Abstract
Background
Various dietary strategies have been proposed for irritable bowel syndrome (IBS) symptom relief; yet, long-term studies and comparative trials remain limited. The literature reports inconsistent findings regarding the nutritional adequacy and global variability of dietary intake in IBS populations, highlighting the need for a broader understanding of how cultural and emerging dietary factors influence symptom expression.
Objective
This narrative review aims to evaluate the effectiveness of predominant dietary interventions for IBS, provide an overview of emerging dietary trends, and examine cultural differences in dietary patterns among IBS populations.
Methods
A comprehensive search was conducted across Google Scholar, Wiley Online Library, PubMed, Elsevier, Scopus, and ScienceDirect for peer-reviewed, English-language articles published after 2000. Studies included clinical guidelines based on systematic reviews, analyses of dietary intakes in IBS populations adopting alternative dietary assessment methods, and reviews of dietary interventions. One hundred and ten studies were included following title, abstract, and full-text screening.
Key findings
Due to the heterogeneous nature of IBS, various dietary strategies are reported. Yet, it remains unclear which is superior, with the low-FODMAP diet being the most studied. Emerging dietary trends show promising potential, but current evidence is preliminary and requires long-term investigations. Internationally, dietary intake patterns vary, with some IBS populations meeting nutritional needs while others exhibit deficiencies, particularly in fibre, calcium, iron, and B vitamins.
Conclusion
Given the variability in IBS responses, tailored dietary management is essential. Furthermore, long-term, comparative, and cross-national studies are needed to address global nutritional gaps and inform standardised dietary guidelines for IBS.
1 Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder characterised by recurrent abdominal pain and altered bowel habits, including diarrhoea, constipation, or a combination of both. The pooled global prevalence is estimated as 11.2% with a cross-national variation of 1.1–45.0% [1,2,3]. One meta-analysis of 57 cross-sectional studies suggests a global prevalence rate of 3.8% on employing the Rome IV symptom-based diagnostic criteria [3]. According to a global study conducted by the Rome Foundation, the prevalence of IBS based on internet surveys ranges between 3 and 5% while the prevalence based on household surveys showed a wider range of 0.2–4.6%, with greater variability observed in the latter [4]. IBS is a significant contributor to healthcare utilisation and impacts the quality of life of millions of individuals. Despite extensive research, its pathophysiology remains complex and poorly understood largely due to its multifactorial nature, involving the gut–brain axis, motility disturbances, visceral hypersensitivity, and alterations in the gut microbiota [5,6,7,8].
Traditional management strategies for IBS include pharmacological interventions aimed at alleviating symptoms [9,10,11] and psychological strategies such as cognitive behavioural therapy and gut-directed hypnotherapy [12]. Over the past years, dietary interventions have gained more attention and included within IBS guidelines in light of the growing body of evidence about the important role that dietary interventions may play in the management of IBS symptoms [13].
Diet presents a potential underlying pathological mechanism as certain foods and eating patterns may trigger symptoms. Food intolerance and allergy, bacterial overgrowth, and postprandially altered colonic flora and GI physiology are amongst the potential mechanisms proposed for triggering food-induced symptoms [14]. Therefore, various dietary strategies have been explored aimed at reducing symptom severity and improving overall GI function [15].
With the growing awareness regarding the potential role that diet can play in the relief of IBS symptoms, emerging dietary trends including artificial intelligence-assisted personalised diets using microbiome testing [16,17,18], plant-based diets (PBDs) [19,20,21,22,23], the Mediterranean diet (MD) [24,25,26,27,28,29], and intermittent fasting (IF) [30,31] amongst others are being explored for their potential effectiveness.
The available literature on the dietary intake of international IBS populations presents heterogeneous conclusions. Some studies suggest that IBS does not adversely affect nutrient intakes, while others report specific nutritional deficiencies [32,33,34,35,36,37,38]. These conflicting findings highlight the complexity of dietary management in IBS and the influence of cultural diversity on dietary intake. Such conclusions emphasise the need for further investigation into the nutritional impact of the disorder across diverse populations.
This narrative review aims to critically evaluate the effectiveness of established dietary interventions in the management of IBS symptoms while acknowledging the inherent complexity and heterogeneity of this disorder of gut-brain interaction. In addition to synthesising current evidence on widely adopted dietary strategies, this review explores emerging dietary trends and their potential therapeutic relevance. This review provides further unique value through the analysis of dietary intake patterns of IBS populations across diverse geographical regions, highlighting the influence of cultural and regional diversity which is often overlooked. Unlike systematic reviews, this narrative synthesis is based on the relevance and contribution of selected studies to the field, rather than on rigid inclusion and exclusion criteria. By integrating findings from various sources, this review contributes to a more nuanced understanding of IBS dietary management and identifies areas for future research.
2 Methodology
A narrative review approach was selected to allow flexibility in synthesising a diverse body of literature, including clinical guidelines, observational studies, and emerging evidence on novel dietary interventions. A thorough literature search was conducted between September 2023 and May 2025 across major databases including Google Scholar, Wiley Online Library, PubMed, Elsevier, Scopus, and ScienceDirect. Studies were categorised based on their primary research focus: dietary management strategies, evaluation of emerging dietary interventions, and assessment of dietary intake in IBS populations.
Study selection was limited to that available in the English language, peer-reviewed, and published after the year 2000 with emphasis on studies published within the last 10 years. Given the challenges to evaluate the dietary intake of such an intricate population, studies of varying sample sizes were included and assessed based on their methodological rigour. Studies selected include clinical guidelines based on systematic reviews, studies comparing cross-sectionally collected dietary intakes or pre-existing dietary data of IBS populations with that of non-IBS counterparts and/or dietary reference values, studies adopting alternative dietary assessment methods, mainly validated food frequency questionnaires and food and beverage diaries, reviews comparing alternative dietary management strategies for IBS and studies assessing the effectiveness of emerging dietary interventions. Studies that specifically investigated the effect of diet on intestinal dysbiosis were excluded since these were outside the scope of this review. Moreover, grey literature was also excluded to maintain the inclusion of peer-reviewed, high-quality sources.
A two-stage screening process was performed: title and abstract review followed by full-text review. All eligible studies were included yielding a total of 110 studies. Fifty-four of such studies evaluated dietary strategies for the management of IBS symptoms, 31 provided evidence for emerging dietary trends, and 25 reported on the dietary intakes of cross-national IBS sufferers.
3 Discussion
Dietary interventions have emerged as a cornerstone in the management of IBS, offering a non-pharmacological alternative to symptom management and improvements in the quality of life [13]. Given the complexity of IBS with its heterogeneous symptomatology and the interplay of physiological and psychological factors together with the myriad of triggers, various dietary strategies have been proposed, each targeting different aspects of the condition. Notably, findings from the CARBIS trial demonstrated that dietary interventions were effective and safe alternatives to pharmacological therapy in reducing IBS symptom severity, reinforcing the role of diet as a safe and viable first-line treatment approach [39]. The most prominent and widely adopted dietary interventions are reviewed in this section, with a focus on their mechanisms of action, efficacy, limitations, clinical applications, and potential directions for future research.
3.1 Traditional dietary advice (TDA)
TDA follows dietary recommendations established by the British Dietetic Association (BDA) and the National Institute for Health and Care Excellence (NICE) [10,40]. In 2016, the BDA provided the first fully comprehensive evidence-based first-line dietary and lifestyle guidelines for the dietary management of IBS with advice related to healthy eating practices, the consumption of alcohol, caffeine, fluids, fat, fibre, dairy, spicy food, and the use of probiotics as summarised in Table 1 [40].
Key recommendations proposed by the BDA [40]
| Food source or group | Mechanism | Impact or response by the body | Recommendation |
|---|---|---|---|
| Alcohol | Influence GI transit, absorption, and intestinal permeability | Abdominal pain, nausea, indigestion, diarrhoea on excessive consumption | Abide by recommended safety limits with even more strict intakes if symptoms worsen |
| Caffeine | Induces gastric acid secretion, colonic motor activity, and rectosigmoid motor activity | Gastroesophageal reflux, dyspepsia, abdominal pain, loose stools | Symptoms should be monitored and intakes restricted accordingly |
| Fluids | May improve stool frequency | Gradual increase in fluid intake aiming 1.5–3 litres daily | |
| Lipids | Stimulate the reflex controlling motility of low GI tract after food intake | Abdominal pain, dyspepsia, flatulence | Reduction in meal fat content |
| Fibre | May improve stool consistency and/or frequency and has beneficial effects on GI microbiota and fermentation by-products | Inadequate or excessive intakes may worsen IBS symptoms of abdominal pain and constipation | If fibre intake is to be improved, a varied consumption of high fibre starchy sources coupled with adequate intake of non-caffeinated and non-alcoholic drinks is encouraged |
| Lactose | Malabsorption in the presence of insufficient amounts of lactase | Bloating, abdominal pain, excessive flatus | Should be considered as part of a low-FODMAP diet |
| Lack of high-quality data to recommend milk-free diets for IBS symptom management | |||
| Spicy food (capsaicin) | Stimulates GI motility | Abdominal pain, oral burning sensations, gastroesophageal reflux | May be useful to investigate other meal components that may trigger symptoms rather than hot spices on their own merit |
| Probiotics | Multi-factorial but not clearly defined | Unlikely to produce IBS symptom benefits | Continue at recommended dose if symptom benefits experienced, although long-term effects uncertain |
| Sweeteners | Poorly absorbed by the gut | May cause laxative effects if consumed in large amounts | Monitor symptoms and adjust intake accordingly |
Evidence suggests that irregular meal patterns, low fruit and vegetable intake, and excessive fast-food consumption may trigger IBS symptoms [10,40]. Therefore, monitoring dietary habits and promoting a balanced diet alongside healthy eating and lifestyle practices is recommended.
Both the BDA and NICE recommend TDA as a first-line treatment prior to consideration of more restrictive diets like the low fermentable oligosaccharide disaccharide monosaccharide and polyols (FODMAP) diet (LFD) [10,40]. Although TDA is widely implemented in clinical practice, the supporting evidence is relatively limited. Most of the recommendations are informed by clinical experience, observational findings, or expert consensus rather than a robust foundation of randomised controlled trials (RCTs). One notable RCT by Eswaran et al. [41] included TDA as a comparator to the LFD and concluded that while TDA may offer symptom improvement for some individuals, the LFD resulted in significantly greater symptom relief. However, the existing lack of large-scale, well-designed trials evaluating TDA as a standalone intervention restricts our ability to draw strong conclusions about its efficacy. Despite these limitations, TDA remains a practical, cost-effective, and nutritionally balanced approach, especially for individuals with milder symptoms particularly when delivered by a skilled dietitian as part of a structured and individualised care plan.
3.2 The low-FODMAP diet
FODMAPs are a group of poorly absorbed carbohydrates that can contribute to the symptoms of IBS. These compounds vary in chain length, with short-chain FODMAPs (lactose, fructose, polyols) being rapidly and incompletely absorbed in the small intestine, where they exert osmotic effects that draw water into the bowel, potentially leading to diarrhoea and bloating. In contrast, long-chain FODMAPs (oligosaccharides) are not absorbed in the small bowel and instead reach the colon intact, where they undergo fermentation by colonic bacteria, producing gas and leading to symptoms such as bloating, flatulence, abdominal pain, and altered bowel habits [42,43,44]. These mechanisms are further detailed in Table 2.
| FODMAP subgroup | Mechanism | IBS symptoms | Examples of dietary sources |
|---|---|---|---|
| Monosaccharide – fructose | As a short-chain FODMAP, it is poorly absorbed in the small bowel, exerting osmotic effects by drawing water into the lumen triggering symptoms | Luminal distension contributes to pain and bloating; Diarrhoea on excessive consumption | Apples, pears, watermelon, mango, sugar snap peas, honey, high fructose corn syrup |
| Disaccharide – lactose | Short-chain FODMAP that requires lactase for digestion. In its absence, lactose is poorly absorbed in the small bowel, exerting osmotic effects | Bloating, abdominal pain, excessive flatus | Milk and products thereof |
| Oligosaccharides – fructans and galacto-oligosaccharides | Long-chain FODMAPs that bypass the small bowel intact and undergo fermentation by colonic bacteria in the large bowel triggering symptoms | Bloating, abdominal pain, excessive flatus | Wheat and rye and products thereof, legumes, nuts, artichokes, onion, and garlic. |
| Polyols – mannitol and sorbitol | Short-chain sugar alcohols that are poorly absorbed in the small bowel exerting osmotic effects. Undigested polyols may reach the large bowel, undergoing partial fermentation | Luminal distension | Apples, pears, stone fruits, mushrooms, cauliflower, snow peas, sugar-free products |
The dietitian-led LFD is recognised as the only recommended second-line treatment for managing IBS when initial dietary interventions are insufficient [40]. This evidence-based approach is designed to alleviate IBS symptoms by reducing the intake of fermentable carbohydrates that are poorly absorbed in the gut and is implemented in three distinct phases – restriction, re-introduction, and personalisation – each playing a crucial role in identifying and managing food triggers specific to the individual [42]. As summarised in Figure 1, these phases are structured to gradually ease IBS symptoms while ensuring long-term dietary sustainability.
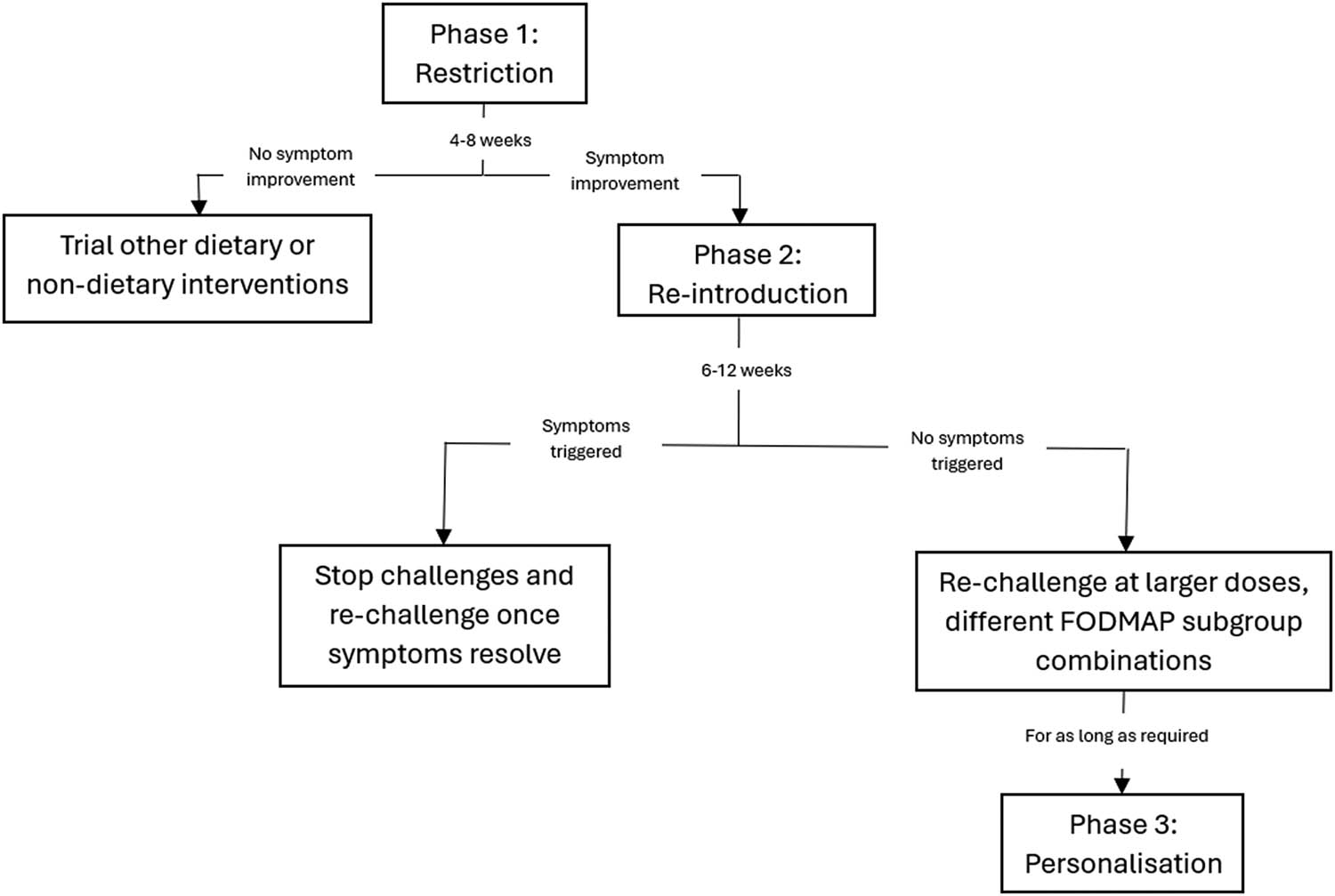
The three phases of the LFD.
The initial restriction phase concerns the global restriction of high-FODMAP food sources for 4–8 weeks with the substitution of suitable low-FODMAP sources to ensure nutritional adequacy. This serves as a diagnostic test for FODMAP sensitivity with a 50–80% positive symptom response. If the patient is non-FODMAP sensitive, alternative dietary interventions are trialled. The second phase reintroduces single FODMAPs over 6–12 weeks in gradually increasing doses over 3 days while the background diet remains low in FODMAPs. Specific challenges include single food containing fructans (wheat-containing bread and garlic), galactans (lentils, legumes), lactose (milk, yoghurt), fructose (honey), sorbitol (dried apricot), and mannitol (mushrooms). This phase is fundamental to prevent unnecessary dietary restrictions and malnutrition in the long term as it determines individual tolerance to specific FODMAPs [45]. In the final phase, the less restrictive personalised diet is formed for the longer term which incorporates low-FODMAP sources and tolerated higher FODMAP sources. However, dietary sources that were not well tolerated should still be re-challenged over time as symptoms may change. It is proposed that a daily threshold of 12 g reduction in dietary FODMAPs results in symptom improvements, keeping in mind individual variability [44,46,47,48,49,50,51].
Commonly consumed food, including wheat-based bread, pasta and pastries, certain fruits (such as apples and stone fruits), vegetables (such as onion, garlic, and cauliflower), legumes, pulses, and dairy are significantly restricted in the first phase. This may compromise nutritional adequacy, as limiting these foods can reduce the intake of key nutrients – calcium from dairy, iron and B vitamins from fortified cereals, and fibre from grains, fruits, vegetables, legumes, and pulses, with several studies looking at adherence to an LFD reporting suboptimal intakes for calcium, iron, magnesium, and B vitamins [5,40,46]. Others have reported a reduced diet quality but not lower nutrient intake [46,51] and reduced energy intakes despite unaffected protein and fat intakes [8]. Oligosaccharide restriction was also attributed to a 4 g/day dietary fibre reduction [40].
A greater symptom improvement is noted with the LFD [55]; however, such an effect may not always be consistent [13,52]. One RCT reported that those following the LFD consumed significantly less energy, carbohydrates, and fibre compared to those following TDA [13,51]. Nevertheless, both the LFD and TDA result in reduced intakes of energy, especially that derived from carbohydrates. After adjusting for the percentage of overall intakes, micronutrient consumption seemed sufficient except for riboflavin [51].
To maintain nutritional adequacy, individuals with IBS are encouraged to follow national dietary recommendations for key nutrients and food groups while incorporating suitable low-FODMAP alternatives. Dietitian-led restriction and personalisation phases of the LFD have been proposed to support overall nutrient intakes. Regular monitoring of body weight is essential and supplementation should be considered for those at risk of nutrient deficiencies [53,54,55,56].
The LFD currently represents the most extensively researched dietary intervention for IBS, with multiple systematic reviews and meta-analyses confirming its effectiveness in managing GI symptoms [57–64]. Evidence consistently demonstrates that the LFD significantly improves global IBS symptoms, with particularly pronounced benefits in individuals with diarrhoea-predominant IBS [62,65–69]. Its efficacy is further enhanced when implemented under the guidance of a dietitian, who plays a vital role in ensuring nutritional adequacy throughout the three-phased LFD [61,66,70–72]. Despite its high short-term efficacy, concerns persist regarding the potential long-term consequences of the diet, especially its impact on gut microbiota composition and metabolic outcomes [59,64,65,68]. Current literature underscores the need for further large-scale, high-quality RCTs to determine their long-term safety and sustainability, to assess their relative effectiveness compared to other dietary, supplemental, or lifestyle-based interventions, and to better characterise patient predictors of response, particularly during the personalised re-introduction phase [59,68,72–74].
While the LFD has shown considerable effectiveness in alleviating IBS symptoms, it is not without some limitations. The diet’s complexity requires extensive food knowledge and label reading, making adherence challenging, especially in social settings [51,53]. Another key concern is the potential impact on gut health, as some FODMAPs act as prebiotics, supporting the growth of beneficial bacteria in the gut, such as Bifidobacteria and Lactobacilli. Prolonged restriction of these fermentable carbohydrates may inadvertently reduce microbial diversity and alter luminal microbiota composition. Furthermore, although one recent systematic review and meta-analysis reports positive improvements of the LFD on microbial regulation, it highlights the need for further high-quality research to confirm these findings within IBS populations [75]. Moreover, while the fermentation of carbohydrates produces short-chain fatty acids, important for colonic health, this process also results in the release of colonic gas further exacerbating GI symptoms [51,53]. Strategies to combat these concerns such as the re-introduction phase, probiotic supplementation, and dietary personalisation may prove fundamental to minimising the effects that the LFD may exert on the microbiome in the longer term. However, further research is warranted to fully comprehend the complex relationship between diet, IBS symptom profiles, and the gut microbiome [76].
To address these challenges, adapted versions of the LFD have been proposed such as the FODMAP gentle approach. These involve a “bottom-up” approach, where only a few specific FODMAP sources are restricted based on a thorough evaluation of dietary history and symptomatology, rather than adopting a blanket restriction of all FODMAP groups [13,51,77–79]. Furthermore, potential microbiota alterations during the restrictive phases of the diet may be reversed in the final re-introduction phase or supported through probiotic supplementation. However, despite these strategies, evidence about the long-term impact of the complete three-phased LFD remains limited and warrants further research.
3.3 Gluten-free diet (GFD)
Wheat contains components such as fructans, gluten, amylose trypsin inhibitors, and other proteins, which may trigger food intolerance symptoms in 23–49% of IBS sufferers, with studies reporting a 58% clinical response to a GFD [13]. The physiologic mechanism of wheat-based fructans is explained in Table 2. Gluten has been primarily associated with symptoms such as abdominal pain, bloating, altered stool consistency, increased gut permeability, immune activation, and fatigue, suggesting that it may be a key driver of symptom onset following wheat consumption [48,80,81]. Supporting this, several studies have reported a reduction in overall symptom severity, including bloating and abdominal pain, in IBS patients following a GFD [82–85].
Nevertheless, the GFD raises dietary concerns including increased fat intake and reduced carbohydrate and fibre intake, along with observed deficiencies in iron, calcium, thiamine, and magnesium. However, data about the nutritional adequacy of GFD in IBS are rather limited [13].
Evidence supporting a GFD for IBS is still lacking predominantly due to the fact that symptom alleviation may not be solely due to gluten exclusion as a GFD also eliminates fructans [13,40,47]. In a placebo-controlled, crossover rechallenge study, patients habitually consuming a GFD in view of non-coeliac gluten sensitivity experienced symptom improvement with an LFD. No independent gluten-specific effects were noted on gluten challenges concluding that FODMAPs are responsible for GI symptoms and not gluten [86]. Moreover, no further benefits were observed when a GFD was introduced to IBS patients already following an LFD [7].
Eliminating consumption of cereals reduces dietary FODMAP content by 50% and may alleviate symptoms [13,40]. Single food challenges are warranted to determine whether such food products trigger symptoms. It remains unclear whether gluten is responsible for IBS symptoms while evidence for fructans as a predominant symptom trigger is accumulating [40]. However, one observational study also reports no correlation between GI symptoms and fructan intake [15]. Further research is warranted to determine the long-term efficacy of a GFD for IBS as most RCTs are short term [13].
3.4 Specific carbohydrate diet (SCD)
The SCD allows only monosaccharide carbohydrates while excluding disaccharides and polysaccharides. It is supplemented with homemade yoghurt fermented for 24 h to minimise the disaccharide lactose. This diet, including a number of allowed and prohibited foods as outlined in Table 3, is proposed as it may reduce inflammation and microbiota dysfunction [87].
Dietary sources allowed and prohibited in the SCD (adapted from [87])
| Food category | Food allowed in the SCD | Food prohibited in the SCD |
|---|---|---|
| Fruit | Any fresh fruit | Any canned fruit with added sugar or ingredients, juices |
| Vegetables | Any fresh vegetables (except for potatoes and corn) | Potatoes, corn, any canned vegetables with added sugar or ingredients |
| Dairy | Homemade prebiotic yoghurt, goat cheese | Most dairy (milk, cheese, most yoghurt, ice cream) |
| Meat, fish, and poultry | Chicken, turkey, meat, game, fish, eggs | Processed meats |
| Cereals | None | All |
| Legumes | Beans, lentils, peas | Soy, chickpea, broad bean |
| Nuts | All | None |
| Spices | All | None |
| Sweeteners | Honey | Sugar and other sweeteners |
As can be determined from Table 3, the SCD shares overlapping mechanisms with the LFD particularly in its exclusion of disaccharides in the form of lactose (found in dairy) and polysaccharides such as starch (found in cereal, potatoes, corn), which are known to be osmotic and fermentable, leading to gas production, bloating and discomfort in IBS patients. Therefore, both diets aim to alleviate IBS symptoms by reducing fermentable carbohydrate intake, but while the LFD takes a broader approach by excluding additional FODMAPs including oligosaccharides and polyols, the SCD is more focused on limiting specific complex carbohydrates. Indeed, a recent randomised non-inferiority study reported that a diet low in starch and sugar shares similar efficacy with the LFD in the reduction of GI symptoms, possibly due to the overlap between the two dietary patterns [88].
Contrastingly, a clinical single-blinded RCT noted that the LFD provided significant improvements in bloating and distension compared to the SCD. Folic acid and vitamin D deficiencies were also observed with the SCD [87]. Such findings support those previously reported in the literature [5]. Given the absence of significant improvements in symptoms, such an exclusion diet is not routinely recommended [40].
3.5 Low lactose or lactose-free diet
Lactose intolerance is common in both the general population and amongst IBS sufferers. Symptoms are triggered due to the inability to form lactase enzymes, unfavourable microbiota compositions, or history of GI disorders. Not all lactose malabsorbers experience symptoms following consumption while symptoms may be heightened at even minimal intakes with visceral hypersensitivity [50]. One systematic review reports the absence of any clear correlations between IBS and lactose intolerance and does not support routine lactose-free diets for all IBS sufferers. Further research is warranted to determine whether any specific components in dairy other than lactose are responsible for IBS complaints [89]. In light of the available evidence, low lactose or lactose-free diets for the management of IBS symptoms should be considered as part of an LFD and not on their own merit.
3.6 Low fructose diet
Over one in three adult IBS sufferers experience fructose malabsorption and its prevalence is similar amongst IBS sufferers and asymptomatic controls [50,90]. The main determinants of fructose malabsorption are presumed to be excessive fructose intake, co-ingestion of fructose and sorbitol, and fructose in excess of glucose as when fructose and glucose are equimolarly ingested, symptomatic improvements were recorded [50,90,91]. Fructose is present in equimolar amounts to glucose in bananas, strawberries, table sugar, and high-fructose corn syrup [91]. Nevertheless, a low fructose diet is rarely acknowledged as effective since fructose malabsorption is a separate disease from and not specific to IBS; it may be uncommon and due to the absence of published dietary guidelines [90]. Should a low fructose diet be warranted for the management of IBS symptoms, this should be considered part of the LFD.
3.7 Comparative analysis
A lack of consensus exists with regard to the comparative efficiency of TDA, LFD, and GFD and it remains unclear which dietary treatment is superior due to the absence of head-to-head trials. RCTs demonstrate similar efficiency between these diets with TDA appearing to be less costly and time consuming and easier to comply with [52,92]. The key uses and limitations of the dietary interventions analysed in this narrative review are compared in Table 4. Table 4 may serve as a practical resource for healthcare professionals guiding decisions as to the most appropriate dietary intervention to adopt.
Comparative analysis of the key uses and limitations of dietary interventions for the management of IBS symptoms
| Dietary approach | Key uses | Limitations |
|---|---|---|
| TDA | Less costly and time consuming and easier compliance compared to alternative dietary interventions. Best suited for those with limited cooking and literacy skills to read food labels and for those with more hectic lifestyles | Some may require a stricter approach in view of a greater level of food sensitivity and intolerance [13,93] |
| Deemed more acceptable and thus recommended as the first-line option [13,40] | ||
| LFD | Better suited for those who require a stricter approach [13,93] | Limited long-term implications data |
| Suitable for those who have minimal knowledge of what food sources or groups trigger symptoms as the three-phased diet enables the determination of FODMAP sensitivity and tolerance to different FODMAP subgroups | May be contraindicated for those with a history of eating disorders, those at risk of malnutrition and paediatric and geriatric populations | |
| Barriers to implement include reliance on ready-made or processed food, frequently disordered eating patterns, limited cooking skills, or control over food preparation and cooking at one’s own household | ||
| FODMAP gentle approach | Suitable for patients with milder IBS symptoms or who consume high FODMAP loads, geriatric and paediatric populations, patients who may be overwhelmed by the full LFD such as those with a history of eating disorders or have low cooking skills [94] | May not identify exclusive FODMAP symptom triggers |
| Absence of robust trial evidence [94] | ||
| GFD | Suitable “bottom-up approach” for those who identify gluten as a predominant trigger [13] | Supporting evidence is still lacking predominantly since symptom alleviation may not be solely due to gluten exclusion but due to fructan elimination [7,80,86] |
| SCD | Proposed as it may reduce inflammation and microbiota dysfunction [87] | Not routinely practised due to absence of significant improvements [40] |
| Low lactose/lactose-free diet | High lactose sensitivity, as may be determined through a three-phased dietetic-led LFD [40] | Absence of any clear correlations between IBS and lactose intolerance warranting further research. Routine lactose-free diets are not recommended to all IBS sufferers [89] |
| Low fructose diet | Fructose sensitivity, as may be determined through a three-phased dietetic-led LFD [40] | Rarely acknowledged as an effective dietary intervention for IBS [90] |
3.8 Emerging dietary trends
With advancements in IBS care, novel dietary interventions show promise for symptom relief. Precision nutrition has the potential to revolutionise GI health by tailoring dietary interventions based on individual gut microbiota profiles offering promising approaches for the management of IBS symptoms [16]. One pilot clinical study compared microbiota compositions of mixed subtypes of IBS patients, as diagnosed by the Rome IV criteria, against that of a control group. Following 6 weeks of either AI-based personalised nutrition or a standard IBS diet, it was concluded that AI-based personalised microbiome modulation through diet improved IBS symptoms and induced favourable microbiome changes, but further large-scale RCTs with long-term follow-up are necessary [17]. A multicentre RCT comparing the effectiveness of microbiome-based AI-assisted personalised diets against the LFD for IBS symptom management concluded that the AI-assisted personalised diet approach demonstrates significant symptom improvements, improved quality of life, and enhanced microbiome diversity [18].
In their hypothesis-driven article, Black and Ford propose a novel framework for the management of IBS that moves beyond traditional symptom-based subtyping. Using latent class analysis, seven distinct patient clusters were identified and characterised by unique constellations of GI symptoms, extraintestinal manifestations, and psychological comorbidities. This classification model demonstrated predictive value for healthcare utilisation, treatment response and quality of life outcomes. By outlining tailored first- and second-line treatment strategies for each cluster, the authors offer a personalised, mechanistically informed approach to IBS care. This model underscores the heterogeneity of IBS and presents a compelling argument for shifting towards stratified treatment pathways, warranting validation through prospective clinical trials [95]. Further three studies support the integration of precision nutrition in IBS care while encouraging future long-term and larger-scale RCTs to fully determine the effectiveness of such digital and personalised dietary interventions [96–98]. As our understanding of the genetic underpinnings of IBS and nutrient interactions expands, precision nutrition may become a cornerstone in the comprehensive management of this complex disorder.
The role of PBDs in managing IBS symptoms has garnered increasing attention, although the existing evidence remains preliminary and warrants further investigation. PBDs may confer GI benefits through the increase in gut bacterial diversity [19,20,21]. One cross-sectional study investigating the association between PBDs and IBS in a large French cohort concluded that long-term PBDs may be associated with IBS but further long-term studies are necessary to confirm this association [22]. Additionally, one systematic review and meta-analysis suggests that PBDs may influence the circulating levels of inflammatory markers and reports a strong association between c-reactive protein, an inflammatory marker, and PBDs. Nevertheless, further investigation is necessary [23]. While PBDs are generally considered beneficial for gut health, some individuals with IBS may experience symptom exacerbation due to increased fibre intake, particularly from high-FODMAP plant foods. Therefore, personalised dietary approaches, possibly integrating elements of PBDs with low-FODMAP principles, may offer a balanced strategy for managing IBS symptoms.
The MD characterised by high consumption of fruits, vegetables, wholegrains, legumes, nuts, seeds, olive oil, and moderate intake of fish and poultry has garnered attention for its potential benefits in managing IBS symptoms. A recent pilot RCT concluded that the MD improves abdominal symptoms for diarrhoea-predominant IBS and mixed subtypes IBS but warrants larger-scale trials to evaluate the effectiveness of the MD against that of LFD and TDA [28]. In agreement, a 6-week RCT involving adults with Rome IV-diagnosed IBS demonstrated that participants adhering to the MD experienced significant reductions in GI and depressive symptoms compared to controls [27]. Similarly, a network meta-analysis identified the MD as one of the most effective dietary interventions for improving IBS symptom severity and quality of life. Nevertheless, it still warrants future large-scale RCTs with head-to-head comparisons to fully comprehend the effectiveness of the MD for IBS populations [29]. These findings are supported by studies indicating that the anti-inflammatory properties of the MD, attributed to components like polyphenols and omega-3 fatty acids, may modulate gut microbiota composition, potentially enhancing gut health and immune function as well as reducing inflammation in IBS patients [24,25,26,27]. Ultimately, while currently available evidence supports the efficacy and feasibility of the MD in IBS symptom management, a combined LFD and MD, or an IBS-modified MD, is recommended for further study and to establish clinical guidelines [99].
The current evidence on IF as a dietary intervention for managing IBS remains preliminary and inconclusive. IF may offer therapeutic benefits through gut microbiota modulation [30,31]. A systematic review of human studies on IF and gut microbiota found that IF can improve gut microbiota richness and alpha diversity which may potentially offer beneficial effects for IBS patients. However, this systematic review highlights the need for further research to clarify and confirm such effects in light of the substantial heterogeneity in results and bacterial strains determined to be statistically significantly influenced by this dietary pattern [30]. While IF shows promise in modulating factors associated with IBS, its clinical application remains uncertain and more rigorous, long-term studies are needed to determine its efficacy and safety in IBS management.
The role of histamine in IBS has garnered increasing attention in recent years, particularly in relation to the emerging concept of histamine intolerance [100]. Although the pathophysiology of IBS is multifactorial, evidence suggests that histamine has the potential to influence gut motility, visceral hypersensitivity, and intestinal inflammation triggering symptoms [101–103]. A positive correlation is identified between mast cells, which release histamine, and IBS symptoms particularly abdominal pain and bloating [104]. Accepted dietary guidelines for a low histamine diet are yet not available, but generally, a low histamine diet involves an elimination-type diet reducing the consumption of hard and semi-hard cheeses, oily fish and shellfish, raw fermented meat products, chicken eggs, fermented soy products, pickled vegetables, fruit and vegetables triggering the release of endogenous histamine, mushrooms, chocolate, wine and beer [103]. However, the role of histamine in IBS is not fully elucidated and the current body of evidence remains limited making it difficult to establish clear clinical guidelines [105]. As such, while a low histamine diet may offer therapeutic potential for certain IBS subtypes, its routine use in clinical practice warrants further investigation supported by high-quality research.
The ketogenic diet, characterised by high fat, adequate protein, and very low carbohydrate intake, has been explored for its therapeutic potential beyond epilepsy and weight management, including in GI disorders such as IBS. The proposed mechanisms through which a ketogenic diet may benefit IBS include modulation of the gut microbiota due to ketone body activity [106–108]. However, the current evidence supporting its efficacy in IBS remains sparse and largely preclinical. Most available studies have been conducted in animal models, particularly rats, demonstrating beneficial changes in gut permeability, inflammation, and microbial composition [106,109]. A limited number of human case studies have suggested that very low carbohydrate diets may reduce symptoms such as bloating, abdominal pain, and altered bowel habits, especially in individuals with diarrhoea-predominant IBS [110]. Moreover, concerns regarding the diet’s restrictiveness, potential nutritional deficiencies, and its impact on gut microbial diversity raise questions about its safety and sustainability for IBS management [108]. While the ketogenic diet may offer symptomatic relief for select patients, there is insufficient high-quality evidence to support its widespread use in IBS treatment and further RCTs are needed to clarify its role.
3.9 Dietary intake of international IBS populations
Nutrient intakes of IBS populations differ across geographical regions with some studies agreeing that IBS does not adversely affect nutrient intakes. A recent systematic review and meta-analysis reports no significant differences in dietary intake between IBS and non-IBS sufferers except for suboptimal intakes of fibre, calcium, and vitamin D [32]. Additionally, a cross-sectional study targeting an English population reported a low risk of nutritional deficiencies and stated that adequate substitutions and supplementation were being practised if specific foods were being excluded [33]. In agreement, a Swedish study reported no significant differences between the dietary intake of IBS and non-IBS sufferers with IBS participants consuming higher dietary fibre intakes compared to the general population. The only suboptimal intakes observed were those for vitamin A, riboflavin, calcium, and potassium mainly due to limited intakes of dairy [34]. An additional RCT also reports nutrient intakes comparable to the general population and even suggests that the LFD may improve overall dietary intake. Vitamin B12 was higher compared to habitual diets, possibly due to increased intakes of eggs and fish [35]. Furthermore, a North American study concluded that IBS sufferers consume significantly higher energy intakes compared to non-IBS counterparts [111].
However, the following studies contraindicate this by arguing that IBS sufferers practise significantly more dietary avoidances. A study performed in a large French population concluded that IBS sufferers consume less milk, yoghurt, and fruits and greater intakes of non-sugar-sweetened beverages [36]. A Norwegian study also reports dairy avoidance in view of lactose malabsorption and intolerance with calcium, potassium, zinc, and B vitamins deficiencies as a consequence [37]. Both studies report a limited consumption of fruit and vegetables due to their potential to generate gas upon fermentation and laxative effects further depleting levels of water-soluble vitamins and minerals. Higher intakes of water and carbonated beverages might be observed in an attempt to compensate for fluids lost through diarrhoea or to prevent constipation. Tea may be avoided as salicylate content may cause constipation and gut symptoms while coffee is related to diarrhoea. A higher intake of carbonated beverages may worsen symptoms due to the potential presence of caffeine. Alternatively, it may be consumed in addition to water with the aim of substituting milk with other fluids to meet fluid requirements as recommended for the management of constipation and diarrhoea. Although alcohol is known to exacerbate symptoms, its consumption may persist in an attempt to relieve severe symptoms [36,37]. Vitamin D levels may also be depleted through the avoidance of fortified dairy [38].
Cultural diets, food availability, and strength of healthcare systems play significant roles in shaping dietary intake and adherence to dietary interventions among IBS populations. Cultural dietary patterns, including staple foods, preparation methods, and meal structures vary widely across regions and often influence the types of food consumed by individuals with IBS. For instance, in regions where high-fibre foods are common, such as in Mediterranean or East Asian diets, IBS patients may experience different symptom management outcomes compared to those in regions with lower fibre intake or a reliance on processed foods such as the Western diet. Moreover, access to food may hinder the IBS patients’ ability to follow specialised diets, such as the LFD or GFD, which may be difficult to adhere to without access to specific food or ingredients. The health system also plays a critical role in IBS management, as its effectiveness in providing education and access to the relevant healthcare professionals, especially dietitians, influences adherence to dietary recommendations. In countries with well-established healthcare infrastructures, IBS patients may have better access to tailored dietary counselling, while in regions with less comprehensive healthcare systems, patients may face challenges in obtaining the necessary support to manage their condition effectively. These factors collectively contribute to variations in nutritional profiles and symptomatology, as cultural, economic, and healthcare-related barriers impact both the quality of the diet and the ability to adhere to tailored dietary interventions.
Table 5 summarises dietary findings of IBS sufferers from different geographical regions and may serve as a useful practical clinical tool for healthcare professionals as it provides an outline of possible dietary deficiencies. It also serves as a valuable resource for improving patient care and facilitating tailored management strategies.
Studies reporting key dietary findings of IBS sufferers from different geographical regions using alternative dietary data tools
| Author | Year | Setting | Study design | No of subjects in study sample | Dietary data tools | Key findings |
|---|---|---|---|---|---|---|
| Malhotra and Olver [112] | 2004 | Northern India | Cross-sectional study | 33 | 72-h dietary recall | IBS patients consumed lower macronutrient and fibre intake compared to healthy controls |
| Monsbakken et al. [113] | 2005 | Norway | Cross-sectional study | 84 | Interview with dietitian | 70% prevalence of food intolerance in IBS subjects |
| McCoubrey et al. [114] | 2008 | UK | Cross-sectional study | 51 | FFQ | Similar energy and macronutrient intakes found for IBS sufferers and controls. However, calcium and iron intakes were significantly lower in IBS sufferers compared to controls |
| Singh et al. [115] | 2008 | India | Cross-sectional study | 81 | Semi-quantitative FFQ | Similar dietary fibre intakes between IBS sufferers and healthy controls. Dietary fibre intake for both groups exceeded national recommendations |
| Prescha et al. [116] | 2009 | Poland | Cross-sectional study | 63 | 24-h dietary recall | IBS sufferers consumed insufficient energy intakes from carbohydrates and insufficient intakes of calcium and vitamin B2 compared to Polish RDAs. Overconsumption of fat and saturated fatty acids was also observed |
| Østgaard et al. [117] | 2011 | Norway | Cross-sectional study | 114 | FFQ | No statistical difference in the intake of energy, carbohydrates, fat, and protein between IBS subjects and controls |
| Williams et al. [33] | 2011 | UK | Cross-sectional study | 104 | Modified version of the EPIC FFQ | IBS participants consumed higher energy, protein, NSP, and micronutrients intake and lower fat and carbohydrate when compared to the UK DRVs |
| IBS participants consumed higher energy, carbohydrate, protein, and micronutrient intakes and lower energy derived from fat when compared to a National Diet and Nutrition Survey | ||||||
| Böhn et al. [34] | 2012 | Sweden | Cross-sectional study | 187 | 4-day FD | Nutrient intake of IBS sufferers appears to be adequate when compared to Nordic Nutrition Recommendations and similar to that of the general population |
| Ligaarden et al. [37] | 2012 | Norway | Cross-sectional study | 388 | FFQ | Subjects with IBS consumed lower intakes of dairy products and potatoes but higher intakes of water, tea, and carbonated beverages compared to subjects without IBS |
| Staudacher et al. [77] | 2012 | UK | RCT | 41 | 7-day FD | Restriction of fermentable short-chain carbohydrates resulted in reductions in overall symptoms and bloating compared to habitual diets. Lower intakes of total carbohydrates, starch, total sugars, and calcium noted when following the diet restricted in fermentable short-chain carbohydrates |
| Omagari et al. [118] | 2013 | Japan | Cross-sectional study | 245 | Semi-quantitative FFQ | Mean daily intakes of energy, carbohydrates, fibre, calcium, and iron were lower while intakes of protein and salt were higher compared to the Dietary Reference Intakes for Japanese |
| Böhn et al. [52] | 2015 | Sweden | Multi-centre, parallel, randomised, controlled, single-blinded, comparative trial | 67 | 4-day FD | LFD did not appear to be superior to TDA as both improved IBS symptoms. A lower energy intake was observed in both dietary interventions |
| Zahedi et al. [93] | 2016 | Iran | RCT, single blind | 101 | 3-day FD | Lower energy intake in both LFD and TDA |
| Tigchelaar et al. [119] | 2017 | Netherlands | Cross-sectional, case-control study | 380 | FFQ | IBS cohort consumed higher intakes of fat and sugar while lower intakes of fibre compared to healthy controls |
| Vincenzi et al. [87] | 2017 | Italy | Clinical, single-blinded randomised trial | 60 | FD | Patients suffering from IBS seem to benefit from an LFD but not from an SCD |
| LFD does not appear to cause vitamin D and folic acid deficiencies compared to SCD | ||||||
| Torres et al. [36] | 2018 | France | Cross-sectional study | 36,448 | 24-h dietary records | IBS subjects had significantly lower intakes of milk, yoghurt, and fruits but higher intakes of non-sugary drinks compared to controls |
| IBS subjects had higher total energy intake with a slightly higher percentage derived from fat and a lower percentage derived from protein compared to controls. Percentage of energy from carbohydrates did not differ between groups but IBS subjects tended to reach the RDA for fibre more than healthy controls | ||||||
| IBS subjects consumed lower intakes of calcium, potassium, zinc, and vitamins B2, B5 and B9 compared to controls | ||||||
| Alharbi et al. [120] | 2019 | Northern Saudi Arabia | Cross-sectional survey | 930 | Purposeful questionnaire | IBS is significantly associated with insufficient fluid and fibre intake. Spicy food consumption is directly associated with IBS |
| Nilholm et al. [121] | 2019 | Sweden | Randomised open clinical trial | 105 | 5-day FD | IBS patients consumed irregular dietary habits with excessive intakes of fast and processed food, cereals, sweets, and sugar-sweetened beverages and low intakes of fish, vegetables, legumes, fruits, berries, and dairy products. Overall dietary intakes did not follow the recommendations of a healthy Nordic diet |
| Staudacher et al. [35] | 2019 | UK | RCT | 130 | 7-day FD | Resultant nutrient intakes of IBS sufferers did not meet respective DRVs. However, overall dietary intake was comparable to the general population |
| Yilmaz and Akbulut [122] | 2019 | Turkey | Cross-sectional study | 70 | 3-day FD + FFQ | IBS participants consumed energy and macronutrient intakes similar to the general Turkish population. However, low intakes of vitamins B9 and 12, potassium, calcium, and magnesium were noted in the majority of IBS participants |
| Hujoel [111] | 2020 | USA | Cross-sectional study | 413 | 24-h dietary recall + FFQ | Despite IBS sufferers reporting significantly more food avoidances, similar macro- and micronutrient intakes were observed between IBS sufferers and controls |
| Shafiee et al. [123] | 2022 | Malaysia | Retrospective case–control study | 306 | FFQ | IBS patients experiencing predominantly constipation consumed significantly lower intakes of wholegrain products, fried foods, dairy products, fruits, and vegetables compared to healthy controls. Daily intakes of energy, certain macronutrients, and micronutrients among IBS-C patients was significantly lower than the healthy subjects. Dietary intake of fibre, vitamins B1, B2, B6, folate, B12, E, and K, and potassium was below the standard RNI |
| Khoo et al. [69] | 2023 | Malaysia | Multi-centre study | 29 | 3-day FD | LFD improves the quality of life and symptoms in IBS sufferers |
| Hillestad et al. [72] | 2024 | Norway | Prospective single-arm intervention study | 36 | 3-day FD | No clinically meaningful changes found in macronutrient and micronutrient intake following a 12-week dietitian-led LFD. However, low diet quality was identified in IBS sufferers prior intervention |
| Alrasheedi et al. [61] | 2025 | Saudi Arabia | Prospective single-arm intervention study | 45 | Semi-quantitative FFQ | LFD results in significant symptom benefits but may result in nutritional deficiencies and malnutrition if not supplemented with adequate low-FODMAP options |
IBS = irritable bowel syndrome, FFQ = food frequency questionnaire, RDA = recommended dietary allowances, NSP = non-starch polysaccharides, DRVs = Dieta45ry reference values, FD = food diary, RCT = randomised controlled trial, LFD = low-FODMAP diet, TDA = traditional dietary advice, SCD = specific carbohydrate diet, IBS-C = irritable bowel syndrome-constipation predominant, RNI = reference nutrient intake, FODMAP = fermentable oligosaccharides, disaccharides, monosaccharides and polyols.
4 Strengths and limitations
This review has highlighted the importance of individualised dietary interventions emphasising the potential benefits of dietary strategies, such as TDA, LFD, and GFD. Despite extensive research, the complexity of IBS and inter-patient variability continues to challenge a clear understanding while still advocating for individually tailored dietary interventions. Although this review was not conducted systematically, it provides a comprehensive overview of dietary interventions for IBS, supported by a rigorous search strategy that minimises selection bias.
Nevertheless, several limitations warrant consideration. A comprehensive understanding of the role of diet in managing IBS symptoms remains complex, primarily due to factors such as small sample sizes, the absence of blinding which may contribute to placebo and nocebo effects and the inherent variability in individual responses. Additionally, the heterogeneity of IBS symptomatology and diagnostic criteria further complicates the interpretation of findings, limiting the ability to draw definitive conclusions.
The discrepancies and similarities in dietary intake among IBS populations across different geographical regions may be attributed to a range of factors. Variations in nutritional assessment methods, such as the use of food frequency questionnaires, 24-h dietary recalls, or food diaries, may influence the accuracy and comparability of data. Sample size and demographic differences, including age, gender, cultural dietary patterns, and healthcare access, also contribute to heterogenous findings. Furthermore, variability in the diagnostic criteria used to define IBS, such as Rome III versus Rome IV, may affect the population characteristics being studied. In some scenarios, the presence of dietitian support or national dietary guidance may promote more balanced substitutions and reduce the risk of deficiencies. Lastly, publication year and local food availability can also shape dietary trends and nutrient intake outcomes. These methodological and contextual differences highlight the need for standardised, cross-national research to better understand and compare the nutritional impact of IBS globally.
5 Conclusion
Diet plays a crucial role in the holistic management of IBS, however, current evidence does not yet support one universally superior dietary intervention. As such, personalised and symptom-targeted dietary strategies remain the cornerstone of effective care. A multidisciplinary approach, led by a qualified dietitian, is essential to guide this process and prevent overly restrictive eating patterns. Keeping a detailed food and symptom diary can assist in identifying potential dietary triggers, reducing the risk of unnecessary exclusions. When symptom patterns are unclear, second-line approaches such as the LFD may be trailed short-term under dietetic supervision. Given the multifactorial nature of IBS, individualised, patient-centred advice continues to be the most appropriate strategy in clinical practice.
Further research is needed to compare dietary interventions and investigate their long-term effects. The LFD shows promise, with evidence suggesting that many individuals may sustain symptom relief with minimal restrictions over time. However, more studies are required to confirm its long-term safety and efficacy. Additionally, a greater understanding is required regarding the role of different fibre types in IBS management. Exploring personalised diets based on genetic, gut microbiome, and metabolic profiles could further refine dietary strategies, enhancing both compliance and therapeutic outcomes. Standardised protocols for dietary interventions would enable healthcare professionals, particularly dietitians, to deliver more effective, individualised care.
Cross-national studies identifying IBS populations with suboptimal dietary intakes are crucial to addressing potential nutrient deficiencies and improving national dietary recommendations. This review underscores the often-overlooked role of cultural influences on dietary patterns in IBS management, which significantly impacts nutritional status and treatment outcomes. Moreover, Table 5 in this narrative review provides a practical clinical tool that healthcare professionals can utilise to guide dietary interventions for IBS patients, offering a valuable resource for improving patient care and tailored management.
Ultimately, while personalised dietary interventions hold great promise, they should be integrated into a holistic treatment approach that accounts for the multifactorial nature of IBS. Combining individualised dietary strategies with pharmacological and psychological therapies can provide a more comprehensive management plan, improving symptom control and enhancing patients’ overall quality of life.
-
Funding information: Authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results, and approved the final version of the manuscript. HG collected and interpreted data and prepared the manuscript, MCGP critically reviewed the manuscript and PJ contributed to conceptualisation, study design, critical review, and editing of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Card T, Canavan C, West J. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6(6):71–80.10.2147/CLEP.S40245Search in Google Scholar PubMed PubMed Central
[2] Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–21.e4.10.1016/j.cgh.2012.02.029Search in Google Scholar PubMed
[3] Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–17.10.1016/S2468-1253(20)30217-XSearch in Google Scholar PubMed
[4] Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2020;160(1):99–114.10.1053/j.gastro.2020.04.014Search in Google Scholar PubMed
[5] Bek S, Teo YN, Tan XH, Fan KHR, Siah KTH. Association between irritable bowel syndrome and micronutrients: A systematic review. J Gastroenterol Hepatol. 2022;37(8):1485–97, https://pubmed.ncbi.nlm.nih.gov/35581170/.10.1111/jgh.15891Search in Google Scholar PubMed
[6] Hayes P, Corish C, O’Mahony E, Quigley EMM. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Dietetics. 2013;27:36–47, https://onlinelibrary.wiley.com/doi/full/10.1111/jhn.12114.10.1111/jhn.12114Search in Google Scholar PubMed
[7] Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407.e5.10.1053/j.gastro.2016.02.031Search in Google Scholar PubMed
[8] Mitchell H, Porter J, Gibson PR, Barrett J, Garg M. Review article: implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment Pharmacol Ther. 2018;49(2):124–39.10.1111/apt.15079Search in Google Scholar PubMed
[9] Jones J. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47(90002):1ii19.10.1136/gut.47.suppl_2.ii1Search in Google Scholar PubMed PubMed Central
[10] NICE. Overview | Irritable bowel syndrome in adults: diagnosis and management | Guidance | NICE [Internet]. Nice.org.uk. NICE; 2008. http://www.nice.org.uk/Guidance/CG61.Search in Google Scholar
[11] Zhou S, Liu X, Wang X, Xi F, Luo X, Yao L, et al. Pharmacological and non-pharmacological treatments for irritable bowel syndrome. Medicine. 2019;98(30):e16446.10.1097/MD.0000000000016446Search in Google Scholar PubMed PubMed Central
[12] Black CJ, Thakur ER, Houghton LA, Quigley EM, Moayyedi P, Ford AC. O61 Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Oral. 2021;69(8):1441–51.10.1136/gutjnl-2020-321191Search in Google Scholar PubMed
[13] Rej A, Avery A, Aziz I, Black CJ, Bowyer RK, Buckle RL, et al. Diet and irritable bowel syndrome: an update from a UK consensus meeting. BMC Med. 2022;20(1):287.10.1186/s12916-022-02496-wSearch in Google Scholar PubMed PubMed Central
[14] Saito YA, Locke GR, Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population-based case-control study. Am J Gastroenterol. 2005;100(12):2743–8.10.1111/j.1572-0241.2005.00288.xSearch in Google Scholar PubMed
[15] Algera JP, Störsrud S, Lindström A, Simrén M, Törnblom H. Gluten and fructan intake and their associations with gastrointestinal symptoms in irritable bowel syndrome: A food diary study. Clin Nutr. 2021;40(10):5365–72.10.1016/j.clnu.2021.09.002Search in Google Scholar PubMed
[16] Ghaffari P, Shoaie S, Nielsen LK. Irritable bowel syndrome and microbiome; Switching from conventional diagnosis and therapies to personalized interventions. J Transl Med. 2022;20(1):173.10.1186/s12967-022-03365-zSearch in Google Scholar PubMed PubMed Central
[17] Karakan T, Gundogdu A, Alagözlü H, Ekmen N, Ozgul S, Tunali V, et al. Artificial intelligence-based personalized diet: A pilot clinical study for irritable bowel syndrome. Gut Microbes. 2022;14(1):2138672.10.1080/19490976.2022.2138672Search in Google Scholar PubMed PubMed Central
[18] Tunali V, Arslan NÇ, Ermiş BH, Hakim GD, Gündoğdu A, Hora M, et al. A Multicenter randomized controlled trial of microbiome-based artificial intelligence-assisted personalized diet vs low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet: a novel approach for the management of irritable bowel syndrome. Am J Gastroenterol. 2024;119(9):1901–12.10.14309/ajg.0000000000002862Search in Google Scholar PubMed PubMed Central
[19] Mazzocchi S, Visaggi P, Baroni L. Plant-based diets in gastrointestinal diseases: Which evidence? Best Pract Res Clin Gastroenterol. 2023;62–63:101829.10.1016/j.bpg.2023.101829Search in Google Scholar PubMed
[20] Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6(47):1–10.10.3389/fnut.2019.00047Search in Google Scholar PubMed PubMed Central
[21] Kim SH, Kang W, Kim M, Hong S, Kim H, Lee JK. Temple stay diet and its impact on gut microbiome and irritable bowel syndrome: a prospective cohort study. Food Funct. 2025;16(12):4894–90310.1039/D4FO06143HSearch in Google Scholar PubMed
[22] Buscail C, Sabate JM, Bouchoucha M, Torres MJ, Allès B, Hercberg S, et al. Association between self-reported vegetarian diet and the irritable bowel syndrome in the French NutriNet cohort. PLOS ONE. 2017;12(8):e0183039.10.1371/journal.pone.0183039Search in Google Scholar PubMed PubMed Central
[23] Menzel J, Jabakhanji A, Biemann R, Mai K, Abraham K, Weikert C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci Rep. 2020;10(1):21736.10.1038/s41598-020-78426-8Search in Google Scholar PubMed PubMed Central
[24] Chen E, Mahurkar-Joshi S, Liu C, Jaffe N, Labus JS, Dong TS, et al. The association between a Mediterranean diet and symptoms of irritable bowel syndrome. Clin Gastroenterol Hepatol. 2023;22(1):164–72.10.1016/j.cgh.2023.07.012Search in Google Scholar PubMed PubMed Central
[25] Kasti A, Petsis K, Lambrinou S, Katsas K, Nikolaki M, Papanikolaou IS, et al. A combination of Mediterranean and low-FODMAP diets for managing IBS symptoms? Ask your gut! Microorganisms. 2022;10(4):751.10.3390/microorganisms10040751Search in Google Scholar PubMed PubMed Central
[26] Kasti AN, Katsas K, Petsis K, Lambrinou S, Synodinou KD, Kapetani A, et al. Is the Mediterranean low fodmap diet effective in managing irritable bowel syndrome symptoms and gut microbiota? An innovative research protocol. Nutrients. 2024;16(11):1592.10.3390/nu16111592Search in Google Scholar PubMed PubMed Central
[27] Staudacher HM, Mahoney S, Kim Ek Canale, Opie R, Loughman A, So D, et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2023;59(4):492–503.10.1111/apt.17791Search in Google Scholar PubMed
[28] Singh P, Dean G, Iram S, Peng W, Chey SW, Rifkin S, et al. Efficacy of Mediterranean diet vs low‐FODMAP diet in patients with nonconstipated irritable bowel syndrome: a pilot randomized controlled trial. Neurogastroenterol Motil. 2025;e70060.10.1111/nmo.70060Search in Google Scholar PubMed
[29] Haghbin H, Hasan F, Gangwani MK, Zakirkhodjaev N, Lee-Smith W, Beran A, et al. Efficacy of dietary interventions for irritable bowel syndrome: a systematic review and network meta-analysis. J Clin Med. 2024;13(24):7531.10.3390/jcm13247531Search in Google Scholar PubMed PubMed Central
[30] Paukkonen I, Törrönen EN, Lok J, Schwab U, El-Nezami H. The impact of intermittent fasting on gut microbiota: a systematic review of human studies. Front Nutr. 2024;11:1342787.10.3389/fnut.2024.1342787Search in Google Scholar PubMed PubMed Central
[31] Teker HT, Ceylani T. Intermittent fasting supports the balance of the gut microbiota composition. Int Microbiol. 2022;26(1):51–7.10.1007/s10123-022-00272-7Search in Google Scholar PubMed
[32] Veraza DI, Calderon G, Jansson-Knodell C, Aljaras R, Foster ED, Xu H, et al. A systematic review and meta-analysis of diet and nutrient intake in adults with irritable bowel syndrome. Neurogastroenterol Motil. 2023;36(1):e14698.10.1111/nmo.14698Search in Google Scholar PubMed PubMed Central
[33] Williams EA, Nai X, Corfe BM. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterology. 2011;11(1):1–7.10.1186/1471-230X-11-9Search in Google Scholar PubMed PubMed Central
[34] Böhn L, Störsrud S, Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil. 2012;25(1):23.e1.10.1111/nmo.12001Search in Google Scholar PubMed
[35] Staudacher HM, Ralph FSE, Irving PM, Whelan K, Lomer MCE. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. J Acad Nutr Dietetics. 2020;120(4):535–47.10.1016/j.jand.2019.01.017Search in Google Scholar PubMed
[36] Torres MJ, Sabate JM, Bouchoucha M, Buscail C, Hercberg S, Julia C. Food consumption and dietary intakes in 36,448 adults and their association with irritable bowel syndrome: Nutrinet-Santé study. Ther Adv Gastroenterol. 2018;11:1756283X1774662.10.1177/1756283X17746625Search in Google Scholar PubMed PubMed Central
[37] Ligaarden SC, Lydersen S, Farup PG. Diet in subjects with irritable bowel syndrome: A cross-sectional study in the general population. BMC Gastroenterol. 2012;12(1):1–8.10.1186/1471-230X-12-61Search in Google Scholar PubMed PubMed Central
[38] Khayyat Y, Attar S. Vitamin D deficiency in patients with irritable bowel syndrome: does it exist? Oman Med J. 2015;30(2):115–8.10.5001/omj.2015.25Search in Google Scholar PubMed PubMed Central
[39] Nybacka S, Törnblom H, Josefsson A, Hreinsson JP, Böhn L, Frändemark Å, et al. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARBIS): a single-centre, single-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9(6):507–20.10.1016/S2468-1253(24)00045-1Search in Google Scholar PubMed
[40] McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O’Sullivan NA, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Dietetics. 2016;29(5):549–75.10.1111/jhn.12385Search in Google Scholar PubMed
[41] Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs modified NICE guidelines in US adults with IBS-D. Am J Gastroenterology. 2016;111(12):1824–32.10.1038/ajg.2016.434Search in Google Scholar PubMed
[42] Barrett JS. How to institute the low-FODMAP diet. J Gastroenterol Hepatol. 2017;32(S1):8–10.10.1111/jgh.13686Search in Google Scholar PubMed
[43] Sultan N, Varney JE, Halmos EP, Biesiekierski JR, Yao CK, Muir JG, et al. How to implement the 3-phase FODMAP diet into gastroenterological practice. J Neurogastroenterol Motil. 2022;28(3):343–56.10.5056/jnm22035Search in Google Scholar PubMed PubMed Central
[44] Bertin L, Zanconato M, Crepaldi M, Marasco G, Cremon C, Barbara G, et al. The role of the FODMAP diet in IBS. Nutrients. 2024;16(3):370.10.3390/nu16030370Search in Google Scholar PubMed PubMed Central
[45] Tuck C, Barrett J. Re-challenging FODMAPs: the low FODMAP diet phase two. J Gastroenterol Hepatol. 2017;32:11–5.10.1111/jgh.13687Search in Google Scholar PubMed
[46] Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2020;160(1):47–62.10.1053/j.gastro.2020.06.099Search in Google Scholar PubMed
[47] El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J. 2015;14(1):36.10.1186/s12937-015-0022-3Search in Google Scholar PubMed PubMed Central
[48] Haller E, Scarlata K. Diet interventions for irritable bowel syndrome. Gastroenterol Clin North Am. 2021;50(3):565–79.10.1016/j.gtc.2021.03.005Search in Google Scholar PubMed
[49] Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Role of FODMAPs in patients with irritable bowel syndrome. Nutr Clin Pract. 2015;30(5):665–82.10.1177/0884533615569886Search in Google Scholar PubMed
[50] Nybacka S, Störsrud S, Lindqvist HM, Törnblom H, Simrén M, Winkvist A. Habitual FODMAP intake in relation to symptom severity and pattern in patients with irritable bowel syndrome. Nutrients. 2020;13(1):27.10.3390/nu13010027Search in Google Scholar PubMed PubMed Central
[51] Wilson B, Cox SR, Whelan K. Challenges of the low FODMAP diet for managing irritable bowel syndrome and approaches to their minimisation and mitigation. Proc Nutr Soc. 2021;80(1):19–28.10.1017/S0029665120006990Search in Google Scholar PubMed
[52] Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–407.e2.10.1053/j.gastro.2015.07.054Search in Google Scholar PubMed
[53] Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32:16–9.10.1111/jgh.13688Search in Google Scholar PubMed
[54] Bellini M, Tonarelli S, Nagy AG, Pancetti A, Costa F, Ricchiuti A, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12(1):148.10.3390/nu12010148Search in Google Scholar PubMed PubMed Central
[55] O’Keeffe M, Jansen C, Martin L, Williams M, Seamark L, Staudacher HM, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil. 2017;30(1):e13154.10.1111/nmo.13154Search in Google Scholar PubMed
[56] Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real‐world experience. Neurogastroenterol Motil. 2019;32(1):e13730.10.1111/nmo.13730Search in Google Scholar PubMed
[57] Cuffe MS, Staudacher HM, Aziz I, Adame EC, Krieger-Grubel C, Madrid AM, et al. Efficacy of dietary interventions in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2025;10(6):520–36.10.1016/S2468-1253(25)00054-8Search in Google Scholar PubMed
[58] Zeraattalab-Motlagh S, Ranjbar M, Mohammadi H, Adibi P. Nutritional interventions in adult patients with irritable bowel syndrome: an umbrella review of systematic reviews and meta-analyses of randomized clinical trials. Nutr Rev. 2025;83(3):1343–54.10.1093/nutrit/nuae107Search in Google Scholar PubMed
[59] Strużek K, Karamus K, Rejmak R, Biłogras J, Borowska-Łygan M, Urban W, et al. Low-FODMAP diet in the treatment of irritable bowel syndrome – efficacy, comparison with other diets, mechanisms, and clinical applications. J Educ Health Sport. 2025;80:59059.10.12775/JEHS.2025.80.59059Search in Google Scholar
[60] Van den Houte K, Colomier E, Routhiaux K, Mariën Z, Schol J, Van den Bergh J, et al. Efficacy and findings of a blinded randomized reintroduction phase for the low FODMAP diet in irritable bowel syndrome. Gastroenterology. 2024;167(2):333–42.10.1053/j.gastro.2024.02.008Search in Google Scholar PubMed
[61] Alrasheedi AA, Jahlan EA, Bakarman MA. The effect of low-FODMAP diet on patients with irritable bowel syndrome. Sci Rep. 2025;15(1):16382.10.1038/s41598-025-01163-3Search in Google Scholar PubMed PubMed Central
[62] Shin A. Expanding the menu of dietary therapies in irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2025;10(6):493–5.10.1016/S2468-1253(25)00070-6Search in Google Scholar PubMed
[63] Kang SJ. Dietary therapy in irritable bowel syndrome. Korean J Gastroenterol. 2025;85(2):97–104.10.4166/kjg.2025.009Search in Google Scholar PubMed
[64] Silva Siqueira M, Marques Vieira L. Impact of a low-FODMAPs diet on gastrointestinal symptoms of patients diagnosed with irritable bowel syndrome: a systematic review of the literature. DEMETRA: Alimentação Nutrição Saúde. 2024;19:81934.10.12957/demetra.2024.81934Search in Google Scholar
[65] Solarz MB, Piekarska J, Solarz JM, Wawrzyńców JT, Cyrkler W, Robak J, et al. The low FODMAP diet in irritable bowel syndrome: Current state of knowledge and therapeutic perspectives. Qual Sport. 2025;40:59768.10.12775/QS.2025.40.59768Search in Google Scholar
[66] Khan Z, Muhammad SA, Amin MS, Gul A. The efficacy of the low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet in irritable bowel syndrome: a systematic review and meta-analysis. Cureus. 2025;17(1):77053.10.7759/cureus.77053Search in Google Scholar PubMed PubMed Central
[67] Wang J, Yang P, Zhang L, Hou X. A low-FODMAP diet improves the global symptoms and bowel habits of adult IBS patients: a systematic review and meta-analysis. Front Nutr. 2021;8:683191.10.3389/fnut.2021.683191Search in Google Scholar PubMed PubMed Central
[68] van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr. 2021;60(6):3505–22.10.1007/s00394-020-02473-0Search in Google Scholar PubMed PubMed Central
[69] Khoo XH, Chong CW, Talha AM, Philip K, Teh CS, Isa AM, et al. The impact of diet and ethnicity on gut microbiota variation in irritable bowel syndrome: A multi‐center study. J Gastroenterol Hepatol. 2023;38(8):1259–68.10.1111/jgh.16174Search in Google Scholar PubMed
[70] Ingvarsdottir IE, Engilbertsdottir S, Halldorsson TI, Bjornsson ES, Gunnarsdottir I. Gastrointestinal symptoms and dietary intake of patients with irritable bowel syndrome following a low FODMAP diet. Laeknabladid. 2024;110(6):298–306.Search in Google Scholar
[71] Hattori Y, Takama M, Izumi C, Hattori M, Tsukahara T. Effectiveness of a low-FODMAP diet intervention for patients with IBS. Can J Diet Pract Res. 2024;85(3):240.Search in Google Scholar
[72] Hillestad EMR, Steinsvik EK, Teige ES, Rasmussen SH, Brønstad I, Lundervold A, et al. Nutritional safety and status following a 12‐week strict low FODMAP diet in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2024;36(7):14814.10.1111/nmo.14814Search in Google Scholar PubMed
[73] Hahn J, Choi J, Chang MJ. Effect of low FODMAPs diet on irritable bowel syndromes: a systematic review and meta-analysis of clinical trials. Nutrients. 2021;13(7):2460.10.3390/nu13072460Search in Google Scholar PubMed PubMed Central
[74] Manning LP, Tuck CJ, Van Den Houte M, Van Oudenhove L, Biesiekierski JR. Nutritional implications of a three-phase low fermentable oligosaccharide, disaccharide, monosaccharide and polyol diet in irritable bowel syndrome. Proc Nutr Soc. 2025;84:E89.10.1017/S0029665125000990Search in Google Scholar
[75] Chu P, He Y, Hu F, Wang X. The effects of low FODMAP diet on gut microbiota regulation: A systematic review and meta‐analysis. J Food Sci. 2025;90(3):70072.10.1111/1750-3841.70072Search in Google Scholar PubMed
[76] Zhang H, Su Q. Low-FODMAP diet for irritable bowel syndrome: insights from microbiome. Nutrients. 2025;17(3):544.10.3390/nu17030544Search in Google Scholar PubMed PubMed Central
[77] Staudacher HM, Lomer MCE, Anderson JL, Barrett JS, Muir JG, Irving PM, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–8.10.3945/jn.112.159285Search in Google Scholar PubMed
[78] Singh P, Chey SW, Nee J, Eswaran S, Lembo A, Chey WD. Is a simplified, less restrictive low FODMAP diet possible? Results from a double-blind, pilot randomized controlled trial. Clin Gastroenterol Hepatol. 2024;23(2):362–4.10.1016/j.cgh.2024.04.021Search in Google Scholar PubMed
[79] Howley T, Cooney L, Doyle SL. Reframing the management of irritable bowel syndrome: investigating the efficacy of a modified low fodmap diet approach on symptom response. Sci Undergrad Res Exper J. 2024;6(1):7.Search in Google Scholar
[80] Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA. 2015;313(9):949.10.1001/jama.2015.0954Search in Google Scholar PubMed
[81] Hadjivasilis A. New insights into irritable bowel syndrome: from pathophysiology to treatment. Ann Gastroenterol. 2019;32(6):554–64.10.20524/aog.2019.0428Search in Google Scholar PubMed PubMed Central
[82] Paduano D, Cingolani A, Tanda E, Usai P. Effect of three diets (low-FODMAP, gluten-free and balanced) on irritable bowel syndrome symptoms and health-related quality of life. Nutrients. 2019;11(7):1566.10.3390/nu11071566Search in Google Scholar PubMed PubMed Central
[83] Pinto-Sanchez MI, Nardelli A, Borojevic R, Palma GD, Calo NC, McCarville J, et al. Gluten-free diet reduces symptoms, particularly Diarrhea, in patients with irritable bowel syndrome and antigliadin IgG. Clin Gastroenterol Hepatol. 2020;19(11):2343–52.10.1016/j.cgh.2020.08.040Search in Google Scholar PubMed
[84] Shahbazkhani B, Sadeghi A, Malekzadeh R, Khatavi F, Etemadi M, Kalantri E, et al. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: a double-blind randomized placebo-controlled trial. Nutrients. 2015;7(6):4542–54.10.3390/nu7064542Search in Google Scholar PubMed PubMed Central
[85] Zanwar VG, Pawar SV, Gambhire PA, Jain SS, Surude RG, Shah VB, et al. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intestinal Res. 2016;14(4):343.10.5217/ir.2016.14.4.343Search in Google Scholar PubMed PubMed Central
[86] Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320–8.e3.10.1053/j.gastro.2013.04.051Search in Google Scholar PubMed
[87] Vincenzi M, Del Ciondolo I, Pasquini E, Gennai K, Paolini B. Effects of a low FODMAP diet and specific carbohydrate diet on symptoms and nutritional adequacy of patients with irritable bowel syndrome: Preliminary results of a single-blinded randomized trial. J Transl Intern Med. 2017;5(2):120–6.10.1515/jtim-2017-0004Search in Google Scholar PubMed PubMed Central
[88] Roth B, Nseir M, Jeppsson H, D’Amato M, Sundquist K, Ohlsson B. A starch- and sucrose-reduced diet has similar efficiency as low FODMAP in IBS – A randomized non-inferiority study. Nutrients. 2024;16(17):3039.10.3390/nu16173039Search in Google Scholar PubMed PubMed Central
[89] Cancarevic I, Rehman M, Iskander B, Lalani S, Malik BH. Is there a correlation between irritable bowel syndrome and lactose intolerance? Cureus. 2020;12(1):e6710.10.7759/cureus.6710Search in Google Scholar PubMed PubMed Central
[90] Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106(10):1631–9.10.1016/j.jada.2006.07.010Search in Google Scholar PubMed
[91] Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109(7):1204–14.10.1016/j.jada.2009.04.012Search in Google Scholar PubMed
[92] Rej A, Sanders DS, Shaw CC, Buckle R, Trott N, Agrawal A, et al. Efficacy and acceptability of dietary therapies in non-constipated irritable bowel syndrome: a randomized trial of traditional dietary advice, the low FODMAP diet and the gluten-free diet. Clin Gastroenterol Hepatol. 2022;20(12):2876–87.10.1016/j.cgh.2022.02.045Search in Google Scholar PubMed
[93] Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. J Gastroenterol Hepatol. 2018;33(6):1192–9.10.1111/jgh.14051Search in Google Scholar PubMed
[94] O'Brien L, Kasti A, Halmos EP, Tuck C, Varney J. Evolution, adaptation, and new applications of the FODMAP diet. JGH Open. 2024;8(5):e13066.10.1002/jgh3.13066Search in Google Scholar PubMed PubMed Central
[95] Black CJ, Ford AC. Personalisation of therapy in irritable bowel syndrome: a hypothesis. Lancet Gastroenterol Hepatol. 2024 Dec;9(12):1162–76.10.1016/S2468-1253(24)00245-0Search in Google Scholar PubMed
[96] Connell J, Toma R, Cleo Hanchen Ho, Shen N, Moura P, Le T, et al. Data-driven precision nutrition improves clinical outcomes and risk scores for IBS, depression, anxiety, and T2D. Am J Lifestyle Med. 2023;15598276231216393.10.1177/15598276231216393Search in Google Scholar
[97] Jactel SN, Olson JM, Wolin KY, Brown J, Pathipati MP, Jagiella VJ, et al. Efficacy of a digital personalized elimination diet for the self-management of irritable bowel syndrome and comorbid irritable bowel syndrome and inflammatory bowel disease. Clin Transl Gastroenterol. 2023;14(1):e00545.10.14309/ctg.0000000000000545Search in Google Scholar PubMed PubMed Central
[98] Zarini G, McLean M. S572 A pilot study for precision nutrition in irritable bowel syndrome: in-vitro stimulation of pro-and anti-inflammatory cytokine release. Am J Gastroenterol. 2022;117(10S):e406.10.14309/01.ajg.0000858928.65055.a2Search in Google Scholar
[99] Kasti AN, Katsas K, Pavlidis DE, Stylianakis E, Petsis KI, Lambrinou S, et al. Clinical Trial: A mediterranean low-FODMAP diet alleviates symptoms of non-constipation IBS – randomized controlled study and volatomics analysis. Nutrients. 2025;17(9):1545.10.3390/nu17091545Search in Google Scholar PubMed PubMed Central
[100] Schnedl WJ, Enko D. Considering histamine in functional gastrointestinal disorders. Crit Rev Food Sci Nutr. 2020;61(17):2960–7.10.1080/10408398.2020.1791049Search in Google Scholar PubMed
[101] Mohamed Siraj H, Usaid M, Shaikh S, Dalain M, Ansari Y, Zehra Naqvi S, et al. Bacterial histamine as a therapeutic target for abdominal pain in irritable bowel syndrome: a literature review. Cureus. 2025;17(4):e82132.10.7759/cureus.82132Search in Google Scholar PubMed PubMed Central
[102] Fabisiak A, Włodarczyk J, Fabisiak N, Storr M, Fichna J. Targeting histamine receptors in irritable bowel syndrome: a critical appraisal. J Neurogastroenterol Motil. 2017;23(3):341–8.10.5056/jnm16203Search in Google Scholar PubMed PubMed Central
[103] Shulpekova YO, Nechaev VM, Popova IR, Deeva TA, Kopylov AT, Malsagova KA, et al. Food intolerance: The role of histamine. Nutrients. 2021;13(9):3207.10.3390/nu13093207Search in Google Scholar PubMed PubMed Central
[104] Krammer L, Sowa AS, Lorentz A. Mast cells in irritable bowel syndrome: a systematic review. J Gastrointest Liver Dis. 2019;28(4):463–72.10.15403/jgld-229Search in Google Scholar PubMed
[105] Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, Latorre-Moratalla M, Vidal-Carou MD. Histamine intolerance: the current state of the art. Biomolecules. 2020;10(8):1181.10.3390/biom10081181Search in Google Scholar PubMed PubMed Central
[106] Orlando A, Chimienti G, Notarnicola M, Russo F. The ketogenic diet improves gut–brain axis in a rat model of irritable bowel syndrome: impact on 5-HT and BDNF systems. Int J Mol Sci. 2022;23(3):1098.10.3390/ijms23031098Search in Google Scholar PubMed PubMed Central
[107] Reddel S, Putignani L, Del Chierico F. The impact of low-FODMAPs, gluten-free, and ketogenic diets on gut microbiota modulation in pathological conditions. Nutrients. 2019;11(2):373.10.3390/nu11020373Search in Google Scholar PubMed PubMed Central
[108] Santangelo A, Corsello A, Immacolata C, Chiara Maria Trovato, Agostoni C, Orsini A, et al. The influence of ketogenic diet on gut microbiota: potential benefits, risks and indications. Nutrients. 2023;15(17):3680.10.3390/nu15173680Search in Google Scholar PubMed PubMed Central
[109] Shalan HT, Fathy RA, Bekheet EA. Effect of ketogenic diet on colonic changes of stress induced irritable bowel syndrome of adult male albino rat model (histological and morphometric study). Ain Shams Med J. 2024;75(3):619–36.10.21608/asmj.2024.311338.1295Search in Google Scholar
[110] Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, et al. A very low-carbohydrate diet improves symptoms and quality of life in Diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7(6):706–8.e1.10.1016/j.cgh.2009.02.023Search in Google Scholar PubMed PubMed Central
[111] Hujoel IA. Nutritional status in irritable bowel syndrome: A North American population‐based study. JGH Open. 2020;4(4):656–62.10.1002/jgh3.12311Search in Google Scholar PubMed PubMed Central
[112] Malhotra R, Olver J. Dietary fiber assessment of patients with irritable bowel syndrome from Northern India. Indian J Gastroenterol. 2004;23(6):217–8.Search in Google Scholar
[113] Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60(5):667–72.10.1038/sj.ejcn.1602367Search in Google Scholar PubMed
[114] McCoubrey H, Parkes GC, Sanderson JD, Lomer MCE. Nutritional intakes in irritable bowel syndrome. J Hum Nutr Diet. 2008;21(4):396–7.10.1111/j.1365-277X.2008.00881_32.xSearch in Google Scholar
[115] Singh N, Makharia GK, Joshi YK. Dietary survey and total dietary fiber intake in patients with irritable bowel syndrome attending a tertiary referral hospital. Indian J Gastroenterol. 2008;27(2):66–70.Search in Google Scholar
[116] Prescha A, Pieczynska J, Ilow R, Poreba J, Neubauer K, Smereka A, et al. Assessment of dietary intake of patients with irritable bowel syndrome. Rocz Panstw Zakl Hig. 2009;60(2):185–9.Search in Google Scholar
[117] Østgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5(6):1382–90.Search in Google Scholar
[118] Omagari K, Murayama T, Tanaka Y, Yoshikawa C, Inoue SI, Ichimura M, et al. Mental, physical, dietary, and nutritional effects on irritable bowel syndrome in young Japanese women. Intern Med. 2013;52(12):1295–301.10.2169/internalmedicine.52.0248Search in Google Scholar PubMed
[119] Tigchelaar EF, Mujagic Z, Zhernakova A, Hesselink MA, Meijboom S, Perenboom CW, et al. Habitual diet and diet quality in Irritable Bowel Syndrome: A case-control study. Neurogastroenterol Motil. 2017;29(12):e13151.10.1111/nmo.13151Search in Google Scholar PubMed
[120] Alharbi SH, Alateeq FA, Alshammari KI, Alshammri ASS, Alabdali NAN, Alsulaiman MAS, et al. Irritable bowel syndrome and dietary habits in Northern Saudi Arabia. Health. 2019;11(3):289–97.10.4236/health.2019.113025Search in Google Scholar
[121] Nilholm C, Larsson E, Roth B, Gustafsson R, Ohlsson B. Irregular dietary habits with a high intake of cereals and sweets are associated with more severe gastrointestinal symptoms in IBS patients. Nutrients. 2019;11(6):1279.10.3390/nu11061279Search in Google Scholar PubMed PubMed Central
[122] Yilmaz B, Akbulut G. The evaluation of the nutritional status in patients with irritable bowel syndrome. Clin Exp Health Sci. 2021;11(1):119–26.10.33808/clinexphealthsci.646176Search in Google Scholar
[123] Shafiee NH, Razalli NH, Mokhtar NM, Tan E, Ali RAR. An evaluation of dietary adequacy among patients with constipation-predominant irritable bowel syndrome in Malaysia. Intestinal Res. 2021;20(1);124–33.10.5217/ir.2020.00050Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Relationship between body mass index and quality of life, use of dietary and physical activity self-management strategies, and mental health in individuals with polycystic ovary syndrome
- Evaluating the challenges and opportunities for diabetes care policy in Nigeria
- Body mass index is associated with subjective workload and REM sleep timing in young healthy adults
- Prediction of hypoglycaemia in subjects with type 1 diabetes during physical activity
- Investigation by the Epworth Sleepiness Scale of daytime sleepiness in professional drivers during work hours
- Understanding public awareness of fall epidemiology in the United States: A national cross-sectional study
- Impact of Covid-19 stress on urban poor in Sylhet Division, Bangladesh: A perception-based assessment
- Impact of the COVID-19 pandemic on mental health, relationship satisfaction, and socioeconomic status: United States
- Psychological factors influencing oocyte donation: A study of Indian donors
- Cervical cancer in eastern Kenya (2018–2020): Impact of awareness and risk perception on screening practices
- Older LGBTQ+ and blockchain in healthcare: A value sensitive design perspective
- Trends and disparities in HPV vaccination among U.S. adolescents, 2018–2023
- Do cell towers help increase vaccine uptake? Evidence from Côte d’Ivoire
- In search of the world’s most popular painkiller: An infodemiological analysis of Google Trend statistics from 2004 to 2023
- Brain fog in chronic pain: A concept analysis of social media postings
- Association between multidimensional poverty intensity and maternal mortality ratio in Madagascar: Analysis of regional disparities
- A “disorder that exacerbates all other crises” or “a word we use to shut you up”? A critical policy analysis of NGOs’ discourses on COVID-19 misinformation
- Smartphone use and stroop performance in a university workforce: A survey-experiment
- Review Articles
- The management of body dysmorphic disorder in adolescents: A systematic literature review
- Navigating challenges and maximizing potential: Handling complications and constraints in minimally invasive surgery
- Examining the scarcity of oncology healthcare providers in cancer management: A case study of the Eastern Cape Province, South Africa
- Dietary strategies for irritable bowel syndrome: A narrative review of effectiveness, emerging dietary trends, and global variability
- The impact of intimate partner violence on victims’ work, health, and wellbeing in OECD countries (2014–2025): A descriptive systematic review
- Nutrition literacy in pregnant women: a systematic review
- Short Communications
- Experience of patients in Germany with the post-COVID-19 vaccination syndrome
- Five linguistic misrepresentations of Huntington’s disease
- Letter to the Editor
- PCOS self-management challenges transcend BMI: A call for equitable support strategies
Articles in the same Issue
- Research Articles
- Relationship between body mass index and quality of life, use of dietary and physical activity self-management strategies, and mental health in individuals with polycystic ovary syndrome
- Evaluating the challenges and opportunities for diabetes care policy in Nigeria
- Body mass index is associated with subjective workload and REM sleep timing in young healthy adults
- Prediction of hypoglycaemia in subjects with type 1 diabetes during physical activity
- Investigation by the Epworth Sleepiness Scale of daytime sleepiness in professional drivers during work hours
- Understanding public awareness of fall epidemiology in the United States: A national cross-sectional study
- Impact of Covid-19 stress on urban poor in Sylhet Division, Bangladesh: A perception-based assessment
- Impact of the COVID-19 pandemic on mental health, relationship satisfaction, and socioeconomic status: United States
- Psychological factors influencing oocyte donation: A study of Indian donors
- Cervical cancer in eastern Kenya (2018–2020): Impact of awareness and risk perception on screening practices
- Older LGBTQ+ and blockchain in healthcare: A value sensitive design perspective
- Trends and disparities in HPV vaccination among U.S. adolescents, 2018–2023
- Do cell towers help increase vaccine uptake? Evidence from Côte d’Ivoire
- In search of the world’s most popular painkiller: An infodemiological analysis of Google Trend statistics from 2004 to 2023
- Brain fog in chronic pain: A concept analysis of social media postings
- Association between multidimensional poverty intensity and maternal mortality ratio in Madagascar: Analysis of regional disparities
- A “disorder that exacerbates all other crises” or “a word we use to shut you up”? A critical policy analysis of NGOs’ discourses on COVID-19 misinformation
- Smartphone use and stroop performance in a university workforce: A survey-experiment
- Review Articles
- The management of body dysmorphic disorder in adolescents: A systematic literature review
- Navigating challenges and maximizing potential: Handling complications and constraints in minimally invasive surgery
- Examining the scarcity of oncology healthcare providers in cancer management: A case study of the Eastern Cape Province, South Africa
- Dietary strategies for irritable bowel syndrome: A narrative review of effectiveness, emerging dietary trends, and global variability
- The impact of intimate partner violence on victims’ work, health, and wellbeing in OECD countries (2014–2025): A descriptive systematic review
- Nutrition literacy in pregnant women: a systematic review
- Short Communications
- Experience of patients in Germany with the post-COVID-19 vaccination syndrome
- Five linguistic misrepresentations of Huntington’s disease
- Letter to the Editor
- PCOS self-management challenges transcend BMI: A call for equitable support strategies


