Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
-
Dongmei Zhao
and Ziwei Gao
Abstract
Alkyl-substituted ferrocenes are typical burning-rate catalysts in composite solid propellants, but their high migration tendency and volatility has impeded their extensive applications. By introducing the concept of ionic energetic compounds, eight new ionic binuclear ferrocenyl compounds, [FcCH2N(CH3)2(CH2)nN(CH3)2CH2Fc]2+ (X–2) (Fc = ferrocenyl; X– = 1,1,3,3-tetracyano propenide; n = 3–10; the compounds being numbered consecutively 1–8), were prepared and characterized. The molecular structures of 1, 2, and 4 were determined by single-crystal X-ray diffraction, and their cations were studied by density functional theory calculations (DFT). Compounds 1–5 show high thermal stability but 6–8 are slightly volatile. The results of cyclic voltammetry studies suggest that each salt exhibits a quasireversible redox system. Catalytic effects of the new salts on thermal degradation of ammonium perchlorate (AP), 1,3,5-trinitro-1,3,5-triaza-cyclo-hexane (RDX), and 1,2,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX), have been investigated by DSC and/or TG methods. The results show that the new compounds can bring the peak temperatures of both AP and RDX down significantly and enhance their heat release dramatically, indicating that the ferrocenyl salts possess high catalytic efficiency for the thermal decomposition of AP and RDX. Their catalytic activities are nearly equal to or higher than that of the corresponding nitrates and picrates, as well as their mononuclear counterparts. Compound 6 also efficiently catalyzes the thermal decomposition of hydroxyl-terminated polybutadiene (HTPB) and 1:1 mixtures of HTPB and AP.
1 Introduction
Ferrocene and its derivatives used as burning-rate (BR) catalysts (combustion modifiers) in solid propellants can be dated to the early 1960s [1–4]. Until now, although a number of other ferrocene derivatives have been prepared to be used as potential BR catalysts, commercially available ferrocene-based BR catalyst are mainly alkyl-substituted ferrocenes such as n-butylferrocene (NBF), tert-butylferrocene (TBF), and 2,2-bis(ethylferrocenyl)propane (Catocene) [2]. The alkylferrocenes, particularly Catocene, show excellent efficiency in increasing the burning rates of hydroxyl-terminated polybutadiene (HTPB)/ammonium perchlorate (AP) composite solid propellants and bring down their pressure index during combustion. However, they migrate easily from propellant grain to insulation during prolonged storage and evaporate and/or sublime conspicuously during propellant fabrications. These drawbacks could give rise to destruction of ballistic performances of the propellants and even to explosion when mixing with ultra-fine AP under certain circumstances [2–4]. A number of low-migratory ferrocene-based BR catalysts were synthesized in this situation, and some of them exhibited very high catalytic activities [5–19]. Nevertheless, some unsolved problems such as bad calibration and high costs have impeded their marketing [13–19].
We introduced recently the concept of ionic compounds into ferrocene-based BR catalysts due to their unique characteristics, such as very low vapor pressures and higher densities, than their atomically similar nonionic analogues [20–22]. A series of mono- and binuclear ionic ferrocenyl compounds with nitrate or picrate as anion have been reported [20–22]. The results revealed that all the new salts have much lower migration rates than NBF and Catocene. In addition, some binuclear salts exhibit highly catalytic effects on the thermal decomposition of AP and 1,3,5-trinitro-1,3,5-triazacyclo-hexane (RDX), even higher than that of Catocene [20].
In our continuing work, a series of polycyano-based ionic ferrocenyl compound were designed and synthesized to improve the energy level of the propellants, motivated by the general rule that nitrogen-rich energetic ionic compounds have higher standard enthalpies of formation than their nitrate analogues, arising from the introduction of nitrogen-rich groups such as tetrazolyl, azido, and/or cyano groups [23–27]. Herein we report eight novel binuclear ionic ferrocenyl compounds with 1,1,3,3-tetracyanopropenide (TCP) as the counter ion (Scheme 1). The new compounds were investigated by spectral and electrochemical methods and 1, 2, and 4 further by theoretical calculations. Finally, their catalytic effects on the thermal degradation of AP, RDX, and 1,2,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX), and that of 6 on hydroxyl-terminated polybutadiene (HTPB) and 1:1 mixtures of HTPB and AP were also evaluated.
![Scheme 1: Molecular structure of N,N′-bis[(ferrocenylmethyl)dimethyl]alkylene-diammonium 1,1,3,3-tetracyanopropenides.](/document/doi/10.1515/znb-2014-0251/asset/graphic/znb-2014-0251_scheme1.jpg)
Molecular structure of N,N′-bis[(ferrocenylmethyl)dimethyl]alkylene-diammonium 1,1,3,3-tetracyanopropenides.
2 Experimental section
AP, RDX, HMX, and HTPB were supplied by Xi’an Modern Chemistry Institute. Dimethylamminomethyl-ferrocene was purchased from Meryer Chemical Technology Co., Ltd. (Shanghai, China) and both I(CH2)nI (n = 3–6) and Br(CH2)nBr (n = 7–10) from Alfa Aesar Chemicals Co., Ltd. (Tianjin, China). Additional reagents and chemicals used in this work were of AR grade (Sinopharm Chemical Reagent Co., Ltd, China) and used as received. All (ferrocenylmethyl)dimethylalkyl ammonium iodides and bromides were synthesized according to the synthetic procedures described by Yamamoto [28]. Potassium 1,1,3,3-tetracyanopropenide was prepared as described [29]. The FT-IR spectra were performed on an EQUINX 55 Spectrometer (Brucher, Germany) in KBr matrix, whereas 1H and 13C NMR spectra were recorded on a Bruker Advance 400 MHz Spectrometer (Brucker, Germany). Elemental analyses were carried out with a Vario EL III Elemental analyzer (Elementar Analysensysteme GmbH, Germany). DSC and TG studies were undertaken on a HS-1 model from Beijing Henven Scientific Instruments (China) and a Q50 model from TA Company (USA), respectively, operating at 5 °C min–1 in a nitrogen atmosphere (50 mL·min–1) with open Al2O3 sample pans. Low-temperature DSC data for compound 7 were obtained on a Q1000DSC+LNCS+FACS Q600SDT thermoanalyzer system from TA Company (USA). Sample masses for both TG and DSC tests were in the range 1–3 mg to prevent damage to the instruments. Combustion catalytic properties of the ionic ferrocenyl compounds for thermal decomposition of the principal components of solid propellants were assessed by DSC and TG techniques. UV/Vis adsorption spectra were recorded on a UV-2450 spectrophotometer from the Shimadzu Corporation (Japan). Cyclic voltammograms were recorded with a CHI660C analyzer (Chenhua Instrument Shanghai Co., Ltd, China). Redox potentials were measured at a scan rate of 100 mV·s–1 in CH3CN containing 0.1 mol·L–1 n-Bu4PF6 as the supporting electrolyte. An Ag/Ag+ reference electrode and a platinum working electrode were used. Theoretical calculations were carried out at the DFT level using the B3LYP functional [30]. Geometry optimization of the singlet ground state was calculated using the 6-31G basis set for C, H, N atoms and the ECP Lanl2dz basis set for the Fe atom [31]. The DFT calculations were performed with the Gaussian 09 program package [32].
2.1 General procedure for synthesis of the ionic ferrocenyl compounds
As the synthetic procedures for all the compounds are similar, the preparation process for N,N′-bis[(ferrocenylmethyl)dimethyl]-1,3-propylenediammonium 1,1,3,3-tetracyano propenide (1) is given as an example. To a 100 mL round-bottom flask containing 1.846 g (2.0 mmol) N,N′-bis[(ferrocenylmethyl)dimethyl]-1,3-propylenediammonium diiodide in 20 mL methanol was added dropwise 0.864 g (4.8 mmol) of potassium 1,1,3,3-tetracyanopropenide in 30 mL water with stirring. A yellow precipitate was formed immediately, and the stirring was continued for another 2 h. The suspension was filtered and washed with both water and methanol three times each. The residue was dried at 40 °C for 24 h under vacuum, and an orange powder was collected:
2.1.1 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,3-propylenediammonium 1,1,3,3-tetracyano-propenide (1)
Orange powder. Yield. 1.49 g (92 %). M. p. 177.8–179.6 °C. – IR(KBr): ν = 3089, 3010, 2875, 2200, 1541, 1474, 1324, 1233, 1105, 1002, 892, 819. – 1H NMR (400 MHz, [D6]DMSO): δ = 7.23 (s, 2H, TCP-H), 4.52 (s, 4H, Fc-H), 4.42 (s, 8H, Fc-H + FcCH2), 4.27 (s, 10H, Fc-H), 3.16 (m, 4H, NCH2), 2.90 (s, 12H, N(CH3)2), 2.15 (m, 2H, CH2). – 13C NMR (100 MHz, [D6]DMSO): δ = 154.55 (NCCC), 119.09 (NC), 115.59 (NC), 72.65, 72.06, 70.12 (C5H4), 69.02 (C5H5), 60.10 (Fc-CH2), 58.95 (NCH2), 50.71 (NCC), 49.05 (NCH3), 16.18 (CH2). – C43H42Fe2N10 (810.6): calcd. C 63.72, H 5.22, N 17.28; found C 63.95, H 5.11, N 17.04 %.
2.1.2 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,4-butylenediammonium 1,1,3,3-tetracyano-propenide (2)
Yellowish powder. Yield. 1.54 g (93 %). M. p. 174.2–176.3 °C. – IR(KBr): ν = 3084, 3009, 2872, 2211, 1538, 1473, 1324, 1230, 1104, 1001, 893, 816, 805. – 1H NMR (400 MHz, [D6]DMSO): δ = 7.23 (s, 2H, TCP-H), 4.52 (t, J = 1.7 Hz, 4H, Fc-H), 4.42 (m, 4H, Fc-H), 4.39 (s, 4H, FcCH2), 4.27 (s, 10H, Fc-H), 3.11 (m, 4H, NCH2), 2.87 (s, 12H, N(CH3)2), 1.67 (m, 4H, CH2). – 13C NMR (100 MHz, [D6]DMSO): δ = 154.55 (NCCC), 119.24 (NC), 115.48 (NC), 72.77, 71.98, 70.08 (C5H4), 69.00 (C5H5), 64.37 (FcCH2), 61.48 (NCH2), 50.71 (NCC), 49.08 (NCH3), 19.12 (NCH2CH2). – C44H44Fe2N10 (824.6): calcd. C 64.09, H 5.38, N 16.99; found C 64.33, H 5.21, N 17.16 %.
2.1.3 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,5-pentylenediammonium 1,1,3,3-tetracyano-propenide (3)
Yellowish powder. Yield. 1.51 g (90 %). M. p. 130.2–131.9 °C. – IR(KBr): ν = 3081, 3008, 2871, 2220, 1536, 1472, 1324, 1229, 1104, 1000, 891, 812, 805. – 1H NMR (400 MHz, [D6]DMSO): δ = 7.21 (s, 2H, TCP-H), 4.51 (m, 4H, Fc-H), 4.40 (d, J = 14.8 Hz, 8H, Fc-H +Fc-CH 2), 4.26 (s, 10H, Fc-H), 3.09 (m, 4H, NCH2), 2.86 (s, 12H, N(CH3)2), 1.73 (m, 4H, NCH2CH2), 1.24 (m, 2H, CH2CH2). – 13C NMR (100 MHz, [D6]DMSO): δ = 154.54 (NCCC), 119.09 (NC), 115.58 (NC), 72.82, 71.95, 70.05 (C5H4), 69.00 (C5H5), 64.13 (FcCH2), 62.11 (NCH2), 50.70 (NCC), 49.04 (NCH3), 22.87 (NCH3CH2), 21.37 (CH2CH2). – C45H46Fe2N10 (838.6): calcd. C 64.45, H 5.53, N 16.70; found C 64.73, H 5.48, N 16.54 %.
2.1.4 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,6-hexylenediammonium 1,1,3,3-tetracyano-propenide (4)
Yellow powder. Yield. 1.53 g (90 %). M. p. 137.6–138.8 °C. – IR(KBr): ν = 3075, 3004, 2869, 2225, 1537, 1475, 1324, 1231, 1104, 1001, 890, 811, 801. – 1H NMR (400 MHz, [D6]DMSO): δ = 7.22 (s, 2H, TCP-H), 4.49 (s, 4H, Fc-H), 4.39 (d, J = 12.9 Hz, 4H, Fc-H), 4.36 (s, 4H, FcCH2), 4.26 (s, 10H, Fc-H), 3.07 (m, 4H, NCH2), 2.85 (s, 12H, N(CH3)2), 1.67 (m, 4H, NCH2CH2), 1.20 (m, 4H, CH2CH2). – 13C NMR (100 MHz, [D6]DMSO): δ = 154.55 (NCCC), 119.09 (NC), 115.58 (NC), 72.71, 71.92, 70.02 (C5H4), 69.00 (C5H5), 64.03 (FcCH2), 62.36 (NCH2), 50.71 (NCC), 48.99 (NCH3), 25.47 (NCH2CH2), 21.67 (CH2CH2). – C46H48Fe2N10 (852.6): calcd. C 64.80, H 5.67, N 16.43; found C 64.53, H 5.52, N 16.37 %.
2.1.5 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,7-heptylenediammonium 1,1,3,3-tetracyano-propenide (5)
Yellowish powder. Yield. 1.49 g (86 %). M. p. 127.5–128.3 °C. – IR(KBr): ν = 3072, 3002, 2866, 2228, 1539, 1472, 1323, 1230, 1102, 1003, 896, 814, 802. – 1H NMR (400 MHz, CD3CN) δ 7.01 (s, 2H, TCP-H), 4.47 (s, 4H, Fc-H), 4.43 (d, J = 10.6 Hz, 4H, Fc-H), 4.38–4.21(m, 14H, Fc-H + Fc-CH2), 3.08 (m, 4H, NCH2), 2.84 (s, 12H, N(CH3)2), 1.75 (m, 4H, NCH2CH2), 1.38–1.20 (d, J = 38.6 Hz, 8H, (CH2CH2)2). – 13C NMR (400 MHz, [D6]DMSO) δ 155.03 (NCCC), 119.59 (NC), 116.06 (NC), 73.40, 72.41, 70.51 (C5H4), 69.50 (C5H5), 64.48 (FcCH2), 62.99 (NCH2), 51.19 (NCC), 49.48 (NCH3), 28.57 (NCH2CH2), 26.26 (CH2CH2), 22.19 (CH2CH2). – C47H50Fe2N10 (866.7): calcd. C 65.14, H 5.82, N 16.16; found C 65.46, H 5.75, N 16.01 %.
2.1.6 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,8-octylenediammonium 1,1,3,3-tetracyano-propenide (6)
Red-brown liquid. Yield. 1.48 g (84 %). M.p. 4.5 °C. – IR(KBr): ν = 3071, 3001, 2868, 2229, 1536, 1475, 1324, 1228, 1100, 1005, 894, 810, 800. – 1H NMR (400 MHz, [D6]DMSO) δ = 7.01 (s, 2H, TCP-H), 4.47 (m, 4H, Fc-H), 4.43 (m, 4H, Fc-H), 4.38 (d, J = 12.8 Hz, 4H, FcCH2), 4.26(s, 10H, Fc-H), 3.08 (m, 4H, NCH2), 2.84 (s, 12H, N(CH3)2), 1.69 (s, 4H, NCH2CH2), 1.28 (d, J = 38.6 Hz, 8H, (CH2CH2)2). – 13C NMR (100 MHz, [D6]DMSO) δ = 154.55 (NCCC), 119.10 (NC), 115.58 (NC), 72.91, 71.92, 70.00 (C5H4), 69.00 (C5H5), 63.91 (FcCH2), 62.55 (NCH2), 50.70 (NCC), 48.96 (NCH3), 28.41 (NCH2CH2), 25.82(CH2CH2CH2), 21.71 (CH2CH2CH2). – C48H52Fe2N10 (880.7): calcd. C 65.46, H 5.95, N 15.90; found C 65.63, H 5.79, N 15.67 %.
2.1.7 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,9-nonylenediammonium 1,1,3,3-tetracyano-propenide (7)
Red-brown liquid. Yield. 1.48 g (83 %). F.p. 0.86 °C. – IR(KBr): ν = 3068, 3002, 2866, 2231, 1537, 1472, 1322, 1225, 1105, 1000, 890, 813, 804. – 1H NMR (400 MHz, [D6]DMSO) δ = 7.21 (s, 2H, TCP-H), 4.49 (m, 4H, Fc-H), 4.39 (m, 4H, Fc-H), 4.36 (s, 4H, FcCH2), 4.26 (s, 10H, Fc-H), 3.08 (m, 4H, NCH2), 2.84 (s, 12H, N(CH3)2), 1.69 (m, 4H, CH2CH2), 1.37–1.19 (m, 8H, (CH2CH2)2CH2). – 13C NMR (100 MHz, [D6]DMSO) δ = 155.09 (NCCC), 119.60 (NC), 116.07 (NC), 73.40, 72.70, 70.50 (C5H4), 69.50 (C5H5), 64.42 (FcCH2), 63.11 (NCH2), 51.20 (NCC), 49.46 (NCH3), 29.20(NCH2CH2), 28.96(N(CH2)2CH2), 26.38 (CH2CH2), 22.26 (CH2CH2). – C49H54Fe2N10 (894.7): calcd. C 65.78, H 6.08, N 15.66; found C 65.53, H 5.99, N 15.84 %.
2.1.8 N,N′-bis[(ferrocenylmethyl)dimethyl]-1,10-dodecylenedia-mmonium 1,1,3,3-tetracyano-propenide (8)
Yellowish powder. Yield. 1.52 g (84 %). M. p. 122.3–123.9 °C. – IR(KBr) ν = 3067, 3004, 2869, 2232, 1536, 1475, 1324, 1224, 1100, 1004, 892, 811., 802. – 1H NMR (400 MHz, [D6]DMSO) δ = 7.21 (s, 2H, TCP-H), 4.48 (m, 4H, Fc-H), 4.39 (m, 4H,Fc-H), 4.36 (s, 4H, Fc-CH2), 4.26 (s, 10H, Fc-H), 3.08 (m, 4H, NCH2), 2.84 (s, 12H, N(CH3)2), 1.70 (m, 4H, CH2CH2), 1.37–1.19 (m, 10H, (CH2CH2CH2)2). – 13C NMR (100 MHz, [D6]DMSO) δ = 154.54 (NCCC), 119.10 (NC), 115.57 (NC), 72.93, 71.91, 69.99 (C5H4), 68.99 (C5H5), 63.86 (FcCH2), 62.61 (NCH2), 50.70 (NCC), 48.94 (NCH3), 28.79(NCH2CH2), 28.55(N(CH2)2CH2), 25.90 (N(CH2)3CH2CH2), 21.76 (N(CH2)4CH2). – C50H56Fe2N10 (908.7): calcd. C 66.08, H 6.21, N 15.41; found C 66.43, H 6.58, N 15.04 %.
2.2 X-ray structure determinations
Data collections were carried out with a Bruker SMART APEX-II CCD detector using graphite monochromated MoKα radiation (λ = 0.71073). Structures were solved by applying Direct Methods using Shelxs-97 [33]. The full-matrix least-squares refinement on F2 included atomic coordinates and anisotropic displacement parameters for all non-H atoms. H atoms were included using a riding model (Shelxl-97 [33]). The crystal structures of the compounds 1, 3, and 4 are illustrated in Fig. 1. Selected crystallographic data and structure refinement parameters of the three compounds are summarized in Table 1. Selected bond lengths and angles are included in Table S1. (Supporting information is available online; see note at the end of this article for availability). Hydrogen bond distances and angles are given in Table S2.
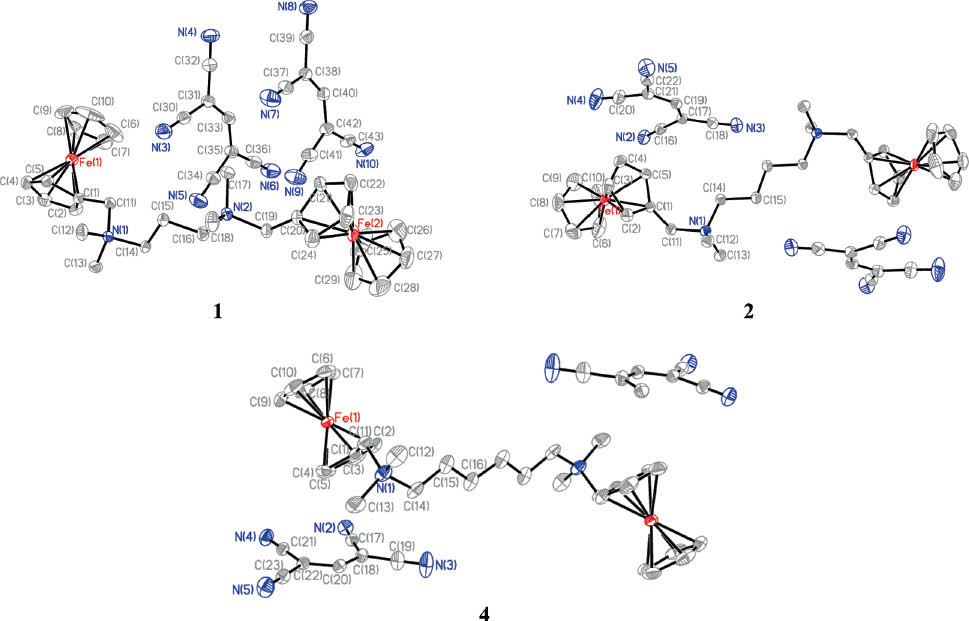
The Ortep views of 1, 2, and 4, with the atoms being partly labeled (displacement elipsoids at the 30 % probability level).
Crystal structure data for 1, 2, and 4.
| 1 | 2 | 4 | |
|---|---|---|---|
| Formula | C43H42Fe2N10 | C44H44Fe2N10 | C46H48Fe2N10 |
| Mr | 810.57 | 824.59 | 852.64 |
| Cryst. size, mm3 | 0.15 × 0.14 × 0.10 | 0.16 × 0.14 × 0.10 | 0.15 × 0.13 × 0.10 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P1̅ | P21/c | P21/c |
| a, Å | 12.7689(11) | 9.6532(4) | 9.7316(4) |
| b, Å | 13.5186(11) | 17.2500(6) | 24.5725(6) |
| c, Å | 9.9543(3) | 12.8391(4) | 13.9705(11) |
| α, deg | 68.548(7) | 90 | 90 |
| β, deg | 73.403(7) | 106.960 | 116.246 |
| γ, deg | 66.195(8) | 90 | 90 |
| V, Å3 | 2026.4(3) | 2044.96(13) | 2134.96(12) |
| Z | 2 | 2 | 2 |
| Dcalcd, g cm–3 | 1.33 | 1.34 | 1.34 |
| μ(MoKα), cm–1 | 0.8 | 0.8 | 0.4 |
| F(000), e | 844 | 860 | 892 |
| hkl range | ±15, ±16, ±17 | ±11, ±21, ±15 | ±11, ±30, –11 → +12 |
| ((sinθ)/λ)max, Å–1 | 0.616 | 0.616 | 0.616 |
| Refl. measured | 40 652 | 20 792 | 21 597 |
| Refl. unique | 7960 | 4016 | 4181 |
| Rint | 0.0388 | 0.0217 | 0.0271 |
| Param. refined | 496 | 253 | 262 |
| R(F)a/wR(F2)b (all refl.) | 0.0553/0.1011 | 0.0433/0.0991 | 0.0461/0.0875 |
| GoF (F2)c | 1.034 | 1.076 | 1.072 |
| Weighting scheme a/bb | 0.0422/0.9356 | 0.0490/0.8133 | 0.0309/1.0830 |
| Δρfin (max/min), e Å–3 | –0.38/0.05 | –0.17/0.04 | –0.24/0.04 |
aR(F) = Σ||Fo| – |Fc||/Σ|Fo|; bwR(F2) = [Σw(Fo2 – Fc2)2/Σw(Fo2)2]1/2, w = [σ2(Fo2) + (aP)2 + bP]–1, where P = (Max(Fo2, 0) + 2Fc2)/3; cGoF = [Σw(Fo2 – Fc2)2/(nobs – nparam)]1/2.
CCDC 973760, 1010676, and 973761 contain the supplementary crystallographic data for this article. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Synthesis and characterization
The new binuclear ferrocenyl salts can be prepared in high yields and purity as they are insoluble in both water and methanol, which were employed for dissolving their reactants. Compounds 1–5 and 8 are solid, but both 6 and 7 are red-brown liquids. Both 1H NMR and 13C NMR spectra show the usual features of the cations and the TCP anion. The FT-IR spectra of the new ionic compounds give characteristic absorption bands of the C≡N groups of the TCP anion centered around 2200 cm–1. The UV/Vis absorption spectra of the new compounds in acetonitrile (Fig. S1: supporting information available online) exhibit absorption bands of the π-π* transitions of the TCP anion centered around 346 nm [34, 35]. The cations of the compounds exert no obvious influence on the absorption position of the TCP anion.
3.2 Crystal structures
To obtain molecular structural information of the binuclear ferrocenyl compounds, single crystals of 1, 2, and 4 suitable for X-ray diffraction were grown and structurally characterized. Compound 1 crystallizes in the triclinic space group P1̅, and both 2 and 4 crystallize in the monoclinic system with space group P21/c. In 1, two crystallographically different anions are located at one side of the cation, whereas the two anions in 2 or 4 are positioned at both sides of the symmetrical cation (see Fig. 1). In compounds of 2 and 4 each cation has an inversion center located in the middle of the C15–C15#1 (symmetry code: 1–x, 1–y, 1–z) bond of 2 and of C16–C16#1 (symmetry code: 2–x, –y, 1–z) bond of 4. The distances between the centroids of the Cp rings and the Fe atoms are in the range of 1.634–1.669 Å. The C(11)–N(1) bond (including the C(19)–N(2) bond in 1) lengths are from 1.491 to 1.534 Å, longer in most cases than the typical C–N single bonds (1.47–1.50 Å), indicating that they are relatively weak. The lengths of the C–N triple bonds in the TCP anions are 1.137–1.147 Å as typical C≡N bond length (1.14–1.16 Å); the C–C bond lengths of the central carbon atoms and their neighboring carbon atoms in the TCP anions are between 1.370 and 1.387 Å, also typical for conjugated C=C double bonds (1.38 Å). The dihedral angles between the planes of the Cp rings (C1–C5) and the TCP anions are 83.7° for 1, 89.9° for 2 and 68.8° for 4, indicating that in 2 the two planes are almost vertical. The dihedral angles between planes of the two Cp rings (C1–C5 and C6–C10) belonging to different ferrocene groups in a compound, are 88.02° in 1, 3.06° in 2, and 1.07° in 4, suggesting that in 1 the two ferrocene groups are almost perpendicular, whereas the two ferrocene units in both 2 and 4 are nearly parallel. The packings of the molecules in the three compounds exhibit an alternating arrangement of anion layers and cation layers in the crystals (Figs. S2–S4: supporting information is available online). The components are linked into high-dimensional networks by hydrogen bonding interactions (Table S2).
3.3 Thermal behavior
Thermal stability of a burning-rate catalyst is one of its important parameters. It is proposed that a ferrocene-based BR catalyst decomposes only at high temperature to produce iron oxide nanoparticles if the catalyst possesses excellent thermal stability. The nanosized iron oxide particles are believed to be the actual catalyst for the combustion process of the solid propellants [2–4]. The thermal decomposition behavior of the new compounds was therefore examined by TG and DSC techniques. It is shown (Fig. 2) that no obvious weight loss is found for 1–5 below 199 °C, but considerable weight loss is observed for 6–8 below 199 °C due to their high volatility; particularly, 8 begins to lose weight from change in room temperature. The decomposition behavior of all the compounds is similar to that of their mononuclear analogs [36]. The first weight loss stage should be ascribed to the loss of the polycyano anions and the organic amine moieties. The second weight loss stages are assigned mainly to the decomposition of the ferrocene moieties. Correspondingly, in their DSC curves (Fig. S5: supporting information available online) two exothermic processes appear for each compound. The two processes are centered in the ranges of 230.4–256.5 °C and 372.8–412.3 °C, respectively. The thermal decomposition behavior demonstrates that 1–5 are thermally very stable, and 6–8 are volatile.
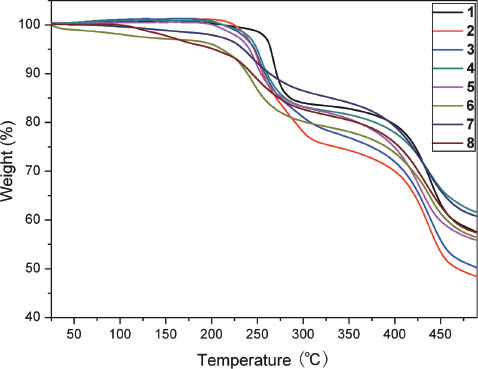
TG curves of compounds 1–8.
3.4 Redox properties
It is known that ferrocene groups are electrochemically active and their electrochemistry has been extensively investigated in various fields [1]. For a ferrocene-based BR catalyst, the redox process of the ferrocene units should be partly responsible for their catalytic performance in the solid propellants [9]. The electrochemistry of the new ionic ferrocenyl compounds in acetonitrile solution was therefore studied by cyclic voltammetry (CV). The results (Fig. 3, Table S4: supporting information available online) indicate that each displays a quasireversible/irreversible process with the potentials in the ranges of 625–646 mV for Ep1/2 and 82–108 mV for ΔEp. The oxidation potentials of the new ferrocenyl salts are higher by 200–300 mV than those of NBF, TBF and Catocene, implying that the new salts cannot be easily oxidized in comparison with the alkylferrocenes and that these salts, similar to their mononuclear analogs [36], exhibit higher antioxidative properties under similar conditions.
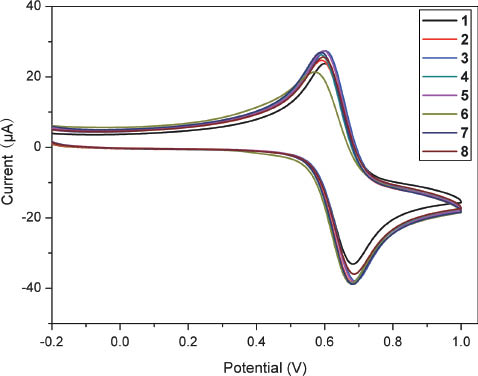
Cyclic voltammograms of 1–8 in 0.1 m n-Bu4PF6-CH3CN with scan rate of 100 mV·s–1.
3.5 Catalytic activities of the new compounds
AP, RDX, HMX, and HTPB are usually employed as principal components in solid propellants. The thermal degradation behavior of these components has close relationship with the combustion process of the solid propellants. Accordingly, it is reasonable to evaluate their catalytic effects on the thermal decomposition of the materials as a guidance for their catalytic performances in solid propellants. The catalytic effects are generally determined by DSC and/or TG techniques. Their catalytic activities are usually assessed by the shifts of the peak temperatures of the materials and the increment of the released heats of the materials with addition of a BR catalyst [37].
The optimum concentrations of the new ferrocenyl compounds in AP, RDX, and HMX were screened initially. The results show that the optimum amounts of the compounds are 5 wt% in AP, 3 wt% in RDX and 1 wt% in HMX (Figs. S6–S8: supporting information is available online). Figure 4 shows the catalytic effects of all the compounds on the thermal degradation of AP. As we observed earlier for other ionic ferrocenes, the new additives have no significant influence on the endothermic phase transition process of the pure AP with a peak at 249 °C, but have great effects on the two exothermic processes, the low-temperature decomposition (LTD) stage and high-temperature decomposition (HTD) stage of AP, particularly on the latter [38–40]. The large temperature span of the pure AP under thermal degradation (178 °C) is narrowed exceedingly (81–101 °C) with the addition of a new salt in 5 wt%, indicating that the new additives can increase the decomposition rate of AP and promote its HTD stage turning to an exothermic process. Moreover, the released heat of AP has a conspicuous improvement with the new compounds as additives. The released heats of the mixture systems are in the range of –1654.6 to –2416.5 J g–1. The released heat of 5 wt% 6 + AP is the highest and is comparable to that of the 5 wt% Catocene + AP [20]. The released heats of the samples are nearly equal to the released heats of those composed of AP and 5 wt% structurally similar nitrates and picrates and higher than the released heats of the mixtures of AP with the corresponding mononuclear analogues [20–22, 36]. Both peak temperatures and released heats of the samples suggest that the new compounds are potential ferrocene-based BR catalysts in HTPB/AP solid propellants.
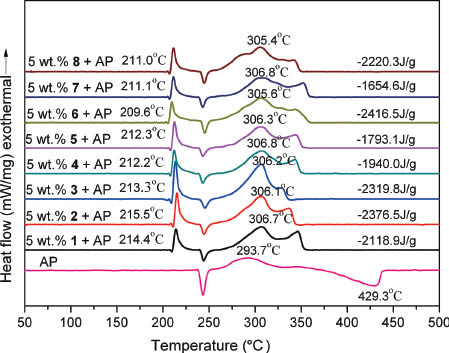
The DSC curves of pure AP and of the mixtures AP + 5 wt% 1–8.
AP is generally added in 68 % weight in a traditional composite solid propellant. The intensive smoke released by the rocket motor during combustion process of the solid propellant decreases dramatically the stealth effect of the propellants. It is therefore necessary to use other oxidizers such as RDX and HMX as partial alternatives. In this context, the catalytic performance of the new ferrocenyl salts for the thermal degradation of both RDX and HMX was investigated. Figures 5 and S9 (supporting information available online) display the DSC curves of the mixtures consisting of RDX or HMX with a new additive, respectively. It is noted that the exothermic decomposition process of RDX peaking at 233.2 °C is shifted downward to 226.7–231.8 °C with 3 wt% additives, of which 4 can bring down the peak temperature of RDX to the lowest temperature of 226.7 °C. Correspondingly, the new compounds promote the released heat of RDX from –803.6 J·g–1 to –979.3 to –1917.1 J·g–1. The results suggest that the binuclear ferrocenyl salts are catalytically highly effective in the thermal decomposition of RDX. As for promoting the thermal degradation of HMX, the results display that the peak temperature of HMX is also shifted when a ferrocenyl salt is added. The released heat of HMX, however, cannot be enhanced in most cases, except with 1 or 6 as additives, which increase the released heat of HMX slightly. Therefore, the results suggest that the new compounds exert no obvious effect on the thermal decomposition of HMX as we reported earlier for other ferrocenyl compounds [20–22, 36].
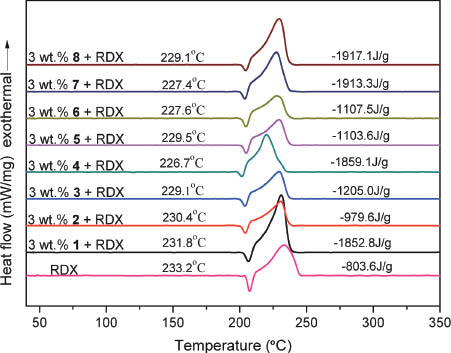
The DSC curves of pure RDX and of the mixtures RDX + 5 wt% 1–8.
The thermal decomposition behavior of HTPB is important for the combustion process of HTPB/AP composite solid propellants because HTPB is the binder in the propellants. In order to represent true state of HTPB in solid propellants, HTPB was copolymerized with approximately 6 wt% 2,4-tolylenediisocyanate (TDI) and grinded into pieces. The thermal degradation process of HTPB, with or without the addition of AP (wt. 1:1) and/or 5 wt% 6, was investigated by TG method. From Fig. 6 it can be noted that for both HTPB and the mixtures of HTPB + AP (1:1) the weight losses start from around 270 °C, but the mass loss of the HTPB + AP (1:1) is much more rapid than that of HTPB between 271 and 362 °C due to the dramatic decomposition of AP. For the mixture of HTPB + 5 wt% 6 the rapid weight-loss starts from 215 °C, disclosing that the initial decomposition temperature of HTPB shifted upon incorporation of 6, which is presumbly due to an increase in its oxidative degradation [41]. It is also known that traces of metals catalyze the homolytic decomposition of hydroperoxides, which are formed during oxidative decomposition of HTPB [42]. For the HTPB + AP + 5 wt% 6 mixture the significant weight loss starts from 199 °C, lower than the initial decomposition temperature of the HTPB + AP, indicating that 6 can advance the thermal decomposition of HTPB + AP mixture. The results are similar to those we observed for the thermal decomposition procedure of the corresponding nitrate analog with non-copolymerized HTPB and AP, implying that TDI has almost no effect on the decomposition behavior. Their degradation behavior is different from that reported for HTPB + AP and HTPB + AP + 1 % CdO, which each has only one distinct weight-loss stage, owing probably to the use of sealed aluminium pans during their DSC determinations [43].
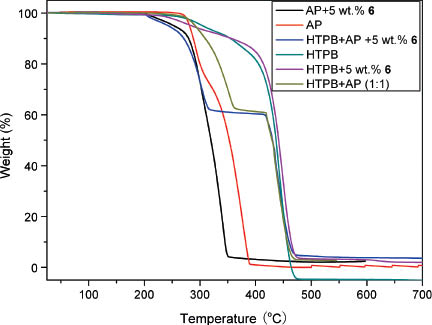
Non isothermal TG curves of AP, HTPB, 1:1 HTBP/AP with and without 5 wt% 6.
3.6 DFT calculations
In order to get deeper insight into the molecular structures and properties of the binuclear compounds at a theoretical level, DFT computational studies were carried out for the cations of 1, 2, and 4. Figures 7 and S10–S14 (supporting information available online) illustrate the structures of the cations of 1, 2, and 4 optimized by DFT and their molecular frontier orbitals. Those of NBF, TBF, and Catocene are also represented for comparison. It can be found that the structures optimized by DFT calculations are identical to those in the crystals and that the LUMO orbitals have contributions from the whole molecule except the Cp ring of a ferrocenyl group for 1 and 2 and by one ferrocenylmethyl group and the adjacent nitrogen atom for 4. The corresponding HOMO orbitals consist mainly of ferrocenyl moieties. It is also worth pointing out that the energies of their HOMO orbitals (–10.12 eV for 1, –10.01 eV for 2 and –9.83 eV for 4) are much lower than those of the HOMO orbitals of NBF (–5.25 eV), TBF (–5.25 eV) and Catocene (–5.14 eV) and are slightly lower than those of the HOMO orbitals of the mononuclear counterparts, indicating that the ferrocenyl groups of the new compounds are more difficult to be oxidized than the ferrocenyl units of alkylferrocenes. The calculated results for HOMO orbital energies could give a reasonable explanation for the high potential of the ionic compounds as catalysts in comparison with that of the alkylferrocenes (Table S3: supporting information is available online).
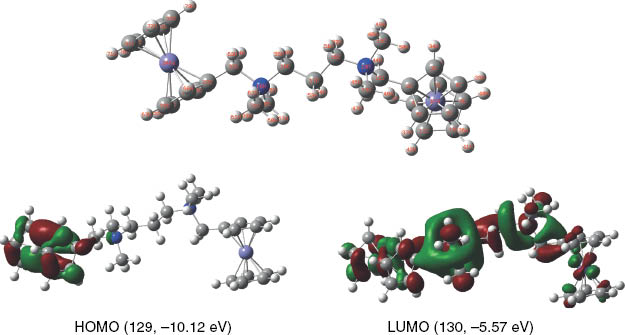
The structure optimized by theoretical calculations and energy diagram of the HOMO and LUMO of the cation of 1.
4 Conclusions
In summary, eight binuclear ferrocenyl salts containing the 1,1,3,3-tetracyanopropenide anion have been synthesized. The structures of 1, 2, and 4 have been confirmed by single-crystal X-ray diffraction. The compounds have also been studied by spectroscopy, electrochemistry, and DFT calculations. The results show that 1–5 have excellent thermal stability and all the compounds are quasireversible redox systems. In addition, the binuclear ferrocenyl derivatives exhibit highly catalytic effects on the thermal degradation of AP, RDX, HTPB, and HTPB + AP (1:1). Their catalytic activities are higher than those of their mononuclear ferrocenyl analogs in thermally degrading AP and RDX. However, most of them cannot enhance the released heat of HMX. The new results will inspire us to design and synthesize other ionic binuclear ferrocenyl compounds with higher nitrogen contents.
5 Supporting information
UV/Vis spectra; DSC curves of the new compounds; DSC curves of mixtures of the compounds with AP, RDX, or HMX; crystal structure plots of 1, 2, and 4; molecular structures optimized from theoretical calculations; and energy diagrams of the HOMOs and LUMOs of the cations of 2, 4 and of NBF, TBF, and Catocene are provided as supporting information available online (DOI: 10.1515/znb-2014-0251).
Acknowledgments
This work is supported by the National Natural Science Foundation of China (21171112, 21401124, 21371112, 21271124) and the program for Changjiang Scholars and Innovative Research Team in University” (IRT_14R33).
References
[1] A. Togni, T. Hayashi, Ferrocenes. Homogeneous Catalysis, Organic Synthesis, Materials Science, Wiley-VCH, Deerfield Beach, FL, 1995.10.1002/9783527615599Search in Google Scholar
[2] X. Zhang, Y. Xia, L. Jia, J. Chi, W. Chang, J. Wang, Chem. Propell. Polym. Mater. 2012, 10, 58.Search in Google Scholar
[3] R. Tong, Y. Zhao, L. Wang, H. Yu, F. Ren, M. Saleem, W. A. Amer, J. Organometal. Chem. 2014, 755, 16.Search in Google Scholar
[4] J. Gao, L. Wang, H. Yu, A. Xiao, W. Ding, Propell. Explos. Pyrotech. 2011, 36, 404.Search in Google Scholar
[5] K. Subramanian, J. Polym. Sci., Part A; Polym. Chem. 1999, 37, 4090.Search in Google Scholar
[6] H. P. Hebekeuser, H. P. Mackowiak, K. Gottlieb, H. Jungbluth, H. Neitsch, U.S. Patent 6313334, 2001. Filed on May 2, 1998; Issued on Nov. 6, 2001.Search in Google Scholar
[7] W. E. Hill, U.S. Patent 3962297, 1976. Filed on Mar. 7, 1969; Issued on June 8, 1976.Search in Google Scholar
[8] D. Saravanakumar, N. Sengottuvelan, V. Narayanan, M. Kandaswamy, T. L. Varghese, J. Appl. Polym. Sci. 2011, 119, 2517.Search in Google Scholar
[9] T. Fu, L. Liu, Z. Gu, Y. Yang, F. Li, Chin. J. Solid Rocket Techn. 2008, 31, 612.Search in Google Scholar
[10] F. J. Xiao, F. F. Feng, L. L. Li, D. Zhang, Propel. Explos. Pyrotech. 2013, 38, 358.Search in Google Scholar
[11] F. Xiao, X. Sun, X. Wu, J. Zhao, Y. Luo, J. Organomet. Chem. 2012, 713, 96.Search in Google Scholar
[12] F. Xiao, M. Shi, L. Peng, Y. Luo, J. Zhao, J. Inorg. Organomet. Polym. 2011, 21, 175.Search in Google Scholar
[13] Y Gao, H. Li, C. Ke, L Xie, B. Wei, Y. Yuan, Appl. Organomet. Chem. 2011, 25, 407.Search in Google Scholar
[14] C. Ke, H. Li, L. Xie, Y. Yuan, Chin. J. Energy Mater. 2011, 19, 19.Search in Google Scholar
[15] W. Liao, Y. Dou, J. Wang, L. Xie, F. Lin, Y. Yuan, Chem. J. Chin. Univ. 2012, 33, 2244.Search in Google Scholar
[16] J. Wang, L. Feng, F. Ma, F. Lin, L. Xie, Y. Yuan, Chin. J. Org. Chem. 2012, 32, 1479.Search in Google Scholar
[17] J. B. Zhuo, H. D. Li, C. X. Lin, L. L. Xie, S. Bai, Y. F. Yuan, J. Mol. Struct. 2014, 1067, 112.Search in Google Scholar
[18] Z. M. Lai, H. M. Ye, Q. Wan, L. L. Xie, S. Bai, Y. F. Yuan, J. Mol. Struct. 2014, 1059, 33.Search in Google Scholar
[19] H. Zhao, L. Guo, S. Chen, Z. Bian, RSC Adv. 2013, 3, 19929.Search in Google Scholar
[20] X.-L. Liu, D.-M. Zhao, F.-Q. Bi, X.-Z. Fan, F.-Q. Zhao, G.-F. Zhang, W.-Q. Zhang, Z.-W. Gao, J. Organomet. Chem. 2014, 762, 1.Search in Google Scholar
[21] Z.-Y. Cheng, G.-F. Zhang, X.-Z. Fan, F.-Q. Bi, F.-Q. Zhao, W.-Q. Zhang, Z.-W. Gao, Inorg. Chim. Acta 2014, 421, 191.10.1016/j.ica.2014.05.043Search in Google Scholar
[22] X.-L. Liu, W.-Q. Zhang, G.-F. Zhang, Z.-W. Gao, New J. Chem. 2015, 39, 155–162.Search in Google Scholar
[23] H. Gao, J. M. Shreeve, Chem. Rev. 2011, 111, 7377.Search in Google Scholar
[24] G. Steinhauser, T. M. Klapötke, Angew. Chem. Int. Ed. 2008, 47, 3330.Search in Google Scholar
[25] H. Gao, Z. Zeng, B. Twamley, J. M. Shreeve, Chem. Eur. J. 2008, 14, 1282.Search in Google Scholar
[26] T. M. Klapötke, C. Miró Sabaté, Z. Anorg. Allg. Chem. 2009, 635, 1812.Search in Google Scholar
[27] M. Dachs, A. A. Dippold, J. Gaar, M. Holler, T. M. Klapötke, Z. Anorg. Allg. Chem. 2013, 639, 2171.Search in Google Scholar
[28] T. Yamamoto, W. Oi, A. Hashidzume, A. Harada. Bull. Chem. Soc. Jpn. 2011, 84, 918.Search in Google Scholar
[29] W. J. Middleton, E. L. Little, D. D. Coffman, V. A. Engelhardt, J. Am. Chem. Soc. 1958, 80, 2795.Search in Google Scholar
[30] P. J. Stephens, F. J. Devlin, C.F. Chabalowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11623.Search in Google Scholar
[31] P. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 270.Search in Google Scholar
[32] M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, A. Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 03, (revision B.04), Gaussian Inc., Wallingford, CT (USA) 2004.Search in Google Scholar
[33] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.Search in Google Scholar
[34] N. Camire, U. T. Mueller-Westerhoff, W. E. Geiger, J. Organomet. Chem. 2001, 637–639, 823.Search in Google Scholar
[35] Y. Miura, F. Shimizu, T. Mochida, Inorg. Chem. 2010, 49, 10032.Search in Google Scholar
[36] X.-N. Gao, J.-Z. Li, Y. Luo, C.-X. Li, F.-Q. Bi, W.-Q. Zhang, G.-F. Zhang, Z.-W. Gao, Z. Anorg. Allg. Chem. 2015, 641, 475–482.Search in Google Scholar
[37] Q. Yang, S. Chen, G. Xie, S. Gao, J. Hazar. Mater. 2011, 197, 199.Search in Google Scholar
[38] A. J. Han, J. J. Liao, M. Q. Ye, Y. Li, X. H. Peng, Chin. J. Chem. Eng. 2011, 19, 1047.Search in Google Scholar
[39] Z. R. Liu, C. M. Yin, Y. H. Kong, F. Q. Zhao, Y. Luo, H. Xiang, Chin. J. Energy Mater. 2000, 8, 75.Search in Google Scholar
[40] A. J. Lang, S. Vyazovkin, Combust. Flame 2006, 145, 779.10.1016/j.combustflame.2006.02.002Search in Google Scholar
[41] R. S. Rastogi, G. Singh, B. L. Dubey, C. S. Shukla, J. Catal. 1980, 65, 25.Search in Google Scholar
[42] P. Srivastava, I. P. S. Kapoor, G. Singh, J. Alloys Compd. 2009, 485, 88.Search in Google Scholar
[43] G. Singh, I. P. S. Kapoor, R. Dubey, P. Srivastava, Mater. Sci. Eng. 2011, B176, 121.Search in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/znb-2014-0251) offers supplementary material, available to authorized users.
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics with ZrNiAl-type structure – a review
- Original Communications
- In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones
- A supramolecular 3-D organic-inorganic hybrid structure based on {PMo12} layers arranged in an alternating mode
- Ionic binuclear ferrocenyl compounds containing 1,1,3,3-tetracyanopropenide anions – synthesis, structural characterization and catalytic effects on thermal decomposition of main components of solid propellants
- Zur Chemie der 1,3,5-Triaza-2-phosphorinan- 4,6-dione. Teil XIV. Darstellung von weiteren P-alkyl- und P-arylsubstituierten 1,3,5-Trimethyl-1,3,5-triaza-2-phosphorinan-4,6-dionen
- Synthesis, biological activity and modeling study of some thiopyrimidine derivatives and their platinum(II) and ruthenium(III) metal complexes
- Synthesis of functionalized benzene using Diels–Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran
- Synthesis, structure, and optical nonlinearity of soluble ternary cadmium copper tellurolate complex [Cd(μ-TeTol)4{Cu(PPh3)2}2] (Tol = 4-tolyl)

