When touch predicts pain: predictive tactile cues modulate perceived intensity of painful stimulation independent of expectancy
-
Daniel S. Harvie
Abstract
Aims
Non-nociceptive somatosensory input, such as tactile or proprioceptive information, alway precedes nociceptive input during a painful event. This relationship provides clear opportunities fo predictive associative learning, which may shape future painful experiences. In this differential classica conditioning study we tested whether pain-associated tactile cues (conditioned stimuli; CS) could altei the perceived intensity of painful stimulation, and whether this depends on duration of the CS—seeing that CS duration might allow or prevent conscious expectation.
Methods
Subjects underwent a classical differential conditioning task in which a tactile cue at locatior A (CS+) preceded painful electrical stimulation at location B (UShigh), whereas a tactile cue at location C (CS–) preceded non-painful electrical stimulation at location B (USlow). At test, we compared the pain evoked by a moderately painful stimulus (USmed) when preceded by either the CS+ or CS–. CS duration was manipulated between subjects. Participants were assigned to one of three groups: Long CS (4s, allowing conscious expectation), Short CS (110 ms) and CS-US indistinguishable (20 ms), preventing conscious expectation). We hypothesised that more pain would be evoked by the US when preceded by the CS+ relative to the CS-, and that the effect would be independent of CS duration.
Results
Fifty-four healthy participants (31 females, age = 26, SD = 9) were included in the analysis. The hypotheses were supported in that more intense pain was evoked by the USmed when paired with the tactile CS+, than when paired with the tactile CS-; mean difference 3 mm on a 150 mm VAS (C 0.4-4.8 mm). CS duration did not moderate the effect. The effect was greater in those participants where calibration was optimal, as indicated by a relatively more painful UShigh.
Conclusions
We conclude that pain-associated tactile cues can influence pain, and that this effect i: not dependent on stimulus duration. This suggests that explicit expectation is not a requirement for predictive cues to modulate pain. That the presence of the CS+ resulted in only a 5.3% higher intensity rating compared with the CS- may reflect a limitation of laboratory studies, where a limited number o trials, an artificial context and the use of experimental pain are likely to reveal only glimpses of what i: clinically possible.
Implications
Pain-associated visual and auditory cues have been shown to enhance pain in laboratory and clinical scenarios, supposedly by influencing expectation of impending harm. We show that pain-associated somatosensory cues can also modulate pain and that this can occur independently of expectation. This points to a larger potential role for associative learning in the development and treatment of pain than has previously been considered. We suggest that research into associative mechanisms underpinning pain, as distinct from those that link pain to pain-related fear and avoidance, is worthwhile.
1 Introduction
Although classical conditioning has long been implicated in the development of pain-related fear and avoidance behaviour in people with chronic pain (see [1]), the possibility of a meaningful role for classical conditioning in the sensory features of chronic pain is less recognised. Nociceptors have higher thresholds and slower transmission than tactile and proprioceptive neurons [2]. Therefore, in physiological situations, nociception is always preceded by non-nociceptive input, providing clear opportunities for associative learning. Sensitisation, a non-associative neural adaptation, enhances synaptic efficiency for both tactile and nociceptive afferents where they synapse with higher order nociceptors [2]. A recent proposal suggests that associative learning may also contribute to the clinical features of sensitisation—hyperalgesia and allodynia, and the development of chronic pain [3]. According to classical conditioning theory, after repeated pairing of a non-nociceptive stimulus (conditioned stimulus; CS) and a nociceptive stimulus (unconditioned stimulus; US), the CS might come to elicit responses that are intrinsically associated with the US—potentially enabling the CS to cause or contribute to pain as a conditioned response (CR) [3]. Indeed pairing a pain-associated cue with nociceptive stimulation seems to facilitate activity in regions responsible for nociceptive processing [4,5,6,7].
A small number of studies have shown that, after a classical conditioning procedure, a nociceptive stimulus is more painful if it is preceded by a pain-associated cue [4,6,7,8]. However, so far, these studies have employed mainly visual and auditory cues and overlooked the somatosensory system, which might be a more functionally and physiologically relevant source of CSs. Preparedness and belongingness theories denote that some stimuli are more easily associated, and more resistant to extinction, than others [9,10,11]. These theories predict that distinct stimuli may have distinct associability because, as a product of evolution, we are efficient at making certain types of associations, and because we learn that certain stimuli have greater ‘belongingess’ [9,10]. Moreover, tactile pathways have a close anatomical relationship with nociceptive pathways including synaptic convergence [12].This affords the tactile system unique opportunities to influence the nociceptive system—as demonstrated by tactile allodynia [13].
Investigations of the influence of pain-associated cues on pain have generally assumed that any effect would be driven by conscious expectation. This is unsurprising—many learning theorists consider that conditioned responding is dependent on high-level cognitive processes, driven by knowledge of the CS-US association [14,15,16]. Others, however, suggest that both conscious and non-conscious processes best account for the evidence [16,15,17]. One investigation of the non-conscious effects of pain-associated cues on nociception-evoked pain reported that the US evoked more pain with CS presented for just 12 ms, 94 ms prior to the US—a time window that precludes conscious processing of the CS, making conscious expectation of the nociceptive signal unlikely [6,8]. That work demonstrated that the effects of associative learning on pain are not always dependent on conscious processes.
We investigated whether tactile CSs could modulate pain, and whether their effect depends on sufficiently delay between stimuli to allow for conscious expectation. We hypothesised that pain evoked by a standard nociceptive stimulus would be greater if it was preceded by a tactile CS+ that predicts very painful stimulation than if it was preceded by a tactile CS-that does not, and that the differential effect would not depend on the CS duration.
2 Methods
2.1 Participants
The study was advertised using flyers, social media (www.bodyinmind.org and university channels) and via word-of-mouth. Participants were reimbursed AU$20 for their participation. Participants were excluded if they reported current pain, or any psychological or neurological impairment. Participants were aware that the study was investigating the relationship between pain and touch but were not informed about the experimental stimulus contingencies or the hypothesis. Sample size was chosen to ensure adequate (80%) power to detect a small to medium effect. Fifty- eight healthy, pain-free individuals (33 females, mean age = 25 years, SD= 9) volunteered. The study was conducted at the University of South Australia and approved by the institutional ethics committee (Protocol #0000031100).
2.2 Stimulus material
Vibrotactile stimuli (CSs) were delivered by 10 mm, 3 V, off- centre, electric motors (tactors). The CS+ and CS-tactors were set to vibrate at the same, easily detectable, non-painful intensity, at 220 Hz. Tactors were not placed over superficial bone. The intensity of the tactors was calibrated on a pilot sample and location of the CS+ and CS-tactors (proximal or distal forearm) was counterbalanced among participants to eliminate any systematic effect on sensation of the anatomical location of the tactor. The tactors were attached to the skin using a double-sided adhesive sticker.
Electrical stimuli (USs) were delivered using two Digitimer DS700 electrical stimulators (Digitimer Ltd, Welwyn Garden City, UK) as 20 ms bursts with instantaneous rise and fall. The US electrodes were placed on the forearm midway between the two CS tactors, which were 8 cm apart (see Fig. 1). This arrangement was chosen to enable spatially distinct conditioned stimuli while maintaining a close anatomical relationship between each CS and the USs.
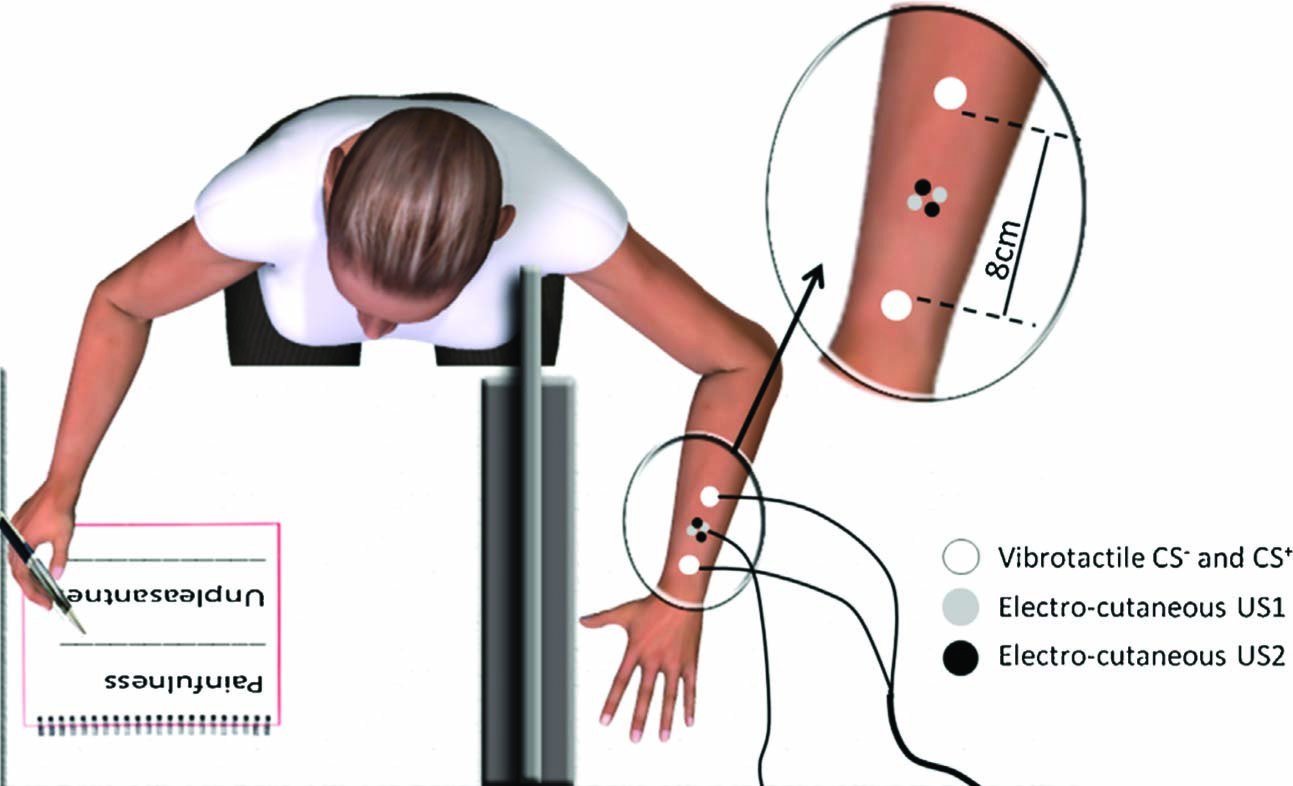
Experimental set-up showing the placement of the tactile and electrocutaneous stimuli. Seated participants rested one arm on a table behind a board, so that it was out of view. Painfulness and unpleasantness of each US were rated using the other hand
The US intensities were individually calibrated, serving as the USlow, USmed and UShigh. Calibration was performed using a staircase method with a single ascending run of 0.1 mA increments. During this procedure, the intensity of the stimulus was rated using a 0-10 numerical rating scale (NRS) where 0 = no sensation at all, 1 = you feel a sensation, but it is not painful, 2 = you feel a strong sensation, but it is not painful, 3 = starts to be painful, but is a very mild pain, 8 = significantly painful and demanding some effort to tolerate, and 10 = the worst imaginable pain. The stimulus intensity (mA) corresponding to ratings of 2, 4 and 8 on this NRS were used as the USlow, USmed (test stimulus) and UShigh, respectively. Participants were free to stop before they reached a rating of 8, but if they did not reach at least 6 for the UShigh, the experiment was discontinued.
2.3 CS duration
Participants were randomised to one of three groups. The only difference between groups was the duration of the CS, which was varied in order to differentiate expectancy and non-expectancy mediated effects (see Fig. 2). In the Long CS group, the onset of the CS+ was 4 s prior to the US. In this condition, any effect could be explained by the participants’ conscious expectation and anticipation of pain. In the Short CS group, the onset of the CS was 110 ms prior to the US. According to Pastor (2004), temporal discrimination of tactile stimuli on the forearm occurs at intervals of greater than 30-50 ms. Therefore, this timing enabled temporal differentiation of the CS and US, yet did not provide sufficient time for participants to deduce and anticipate the outcome. That is, the duration of the CS was less than that which would enable it to be recognised and analysed with respect to past experience, a process that would be required before an outcome could be consciously predicted (common estimates of pre-conscious processing are in the order of 250 ms [18]). In the CS-US Indistinguishable group, the onset of the CS was only 20 ms prior to the US. In this condition, not only could the US not be consciously predicted on the basis of the CS, the two stimuli could not even be temporally differentiated. As can be seen in Fig. 2, the CS tactors overlapped with the 20 ms US (delay conditioning), probably more closely mimicking the clinical relationship between somatosensory and nociceptive input.
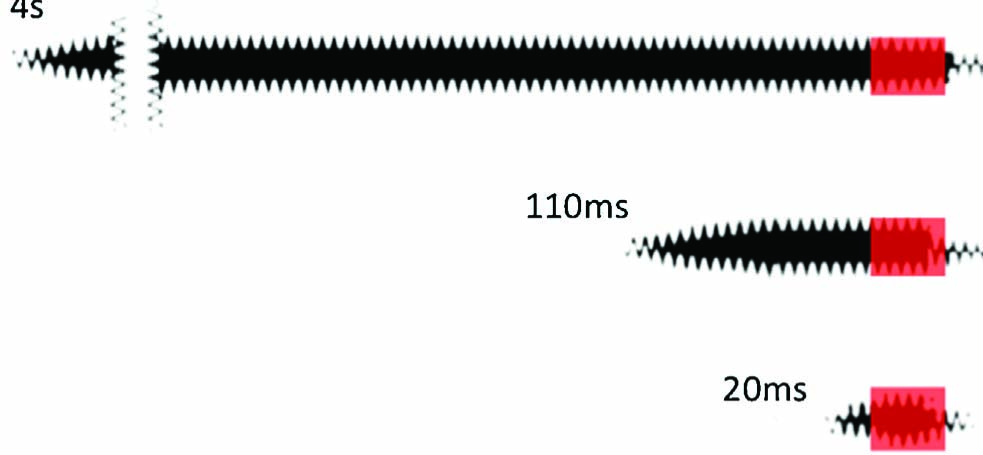
Duration and timing of the vibration and electrical stimuli in the three CS duration groups. The black bar represents the vibration and the red bar represents the 20 ms electrocutaneous stimulus.
2.4 Experimental design
We used a differential classical conditioning design with stimulus type (CS+ vs. CS-) as the within-subjects factor, and CS duration (4 s, 110 ms or 20 ms) as a between-subjects factor.The experiment consisted of two phases: the acquisition and the test phases. During the acquisition phase, a tactile cue at one location on the forearm (CS+) was always followed by a high intensity electrical stimulus (UShigh), and a tactile cue at another location on the forearm (CS-), was always followed by a low intensity electrical stimulus (USlow). During the subsequent test phase, the effects of the CS+ and CS- on pain evoked by a medium intensity painful stimulus (USmed) were determined.
During the acquisition phase, the CS+/UShigh and CS-/USlow pairs were presented 12 times each. During the test phase, the CS-/USmed were paired 10 times, while the CS+ was paired 5 times with the USmed, and 5 times with the UShigh. In this phase, both CSs were paired with the USmed so that their modulating effect on the pain evoked by an identical test stimulus could be determined. We included CS+/UShigh presentations during the test phase as ongoing reinforcement, to prolong differential learning and prevent extinction. Including CS-/USlow stimuli might have assisted this further, but the experimental set-up restricted us to two US intensities per phase. Stimuli were delivered according to three pre-selected, semi-randomised sequences, with the restriction that no stimulus pair could be presented in more than three consecutive trials. Participants were allocated to one of the three sequences according to a pre-randomised order, with each sequence allocated to six people in each group. Each CS-US paired presentation was separated by as inter-trial interval.
2.5 Measurement
During the experiment, participants were asked to rate pain intensity and unpleasantness for each electrical stimulus, immediately after the stimulus presentation, during the inter-trial interval. Participants recorded their pain intensity ratings on a 150 mm visual analogue scale (VAS) where 0 = no pain and 150 mm = worst imaginable pain. Participants similarly recorded their pain unpleasantness ratings on a 150 mm VAS where 0 = not at all unpleasant and 150 mm = most unpleasantness imaginable. Data were extracted and entered by an assessor who was blinded to stimulus type.
2.6 Protocol
After being informed of the broad nature of the study, participants gave signed informed consent. The electrodes and tactors were then attached (see Fig. 1) and calibration of the different US types was initiated. Once the acquisition phase was complete, the intensity of the current stimulator delivering the USlow was increased to the pre-calibrated USmed intensity for the test phase. Thus there were no USlow presentations in the test phase.
2.7 Software and apparatus
The experiment was executed using a custom-built, Arduino™- based module, which controlled the timing and intensity of the two tactors, and triggered the two Digitimer DS700 electrical stimulators via TTL signalling. The pre-randomised stimulus presentation sequences were uploaded to the Arduino module using customised Lab VIEWTM software. Electrodes and tactors were placed on the left arm, which was positioned behind a screen to eliminate visual feedback. Participants wore white-noise-emitting headphones to eliminate auditory feedback and background noise. During the procedure, the experimenter was in a partitioned section of the laboratory and did not interact with the subject.
2.8 Differential conditioning manipulation checks
Because potential limitations in the experiment set-up could influence differential learning, aspects of the set-up were assessed in order that their effect on conditioned responding could be discussed and accounted for by secondary analyses. These potential limitations were: 1. insufficiently distinct USlow and UShigh to enable differential learning, and 2. inability to spatially discriminate between the conditioning stimuli. Furthermore, because evidence suggests that differential conditioning is highly correlated with propositional learning [14,15], awareness of the contingency between tactile cues and painful stimulation was also assessed.
In order to assess whether the USlow and UShigh were sufficiently distinct with respect to quality (non-painful vs. painful) and intensity, a criterion was set to score this feature of calibration as ‘valid’ or ‘not valid’. To be valid, the mean USlow had to be less than 30 mm on the 150 mm VAS, because this was considered to be relatively non-painful, and the mean UShigh had to be at least 30 mm greater than the USlow. Thus, only subjects whose calibration was classified as ‘valid’ were expected to show a meaningful differential effect. In order to ensure that the CSs were spatially distinct, each participant’s ability to discriminate between CSs was confirmed upon completion of the study by stimulating each location, asking participants to identify the locations of the stimuli as the same or different, and to correctly identify each location (proximal or distal).
In order to assess post-experimental propositional knowledge of the CS-US association, participants were exposed to the CS stimuli and asked: “Are you aware which of these stimuli were most often paired with higher pain intensity and, if so, which?” If participants were aware and correctly identified the CS+, they were classified as contingency aware.
2.9 Statistical analysis overview
2.9.1 Primary analysis
In order to test the primary hypotheses—(i) that pain evoked by a nociceptive stimulus would be greater if the nociceptive stimulus was preceded by a tactile cue that predicts painful stimulation than if it was preceded by a tactile cue that does not, and (ii) that this effect does not depend on the duration of the tactile cue the mean pain intensities for the CS+/USmed pair and CS-/USmed were compared within subjects and between groups, using a 3 (Group: CS 4 s vs. CS 110 ms vs. CS 20 ms) × 2 (CS-type: CS+ vs. CS-) mixed ANOVA.
2.9.2 Secondary analyses
In order to test whether the unpleasantness of a nociceptive stimulus would be greater if preceded by a tactile cue predicting painful stimulation, and whether this effect depended on the duration of the tactile cue the mean unpleasantness for the CS+/USmed pair and CS-/USmed were compared within subjects and between groups, using a 3 (Group: CS 4 s vs. CS 110 ms vs. CS 20 ms) × 2 (CS-type: CS+ vs. CS-) mixed ANOVA.
In order to assess whether there was an effect of US intensity (sufficiently low USlow and distinct UShigh), the differences between the mean CS+/USmed and CS-/USmed ratings were compared between groups classified as having had a ‘valid’ or ‘non-valid’ calibration. This was done using a 2 (Validity Group: ‘valid’ vs. ‘non-valid’) × 2 (CS type: CS+ vs. CS-) mixed ANOVA.
In order to assess whether the differential effect related to contingency awareness, the mean pain intensity for the CS+/USmed pair and CS-/USmed were compared within subjects and between groups, using a 2 (Contingency Group: contingency aware vs. unaware) × 2 (CS type: CS+ vs. CS-) mixed ANOVA.
In order to determine whether failure to learn the contingency was related to a general increase in sensitivity, we compared the change in UShigh evoked pain (change = mean test phase UShigh pain intensity—mean acquisition phase UShigh pain intensity) between individuals who were contingency aware vs. contingency unaware, using an independent samples T-test.
Alpha was set at p = 0.05. Effect sizes—partial eta squared η2p-were interpreted using Cohen’s guidelines (0.01= small, 0.059 = medium and 0.138 = large) [34].
3 Results
3.1 Participants
One participant was excluded because of complete loss of electrode conductance. Three participants were excluded because of technical failure. As such, fifty-four healthy, pain-free individuals (31 females, mean age = 26 years, SD = 9) completed the study.
3.2 Stimulus characteristics
The perceived intensities of the electrical stimuli for each phase are shown in Fig. 3. On average, the UShigh was rated more painful that the USlow (p < 0.001). The USmed was successfully delivered at an intensity between the intensities of the UShigh and the USlow. All participants were able to discriminate between the CS locations when individually presented post-experimentally.
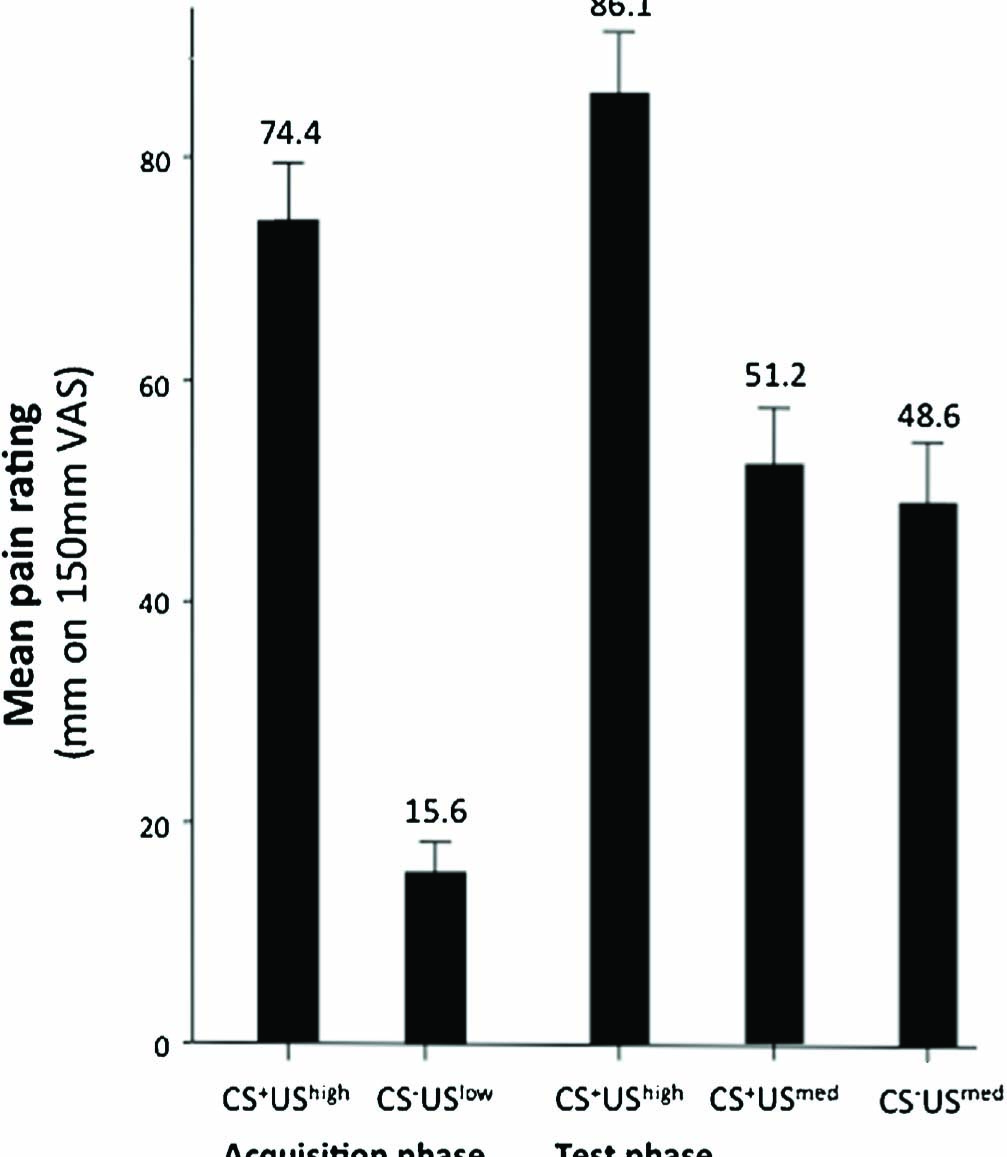
Mean pain intensity ratings for each stimulus type. Intensity ratings measured in millimetres on a 150 mm VAS. Error bars denote SE.
3.3 Testing the primary hypotheses: effect of predictive cues and CS duration on pain intensity
Pain evoked by a moderately painful stimulus was greater when it followed a tactile cue that had predicted a high intensity stimulation during acquisition, than when it followed a tactile cue that had predicted a low intensity stimulus during acquisition. That is, there was a main effect of CS type; F (1, 51) = 8.1, p = .022, η2p = 0.1. The relative duration of the tactile CS did not significantly alter the effect of CS on perceived pain (CS type x Group interaction; F (2, 51) = 0.9, p =0.4, n.s. (Fig. 4, panel 1). Mean (95% CI) difference in pain intensity between the CS+/USmed combination and the CS-/USmed combination was 3 mm (CI 0.4-4.8 mm). Post hoc comparisons examining the effect of predictive cue (CS-type) on pain intensity ratings within each CS duration group did not support the main finding (Holm-Bonferroni corrected p = 0.24,0.26 and 0.09 for the 4 s, 110 ms and 20 ms durations respectively).
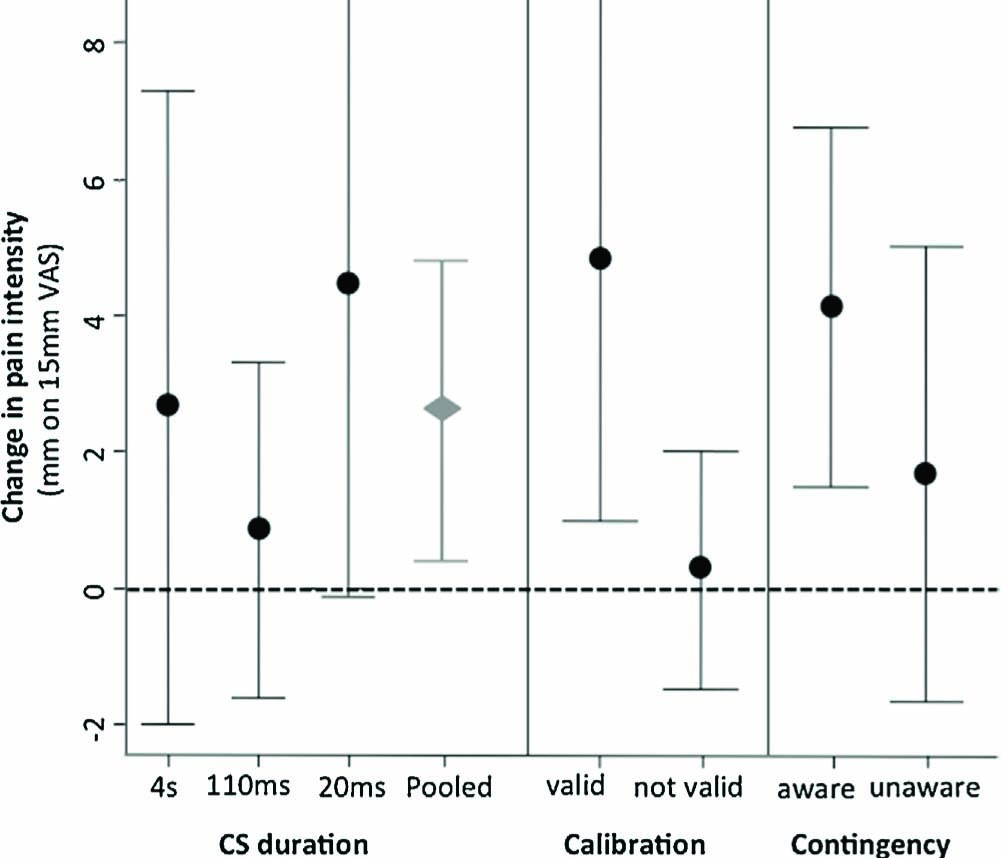
Panel one shows the mean test phase difference between CS+/USmed and CS-/USmed VAS pain ratings (mm) for each CS duration group (4 s, 110 ms and 20 ms) and overall (pooled). The second and third panels show the mean test phase difference between CS+/USmed and CS-/USmed pain ratings (mm) for subgroups where calibration was classified as ‘valid’ or ‘not valid’ and for participants who were aware or not aware of the CS-US contingencies. Error bars denote 95% CI.
3.4 Secondary analyses—unpleasantness
Perceived unpleasantness of the USmed was not significantly different following a tactile cue that had predicted a high intensity stimulation during acquisition, than following a tactile cue that had predicted a low intensity stimulus during acquisition (Fig. 5). That is, there was no main effect of CS type on unpleasantness; F (1, 51) = .83, p = .37. Post hoc comparisons examining the effect of predictive cue (CS-type) on pain unpleasantness ratings within each CS duration group were also non-significance (Holm-Bonferroni corrected p = 0.42,0.42 and 0.14 for the 4 s, 110 ms and 20 ms durations respectively).
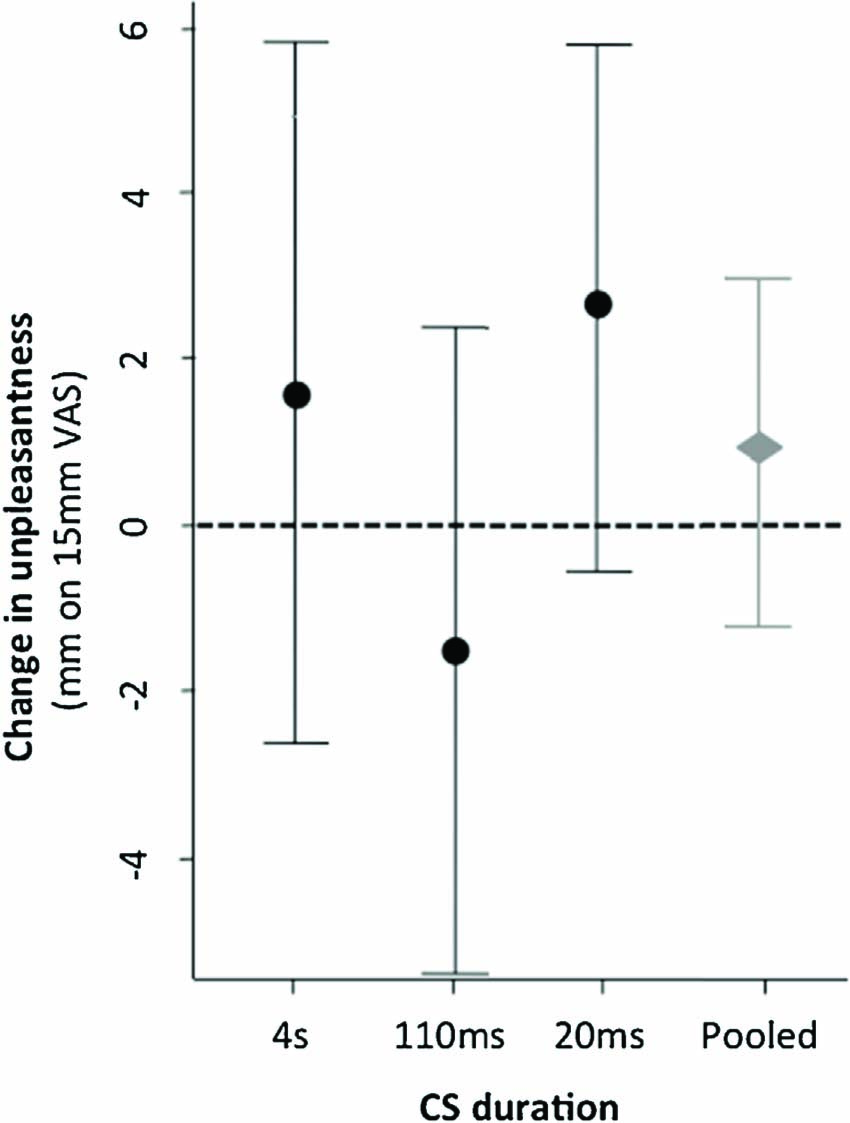
Mean test phase difference between CS+/USmed and CS-/USmed unpleasantness ratings (mm) overall and for each CS duration group. Error bars denote 95% CI.
3.5 Effect of US intensity calibration
Twenty nine of the 54 participants met the criteria for successful calibration (USlow <30 mm, UShigh >30 mm with respect to the USlow) and were classified as valid (see Table 1). The mean CS+/USmed vs. CS-/USmed difference was 4.8 mm (CI 0.9-8.7) in the valid group and 0.2 mm (CI -1.5 to 2) in the non-valid group (Fig. 4, panel 2). Repeated measures ANOVA [2 (CS type: CS+ vs. CS-) × 2 (Validity Group: ‘valid’ vs. ‘non-valid’)] confirmed the interaction between the observed effect and validity of calibration, F (1,1) = 4.7, p = .034, η2p = 0.1. Post hoc t-tests revealed that the ‘valid’ group showed a differential effect, t (27) = 2.6, p = .01, whereas the ‘non- valid’ group did not t (25) = .26, p = .4.
3.6 Relationship between contingency learning and pain
Twenty-two of the 54 participants were able to report the contingency verbally (six, nine and seven people in the 4 s, 110 ms and 20 ms groups respectively). Interestingly, while awareness did not relate to longer CS duration, it seemed to relate distinctiveness of the UShigh and USlow. That is, of the 22 aware participants, were also classified as having a ‘valid’ calibration defined by high distinctiveness of the UShigh and USlow. While the aware participants had, on average, a greater conditioning effect (see Fig. 4, panel 3), the RM ANOVA [2 (CS type: CS+ vs. CS-) × 2 (Contingency Group: ‘aware’ vs. ‘non-aware’)] did not demonstrate an interaction between contingency awareness and stimulus type F (1, 52) = 1.2, p = .28. Interestingly, those who did not become aware of the CS- US contingency experienced a significantly greater increase in pain responses in the test phase with respect to the UShigh (see Fig. 6), t(52) = 2.21, p = .03.
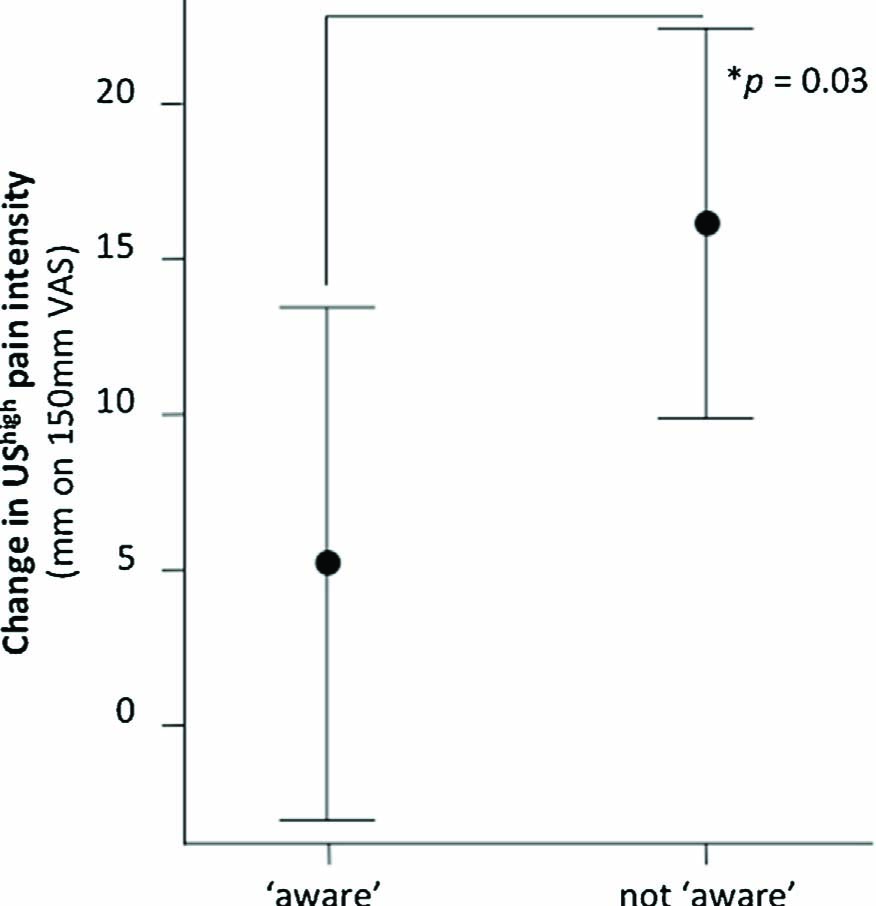
Mean differences between UShigh ratings in acquisition vs. test phase for participants who became aware of the contingencies and those who did not. Error bars denote 95% CI.
4 Discussion
We hypothesised that pain evoked by a nociceptive stimulus would be greater if the nociceptive stimulus was preceded by a tactile cue that predicts painful stimulation than if it was preceded by a tactile cue that does not, and that this effect would not depend on the duration of the tactile stimulus. Our hypotheses were supported, as evidenced by (i) higher pain ratings when the CS+ preceded the moderately painful stimulus than when the CS-did, and (ii) no difference in the effect between groups for which the delay between the predictive cue and the painful stimulus varied from 20 ms to 4 s. We conclude that tactile cues can influence pain independent of an expectancy effect. Given that the tactile cues modulated pain based on learning history, we refer to the effect as ‘classically conditioned modulation of pain intensity’.
4.1 Pain-predicting cues modulate pain intensity independent of expectancy
Previous studies that have also shown that pain can be enhanced by the sensory cues that predict the painful stimulus [4,7,8] have attributed the effect to conscious expectancy. One attempt to isolate the non-conscious effects of pain-associated cues on pain intensity used a paradigm in which one facial expression was used to signal the UShigh and another the USlow [6,8]. In the test phase these faces were presented only 94 ms prior to painful stimulation, for just 12 ms. Because of this short duration, participants were unable to consciously discriminate the CSs, yet the CS+/USmed evoked significantly more pain than the CS–/USmed.
The current study extends that finding to the tactile domain. Here, the effect seen in two of the three groups (where the difference in stimulus onset was just 110 ms and 20 ms) could not reasonably be explained by conscious expectation [18]. Indeed, the lack of significant interaction between learning the contingency and the observed effect also supports that conscious learning was not a critical factor in this study. The magnitude of the effect in this study was smaller than that reported by Jensen et al. [6,8]. That we did not have sufficient power to detect the effect in post hoc tests in individual groups emphasises the small effect. This suggests that tactile cues do not have greater associability with noxious stimuli compared with visual cues such as those used by Jensen et al. [6,8]. That we used fewer acquisition trials (24 pairings rather than 50); and that they used visual stimuli with sociological salience and complexity (pictures of faces), may also explain the differences.
Surprisingly, the statistically supported modulation of pain intensity was not paralleled in the domain of pain unpleasantness. It may be premature, however, to conclude that pain intensity may be preferentially modulated by this procedure; particularly because intensity and unpleasantness results were comparable among two of the three groups.
4.2 Theoretical considerations
The effects can be explained by classical conditioning theory. Most applications of classical conditioning to pain research assign pain as the US and assign a behavioural response consistent with fear as the unconditioned response (UR) (e.g. [19]). However, the view of pain as a response [20] invites us to consider a parallel process, whereby the nociceptive stimulus assumes the role of the US and pain assumes the role of a perceptual UR—which may later be contributed to, or perhaps evoked, by a previously inert CS [3]. The ability of a CS to influence US-related responses is thought to develop through adaptation at synapses where neurons encoding the CS converge with those encoding the US [21].
4.3 Adaptive and maladaptive modulation of pain intensity
Like nociception, innocuous pain-associated stimuli signal bodily danger. Where this suggestion of danger is accurate and proportionately enhances pain, the effect might be adaptive, and subserve protection. Where the effect persists, is disproportionate or not specific to the cues truly predicting danger, the effect may constitute maladaptation, and contribute to pain states. The potential for, and determinants of, these maladaptations may be specifically considered in future studies. Notably, the UShigh increased in painfulness between acquisition and test phases by a much greater amount in participants who did not learn the contingency than those who did not (VAS change 16 mm vs. 5 mm, see Fig. 6). That contingency naive participants experienced greater increases in pain and had reduced differential (CS+ vs. CS-) conditioning suggests that they experienced a general rather than CS+-specific increase in pain sensitivity in response to classical conditioning. This corroborates the previous finding from our group that failure to learn the predictors of pain results in a non-specific increase in pain responses, likely because of contextual anxiety [33]. That the general pain modulation associated with not learning the contingency was greater than the specific effect of the CS+ relative to the CS-suggests that non-selective learning may be a key maladaptation.
4.4 Clinical relevance
Non-nociceptive cues that might act as predictors of harm have been shown to reduce pain threshold during movement [22] as well as during touch [23], possibly via a classical conditioning pathway [3]. In this sense, the current findings are consistent with the Imprecision Hypothesis of chronic pain [3], which outlines an associative pathway to chronic pain, mediated by maladaptive over-generalisation of conditioned responses.
Because pain-associated cues can enhance later painful experiences, procedures aiming to extinguish these associations may help treat persistent pain. Such treatments could aim to exercise neural networks that encode the non-nociceptive cues independent of those that encode nociception (for example, graded motor imagery [24])—or could aim to disassociate the cues from threat through exposure therapy [25] or pain education [26,30]. The use of approaches to understanding and treating chronic pain that are founded in learning theory is strengthened by evidence suggesting that people with chronic pain may show learning characteristics consistent with maladaptive pain-related learning [27,31,32]
4.5 Limitations
Behavioural studies such as this one rely on self-report of pain as a primary outcome. Although self-report is the gold standard approach to measuring pain, the inclusion of a physiological correlate of pain, for example via brain imaging, would add weight to our finding. Unfortunately, the current design does not allow us to confidently interpret the result as either a hyperalgesic effect of the CS+ or a hypoalgesic effect of the CS-. In a similar study using visual conditioning stimuli, Jensen et al. found that the CS+ resulted in higher pain and the CS- resulted in less pain, relative to a previously unpaired control stimulus [6,8]. They interpreted this to mean that the CS+ vs. CS- difference was due to both hyper- and hypo-algesic effects (i.e. CS+ related nocebo and CS- related placebo effects). There are several reasons, however, that cast doubt over this interpretation. Firstly, the novel control stimulus is not neutral, but rather one that is likely associated with surprise and uncertainty. Secondly, both CS+ and CS-were paired with painful stimuli, and thus both predicted aversive stimulation. Therefore, it is possible that what is actually being observed is three levels of pain amplification by pain-predicting and surprising stimuli. Therefore, while inclusion of a novel control stimulus in our study might have been satisfying, it could not have assisted in answering this question. Another possibility would be to compare the painfulness of the USmed alone (either at calibration or test phase) to when paired with the CS+ and CS-. Unfortunately, comparison to baseline ratings would be confounded by changes in sensitivity and response criteria over time, such as those due to repeated stimulation and emotional modulation. Within-phase comparison to USmed alone, on the other hand, would be confounded by the lack of concurrent tactile stimuli that might modulate pain via neural, attention mechanisms.
Another limitation of this study that could be investigated in future designs is the possibility that the perception of the CSs themselves may have been altered by the conditioning process. That is, the CS+ may evoke more unpleasant or intense perceptions than the CS-, because of its association with the aversive US. This question, however, has been investigated using electrocutaneous CSs with no apparent effect [5]. This suggests that more lengthy and/or aggressive conditioning procedures, than typically used in laboratory studies, may be needed to shift the sensory qualities of somatosensory CSs, if they can indeed be shifted.
Retrospective measurement of contingency learning was used in this study so as to prevent confounding of the primary outcome by experimental demand [28]. Furthermore, there was no way to introduce questions about variables such as expectancy awareness in-between CS and US presentations, because timing was critical to this study. Nonetheless, offline ratings do not necessarily relate well to online measures [29], which may be more sensitive [15].
We also note that we did not account for individual differences in psychological variables that may interact with the conditioning process and with pain reports. Given the limited sample size within each group, these differences may not have been accounted for in the randomization process. This reduces our confidence in the between-group comparisons and should be considered in future studies.
Finally, the magnitude of the induced change in pain observed in the primary outcome may not be clinically meaningful. That is, the presence of the CS+ resulted in only a 5.3% higher intensity rating compared with the CS-. However, this too reflects our aim and a broader limitation of laboratory studies, where a limited number of trials, an artificial context and the use of experimental pain are likely to reveal only glimpses of what is clinically possible. Available data suggest that pain-associated cues may be more potent in clinical states [22,23]. Nonetheless, on the basis of our data, it remains possible that the potential effect of pain-associated tactile cues is not clinically relevant. It should be noted, however, that the effects seen in subgroups of people where the calibration was optimal and where the contingency was learned produced greater effects.
5 Conclusion
Pain-associated tactile cues appear able to modulate pain with or without explicit expectation. That classical conditioning might explain this effect raises the possibility of an associative pathway by which pain may be evoked by non-nociceptive stimuli—a kind of ‘associative sensitisation’. Given that current explanations for chronic pain fall short of accounting for the breadth of clinical observations, we suggest that research into associative mechanisms underpinning pain, as distinct from those that link pain to pain-related fear and avoidance, is worthwhile.
Highlights
We tested whether pain-associated tactile cues could modulate perceived intensity of painful stimulation.
More intense pain was evoked by painful stimuli when preceded by a pain-associated cue.
The effect did not appear dependent on explicit expectation.
This points to a larger potential role for associative learning in the development and treatment of pain than previously considered.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2015.11.012.
-
Author contributionsD.S.H, V.J.M and G.L.M. developed the study concept, contributed to design, data interpretation, and write-up. D.P. and D.S.H collected the data. R.B. designed and constructed the vibro-tactile device. S.H. and A.M. contributed to interpretation and write-up. All authors approved the final version of the manuscript for submission.
-
Conflicts of interestThe authors declare no conflicts of interest.
References
[1] Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. PAIN 2000;85:317–32.Search in Google Scholar
[2] McMahon S, Koltzenburg M. Melzack’s textbook of pain. Philadelphia, PA: Elsevier/Churchill Livingstone; 2006.Search in Google Scholar
[3] Moseley GL, Vlaeyen JW. Beyond nociception: the imprecision hypothesis of chronic pain. PAIN 2015;156:35–8.Search in Google Scholar
[4] Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci 2010;30:12964–77.Search in Google Scholar
[5] Diesch E, Flor H. Alteration in the response properties of primary somatosensory cortex related to differential aversive Pavlovian conditioning. PAIN 2007;131:171–80.Search in Google Scholar
[6] Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cerebral Cortex 2014, bhu275.Search in Google Scholar
[7] Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 2006;26:4437–43.Search in Google Scholar
[8] Jensen KB, KaptchukTJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci USA 2012;109:15959–64.Search in Google Scholar
[9] Hamm AO, Vaitl D, Lang PJ. Fear conditioning, meaning, and belongingness: a selective association analysis. J Abnorm Psychol 1989;98:395.Search in Google Scholar
[10] Seligman ME. Phobias and preparedness. Behav Ther 1971;2:307–20.Search in Google Scholar
[11] LoLordo VM. Selective associations. Mechanisms of learning and motivation: a memorial volume to Jerzy Konorski; 1979. p. 367–98.Search in Google Scholar
[12] Cervero F. Spinal cord mechanisms of hyperalgesia and allodynia: role of peripheral input from nociceptors. Prog Brain Res 1996;113:413–22.Search in Google Scholar
[13] Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152:S2–15.Search in Google Scholar
[14] Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol: Anim Behav Process 2002;28:3.Search in Google Scholar
[15] Mitchell CJ, De Houwer J, Lovibond PF. The propositional nature of human associative learning. Behav Brain Sci 2009;32:183–98.Search in Google Scholar
[16] Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: separate or connected processes? Psychol Conscious: Theory Res Pract 2014;1:51.Search in Google Scholar
[17] Moors A, De Houwer J. Automaticity: a theoretical and conceptual analysis. Psychol Bull 2006;132:297.Search in Google Scholar
[18] Velmans M. When perception becomes conscious. Br J Psychol 1999;90: 543–66.Search in Google Scholar
[19] Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci 2013;7.Search in Google Scholar
[20] Moseley G. Reconceptualising pain according to its underlying biology. Phys TherRev 2007;12:169–78.Search in Google Scholar
[21] Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol 2005;56:207–34.Search in Google Scholar
[22] Harvie DS, Broecker M, Smith RT, Meulders A, Madden VJ, Moseley GL. Bogus visual feedback alters onset of movement-evoked pain in people with neck pain. Psychol Sci 2015;26:385–92.Search in Google Scholar
[23] Acerra NE, Moseley GL. Dysynchiria: watching the mirror image of the unaffected limb elicits pain on the affected side. Neurology 2005;65:751–3.Search in Google Scholar
[24] Moseley GL, Butler DS, Beames TB, Giles TJ. The graded motor imagery handbook. Adelaide: Noigroup Publications; 2012.Search in Google Scholar
[25] Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther 2001;39:151–66.Search in Google Scholar
[26] Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain 2004;20:324–30.Search in Google Scholar
[27] Meulders A, Jans A, Vlaeyen JW. Differences in pain-related fear acquisition and generalization: an experimental study comparing fibromyalgia patients and healthy controls. PAIN 2015;156:108–22.Search in Google Scholar
[28] Boddez Y, Baeyens F, Luyten L, Vansteenwegen D, Hermans D, Beckers T. Rating data are underrated: validity of US expectancy in human fear conditioning. J Behav Ther Exp Psychiatry 2013;44:201–6.Search in Google Scholar
[29] Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry 2008;165: 898–904.Search in Google Scholar
[30] Butler DS, Moseley GL. Explain pain (revised and updated). Adelaide: Noigroup Publications; 2013.Search in Google Scholar
[31] Jenewein J, Moergeli H, Sprott H, Honegger D, Brunner L, Ettlin D,..., Schwegler K. Fear-learning deficits in subjects with fibromyalgia syndrome? Eur J Pain 2013;17:1374–84.Search in Google Scholar
[32] Meulders A, Harvie DS, Bowering KJ, Caragianis S, Vlaeyen JW, Moseley GL. Contingency learning deficits and generalization in chronic unilateral hand pain patients. J Pain 2014;15:1046–56.Search in Google Scholar
[33] Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un) predictable painful electrocutaneous stimulation: evidence for mediation by fear of pain. PAIN 2012;153: 1030–41.Search in Google Scholar
[34] Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988.Search in Google Scholar
© 2015 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- Psychophysiological effects of threatening a rubber hand that is perceptually embodied in healthy human subjects
- Original experimental
- A preliminary investigation into psychophysiological effects of threatening a perceptually embodied rubber hand in healthy human participants
- Editorial comment
- Analysis of C-reactive protein (CRP) levels in pain patients – Can biomarker studies lead to better understanding of the pathophysiology of pain?
- Clinical pain research
- Serum C-reactive protein levels predict regional brain responses to noxious cold stimulation of the hand in chronic whiplash associated disorders
- Editorial comment
- Importance of early diagnosis of complex regional pain syndrome (CRPS-1 and CRPS-2): Delayed diagnosis of CRPS is a major problem
- Clinical pain research
- Delayed diagnosis and worsening of pain following orthopedic surgery in patients with complex regional pain syndrome (CRPS)
- Editorial comment
- Associative learning mechanisms may trigger increased burden of chronic pain; unlearning and extinguishing learned maladaptive responses should help chronic pain patients
- Original experimental
- When touch predicts pain: predictive tactile cues modulate perceived intensity of painful stimulation independent of expectancy
- Editorial comment
- Low back pain among nurses: Common cause of lost days at work and contributing to the worldwide shortage of nurses
- Observational study
- Pain-related factors associated with lost work days in nurses with low back pain: A cross-sectional study
- Editorial comment
- Assessment of persistent pelvic pain after hysterectomy: Neuropathic or nociceptive?
- Clinical pain research
- Characterization of persistent pain after hysterectomy based on gynaecological and sensory examination
- Editorial comment
- Transmucosal fentanyl for severe cancer pain: Nasal mucosa superior to oral mucosa?
- Original experimental
- Facilitation of accurate and effective radiation therapy using fentanyl pectin nasal spray (FPNS) to reduce incidental breakthrough pain due to procedure positioning
- Editorial comment
- Why do we have opioid-receptors in peripheral tissues? Not for relief of pain by opioids
- Clinical pain research
- Peripheral morphine reduces acute pain in inflamed tissue after third molar extraction: A double-blind, randomized, active-controlled clinical trial
- Editorial comment
- Chronic pain and psychological distress among long-term social assistance recipients – An intolerable burden on those on the lowest steps of the socioeconomic ladder
- Clinical pain research
- The co-occurrence of chronic pain and psychological distress and its associations with salient socio-demographic characteristics among long-term social assistance recipients in Norway
- Editorial comment
- Fifty years on the Visual Analogue Scale (VAS) for pain-intensity is still good for acute pain. But multidimensional assessment is needed for chronic pain
- Clinical pain research
- Patient reported outcome measures of pain intensity: Do they tell us what we need to know?
- Editorial comment
- Postoperative pain documentation 30 years after
- Topical review
- Postoperative pain documentation in a hospital setting: A topical review
- Editorial comment
- Aspects of pain attitudes and pain beliefs in children: Clinical importance and validity
- Observational study
- The Survey of Pain Attitudes: A revised version of its pediatric form
- Editorial comment
- The role of social anxiety in chronic pain and the return-to-work process
- Clinical pain research
- Social Anxiety, Pain Catastrophizing and Return-To-Work Self-Efficacy in chronic pain: a cross-sectional study
- Editorial comment
- Advances in understanding and treatment of opioid-induced-bowel-dysfunction, opioid-induced-constipation in particular Nordic recommendations based on multi-specialist input
- Topical review
- Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction–Recommendations of the Nordic Working Group
- Observational study
- Opioid-induced constipation, use of laxatives, and health-related quality of life
- Editorial comment
- Migraine headache and bipolar disorders: Common comorbidities
- Systematic review
- Migraine headache and bipolar disorder comorbidity: A systematic review of the literature and clinical implications
- Editorial comment
- The role of catastrophizing in the pain–depression relationship
- Clinical pain research
- The mediating role of catastrophizing in the relationship between pain intensity and depressed mood in older adults with persistent pain: A longitudinal analysis
- Announcement
- May 26-27, 2016 Scandinavian Association for the Study of Pain, Reykjavik, Iceland May 25, 2016 PhD course
Articles in the same Issue
- Editorial comment
- Psychophysiological effects of threatening a rubber hand that is perceptually embodied in healthy human subjects
- Original experimental
- A preliminary investigation into psychophysiological effects of threatening a perceptually embodied rubber hand in healthy human participants
- Editorial comment
- Analysis of C-reactive protein (CRP) levels in pain patients – Can biomarker studies lead to better understanding of the pathophysiology of pain?
- Clinical pain research
- Serum C-reactive protein levels predict regional brain responses to noxious cold stimulation of the hand in chronic whiplash associated disorders
- Editorial comment
- Importance of early diagnosis of complex regional pain syndrome (CRPS-1 and CRPS-2): Delayed diagnosis of CRPS is a major problem
- Clinical pain research
- Delayed diagnosis and worsening of pain following orthopedic surgery in patients with complex regional pain syndrome (CRPS)
- Editorial comment
- Associative learning mechanisms may trigger increased burden of chronic pain; unlearning and extinguishing learned maladaptive responses should help chronic pain patients
- Original experimental
- When touch predicts pain: predictive tactile cues modulate perceived intensity of painful stimulation independent of expectancy
- Editorial comment
- Low back pain among nurses: Common cause of lost days at work and contributing to the worldwide shortage of nurses
- Observational study
- Pain-related factors associated with lost work days in nurses with low back pain: A cross-sectional study
- Editorial comment
- Assessment of persistent pelvic pain after hysterectomy: Neuropathic or nociceptive?
- Clinical pain research
- Characterization of persistent pain after hysterectomy based on gynaecological and sensory examination
- Editorial comment
- Transmucosal fentanyl for severe cancer pain: Nasal mucosa superior to oral mucosa?
- Original experimental
- Facilitation of accurate and effective radiation therapy using fentanyl pectin nasal spray (FPNS) to reduce incidental breakthrough pain due to procedure positioning
- Editorial comment
- Why do we have opioid-receptors in peripheral tissues? Not for relief of pain by opioids
- Clinical pain research
- Peripheral morphine reduces acute pain in inflamed tissue after third molar extraction: A double-blind, randomized, active-controlled clinical trial
- Editorial comment
- Chronic pain and psychological distress among long-term social assistance recipients – An intolerable burden on those on the lowest steps of the socioeconomic ladder
- Clinical pain research
- The co-occurrence of chronic pain and psychological distress and its associations with salient socio-demographic characteristics among long-term social assistance recipients in Norway
- Editorial comment
- Fifty years on the Visual Analogue Scale (VAS) for pain-intensity is still good for acute pain. But multidimensional assessment is needed for chronic pain
- Clinical pain research
- Patient reported outcome measures of pain intensity: Do they tell us what we need to know?
- Editorial comment
- Postoperative pain documentation 30 years after
- Topical review
- Postoperative pain documentation in a hospital setting: A topical review
- Editorial comment
- Aspects of pain attitudes and pain beliefs in children: Clinical importance and validity
- Observational study
- The Survey of Pain Attitudes: A revised version of its pediatric form
- Editorial comment
- The role of social anxiety in chronic pain and the return-to-work process
- Clinical pain research
- Social Anxiety, Pain Catastrophizing and Return-To-Work Self-Efficacy in chronic pain: a cross-sectional study
- Editorial comment
- Advances in understanding and treatment of opioid-induced-bowel-dysfunction, opioid-induced-constipation in particular Nordic recommendations based on multi-specialist input
- Topical review
- Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction–Recommendations of the Nordic Working Group
- Observational study
- Opioid-induced constipation, use of laxatives, and health-related quality of life
- Editorial comment
- Migraine headache and bipolar disorders: Common comorbidities
- Systematic review
- Migraine headache and bipolar disorder comorbidity: A systematic review of the literature and clinical implications
- Editorial comment
- The role of catastrophizing in the pain–depression relationship
- Clinical pain research
- The mediating role of catastrophizing in the relationship between pain intensity and depressed mood in older adults with persistent pain: A longitudinal analysis
- Announcement
- May 26-27, 2016 Scandinavian Association for the Study of Pain, Reykjavik, Iceland May 25, 2016 PhD course

