Abstract
Objectives
The results of existing studies on bisphenol A (BPA) and puberty timing did not reach a consensus. Thereby we performed this meta-analytic study to explore the association between BPA exposure in urine and puberty timing.
Methods
Meta-analysis of the pooled odds ratios (OR), prevalence ratios (PR) or hazards ratios (HR) with 95% confidence intervals (CI) were calculated and estimated using fixed-effects or random-effects models based on between-study heterogeneity.
Results
A total of 10 studies involving 5621 subjects were finally included. The meta-analysis showed that BPA exposure was weakly associated with thelarche (PR: 0.96, 95% CI: 0.93–0.99), while no association was found between BPA exposure and menarche (HR: 0.99, 95% CI: 0.89–1.12; OR: 1.02, 95% CI: 0.73–1.43), and pubarche (OR: 1.00, 95% CI: 0.79–1.26; PR: 1.00, 95% CI: 0.95–1.05).
Conclusions
There was no strong correlation between BPA exposure and puberty timing. Further studies with large sample sizes are needed to verify the relationship between BPA and puberty timing.
Introduction
Puberty is a critical period of the development of reproductive maturity including gonads maturation, genital development and the appearance of secondary sexual characteristics [1]. Precocious puberty has been very common in developed countries since the late 19th century and in recent years also has been detected in developing countries, affecting one in 5000 children especially in girls [2]. The early sexual maturation might be associated with genetic, ethnicity, pediatric obesity, psychosocial stress and exposure to environmental endocrine disruptors (EEDs) [3]. Bisphenol A (BPA) is one of the classes of EEDs, which is widely used in the plastics production that directly contacts with food and in inner coatings of cans and jar caps. Human can expose to BPA though oral, inhalation or transdermal [4]. Population studies [5] have indicated that most children were exposed to BPA. Multiple studies have indicated that BPA exposure was associated with precocious puberty. Animal studies have confirmed that BPA with properties similar to estrogen, has endocrine-disrupting effects [6]. Case-control studies have showed that BPA might be a risk factor for precocious puberty [7]. However, most but not all cohort studies of BPA exposure and puberty timing have reported non-significant associations. In recent studies, research findings have not achieved a consensus so far. Therefore, it is necessary to perform meta-analysis to synthesize current study evidence and identity the association between BPA exposure and puberty timing.
Methods
Selection criteria
Inclusion criteria: (1) epidemiological studies on humans such as cohort studies, case-control studies and cross-sectional studies; (2) the exposure of interest was urine levels of BPA in children; (3) the outcomes of interest were occurrence of secondary sexual characteristics, including thelarche, pubarche and menarche; (4) studies were published in English or Chinese language in journals.
Exclusion criteria: (1) children had idiopathic central precocious puberty (ICPP) or any diseases which would affect pubertal development, such as congenital gonadal dysplasia, adrenal cortical tumors; (2) studies defined the pubertal development by childhood growth (e.g. height growth spurt) rather than secondary sexual characteristics; (3) odds ratios (OR), prevalence ratios (PR) or hazards ratios (HR) with 95% confidence intervals (CIs) were not reported or the number of the same ratio category studies with one pubertal timing indicator was limited; and (4) studies with outcomes compared with 1-unit increase in urinary BPA.
Search strategy
We searched the following databases up to October 2020: PubMed, Web Of Science and EBSCO, using both the MeSH terms and free terms: “bisphenol A” or “BPA” or “4, four isopropylidenediphenol” or “environmental endocrine disruptors” or “EEDs” or “endocrine disruptor chemicals” or “EDCs”, in combination with “pubertal timing” or “puberty timing” or “puberty” or “pubarche” or “thelarche” or “menarche” or “pubic hair development” or “breast development”. In addition, the reference lists from retrieved articles were screened manually for further relevant articles.
Data extraction
The following data were extracted from included studies using a predesigned extraction form: (1) general information, including authors, publication year, country and age interval; (2) study design and sample size; (3) BPA unit and the source of sample; (4) adjusted ORs, HRs or PRs with corresponding 95% confidence intervals, adjustment in model and primary outcomes. If there was disagreement about eligibility of the article, it would be resolved through discussion.
Quality assessment
To assess the methodology quality of observational cohort and cross-sectional studies, an evaluation system based on the National Institutes of Health’s Quality Assessment Tool [8] was adopted. There were 14 criteria and total scores ranged from 0 to 14. For each criterion, a score of one was obtained if “yes” was the response, whereas a score of zero was assigned otherwise (i.e. an answer of “no”, “not applicable”, “not reported” or “cannot determine”).
Statistical analysis
Odds ratios, prevalence ratios or hazards ratios with corresponding 95% confidence intervals were obtained from the selected articles to assess the association of BPA and pubertal timing. Heterogeneity among studies was evaluated by I2 statistic tests. Once the effect was found to be heterogeneous (I2 > 50% or p < 0.05), the random effects model was used. Otherwise, the fixed effects model was adopted. Sensitivity analyses were conducted to investigate the stability of the results with the leave-one-out method. Publication bias was estimated using Egger’s regression asymmetry test. It existed, the p-value < 0.05. Statistical analyses were performed with STATA version 12 (Stata Corporation, College Station, Texas, USA).
Results
Search results
A flow diagram of the selection process was shown in Figure 1. The 10 studies were searched using a systematic search strategy. Finally, six cohort studies [9], [10], [11], [12], [13], [14] and four cross-sectional studies [15], [16], [17], [18] involving 5621 participants were identified through a systematic search strategy. And there are four studies [9], [10], [14], [18] reported OR, four studies [13], [15], [16], [17] reported PR and two studies [11], [12] reported HR.
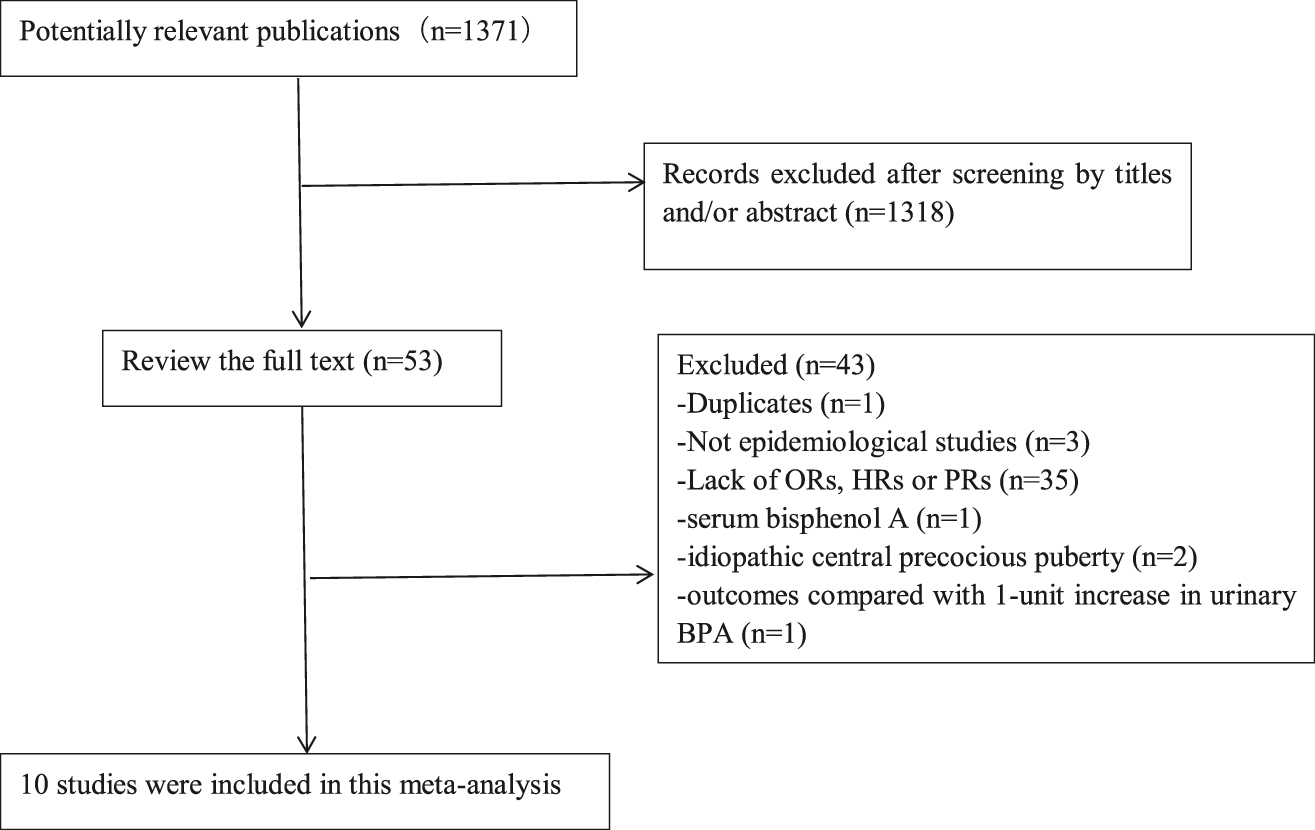
Flow diagram of the literature search.
Characteristics of included studies
Primary characteristics of included studies were presented in Table 1. All the studies were published between 2008 and 2018, with sample size ranging from 113 to 1151. Among the 10 studies, four studies [11], [13], [17], [18] were performed in the America, two [15], [16] were performed in China, two [9], [14] were performed in Mexico, while the other two studies were performed in Germany [10] and Chile [12], respectively. Of those studies, two studies [9], [16] were only boys, seven studies [11], [12], [13], [14], [15], [17], [18] were only girls and one study [10] was both boys and girls. Among those studies, five [10], [13], [14], [15], [17] reported the girls with thelarche, five [10], [11], [12], [14], [18] reported the outcome of menarche and three [9], [10], [16] reported the number of boys with pubarche, while in girls the number was five [10], [13], [14], [15], [17].
Characteristics of studies included in the meta-analysis.
| Study | Country | Age interval | Source of sample | Study design | Sample size | BPA unit | Ratio category | OR/PR/HR (95%CI) | Primary outcomes | Adjustment in model | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferguson et al. 2014 [9] | Mexico | 8–14 | Boys | Cohort study | 113 | Ln-ng/mL | OR | 1.01 (0.50–2.04) | PH2+ vs. PH1 | Urinary specific gravity, child age, and child body mass index Z-score | 9 |
| Miao et al. 2017 [15] | China | 9–18 | Girls | Cross-sectional study | 655 | Ln-μg/gC | PR | 1.17 (0.60–2.25) 0.91 (0.80–1.04) |
Age at PH2 Age at B2 |
Age, BMI, household income, sleep quality, sports activity, unbalanced diet, and depression score | 9 |
| Wang et al. 2017 [16] | China | 9–18 | Boys | Cross-sectional study | 671 | Ln-μg/gC | PR | 1.11 (0.86–1.42) | PH2+ vs. PH1 | Age, height, BMI, household income, parental education, sports activity, and intake of fruits and vegetables, soy-based foods, dairy products, cereals, fish, meat, and junk foods | 10 |
| Kasper-Sonnenberg et al. 2017 [10] | Germany | 8–13 | Girls | Cohort study | 222 | Ln-μg/L | OR | 0.79 (0.57–1.10) | Breast developmenta | Age (years), BMI (kg/m2), creatinine (mg/dl) | 10 |
| 1.18 (0.87–1.60) | Pubic hairb | ||||||||||
| 1.25 (0.84–1.88) | Menarche onset (yes vs. no) | ||||||||||
| Boys | 250 | 0.73 (0.47–1.14) | Public hairb | ||||||||
| Wolff et al. 2017 [11] | America | 6–8 | Girls | Cohort study | 1051 | Ln-μg/gC | HR | 1.04 (0.96–1.12) | Age at menarche | Ln-urine creatinine, race/ethnicity and caregiver education | 11 |
| Binder et al. 2018 [12] | Chile | 6–10 | Girls | Cohort study | 200 | Ln-ng/ml | HR | 0.92 (0.79–1.06) | Age at menarche | BMI Z-score, and maternal education | 12 |
| Wolff et al. 2008 [17] | America | 9 | Girls | Cross-sectional study | 192 | Ln-μg/gC | PR | 0.96 (0.92–1.01) | B2+ vs. B1 | Height and Black race | 10 |
| 0.98 (0.89–1.08) | PH2+ vs. PH1 | ||||||||||
| Wolff et al. 2010 [13] | America | 6–8 | Girls | Cohort study | 1151 | Ln-μg/gC | PR | 0.97 (0.91–1.03) | B2+ vs. B1 | Age, race/ethnicity, sex- and age-specific BMI%, guardian education, season of urine collection, and site | 10 |
| 1.00 (0.94–1.07) | PH2+ vs. PH1 | ||||||||||
| McGuinn et al. 2015 [18] | America | 12–19 | Girls | Cross-sectional study | 987 | Ln-ng/mL | OR | 0.77 (0.38–1.55) | Age at menarche | Race/ethnicity, parental education, country of birth, and body size | 9 |
| Watkins et al. 2014 [14] | Mexico | 8–13 | Girls | Cohort study | 129 | Ln-μg/L | OR | 0.99 (0.33–2.96) | B2+ vs. B1 | Age, BMI Z-score, and specific gravity | 9 |
| 0.68 (0.20–2.33) | PH2+ vs. PH1 | ||||||||||
| 0.44 (0.14–1.36) | Menarche onset |
-
OR, odds ratio; PR, prevalence ratios; HR, hazard ratios; CI, credibility interval; PH2+, pubic hair stage 2 or higher; PH1, no pubic hair development; μg/gC, micrograms-per-gram creatinine; B2, breast development stage 2; B1, no breast development; BMI, body mass index.
aDefinitely started and higher vs. not yet happened or barely started in breast development.
bDefinitely started and higher vs. not yet happened or barely started in pubic hair development.
In this meta-analysis, we only included papers with outcome compared with IQR or high/low to reach comparability. The levels of BPA are low among studies included in our meta-analysis. Among the 10 studies included in our meta-analysis, five studies reported with geometric mean of BPA concentration with ng/ml or μg/l of BPA units and the values ranged from 1.05 to 2.64 ng/ml. All studies had limits of detection (LOD), and five studies defined the values below the LOD were replaced with
BPA exposure and puberty timing
This meta-analysis based on 5621 subjects in 10 studies indicated that BPA exposure was not strongly correlated with puberty timing.
Thelarche
Five studies [10], [13], [14], [15], [17] comprising 2349 girls were included in the analysis of BPA exposure and thelarche. The pooled PR showed that BPA exposure was weakly associated with the thelarche (PR: 0.96, 95% CI: 0.93–0.99), while no difference was detected in BPA exposure and thelarche with a pooled OR of 0.80 (95% CI: 0.59–1.10). There was little statistical heterogeneity among studies (PR: p=0.69, I2<0.1%; OR: p=0.70, I2<0.1%), consequently, a fixed effects model was used (Figure 2.).
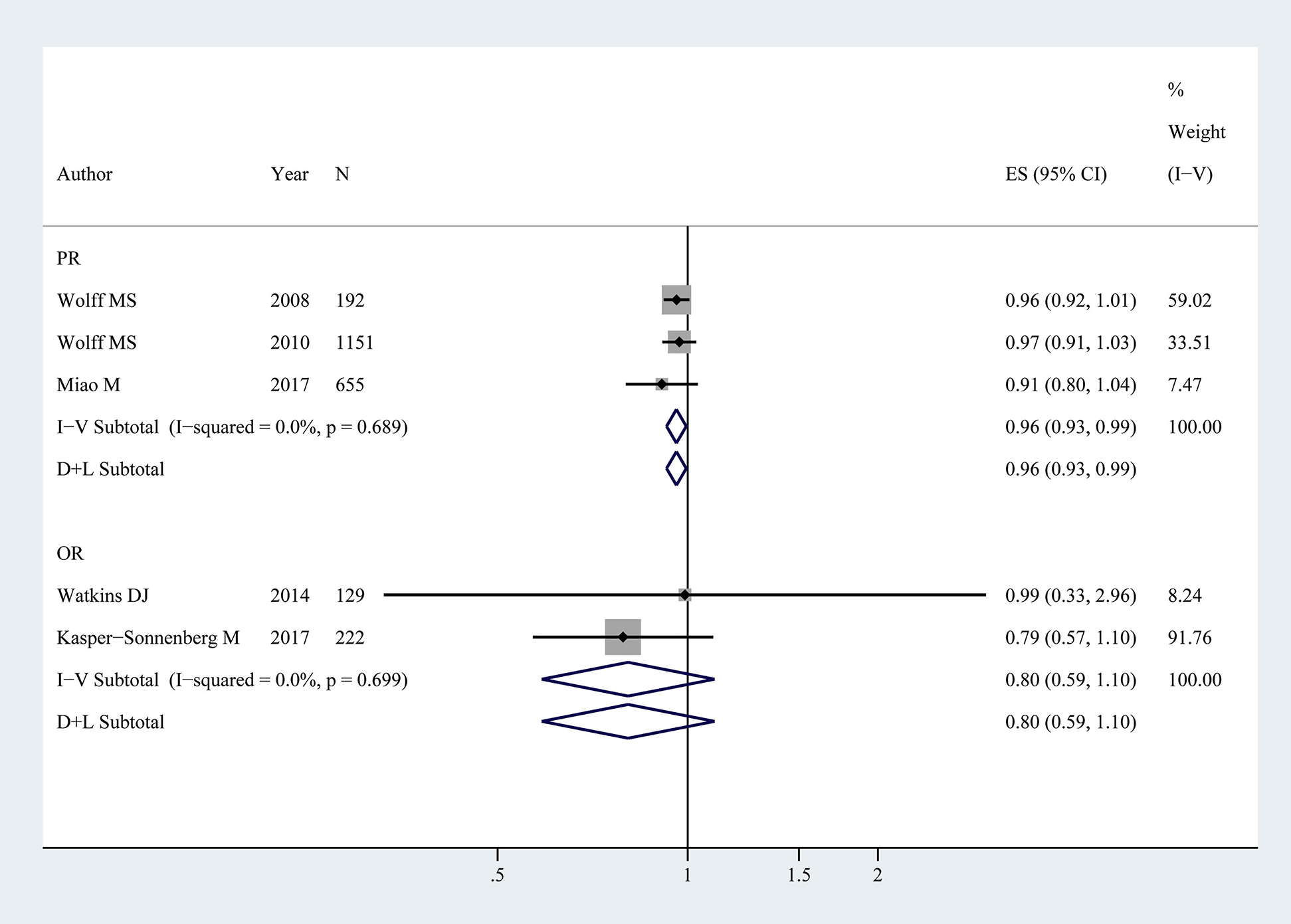
Forest plot for BPA exposure and thelarche in children (NOR=351, NPR=1998).
The association represents age of thelarche vs. log BPA. OR, PR and ES represent odds ratios, prevalence ratios and effect size with 95% confidence interval (CI) from the studies included in the current meta-analysis, respectively. % weight represents the percentage of study weight in the current meta-analysis.
Menarche
There were five publications [10], [11], [12], [14], [18] reported menarche based on 2589 girls. No evident association was detected in BPA exposure and menarche (HR: 0.99, 95% CI: 0.89–1.12; OR: 1.02, 95% CI: 0.73–1.43). A random model and fixed model were adopted in pooled HR and OR, respectively (HR: p=0.15, I2=52.3%; OR: p=0.16, I2=45.9%) (Figure 3.).
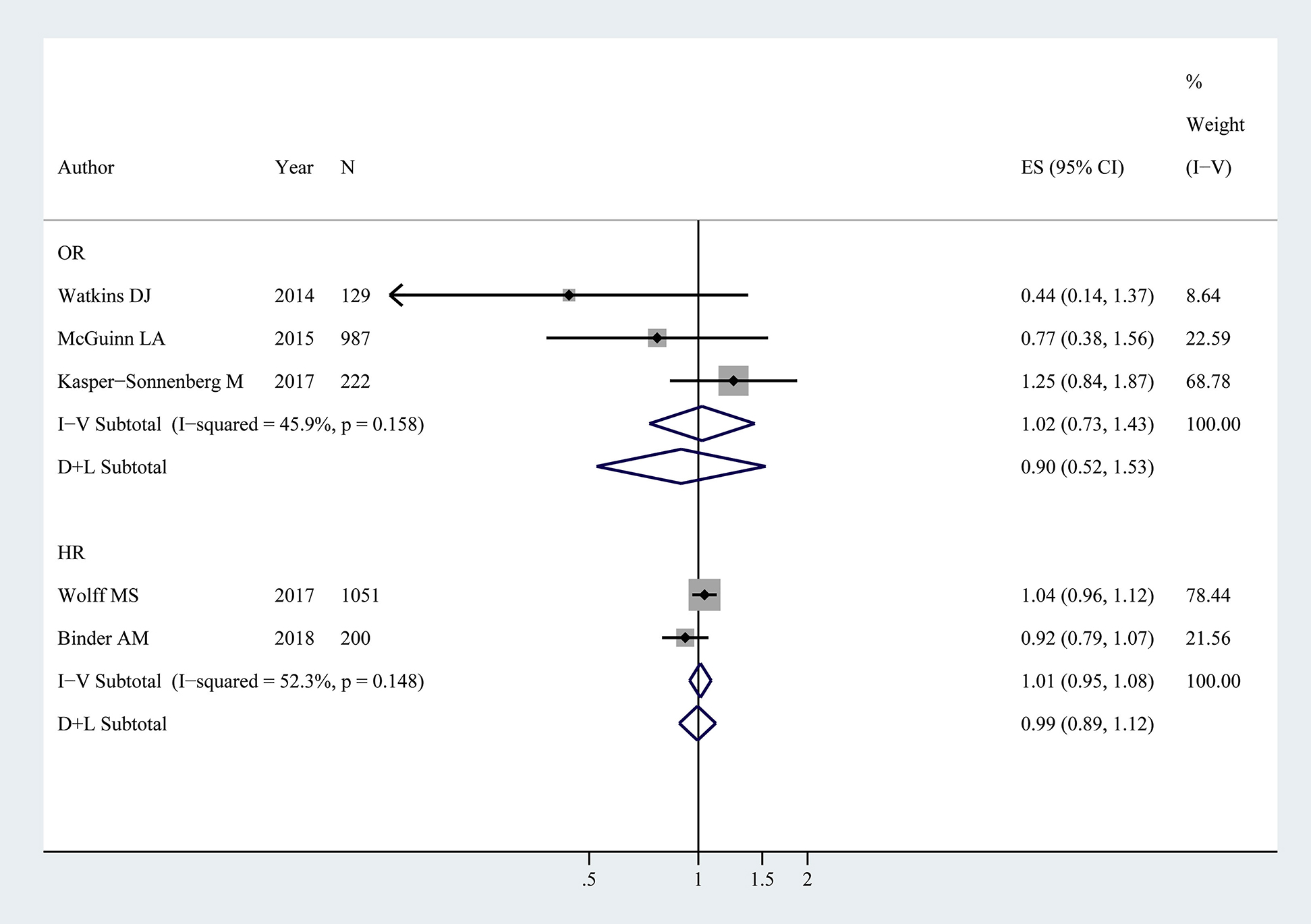
Forest plot for BPA exposure and menarche in children (NOR=1338, NHR=1251).
The association represents age of menarche vs. log BPA. OR, HR and ES represent odds ratios, hazards ratios and effect size with 95% confidence interval (CI) from the studies included in the current meta-analysis, respectively. % weight represents the percentage of study weight in the current meta-analysis.
Pubarche
Seven studies [9], [10], [13], [14], [15], [16], [17] reported the pubarche based on 1034 boys and 2349 girls, showing that there was no statistical difference on the relationship between BPA exposure and pubarche (OR: 1.00, 95% CI: 0.79–1.26; PR: 1.00, 95% CI: 0.95–1.05). A fixed-effects model was used because there was not significant heterogeneity among studies (OR: p=0.33, I2=13.1%; PR: p=0.79, I2<0.1%) (Figure 4.).
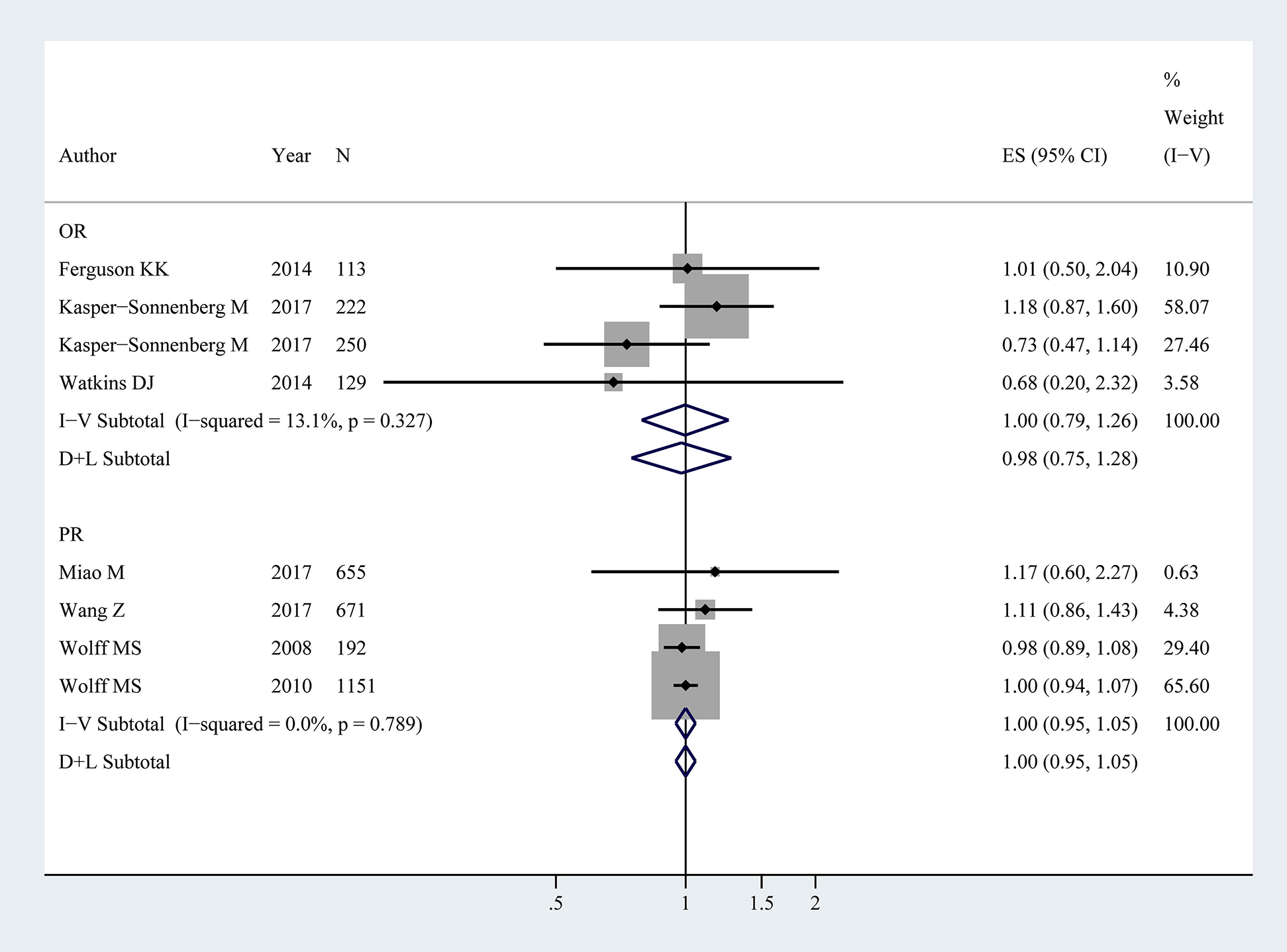
Forest plot for BPA exposure and pubarche in children (NOR=714, NPR=2669).
The association represents age of pubarche vs. log BPA. OR, PR and ES represent odds ratios, prevalence ratios and effect size with 95% confidence interval (CI) from the studies included in the current meta-analysis, respectively. % weight represents the percentage of study weight in the current meta-analysis.
Sensitivity analysis and publication bias
Sensitivity analysis suggested that no individual study had excessive influence on the above-mentioned pooled effect. The Egger test showed no evidence of significant publication bias for the analysis between BPA exposure and pubertal timing (thelarche: p=0.23; menarche: p=0.40; pubarche: p=1.00) (Supplemental Figures 1–3).
Discussion
In this meta-analysis, BPA exposure was a weak protective factor for thelarche. However, no statistically significant association was found in other pubertal timing indicators with puberty timing.
The exposure of BPA was not statistically associated with pubarche and menarche, which may not necessarily mean that there was no relationship among them. As the number of sample size increasing, maybe we can find that BPA exposure in urine is weakly associated with pubarche and menarche like thelarche. Meanwhile, the results may be related to BPA concentration, and one study [16] conducted by Wang indicated that moderate BPA (limit of detection(LOD) -75th) was related to prematurity of genital and pubic hair and the adjusted PRs were 1.13 (95% CI: 1.03, 1.68) and 1.28 (95% CI: 1.02, 1.60), respectively. The same result was reported by another study [18] which showed that girls with moderate BPA (1.9–4.1 ng/ml) were more likely to have delayed menarche compared with the lowest BPA with OR of 0.55 (95% CI: 0.31, 0.99) after adjusting model one for urinary creatinine (log-transformed mg/dl), age, and NHANES cycle, while, after adjusting model two for race/ethnicity, parental education, country of birth, and body size in addition to model one covariates, no statistical association was found between menarche and moderate BPA with OR of 0.57 (95% CI: 0.30, 1.08). However, in most present study, we did not observe a stronger association among children with moderate BPA exposure compared with those with highest exposure.
Although most of the molecular mechanisms of BPA effects were still unknown, an animal experiment [19] showed that BPA induced premature puberty resulted from decreased inhibition of gonadotropin releasing hormone (GnRH) neurons. Meanwhile, another study [20] indicated that BPA can alter the GnRH network to induce precocious puberty by activating stimulatory and repressing inhibitory in puberty activation. Moreover, many studies [21] showed that its effects might due to its estrogen-like action. The BPA could bind to estrogen receptors (αER, βER) in vitro and in vivo [22]. Furthermore, it could inhibit the activity of endogenous estrogen and interfere with estrogen nuclear receptors [23].
To the best of our knowledge, this is the first meta-analysis to examine the association between BPA exposure and puberty timing in both girls and boys. Besides, the amounts of subjects of 5621 was pretty substantial in this meta-analysis. Despite a rigorous approach of meta-analysis, several potential limitations should be considered. Firstly, heterogeneity that came from race diversity, body mass index (BMI), age variation and diet might be potential effects. One study [11] found that age at thelarche differed by BMI and race by 6–12 months. Another study showed that girls with a higher BMI were at an increased risk for earlier pubertal maturation [24]. Moreover, two longitudinal studies found that fiber intake was a protective effect for age at menarche [25], [26]. Although these factors were important, still not all included studies of the meta-analysis adjusted these factors. Secondly, only single spot urine samples were collected in most of our studies to reflect BPA exposure, which might not account for the within-individual variation and the day-to-day-variability of BPA exposure due to the short half-life of BPA. Nonetheless, precious studies have shown that single spot urine samples of BPA might be representative of long-term averages [27], [28], [29]. Thirdly, this meta-analysis did not account for prenatal exposure of BPA on puberty timing. Animal evidence for a relationship between in utero BPA exposure and anogenital distance was conflicting [30], [31]. A cohort study [32] showed that BPA exposure might influence the female reproductive development in specific critical periods of utero development. Thus, prenatal exposure of BPA might have modified our observed associations with pubertal development. Future studies are needed to further identify the relationship of early life BPA exposure and childhood outcomes in puberty timing. Fourthly, the difficulty of accurately recalling the age of menarche pubarche and thelarche might contribute to bias in meta-analysis. Fifthly, there are four included studies using logistic regression analysis in this meta-analysis, which may cause bias of statistics. From a statistical perspective, we believed that poisson regression is more appropriate than logistic regression based on the characteristics of these studies. In addition, most included studies were cross-sectional researches in human and the sample number of the studies was small, which increased risk of bias and downgraded the quality of evidence. Therefore, further studies with higher quality and larger sample size are needed in future.
Conclusions
In conclusion, no strong correlation was observed in the relationship between BPA exposure in urine and puberty timing. Our findings of this meta-analysis demonstrated that BPA exposure might be weakly associated with thelarche. However, there was no association between BPA exposure and other pubertal timing indicators. Furthermore, prospective epidemiological studies with larger sample size, well designed methods that exclude potential contamination of samples are needed.
Funding source: China Postdoctoral Science Foundation 10.13039/501100010008
Award Identifier / Grant number: 2019M660161
-
Research funding: This study was sponsored by the Postdoctoral Science Foundation of China grant 2019M660161 to Dr Zhou.
-
Author contributions: Yunping Zhou participated in the design of the study. Hui Meng and Yunxia Jiang collected, selected and extracted the data. Yunping Zhou performed the statistical analysis. Hui Meng drafted the manuscript and Yunping Zhou and Yunxia Jiang revised the manuscript. All authors read and approved the final manuscript.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent is not applicable.
-
Ethical approval: The conducted research is not related to either human or animal use.
References
1. Kane, L, Ismail, N. Puberty as a vulnerable period to the effects of immune challenges: focus on sex differences. Behav Brain Res 2017;320:374–82. https://doi.org/10.1016/j.bbr.2016.11.006.Search in Google Scholar PubMed
2. Teilmann, G, Pedersen, CB, Jensen, TK, Skakkebaek, NE, Juul, A. Prevalence and incidence of precocious pubertal development in Denmark: an epidemiologic study based on national registries. Pediatrics 2005;116:1323–8. https://doi.org/10.1542/peds.2005-0012.Search in Google Scholar PubMed
3. Cesario, SK, Hughes, LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs 2007;36:263–74. https://doi.org/10.1111/j.1552-6909.2007.00145.x.Search in Google Scholar PubMed
4. Konieczna, A, Rutkowska, A, Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz Panstw Zakl Hig 2015;66:5–11.Search in Google Scholar
5. Shelby, MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Ntp cerhr mon 2008. 1–64. v, vii-ix. passim.Search in Google Scholar
6. Nah, WH, Park, MJ, Gye, MC. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin Exp Reprod Med 2011;38:75–81. https://doi.org/10.5653/cerm.2011.38.2.75.Search in Google Scholar PubMed PubMed Central
7. Durmaz, E, Asci, A, Erkekoglu, P, Akcurin, S, Gumusel, BK, Bircan, I. Urinary bisphenol a levels in girls with idiopathic central precocious puberty. J Clin Res Pediatr Endocrinol 2014;6:16–21. https://doi.org/10.4274/jcrpe.1220.Search in Google Scholar
8. National Heart, Lung, Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies; 2014. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/. cardiovascular-risk-reduction/tools/cohort.Search in Google Scholar
9. Ferguson, KK, Peterson, KE, Lee, JM, Mercado-Garcia, A, Blank-Goldenberg, C, Tellez-Rojo, MM, et al.. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol 2014;47:70–6. https://doi.org/10.1016/j.reprotox.2014.06.002.Search in Google Scholar PubMed PubMed Central
10. Kasper-Sonnenberg, M, Wittsiepe, J, Wald, K, Koch, HM, Wilhelm, M. Pre-pubertal exposure with phthalates and bisphenol A and pubertal development. PLoS One 2017;12: e0187922. https://doi.org/10.1371/journal.pone.0187922.Search in Google Scholar PubMed PubMed Central
11. Wolff, MS, Pajak, A, Pinney, SM, Windham, GC, Galvez, M, Rybak, M, et al.. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod Toxicol 2017;67:56–64. https://doi.org/10.1016/j.reprotox.2016.11.009.Search in Google Scholar PubMed PubMed Central
12. Binder, AM, Corvalan, C, Calafat, AM, Ye, X, Mericq, V, Pereira, A, et al.. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ Health 2018;17:32. https://doi.org/10.1186/s12940-018-0376-z.Search in Google Scholar PubMed PubMed Central
13. Wolff, MS, Teitelbaum, SL, Pinney, SM, Windham, G, Liao, L, Biro, F, et al.. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect 2010;118:1039–46. https://doi.org/10.1289/ehp.0901690.Search in Google Scholar PubMed PubMed Central
14. Watkins, DJ, Tellez-Rojo, MM, Ferguson, KK, Lee, JM, Solano-Gonzalez, M, Blank-Goldenberg, C, et al.. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res 2014;134:233–41. https://doi.org/10.1016/j.envres.2014.08.010.Search in Google Scholar PubMed PubMed Central
15. Miao, M, Wang, Z, Liu, X, Liang, H, Zhou, Z, Tan, H, et al.. Urinary bisphenol A and pubertal development in Chinese school-aged girls: a cross-sectional study. Environ Health 2017;16:80. https://doi.org/10.1186/s12940-017-0290-9.Search in Google Scholar PubMed PubMed Central
16. Wang, Z, Li, D, Miao, M, Liang, H, Chen, J, Zhou, Z, et al.. Urine bisphenol A and pubertal development in boys. Int J Hyg Environ Health 2017;220:43–50. https://doi.org/10.1016/j.ijheh.2016.10.004.Search in Google Scholar PubMed
17. Wolff, MS, Britton, JA, Boguski, L, Hochman, S, Maloney, N, Serra, N, et al.. Environmental exposures and puberty in inner-city girls. Environ Res 2008;107:393–400. https://doi.org/10.1016/j.envres.2008.03.006.Search in Google Scholar PubMed PubMed Central
18. McGuinn, LA, Ghazarian, AA, Joseph Su, L, Ellison, GL. Urinary bisphenol A and age at menarche among adolescent girls: evidence from NHANES 2003–2010. Environ Res 2015;136:381–6. https://doi.org/10.1016/j.envres.2014.10.037.Search in Google Scholar PubMed PubMed Central
19. Losa-Ward, SM, Todd, KL, McCaffrey, KA, Tsutsui, K, Patisaul, HB. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod 2012;87:28. https://doi.org/10.1095/biolreprod.112.100826.Search in Google Scholar PubMed PubMed Central
20. Mueller, JK, Heger, S. Endocrine disrupting chemicals affect the gonadotropin releasing hormone neuronal network. Reprod Toxicol 2014;44:73–84. https://doi.org/10.1016/j.reprotox.2013.10.011.Search in Google Scholar PubMed
21. Caserta, D, Di Segni, N, Mallozzi, M, Giovanale, V, Mantovani, A, Marci, R, et al.. Bisphenol A and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol: RBE (Rev Bras Entomol) 2014;12:37. https://doi.org/10.1186/1477-7827-12-37.Search in Google Scholar PubMed PubMed Central
22. Caserta, D, Mantovani, A, Marci, R, Fazi, A, Ciardo, F, La Rocca, C, et al.. Environment and women’s reproductive health. Hum Reprod Update 2011;17:418–33. https://doi.org/10.1093/humupd/dmq061.Search in Google Scholar PubMed
23. Wetherill, YB, Akingbemi, BT, Kanno, J, McLachlan, JA, Nadal, A, Sonnenschein, C, et al.. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 2007;24:178–98. https://doi.org/10.1016/j.reprotox.2007.05.010.Search in Google Scholar PubMed
24. Biro, FM, Khoury, P, Morrison, JA. Influence of obesity on timing of puberty. Int J Androl 2006;29:272–7. discussion 86–90. https://doi.org/10.1111/j.1365-2605.2005.00602.x.Search in Google Scholar PubMed
25. Koo, MM, Rohan, TE, Jain, M, McLaughlin, JR, Corey, PN. A cohort study of dietary fibre intake and menarche. Publ Health Nutr 2002;5:353–60. https://doi.org/10.1079/phn2002261.Search in Google Scholar
26. de Ridder, CM, Thijssen, JH, Van ’t Veer, P, van Duuren, R, Bruning, PF, Zonderland, ML, et al.. Dietary habits, sexual maturation, and plasma hormones in pubertal girls: a longitudinal study. Am J Clin Nutr 1991;54:805–13. https://doi.org/10.1093/ajcn/54.5.805.Search in Google Scholar PubMed
27. Vandenberg, LN, Hauser, R, Marcus, M, Olea, N, Welshons, WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–77. https://doi.org/10.1016/j.reprotox.2007.07.010.Search in Google Scholar PubMed
28. Christensen, KL, Lorber, M, Koch, HM, Kolossa-Gehring, M, Morgan, MK. Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24- or 48-h collections. J Expo Sci Environ Epidemiol 2012;22:632–40. https://doi.org/10.1038/jes.2012.52.Search in Google Scholar PubMed
29. Teitelbaum, SL, Britton, JA, Calafat, AM, Ye, X, Silva, MJ, Reidy, JA, et al.. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 2008;106:257–69. https://doi.org/10.1016/j.envres.2007.09.010.Search in Google Scholar PubMed
30. Kobayashi, K, Miyagawa, M, Wang, RS, Sekiguchi, S, Suda, M, Honma, T. Effects of in utero and lactational exposure to bisphenol A on somatic growth and anogenital distance in F1 rat offspring. Ind Health 2002;40:375–81. https://doi.org/10.2486/indhealth.40.375.Search in Google Scholar PubMed
31. Christiansen, S, Axelstad, M, Boberg, J, Vinggaard, AM, Pedersen, GA, Hass, U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2014;147:477–87. https://doi.org/10.1530/rep-13-0377.Search in Google Scholar
32. Watkins, DJ, Sánchez, BN, Téllez-Rojo, MM, Lee, JM, Mercado-García, A, Blank-Goldenberg, C, et al.. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res 2017;159:143–51. https://doi.org/10.1016/j.envres.2017.07.051.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/reveh-2020-0091).
© 2020 Hui Meng et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Short Communication
- Greater than the sum of its parts: focusing SRP research through a systems approach lens
- Review Articles
- Association of bisphenol A with puberty timing: a meta-analysis
- Pesticide applicators and cancer: a systematic review
- Current knowledge on urease and nitrification inhibitors technology and their safety
- Environment as the risk factor for tuberculosis in Malaysia: a systematic review of the literature
- Effects of ambient air pollution on psychological stress and anxiety disorder: a systematic review and meta-analysis of epidemiological evidence
- The possible role of arsenic and gene-arsenic interactions in susceptibility to breast cancer: a systematic review
- Ambient air pollution and multiple sclerosis: a systematic review
- Mitochondria and traffic-related air pollution linked coronary artery calcification: exploring the missing link
- Air pollutants and impairments of male reproductive health-an overview
- Exposure to cadmium and head and neck cancers: a meta-analysis of observational studies
- Lost opportunities for cancer prevention: historical evidence on early warnings with emphasis on radiofrequency radiation
- Health protection messaging for populations susceptible to air pollution during landscape fire smoke events: an integrative review
Articles in the same Issue
- Frontmatter
- Short Communication
- Greater than the sum of its parts: focusing SRP research through a systems approach lens
- Review Articles
- Association of bisphenol A with puberty timing: a meta-analysis
- Pesticide applicators and cancer: a systematic review
- Current knowledge on urease and nitrification inhibitors technology and their safety
- Environment as the risk factor for tuberculosis in Malaysia: a systematic review of the literature
- Effects of ambient air pollution on psychological stress and anxiety disorder: a systematic review and meta-analysis of epidemiological evidence
- The possible role of arsenic and gene-arsenic interactions in susceptibility to breast cancer: a systematic review
- Ambient air pollution and multiple sclerosis: a systematic review
- Mitochondria and traffic-related air pollution linked coronary artery calcification: exploring the missing link
- Air pollutants and impairments of male reproductive health-an overview
- Exposure to cadmium and head and neck cancers: a meta-analysis of observational studies
- Lost opportunities for cancer prevention: historical evidence on early warnings with emphasis on radiofrequency radiation
- Health protection messaging for populations susceptible to air pollution during landscape fire smoke events: an integrative review

