biofuels and fine chemicals [14]. Beyond production, microalgae are applied in waste industry such as the treatment of industrial or municipal wastewater or they ensure water quality in aquaculture [15], [16]. In comparison to other widely used microorganisms in industrial production, microalgae offer some advantages. Unlike yeast or bacteria, microalgae don’t rely on energy-rich, high-quality carbon sources such as sugars or proteins; instead, they grow in medium with minimal nutrient requirements. They use CO2 and solar energy to produce sugar and release O2 to the atmosphere while still achieving rapid growth rates [2], [12], [17]. By using CO2 for metabolism and growth, they efficiently absorb it from atmosphere, making them a promising solution to achieve CO2 neutrality or even positive atmospheric CO2 removal [7], [17].
Despite the considerable potential of microalgae, the prospect of large-scale production is hindered by quite a few issues which require more careful scientific research. These obstacles encompass suitable strain selection, cultivation techniques, harvesting methods, the high production costs, and the complexity of metabolic regulation in the cell [18].
The fabrication and commercialization of algal products face regulations. As a consequence, only a limited number of microalgae species are reckoned “generally recognized as safe” (GRAS), allowing their direct approval for human consumption. The approval process for all other products, especially new algae species or products, tends to be prolonged and complicated. Engineered microalgae, often falling under the category of gene-modified organisms (GMO), are subjected to even more stringent controls. These regulatory barriers set a significant obstacle to develop innovative industrial production opportunities, particularly in highly regulated regions such as the European Union [5].
The rapid advancement of modern technologies includes the promise to revolutionize cultivation procedures of microalgae and unlock their previously untapped potentials. Ongoing research is focused on several dynamically evolving fields, including the refinement of cultivation techniques, the reduction of energy consumption and production cost, and the biological enhancement of production strains (Figure 2).

Photobioreactor for research. Flexible regulation and control of growth parameters for an optimal study of microalgae.
To date, microalgae were primarily cultivated in open pond systems, offering low production costs but prone to contamination and inconsistent biomass yields caused by variable environmental conditions such as temperature, pH, and light [11], [19]. To overcome these challenges, sophisticated closed photobioreactors are in favour. These precisely engineered systems allow meticulous control on factors such as temperature, light, or flow, ensuring consistent yields and minimizing contamination [20] (Figure 3A). Moreover, insights from hydrodynamics were applied to optimize photobioreactors. By leveraging the behavior of liquids it becomes possible to refine mixing processes, optimize fluid flow dynamics, mitigate shear forces, and enhance the transfer of both gas and light energy to microalgae cells [21] (Figure 3C). Modern materials also allow lighter, sturdier photobioreactors with improved light transparency and reduced susceptibility to fouling from microalgae growth [22] (Figure 3B).
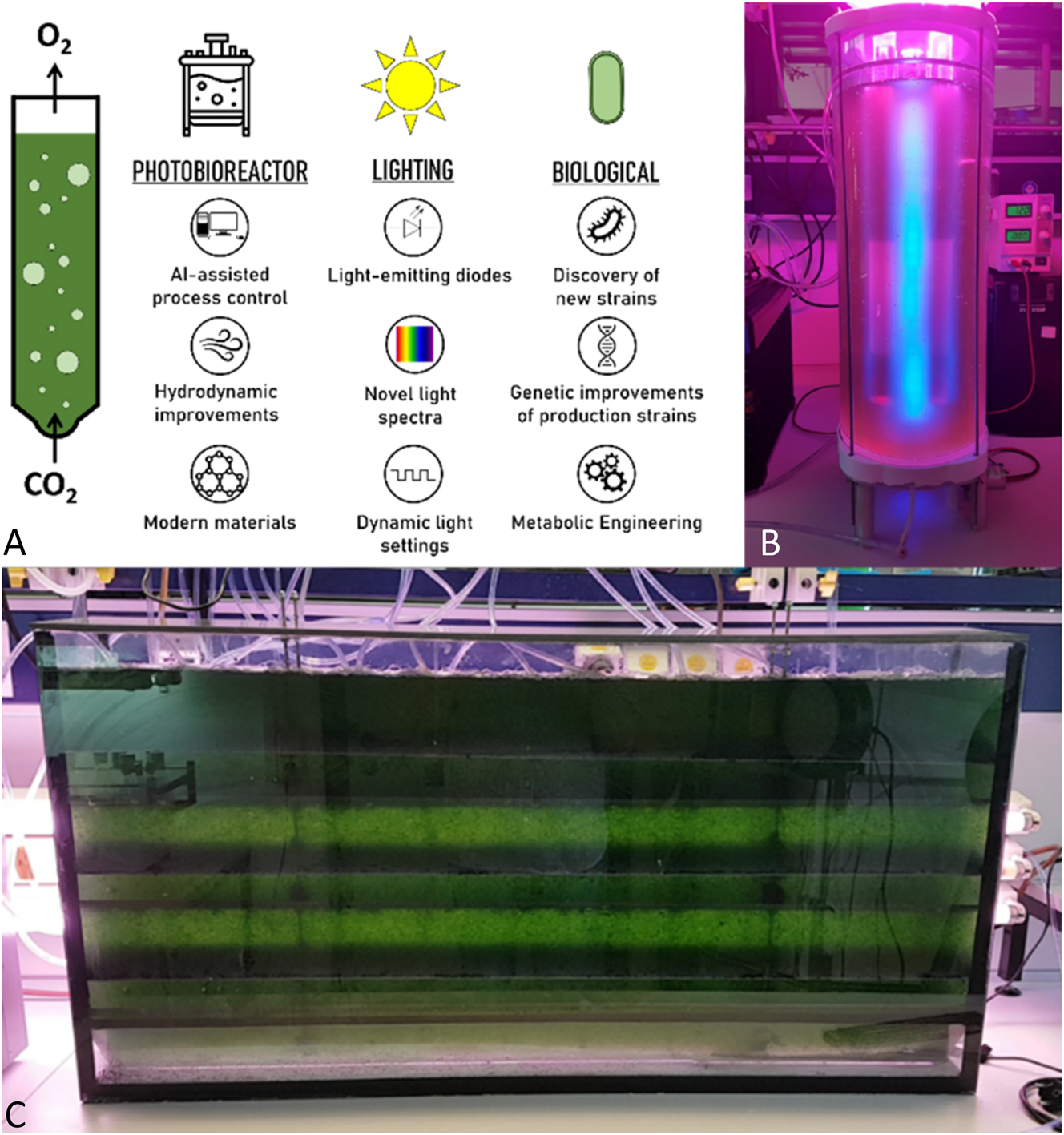
Development of cultivation solutions. (A) Key innovations in microalgae cultivation practice; (B) prototype of a photobioreactor with internal blue light and external red/blue light lighting; (C) model of a flat-panel photobioreactor with an internal system for turbulent flow.
Cultivating microalgae under sunlight is feasible, it proves most practical in regions near the equator with ample daily sunlight hours, only. Conversely, in countries of Northern Europe, artificial light sources become indispensable in algal production albeit at the cost of increased production costs [23]. Here, modern technologies offer a pivotal solution to reduce overall production expenses. For example, employing light-emitting diodes (LED) technology allows targeted manipulation of cell biology. By adjusting the intensity, wavelength, frequency, and dynamics of the illumination, significant improvements can be achieved. This includes bolstering photosynthetic efficiency, fostering better growth, enhancing light penetration into cell cultures, and elevating the production of specific desired substances [23]–[25].
Looking at future developments, there is considerable potential for improving the productivity/economic viability of microalgae via biological enhancement. High-throughput phenotyping technologies hold the promise of identifying previously undiscovered strains with superior characteristics such as faster growth rates, greater robustness, and the ability to produce high-value products [26]. Furthermore, there are opportunities to enhance the value of already established ones. Due to their comparable simple but still complex construction and single cell based biology, microalgae can be manipulated and optimized for production. Employing modern genetics and metabolic engineering techniques enables the precise “engineering” of a species. This includes improvement of favorable traits (e.g. robust growth, valuable metabolites), eliminating undesirable ones (e.g. toxin production, thick cell wall), and even introduction of novel biosynthetic pathways, leading to the production of high-value products not native to the species [27], [28].
The future of microalgae cultivation holds immense promises as researchers continue to explore innovative solutions in modern cultivation practices, energy efficiency, and biological enhancement. The ongoing convergence of technology and biology is paving the way for a more sustainable and economically viable exploitation of microalgae’s unexploited potentials [29], [30], [31].
3 Case study: metabolic engineering of microalgae for production of high-value carotenoids
Carotenoids, a diverse group of water-insoluble pigments, are prevalent in the natural world, being synthesized by virtually all photosynthetically active organisms and by many bacteria and fungi. In the realm of microalgae, more than 30 distinct carotenoids are synthesized and play pivotal roles in photosynthesis. They act as pigments for light absorption and offer protection against photo-oxidative damage [32], [33]. Beyond their fundamental biological functions, carotenoids have also found widespread application in industrial settings. Their importance in the food and pharmaceutical industries is underscored by their vibrant colours, antioxidant properties, anti-aging attributes, and stress-relieving effects coupled with the role as precursors of essential vitamins. Among these carotenoids, astaxanthin, a deep red pigment, distinguishes with remarkable antioxidant potency – about ten times higher estimated biological activity than other carotenoids and a thousand times more potent than vitamin E [13]. Presently, astaxanthin is predominantly utilized in animal feed for aquaculture for enhancing pigmentation in salmon, trout, and crab flesh while also conferring additional health benefits to these aquatic species. However, the attraction of astaxanthin doesn’t end in aquaculture. It’s extraordinary qualities pushed it into spotlight, leading to an increasing demand for its inclusion in pharmaceutical and cosmetic products [13], [32], [33].
In 2019, the global market for astaxanthin reached a value of USD 1.0 billion, and it is anticipated to experience a robust growth rate of 16.2 % until 2027 [34]. Within this market, synthetic astaxanthin, primarily derived from chemical synthesis, dominates the fish and animal feed sector due to its comparatively low cost at USD 1000 per kilogram. Conversely, the food, pharmaceutical, and cosmetics industries exhibit a preference for natural astaxanthin sourced from biological origins. Despite its higher price tag of USD 5000 per kilogram compared to synthetic alternatives, natural astaxanthin is favoured due to the significantly enhanced and proven health effects [13], [34].
Various species spontaneously produce astaxanthin, with Haematococcus pluvialis being the most prominent and well-established source. This freshwater algae species exhibits an impressive ability to produce up to 5 % of its dry mass astaxanthin [35]. However, cultivation is not that simple. This arises from the fact that astaxanthin is produced in high quantities only during a specific stage of the cell’s life cycle. Therefore, a two-stage production process is mandatory, which makes production in industrial scale difficult. In addition, the growth rate of H. pluvialis is notably slower than that of other microalgae species. To overcome these challenges, current research aiming to enhance astaxanthin production focusses on three key strategies: (1) continuous production route in fast-growing cells; (2) increase the dry weight percentages of astaxanthin; and (3) fine-tuning cellular and metabolic processes to facilitate more efficient harvesting and purification.
Metabolic engineering is the tool of choice for these strategies. Here, in some examples biosynthetic enzymes from astaxanthin-producing microalgae or bacteria, such as Haematococcus pulvialis, Chlamydomonas reinhardtii, or Brevundimonas spp. [13], [35], were integrated into the existing carotenoid biosynthetic network of fast-growing microalgae species. In theory, this pathway extension seems simple yet proves highly effective. In principle, astaxanthin production in microalgae that also rapidly generate large amounts of biomass is the goal. In practice, however, metabolic engineering is a highly complex process. Challenges include the establishment of stable genetic modifications and selection of suitable combinations of enzymes and their interactions. Moreover, expanding the synthesis pathway is just the first step. Subsequent pathway refinements are crucial for achieving high yields. Despite these complexities, the astaxanthin biosynthetic pathway has been successfully introduced into some microalgae species, such as Synechocystis sp. PCC6803 and C. reinhardtii [36]–[38]. A notable breakthrough includes our recent establishment of the astaxanthin biosynthetic pathway in Synechococcus sp. PCC7002, a very fast-growing cyanobacterium naturally synthesizing only β-carotene and small amounts of zeaxanthin, an astaxanthin precursor. By integrating three distinct components – (1) an artificial, optimized astaxanthin biosynthetic pathway, (2) an engineered carotenoid biosynthetic pathway with increased output, and (3) cellular transport system – into a single organism, we have successfully achieved the continuous production and extracellular export of a substantial amount of astaxanthin into the growth medium (Figure 4A). This breakthrough marks the first-ever achievement of astaxanthin secretion by microalgae, which not only streamlines the extraction process, but also promises to significantly reduce costs [39]. These achievements highlight the ongoing advancements in conquering the complexities of metabolic engineering for astaxanthin production in microalgae and underscores the enormous potential of this pioneering approach (Figure 4).
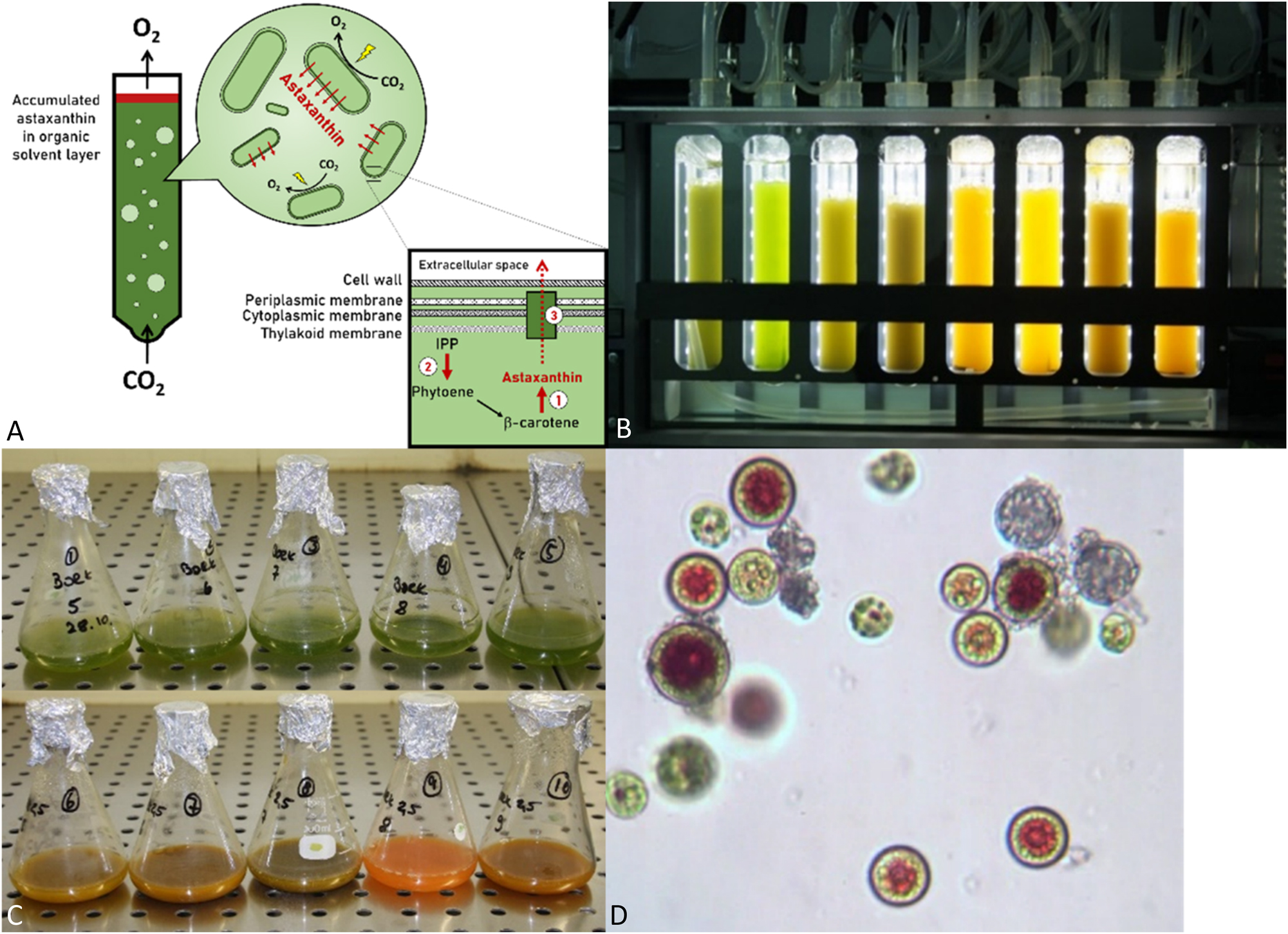
Metabolic engineering of Synechococcus sp. PCC7002 as a platform for astaxanthin production. (A) The organism has been engineered to include three main components: (1) an optimized astaxanthin biosynthetic pathway, (2) a carotenoid biosynthetic pathway optimized for enhanced performance, and (3) a cellular transport system to facilitate astaxanthin production and secretion; (B) astaxanthin-producing microalgae in a photobioreactor; (C) comparison between controls (top) and an engineered strain that produces astaxanthin (bottom); (D) microscope view of astaxanthin producing microalgae cells.
4 Case study: engineering of microalgae for carbon sequestration
In our world today, with escalating challenges posed by climate change, there is an increasingly urgent need to transition towards a more sustainable and resilient future. At the heart of these efforts lies a fundamental question: how can we effectively halt the increase of atmospheric CO2 concentrations, the primary driver of global warming [40]? More than 100 countries have either adopted, announced, or are actively discussing net-zero commitments, collectively representing over two-thirds of global greenhouse gas (GHG) emissions [40], [41]. While most of these nations are targeting net-zero emissions by 2050, some, like Finland, have set even more ambitious goals, aiming for carbon neutrality by 2035 [42].
In our pursuit for net-zero emissions, microalgae present a valuable opportunity due to their unique capability to remove CO2 from the atmosphere by photosynthesis. This process, shared by algae and plants, uses light energy to convert CO2 and water into biomass and oxygen. What raises some microalgae species out of a lot is their remarkable ability, known as carbon concentration mechanisms (CCMs), to actively accumulate CO2 within specialized cellular compartments. This significantly enhances their photosynthetic efficiency and CO2 removal capacity [43]. The photosynthetic activities of oceanic microalgae alone contributes up to 40 % of the total CO2 absorption in the global carbon cycle, far superior to their terrestrial plant counterparts and even though microalgae make up just no more than 2 % of the world’s total plant biomass [44]. Ongoing research is focused on developing and refining algae-based carbon capture and storage technology. This process involves the cultivation of algae, which can absorb CO2 through photosynthesis. The captured CO2 can be utilized in various carbon-neutral applications or stored in a manner that prevents its release into the atmosphere. Moreover, the substantial profit generated by algal products, including biofuels and other valuable compounds, would also help offset the expenses associated with CO2 sequestration [45].
Despite the potential for algae-based carbon capture and storage, there is work ahead to enhance current efficiency levels. This can be achieved by various methods, for example the identification and characterization of new algal species for more efficient photosynthesis or robust growth [36], [46]. Genetic engineering techniques can further enhance the capability of existing species [47]. Moreover, efforts are underway to increase the capacity of the carbon concentration mechanism (CCM) [47] and reduce the decline in photosynthetic efficiency under unfavourable conditions [40]. Improved production of diverse algal products can reduce costs and by this support CO2 sequestration efforts. From a technical perspective, fulfilling these tasks is essential to establish economically viable and sustainable algae-based CO2 sequestration. These challenges include minimizing water footprint [48], reducing energy consumption and carbon footprint in industrial production – particularly concerning lighting and biomass harvesting [22], and optimizing bioreactor design [49]. Researchers are actively exploring innovative solutions, such as the use of artificial LED lighting for algae cultivation, which allows the implementation of novel light settings like unique light spectra, flashing light, and dynamic light profiles [23]. Integrating bioreactors into CO2-rich sources, such as flue gas, also remains a pivotal focus [50].
In 2021, the city of Belgrade, Serbia, took a significant step by installing a 600-liter solar-powered algae bioreactor in a busy urban area with considerable air pollution. Dubbed “Liquid Tree” is expected to absorb up to 20 kg of CO2 per day, equivalent to the amount absorbed by two 10-year-old trees or 200 square meters of lawn [51]. While this is a prove of concept, it suggests that larger and more powerful algae-based CO2 capture devices could well become a reality in not-too-far future.
5 Applications of machine learning in microalgae research and production
Machine Learning (ML) is a cutting-edge technology that empowers computer algorithms to extract valuable insights from intricate data, uncover hidden patterns, and deliver precise predictions. The Machine Learning Revolution, which gained momentum in the 2010s, has reshaped countless industries and sparked innovation across a wide array of applications. In microalgae research, ML technologies are driving innovation, revolutionizing cultivation techniques, and expanding our knowledge of these species.
Machine learning advance greatly benefits from the recent remarkable progress in various so called “omics” disciplines. These deal with the comprehensive study of an organism’s entire set of genes and genetic elements (genomics and epigenomics), gene activities (transcriptomics), protein profiles (proteomics), or the intricate web of metabolic processes (metabolomics, lipidomics, fluxomics). Together, they offer an unprecedented wealth of data that provides valuable comprehensive insights into the biology of an organism [52]. The integration of ML algorithms with “omics” data is truly revolutionizing biology in general and algal research in particular, unlocking significant potential for both fundamental research and industrial applications: expedite the identification of new species [53], enhancing the selection of promising cell lines for diverse production purposes [54], and rapidly discovering biomarkers linked to desired traits, such as robust growth or high production yield [55]. Furthermore, it opens doors to unravel novel metabolic pathways and, if combined with precision genome editing techniques like CRISPR-Cas, enables rational and highly precise genetic engineering [56]. ML also empower the development of highly capable predictive models to anticipate the impact of genetic variations and environmental factors on biological systems such as in photobioreactors [57]. This predictive capability is crucial for the industrial production of microalgae, ensuring precise control over cultivation and early detection of stress, disease, or contamination.
ML also has the potential to revolutionize the way we collect biological data. While traditional analytical methods are known for their precision, they are often expensive and time-consuming. To overcome these limitations, researchers are actively exploring innovative alternatives such as remote sensing and non-invasive or minimally invasive sensors. These methods collect parameter that are technically easy to measure, such as reflectance [58], fluorescence [59], or electrical signals [60], and use this data to predict critical biological information. In this context, ML plays a key role by leveraging its ability to analyse large data sets and uncover meaningful patterns and trends that would be difficult for humans to detect. Moreover, the wealth of data generated by these simple measurement techniques enhances the accuracy and resilience of ML predictions. This synergy between innovative data collection methods and ML provides cost-effective and efficient solutions for biological and physiological analysis. Ultimately, it significantly improves both the quantity and quality of biological data and promises to have a transformative impact on the field.
Soon, we can anticipate the emergence of fully automated real-time monitoring and control systems for microalgae cultivation, all because of the power of ML (Figure 5). These systems are set to revolutionize the way we foster the microscopic powerhouse microalgae. Operating as closed-loop ecosystems, ML-devices continuously gather data from an array of sensors, ranging from temperature, pH, and nutrient levels to various vital physiological parameters of microalgae. These data are analysed by ML algorithms, provide immediate insights into the growth and overall physiological status of microalgae. Upon the detection of any irregularities or less-than-ideal conditions, these automated control systems swiftly and seamlessly adjust variables such as lighting, temperature, and nutrient dosing to create the perfect environmental conditions. Real-time monitoring and control approach asserts the ability to identify issues before they escalate into serious problems, securing the steadfast stability and reliability of microalgae production. As we look ahead, it becomes evident that these systems will be an indispensable element in the field of large-scale, commercial cultivation endeavours.

An innovative concept for a fully automated real-time monitoring and control system for microalgae cultivation. Sensor data (1) is continuously collected and analysed using machine learning algorithms (2) to determine parameters related to growth conditions and cell physiology (3). This information is then used by an AI-driven central control unit (4) to make real-time adjustments (5) to ensure effective microalgae growth and productivity.
6 Conclusion and outlook
Microalgae are truly fascinating organisms. At first glance, their potential seems boundless: they grow rapidly and efficiently, offer a diverse array of valuable products and applications, and even have the power to combat climate change by capturing CO2 from atmosphere. Yet, considering today’s global microalgae production, the total value remains small with a focus predominantly on the food and feed industries. Algae-derived pharmaceuticals and nutraceuticals represent only a fraction of the market, while promising sectors like biofuels and carbon sequestration have not yet found commercial success [61]. The primary roadblock seems the high investment and production costs [62].
Back in the 1950s and 1960s, there was another wave of excitement about microalgae, especially regarding their potential as a future food source. However, various challenges in the production processes, combined with the significant advancements in traditional agriculture thanks to new technologies, hindered the widespread adoption of algae production [63]. Microalgae might forever remain a niche market unless the persistent production challenges can be addressed. Fortunately, we are currently witnessing a transformative shift in technologies. Modern techniques, including genetic and metabolic engineering, machine learning, and more, promise to revolutionize microalgae production and finally unlock their full potential. This underscores the need for robust support frameworks from governments, research institutes and international organisations to foster extensive multidisciplinary collaboration across different scientific fields. Only through such collaborative efforts we can ensure the effective integration of microalgae as an essential element of sustainable solutions, thereby securing a resilient and sustainable future for our global community.
About the authors

Simon Greulich is currently a PhD candidate, having successfully completed an MSc in Biomolecular Engineering in 2021. His previous work has focused on the production of carotenoids in cyanobacteria. During his graduate studies, he worked in the field of bioreactor design and optimisation.

Dr. Nam Trung Tran completed his PhD in 2019 with a thesis on Metabolic Engineering of microalgae for the production of valuable carotenoids. Following his doctoral studies, he has been exploring the effects of various light spectra on plant physiology. He has a keen interest in leveraging Machine Learning technology to address biological research inquiries.

Prof. Ralf Kaldenhoff Career History: Since 2003 Professor for Botany, Applied Plant Science, Technische Universität Darmstadt, Germany. Earlier appointments: Professor, Molecular Plant Physiology, University Würzburg, Employee University Hannover, Fellowship Max-Planck-Society, Berlin and Yamada Science Foundation, Osaka, Japan. Guest scientist at Ohio State University, Columbus, USA.
Acknowledgments
We would like to thank Professor Jürgen Adamy, Professor Heinz Köppl and Professor Georg Bretthauer for the invitation to write this review.
-
Research ethics: Not applicable.
-
Author contributions: Conceptualization N.T.T, S.G, R.K. Manuscript writing: N.T.T, S.G. Proofreading: N.T.T, S.G, R.K. All authors accept responsibility for the entire content of this manuscript and approve its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
[1] Y. K. Dasan, M. K. Lam, S. Yusup, J. W. Lim, and K. T. Lee, “Life cycle evaluation of microalgae biofuels production: effect of cultivation system on energy, carbon emission and cost balance analysis,” Sci. Total Environ., vol. 688, no. 14, pp. 112–128, 2019. https://doi.org/10.1016/j.scitotenv.2019.06.181.Suche in Google Scholar PubMed
[2] M. Kumar, et al.., “Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges,” Sci. Total Environ., vol. 716, no. 89, p. 137116, 2020. https://doi.org/10.1016/j.scitotenv.2020.137116.Suche in Google Scholar PubMed
[3] D. L. Medeiros, E. A. Sales, and A. Kiperstok, “Energy production from microalgae biomass: carbon footprint and energy balance,” J. Cleaner Prod., vol. 96, no. 48, pp. 493–500, 2015. https://doi.org/10.1016/j.jclepro.2014.07.038.Suche in Google Scholar
[4] K. Heimann and R. Huerlimann, “Microalgal classification,” in Handbook of Marine Microalgae, 1st ed. Amsterdam, Netherlands, Elsevier, 2015, pp. 25–41.10.1016/B978-0-12-800776-1.00003-0Suche in Google Scholar
[5] J. Rumin, E. Nicolau, R. G. D. O. Junior, C. Fuentes-Grünewald, and L. Picot, “Analysis of scientific research driving microalgae market opportunities in Europe,” Mar. Drugs, vol. 18, no. 5, p. 264, 2020. https://doi.org/10.3390/md18050264.Suche in Google Scholar PubMed PubMed Central
[6] J. Singh and R. C. Saxena, “An introduction to microalgae,” in Handbook of Marine Microalgae, Amsterdam, Netherlands, Elsevier, 2015, pp. 11–24.10.1016/B978-0-12-800776-1.00002-9Suche in Google Scholar
[7] S. Maity and N. Mallick, “Trends and advances in sustainable bioethanol production by marine microalgae: a critical review,” J. Cleaner Prod., vol. 345, no. 54, p. 131153, 2022. https://doi.org/10.1016/j.jclepro.2022.131153.Suche in Google Scholar
[8] G. M. Hallegraeff, “Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge,” J. Phycol., vol. 46, no. 2, pp. 220–235, 2010. https://doi.org/10.1111/j.1529-8817.2010.00815.x.Suche in Google Scholar
[9] P. Spolaore, C. Joannis-Cassan, E. Duran, and A. Isambert, “Commercial applications of microalgae,” J. Biosci. Bioeng., vol. 101, no. 2, pp. 87–96, 2006. https://doi.org/10.1263/jbb.101.87.Suche in Google Scholar PubMed
[10] T. Mutanda, D. Naidoo, J. K. Bwapwa, and A. Anandraj, “Biotechnological applications of microalgal oleaginous compounds: current trends on microalgal bioprocessing of products,” Front. Energy Res., vol. 8, 2020, Art. no. 598803. https://doi.org/10.3389/fenrg.2020.598803.Suche in Google Scholar
[11] R. Harun, M. Singh, G. M. Forde, and M. K. Danquah, “Bioprocess engineering of microalgae to produce a variety of consumer products,” Renewable Sustainable Energy Rev., vol. 14, no. 3, pp. 1037–1047, 2010. https://doi.org/10.1016/j.rser.2009.11.004.Suche in Google Scholar
[12] F. G. Acién, J. M. Fernández, J. J. Magán, and E. Molina, “Production cost of a real microalgae production plant and strategies to reduce it,” Biotechnol. Adv., vol. 30, no. 6, pp. 1344–1353, 2012. https://doi.org/10.1016/j.biotechadv.2012.02.005.Suche in Google Scholar PubMed
[13] A. K. Patel, et al.., “Recent advancements in astaxanthin production from microalgae: a review,” Bioresour. Technol., vol. 364, no. 74, p. 128030, 2022. https://doi.org/10.1016/j.biortech.2022.128030.Suche in Google Scholar PubMed
[14] S. Jagadevan, et al.., “Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production,” Biotechnol. Biofuels, vol. 11, no. 185, p. 185, 2018. https://doi.org/10.1186/s13068-018-1181-1.Suche in Google Scholar PubMed PubMed Central
[15] C. Viegas, L. Gouveia, and M. Gonçalves, “Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy,” J. Environ. Manage., vol. 286, no. 4, p. 112187, 2021. https://doi.org/10.1016/j.jenvman.2021.112187.Suche in Google Scholar PubMed
[16] F. Wollmann, et al.., “Microalgae wastewater treatment: biological and technological approaches,” Eng. Life Sci., vol. 19, no. 12, pp. 860–871, 2019. https://doi.org/10.1002/elsc.201900071.Suche in Google Scholar PubMed PubMed Central
[17] W. Zhou, et al.., “Bio-mitigation of carbon dioxide using microalgal systems: advances and perspectives,” Renewable Sustainable Energy Rev., vol. 76, no. 82, pp. 1163–1175, 2017. https://doi.org/10.1016/j.rser.2017.03.065.Suche in Google Scholar
[18] M. Mofijur, S. M. Ashrafur Rahman, L. N. Nguyen, T. M. I. Mahlia, and L. D. Nghiem, “Selection of microalgae strains for sustainable production of aviation biofuel,” Bioresour. Technol., vol. 345, no. 58, p. 126408, 2022. https://doi.org/10.1016/j.biortech.2021.126408.Suche in Google Scholar PubMed
[19] A. Udayan, R. Sirohi, N. Sreekumar, B.-I. Sang, and S. J. Sim, “Mass cultivation and harvesting of microalgal biomass: current trends and future perspectives,” Bioresour. Technol., vol. 344, no. Pt B, p. 126406, 2022. https://doi.org/10.1016/j.biortech.2021.126406.Suche in Google Scholar PubMed
[20] J. Legrand, A. Artu, and J. Pruvost, “A review on photobioreactor design and modelling for microalgae production,” React. Chem. Eng., vol. 6, no. 7, pp. 1134–1151, 2021. https://doi.org/10.1039/d0re00450b.Suche in Google Scholar
[21] R. Sirohi, et al.., “Design and applications of photobioreactors- a review,” Bioresour. Technol., vol. 349, no. 4, p. 126858, 2022. https://doi.org/10.1016/j.biortech.2022.126858.Suche in Google Scholar PubMed
[22] W. Blanken, M. Cuaresma, R. H. Wijffels, and M. Janssen, “Cultivation of microalgae on artificial light comes at a cost,” Algal Res., vol. 2, no. 4, pp. 333–340, 2013. https://doi.org/10.1016/j.algal.2013.09.004.Suche in Google Scholar
[23] M. Glemser, et al.., “Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives,” Appl. Microbiol. Biotechnol., vol. 100, no. 3, pp. 1077–1088, 2015. https://doi.org/10.1007/s00253-015-7144-6.Suche in Google Scholar PubMed
[24] B. R. D. A. Moreira, Y. A. Frias, E. W. de Lima, V. H. Cruz, P. R. M. Lopes, and R. D. S. Viana, “Algae-specific colorful LEDs: biotechnological drivers to biorefinery and photobiological platforms,” J. Cleaner Prod., vol. 316, no. 91, p. 128350, 2021. https://doi.org/10.1016/j.jclepro.2021.128350.Suche in Google Scholar
[25] P. S. C. Schulze, R. Guerra, H. Pereira, L. M. Schüler, and J. C. S. Varela, “Flashing LEDs for microalgal production,” Trends Biotechnol., vol. 35, no. 11, pp. 1088–1101, 2017. https://doi.org/10.1016/j.tibtech.2017.07.011.Suche in Google Scholar PubMed
[26] D. L. Sutherland, J. Burke, and P. J. Ralph, “High-throughput screening for heterotrophic growth in microalgae using the Biolog Plate assay,” New Biotechnol., vol. 65, no. 6, pp. 61–68, 2021. https://doi.org/10.1016/j.nbt.2021.08.001.Suche in Google Scholar PubMed
[27] N. K. Kang, K. Baek, H. G. Koh, C. A. Atkinson, D. R. Ort, and Y.-S. Jin, “Microalgal metabolic engineering strategies for the production of fuels and chemicals,” Bioresour. Technol., vol. 345, no. 15, p. 126529, 2022. https://doi.org/10.1016/j.biortech.2021.126529.Suche in Google Scholar PubMed
[28] N. T. Tran and R. Kaldenhoff, “Achievements and challenges of genetic engineering of the model green alga Chlamydomonas reinhardtii,” Algal Res., vol. 50, no. 9, p. 101986, 2020. https://doi.org/10.1016/j.algal.2020.101986.Suche in Google Scholar
[29] F. Fasaei, J. H. Bitter, P. M. Slegers, and A. J. B. van Boxtel, “Techno-economic evaluation of microalgae harvesting and dewatering systems,” Algal Res., vol. 31, no. 39, pp. 347–362, 2018. https://doi.org/10.1016/j.algal.2017.11.038.Suche in Google Scholar
[30] Z. Zhao, J. Blockx, K. Muylaert, W. Thielemans, A. Szymczyk, and I. F. J. Vankelecom, “Exploiting flocculation and membrane filtration synergies for highly energy-efficient, high-yield microalgae harvesting,” Sep. Purif. Technol., vol. 296, no. 36, p. 121386, 2022. https://doi.org/10.1016/j.seppur.2022.121386.Suche in Google Scholar
[31] L. Mennella, et al.., “Perspectives and challenges of small scale plant microalgae cultivation. Evidences from Southern Italy,” Algal Res., vol. 45, no. 47, p. 101693, 2020. https://doi.org/10.1016/j.algal.2019.101693.Suche in Google Scholar
[32] J. C. Varela, H. Pereira, M. Vila, and R. León, “Production of carotenoids by microalgae: achievements and challenges,” Photosynth. Res., vol. 125, no. 3, pp. 423–436, 2015. https://doi.org/10.1007/s11120-015-0149-2.Suche in Google Scholar PubMed
[33] F. Nabi, et al.., “Health benefits of carotenoids and potential application in poultry industry: a review,” J. Anim. Physiol. Anim. Nutr., vol. 104, no. 6, pp. 1809–1818, 2020. https://doi.org/10.1111/jpn.13375.Suche in Google Scholar PubMed
[34] B. Stachowiak and P. Szulc, “Astaxanthin for the food industry,” Molecules, vol. 26, no. 9, p. 2666, 2021. https://doi.org/10.3390/molecules26092666.Suche in Google Scholar PubMed PubMed Central
[35] X. Wan, et al.., “Reprogramming microorganisms for the biosynthesis of astaxanthin via metabolic engineering,” Prog. Lipid Res., vol. 81, no. 5, p. 101083, 2021. https://doi.org/10.1016/j.plipres.2020.101083.Suche in Google Scholar PubMed
[36] Y. Liu, Y. Cui, J. Chen, S. Qin, and G. Chen, “Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin,” Algal Res., vol. 44, no. 10, p. 101679, 2019. https://doi.org/10.1016/j.algal.2019.101679.Suche in Google Scholar
[37] N. Shimada, Y. Okuda, K. Maeda, D. Umeno, S. Takaichi, and M. Ikeuchi, “Astaxanthin production in a model cyanobacterium Synechocystis sp. PCC 6803,” J. Gen. Appl. Microbiol., vol. 66, no. 2, pp. 116–120, 2020. https://doi.org/10.2323/jgam.2020.01.003.Suche in Google Scholar PubMed
[38] S. Amendola, et al.., “Metabolic engineering for efficient ketocarotenoid accumulation in the green microalga Chlamydomonas reinhardtii,” ACS Synth. Biol., vol. 12, no. 3, pp. 820–831, 2023. https://doi.org/10.1021/acssynbio.2c00616.Suche in Google Scholar PubMed
[39] G. Simon and R. Kaldenhoff, “Astaxanthin synthesis in synechococcus pcc7002: optimization and productsecretion to culture medium,” Int. J. Adv. Res., vol. 12, no. 32, pp. 255–263, 2024. https://doi.org/10.21474/IJAR01/18390.Suche in Google Scholar
[40] S. Solomon, G.-K. Plattner, R. Knutti, and P. Friedlingstein, “Irreversible climate change due to carbon dioxide emissions,” Proc. Natl. Acad. Sci. U. S. A., vol. 106, no. 6, pp. 1704–1709, 2009. https://doi.org/10.1073/pnas.0812721106.Suche in Google Scholar PubMed PubMed Central
[41] Sixth Assessment Report, Intergovernmental Panel on Climate Change (IPCC) – 20 March 2023.Suche in Google Scholar
[42] Climate Act (423/2022), Finish Ministry of the Environment.Suche in Google Scholar
[43] G. D. Price, “Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism,” Photosynth. Res., vol. 109, nos. 1–3, pp. 47–57, 2011. https://doi.org/10.1007/s11120-010-9608-y.Suche in Google Scholar PubMed
[44] P. G. Falkowski, “The role of phytoplankton photosynthesis in global biogeochemical cycles,” Photosynth. Res., vol. 39, no. 3, pp. 235–258, 1994. https://doi.org/10.1007/bf00014586.Suche in Google Scholar
[45] M. A. Borowitzka, “High-value products from microalgae—their development and commercialisation,” J. Appl. Phycol., vol. 25, no. 3, pp. 743–756, 2013. https://doi.org/10.1007/s10811-013-9983-9.Suche in Google Scholar
[46] V. E. J. Jassey, et al.., “Contribution of soil algae to the global carbon cycle,” New Phytol., vol. 234, no. 1, pp. 64–76, 2022. https://doi.org/10.1111/nph.17950.Suche in Google Scholar PubMed
[47] S. Negi, et al.., “Light regulation of light‐harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae,” Plant J., vol. 103, no. 2, pp. 584–603, 2020. https://doi.org/10.1111/tpj.14751.Suche in Google Scholar PubMed
[48] E. Nogueira Junior, M. Kumar, S. Pankratz, A. O. Oyedun, and A. Kumar, “Development of life cycle water footprints for the production of fuels and chemicals from algae biomass,” Water Res., vol. 140, no. 31, pp. 311–322, 2018. https://doi.org/10.1016/j.watres.2018.04.046.Suche in Google Scholar PubMed
[49] R. N. Singh and S. Sharma, “Development of suitable photobioreactor for algae production – a review,” Renewable Sustainable Energy Rev., vol. 16, no. 4, pp. 2347–2353, 2012. https://doi.org/10.1016/j.rser.2012.01.026.Suche in Google Scholar
[50] G. Vunjak-Novakovic, Y. Kim, X. Wu, I. Berzin, and J. C. Merchuk, “Air-lift bioreactors for algal growth on flue gas: mathematical modeling and pilot-plant studies,” Ind. Eng. Chem. Res., vol. 44, no. 16, pp. 6154–6163, 2005. https://doi.org/10.1021/ie049099z.Suche in Google Scholar
[51] 2021. Available at: https://www.euronews.com/2021/12/07/this-liquid-tree-in-belgrade-is-fighting-back-against-air-pollution.Suche in Google Scholar
[52] I. Subramanian, S. Verma, S. Kumar, A. Jere, and K. Anamika, “Multi-omics data integration, interpretation, and its application,” Bioinf. Biol. Insights, vol. 14, 2020, Art. no. 117793221989905. https://doi.org/10.1177/1177932219899051.Suche in Google Scholar PubMed PubMed Central
[53] X. Lin, “The application of transcriptomics, metagenomics, and metatranscriptomics in algal research,” in Research Methods of Environmental Physiology in Aquatic Sciences, Singapore, Springer, 2020, pp. 285–291.10.1007/978-981-15-5354-7_34Suche in Google Scholar
[54] A. Coşgun, M. E. Günay, and R. Yıldırım, “Exploring the critical factors of algal biomass and lipid production for renewable fuel production by machine learning,” Renewable Energy, vol. 163, no. 87, pp. 1299–1317, 2021. https://doi.org/10.1016/j.renene.2020.09.034.Suche in Google Scholar
[55] F. V. Winck, D. O. P. Melo, D. M. Riaño-Pachón, M. C. M. Martins, C. Caldana, and A. F. G. Barrios, “Analysis of sensitive CO2 pathways and genes related to carbon uptake and accumulation in Chlamydomonas reinhardtii through genomic scale modeling and experimental validation,” Front. Plant Sci., vol. 7, 2016, Art. no. 43. https://doi.org/10.3389/fpls.2016.00043.Suche in Google Scholar PubMed PubMed Central
[56] S. Y. Teng, G. Y. Yew, K. Sukačová, P. L. Show, V. Máša, and J.-S. Chang, “Microalgae with artificial intelligence: a digitalized perspective on genetics, systems and products,” Biotechnol. Adv., vol. 44, no. 15, p. 107631, 2020. https://doi.org/10.1016/j.biotechadv.2020.107631.Suche in Google Scholar PubMed
[57] R. SupriyantoNoguchi, et al.., “Artificial neural networks model for estimating growth of polyculture microalgae in an open raceway pond,” Biosyst. Eng., vol. 177, no. 12, pp. 122–129, 2019. https://doi.org/10.1016/j.biosystemseng.2018.10.002.Suche in Google Scholar
[58] T. Q. Vinh, et al.., “Light reflection spectra as a tool for direct and real-time determination of biomass and pigments in the microalgae Microchloropsis salina,” Lighting Res. Technol., vol. 53, no. 2, pp. 171–184, 2020. https://doi.org/10.1177/1477153520958455.Suche in Google Scholar
[59] J. L. Deglint, C. Jin, A. Chao, and A. Wong, “The feasibility of automated identification of six algae types using feed-forward neural networks and fluorescence-based spectral-morphological features,” IEEE Access, vol. 7, pp. 7041–7053, 2019. https://doi.org/10.1109/access.2018.2889017.Suche in Google Scholar
[60] E.-H. Lee, S.-W. Lee, and R. F. Saraf, “Noninvasive measurement of membrane potential modulation in microorganisms: photosynthesis in green algae,” ACS Nano, vol. 8, no. 1, pp. 780–786, 2013. https://doi.org/10.1021/nn405437z.Suche in Google Scholar PubMed
[61] Business Growth Reports, Algae Products Global Market Report, Hyderabad, India, The Business Research Company, 2023.Suche in Google Scholar
[62] S. A. Scott, et al.., “Biodiesel from algae: challenges and prospects,” Curr. Opin. Biotechnol., vol. 21, no. 3, pp. 277–286, 2010. https://doi.org/10.1016/j.copbio.2010.03.005.Suche in Google Scholar PubMed
[63] W. Belasco, “Algae burgers for a hungry world? The rise and fall of chlorella cuisine,” Technol. Cult., vol. 38, no. 3, p. 608, 1997. https://doi.org/10.2307/3106856.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Survey
- Biological engineering – an engineering discipline crucial to the future of our civilization
- Forum
- New biological solutions to the many problems of our time
- Survey
- Biological engineering as a driver of innovation: implications for the economy
- Advancing vertical farming with automation for sustainable food production
- Harnessing microalgae: from biology to innovation in sustainable solutions
- Generation of molecular hydrogen (H2) by microalgae and their biocatalysts
- Biocatalytic approaches for plastic recycling
- Engineered living materials: pushing the boundaries of materials sciences through biological engineering
- The fabrication-assembly challenge in tissue engineering
- Evolution of biofabrication and 3D-bioprinting technologies – from market pull to technology push
- A bio-engineering approach to generate bioinspired (spider) silk protein-based materials
- RNA aptamers: promising tools in synthetic biology
- Automated handling of biological objects with a flexible gripper for biodiversity research
- Building with renewable materials
- Growing new types of building materials: mycelium-based composite materials
- Façade greening – from science to school
Artikel in diesem Heft
- Frontmatter
- Survey
- Biological engineering – an engineering discipline crucial to the future of our civilization
- Forum
- New biological solutions to the many problems of our time
- Survey
- Biological engineering as a driver of innovation: implications for the economy
- Advancing vertical farming with automation for sustainable food production
- Harnessing microalgae: from biology to innovation in sustainable solutions
- Generation of molecular hydrogen (H2) by microalgae and their biocatalysts
- Biocatalytic approaches for plastic recycling
- Engineered living materials: pushing the boundaries of materials sciences through biological engineering
- The fabrication-assembly challenge in tissue engineering
- Evolution of biofabrication and 3D-bioprinting technologies – from market pull to technology push
- A bio-engineering approach to generate bioinspired (spider) silk protein-based materials
- RNA aptamers: promising tools in synthetic biology
- Automated handling of biological objects with a flexible gripper for biodiversity research
- Building with renewable materials
- Growing new types of building materials: mycelium-based composite materials
- Façade greening – from science to school

