A 58-year-old male presented with an 8-day history of severe upper abdominal pain and vomiting. His medical history was unremarkable, with a normal abdominal computed tomography (CT) six months prior. Laboratory tests revealed elevated serum amylase, lipase, C-reactive protein, and D-dimer levels. CT scan identified a well-circumscribed 36 mm × 26 mm cystic lesion within the pancreatic head with peripancreatic exudation (Figure 1A). Despite supportive treatment for pancreatitis, symptoms persisted. Over two weeks, the lesion progressively enlarged to 67 mm × 49 mm on CT and magnetic resonance imaging (MRI), with branch pancreatic duct dilation (Figure 1B-C). Conventional etiologies such as biliary obstruction, hypertriglyceridemia, alcohol, drugs, or infection were excluded. An autoimmune workup revealed a normal immunoglobulin G4 (IgG4) level but positive antiphospholipid antibodies (aPL: Lupus anticoagulant (LA) 1.41 [positive: > 1.20]; anticardiolipin antibody (ACL)-IgM, 12.5 IgM phospholipid units (MPLU)/mL [positive: > 12.0]; beta-2 glycoprotein 1 (β2GP1)-IgM 60.1 AU/mL [positive: > 24.0]). The cystic lesion, inconsistent with intraductal papillary mucinous neoplasm (IPMN) or pseudocyst, led to the diagnosis of aPL-associated pancreatic cystic lesion with concurrent acute pancreatitis. Thromboembolic screening was negative. Methylprednisolone (40 mg/day) and low-molecular-weight heparin (4000 U/day) were administered, along with biliary and pancreatic duct stenting. As symptoms resolved, medications were tapered. Follow-up imaging demonstrated marked cyst regression (Figure 1D). Repeated testing after three months confirmed persistent aPL positivity (LA 1.28, ACL-IgM 14.9 MPLU/mL, β2GP1-IgM 42.6 AU/mL).
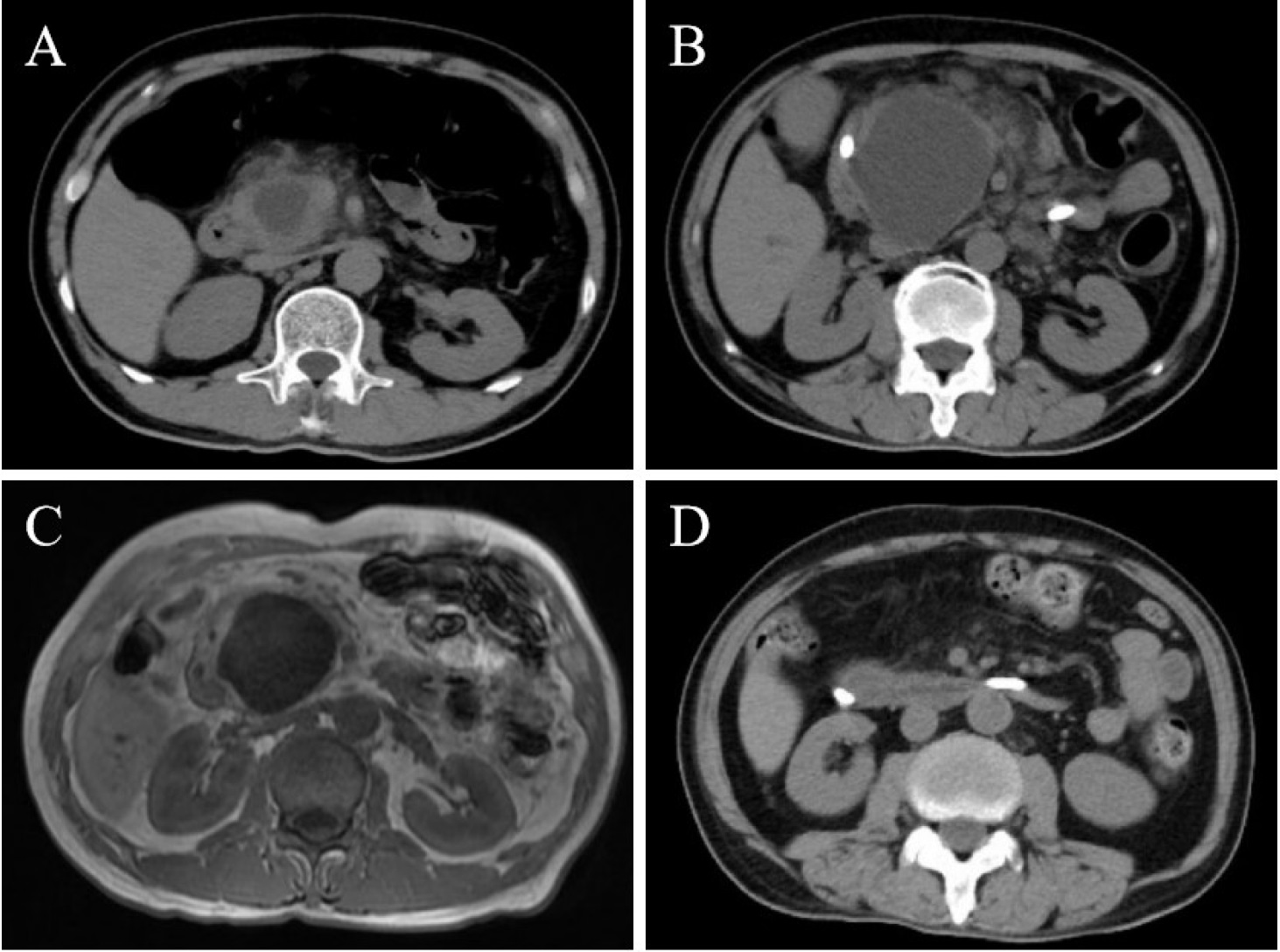
Images of the patient’s pancreas. Initial CT scan on admission (A) demonstrated a well-circumscribed cystic lesion (36 mm × 26 mm) within the pancreatic head, accompanied by peripancreatic exudation. CT scan after two weeks (B) showed progressive enlargement of the cyst to 67 mm × 49 mm, accompanied by dilation of the branch pancreatic ducts. Contrast-enhanced MRI (C) revealed hypointense on T1-weighted images and hyperintense on T2-weighted images within the pancreatic head, with no abnormal enhancement post-contrast administration. Follow-up CT scan after two weeks (D) demonstrated a significant reduction in cyst lesion size and resolution of peripancreatic exudation.
This case highlights a rare manifestation of patients with aPL positivity, as pancreatic involvement occurs in ~0.5% of antiphospholipid syndrome (APS) patients.[1] While pancreatitis is common, aPL-associated cystic lesions are unusual but clinically significant.[2, 3, 4] The proposed mechanism involves aPL-mediated microvascular thrombosis, leading to ischemic necrosis, inflammation, and cystogenesis.[1,5] Persistent aPL positivity and therapeutic responsiveness further support aPLs as the etiology. This case underscores the importance of recognizing the diverse organ involvement and micro-vascular pathology of APS. Early identification and targeted treatment addressing inflammation and thrombosis can significantly benefit patients in such scenarios.
Funding statement: This study was supported by the Chinese National Key Technology R& D Program, Ministry of Science and Technology (2021YFC250130), Beijing Municipal Science& Technology Commission (No. Z201100005520022, 23, 25-27), CAMS Innovation Fund for Medical Sciences (CIFMS)(2023-I2M-C& T-B-051).
Acknowledgements
None.
-
Author contributions
Junxian Hong: Writing-Original draft preparation. Shikai Hu and Yangzhong Zhou: Writing-Reviewing and Editing. Jiuliang Zhao and Yang-zhong Zhou: Supervision. All authors have read and agreed to the published version of the manuscript.
-
Ethical approval
Not applicable.
-
Informed consent
Informed consents have been obtained. The patient has given the consent for the images and other clinical information to be reported in the journal.
-
Conflict of interest
Jiuliang Zhao is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of the editor and his research groups.
-
Use of large language models, AI and machine learning tools
None declared.
-
Data availability statement
Data can be available on demand.
References
[1] Zhang J, Li C, Han X, et al. The digestive system involvement of antiphospholipid syndrome: pathophysiology, clinical characteristics, and treatment strategies. Ann Med. 2021;53:1328–1339.10.1080/07853890.2021.1962964Search in Google Scholar PubMed PubMed Central
[2] Nesher G, Breuer GS, Temprano K, et al. Lupus-associated pancreatitis. Semin Arthritis Rheum. 2006;35:260–267.10.1016/j.semarthrit.2005.08.003Search in Google Scholar PubMed
[3] Nguyen HC, Dimou A, Govil A, et al. Primary antiphospholipid syndrome and necrotizing pancreatitis: a diagnostic challenge. J Clin Rheumatol. 2013;19:348–350.10.1097/RHU.0b013e31829cef33Search in Google Scholar PubMed
[4] Savey L, Piette JC, Bellanger J, et al. Catastrophic antiphospholipid syndrome (CAPS)-induced ischemic pancreatic ducts injury mimicking intraductal papillary mucinous neoplasm (IPMN). Se-min Arthritis Rheum. 2018;47:565–568.10.1016/j.semarthrit.2017.07.001Search in Google Scholar PubMed
[5] Dellamonica J, Tieulie N, Bernardin G. Pancreatitis due to catastrophic antiphospholipid syndrome. Pancreas. 2007;34:380–381.10.1097/MPA.0b013e3180325bc1Search in Google Scholar PubMed
© 2025 Junxian Hong, Shikai Hu, Jiuliang Zhao, Yangzhong Zhou, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Original Article
- Angular assessment of joints in juvenile idiopathic arthritis
- Circ_0088200 acts as a sponge for miR-127-5p to promote the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes
- Insights into familial Mediterranean fever: Chronic disease correlations with arthralgia and current health status of patients with familial Mediterranean fever in Jordan
- Nitazoxanide alleviates CFA-induced rheumatoid arthritis in Wistar rats by modulating the STAT-3 and NF-κB pathways
- The causal relationship between obstructive sleep apnea and rheumatic disease: A bidirectional Mendelian randomization study
- Efficacy and safety of 2% isosorbide cream in systemic sclerosis patients with digital ulcers and Raynaud’s phenomenon
- Letter to the Editor
- Insights into the clinical and immunological significance of anti-α-fodrin antibodies in systemic lupus erythematosus
- Images
- Antiphospholipid antibody-associated cystic lesion of the pancreatic head with concurrent acute pancreatitis
Articles in the same Issue
- Original Article
- Angular assessment of joints in juvenile idiopathic arthritis
- Circ_0088200 acts as a sponge for miR-127-5p to promote the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes
- Insights into familial Mediterranean fever: Chronic disease correlations with arthralgia and current health status of patients with familial Mediterranean fever in Jordan
- Nitazoxanide alleviates CFA-induced rheumatoid arthritis in Wistar rats by modulating the STAT-3 and NF-κB pathways
- The causal relationship between obstructive sleep apnea and rheumatic disease: A bidirectional Mendelian randomization study
- Efficacy and safety of 2% isosorbide cream in systemic sclerosis patients with digital ulcers and Raynaud’s phenomenon
- Letter to the Editor
- Insights into the clinical and immunological significance of anti-α-fodrin antibodies in systemic lupus erythematosus
- Images
- Antiphospholipid antibody-associated cystic lesion of the pancreatic head with concurrent acute pancreatitis

