Abstract
An effective one-pot synthesis of benzene derivatives using a Diels–Alder reaction of activated acetylenes with phosphoryl-2-oxo-2H-pyrans is described. The latter compounds were synthesized in good yield via the reaction of dialkyl acetylenedicarboxylates and alkyl bromides in the presence of trialkyl phosphites under solvent-free conditions at 50 oC.
1 Introduction
Functionalized benzene derivatives are frequently favored in organic chemistry, natural product chemistry, and materials science [1–4]. The multicomponent reaction of benzene ring is an interesting option in benzene synthesis. For instance, [2 + 2 + 2] syntheses have been achieved by transition metal-catalyzed reactions [5–8], and [4 + 2] syntheses are also catalyzed by transition metals reactions or induced thermally [5, 9, 10]. The chemistry of aromatic compound bearing carboxylate groups has obtained considerable notice due to the diversity of bridging facilities of these compounds in the creation of inorganic-organic skeletons. Especially, benzenecarboxylate ligands have been applied as building blocks in the design of metal-organic materials with wanted topologies owing to their full coordination modes [11–14]. Carbon nucleophiles (anions of carboxylic acids, organometallics, ylides, enamines, enol ethers, etc.) frequently add to alkynes only in the presence of some activating substituent, reaction-facilitating solvents, specific coordination sites, or catalysts [15]. In many cases, cyclic products such as benzene derivatives are achieved. Consequently, in this report, we investigate the synthesis of benzene derivatives using the reaction of phosphoryl-2-oxo-2H-pyrans with dialkyl acetylenedicarboxylate without any catalyst.
2 Results and discussion
At first, we describe the synthesis of dialkoxy 3-phosphoryl-2-oxo-2H-pyrans 4 under solvent-free conditions through the reaction of trivalent phosphorus nucleophile 1 with dialkyl acetylenedicarboxylate 2 and alkyl bromides 3 (Scheme 1).
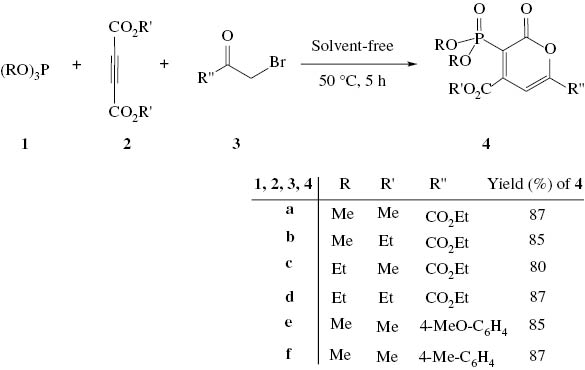
Synthesis of phosphoryl-2-oxo-2H-pyrans 4.
The 1H NMR spectrum of 4a showed one singlet for methoxy protons at δ = 3.78 ppm and one singlet for a methine proton at δ = 7.62 ppm. The two methoxy groups of the phosphoryl moiety display one doublet at 3.75 ppm (d, 3JHP = 11.5 Hz). The 13C NMR spectrum of 4a showed one doublet for the two methoxy groups of the phosphoryl moiety at 52.4 ppm (d, 2JPC = 8.2 Hz) and the resonance of a methine group at 121.4 ppm (d, 3JPC = 23.2 Hz, CH), along with resonances of carbonyl groups at 159.4 ppm (d, 2JPC = 5.4 Hz), 162.4 ppm, 167.2 ppm (d, 3JPC = 24.3 Hz) in agreement with the proposed structure. The 31P NMR signals was found at δ = 17.8 ppm. On the basis of the well-established chemistry of trivalent phosphorus nucleophiles, it is reasonable to guess that phosphoryl-2-oxo-2H-pyrans 4 results from the initial addition of trialkyl phosphite to the acetylenic compound and subsequent attack of the resulting anion 5 to the carbon of alkyl bromides 3 to yield intermediate 6, which apparently cyclizes under the reaction conditions employed to generate the phosphonate derivatives 4 (Scheme 2).
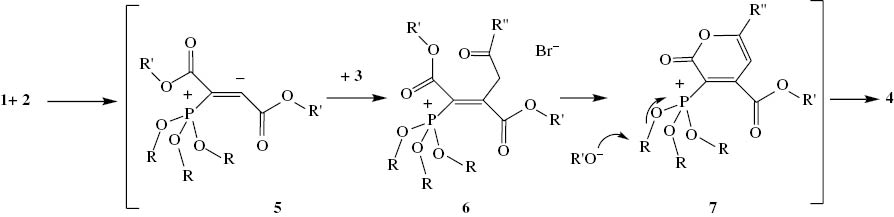
Proposed mechanism for the formation of 4.
After the synthesis of phosphoryl-2-oxo-2H-pyrans, synthesis of benzene derivatives 9 is performed using the Diels–Alder reaction of phosphoryl-2-oxo-2H-pyran 4 with dialkyl acetylenedicarboxylate 2 in toluene under reflux conditions (Scheme 3).
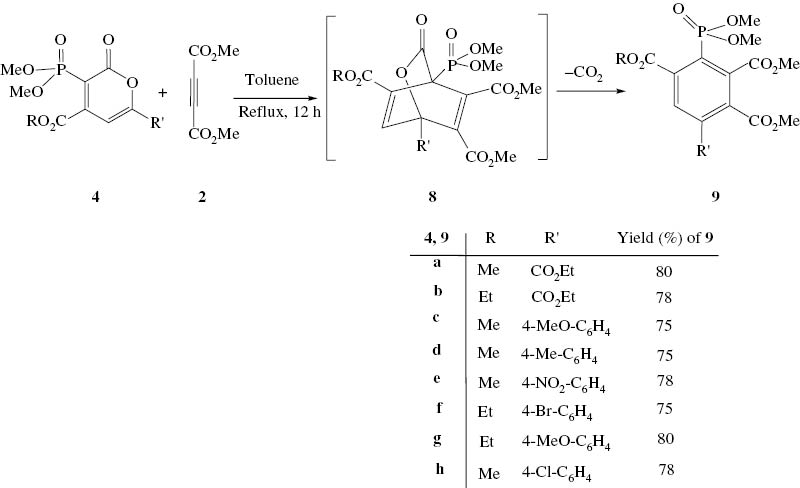
Dielz-Alder reaction for synthesis of benzene derivatives.
The 1H NMR spectrum of 9a showed one doublet at δ = 3.78 ppm (d, 3JHP = 11.8 Hz) for two methoxy groups of the phosphoryl moiety, three singlets at δ = 3.85, 3.87 and 3.92 ppm for methoxy protons, and one singlet at 8.72 ppm for a methine proton. The 13C NMR spectrum of 9a showed three singlets at δ = 51.8, 52.2, and 52.6 ppm for methoxy groups and one doublet for two methoxy groups of the phosphoryl moiety at 53.7 ppm (d, 2JPC = 11.2 Hz) and the resonance of methine group at 133.8 ppm (d, 3JPC = 21.7 Hz) along with the resonance of carbonyl groups at 160.2 ppm (d, 3JPC = 24.2 Hz), 161.4 ppm, 168.7 ppm (d, 3JPC = 19.7 Hz), and 169.4 ppm in agreement with the proposed structure.
3 Conclusion
In summary, the three-component reaction of trialkyl phosphite, dialkyl acetylenedicarboxylate, and alkyl bromides under solvent-free conditions provided 3-phosphoryl-2-oxo-2H-pyran derivatives in good yields. Diels–Alder reaction of 3-phosphoryl-2-oxo-2H-pyran derivatives with dialkyl acetylenedicarboxylates produced benzene derivatives under reflux condition in toluene in good yield. The advantages of these reactions involve good yield and easy reaction workup procedures.
4 Experimental
4.1 General
Melting points were taken on a Kofler hot stage apparatus and are uncorrected. 1H, 13C, and 31P NMR spectra were obtained with a Bruker FT-500 spectrometer in CDCl3, and tetramethylsilane (TMS) was used as an internal standard or 85 % H3PO4 as external standard. Mass spectra were recorded with a Finnigan Mat TSQ-70 spectrometer. Infrared (IR) spectra were acquired on a Nicolet Magna 550-FT spectrometer. Elemental analyses were carried out with a Perkin–Elmer model 240-C apparatus. The results of elemental analyses (C, H, N) were within ±0.4 % of the calculated values. Acetylenic ester, phenacyl bromide or its derivatives, and phosphites were obtained from Fluka and were used without further purification.
4.2 General procedure for the preparation of pyrane derivatives 4
To a stirred mixture of an alkyl bromide 3 (2 mmol) and a dialkyl acetylenedicarboxylate 2 (2 mmol) was added trialkyl phosphite 1 (2 mmol) at 50 °C. The reaction mixture was stirred for 5 h at 50 °C. After completion of the reaction (monitored by TLC), 15 mL of H2O was poured into the reaction mixture, and the solid residue was filtered and washed with cold diethylether (Et2O) to afford 4.
4.3 6-Ethyl-4-methyl-3-(dimethoxyphosphoryl)-2-oxo-2H-pyran-4,6-dicarboxylate (4a)
Yellow powder; yield: 0.58 g (87 %). – IR (KBr): νmax = 1742, 1737, 1565, 1478, 1263, 1157 cm-1. – MS: m/z (%) = 334 (15) [M]+, 303 (58), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.27 (3 H, t, 3JHH = 7.5 Hz, Me), 3.75 (6 H, d, 3JHP = 11.5 Hz, 2 MeO), 3.87 (3 H, s, MeO), 4.22 (2 H, q, 3JHH = 7.5 Hz, CH2O), 7.62 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 13.8 (Me), 52.4 (d, 2JPC = 8.2 Hz, 2 MeO), 53.7 (MeO), 61.4 (CH2O), 118.4 (d, 1JPC = 142.4 Hz, C), 121.4 (d, 3JPC = 23.2 Hz, CH), 148.5 (C), 152.3 (d, 2JPC = 9.4 Hz, C), 159.4 (d, 2JPC = 5.4 Hz, C=O), 162.4 (C=O), 167.2 (d, 3JPC = 24.3 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 17.8 ppm. – Anal. calcd. for C12H15O9P (334.22): C 43.13, H 4.52; found: C 43.24, H 4.63 %.
4.4 Diethyl-3-(dimethoxyphosphoryl)-2-oxo-2H-pyran-4,6-dicarboxylate (4b)
Yellow powder, yield: 0.59 g (85 %). – IR (KBr): νmax = 1745, 1738, 1574, 1483, 1276, 1195 cm-1. – MS: m/z (%) = 348 (10) [M]+, 317 (64), 45 (88), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.28 (3 H, t, 3JHH = 7.4 Hz, Me), 1.32 (3 H, t, 3JHH = 7.3 Hz, Me), 3.78 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 4.23 (2 H, q, 3JHH = 7.4 Hz, CH2O), 4.26 (2 H, q, 3JHH = 7.3 Hz, CH2O), 7.67 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 14.0 (Me), 14.2 (Me), 51.8 (d, 2JPC = 8.5 Hz, 2 MeO), 61.5 (CH2O), 62.3 (CH2O), 119.2 (d, 1JPC = 140.4 Hz, C), 122.1 (d, 3JPC = 22.8 Hz, CH), 149.2 (C), 151.8 (d, 2JPC = 8.7 Hz, C), 158.4 (d, 2JPC = 6.2 Hz, C=O), 163.2 (C=O), 166.5 (d, 3JPC = 22.3 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 18.4 ppm. – Anal. calcd. for C13H17O9P (348.24): C 44.84, H 4.92; found: C 44.72, H 4.83 %.
4.5 6-Ethyl-4-methyl-3-(diethoxyphosphoryl)-2-oxo-2H-pyran-4, 6-dicarboxylate (4c)
Yellow powder; yield: 0.58 g (80 %). – IR (KBr): νmax = 1747, 1742, 1587, 1495, 1234, 1147 cm-1. – MS: m/z (%) = 362 (15) [M]+, 331 (88), 317 (82), 45 (100), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.23 (3 H, t, 3JHH = 7.5 Hz, Me), 1.27 (3 H, t, 3JHH = 7.5 Hz, Me), 1.34 (3 H, t, 3JHH = 7.4 Hz, Me), 3.87 (MeO), 4.12 (2 H, m, CH2O), 4.22 (2 H, m, CH2O), 4.28 (2 H, q, 3JHH = 7.5 Hz, CH2O), 7.72 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 13.7 (Me), 14.0 (d, 3JPC = 21.8 Hz, Me), 14.3 (d, 3JPC = 21.8 Hz, Me), 52.3 (MeO), 61.4 (CH2O), 62.3 (d, 2JPC = 9.8 Hz, CH2O), 63.2 (d, 2JPC = 9.8 Hz, CH2O), 118.4 (d, 1JPC = 138.7 Hz, C), 124.2 (d, 3JPC = 23.4 Hz, CH), 148.6 (C), 152.3 (d, 2JPC = 9.4 Hz, C), 156.2 (d, 2JPC = 6.5 Hz, C=O), 163.7 (C=O), 167.2 (d, 3JPC = 22.7 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 18.7 ppm. – Anal. calcd. for C14H19O9P (362.23): C 46.42, H 5.29; found: C 46.53, H 5.34 %.
4.6 Diethyl-3-(diethoxyphosphoryl)-2-oxo-2H-pyran-4,6-dicarboxylate (4d)
Yellow powder; yield: 0.65 g (87 %). – IR (KBr): νmax = 1740, 1737, 1592, 1486, 1264, 1183 cm-1. – MS: m/z (%) = 376 (15) [M]+, 331 (86), 45 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.25 (3 H, t, 3JHH = 7.3 Hz, Me), 1.30 (3 H, t, 3JHH = 7.3 Hz, Me), 1.36 (3 H, t, 3JHH = 7.4 Hz, Me), 1.42 (3 H, t, 3JHH = 7.5 Hz, Me), 4.15 (2 H, m, CH2O), 4.26 (2 H, m, CH2O), 4.32 (2 H, q, 3JHH = 7.5 Hz, CH2O), 4.40 (2 H, q, 3JHH = 7.5 Hz, CH2O), 7.78 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 13.8 (Me), 14.2 (Me), 14.8 (d, 3JPC = 19.2 Hz, Me), 15.3 (d, 3JPC = 19.3 Hz, Me), 60.4 (CH2O), 61.2 (CH2O), 62.0 (d, 2JPC = 10.2 Hz, CH2O), 62.8 (d, 2JPC = 10.2 Hz, CH2O), 116.3 (d, 1JPC = 139.4 Hz, C), 124.7 (d, 3JPC = 23.5 Hz, CH), 149.2 (C), 153.4 (d, 2JPC = 9.8 Hz, C), 159.4 (d, 2JPC = 6.8 Hz, C=O), 164.2 (C=O), 167.5 (d, 3JPC = 21.2 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 19.4 ppm. – Anal. calcd. for C15H21O9P (376.29): C 47.88, H 5.63; found: C 74.92, H 5.74 %.
4.7 Methyl-3-(dimethoxyphosphoryl)-6- (4-methoxyphenyl)-2-oxo-2H-pyran-4-carboxylate (4e)
Yellow powder; yield: 0.63 g (85 %). – IR (KBr): νmax = 1732, 1692, 1585, 1426, 1254, 1125 cm-1. – MS: m/z (%) = 368(20) [M]+, 337 (78), 107 (48), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 3.67 (6 H, d, 3JHP = 12.2 Hz, 2 MeO), 3.78 (3 H, s, MeO), 3.89 (3 H, s, MeO), 6.87 (1 H, s, CH), 7.34 (2 H, d, 3JHH = 7.8 Hz, 2 CH), 7.62 (2 H, d, 3JHH = 7.8 Hz, 2 CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 52.4 (MeO), 53.2 (d, 2JPC = 8.7 Hz, 2 MeO), 55.6 (MeO), 100.2 (d, 3JPC = 22.4 Hz, CH), 113.2 (2 CH), 115.4 (d, 1JPC = 143.2 Hz, C), 124.2 (2 CH), 136.4 (C), 153.5 (d, 2JPC = 9.8 Hz, C), 156.2 (C), 159.6 (d, 2JPC = 6.3 Hz, C=O), 161.4 (C), 164.2 (d, 3JPC = 23.8 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 20.3 ppm. – Anal. calcd. for C16H17O8P (368.28): C 52.18, H 4.65; found: C 52.24, H 4.76 %.
4.8 Methyl-3-(dimethoxyphosphoryl)-6- (4-methylphenyl)-2-oxo-2H-pyran-4-carboxylate (4f)
Yellow powder; yield: 0.63 g (87 %). – IR (KBr): νmax = 1738, 1687, 1574, 1453, 1284, 1163 cm-1. – MS: m/z (%) = 352 (15) [M]+, 321 (64), 91 (56), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 2.36 (Me), 3.72 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 3.85 (3 H, s, MeO), 6.92 (1 H, s, CH), 7.28 (2 H, d, 3JHH = 7.6 Hz, 2 CH), 7.58 (2 H, d, 3JHH = 7.6 Hz, 2 CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 22.3 (Me), 51.2 (d, 2JPC = 9.6 Hz, 2 MeO), 52.7 (MeO), 98.6 (d, 3JPC = 20.7 Hz, CH), 115.8 (d, 1JPC = 139.4 Hz, C), 123.7 (2 CH), 128.4 (2 CH), 135.2 (C), 137.5 (C), 152.7 (d, 2JPC = 10.4 Hz, C), 158.4 (d, 2JPC = 7.0 Hz, C=O), 162.3 (C), 165.6 (d, 3JPC = 22.7 Hz, C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 21.4 ppm. – Anal. calcd. for C16H17O7P (352.28): C 54.55, H 4.86; found: C 54.42, H 4.73 %.
4.9 General procedure for the preparation of benzene derivatives 9
The solution of a dialkoxy phosphoryl-2-oxo-2H-pyran 4 (2 mmol) and dimethyl acetylenedicarboxylate 2 (2 mmol) in toluene was stirred under reflux conditions for 12 h. The solvent is evaporated, and the viscous residue was purified by column chromatography on silica gel (Merck 230–400 mesh) using n-hexane-EtOAc (7:1) as eluent to afford 9.
4.10 1-Ethyl-2,3,5-trimethyl 4-(dimethoxyphosphoryl)-1,2,3,5-benzenetetracarboxylate (9a)
Pale yellow powder; yield: 0.69 g (80 %). – IR (KBr): νmax = 1745, 1740, 1738, 1697, 1587, 1469, 1357, 1284, 1129 cm-1. MS: m/z (%) = 432 (10) [M]+, 401 (86), 45 (88), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.32 (3 H, t, 3JHH = 7.4 Hz, Me), 3.78 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 3.85 (3 H, s, MeO), 3.87 (3 H, s, MeO), 3.92 (3 H, s, MeO), 4.26 (2 H, q, 3JHH = 7.4 Hz, CH2O), 8.72 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 14.0 (Me), 51.8 (MeO), 52.2 (MeO), 52.6 (MeO), 53.7 (d, 2JPC = 11.2 Hz, 2 MeO), 61.5 (CH2O), 133.2 (d, 2JPC = 10.8 Hz, C), 133.8 (d, 3JPC = 21.7 Hz, CH), 134.8 (d, 2JPC = 11.5 Hz, C), 138.2 (d, 3JPC = 21.4 Hz, C), 139.7 (C), 147.5 (d, 1JPC = 138.7 Hz, C), 160.2 (d, 3JPC = 24.2 Hz, C=O), 161.4 (C=O), 168.7 (d, 3JPC = 19.7 Hz, C=O), 169.4 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 19.2 ppm. – Anal. calcd. for C17H21O11P (432.32): C 47.23, H 4.90; found: C 47.36, H 5.06 %.
4.11 1,5-Diethyl 2,3-dimethyl 4-(dimethoxyphosphoryl)-1,2,3,5-benzenetetracarboxylate (9b)
Yellow powder; yield: 0.67 g (75 %). – IR (KBr): νmax = 1744, 1739, 1695, 1487, 1376, 1295 cm-1. – MS: m/z (%) = 446 (15) [M]+, 415 (66), 45 (68), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.25 (3 H, t, 3JHH = 7.5 Hz, Me), 1.34 (3 H, t, 3JHH = 7.4 Hz, Me), 3.79 (6 H, d, 3JHP = 11.5 Hz, 2 MeO), 3.84 (MeO), 3.87 (MeO), 4.28 (2 H, q, 3JHH = 7.5 Hz, CH2O), 4.32 (2 H, q, 3JHH = 7.4 Hz, CH2O), 8.54 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 13.6 (Me), 14.0 (Me), 51.7 (MeO), 52.4 (MeO), 53.6 (d, 2JPC = 9.2 Hz, 2 MeO), 61.2 (CH2O), 62.5 (CH2O), 132.4 (d, 2JPC = 8.7 Hz, C), 133.5 (d, 3JPC = 21.4 Hz, CH), 134.3 (d, 2JPC = 9.5 Hz, C), 137.4 (d, 2JPC = 10.2 Hz, C), 139.8 (C), 147.5 (d, 1JPC = 140.2 Hz, C), 9.2 (C), 159.6 (d, 3JPC = 21.7 Hz, C=O), 160.7 (C=O), 162.8 (d, 3JPC = 22.5 Hz, C=O), 167.4 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 18.8 ppm. – Anal. calcd. for C18H23O11P (446.34): C 48.44, H 5.19; found: C 48.52, H 5.32 %.
4.12 Trimethyl-3-(dimethoxyphosphoryl)-6-(4-methoxyphenyl)-1,2,4-benzenetricarboxylate (9c)
Yellow powder; yield: 0.73 g (75 %). – IR (KBr): νmax = 1742, 1738, 1735, 1697, 1587, 1464, 1373, 1225 cm-1. – MS: m/z (%) = 466 (20) [M]+, 435 (88), 107 (68), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 3.72 (6 H, d, 3JHP = 12.5 Hz, 2 MeO), 3.85 (3 H, s, MeO), 3.87 (3 H, s, MeO), 3.90 (MeO), 3.94 (MeO), 7.32 (2 H, d, 3JHH = 7.6 Hz, 2 CH), 7.75 (2 H, d, 3JHH = 7.6 Hz, 2 CH), 8.62 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 51.4 (MeO), 52.0 (MeO), 52.3 (MeO), 53.6 (d, 2JPC = 9.4 Hz, 2 MeO), 55.4 (MeO), 112.8 (2 CH), 124.8 (2 CH), 125.6 (d, 2JPC = 9.7 Hz, C), 126.2 (C), 126.6 (d, 3JPC = 21.8 Hz, C), 127.2 (d, 3JPC = 22.5 Hz, CH), 127.8 (d, 2JPC = 8.7 Hz, C), 144.2 (d, 1JPC = 141.2 Hz, C), 146.8 (C), 155.7 (C), 159.7 (d, 3JPC = 21.4 Hz, C=O), 160.7 (d, 3JPC = 22.3 Hz, C=O), 167.4 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 19.8 ppm. – Anal. calcd. for C21H23O10P (466.38): C 54.08, H 4.97; found: C 54.23, H 5.18 %.
4.13 Trimethyl-3-(dimethoxyphosphoryl)-6-(4-methylphenyl)-1,2,4-benzenetricarboxylate (9d)
Yellow powder; yield: 0.63 g (75 %). – IR (KBr): νmax = 1745, 1740, 1738, 1695, 1587, 1465, 1357, 1215 cm-1. – MS: m/z (%) = 450 (15) [M]+, 419 (66), 91 (86), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 2.28 (Me), 3.75 (6 H, d, 3JHP = 11.5 Hz, 2 MeO), 3.84 (3 H, s, MeO), 3.87 (3 H, s, MeO), 3.92 (3 H, s, MeO), 7.32 (2 H, d, 3JHH = 7.5 Hz, 2 CH), 7.75 (2 H, d, 3JHH = 7.5 Hz, 2 CH), 8.57 (1 H, s, CH), ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 22.5 (Me), 51.4 (MeO), 52.3 (MeO), 52.7 (MeO), 53.7 (d, 2JPC = 10.2 Hz, 2 MeO), 121.8 (C), 123.5 (2 CH), 125.4 (2 CH), 125.8 (d, 2JPC = 11.2 Hz, C), 126.2 (d, 3JPC = 21.2 Hz, C), 127.2 (d, 3JPC = 21.4 Hz, CH), 127.8 (d, 2JPC = 10.8 Hz, C), 133.4 (C), 134.7 (C), 144.3 (d, 1JPC = 138.7 Hz, C), 160.2 (d, 3JPC = 23.2 Hz, C=O), 165.3 (d, 3JPC = 22.4 Hz, C=O), 167.8 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 20.7 ppm. – Anal. calcd. for C21H23O9P (450.38): C 56.00, H 5.15; found: C 56.22, H 5.28 %.
4.14 Trimethyl-3-(dimethoxyphosphoryl)-6-(4-Nitrophenyl)-1,2,4-benzenetricarboxylate (9e)
Yellow powder; yield: 0.63 g (78 %). – IR (KBr): νmax = 1742, 1738, 1735, 1697, 1562, 1487, 1352, 1295 cm-1. – MS: m/z (%) = 481 (10) [M]+, 450 (86), 122 (82), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 3.82 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 3.85 (3 H, s, MeO), 3.88 (3 H, s, MeO), 3.93 (3 H, s, MeO), 7.57 (2 H, d, 3JHH = 7.8 Hz, 2 CH), 8.62 (1 H, s, CH), 8.22 (2 H, d, 3JHH = 7.8 Hz, 2 CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 51.8 (MeO), 52.2 (MeO), 52.8 (MeO), 53.5 (d, 2JPC = 10.5 Hz, 2 MeO), 120.1 (2 CH), 122.8 (d, 3JPC = 20.8 Hz, C),123.6 (d, 3JPC = 21.5 Hz, CH), 125.6 (2 CH), 126.2 (d, 2JPC = 11.5 Hz, C), 127.2 (C), 127.6 (d,2JPC = 11.4 Hz, C), 140.2 (C), 144.5 (C), 145.8 (d, 1JPC = 139.2 Hz, C), 160.4 (d, 3JPC = 22.3 Hz, C=O), 164.8 (d, 3JPC = 21.7 Hz, C=O), 168.4 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 22.4 ppm. – Anal. calcd. for C20H20NO11P (481.35): C 49.90, H 4.19, N 2.91; found: C 49.78, H 4.02, N 2.75 %.
4.15 4-Ethyl-1,2-dimethyl3-(dimethoxyphosphoryl)-6- (4-bromophenyl)-1,2,4-benzenetricarboxylate (9f)
Pale yellow powder; yield: 0.79 g (75 %). – IR (KBr): νmax = 1738, 1735, 1730, 1695, 1578, 1457, 1355, 1298 cm-1. – 1H NMR (500 MHz, CDCl3): δ = 1.28 (3 H, t, 3JHH = 7.5 Hz, Me), 3.85 (6 H, d, 3JHP = 11.5 Hz, 2 MeO), 3.87 (3 H, s, MeO), 3.92 (3 H, s, MeO), 4.26 (2 H, q, 3JHH = 7.4 Hz, CH2O), 7.38 (2 H, d, 3JHH = 7.6 Hz, 2 CH), 7.45 (2 H, d, 3JHH = 7.6 Hz, 2 CH), 8.58 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 13.7 (Me), 51.6 (MeO), 52.4 (MeO), 53.8 (d, 2JPC = 10.8 Hz, 2 MeO), 61.2 (CH2O), 116.7 (C), 124.2 (2 CH), 125.2 (d, 2JPC = 10.2 Hz, C), 125.8 (C), 126.5 (d, 3JPC = 21.8 Hz, C), 126.8 (d, 3JPC = 22.4 Hz, CH), 127.2 (2 CH), 127.8 (d, 2JPC = 10.8 Hz, C), 135.4 (C), 144.2 (d, 1JPC = 138.6 Hz, C), 161.7 (d, 3JPC = 22.8 Hz, C=O), 165.2 (d, 3JPC = 22.3 Hz, C=O), 168.7 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 22.8 ppm. – MS: m/z (%) = 529 (15) [M]+, 498 (78), 156 (68), 31 (100). Anal. calcd. for C21H22BrO9P (529.27): C 47.66, H 4.19; found: C 47.74, H 4.32 %.
4.16 4-Ethyl-1,2-dimethyl3-(dimethoxyphosphoryl)-6- (4-methylphenyl)-1,2,4-benzenetricarboxylate (9g)
Yellow powder; yield: 0.77 g (80 %). – IR (KBr): νmax = 1742, 1740, 1737, 1698, 1567, 1455, 1356, 1245 cm-1. – MS: m/z (%) = 480 (15) [M]+, 449 (68), 108 (88), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 1.30 (3 H, t, 3JHH = 7.6 Hz, Me), 3.64 (MeO), 3.78 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 3.86 (3 H, s, MeO), 3.90 (3 H, s, MeO), 4.32 (2 H, q, 3JHH = 7.6 Hz, CH2O), 6.92 (2 H, d, 3JHH = 7.5 Hz, 2 CH), 7.34 (2 H, d, 3JHH = 7.5 Hz, 2 CH), 8.62 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 14.0 (Me), 51.6 (MeO), 52.7 (MeO), 53.6 (d, 2JPC = 11.4 Hz, 2 MeO), 55.4 (MeO), 62.4 (CH2O), 113.4 (2 CH), 124.5 (2 CH), 126.2 (d, 2JPC = 11.4 Hz, C), 126.8 (C), 127.0 (d, 3JPC = 21.5 Hz, C), 127.5 (d, 3JPC = 21.5 Hz, CH), 128.2 (d, 2JPC = 10.4 Hz, C), 144.5 (d 1JPC = 137.5 Hz, C), 146.2 (C), 156.7 (C), 160.4 (d, 3JPC = 22.8 Hz, C=O), 164.2 (d, 3JPC = 22.8 Hz, C=O), 167.2 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 21.4. – Anal. calcd. for C20H25O10P (480.40): C 55.0, H 5.25; found: C 55.24, H 5.38 %.
4.17 Trimethyl-3-(dimethoxyphosphoryl)-6-(4-chlorophenyl)-1,2,4-benzenetricarboxylate (9h)
Yellow powder; yield: 0.73 g (78 %). – IR (KBr): νmax = 1742, 1739, 1735, 1697, 1587, 1467, 1398, 1247 cm-1. – MS: m/z (%) = 470 (20) [M]+, 439 (86), 111 (66), 31 (100). – 1H NMR (500 MHz, CDCl3): δ = 3.78 (6 H, d, 3JHP = 11.8 Hz, 2 MeO), 3.87 (3 H, s, MeO), 3.89 (3 H, s, MeO), 3.94 (3 H, s, MeO), 7.68 (2 H, d, 3JHH = 7.8 Hz, 2 CH), 7.85 (2 H, d, 3JHH = 7.8 Hz, 2 CH), 8.63 (1 H, s, CH) ppm. – 13C NMR (125.7 MHz, CDCl3): δ = 51.8 (MeO), 52.0 (MeO), 52.4 (MeO), 53.7 (d, 2JPC = 10.6 Hz, 2 MeO), 123.4 (C), 124.6 (2 CH), 126.2 (2 CH), 126.8 (d, 2JPC = 11.5 Hz, C), 127.2 (d, 3JPC = 21.5 Hz, C), 127.8 (d, 3JPC = 21.5 Hz, CH), 128.3 (d, 2JPC = 11.4 Hz, C), 131.2 (C), 132.6 (C), 143.8 (d, 1JPC = 139.2 Hz, C), 160.5 (d, 3JPC = 23.5 Hz, C=O), 164.8 (d, 3JPC = 21.7 Hz, C=O), 168.2 (C=O) ppm. – 31P NMR (202 MHz, CDCl3): δ = 22.7 ppm. – Anal. calcd. for C20H20ClO9P (470.79): C 51.02, H 4.28; found: C 51.23, H 4.37 %.
Acknowledgments
We gratefully acknowledge financial support from the Islamic Azad University of Qaemshahr and Firoozkooh and Gonbad Kavous University.
References
[1] J. Li, H. Jiang, M. Chen, J. Org. Chem. 2001, 66, 3627.Search in Google Scholar
[2] D. Y. Park, S. Gowrisankar, J. N. Kim, Tetrahedron Lett. 2006, 47, 6641.Search in Google Scholar
[3] A. S. Münch, F. Katzsch, E. Weber, O. R. L. F. Mertens, J. Mol. Struct. 2013, 1043, 103.Search in Google Scholar
[4] S. Sato, H. Isobe, T. Tanaka, T. Ushijima, E. Nakamura, Tetrahedron 2005, 61, 11449.10.1016/j.tet.2005.09.011Search in Google Scholar
[5] S. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901.Search in Google Scholar
[6] K. P. C. Vollhardt, Angew. Chem., Int. Ed. Engl. 1984, 23, 539.Search in Google Scholar
[7] H. tom Dieck, C. Munz, C. Müller, J. Organomet. Chem. 1990, 384, 243.Search in Google Scholar
[8] R. Takeuchi, Y. Nakaya, Org. Lett. 2003, 5, 3659.Search in Google Scholar
[9] V. Gevorgyan, Y. Yamamoto, J. Organomet. Chem. 1999, 576, 232.Search in Google Scholar
[10] W. Carruthers, Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford, 1990, p. 91.Search in Google Scholar
[11] R. Cao, Q. Shi, D. Sun, M. Hong, W. Bi, Y. Zhao, Inorg. Chem. 2002, 41, 6161.Search in Google Scholar
[12] Q. Shi, R. Cao, D.-F. Sun, M.-C. Hong, Y.-C. Liang, Polyhedron 2001, 20, 3287.10.1016/S0277-5387(01)00945-7Search in Google Scholar
[13] Y. Li, N. Hao, Y. Lu, E.Wang, Z. Kang, C. Hu, Inorg. Chem. 2003, 42, 3119.Search in Google Scholar
[14] Y. Li, H. Zhang, E.Wang, N. Hao, C. Hu, Y. Yan, D. Hall, New J. Chem. 2002, 26, 1619.Search in Google Scholar
[15] J. I. Dickstein, S. I. Miller in The Chemistry of the Carbon-Carbon Triple Bond, (Ed.: S. Patai), J. Wiley & Sons, New York, 1978, chapter 19, p. 813.Search in Google Scholar
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)

