Abstract
An organic-inorganic hybrid compound based on {PMo12} layers, [Ag(pz)2]6[PMo12O40]2·3H2O 1 (pz = pyrazole), has been hydrothermally synthesized and characterized by elemental analysis, TG, IR spectroscopy, and single-crystal X-ray diffraction. Two crystallographically independent [PMo12O40]3– clusters are arranged in an AB–AB mode to give two kinds of supramolecular layers. The layers are further connected in a special alternating mode by [Ag(pz)2]+ linkers to form complex 3-D supramolecular network. Compound 1 exhibits good electrocatalytic activity in the reduction of hydrogen peroxide and shows fluorescence in the solid state at room temperature.
1 Introduction
Polyoxometalates (POMs), as significant metal-oxygen clusters with controllable shape, size, highly negative charges, and O-enriched surfaces have been widely employed as inorganic building units for constructing functional inorganic–organic hybrid materials [1–3]. Currently POM-based hybrid assemblies involve the selection and design of appropriate POM building blocks, organic ligands, and transition metals. Even though POM chemistry has been known for more than two centuries, it still attracts wide interest, because POMs show promising properties in the area of catalysis, magnetism, electronics, and photochemistry [3–6]. POMs with various structures have been known. As a class of well-defined oxo nanoclusters, Keggin-type POMs not only exhibit a wide variety of properties, remarkable coordination ability and modes, but also contain abundant terminal and bridging oxygen atoms, which can act as multi-potential hydrogen bonding acceptors. The introduction of organic ligands and transition metals into Keggin POM supramolecular assembles through hydrogen bonding or supramolecular interactions is an effective synthetic strategy to construct new functional materials [7–9]. Some Keggin-based inorganic-organic hybrid frameworks have been reported [10–14]. However, examples with pyrazol ligands as linkers to assemble Keggin units have remained relatively undeveloped.
SilverI is a good candidate for the assembly of mononuclear complexes due to its flexible coordination modes and high affinity to O- and N-containing ligands. It can provide various coordination modes, such as “seesaw,” “linear,” “square-pyramidal,” “T-type,” “trigonal-bipyramidal,” and “octahedral” geometries [15, 16]. AgI (d10) ions are often used as metals in building organic–inorganic hybrids [17, 18]. The application perspectives stimulated our interest in Keggin-shaped POMs modified by silver or silver complexes. Therefore, we attempted to introduce AgI cations and pyrazol ligands as linkers into the Keggin-containing reaction systems in order to explore new Keggin-based supramolecular assemblies.
On the basis of the previous work [19–22], we have synthesized the compound: [Ag(pz)2]6[PMo12O40]2·3H2O (1). Its electrochemical performance, electrocatalytic behavior, and solid-state fluorescence at room temperature have been investigated.
2 Results and discussion
2.1 Crystal structure of 1
X-ray diffraction analysis has revealed that compound 1 is constructed from six AgI cations, two Keggin-type [PMo12O40]3– polyanions, 12 pz ligands and three coordinating water molecules (Fig. 1). The polyoxoanions of 1 are composed of a central PO4 tetrahedron surrounded by four vertex-sharing Mo3O13 units, which emanate from the association of three edge-sharing MoO6 octahedra. The P–O distances range from 1.533(4) to 1.547(8) Å and the O–P–O bond angles are 109.17(19)° and 109.77(19)°. The Mo–O distances fall into three classes: Mo–Od (terminal), 1.674(5)–1.695(5) Å, Mo–Ob/c (bridge), 1.821(5)–2.006(5) Å, Mo–Oa (central) 2.420(5)–2.452(5) Å.
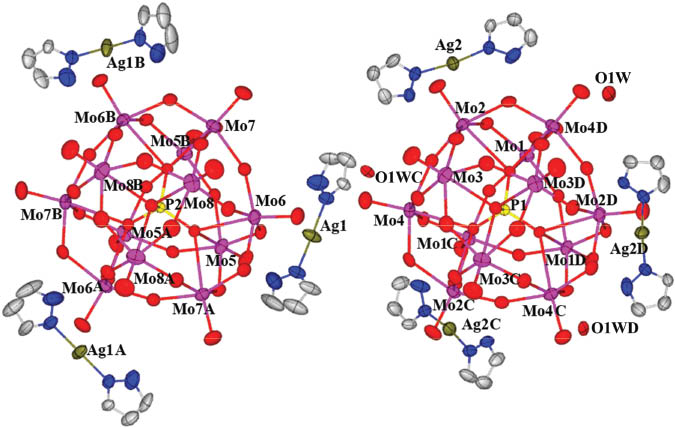
Molecular structure of compound 1 in the crystal.
Each AgI cation displays a linear coordination geometry to join two N atoms from two pz ligands via Ag–N bonds to form a short rod [Ag(pz)2]+. The Ag–N distances range from 2.111(8) to 2.169(9) Å. Three such short rods are connected to three water molecules in an alternating mode via hydrogen bonding interactions (N6···O1W 2.975(11) Å and N8···O1W 2.739(10) Å) to form macrocycles (Fig. 2). Two crystallographically independent Keggin clusters, [P(1)Mo12O40]3– and [P(2)Mo12O40]3–, are placed at the center of the ring via hydrogen bonding interactions (hydrogen bonds O2···O1W 2.825(8) Å, O17···N8 3.008(10) Å), in two kinds of subunits (Fig. 3). O1W links three short rods [Ag(2)(pz)2]+ to form a supramolecular ring system and further acts to connect Keggin clusters.
![Fig. 2: Supramolecular layer constructed of [P(1)Mo12O40]3– clusters and hydrogen bonding in a macrocycle (all H atoms omitted for clarity).](/document/doi/10.1515/znb-2014-0239/asset/graphic/znb-2014-0239_fig2.jpg)
Supramolecular layer constructed of [P(1)Mo12O40]3– clusters and hydrogen bonding in a macrocycle (all H atoms omitted for clarity).
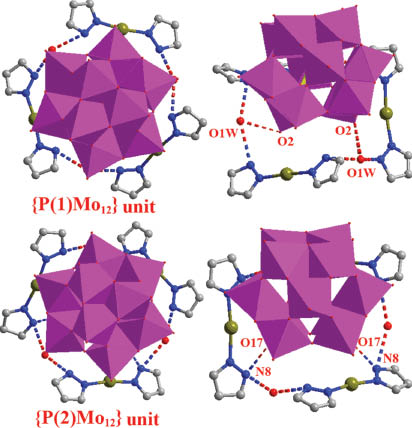
Hydrogen bonding between crystallographically independent Keggin clusters and the macrocycle.
Each [P(1)Mo12O40]3– subunit is connected to three adjacent [P(1)Mo12O40]3– units in AB–AB mode via supramolecular interactions (O11···O11 3.002 Å) to lead to supramolecular layers (Fig. 2) with hexagonal holes. The dimension of the holes is 13.34 × 13.34 Å (O(5)–O(5) distance). Each [P(2)Mo12O40]3– is further connected to three adjacent [P(2)Mo12O40]3– units in AB–AB mode via two [Ag(2)(pz)2]+ linkers to form another supramolecular layer along the c axis (Fig. 4). The main interactions are Ag2···O17 2.785 Å and Ag2···O22 3.155 Å. Another kind of hexagonal pores has dimensions of 13.53 × 13.53 Å (O(19)–O(19) distance). Two kinds of layers are further connected in a special alternating type (A-B-A-A-B-A-B-B) by [Ag(pz)2]+ linkers to form a complex 3-D supramolecular network (Fig. 5). The main supramolecular interactions are Ag2···O2 2.918 Å, Ag1···O19 2.791 Å, Ag1···O9 3.237 Å Ag1···O5 3.120 Å. Water molecules play an essential role in forming two kinds of hydrogen bonds (O1W···O2 2.825 Å, O1W···O17 3.076 Å).
![Fig. 4: A supramolecular layer constructed of [P(2)Mo12O40]3– clusters. Some Ag(pz)2 units are omitted for clarity.](/document/doi/10.1515/znb-2014-0239/asset/graphic/znb-2014-0239_fig4.jpg)
A supramolecular layer constructed of [P(2)Mo12O40]3– clusters. Some Ag(pz)2 units are omitted for clarity.
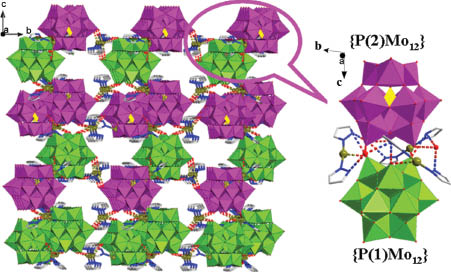
The 3D structure of compound 1 based on {P(1)Mo12} and {P(2)Mo12} layers arranged in an alternating mode (A-B-A-A-B-A-B-B).
According to bond valence sum calculations, all Mo atoms exhibit the +VI oxidation state (average calculated value = 5.99) and the Ag centers are in the +I oxidation state (average calculated value = 0.96), consistent with the overall charge balance.
2.2 Thermal analysis
The thermal stability of compound 1 was determined under nitrogen atmosphere by thermogravimetric analysis (TGA) from 25 to 800 °C. It shows two weight-loss steps: the first weight loss of 1.1 % in the temperature range of 60–93 °C corresponds to the release of the water molecules, which is in accordance with the calculated value of 1.0 % (∼3 H2O). The second weight loss of 15.3 % in the temperature range of 130–590 °C is attributed to the loss of all pz ligands, which is close to the calculated value of 15.8 % (∼12 pz).
2.3 Fluorescence
When excited at 397 nm, compound 1 exhibits a strong emission at 482 nm in the solid state, which can be assigned to charge transfer between AgI and the pz ligand [15]. The result indicates that the title compound is a potential fluorescence-emitting material (Fig. 6).
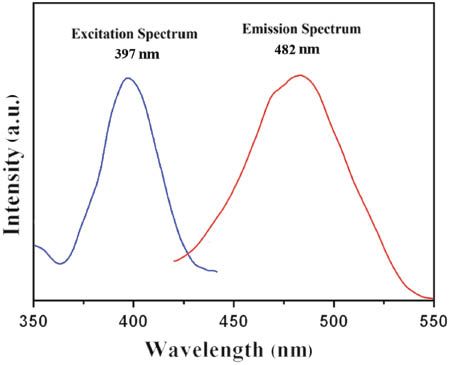
Fluorescence spectra of compound 1 at room temperature.
2.4 Voltammetric behavior of 1-CPE in aqueous electrolyte
Owing to the insolubility of compound 1 in water, its performance in carbon paste electrodes (CPE) can be studied. The cyclic voltammograms for 1-CPE in 1 m H2SO4 aqueous solution at different scan rates were recorded. As shown in Fig. 7a, three reversible redox peaks appear in the potential range –1.0 to 1.0 V. The half-wave potentials E1/2 = (Epa + Epc)/2 (scan rate: 20 mV·s–1) are –0.541 (I–I′), –0.353 (II–II′) and 0.123 (III–III′)V, in accordance with three consecutive two-electron processes of the Mo(VI/V) couples in {PMo12} [23, 24]. When the scan rates varied from 20 to 500 mV·s–1, the peak potentials changed gradually: the cathodic peak potentials (Epc) shifted slightly to negative values and the anodic peak potentials (Epa) shifted slightly to positive values with increasing scan rates. The peak-to-peak separations between the corresponding anodic and cathodic peaks increased, but the average peak potentials did not change. The behavior is consistent with a reversible but nonideal redox process [25, 26].
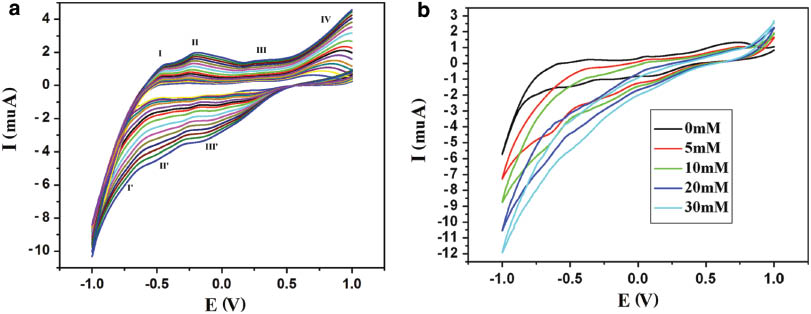
(a) Cyclic voltammograms of the 1-CPE in 1 m H2SO4 solution at different scan rates (from inner to outer: 20, 30, 40, 50, 60, 80, 100, 120, 150, 200, 250, 300, 350, 400, 450, and 500 mV·s–1); (b) Cyclic voltammograms of the 1-CPE in 1 m H2SO4 solution containing H2O2 at different concentrations (potentials vs. SCE; scan rate: 60 mV s–1).
2.5 Electrocatalytic activity of 1-CPE for the reduction of H2O2
The cyclic voltammograms for the electrocatalytic reduction of hydrogen peroxide in 1 m H2SO4 aqueous solution in the potential range from –1.0 to 1.0 V for 1 are shown in Fig. 7b. It can be clearly seen that with increasing H2O2 concentration (from 0.0 to 30 mm), all three reduction peak currents increased while the corresponding oxidation peak currents dramatically decreased, suggesting that H2O2 was reduced by the PMo12O403– polyanions. The results indicate that 1-CPE has good electrocatalytic activity toward the reduction of H2O2.
3 Conclusions
In summary, [Ag(pz)2]+ segments are first introduced into {PMo12} hybrids system to yield a complex 3-D supramolecular network constructed by two kinds of supramolecular layer with two types of hexagonal holes. The compound may provide a new model for the construction of POM-based organic–inorganic hybrid systems, and further studies are being conducted in our laboratory.
4 Experimental
4.1 Materials and measurements
All reagents were purchased commercially and used without further purification. H3PMo12O40·13H2O was prepared according to a literature method [27, 28] and verified by the IR spectrum. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer 2400 CHN elemental analyzer. P, Mo, and Ag analyses were performed on a PLASMA-SPEC (I) inductively coupled plasma atomic emission spectrometer. The IR spectra were obtained on an Alpha Centaurt FT/IR spectrometer with KBr pellets in the 400–4000 cm–1 region. The thermogravimetric analyses (TGA) were carried out in N2 on a Perkin-Elmer DTA 1700 differential thermal analyzer with a rate of 10 °C min–1. Fluorescence spectra were performed on a Hitachi F-4500 fluorescence/phosphorescence spectrophotometer with a 450 W xenon lamp as the excitation source. Electrochemical measurements were performed with a CHI660 electrochemical workstation. A conventional three-electrode system was used, with a modified carbon paste electrode (CPE) as a working electrode, a twisted platinum wire as counter electrode, and a commercial Ag/AgCl electrode as reference electrode.
4.2 Synthesis
A mixture of H3PMo12O40·13H2O (0.515 g, 0.25 mmol), V2O5 (0.045 g, 0.25 mmol), AgNO3 (0.085 g, 0.5 mmol), pz (0.034 g, 0.5 mmol), and H2O (10 mL) was stirred for 30 min in air until it was homogeneous. The mixture was then transferred to a Teflon-lined stainless steel autoclave (30 mL) and kept at 180 °C for 2 days. After the autoclave was cooled to room temperature, red block-like crystals were obtained in a yield of 25 % (based on Mo). – Anal. C36H54Ag6Mo24N24O83P2 (5162.75): calcd. C 8.37, H 1.05, N 6.51, Ag 12.54, Mo 44.60, P 1.20; found C 8.36, H 1.04, N 6.50, Ag 12.55, Mo 44.62, P 1.23 %. – IR (KBr pellet, cm–1): 3468 (br), 1613 (s), 1404 (m), 1067 (s), 968 (m), 876 (s), 794 (s).
4.3 X-ray crystallographic studies
The crystal structure of compound 1 was determined from single-crystal X-ray diffraction data. Intensity data were collected on a Bruker SMART CCD diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073Å). The structure was solved by Direct Methods and difference Fourier maps and refined by full-matrix least-squares techniques on F2 (shelxs/l-97 [29, 30]). Anisotropic displacement parameters were used to refine all nonhydrogen atoms. The positions of hydrogen atoms of the organic molecules were calculated in idealized positions and refined using a riding model. Hydrogen atoms of water molecules were not included. Crystal data and refinement parameters are summarized in Table 1. Selected bond lengths and bond angles of compound 1 are listed in Table 2 and hydrogen bond parameters in Table 3.
Crystal data and numbers pertinent to data collection and structure refinement of 1.
| Chemical formula | C36H54Ag6Mo24N24O83P2 |
| Formula weight | 5162.75 |
| T, K | 296(2) |
| Crystal size, mm3 | 0.20 × 0.18 × 0.17 |
| Crystal system | Rhombohedral (hexagonal axes) |
| Space group | R3̅ |
| a, Å | 20.437(2) |
| c, Å | 48.003(1) |
| V, Å3 | 17 363(4) |
| Z | 6 |
| Dcalcd, g cm–3 | 2.96 |
| μ, mm–1 | 3.6 |
| F(000), e | 14 460 |
| λ, Å | 0.71073 |
| θ range, deg | 1.23–28.22 |
| Reflections collected/unique/Rint | 36 736/9549/0.030 |
| Data/ref. parameters | 9488/526 |
| GOF on F2 | 1.058 |
| Final R1/wR2 [I> 2 σ(I)] | 0.0521/0.1646 |
| Final R1/wR2 (all data) | 0.0717/0.1835 |
| Δρfin (max/min), e Å–3 | 3.31/–2.69 |
Selected bond lengths (Å) and bond angles (deg) of compound 1.a
| P(1)–O(14)#1 | 1.533(4) | P(1)–O(14) | 1.533(4) |
| P(1)–O(14)#2 | 1.533(4) | P(1)–O(13) | 1.547(8) |
| P(2)–O(28) | 1.536(8) | P(2)–O(25)#4 | 1.540(5) |
| P(2)–O(25)#3 | 1.540(5) | P(2)–O(25) | 1.540(5) |
| Ag(1)–N(1) | 2.111(8) | Ag(1)–N(3) | 2.132(9) |
| Ag(2)–N(5) | 2.157(9) | Ag(2)–N(7) | 2.169(9) |
| O(14)#1–P(1)–O(14)#2 | 109.27(19) | O(14)#1–P(1)–O(14) | 109.27(19) |
| O(14)#1–P(1)–O(13) | 109.67(19) | O(28)–P(2)–O(25)#3 | 109.75(19) |
| O(28)–P(2)–O(25) | 109.75(19) | O(28)–P(2)–O(25)#4 | 109.8(2) |
| N(1)–Ag(1)–N(3) | 175.5(3) | N(5)–Ag(2)–N(7) | 172.5(3) |
aSymmetry transformations used to generate equivalent atoms: #1–y+ 1, x– y, z; #2–x + y + 1, –x + 1, z; #3y + 2, x– y + 1, z; #4–x + y + 1, –x + 2, z.
Selected hydrogen bond lengths (Å) and bond angles (deg) of compound 1.a
| D–H···A | d(D–H) | d(H···A) | <(D–H···A) | d(D···A) |
|---|---|---|---|---|
| N6–H7···O2#5 | 0.86 | 2.51 | 127.8 | 3.112(11) |
| N6–H7···O1W#6 | 0.86 | 2.38 | 127.2 | 2.975(11) |
| N8–H4···O1#7 | 0.86 | 2.38 | 164.3 | 3.213(9) |
| O1W–H1WB···O17#2 | 0.85 | 2.55 | 121.4 | 3.076(8) |
| O1W–H1WB···N8#2 | 0.85 | 2.00 | 145.2 | 2.739(10) |
| O1W–H1WA···O2#8 | 0.85 | 1.99 | 168.3 | 2.825(8) |
| N8–H4···O17 | 0.86 | 2.60 | 110.4 | 3.008(10) |
| N4–H3···O26 | 0.86 | 2.64 | 151.8 | 3.419(17) |
| N4–H3···O20 | 0.86 | 2.60 | 136.9 | 3.28(2) |
aSymmetry codes: #2–x + y + 1, –x + 1, z; #5–x + 5/3, –y + 4/3, –z + 1/3; #6x, y + 1, z; #7y + 2/3, –x + y + 4/3, –z + 1/3; #8–x + 5/3, –y + 1/3, –z + 1/3.
CCDC 988814 contains the supplementary crystallographic data for this article. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
This work was supported the National Natural Science Foundation of China (Grants Nos. 21271056 and 21371042), the Ministry of Education and Specialised Research Fund for the Doctoral Program of Higher Education (20122329110001), the Natural Science Foundation of Heilongjiang Province (B201216), Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education, Doctoral initiation Foundation of Harbin Normal University (No. KGB201214), and Program for Scientific and Technological Innovation Team Construction in Universities of Heilongjiang Province (No. 2011TD010).
References
[1] C. L. Hill, Chem. Rev.1998, 98, 1.Search in Google Scholar
[2] H. Y. An, E. B. Wang, D. R. Xiao, Y. G. Li, Z. M. Su, L. Xu, Angew. Chem. Int. Ed.2006, 45, 904.Search in Google Scholar
[3] D. L. Long, E. Burkholder, L. Cronin, Chem. Soc. Rev. 2007, 36, 105.Search in Google Scholar
[4] Y. F. Song, R. Tsunashima, Chem. Soc. Rev. 2012, 41, 7384.Search in Google Scholar
[5] C. L. Hill, G. S. Kim, C. M. Prosser-McCartha, D. Judd, Mol. Eng. 1993, 3, 263.Search in Google Scholar
[6] E. Coronado, C. Giménez-Saiz, C. Gómez-García, Coord. Chem. Rev. 2005, 249, 1776.Search in Google Scholar
[7] J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. I. Hardcastle, C. L. Hill, J. Am. Chem. Soc. 2011, 133, 16839.Search in Google Scholar
[8] Y. Sawada, W. Kosaka, Y. Hayashi, H. Miyasaka, Inorg. Chem. 2012, 51, 4824.Search in Google Scholar
[9] G. C. Liu, Y. F. Wang, A. X. Tian, X. L. Wang, J. J. Cao, S. Yang, H. Y. Lin, Z. Anorg. Allg. Chem.2013, 639, 148.Search in Google Scholar
[10] Y. Bai, G. Q. Zhang, D. B. Dang, P. T. Ma, H. Gao, J. Y. Niu, Cryst. Eng. Comm.2011, 13, 4181.Search in Google Scholar
[11] B. X. Han, C. Z. Wang, Y. Zhao, K. Chen, X. Xiao, Q. J. Zhu, S. F. Xue, Y. Q. Zhang, Z. Tao, Eur. J. Org. Chem. 2014, 5, 831.Search in Google Scholar
[12] X. L. Wang, J. Li, A. X. Tian, D. Zhao, G. C. Liu, H. Y. Lin, Cryst. Growth Des.2011, 11, 3456.Search in Google Scholar
[13] X. Q. Huang, X. M. Zhang, D. Zhang, S. Yang, X. Feng, J. K. Li, Z. G. Lin, J. Cao, R. Pan, Y. N. Chi, B. Wang, C. W. Hu, Chem. Eur. J.2014, 20, 2557.Search in Google Scholar
[14] S. Jones, A. Aldous, E. Burkholder, J. Zubieta, Polyhedron. 2013, 52, 582.Search in Google Scholar
[15] J. Q. Sha, L. Y. Liang, J. W. Sun, A. X. Tian, P. F. Yan, G. M. Li, C. Wang, Cryst. Growth Des. 2012, 12, 894.Search in Google Scholar
[16] H. J. Pang, J. Chen, J. Peng, J. Q. Sha, Z. Y. Shi, A. X. Tian, P. P. Zhang, Solid State Sci. 2009, 11, 824.Search in Google Scholar
[17] X. L. Wang, N. Li, A. X. Tian, J. Ying, G. C. Liu, H. Y. Lin, J. W. Zhang, Y. Yang, Dalton Trans. 2013, 42, 14856.Search in Google Scholar
[18] A. X. Tian, X. L. Lin, N. Sun, J. Ying, J. W. Zhang, N. Li, X. L. Wang, Inorg. Chem. Commun.2014, 40, 51.Search in Google Scholar
[19] M. L. Qi, K. Yu, Z. H. Su, C. X. Wang, C. M. Wang, B. B. Zhou, C. C. Zhu, Inorg. Chim. Acta2013, 400, 59.10.1016/j.ica.2013.01.030Search in Google Scholar
[20] M. L. Qi, K. Yu, Z. H. Su, C. X. Wang, C. M. Wang, B. B. Zhou, C. C. Zhu, Inorg. Chem. Commun. 2013, 30, 173.Search in Google Scholar
[21] M. L. Qi, K. Yu, Z. H. Su, C. X. Wang, C. M. Wang, B. B. Zhou, C. C. Zhu, Dalton Trans. 2013, 42, 7586.Search in Google Scholar
[22] H. Zhang, K. Yu, S. Gao, C. M. Wang, C. X, Wang, H. Y. Wang, B. B. Zhou, Inorg. Chem. Commun. 2014, 44, 91.Search in Google Scholar
[23] P. Wang, Y. Yuan, Z. B. Han, G. Y. Zhu, J. Mater. Chem. 2001, 11, 549.Search in Google Scholar
[24] X. L. Wang, H. Y. Lin, Y. F. Bi, B. K. Chen, G. C. Liu, J. Solid State Chem. 2008, 181, 556.Search in Google Scholar
[25] S. P. Liu, F. Y. Li, W. H. Guo, Y. Xing, Z. X. Sun, Electrochim. Acta. 2011, 56, 8156.Search in Google Scholar
[26] S. Q. Liu, D. G. Kurth, B. Bredenkötter, D. Volkmer, J. Am. Chem. Soc. 2002, 124, 12279.Search in Google Scholar
[27] C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck, R. Thouvenot, Inorg. Chem.1983, 22, 207.Search in Google Scholar
[28] T. F. S. Silva, L. M. D. R. S. Martins, M. F. C. G. D. Silva, M. L. Kuznetsov, A. R. Fernandes, A. Silva, C. J. Pan, J. F. Lee, B. J. Hwang, A. J. L. Pombeiro, Chem. Asian J. 2014, 9, 1132.Search in Google Scholar
[29] G. M. Sheldrick, shelxs/l-97, Programs for Crystal Structure Determination, University of Göttingen, Göttingen (Germany) 1997.Search in Google Scholar
[30] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.Search in Google Scholar
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)

