Abstract
Background
From 2018 to 2023, Human papillomavirus (HPV) vaccination coverage in the United States was shaped by both proactive immunization efforts and the disruptions caused by the COVID-19 pandemic, leading to the first national decline in nearly a decade. This study aimed to assess vaccination trends over time and across regions to identify coverage gaps and inform health policy best practices for achieving optimal HPV vaccination rates.
Methods
We used provider-verified data from the National Immunization Survey–Teen for adolescents aged 13–17, focusing on vaccine initiation (≥1 dose) and up-to-date (UTD) status, as defined by Centers for Disease Control and Prevention guidelines. We used Cochran-Armitage trend tests to assess changes across the pre-pandemic, pandemic, and post-pandemic periods. We stratified our analyses by sex, race/ethnicity, and state.
Results
Initiation increased from 68.1% in 2018 to 76.9% in 2021, then declined to 76.8% in 2023. UTD status rose from 51.1 to 62.6% by 2022 but fell to 61.4% in 2023. Females and Hispanic/Black adolescents consistently had higher coverage than males and White adolescents. Eighteen states, mainly in the Northeast and Upper Midwest, achieved ≥80% initiation by 2023, while Southern states lagged.
Conclusion
Best practices for improving HPV vaccination include: (1) strengthening vaccination infrastructure in low-performing Southern states, (2) targeting male and White adolescents, (3) maintaining robust delivery systems during crises, and (4) replicating high-performing regional models. These strategies can improve vaccine equity and contribute to achieving national targets over time and space.
1 Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with approximately 43 million infections occurring annually and nearly all sexually active individuals acquiring HPV at some point in their lives [1]. Despite the availability of highly effective vaccines capable of preventing more than 32,000 HPV-related cancers each year, approximately 37,800 new cases continue to occur annually [2]. As of 2023, only 61.4% of U.S. adolescents were up-to-date (UTD) on the HPV vaccine series, representing the first national decline in coverage rates in nearly a decade and falling substantially short of the Healthy People 2030 target of 80% [3,4]. This coverage gap reflects more than missed clinical opportunities; it represents a critical vulnerability in population-level protection that has been further exposed by the unforeseen disruptions of the COVID-19 pandemic [5–8].
1.1 Theoretical framework: Optimal vaccination coverage and herd immunity thresholds
The concept of herd immunity provides the theoretical foundation for understanding optimal vaccination rates in populations. The herd immunity threshold is mathematically defined as (
1.2 Lessons from COVID-19: Vaccination program resilience and coverage thresholds
The COVID-19 pandemic provided an unprecedented natural experiment in vaccination program implementation and revealed critical insights about achieving optimal coverage rates under real-world conditions. Studies suggested that 60–70% population coverage would achieve herd immunity for SARS-CoV-2, with some estimates reaching 90% for highly transmissible variants [13–15]. However, practical experience demonstrated significant gaps between theoretical thresholds and achievable coverage rates, with most high-income countries reaching a practical ceiling of approximately 75–80% vaccination coverage despite resource mobilization [16].
The pandemic experience revealed several critical lessons relevant to HPV vaccination programs. First, achieving high vaccination coverage requires more than mathematical modeling of herd immunity thresholds; it demands robust delivery systems, sustained community engagement, adaptive program design, and crisis-resilient infrastructure capable of maintaining services during public health emergencies [17,18]. Second, vaccination programs are particularly vulnerable to disruptions that compound existing health disparities, with rural communities, racial and ethnic minorities, and socioeconomically disadvantaged populations experiencing disproportionate barriers to vaccine access [19,20]. These lessons reveal the importance of understanding how vaccination programs perform under stress and which populations are most vulnerable to coverage disruptions during public health emergencies.
1.3 Literature gap and study rationale
While extensive research has documented HPV vaccination disparities and coverage trends, a critical gap remains in understanding how coverage evolved before, during, and after the COVID-19 crisis. Most existing studies rely on cross-sectional, single-year data, or focus on limited geographic areas, limiting insight into long-term national trends and program resilience during major public health disruptions [21–23]. Although previous research confirms that COVID-19 significantly disrupted routine childhood vaccinations globally [24], HPV-specific coverage patterns across distinct pandemic phases remain insufficiently explored.
This knowledge gap is particularly important given the unique characteristics of HPV vaccination programs. Unlike routine childhood vaccines administered in early infancy, HPV vaccines are delivered to adolescents who may have delayed or missed healthcare visits during pandemic-related disruptions [24]. Additionally, HPV vaccination often requires multiple healthcare encounters and faces distinctive challenges related to vaccine hesitancy and parental decision-making that may have been worsened during the pandemic [25,26].
Furthermore, the timing of the first national decline in U.S. HPV vaccination rates in nearly a decade occurred during this period [3], yet no comprehensive analysis has examined how this decline varied across demographic and geographic populations, or what these patterns reveal about strategies for achieving optimal vaccination coverage during normal and crisis conditions.
Understanding these patterns is essential for three critical reasons. First, they inform targeted strategies to not only recover but improve upon pre-pandemic coverage levels. Second, they reveal structural vulnerabilities that hinder progress toward optimal population protection. Third, they offer guidance for designing vaccination systems resilient enough to sustain herd immunity goals during future public health crises.
1.4 Research questions and study objectives
To address this critical gap in the literature, this study examines HPV vaccination coverage trends from 2018 to 2023, covering the pre-pandemic, pandemic, and post-pandemic periods. This timeframe offers a natural experiment for evaluating how coverage patterns responded to public health system stress and for assessing progress toward herd immunity coverage thresholds.
Specifically, this study addresses three interconnected research questions that bridge theoretical vaccination coverage models with real-world program implementation:
Coverage trajectory analysis: How did HPV vaccination coverage change before, during, and after the COVID-19 pandemic? How do these trends compare to the Healthy People 2030 TARGETS for optimal population protection?
Disparities in coverage: Which demographic and geographic groups experienced the greatest shifts in vaccination coverage during this period?
Path to optimal coverage: What do these observed patterns reveal about program vulnerabilities and strategies needed to achieve sustained, high coverage?
Using provider-verified national surveillance data, this study generates evidence-based recommendations for reaching the 80% national coverage target, strengthening pandemic-resilient vaccination systems, and addressing persistent equity gaps that hinder progress toward HPV-related cancer prevention. Findings will inform both immediate recovery efforts and longer-term strategies aligned with theoretical thresholds for population-level protection.
2 Methods
2.1 Sample and data source
This study used data from the National Immunization Survey–Teen (NIS-Teen), collected annually from 2018 to 2023. The NIS-Teen is a nationally representative surveillance system administered by the Centers for Disease Control and Prevention (CDC) to monitor vaccination coverage among U.S. adolescents aged 13–17 years. Data are collected in two phases. In the first phase, parents or guardians participate in a structured telephone interview that gathers demographic and health information. In the second phase, immunization providers identified during the interview are contacted to verify the adolescent’s vaccination history. Only adolescents with provider-verified HPV vaccination records were included in this analysis to ensure accurate assessment of vaccination status. To evaluate changes in HPV vaccination coverage across the COVID-19 pandemic, the six survey years were divided into three time periods: before the pandemic (2018–2019), during the pandemic (2020–2021), and after the COVID-19 pandemic (2022–2023). We adopted this categorization based on a framework used in recent vaccine coverage study [27], which defined distinct pandemic phases to assess time-specific impacts of public health emergencies on immunization programs. This structure allowed us to track how vaccination rates shifted in response to disruptions in healthcare delivery and public health efforts.
2.2 Measures of variables
The study’s two primary outcomes were HPV vaccine initiation and UTD status. Initiation was defined as receiving at least one dose of the HPV vaccine. UTD status followed CDC guidelines and was defined as receiving either two doses when the first dose was given before age 15 and spaced at least 5 months minus 4 days apart, or three or more doses if the series was initiated at age 15 or older, or if the adolescent was immunocompromised [28].
The main explanatory variables included age (13–17 years), sex (male or female), race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or non-Hispanic others), and state of residence (based on respondent-reported U.S. state). These variables were chosen based on their relevance to vaccination disparities and their ability to help answer the study’s central questions on temporal trends and subgroup differences.
2.3 Data analysis procedure
All analyses accounted for the complex sampling design of the NIS-Teen using CDC-provided provider-phase weights. These weights correct for unequal probabilities of selection, nonresponse at both household and provider levels, and post-stratification adjustments. Weighted proportions were calculated for HPV vaccine initiation and UTD coverage, stratified by survey year and demographic subgroups.
Trend analysis was performed using the Cochran–Armitage test for trend, which assesses whether changes in vaccination rates over time followed a linear pattern across the three pandemic periods. This method was applied separately for both outcomes. Subgroup trends were examined descriptively; however, this study did not use multivariable regression models, as the primary objective was to identify temporal and spatial patterns rather than estimate causal effects.
All analyses were conducted in R (v4.4.1) using survey-weighted functions, excluding <1% of adolescents with missing primary outcomes data.
2.4 Ethical considerations
This study used publicly available, de-identified data from the NIS-Teen surveillance system. The original data collection was approved by the National Center for Health Statistics Research Ethics Review Board. No additional institutional review board approval was required for this secondary data analysis.
-
Informed consent: Not applicable. This study analyzed de-identified, publicly available data.
-
Ethical approval: Not applicable. The National Immunization Survey–Teen (NIS-Teen) dataset used in this research is publicly available and fully anonymized. No additional ethical approval was required.
3 Results
The unweighted sample sizes of adolescents aged 13–17 years with provider-verified HPV vaccination records in the NIS-Teen were 18,700 in 2018, 18,788 in 2019, 20,163 in 2020, 18,002 in 2021, 16,043 in 2022, and 16,568 in 2023.
Between 2018 and 2023, national HPV vaccination coverage initially improved but declined following the pandemic (Figure 1). Initiation rates rose from 68.1% in 2018 to a peak of 76.9% in 2021, then dropped slightly to 76.0% in 2022, with a modest rebound to 76.8% in 2023. UTD coverage showed a similar pattern, increasing from 51.1% in 2018 to 62.6% in 2022 before falling to 61.4% in 2023. Despite these gains, both initiation and UTD rates remained below the Healthy People 2030 target of 80%. These trends were statistically significant (Cochran–Armitage trend test, p < 0.001), indicating a reversal of progress after the COVID-19 pandemic.
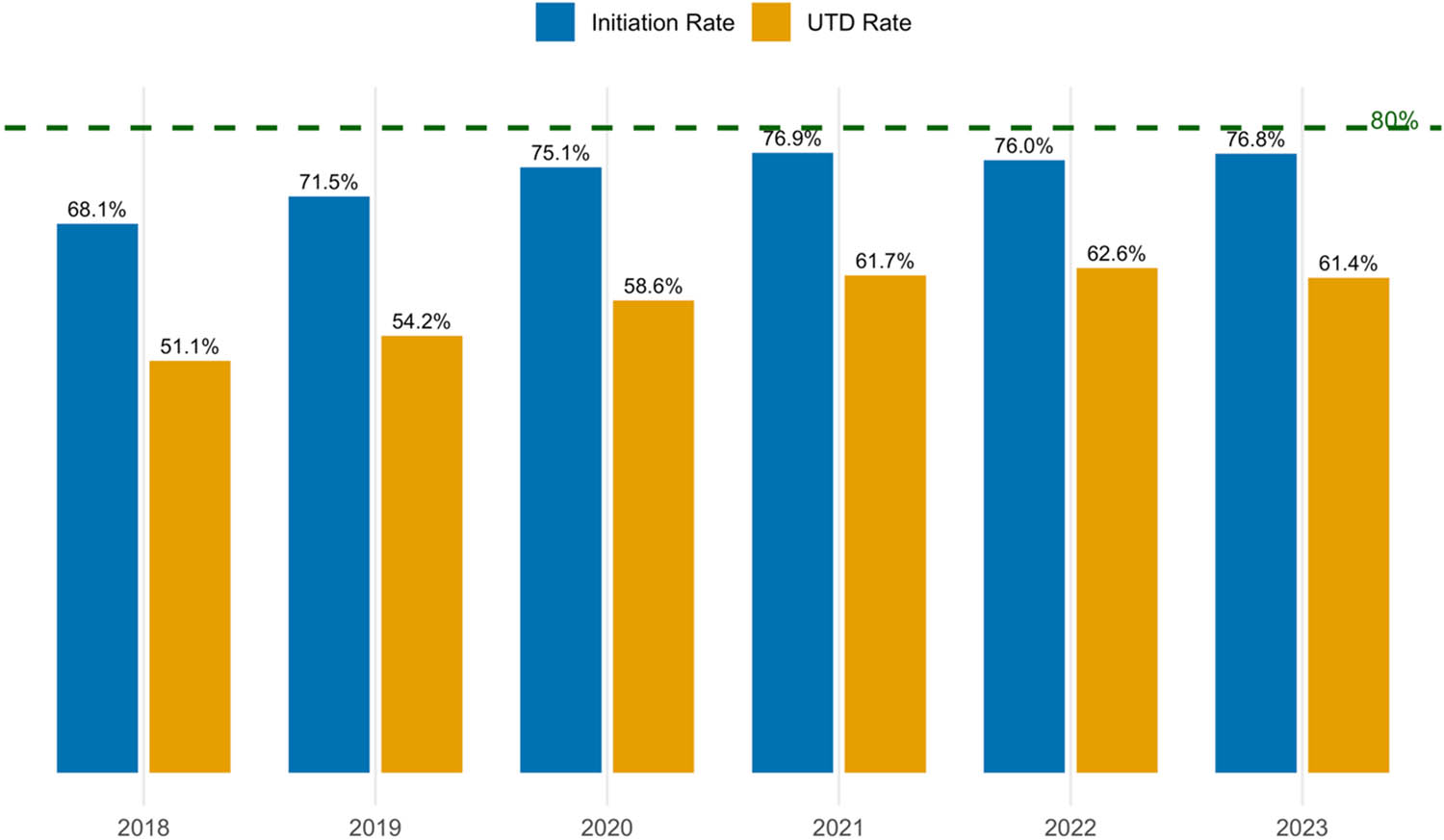
National HPV vaccination initiation and UTD rates among U.S. adolescents from 2018 to 2023. The horizontal green dashed line indicates the Healthy People 2030 target for HPV vaccination coverage.
Across all years, females consistently had higher HPV vaccination coverage than males (Figure 2a and b). Initiation among females rose from 70.7% in 2018 to 80.0% in 2022 before declining to 78.6% in 2023, while male rates increased from 66.0% in 2018 to 77.3% in 2023. UTD coverage followed a similar pattern, with females increasing from 54.5% in 2018 to 66.0% in 2022 before declining to 65.0% in 2023, while males showed steady improvement from 49.5% in 2018 to 62.6% in 2023.
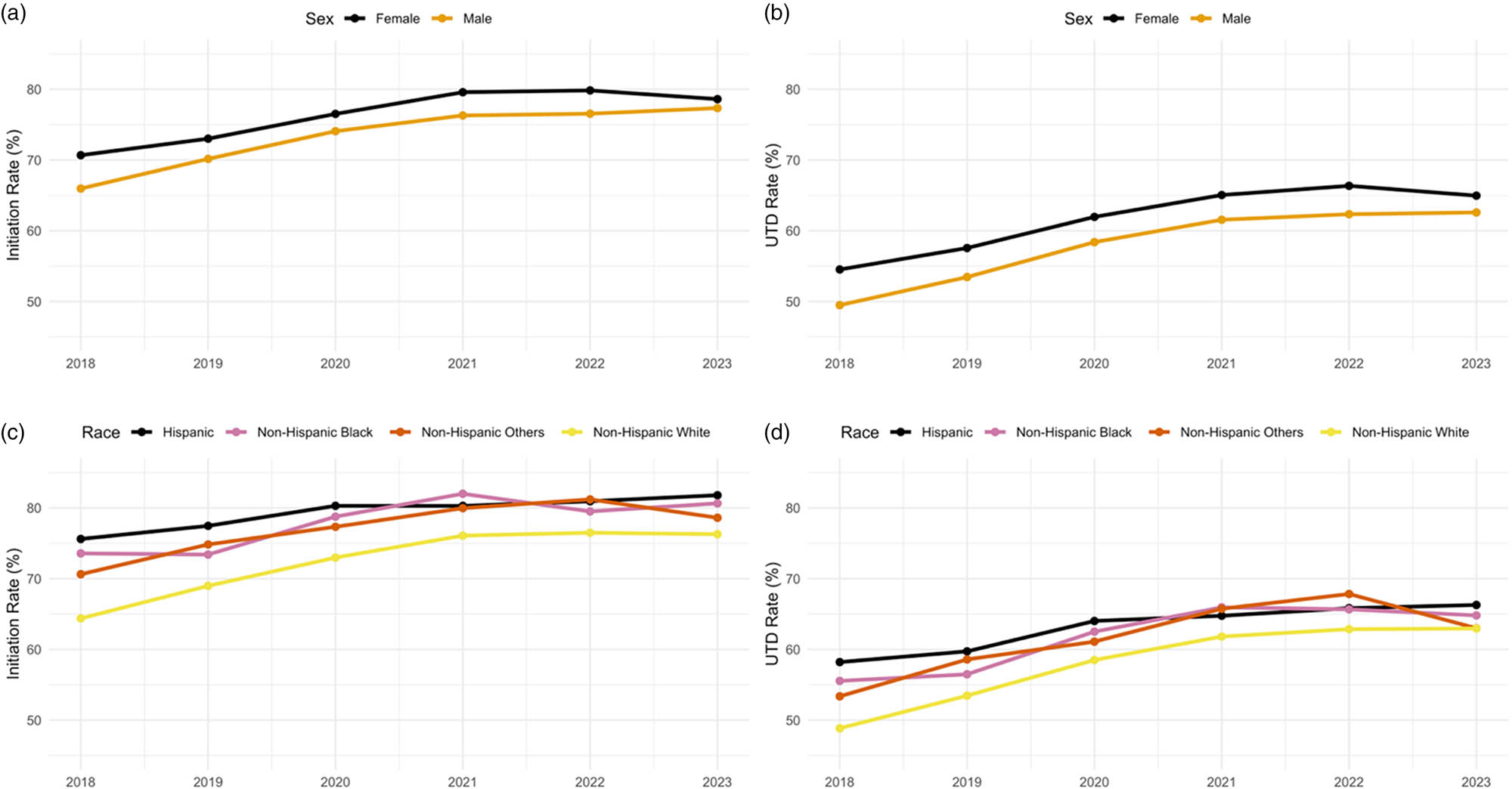
Trends in HPV vaccine initiation and UTD rates by sex and race/ethnicity among U.S. adolescents aged 13–17, 2018–2023: (a) Initiation rates by sex, (b) UTD rates by sex, (c) initiation rates by race/ethnicity, and (d) UTD rates by race/ethnicity.
Apparent racial and ethnic gaps in HPV vaccine coverage persisted across the 6 years despite overall gains in coverage (Figure 2c and d). In 2018, initiation rates were highest among Hispanic adolescents (75.6%), followed by non-Hispanic Black (73.6%), non-Hispanic Other adolescents (70.6%), and non-Hispanic White adolescents (64.4%). By 2023, all groups had made substantial improvements. However, non-Hispanic White adolescents continued to lag, with an initiation rate of 76.3% compared to 81.8% among Hispanic, 80.6% among non-Hispanic Black, and 78.6% among non-Hispanic Other adolescents.
UTD rates followed a similar pattern, with Hispanic adolescents maintaining the highest coverage throughout the study period. UTD coverage among Hispanic adolescents increased from 59.7% in 2018 to 66.3% in 2023, while non-Hispanic Black adolescents improved from 55.6% in 2018 to 65.3% in 2023. Among non-Hispanic White adolescents, UTD coverage increased from 50.3% in 2018 to 63.0% in 2023. Non-Hispanic Other adolescents showed improvement, increasing from 53.5% in 2018 to 63.0% in 2023.
While racial and ethnic gaps in coverage began to narrow by 2023, differences remained depending on where adolescents lived. HPV vaccination rates varied across states, with clear regional patterns in both initiation and UTD coverage (Figures 3 and 4). In 2018, HPV vaccination initiation rates varied widely across states, with only Rhode Island achieving the 80% threshold recommended by Healthy People 2030. Most states (42 states) fell within the 60.0–79.9% range, while six states – Wyoming, Arkansas, Mississippi, Tennessee, Kentucky, and West Virginia – had initiation rates below 60%. By 2023, 18 states reached or exceeded 80% initiation coverage: Washington, Oregon, North Dakota, South Dakota, New Mexico, Nebraska, Minnesota, Wisconsin, Illinois, Iowa, Pennsylvania, Connecticut, Rhode Island, Massachusetts, Vermont, New Hampshire, New Jersey, and Hawaii. These high-performing states are clustered primarily in the Northeast and Upper Midwest. In contrast, Mississippi remained the only state with coverage below 60%.
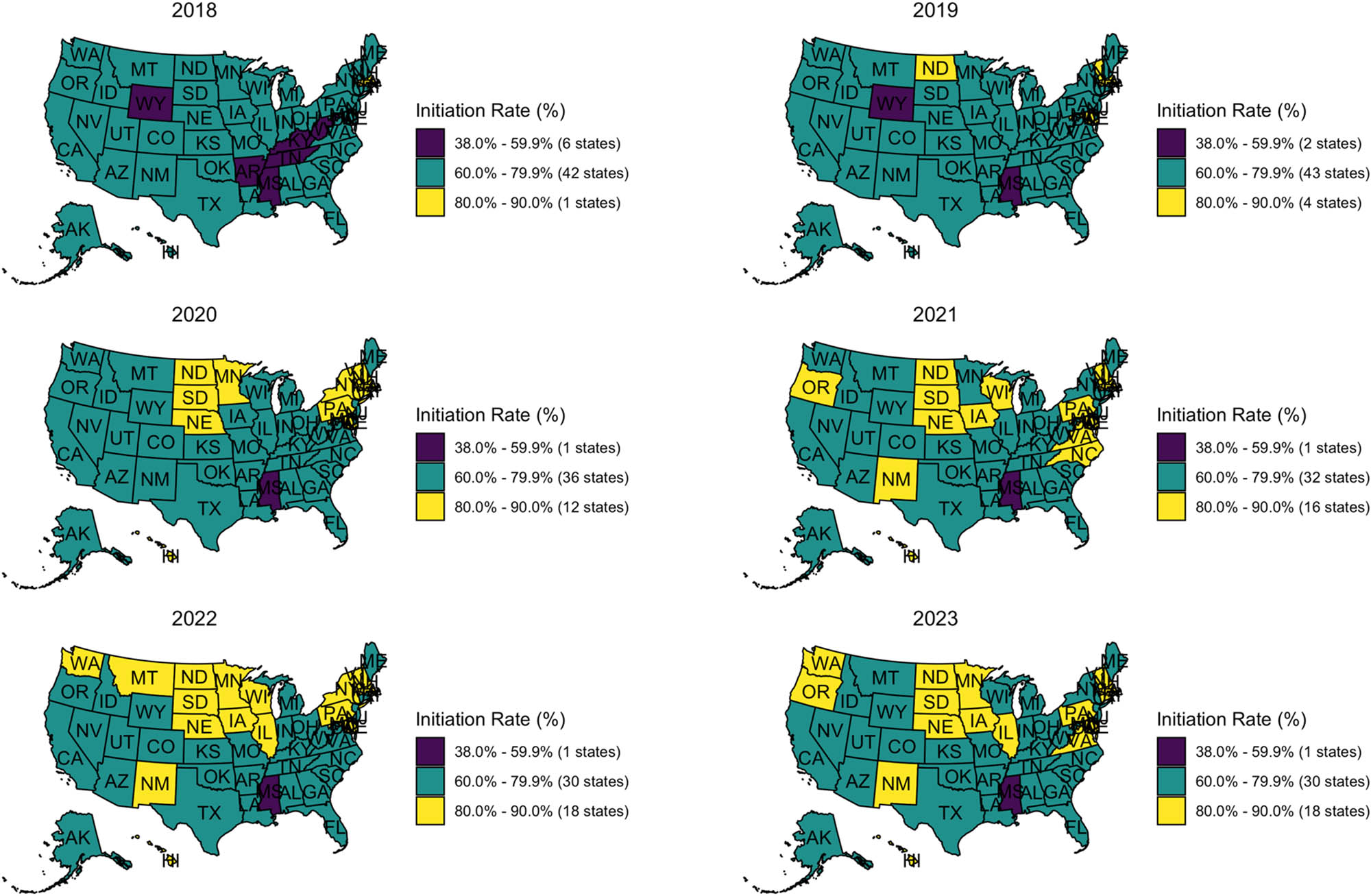
State-level HPV vaccine initiation rates among U.S. adolescents aged 13–17 from 2018 to 2023. States are colored by initiation rate categories: Purple (38.0–59.9%), teal (60.0–79.9%), and yellow (80.0–90.0%). Each panel represents a single year, arranged chronologically from top to bottom, left to right. Source: Created by the authors.
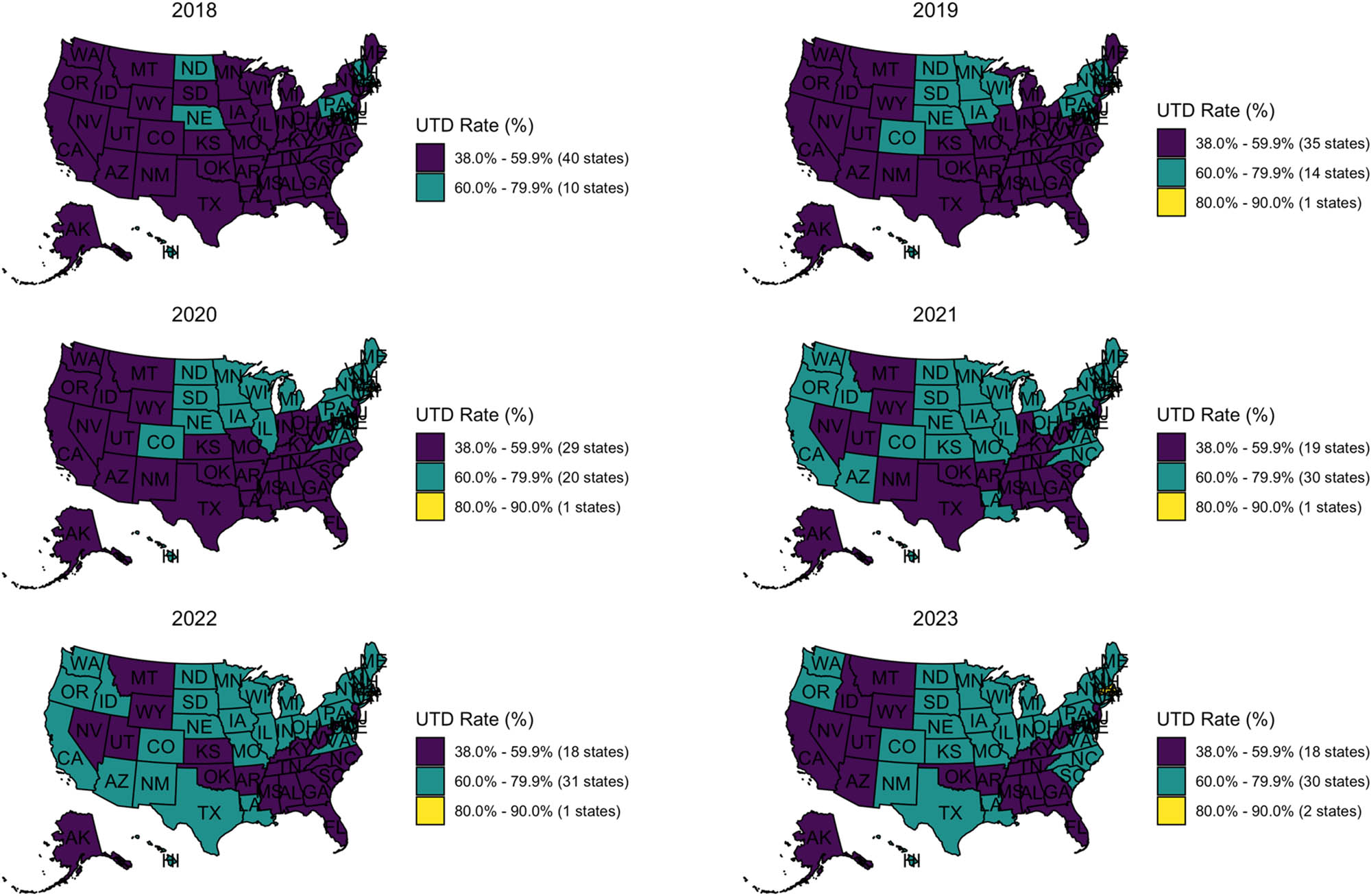
State-level HPV vaccine UTD rates among U.S. adolescents aged 13–17 from 2018 to 2023. States are colored by UTD rate categories: Purple (38.0–59.9%), teal (60.0–79.9%), and yellow (80.0–90.0%). Each panel represents 1 year, arranged chronologically from top to bottom, left to right. Source: Created by the authors.
UTD coverage trends revealed a slower pace of progress compared to the initiation of the vaccine. In 2018, 40 states fell below 60% coverage, with just ten states in the 60–79.9% range and none reaching 80%. By 2023, progress was still limited. Only Rhode Island and Massachusetts crossed the 80% threshold. Thirty states still had UTD rates between the 60 and 79.9% range, showing some movement in the right direction. However, 18 states still fell below 60%, with most of them concentrated in the South and Mountain West. These included California, Nevada, Arizona, Utah, Montana, Wyoming, Idaho, Alaska, Oklahoma, Arkansas, Mississippi, Alabama, Florida, Georgia, Tennessee, Kentucky, West Virginia, and New Jersey. The map tells a clear story: even as more states made progress on HPV vaccine initiation, many struggled to convert that progress into higher UTD coverage. The Deep South and Mountain West, in particular, lagging.
4 Discussion
4.1 Synthesis of main findings
Summary of key findings, 2018–2023
| Outcome | Before pandemic (2018–2019) | During pandemic (2020–2021) | After pandemic (2022–2023) | Key trends |
|---|---|---|---|---|
| National initiation coverage | 68.1% → 69.8% | 73.1% → 76.9% | 76.0% → 76.8% | Steady increase, then plateau |
| National UTD coverage | 51.1% → 54.1% | 58.3% → 61.9% | 62.6% → 61.4% | Consistent growth, then decline |
| Sex disparities | Female advantage: 4.7 pp | Female advantage: 3.9 pp | Female advantage: 1.3 pp | Gap narrowing over time |
| Racial disparities | White adolescents lowest | Pattern maintained | White adolescents still lowest | Persistent but narrowing gaps |
| Geographic coverage | 1 state ≥80% initiation, 1 state ≥80% UTD | 10 states ≥80% initiation, 1 state ≥80% UTD | 18 states ≥80% initiation, 2 states ≥80% UTD | Regional clustering evident, Southern States suffers |
Note: pp = percentage points; UTD = up-to-date.
4.2 Contribution to existing literature
This study provides the first comprehensive national analysis of HPV vaccination trends across the COVID-19 pandemic period, addressing critical knowledge gaps in our understanding of vaccination resilience during public health emergencies. Unlike previous studies that examined single-year snapshots or localized populations [29,30], our findings reveal complex temporal patterns that challenge conventional assumptions about pandemic impacts on routine immunization.
Our results contradict the prevailing narrative that COVID-19 universally disrupted childhood vaccination programs [31–33]. While another study documented drop in HPV vaccine uptake in 2020 [34], our national data show that initiation rates actually increased during the pandemic period, suggesting that the U.S. vaccination system demonstrated remarkable adaptability. The persistence of racial and ethnic disparities in our data confirms patterns identified in pre-pandemic studies, where Hispanic and Black adolescents consistently showed higher vaccination rates than their White counterparts [8,35]. These finding challenges common assumptions about health equity and represents what research has termed “reverse disparities” in HPV vaccination, where racial/ethnic minorities demonstrate higher uptake rates compared to White adolescents [36]. These reverse disparities are unique, as racial and ethnic minorities are generally less likely to receive adolescent preventive services [37], but the HPV vaccination context appears to be an exception to this pattern.
4.3 Expected and unexpected findings
This study confirmed several known trends in HPV vaccination, while also uncovering surprising findings that challenge assumptions. We found that uptake remains lower among White adolescents, a trend mirrored in national surveys showing higher HPV initiation among Hispanic and Black teens compared to non-Hispanic Whites [38,39]. Similarly, the continued sex disparities were expected, reflecting the HPV vaccine’s initial introduction as a cervical cancer prevention tool for females [40]. The narrowing of these gaps over time was expected following the 2011 expansion of recommendations to include males [41], though the persistence of disparities suggests that parent attitudes may lag behind policy changes [42]. These patterns address the research questions from our Introduction about whether demographic differences persist.
Contrary to global trends, we found the U.S. achieved an unexpected improvement in HPV coverage during the COVID-19 pandemic (2020–2021). While studies in Europe and low- to middle-income countries have reported declines in coverage, current levels range between 50% and 65% [43], U.S. rates not only rebounded but surpassed 2019 levels [24]. High-performing regions, specifically the Northeast and Upper Midwest also met expectations, reflecting longstanding strengths in public health infrastructure, provider practices, and overall vaccination uptake [44–46].
The most surprising finding was that HPV vaccination coverage improved during the pandemic period (2020–2021) rather than declining as anticipated. This contradicts the widespread disruption to healthcare services documented globally [32]. Several factors may explain this unexpected trend, including the rapid implementation of telehealth services and modified care delivery models that improved access for some populations. For example, during June–November 2020, approximately 30% of weekly health center visits occurred via telehealth, enabling continued access to care during pandemic disruptions [47]. The pandemic may have also heightened public awareness of vaccine-preventable diseases, which in turn increased acceptance of routine immunizations [48]. Additionally, targeted public health campaigns and catch-up vaccination efforts launched by the CDC and partner agencies played a role in mitigating missed doses and sustaining adolescent vaccination coverage [49]. These actions reflect the U.S. healthcare system’s adaptability and resilience during a national crisis.
Yet, this national resilience masked troubling state-level gaps, nowhere more evident than in Mississippi. Despite federal support and widespread catch-up efforts, Mississippi’s HPV vaccination coverage consistently remained below 60%, making it the worst-performing state in the country. This failure is not incidental; it highlights deeply rooted issues in healthcare access, provider engagement, and community trust [50]. In contrast to high-performing states like Rhode Island, where strong infrastructure and proactive public health strategies drove coverage above 80% [50]. Mississippi’s stagnation suggests that national campaigns alone are insufficient. Addressing such extreme disparities will require tailored, on-the-ground interventions that go beyond broad messaging and confront the specific cultural, political, and structural barriers limiting vaccine uptake in the most vulnerable regions.
Another concern was the decline in UTD HPV vaccination coverage observed in 2023, which disrupted the upward trend established during the pandemic period. Modeling studies have shown that even reductions in vaccination coverage can result in increased incidence of HPV-related cancers over time if left unaddressed [51]. While the long-term effects of this decline remain to be fully seen, the reversal signals that short-term gains in coverage may not be sustainable without continuous public health attention. These findings reinforce the importance of maintaining vaccination infrastructure and targeted interventions, particularly during periods of shifting public health priorities.
4.4 New insights and contributions
This study confirms established disparities in race, sex, and geography while providing critical context. Our findings reveal the U.S. vaccination system’s capacity to maintain HPV vaccination rates during COVID-19, contrasting with global declines in routine immunizations. However, the data also expose the fragility of coverage gains post-pandemic. By linking these patterns to regional HPV prevalence data, we demonstrate how policy responses and healthcare system adaptations directly shape public health outcomes.
4.5 Broader implications
Our results offer important lessons for high-income countries. Adaptive healthcare infrastructure can sustain preventive services during crises, but equity requires deliberate action rather than emerging naturally. Even healthcare systems with universal coverage face persistent disparities rooted in cultural factors, provider practices, and incomplete vaccination series. While other nations can draw insights from the U.S. experience, they should implement protective measures to prevent post-crisis rollbacks in preventive care.
4.6 Policy recommendations
Incentive system theory provides a practical framework for improvement. Federal policy should tie provider incentives to series UTD rather than just initiation, particularly targeting high-risk populations. Research has shown that financial incentives and systematic reminders increase HPV vaccination uptake [52]. Designating HPV vaccination as a national healthcare priority, with routine public reporting of state-level performance metrics, would maintain focus and drive accountability across regions.
Real-time surveillance systems could identify underperforming areas early, enabling rapid deployment of mobile clinics, targeted provider training, and community outreach programs. State and local interventions should address specific barriers through evidence-based approaches like school-based reminder systems and streamlined consent processes, particularly in communities with low completion rates.
Healthcare systems should implement comprehensive reminder systems through electronic health records, automatic appointment scheduling, and consent tracking to reduce follow-up gaps. Provider communication represents an equally critical component. Training programs in narrative education and motivational interviewing can address persistent vaccine hesitancy and clearly communicate benefits to families. Integrating these practices into annual quality improvement cycles ensures sustained implementation and continuous refinement.
5 Conclusion
This study addresses a critical gap in vaccination research by providing the first comprehensive national analysis of HPV vaccination trends across the COVID-19 pandemic period. While existing literature has extensively documented pre-pandemic HPV vaccination disparities and COVID-19’s general impact on childhood immunizations separately, our research uniquely examines their intersection within the U.S. healthcare system from 2018 to 2023. Our findings challenge prevailing assumptions about pandemic vulnerability by demonstrating that HPV vaccination rates actually improved during 2020–2021, contrasting sharply with global declines in routine immunizations documented elsewhere.
The larger significance of this research extends beyond HPV vaccination to inform pandemic preparedness strategies and health equity frameworks. By revealing that system-level resilience does not automatically translate to equitable outcomes, we demonstrate how established disparities can persist even when overall coverage improves. This work contributes to health systems theory by illustrating the complex relationship between healthcare infrastructure adaptability and population-level health equity during crisis periods. Our analysis of the post-pandemic decline in UTD coverage (from 62.6% in 2022 to 61.4% in 2023) reveals the fragility of public health gains when attention shifts away from routine preventive services.
However, several important limitations constrain our conclusions and generalizability. Our reliance on the NIS-Teen provides robust national estimates but cannot capture individual-level decision-making processes, provider–patient interactions, or the specific mechanisms driving observed trends. The aggregated nature of surveillance data obscures nuanced factors such as parental attitudes, provider communication strategies, and community-level influences that likely shaped vaccination behaviors during the pandemic. Additionally, our geographic analysis focuses on state-level patterns but cannot account for intra-state variations or local policy differences that may have influenced coverage rates.
The temporal framework dividing the study period into before, during, and after pandemic phases, while analytically useful, may oversimplify the complex and evolving nature of pandemic impacts. Our analysis cannot fully disentangle the effects of COVID-19 disruptions from concurrent policy changes, public health campaigns, or broader social factors affecting vaccination acceptance. Furthermore, the study’s focus on the U.S. healthcare system limits generalizability to countries with different healthcare financing mechanisms, regulatory structures, or cultural contexts around vaccination.
Additional methodological limitations include our reliance on provider-verified vaccination records, which may underestimate coverage if some providers did not respond to verification requests or if adolescents received vaccines from multiple providers. The cross-sectional nature of the NIS-Teen data prevents us from tracking individual adolescents over time, limiting our ability to understand vaccination trajectories or identify factors associated with series completion versus discontinuation. Our analysis also excludes adolescents without provider-verified records, potentially introducing selection bias if excluded adolescents differed systematically from those included in the analysis.
The study’s focus on demographic variables such as race, ethnicity, and sex, while important for understanding disparities, may not capture other relevant factors such as parental education, household income, insurance status, or access to healthcare that could influence vaccination decisions. Our state-level geographic analysis, while comprehensive, cannot account for urban–rural differences, local healthcare capacity, or community-level factors that may drive vaccination patterns. Additionally, the observational nature of this study design limits causal inferences about the relationships between pandemic timing and vaccination trends.
Finally, our analysis period ends in 2023, potentially missing longer-term effects of the pandemic on HPV vaccination behaviors. The definition of “after pandemic” as 2022–2023 may be premature, as ongoing effects of COVID-19 on healthcare systems and public health priorities continue to evolve. This temporal limitation may affect our conclusions about the sustainability of coverage gains and the trajectory of post-pandemic recovery.
5.1 Theoretical implications
Our findings fundamentally challenge existing theoretical frameworks about healthcare system vulnerability during public health emergencies. Traditional disaster preparedness models predict uniform degradation of non-essential services during crises, yet our data demonstrate what we term “selective resilience” – the capacity for well-supported preventive services to maintain or even improve performance while other healthcare components face significant disruption. This concept extends beyond simple system robustness to encompass adaptive capacity that can leverage crisis conditions to accelerate existing improvement trends.
The persistence of racial and ethnic disparities during a period of overall coverage improvement reveals critical theoretical insights about the relationship between system-level performance and health equity. Our results suggest that healthcare infrastructure resilience and population-level equity operate through fundamentally different mechanisms, requiring distinct theoretical frameworks. These findings challenge health services research assumptions that system improvements automatically benefit all populations equally, instead supporting theories of “differential access” where structural advantages and disadvantages are maintained or even amplified during periods of change.
The emergence and persistence of “reverse disparities” in HPV vaccination – where racial and ethnic minorities demonstrate higher uptake than White adolescents – represents a unique phenomenon in public health that deserves greater theoretical attention. This pattern contradicts standard minority health frameworks and suggests that vaccine-specific factors, historical public health messaging, and community trust dynamics create distinct pathways for health behavior adoption that may not generalize to other preventive services.
Our temporal analysis also contributes to implementation science theory by demonstrating how external shocks can create windows of opportunity for accelerating existing public health initiatives. The pandemic period’s improved HPV vaccination rates suggest that crisis conditions can enhance system adaptability and community receptiveness to preventive interventions when supported by appropriate infrastructure and messaging.
5.2 Managerial and policy implications
Healthcare administrators and policymakers can derive several actionable insights from our findings that extend beyond HPV vaccination to broader preventive care delivery. First, investment in robust preventive care infrastructure – including electronic health record systems, automated reminder protocols, telehealth capabilities, and provider training programs – provides returns that extend beyond normal operations by creating system resilience during emergencies. Healthcare organizations should conceptualize these elements as essential components of emergency preparedness rather than merely quality improvement initiatives.
Second, our results demonstrate that maintaining aggregate population-level performance metrics is insufficient for achieving health equity goals. The persistence of state-level disparities, exemplified by Mississippi’s consistently poor performance despite national improvements, reveals the limitations of broad-based policy approaches. Policymakers must implement active monitoring systems that track disparities in real-time and deploy targeted interventions when geographic or demographic gaps persist or widen. This requires fundamental shifts from population-level performance metrics to equity-focused accountability measures that incentivize providers and health systems to address disparities proactively.
The post-pandemic decline in UTD coverage observed in 2023 highlights the critical importance of sustained attention to preventive services. Policymakers should develop mechanisms to maintain focus on routine immunizations even when public health priorities shift toward emerging threats. This could include establishing dedicated funding streams for preventive services that are protected from reallocation during crisis periods, or implementing automatic triggers that deploy additional resources when coverage rates decline.
For provider-level interventions, our findings support transitioning from initiation-focused metrics to UTD -focused accountability measures. The persistent gap between initiation and UTD coverage across all demographic groups suggests that current systems successfully engage families initially but fail to support series completion. Healthcare systems should implement comprehensive tracking mechanisms that monitor patients throughout the vaccination series and deploy targeted interventions when follow-up appointments are missed.
International policymakers can learn from both the successes and limitations of the U.S. experience. While strong healthcare infrastructure enabled resilience during the pandemic, the persistence of geographic and demographic disparities reveals that infrastructure alone is insufficient. Countries developing HPV vaccination programs should prioritize building equitable delivery systems from the outset rather than attempting to address disparities after achieving high aggregate coverage rates.
5.3 Ideas for future research
Several critical research directions emerge from our findings that could significantly advance understanding of preventive care delivery, health equity, and pandemic preparedness. First, mixed-methods longitudinal studies following individual patients and families through the pandemic period could illuminate decision-making processes that aggregate surveillance data cannot capture. Such research should examine how pandemic experiences shaped attitudes toward preventive care, whether families who delayed care eventually caught up, and how provider communication strategies evolved during different phases of the crisis.
Second, comparative international research examining HPV vaccination patterns during COVID-19 across different healthcare systems could test the generalizability of our findings about selective resilience. Cross-national studies could identify specific system characteristics that promote equitable preventive care delivery during emergencies and inform policy transfer efforts between countries with different organizational and financing structures. Such research should particularly focus on countries that maintained both high coverage and low disparities during the pandemic to identify best practices.
Third, implementation science research should examine the specific organizational adaptations that enabled some healthcare systems to maintain or improve HPV vaccination delivery during the pandemic. Case studies of high-performing health systems, states, and individual practices could identify transferable strategies for maintaining equity-focused preventive care during emergencies. This research should investigate how successful organizations modified workflows, communication strategies, and performance monitoring systems to sustain vaccination services.
Fourth, behavioral economics research could explore how crisis conditions alter decision-making around preventive health behaviors. The unexpected improvement in HPV vaccination rates during 2020–2021 suggests that pandemic conditions may have created unique psychological or social dynamics that enhanced vaccine acceptance. Understanding these mechanisms could inform strategies for promoting preventive behaviors during future emergencies or even during normal circumstances.
Fifth, health disparities research should investigate the mechanisms underlying “reverse disparities” in HPV vaccination and whether similar patterns exist for other preventive services. Such studies could examine how historical public health messaging, community trust dynamics, and provider practices interact to create differential uptake patterns across racial and ethnic groups. This research could inform more effective communication strategies and help predict which populations might be most responsive to specific intervention approaches.
Finally, health economics research should evaluate the cost-effectiveness of different strategies for maintaining preventive services during public health emergencies. Our findings suggest that infrastructure investments enabling pandemic resilience may provide substantial returns, but quantitative analyses are needed to guide resource allocation decisions. Such studies should examine both the direct costs of maintaining services and the long-term health and economic consequences of coverage disruptions.
These research directions collectively could inform the development of more resilient, equitable, and effective preventive care systems capable of maintaining high-quality services regardless of external circumstances while actively addressing persistent health disparities. The COVID-19 pandemic provided a good experiment in healthcare system adaptation that researchers should continue to mine for insights applicable to future public health challenges.
Acknowledgments
The authors would like to thank the Centers for Disease Control and Prevention (CDC) for making the National Immunization Survey–Teen (NIS-Teen) data publicly available. They also acknowledge the healthcare providers and families who contributed to the survey and made this analysis possible.
-
Funding information: Authors state no funding involved.
-
Author contributions: All authors accepted responsibility for the content of this manuscript and consented to its submission. V.I.A. conceived the study, led the statistical analysis, and drafted the manuscript. I.A.U. contributed to the statistical analysis, contextualized the findings, and assisted in manuscript revision. A.A. performed data preparation, modeling, and figure development. T.K.A. contributed to coding, map visualization, and interpretation of geographic trends. All authors reviewed the results and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data used in this study are publicly available through the Centers for Disease Control and Prevention (CDC) National Immunization Survey–Teen portal at: https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html.
References
[1] Brooks EM, Fugate-Laus K, Webel B, Naavaal S. Perceptions of a state-level HPV vaccine mandate and exemption option in rural virginia: a qualitative study. Vaccines. 2024 Apr [cited 2025 Jun 14];12(4):401, https://www.mdpi.com/2076-393X/12/4/401.10.3390/vaccines12040401Search in Google Scholar PubMed PubMed Central
[2] CDC. Cancers linked with HPV each year. Cancer. 2025, [cited 2025 Jun 14] https://www.cdc.gov/cancer/hpv/cases.html.Search in Google Scholar
[3] Burki T. Continued suboptimal HPV vaccine coverage in the USA. Lancet Oncol. 2024 Oct [cited 2025 Jun 14];25(10):1257, https://linkinghub.elsevier.com/retrieve/pii/S1470204524003942.10.1016/S1470-2045(24)00394-2Search in Google Scholar PubMed
[4] Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID‑08 - Healthy People 2030 | odphp.health.gov. [cited 2025 Jun 25]. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08. Search in Google Scholar
[5] Sokale I, Montealegre J, Amuta AO, Oluyomi A, Thrift AP. Racial and ethnic disparities in human papillomavirus vaccination among US-born and foreign-born adults aged 18 to 26 years in the United States. Vaccines. 2025 Jan [cited 2025 Jun 14];13(2):98, https://www.mdpi.com/2076-393X/13/2/98.10.3390/vaccines13020098Search in Google Scholar PubMed PubMed Central
[6] Sonawane K, Zhu Y, Damgacioglu H, Garg A, Graboyes EM, Montealegre JR, et al. Factors associated with parental human papillomavirus vaccination intentions among adolescents from socioeconomically advantaged versus deprived households: a nationwide, cross-sectional survey. Lancet Reg Health Am. 2024 Mar [cited 2025 Jun 14];31:100694, https://linkinghub.elsevier.com/retrieve/pii/S2667193X24000218.10.1016/j.lana.2024.100694Search in Google Scholar PubMed PubMed Central
[7] McNeil CJ, Barr B, Munawar I, DeWitt ME, Myers JS, Shetty AK. Assessing barriers to human papillomavirus (hpv) vaccination in at-risk rural communities of western North Carolina, United States. Vaccines. 2023 Nov [cited 2025 Jun 14];11(12):1785, https://www.mdpi.com/2076-393X/11/12/1785.10.3390/vaccines11121785Search in Google Scholar PubMed PubMed Central
[8] Chido-Amajuoyi OG, Talluri R, Wonodi C, Shete S. Trends in HPV vaccination initiation and completion within ages 9–12 years: 2008–2018. Pediatrics. 2021 Jun [cited 2025 Jun 14];147(6):e2020012765, https://publications.aap.org/pediatrics/article/147/6/e2020012765/180281/Trends-in-HPV-Vaccination-Initiation-and.10.1542/peds.2020-012765Search in Google Scholar PubMed PubMed Central
[9] Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. 2011 Apr [cited 2025 Jun 14];52(7):911–6. 10.1093/cid/cir007.Search in Google Scholar PubMed
[10] On the Elimination of Infections Related to Oncogenic Human Papillomavirus: An Approach Using a Computational Network Model. [cited 2025 Jun 14] https://www.mdpi.com/1999-4915/13/5/906.10.3390/v13050906Search in Google Scholar PubMed PubMed Central
[11] Jit M, Prem K, Benard E, Brisson M. From cervical cancer elimination to eradication of vaccine-type human papillomavirus: feasibility, public health strategies and cost-effectiveness. Prev Med. 2021 Mar [cited 2025 Jun 14];144:106354, https://www.sciencedirect.com/science/article/pii/S0091743520303856.10.1016/j.ypmed.2020.106354Search in Google Scholar PubMed PubMed Central
[12] Roman BR, Aragones A. Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol. 2021 [cited 2025 Jun 14];124(6):920–2. 10.1002/jso.26687.Search in Google Scholar PubMed PubMed Central
[13] Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020 Nov [cited 2025 Jun 25];324(20):2095–6. 10.1001/jama.2020.20892.Search in Google Scholar PubMed
[14] Takefuji Y. A herd immunity approach to the COVID-19 pandemic? Health Technol. 2022 Sep [cited 2025 Jun 25];12(5):1037–41. 10.1007/s12553-022-00676-5.Search in Google Scholar PubMed PubMed Central
[15] Plans-Rubió P. Percentages of vaccination coverage required to establish herd immunity against SARS-CoV-2. Vaccines. 2022 May [cited 2025 Jun 25];10(5):736, https://www.mdpi.com/2076-393X/10/5/736.10.3390/vaccines10050736Search in Google Scholar PubMed PubMed Central
[16] Aw J, Seng JJB, Seah SSY, Low LL. COVID-19 vaccine hesitancy – a scoping review of literature in high-income countries. Vaccines. 2021 Aug [cited 2025 Jun 25];9(8):900, https://www.mdpi.com/2076-393X/9/8/900.10.3390/vaccines9080900Search in Google Scholar PubMed PubMed Central
[17] Coccia M. Optimal levels of vaccination to reduce COVID-19 infected individuals and deaths: a global analysis. Environ Res. 2022 Mar [cited 2025 Jun 14];204:112314, https://linkinghub.elsevier.com/retrieve/pii/S0013935121016157.10.1016/j.envres.2021.112314Search in Google Scholar PubMed PubMed Central
[18] Ismail SA, Lam ST, Bell S, Fouad FM, Blanchet K, Borghi J. Strengthening vaccination delivery system resilience in the context of protracted humanitarian crisis: a realist-informed systematic review. BMC Health Serv Res. 2022 Oct [cited 2025 Jun 25];22(1):1277. 10.1186/s12913-022-08653-4.Search in Google Scholar PubMed PubMed Central
[19] Santangelo OE, Provenzano S, Di Martino G, Ferrara P. COVID-19 vaccination and public health: addressing global, regional, and within-country inequalities. Vaccines. 2024 Aug [cited 2025 Jun 25];12(8):885, https://www.mdpi.com/2076-393X/12/8/885.10.3390/vaccines12080885Search in Google Scholar PubMed PubMed Central
[20] Savoia E, Masterson E, Olander DR, Anderson E, Mohamed Farah A, Pirrotta L. Determinants of vaccine hesitancy among African American and Black individuals in the United States of America: a systematic literature review. Vaccines. 2024 Mar [cited 2025 Jun 25];12(3):277, https://www.mdpi.com/2076-393X/12/3/277.10.3390/vaccines12030277Search in Google Scholar PubMed PubMed Central
[21] Nelson EJ, Hughes J, Oakes JM, Pankow JS, Kulasingam SL. Geospatial patterns of human papillomavirus vaccine uptake in Minnesota. BMJ Open. 2015 Aug [cited 2025 Jun 25];5(8):e008617, https://bmjopen.bmj.com/content/5/8/e008617.10.1136/bmjopen-2015-008617Search in Google Scholar PubMed PubMed Central
[22] Conrey R, Valencia V, Cioletti A, Williams-Brown MY. Regional variation in human papillomavirus vaccination uptake and completion among adolescents 13–17 in the state of Texas. Vaccine. 2020 May [cited 2025 Jun 25];38(25):4119–24, https://www.sciencedirect.com/science/article/pii/S0264410X20304485.10.1016/j.vaccine.2020.03.059Search in Google Scholar PubMed
[23] Adekanmbi V, Sokale I, Guo F, Ngo J, Hoang TN, Hsu CD, et al. Human papillomavirus vaccination and human papillomavirus-related cancer rates. JAMA Netw Open. 2024 Sep [cited 2025 Jun 25];7(9):e2431807. 10.1001/jamanetworkopen.2024.31807.Search in Google Scholar PubMed PubMed Central
[24] Pingali C. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2020. MMWR Morb Mortal Wkly Rep. 2021 [cited 2025 Jun 23];70:5. https://www.cdc.gov/mmwr/volumes/70/wr/mm7035a1.htm.10.15585/mmwr.mm7035a1Search in Google Scholar PubMed PubMed Central
[25] Shin MB, Sloan KE, Martinez B, Soto C, Baezconde-Garbanati L, Unger JB, et al. Examining multilevel influences on parental HPV vaccine hesitancy among multiethnic communities in Los Angeles: a qualitative analysis. BMC Public Health. 2023 Mar [cited 2025 Jun 25];23(1):545. 10.1186/s12889-023-15318-2.Search in Google Scholar PubMed PubMed Central
[26] Chido-Amajuoyi OG, Pande M, Agbajogu C, Yu RK, Cunningham S, Shete S. HPV vaccination uptake, hesitancy, and refusal: observations of health-care professionals during the COVID-19 pandemic. JNCI Cancer Spectr. 2022 Aug [cited 2025 Jun 25];6(4):pkac053. 10.1093/jncics/pkac053.Search in Google Scholar PubMed PubMed Central
[27] Nguyen P, Calderon-Mora J, Singh V, Hernandez A, Roy S, Molokwu J. Impact of the COVID-19 pandemic on HPV vaccine uptake in a predominantly hispanic border community: a retrospective cross-sectional analysis of the “Tiempo de Vacunarte Program. Arch Public Health. 2024 Jun [cited 2025 Jun 23];82(1):96. 10.1186/s13690-024-01318-0.Search in Google Scholar PubMed PubMed Central
[28] CDC. HPV Vaccine Recommendations. Human Papillomavirus (HPV). 2025. [cited 2025 Jun 23]. https://www.cdc.gov/hpv/hcp/vaccination-considerations/index.html.Search in Google Scholar
[29] Turner K, Brownstein NC, Whiting J, Arevalo M, Vadaparampil S, Giuliano AR, et al. Impact of the COVID-19 pandemic on human papillomavirus (HPV) vaccination among a national sample of United States adults ages 18–45: a cross-sectional study. Prev Med Rep. 2023 Feb [cited 2025 Jun 23];31:102067, https://www.sciencedirect.com/science/article/pii/S2211335522003746.10.1016/j.pmedr.2022.102067Search in Google Scholar PubMed PubMed Central
[30] Schelbar N, Ward CN, Phillips E, Herr MJ, Acevedo S, Conner H, et al. Impact of COVID-19 pandemic and vaccine perceptions on HPV vaccine hesitancy. Am J Otolaryngol. 2024 Mar [cited 2025 Jun 23];45(2):104172, https://www.sciencedirect.com/science/article/pii/S0196070923003861.10.1016/j.amjoto.2023.104172Search in Google Scholar PubMed
[31] COVID-19 pandemic leads to major backsliding on childhood vaccinations, new WHO, UNICEF data shows. [cited 2025 Jun 23]. https://www.unicef.org/press-releases/covid-19-pandemic-leads-major-backsliding-childhood-vaccinations-new-who-unicef-data.Search in Google Scholar
[32] Cardoso Pinto AM, Ranasinghe L, Dodd PJ, Budhathoki SS, Seddon JA, Whittaker E. Disruptions to routine childhood vaccinations in low- and middle-income countries during the COVID-19 pandemic: a systematic review. Front Pediatr. 2022 Aug [cited 2025 Jun 23]. https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2022.979769/full.10.3389/fped.2022.979769Search in Google Scholar PubMed PubMed Central
[33] Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin A, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. 2021 Aug [cited 2025 Jun 23];398(10299):522–34, https://www.thelancet.com/article/S0140-6736(21)01337-4/fulltext.10.1016/S0140-6736(21)01337-4Search in Google Scholar PubMed PubMed Central
[34] Patel B. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations – 10 U.S. jurisdictions, March–September 2020. MMWR Morb Mortal Wkly Rep. 2021 [cited 2025 Jun 23];70:1. https://www.cdc.gov/mmwr/volumes/70/wr/mm7023a2.htm.10.15585/mmwr.mm7023a2Search in Google Scholar PubMed PubMed Central
[35] Spencer JC, Calo WA, Brewer NT. Disparities and reverse disparities in HPV vaccination: a systematic review and meta-analysis. Prev Med. 2019 Jun [cited 2025 Jun 23];123:197–203, https://www.sciencedirect.com/science/article/pii/S0091743519301148.10.1016/j.ypmed.2019.03.037Search in Google Scholar PubMed PubMed Central
[36] Ramphul R, Zamorano AS, Upadhyay S, Desai M, Bauer C. Spatiotemporal analysis of HPV vaccination and associated neighborhood-level disparities in Texas – an ecological study. Front Public Health. 2024 Jun [cited 2025 Jun 23];12:1418526. 10.3389/fpubh.2024.1418526/full.Search in Google Scholar
[37] Tabet M, Kirby RS, Xaverius P. Racial and ethnic differences in factors associated with delayed or missed pediatric preventive care in the US due to the COVID-19 pandemic. JAMA Network Open. 2023 Jul [cited 2025 Jun 23];6(7):e2322588. 10.1001/jamanetworkopen.2023.22588.Search in Google Scholar PubMed PubMed Central
[38] Jeudin P, Liveright E, Carmen MG, del Perkins RB. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clin Ther. 2014 Jan [cited 2025 Jun 23];36(1):24–37, https://www.clinicaltherapeutics.com/article/S0149-2918%2813%2901070-9/fulltext.10.1016/j.clinthera.2013.11.001Search in Google Scholar PubMed
[39] Finney Rutten LJ, Wilson PM, Jacobson DJ, Agunwamba AA, Radecki Breitkopf C, Jacobson RM, et al. A population-based study of sociodemographic and geographic variation in HPV vaccination. Cancer Epidemiol Biomarkers Prev. 2017 Apr [cited 2025 Jun 23];26(4):533–40. 10.1158/1055-9965.EPI-16-0877.Search in Google Scholar PubMed PubMed Central
[40] Rathod S, Potdar J, Gupta A, Sethi N, Dande A, Rathod DS, et al. Empowering women’s health: insights into HPV vaccination and the prevention of invasive cervical cancer. Cureus. 2023 Nov [cited 2025 Jun 23];15(11):2. https://www.cureus.com/articles/209470-empowering-womens-health-insights-into-hpv-vaccination-and-the-prevention-of-invasive-cervical-cancer.10.7759/cureus.49523Search in Google Scholar PubMed PubMed Central
[41] Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males — Advisory Committee on Immunization Practices (ACIP), 2011. [cited 2025 Jun 23]. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6050a3.htm.Search in Google Scholar
[42] Victory M, Do TQN, Kuo YF, Rodriguez AM. Parental knowledge gaps and barriers for children receiving human papillomavirus vaccine in the Rio Grande Valley of Texas. Hum Vaccines Immunother. 2019 Aug [cited 2025 Jun 23];15(7–8):1678–87. 10.1080/21645515.2019.1628551.Search in Google Scholar PubMed PubMed Central
[43] Casey RM, Akaba H, Hyde TB, Bloem P. Covid-19 pandemic and equity of global human papillomavirus vaccination: descriptive study of World Health Organization-Unicef vaccination coverage estimates. BMJ Med. 2024 Jan [cited 2025 Jun 23];3(1):3. https://bmjmedicine.bmj.com/content/3/1/e000726.10.1136/bmjmed-2023-000726Search in Google Scholar PubMed PubMed Central
[44] Hirth J. Disparities in HPV vaccination rates and HPV prevalence in the United States: a review of the literature. Hum Vaccines Immunother. 2019 Jan [cited 2025 Jun 23];15(1):146–55. 10.1080/21645515.2018.1512453.Search in Google Scholar PubMed PubMed Central
[45] These States Have the Best Health Care. [cited 2025 Jun 23]. https://www.usnews.com/news/best-states/rankings/health-care Search in Google Scholar
[46] Flourish | Data Visualisation & Storytelling. Flourish. [cited 2025 Jun 23]. https://public.flourish.studio/story/1939127/.Search in Google Scholar
[47] Demeke HB. Trends in use of telehealth among health centers during the COVID-19 pandemic – United States, June 26–November 6, 2020. MMWR Morb Mortal Wkly Rep. 2021 [cited 2025 Jun 23];70:1. https://www.cdc.gov/mmwr/volumes/70/wr/mm7007a3.htm.10.15585/mmwr.mm7007a3Search in Google Scholar PubMed PubMed Central
[48] Hamson E, Forbes C, Wittkopf P, Pandey A, Mendes D, Kowalik J, et al. Impact of pandemics and disruptions to vaccination on infectious diseases epidemiology past and present. Hum Vaccines Immunother. 2023 Aug [cited 2025 Jun 23];19(2):2219577. 10.1080/21645515.2023.2219577.Search in Google Scholar PubMed PubMed Central
[49] CDC. Catch-up immunization schedule for children and adolescents. Vaccines Immunizations. 2025 [cited 2025 Jun 23]. https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-catch-up.html.Search in Google Scholar
[50] A tale of two states: examining the best and worst performing states for HPV vaccination. HMP Global Learn Network. 2024;10(1) [cited 2025 Jun 23]. https://www.hmpgloballearningnetwork.com/site/jcp/blog/tale-two-states-examining-best-and-worst-performing-states-hpv-vaccination.Search in Google Scholar
[51] Daniels V, Saxena K, Roberts C, Kothari S, Corman S, Yao L, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021 May [cited 2025 Jun 23];39(20):2731–5, https://www.sciencedirect.com/science/article/pii/S0264410X2100428X.10.1016/j.vaccine.2021.04.003Search in Google Scholar PubMed PubMed Central
[52] Mantzari E, Vogt F, Marteau TM. Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial. Health Psychol. 2015;34(2):160–71.10.1037/hea0000088Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Relationship between body mass index and quality of life, use of dietary and physical activity self-management strategies, and mental health in individuals with polycystic ovary syndrome
- Evaluating the challenges and opportunities for diabetes care policy in Nigeria
- Body mass index is associated with subjective workload and REM sleep timing in young healthy adults
- Prediction of hypoglycaemia in subjects with type 1 diabetes during physical activity
- Investigation by the Epworth Sleepiness Scale of daytime sleepiness in professional drivers during work hours
- Understanding public awareness of fall epidemiology in the United States: A national cross-sectional study
- Impact of Covid-19 stress on urban poor in Sylhet Division, Bangladesh: A perception-based assessment
- Impact of the COVID-19 pandemic on mental health, relationship satisfaction, and socioeconomic status: United States
- Psychological factors influencing oocyte donation: A study of Indian donors
- Cervical cancer in eastern Kenya (2018–2020): Impact of awareness and risk perception on screening practices
- Older LGBTQ+ and blockchain in healthcare: A value sensitive design perspective
- Trends and disparities in HPV vaccination among U.S. adolescents, 2018–2023
- Do cell towers help increase vaccine uptake? Evidence from Côte d’Ivoire
- In search of the world’s most popular painkiller: An infodemiological analysis of Google Trend statistics from 2004 to 2023
- Brain fog in chronic pain: A concept analysis of social media postings
- Association between multidimensional poverty intensity and maternal mortality ratio in Madagascar: Analysis of regional disparities
- A “disorder that exacerbates all other crises” or “a word we use to shut you up”? A critical policy analysis of NGOs’ discourses on COVID-19 misinformation
- Smartphone use and stroop performance in a university workforce: A survey-experiment
- Review Articles
- The management of body dysmorphic disorder in adolescents: A systematic literature review
- Navigating challenges and maximizing potential: Handling complications and constraints in minimally invasive surgery
- Examining the scarcity of oncology healthcare providers in cancer management: A case study of the Eastern Cape Province, South Africa
- Dietary strategies for irritable bowel syndrome: A narrative review of effectiveness, emerging dietary trends, and global variability
- The impact of intimate partner violence on victims’ work, health, and wellbeing in OECD countries (2014–2025): A descriptive systematic review
- Nutrition literacy in pregnant women: a systematic review
- Short Communications
- Experience of patients in Germany with the post-COVID-19 vaccination syndrome
- Five linguistic misrepresentations of Huntington’s disease
- Letter to the Editor
- PCOS self-management challenges transcend BMI: A call for equitable support strategies
Articles in the same Issue
- Research Articles
- Relationship between body mass index and quality of life, use of dietary and physical activity self-management strategies, and mental health in individuals with polycystic ovary syndrome
- Evaluating the challenges and opportunities for diabetes care policy in Nigeria
- Body mass index is associated with subjective workload and REM sleep timing in young healthy adults
- Prediction of hypoglycaemia in subjects with type 1 diabetes during physical activity
- Investigation by the Epworth Sleepiness Scale of daytime sleepiness in professional drivers during work hours
- Understanding public awareness of fall epidemiology in the United States: A national cross-sectional study
- Impact of Covid-19 stress on urban poor in Sylhet Division, Bangladesh: A perception-based assessment
- Impact of the COVID-19 pandemic on mental health, relationship satisfaction, and socioeconomic status: United States
- Psychological factors influencing oocyte donation: A study of Indian donors
- Cervical cancer in eastern Kenya (2018–2020): Impact of awareness and risk perception on screening practices
- Older LGBTQ+ and blockchain in healthcare: A value sensitive design perspective
- Trends and disparities in HPV vaccination among U.S. adolescents, 2018–2023
- Do cell towers help increase vaccine uptake? Evidence from Côte d’Ivoire
- In search of the world’s most popular painkiller: An infodemiological analysis of Google Trend statistics from 2004 to 2023
- Brain fog in chronic pain: A concept analysis of social media postings
- Association between multidimensional poverty intensity and maternal mortality ratio in Madagascar: Analysis of regional disparities
- A “disorder that exacerbates all other crises” or “a word we use to shut you up”? A critical policy analysis of NGOs’ discourses on COVID-19 misinformation
- Smartphone use and stroop performance in a university workforce: A survey-experiment
- Review Articles
- The management of body dysmorphic disorder in adolescents: A systematic literature review
- Navigating challenges and maximizing potential: Handling complications and constraints in minimally invasive surgery
- Examining the scarcity of oncology healthcare providers in cancer management: A case study of the Eastern Cape Province, South Africa
- Dietary strategies for irritable bowel syndrome: A narrative review of effectiveness, emerging dietary trends, and global variability
- The impact of intimate partner violence on victims’ work, health, and wellbeing in OECD countries (2014–2025): A descriptive systematic review
- Nutrition literacy in pregnant women: a systematic review
- Short Communications
- Experience of patients in Germany with the post-COVID-19 vaccination syndrome
- Five linguistic misrepresentations of Huntington’s disease
- Letter to the Editor
- PCOS self-management challenges transcend BMI: A call for equitable support strategies


