A118G polymorphism in the μ-opioid receptor gene and levels of β-endorphin are associated with provoked vestibulodynia and pressure pain sensitivity
-
Ulrika Heddini
, Ulrika Johannesson
Abstract
Background and aims
Provoked vestibulodynia (PVD) is the most common cause of dyspareunia among young women. The aetiology is largely unknown and treatment is often extensive and longstanding with varying outcomes. Patients display general pain hypersensitivity and there are correlations with other chronic pain syndromes such as fibromyalgia later in life. The A118G polymorphism in the μ-opioid receptor (OPRM1) gene influences endogenous pain regulation and pain sensitivity, but has not been studied in this patient group before. We aimed to investigate a possible association between A118G polymorphism and PVD, with correlation to plasma levels of β-endorphin, and to explore relationships between this polymorphism and pain sensitivity among women with PVD and healthy controls.
Methods
This case-control study included 98 women with PVD and 103 controls. Participants filled out study-specific questionnaires and underwent quantitative sensory testing of pressure pain thresholds on the arm, leg and in the vestibular area. Levels of β-endorphin were analyzed by radioimmunoassay using the EURIA-beta-endorphin kit, and the A118G single-nucleotide polymorphism (SNP; rs1799971) in the OPRM1 gene was analyzed using the TaqMan SNP genotyping assay.
Results
The 118G allele was more common in controls (44%) than in patients (30%) (p = 0.042). The odds ratio of having PVD was 1.8 in participants carrying the 118A allele compared to participants hetero- or homozygous for the 118G allele (OR = 1.846, CI: 1.03-3.31, p = 0.039). Pressure pain thresholds on the leg were higher for participants carrying the 118G allele (mean 480 kPa, SD 167.5) than for those carrying the 118A allele (mean 419, SD 150.4, p = 0.008). Levels of β-endorphin were higher in patients (mean 17.9 fmol/ml, SD 4.71) than in controls (mean 15.8 fmol/ml, SD 4.03) (p < 0.001).
Conclusion
We found an association between the A118G polymorphism in the OPRM1 gene and an increased risk of PVD and increased pain sensitivity among participants carrying the 118A allele. PVD patients were more sensitive to pressure pain and had higher levels of plasma β-endorphin than controls. The results indicate that differences in endogenous pain modulation involving the opioid system could contribute to the pathophysiology of PVD and the general pain hypersensitivity seen in these women.
Implications
The data support the conceptualization of PVD as part of a general pain disorder with a possible genetic predisposition. The age of onset of PVD is usually between 18 and 25 years and already at this age general pain hypersensitivity is present but rarely causing disability. We believe that early recognition and treatment, with the risk of further development of chronic pain taken into consideration, might prevent future aggravated pain problems in this patient group.
1 Introduction
Provoked vestibulodynia (PVD) is a pain disorder characterized by pain provoked by touch, pressure and stretch of the mucosa around the vaginal opening, often resulting in inability to engage in vaginal intercourse [1]. The aetiology is thought to be multifactorial, including both biomedical and psychosexual triggers such as recurrent vulvovaginal candidiasis, hormonal effects, and lack of sexual arousal [1,2,3,4]. There is evidence of both peripheral and central pain mechanisms being involved in the pathogenesis [5,6,7]. Women with PVD display general pain hypersensitivity with lower pain thresholds also in other body areas. Complaints of concomitant bodily pain are common and correlations with other pain conditions such as fibromyalgia, dysmenorrhea, and irritable bowel syndrome have been reported [2,8,9,10].
A genetic predisposition of PVD has been proposed. Studies have found PVD-associated polymorphisms of genes affecting the pro-inflammatory immune response [11,12,13]. However, few studies have investigated the influence of genetic polymorphism on endogenous pain modulation in these patients. We recently reported a lack of association between a specified single-nucleotide polymorphism (SNP) combination in the guanosine triphosphate cyclohydrolase (GCH1) gene and PVD [14]. The contribution of genetics to other pain syndromes associated with PVD, has been estimated as approximately 50% [15,16].
The SNPA118G (rs1799971) in the μ-opioid receptor 1 (OPRM1) gene causes a substitution from asparagine (Asn) to aspartic acid (Asp) at amino acid 40, with the resultant removal of a putative N-linked glycosylation site in the receptor and effects on endogenous pain modulation [17]. The endogenous agonist of the μ-opioid receptor, β-endorphin, is synthesized in the anterior pituitary gland and secreted into peripheral blood in response to pain and other stressful stimuli. Increased β-endorphin potency and increased receptor binding affinity between β-endorphin and the variant 118G receptor have been described [17], however results are equivocal. Beyer et al. reported similar β-endorphin binding affinities and potencies for both receptor variants [18]. Moreover, elevated plasma levels of β-endorphin have been suggested as a biomarker for reduced endogenous opioid anti-nociceptive function in chronic pain patients [19].
Initially, the 118G allele was thought to be pain protective. Fillingim et al. reported that healthy individuals with heterozygous (AG) and minor homozygous (GG) genotypes had higher pressure pain thresholds than individuals with the major homozygous (AA) genotype and there were findings of carriers having less chronic pain than non-carriers [20,21]. However, recent studies show a more complex picture with somewhat conflicting results; the association between A118G polymorphism and pain sensitivity seems to be influenced by several factors such as sex, ethnicity, and pain modality. For example women homo- or heterozygous for the 118G allele showed increased pain intensity in the first year after lumbar disc herniation and reported more pain following caesarean section compared to 118A carriers [22,23,24,25].
The aim of this study was to investigate the possible association between the A118G polymorphism in the OPRM1 gene and PVD, with correlation to plasma levels of β-endorphin. In addition, we aimed to explore relationships between this polymorphism and pain sensitivity among women with PVD and healthy controls.
2 Methods
2.1 Participants
Ninety-eight women with PVD were recruited. The inclusion criteria for patients were: age ≥18 years, PVD defined as pain at vestibular contact and vaginal entry, with duration of symptoms ≥6 months, based on the initial exam at the time of diagnosis. The exclusion criteria were: local infection or dermatological causes for dyspareunia, major psychiatric or medical disease, or pregnancy. The controls were 103 healthy women in the same age range, with regular menstruation. Exclusion criteria for the controls were: dyspareunia, regular use of analgesics or anti-depressants, major medical or psychiatric disease, or pregnancy. All participants received oral and written information about the study and provided informed consent; the study was approved by the local ethical committee.
2.2 Questionnaires and quantitative sensory testing
Testing was carried out on one occasion during days 3-13 of the menstrual cycle. Participants filled out comprehensive questionnaires for psychosocial, medical and gynaecological history, including reports of other frequent bodily pain disorders. A bodily pain score with a range of 0-5 was created for each participant, using the number of pain disorders reported. Patients engaging in vaginal intercourse were asked to score the intensity of coital pain during the last month on a visual analogue scale (VAS) with a range of 0-100, where 0 represents no pain and 100 represents the worst pain imaginable. PPTs on the arm and leg were measured using a pressure algometer (Somedic Sales AB, Hörby, Sweden) according to a previously described procedure [14]. In brief, the arm was tested on the deltoid muscle, 3 cm proximal to the tendon insertion for the muscle, and the leg was tested on the anterior tibial muscle, approximately 5 cm below and 3 cm lateral to the tibial tuberosity. The pressure was increased until the participant reported the PPT, defined as the first sensation of pain. All participants received an explanation of the procedure and a training session on the opposite arm before testing. The examiner was, to this point, blinded to whether the participant belonged to the patient or control group as well as to the participant’s genotype.
At this stage, the patient or control status was revealed and PPTs in the vestibular mucosa were measured in patients only, using vulvar algesiometers exerting a pressure ranging from 3 to 1000 g [26]. Two areas of the vestibule on the right side of the vaginal opening were tested: area A, in the anterior vestibule close to the urethra; and area B, in the posterior vestibule close to the opening of the Bartholin’ glands. Again, pressure was increased until the PPT was reported. The mean value of two measurements was used for analysis for all the PPTs. Coital pain and PPTs in the vestibular area were not measured among controls since absence of coital pain was an inclusion criterion.
2.3 Sample collection
Venous blood samples were collected from all participants in EDTA tubes. Whole blood samples were centrifuged for 10 min at 3000 rpm, and plasma was collected for radioimmunoassay analysis of β-endorphin levels. Samples were stored at –70°C until further processing.
2.4 DNA isolation
Total genomic DNA was isolated from the whole blood samples using the Magtration 12GC system (Precision System Science, Chiba, Japan) and the Magazorb® DNA Common Kit-200 (PSS, Chiba, Japan). The concentration of DNA was determined using a Nano drop Spectrophotometer (Nano Drop Technologies Inc., Wilmington, DE, USA).
2.5 Genotyping
The A118G SNP (rs1799971) in the OPRM1 gene was analyzed using the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, USA), with manufacturer-designed primers and allele-specific probes. Briefly, genomic DNA (5 ng), water, TaqMan Universal PCR master mix and TaqMan genotyping assay mix were placed in each well of a 384-well plate to a total volume of 5 μl. The assay mix included target-specific PCR primers and TaqMan MGB probes labelled with two special dyes: FAM and VIC. The reaction was performed according to the manufacturer’s instructions using the ABI7900HT genetic detection system (Applied Biosystem, Foster City, USA) with the following amplification protocol: 10 min at 95 °C and 40 cycles of 15 s at 92 °C and 1 min at 60°C.
2.6 Radioimmunoassay
The frozen plasma samples were thawed on ice and centrifuged at 4 °C for 10 min at 3000 × g. The supernatants were collected, diluted (1:5) with 0.1 M formic acid and 0.018 M pyridine (buffer I), and separated on minicolumns (1 ml) packed with SP-Sephadex C-25 gel. The columns were washed with 10 ml buffer I prior to sample application, and 10 ml buffer I and 5 ml 0.1 M formic acid/0.1 M pyridine (pH 4.1; buffer II) after sample application. The peptide-containing fractions were then eluted with 4 ml 1.6 M formic acid/1.6 M pyridine (pH 4.1; buffer V). All buffers contained 0.01% mercaptoethanol. The eluted samples were evaporated in a Speed Vac centrifuge (Savant, Hicksville, NY, USA).
The EURIA-beta-endorphin kit (EURO-DIAGNOSTICA AB, Sweden) was used for the β-endorphin radioimmunoassay (RIA), which is based on a double antibody precipitation. The evaporated samples were diluted with 220 μl diluent (0.05 M phosphate pH 7.4, 0.25% human serum albumin, 0.05% sodium azide, 0.25% EDTA and 500 KIU Trasylol®/ml) and incubated with 100 μl of anti-beta-endorphin antiserum for 24 h at 4 °C. After incubation the labelled peptide, 125I-β-endorphin, was added to each sample and incubated for an additional 24 h at 4 °C. Thereafter, the double antibody PEG was added, and the tubes were incubated for 60 min and then centrifuged for 15min at 12,000rpm and 4°C. The supernatants were thereafter decanted and the radioactivity of the precipitates was counted in a gamma counter.
2.7 Statistics
The Statistica programme (version 10, StatSoft Inc., Tulsa, OK, USA) and the Statistical package for the Social Sciences programme (version 20, SPSS Inc., Chicago, IL, USA) were used to analyze the data. The student t-test or, for ordinal and non-normally distributed data, the Mann-Whitney U-test were used for comparisons between groups regarding age, PPTs and β-endorphin levels. The Chi2 test and Fishers exact test were used for frequencies of pain disorders and SNPs. The data were analyzed using logistic regression to explore a possible association between the A118G polymorphism and a diagnosis of PVD and the Spearman rank method was used to investigate possible correlations between β-endorphin levels and pain measurements. A significance level of p < 0.05 was used for all statistical tests and a confidence interval of 95% was used for the logistic regression analyses. The initial sample size of 100 participants in each group were estimated to give sufficient power to detect a possible difference in SNP frequencies between patients and controls based on a method to optimize sample size in candidate gene studies described by Belfer et al. [27]. To detect allele linked differences in pain sensitivity Huang et al. [24] assumed based on previous findings, an effect size of 0.6 for the A118G SNP effect on PPTs. Thus, with a significance level of 0.05, 35 subjects were needed in each group to ensure a power of 0.8.
3 Results
3.1 Clinical background data
Clinical background data are shown in Table 1. Patients displayed significantly more frequent pain symptoms than controls in all the pain modalities surveyed: dysmenorrhea 71% vs 54% (p= 0.02), headache (tension type and migraine) 60% vs 29%, stomach pain (gastritis and irritable bowel syndrome) 54% vs 22%, back pain 49% vs 20%, and muscle pain 32% vs 2% (all p < 0.001). See also the bodily pain score in Table 3 for mean number of pain problems and statistical analysis. There were no significant differences in frequencies of pain symptoms between carriers of the 118G or 118A alleles in patients or controls respectively.
Clinical characteristics of participants.
| Variables | Patients (n = 98) | Controls (n = 103) | p-Value |
|---|---|---|---|
| Current age, years (range) | 29 (19–44) | 24 (18–35) | <0.001 |
| Number with Caucasian ethnicity (%) | 95 (97) | 99 (96) | ns |
| Duration of PVD, years (range) | 8 (0.5–18) | - | - |
| Sample taken on menstrual cycle day (range) | 7.9 (4–13) | 8.0 (3–13) | ns |
-
PVD, provoked vestibulodynia.
3.2 Genotyping
Genotyping for the studied A118G SNP (rs1799971) in the OPRM1 gene was completed in 201 subjects. Frequencies are shown in Table 2. All frequencies were in accord with the Hardy-Weinberg equilibrium (χ2 = 2.84, p = 0.09). Subjects with the minor homozygous (GG) and heterozygous (AG) subjects were combined to form the rare allele genotype (118G) group for further analysis in accordance with previous studies [20,24]. The rare 118G allele was significantly more common among controls than among patients.
Allele frequencies of the OPRM1 A118G polymorphism.
| 118A | 118G | p-Value | ||
|---|---|---|---|---|
|
|
||||
| AA no (%) | AG no (%) | GG no (%) | ||
| All participants (n = 201) | 127 (63) | 58 (29) | 16 (8) | |
| Patients (n = 98) | 69 (70) | 24 (25) | 5 (5) | p = 0.042 |
| Controls (n =103) | 58 (56) | 34 (33) | 11 (11) | |
3.3 A118G polymorphism in relation to PVD
The odds ratio of having PVD was 1.8 among participants carrying the 118A allele compared to participants who were hetero- or homozygous for the 118G allele (OR= 1.846, CI: 1.03–3.31, p = 0.039).
3.4 Pain measurements
There were significantly higher PPTs on the arm and leg among controls than among patients. Additionally, controls had lower selfreported bodily pain scores. See Table 3.
Pain measurements for patients vs controls.
| Patients (n = 98) | Controls (n =103) | p-Value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean (SD) | Median (Q1-Q3) | Mean (SD) | Median (Q1-Q3) | ||
| PPT leg (kPa) | 405 (161) | 390 (299–499) | 474 (152) | 457 (361–575) | 0.002 (t-test), 0.001 (M-WU) |
| PPT arm (kPa) | 268 (124) | 238 (189–331) | 309 (116) | 298 (227–355) | 0.018 (t-test), 0.002 (M-WU) |
| Bodily pain score (0–5) | 2.1 (1.2) | 2 (1–3) | 0.7 (0.9) | 0 (0–1) | <0.001 (M-WU) |
| PPT vestibulum A (g) | 48 (31) | 40 (25–60) | - | - | - |
| PPT vestibulum B (g) | 42 (44) | 28 (15–50) | - | - | - |
| Coital VAS pain (0–100) | 53 (32) | 54 (23–78) | - | - | - |
-
PPT, pressure pain threshold; kPa, kilopascal; A, anterior vestibule; B, posterior vestibule; VAS, visual analogue scale; M-WU, Mann-Whitney U-test.
3.5 A118G polymorphism in relation to pain
As shown in Table 4 and Fig. 1, significantly higher PPTs were obtained on the leg in all participants carrying the 118G genotype than in participants carrying the 118A genotype. There were no differences in the other pain measurements between carriers of the 118G and 118A genotypes in both groups combined. When patients and controls were analyzed separately there were significantly higher PPTs on the leg and arm among individuals carrying the 118G genotype compared to 118A carriers. Among patients there were no significant differences.
Allele linked pain measurements.
| 118A | 118G | p-Value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean (SD) | Median (Q1-Q3) | Mean (SD) | Median (Q1-Q3) | ||
|
|
|||||
| All participants (n = 127) | All participants (n = 74) | ||||
| PPT leg (kPa) | 419 (150) | 393 (324–499) | 476 (170) | 460 (366–590) | 0.01 (t-test), 0.01 (M-WU) |
| PPT arm (kPa) | 280 (119) | 264 (194–342) | 304 (126) | 283 (220–352) | ns |
| Bodily pain score (0–5) | 1.46 (1.3) | 1 (0–2) | 1.34 (1.3) | 1 (0–2) | ns |
| Controls (n = 58) | Controls (n = 45) | ||||
| PPT leg (kPa) | 430 (125) | 406 (348–507) | 530 (165) | 491 (401–628) | <0.001 (t-test), 0.002 (M-WU) |
| PPT arm (kPa) | 283 (92) | 278 (213–341) | 342 (135) | 316 (245–378) | 0.009 (t-test), 0.02 (M-WU) |
| Bodily pain score (0–5) | 0.7 (0.9) | 0 (0–1) | 0.8 (0.9) | 1 (0–1) | ns |
| Patients (n =69) | Patients (n = 29) | ||||
| PPT leg (kPa) | 409 (169) | 377 (299–473) | 393 (143) | 402 (299–505) | ns |
| PPT arm (kPa) | 278 (139) | 246 (189–342) | 240 (77) | 220 (191–279) | ns |
| Bodily pain score (0–5) | 2.1 (1.3) | 2 (1–3) | 2.2 (1.2) | 2 (1–3) | ns |
| PPT vestibulum A (g) | 48 (32) | 40 (25–63) | 47 (30) | 40 (23–60) | ns |
| PPT vestibulum B (g) | 45 (50) | 30 (15–50) | 36 (29) | 25 (20–50) | ns |
| Coital VAS pain (0–100) | 55 (33) | 58 (23–82) | 48 (28) | 49 (25–68) | ns |
-
PPT, pressure pain threshold; kPa, kilopascal; A, anterior vestibule; B, posterior vestibule; VAS, visual analogue scale; M-WU, Mann-Whitney U-test.
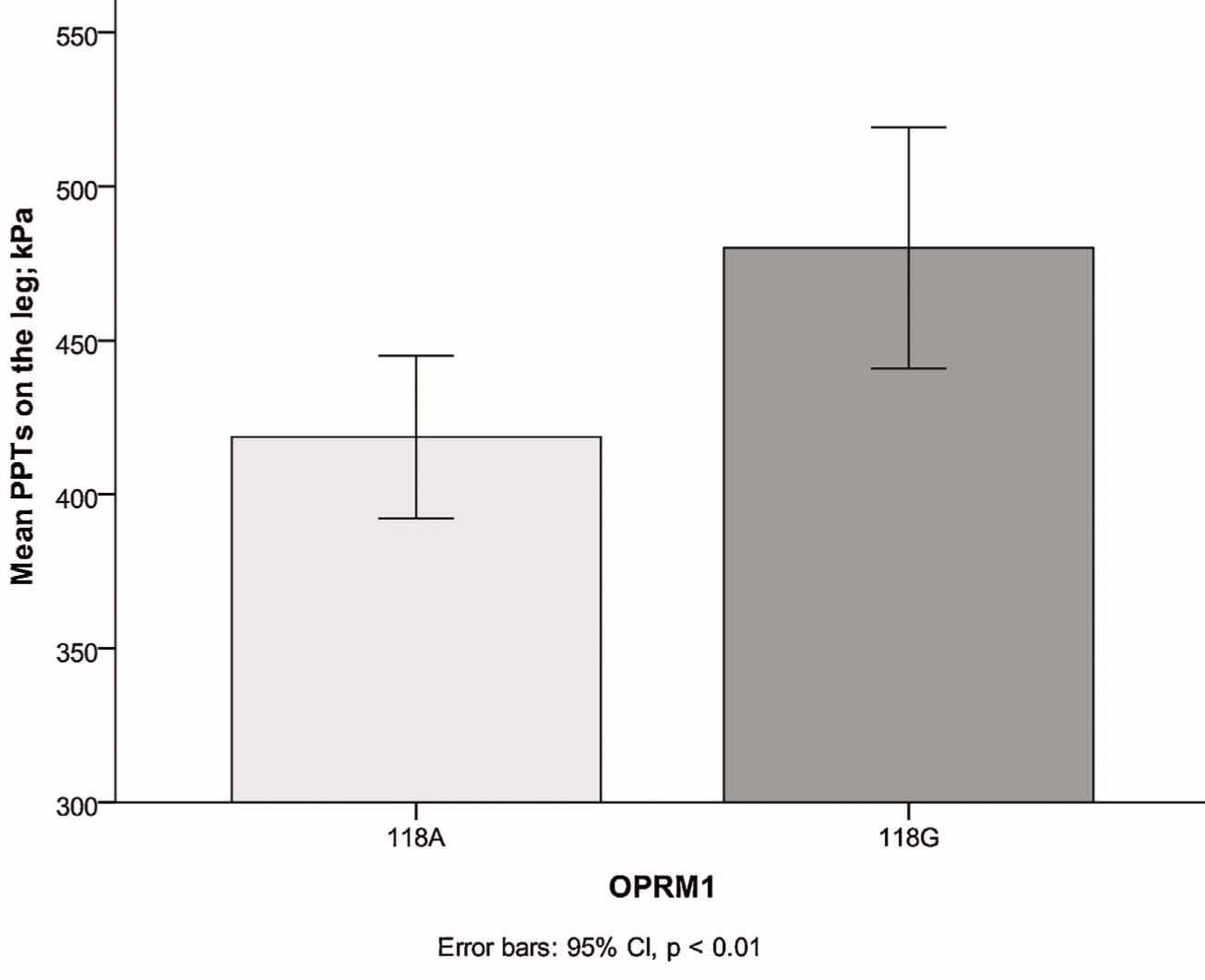
Lower pain sensitivity (higher pressure pain thresholds) seen in carriers ofthe 118G-allele of the OPRMI-gene. PPT, pressure pain threshold.
3.6 β-Endorphin levels
We found significantly higher levels of β-endorphin in patients (mean 17.9 fmol/ml, SD 4.71, n = 80) than in controls (mean 15.8 fmol/ml, SD 4.03, n = 95; z = –3.61, p < 0.001), see Fig. 2.
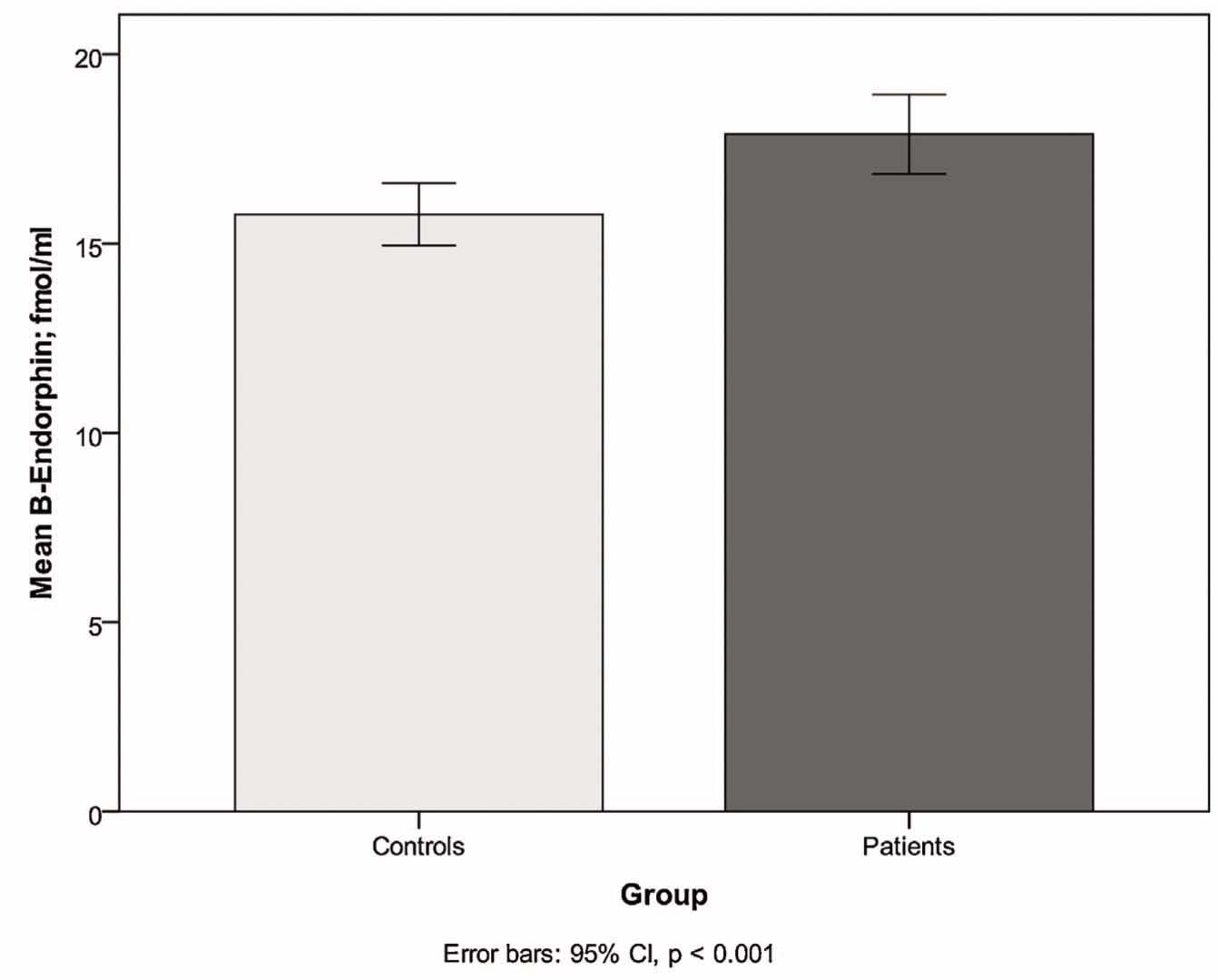
Higher plasma concentrations of β-endorphin in patients compared to controls.
Mean levels of β-endorphin were lower in carriers of the 118G genotype (mean 16.0 fmol/ml, SD 4.26, n = 64) than in carriers of the 118A genotype (mean 17.2 fmol/ml, SD 4.55, n = 111), with a tendency towards a significant difference (z =1.92, p = 0.055).
3.7 β-Endorphin levels in relation to pain
There was a significant correlation between plasma levels of β-endorphin and pain score (rho = 184, p = 0.015) with higher levels of β-endorphin among participants with more concomitant pain disorders. There were no significant correlations between plasma levels of β-endorphin and the different PPTs measured or the coital VAS score. Further, there were no interaction effects between β-endorphin levels and gene variants on the pain measurements.
4 Discussion
The aim of this study was to investigate a possible association between the A118G polymorphism in the OPRM1 gene and a diagnosis of PVD. The frequency of the 118G allele was lower in patients than in healthy controls and the risk of a diagnosis of PVD in noncarriers was almost twice as high as in 118G carriers. These findings indicate that differences in the endogenous opioid analgesic function might contribute to the patho-physiology of this provoked, localized pain condition in young women.
Pain sensitivity was also lower, with higher PPTs on the leg, among carriers of the 118G allele. However, when analyzed separately the findings were only consistent among controls that had higher PPTs on the leg as well as on the arm but among patients there were no significant differences. Although many studies have investigated the pain modulatory effects of the A118G SNP in the μ-opioid receptor, the results have been somewhat conflicting. Our findings are in line with previous results indicating a pain protective effect of the 118G allele [20,21] but are contradictory to the findings by Huang et al. who found no statistically significant allele linked differences in PPTs in healthy women [24]. The initial finding of higher PPTs among 118G carriers by Fillingim et al. was most evident in men, and several later studies showed a sex-genotype interaction where, in contrast to our results, women with the 118G allele had higher pain sensitivity than those with the 118A allele [22,23]. However, these findings were linked with clinical pain and pain after surgery rather than experimental PPTs and possibly the pain modality might affect the result. The fact that only one of the measured PPTs was associated with genotype and that the finding of an association only was consistent in the control group raises questions. There could be several explanations to this fact. Controls displayed higher PPT as compared to patients and therefore a possible association could be more evident in this group. By dividing the material in several subgroups the power to detect an association might also be reduced. Apart from these factors the impact of a single gene polymorphism on the general pain hypersensitivity seen among patients is expected to be modest and therefore difficult to statistically establish.
In our study, patients also had significantly higher levels of the endogenous μ-opioid receptor agonist, β-endorphin. To our knowledge, no other clinical studies have analyzed the μ-opioid receptor polymorphism in combination with its agonist. Another interesting finding was the positive correlation between levels of plasma β-endorphin and the number of concomitant pain symptoms among patients. There is little information about the relationship between resting plasma levels of β-endorphin and endogenous pain modulation and the function of the opioid system. Analgesic pathways for plasma β-endorphin are less clear than the central effects of β-endorphin in the cerebrospinal fluid (CSF). β-endorphin levels in plasma and CSF do not necessarily correspond [28]. The results from a study by Bruehl et al. suggest that higher levels of resting plasma β-endorphin are associated with lower endogenous opioid analgesia [19]. It could be speculated that this finding is consistent with previous findings of a higher binding affinity of β-endorphin to the 118G variant receptor than to the 118A receptor [17]. Although statistical significance was not reached in this study regarding differences in β-endorphin levels between carriers and non-carriers of the 118G allele, there was a tendency towards lower β-endorphin levels among carriers, which could add to this speculation. Notably, the fact that patients displayed many other pain symptoms might confound the results since the elevated levels of β-endorphin as well as the lower frequency of the 118G allele could in fact be associated to these additional pains rather than the PVD. However, we could not see any significant differences in the frequency of other pain symptoms among the carriers of the different alleles in our material. Furthermore, since evidence point towards PVD being part of a general pain hypersensitivity condition it is likely that the same genetic polymorphisms affect all of these symptoms. The elevated levels of β-endorphin could have several other explanations as well. For example, physical exercise has been shown to activate endogenous opioid systems [29]. No information about the training habits of the participants was collected in our study.
Additionally, β-endorphin shares a common precursor, proopiomelanocortin (POMC), with adrenocorticotrophic hormone (ACTH) and release of β-endorphin from the pituitary gland into the circulation is part of the systemic stress response. Investigation of the possible effects of the A118G polymorphism on the activation of the hypothalamus pituitary adrenal (HPA)-axis and cortisol release found higher cortisol concentrations at baseline and after naloxone infusion among 118G carriers [30]. Specific psychological traits are associated with PVD, such as higher levels of anxiety, catastrophizing, and low sexual desire [1,2,3]. Also, morning awakening cortisol appears to be blunted in these women, indicating chronic stress [31]. The mechanism of interaction is, however, not known and there is discussion of whether these traits precede the diagnosis of PVD or occur as a result of the longstanding pain. The endogenous opioid system and β-endorphin have been shown in many studies to play a role in anxiety, stress response and sexual behaviour [28]. It is therefore interesting to speculate whether the differences in μ-opioid receptor polymorphism and β-endorphin levels between patients and controls might partly explain these differences in psychological traits as well as differences in pain sensitivity.
Among the strengths of this study were the well defined study population; all patients were examined and diagnosed at the same vulvar open care clinic, and the fact that all participants were examined in the same menstrual phase. However, there are some limitations to take into consideration when interpreting our results; regular use of analgesic drugs was an exclusion criterion only for controls, which could have resulted in a selection bias, with the control group having lower pain sensitivity than the normal population. The controls were significantly younger than the patients; however, a mean age difference of 5 years is unlikely to affect the results. The patient sample, although quite large in the field of PVD, is limited and we do not have any external validation sample. Furthermore, the impact of a single gene polymorphism on the complex phenomenon of chronic pain can only be expected to be modest and future studies investigating possible gene-gene interactions would be of value.
5 Conclusions
PVD is a common disorder that carries a great clinical challenge. The aetiology is largely unknown and treatment is often extensive and longstanding with varying outcomes. The results of this study indicate that differences in endogenous pain modulation involving the opioid system could contribute to the aetiology of PVD and the general pain hypersensitivity seen in these women. The 118A allele of the OPRM1 gene was more common and levels of the μ-opioid receptor agonist β-endorphin were higher in PVD patients than in healthy women. The data supports the conceptualization of PVD as part of a general pain disorder with a possible genetic predisposition. The results also contribute to our understanding of the pain modulatory effects of the A118G polymorphism in the μ-opioid receptor, which could be relevant in other chronic pain disorders as well. The age of onset of PVD is usually between 18 and 25 years and already at this age general pain hypersensitivity is present but rarely causing disability. We believe that early recognition and treatment, with the risk of further development of chronic pain taken into consideration, might prevent future aggravated pain problems in this patient group. It remains for research to continue to elucidate the pain mechanisms involved in PVD and other chronic pain conditions in order to improve treatments.
Highlights
The 118A allele in the OPRM1 gene was more common in provoked vestibulodynia (PVD) patients than in controls.
Levels of β-endorphin were higher in PVD patients than in healthy women.
The odds ratio of PVD was 1.8 in 118A carriers compared to 118G carriers.
Carriers of the 118A allele had higher pain sensitivity than 118G carriers.
Differences in opioid system function might contribute to the aetiology of PVD.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2013.11.006
-
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Hilde Larsson for assistance in collecting data, and Britt-Marie Johansson and Uppsala Genome Centre, Rudbeck Laboratory, Uppsala, for valuable technical assistance. This study was supported by the National Vulvodynia Association and the Karolinska Institute Research Fund. The funders had no involvement in designing or performing the study or in writing and publishing of the report.
References
[1] vanLankveld JJ, Granot M, Weijmar Schultz WC, Binik YM, Wesselmann U, Pukall CF, Bohm-Starke N, Achtrari C. Women’s sexual pain disorders. J Sex Med 2010;7:615–31.Search in Google Scholar
[2] Danielsson I, Eisemann M, Sjoberg I, Wikman M. Vulvar vestibulitis: a multifactorial condition. BJOG 2001;108:456–61.Search in Google Scholar
[3] Granot M, Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther 2005;31:285–302.Search in Google Scholar
[4] Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol 2002;156:254–61.Search in Google Scholar
[5] Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest 1998;46:256–60.Search in Google Scholar
[6] Bornstein J, Goldschmid N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol Obstet Invest 2004;58:171–8.Search in Google Scholar
[7] Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain 2001;94:177–83.Search in Google Scholar
[8] Granot M, Friedman M, Yarnitsky D, Zimmer EZ. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. BJOG 2002;109:863–6.Search in Google Scholar
[9] Bohm-Starke N, Brodda-Jensen G, Linder J, Danielsson I. The result of treatment on vestibular and general pain thresholds in women with provoked vestibulo-dynia. Clin J Pain 2007;23:598–604.Search in Google Scholar
[10] Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol 2006;107:617–24.Search in Google Scholar
[11] Foster DC, Sazenski TM, Stodgell CJ. Impact of genetic variation in interleukin-1 receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome. J Reprod Med 2004;49:503–9.Search in Google Scholar
[12] Babula O, Danielsson I, Sjoberg I, Ledger WJ, Witkin SS. Altered distribution of mannose-binding lectin alleles at exon I codon 54 in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol 2004;191:762–6.Search in Google Scholar
[13] Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflamma some component NALP3 and recurrent vulvovaginal candidiasis in women with vulvarvestibulitis syndrome. Am J Obstet Gynecol 2009;200:e1–6.Search in Google Scholar
[14] Heddini U, Bohm-Starke N, Grönbladh A, Nyberg F, Nilsson KW, Johannesson U. GCH1-polymorphism and pain sensitivity among women with provoked vestibulodynia. Mol Pain 2012;8:68.Search in Google Scholar
[15] Treloar SA, Martin NG, Heath AC. Longitudinal genetic analysis of menstrual flow, pain and limitation in a sample of Australian twins. Behav Genet 1998;28:107–16.Search in Google Scholar
[16] Bengtsson B, Thorson J. Back pain: a study of twins. Acta Genet Med Gemellol 1991;40:83–90.Search in Google Scholar
[17] Bond C, Laforge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA 1998;95:9608–13.Search in Google Scholar
[18] Beyer A, Koch T, Schröder H, Schulz S, Höllt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem 2004;89:553–60.Search in Google Scholar
[19] Bruehl S, Burns JW, Chung OY, Chont M. What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function. Eur J Pain 2012;16:370–80.Search in Google Scholar
[20] Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wal- lace MR. TheA118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain 2005;6:159–67.Search in Google Scholar
[21] Janicki PK, Schuler G, Francis D, Bohr A, Gordin V, Jarzembowski T, Velasco VR, Mets B. A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain. AnesthAnalg 2006;103:1011–7.Search in Google Scholar
[22] Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, Teo YY, Tan EC. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 2008;109:520–6.Search in Google Scholar
[23] Belland-Olsen M, Jacobsen LM, Schistad El, Pedersen LM, Rygh LJ, Roe C, Gjerstad J. Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci 2012;32:9831–4.Search in Google Scholar
[24] Huang CJ, Liu HF, Su NY, Hsu YW, Yang CH, Chen CC, Tsai PS. Association between human opioid receptor genes polymorphisms and pressure pain sensitivity in females. Anaesthesia 2008;63:1288–95.Search in Google Scholar
[25] Hastie BA, Riley3rd JL, Kaplan L, Herrera DG, Campbell CM, Virtusio K, Mogil JS, Wallace MR, Fillingim RB. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. Pain 2012;153:1610–9.Search in Google Scholar
[26] Pukall CF, Binik YM, Khalife S. A new instrument forpain assessment in vulvar vestibulitis syndrome. J Sex Marital Ther 2004;30:69–78.Search in Google Scholar
[27] Belfer I, Wu T, Kingman A, Krishnaraju RK, Goldman D, Max MB. Candidate gene studies of human pain mechanisms: methods for optimizing choice of polymorphisms and sample size. Anesthesiology 2004;100:1562–72.Search in Google Scholar
[28] Veening JG, Gerrits PO, Barendregt HP. Volume transmission ofbeta-endorphin via the cerebrospinal fluid: a review. Fluids Barriers CNS 2012;9:16.Search in Google Scholar
[29] Glamsta EL, Morkrid L, Lantz I, Nyberg F. Concomitant increase in blood plasma levels of immunoreactive hemorphin-7 and beta-endorphin following long distance running. Regul Pept 1993;49:9–18.Search in Google Scholar
[30] Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid locus (OPRM1). Am J Med Genet B NeuropsychiatrGene 2003;118B:60–5.Search in Google Scholar
[31] Ehrstrom S, Kornfeld D, Rylander E, Bohm-Starke N. Chronic stress in women with localized provoked vulvodynia. J Psycosom Obstet Gynaecol 2009;30:73–9.Search in Google Scholar
© 2013 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- High risk of depression and suicide attempt among chronic pain patients: Always explore catastrophizing and suicide thoughts when evaluating chronic pain patients
- Clinical pain research
- Suicide attempts in chronic pain patients. A register-based study
- Editorial comment
- Polymorphism in the μ-opioid receptor gene OPRM1 A118G —An example of the enigma of genetic variability behind chronic pain syndromes
- Original experimental
- A118G polymorphism in the μ-opioid receptor gene and levels of β-endorphin are associated with provoked vestibulodynia and pressure pain sensitivity
- Editorial comment
- Genital pain related to sexual activity in young women: A large group who suffer in silence
- Original experimental
- Living with genital pain: Sexual function, satisfaction, and help-seeking among women living in Sweden
- Editorial comment
- The Norwegian version of the Neck Disability Index (NDI) is reliable and sensitive to changes in pain-intensity and consequences of pain-in-the-neck
- Clinical pain research
- Reliability and responsiveness of the Norwegian version of the Neck Disability Index
- Editorial comment
- Quality of life in low back pain patients with MRI-lesions in spinal bone marrow and vertebral endplates (Modic-changes): Clinical significance for outcome of spinal surgery?
- Clinical pain research
- Association of Modic changes with health-related quality of life among patients referred to spine surgery
- Editorial comment
- Warming and alkalinisation of lidocaine with epinephrine mixture: Some useful aspects at first glance, but not so simple?
- Clinical pain research
- Warmed and buffered lidocaine for pain relief during bone marrow aspiration and biopsy. A randomized and controlled trial
- Acknowledgement of Reviewers
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- High risk of depression and suicide attempt among chronic pain patients: Always explore catastrophizing and suicide thoughts when evaluating chronic pain patients
- Clinical pain research
- Suicide attempts in chronic pain patients. A register-based study
- Editorial comment
- Polymorphism in the μ-opioid receptor gene OPRM1 A118G —An example of the enigma of genetic variability behind chronic pain syndromes
- Original experimental
- A118G polymorphism in the μ-opioid receptor gene and levels of β-endorphin are associated with provoked vestibulodynia and pressure pain sensitivity
- Editorial comment
- Genital pain related to sexual activity in young women: A large group who suffer in silence
- Original experimental
- Living with genital pain: Sexual function, satisfaction, and help-seeking among women living in Sweden
- Editorial comment
- The Norwegian version of the Neck Disability Index (NDI) is reliable and sensitive to changes in pain-intensity and consequences of pain-in-the-neck
- Clinical pain research
- Reliability and responsiveness of the Norwegian version of the Neck Disability Index
- Editorial comment
- Quality of life in low back pain patients with MRI-lesions in spinal bone marrow and vertebral endplates (Modic-changes): Clinical significance for outcome of spinal surgery?
- Clinical pain research
- Association of Modic changes with health-related quality of life among patients referred to spine surgery
- Editorial comment
- Warming and alkalinisation of lidocaine with epinephrine mixture: Some useful aspects at first glance, but not so simple?
- Clinical pain research
- Warmed and buffered lidocaine for pain relief during bone marrow aspiration and biopsy. A randomized and controlled trial
- Acknowledgement of Reviewers

