Abstract
For more than two decades, peripheral administration of GLP-1 or GLP-1 receptor (GLP-1R) agonist (GLP-1RA) curbs appetite and reduce body weight gain in animal models. More importantly, the body weight lowering effect has been effectively observed in clinical practice. There is no doubt that the target sites for GLP-1 or GLP-1RAs to exert those functions are located in the brain. It, however, remains controversy on exactly how these drugs access their targets in the brain. Here, we have discussed literatures on whether peripheral GLP-1 or GLP-1RAs enters the brain via crossing the blood-brain barrier, or the blood cerebrospinal fluid barrier, or given circumventricular organs. We have then commented the view or opinion that peripheral GLP-1RAs may exert their brain functions via organ-organ communications without entering the brain.
Introduction
Incretins are defined as gut-produced hormones that facilitate postprandial insulin secretion from pancreatic islet β-cells. [1, 2, 3, 4, 5, 6, 7, 8] Such facilitation is tightly controlled in glucose concentration dependent manners, without causing hypoglycemia. The gut in mammalian species is known to produce two types of incretins. The first one is gastric inhibitory polypeptide (GIP) produced by gut endocrine K cells (also known as glucose-dependent insulinotropic polypeptide), mostly found in the duodenum. The second one is glucagon-like peptide-1 (GLP-1) produced by gut endocrine L cells, mostly found in the distal ileum and colon.[1,2,9, 10, 11, 12, 13]
GIP, as the first incretin hormone, was identified by the Canadian scientist John Brown in University of British Columbia (UBC) and his colleagues in UBC and elsewhere back to later 1960 s and early 1970 s.[14, 15, 16, 17, 18, 19, 20] For detailed information on the serendipity discovery of GIP and its functional studies, please see nice review articles and other publications elsewhere,[21, 22, 23] including the “In Memoriam” composed by Timothy Kieffer (a formal PhD student of late professor John Brown) in 2017.[24] We have yet seen the final success on developing GIP-based pharmaceutical agents for diabetes or other metabolic disorders. Mechanistic functional studies on GIP and its G-protein coupled receptor, GIPR. However, they have contributed to the development of dipeptidyl peptidase-4 (DPP-4) inhibitor Sitagliptin (commercially known as Januvia®) as a diabetes drug by Merck & Co. in 2006.[25,26] More recently, the dual GIP/GLP-1 receptor agonist Tirzepatide, has been shown to be more potent than a pure GLP-1 receptor agonist (GLP-1RA) in clinical trials on reducing body weight.[27] Currently, Tirzepatide is sold under the brand name Mounjaro for type 2 diabetes (T2D) treatment and Zepbound for weight management and the treatment of obstructive sleep apnea.
In the late 1980 s, studies conducted by Habener and his colleagues at Harvard University [28, 29, 30] and by Holst and his colleagues at University of Copenhagen,[31, 32, 33] demonstrated that GLP-1 is the 2nd incretin hormone. Since then, efforts made by scientific researchers across multiple disciplines have led to the development of various GLP-1-based drugs or GLP-1RAs as therapeutic agents for T2D.
Active GLP-1 molecules include GLP-17–37 and GLP-17–36 amide.[28, 29, 30, 31, 32, 33] They cannot serve as therapeutic agents per se, as their half-lives are only approximately 1.5–2.0 min in the circulation. The rapid degradation of the native GLP-1 molecules is mainly attributed to ubiquitously expressed endogenous enzymes known as DPP-4, which converts active GLP-17–37 and GLP-17–36 amide into inactive GLP-19–37 and GLP-19–36 amide. Another enzyme namely neutral endopeptidase 24.11 (NEP 24.11) can further cleave GLP-19–37 and GLP-19–36 amide into even smaller peptides.[34, 35, 36] Paradoxically, certain smaller peptides, such as GLP-128–36 amide and GLP-132–36 amide were shown to possess certain metabolic beneficial effect.[34, 35, 36, 37, 38, 39]
Exendin-4 (Ex-4) is a polypeptide with 39 amino acid residues, isolated from the venom of the Gila monster (Heloderma suspectum).[40] Ex-4 shares 53% of its amino acid residue identity with mammalian GLP-1, while its half-life is much longer (∼10 min) due to its resistance against the enzymatic cleavage mediated by DPP-4. In 1992, Eng’s team demonstrated the incretin feature of Ex-4 in both rat and guinea pig models.[41,42] In 1993, Goke et al. (in Eng’s team) further demonstrated that Ex-4 is a potent agonist of GLP-1R.[43] Truncated Ex-4 known as Exendin (9–39) serves as a powerful antagonist of GLP-1R, which has been broadly utilized in functional studies of GLP-1/GLP-1R signaling cascade in the brain and elsewhere. GLP-1R is also a G-protein-coupled receptor. Both protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac) have been shown to mediate functions of GLP-1/GLP-1R signaling activation in pancreatic islets and elsewhere.[7,44, 45, 46]
A synthetic version of Ex-4, namely Exenatide, developed by Amylin Pharmaceuticals and Eli Lilly and Co., was the first GLP-1-based therapeutic agent for T2D. It was approved by the Food and Drug Administration (FDA) in 2005, with the brand name Byetta®. Over the past two decades, nearly a dozen chemically modified versions of human GLP-1 have been developed as therapeutic agents, including Liraglutide (Victoza®) and Semaglutide (Ozempic®), the products of Novo Nordisk Inc.
Extensive investigations over the past three decades have shown that GLP-1 and GLP-1RAs exert their metabolic and other beneficial functions far beyond serving as an incretin hormone. In other word, functions of GLP-1 do not entirely dependent on insulin. This explains why GLP-1RAs are effective in T2D patients with insulin resistance.[9] Indeed, GLP- 1 is a pluripotent hormone, targeting various organs including the brain, gut, heart, liver, lung, and adipose tissues. For extra-pancreatic functions of GLP-1 and its based diabetes drugs, please see nice review and other publications elsewhere.[2,4,6,47, 48, 49, 50, 51, 52, 53, 54, 55]
It is also worth mentioning that GLP-1 based research has been drawing extensive global attention. Over the past five years, top scientists in this field, including Joel F. Habener, Jens J. Holst, Daniel J. Drucker, Svetlana Mojsov, and Lotte B. Knudsen, have been recognized by a battery of prestigious international awards, commented recently by our team and by others.[5,56, 57, 58, 59, 60] The top awards include Warren Alpert Foundation Prize, Canada Gairdner International Award, Wolf Prize in Medicine, Tang Prize in Medicine, Lasker in Clinical Research, and most recently, the Breakthrough Prize in Life Sciences Award.
GLP-1 based drugs also promote weight loss
Obesity and overweight may lead to the development of various metabolic diseases including T2D, heart and cardiovascular disorders, stroke, metabolic associated fatty liver disease (MAFLD), infectious diseases, and others.[61, 62, 63, 64, 65] Based on World Health Organization (WHO), worldwide adult obesity has more than doubled since 1990, while adolescent obesity has quadrupled. In 2022, 43% of adults in the world were overweight and 16% of our world population were living with obesity. We have also seen even astonishing elevation on children and adolescents overweight and obesity. These changes have not only increased the economic burdens globally but also worsened the overall quality of our lives. Hence, brain function of GLP-1 and its based drugs has been drawing significant global attention.
Brain function of GLP-1 was initially demonstrated by Bloom’s team in Imperial College London back to 1996 in a rat model with the brain 3rd ventricle native GLP-1 injection,[66,67] followed by intensive investigations in North America, Asian countries, European nations, and elsewhere. For further updated knowledge on the anorexic function and neuroprotective effects of GLP-1 and GLP-1RAs in animal models and in clinical studies, please see excellent review articles elsewhere.[51,68, 69, 70, 71, 72]
Liraglutide (Saxenda®) is the first GLP-1RA therapeutic agent being approved by FDA for adults and adolescents (ages ≥ 12) with obesity or overweight and comorbidities. In a 56-week, randomized, placebo-controlled trial (SCALE trial), 3.0 mg of daily Liraglutide shown mean body weight loss of 8.0%.[73] In 2021, the second GLP-1RA, Semaglutide (Wegovy®) became approved by FDA for the treatment of obesity or overweight. Superior efficacy of Semaglutide (Wegovy®) was demonstrated in STEP 1 trial, whereas mean body weight lost nearly achieved 15% over 68-week trial by giving 2.4 mg once per week.[74] It’s interesting to note that Semaglutide (Ozempic®) has been approved for T2D and cardiovascular risk reduction but not yet for weight loss application. The dual GIP/GLP-1 receptor agonist Tirzepatide (Zepbound®) was approved in 2023 and is the most effective GLP-1RA to date for body weight reduction in individuals with obesity or overweight.[75] When compared with Semaglutide, Tirzepatide treatment results 5% or greater weight loss with similar extent of gastrointestinal adverse events.[76] Several clinical trials on additional GLP-1-based agents including triple agonists (Retatrutide),[77] oral formation (Orforglipron),[78] and combination therapy (CagriSema) are in the late phase of trials.
The strong body weight lowering effect of above GLP-1RAs, as well as the dual GIP/GLP-1 receptor agonist Tirzepatide, expanded the application of incretin-based therapeutics into the novel avenues including the anti-obesity. Even with the profound body weight lowering effects seen in preclinical studies and clinical trials, it is still puzzling to many researchers and clinicians on the mechanism of action of those GLP-1-based therapeutics. There is no doubt that target sites for GLP-1 or GLP-1RAs to exert their anti-obesity function are located with the brain. It, however, remains to be determined exactly how gut produced GLP-1 or peripherally administrated GLP-1RAs enter the brain to exert their anorectic and other beneficial functions. Alternatively, can gut produced native GLP-1 or peripherally administrated GLP-1RAs as therapeutic agents exert their brain function without physically entering the brain, but via other means. An alternative mean is via the vagal afferent neuron mediated organ-organ communication. Here we will discuss some key literatures on addressing the above questions during the past two decades, including the highlight of controversies in this active field. We have then composed a summary, along with presenting our perspective view.
Barriers between the brain and the peripheral circulation system
There are three barriers between the brain and the peripheral circulation system, known as the blood brain barrier (BBB), the blood-cerebrospinal fluid (CSF) barrier defined here as BCB, and the brain circumventricular organs (CVOs) (Figure 1).[79] We have presented a brief introduction on each of them in below.
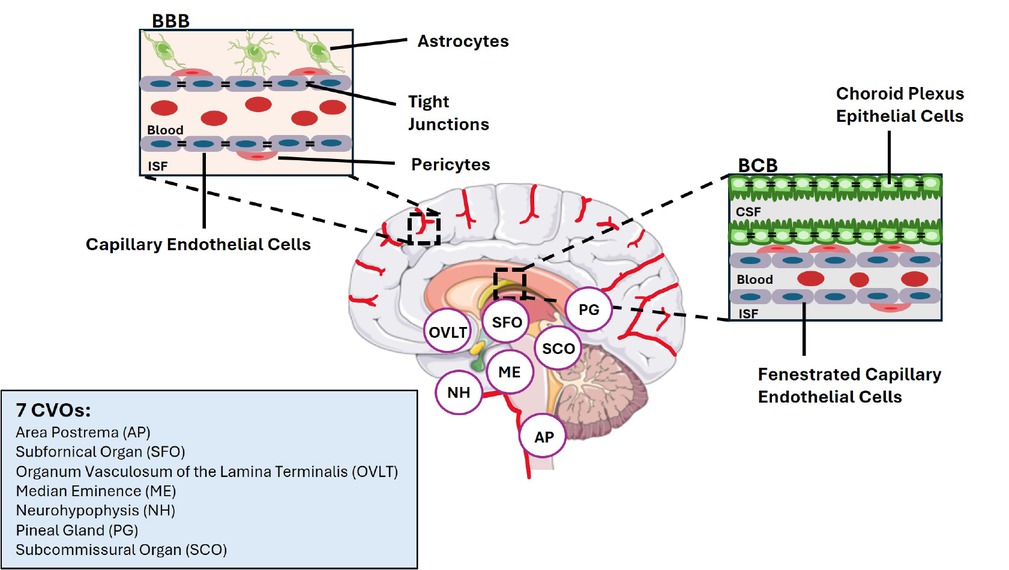
Structures of blood-brain barrier and blood cerebrospinal fluid, and locations of the seven circumventricular organs in the brain. BBB: blood-brain barrier; BCB: blood cerebrospinal fluid (CSF) barrier (BCB); CVOs: circumventricular organs; ISF: interstitial fluid.
In mammals, BBB is a semipermeable border of endothelial cells that control the transfer of chemicals or solutes between the peripheral circulatory system and the central nervous system. Such a barrier effectively protects the brain from damages that can be induced by harmful substances in the peripheral circulation. In normal physiological conditions, BBB passage of circulating molecules can be achieved via either the paracellular route or via the transcellular route. The passive paracellular route only allows the passage of molecules that are smaller than 500 Da. Native GLP-1 and Liraglutide are, however, 3298.7 Da and 3751.2 Da, respectively. The long-term effective GLP-1RA Semaglutide is 4113.6 Da. The transcellular route refers to either transporter- or receptor-medicated access that across the BBB. A few early studies have suggested that peripheral leptin administration may across BBB via such transcellular mechanism,[80, 81, 82] raising the possibility that GLP-1 may also enter the brain utilizing a similar mechanism.
Another way for circulating substance to enter the brain is via BCB. By crossing BCB, the circulating substance can enter CSF. We have learned that the choroid plexus (CP) of BCB displays fundamentally different properties from that of BBB.[83] With relatively high permeable capillaries, CP provides the central nerves system (CNS) with a high turnover rate of fluid that contain micronutrients, peptides, and hormones for the neuronal networks. It has also been reported that peripherally produced leptin can bind to leptin receptors that are expressed in ependymal cells, crossing the ependymal barrier.[84] If GLP-1 or GLP-1RAs can enter the brain via BCB, one would anticipate that GLP-1RA level in CSF would increase following a long-term GLP-1RA treatment.
Additional sites for circulating molecules to enter the brain are through various CVOs, bordering the 3rd and 4th ventricles of the brain.[85] Those structures in the brain are characterized by highly permeable capillaries, unlike those in the rest of the brain area where BBB is present at the capillary level. Although the term “CVO” was proposed in 1958 by Dr. Helmut O. Hofer, the penetration of blood-borne dyes into small specific CVO regions was demonstrated in the early 20th century. The permeable CVOs enabling the rapid neurohumoral exchange include the subfornical organ (SFO), the area postrema (AP), the vascular organ of lamina terminalis (VOLT), the median eminence (ME), neurohypophysis (NH), the pineal gland (PG), and subcommissural organ (SCO). Figure 1 illustrates the structures of BBB, BCB and the locations of the seven CVOs, along with their abbreviations. For detailed functions of these CVOs, please see review articles elsewhere.[86,87]
Can native gut produced GLP-1 and peripherally administrated GLP-1RAs access the brain via BBB
Back to 2002, Kastin and colleagues have assessed the influx of radioisotope labelled [Ser8] GLP-1 into the brain following its peripheral administration [i.e., intravenous (iv) injection).[88] [Ser8] GLP-1 was demonstrated to possess similar biological effects when compared with the native GLP-1 but has greater stability.[88] The authors reported that they have detected a rapid influx with the multiple-time regression analysis when 125I-[Ser8] GLP-1 was peripherally administrated.[88] Furthermore, there was no self inhibition by excess doses of the unlabeled [Ser8] GLP-1 either via iv injection or via in situ brain perfusion. In addition, the authors reported that they observed no inhibition of influx by the GLP-1R antagonist Exendin (9–39). Based on the lack of inhibition of the influx of radiolabelled GLP-1 with unlabelled GLP-1R antagonist, the authors made their suggestion that the rapid entry of those radiolabelled GLP-1 into the brain does not require the participation of GLP-1R.[88] Similar investigations was then performed with the GLP-1RA Exenatide by the same research group.[89] Brain access of relatively larger sized GLP-1RAs (human origin GLP-1 with structural modifications) such as Liraglutide has also been assessed recently, utilizing the similar radioisotope labeling approaches.[90, 91, 92, 93, 94]
Although Kastin and colleagues have suggested that GLP-1 and Ex-4 can rapidly access the brain parenchyma in a receptor-independent manner,[88,89] their suggestion is against the classical dogma that BBB only allows its paracellular access of molecules smaller than 500 Da, regardless of receptor presence.
In 2020, Fu and colleagues presented their investigation, showing a set of totally different observations along with their different conclusions.[95] They have iv injected fluorescence (FAM) labeled-GLP-1 or Ex-4 into the rats, followed by the detection of their appearance as well as PKA activity in various brain regions. They have also demonstrated that rat brain microvascular endothelial cells can rapidly uptake fluorescence-labeled GLP-1, the event can be blunted with the utilization of the GLP-1R antagonist Exendin (9–39). Hence, they concluded that GLP-1 crosses the BBB through active trans-endothelial transport, which requires the GLP-1R. This observation is somehow like brain access of peripheral leptin, via a receptor mediated transcellular mechanism, as we have mentioned above.[80, 81, 82] This conclusion, however, is not supported by recent studies, indicating that hypothalamic endothelial cells do not express GLP-1R,[96, 97, 98] including one review article discussed by Buller and Blouet.[99]
In one of the above investigations led by Imbernon and Saponaro et al., the team utilized fluorescently labeled Liraglutide and reported that endothelial cells of BBB do not express GLP-1R. This was further supported by employing in vitro BBB models and radiolabeled compounds 125I-GLP1 and 125I-Liraglutide. Instead, they showed that Liraglutide enters the ME (one of seven CVOs) via tanycyte-mediated transcytosis as early as 60 seconds following iv injection. Inhibiting tanycytic transcytosis by botulinum toxin or selectively knock down of Glp1r expression in tanycytes by AAV-mediated shRNA delivery indicated the loss of Liraglutide transport into the brain. This impaired transport also led to the diminished metabolic benefits mediated by Liraglutide.[96] The other two studies failed in the detection of GLP-1R immunoreactivity on brain endothelial cells using a highly selective GLP-1R antibody with confocal or electron microscopy.[97,98] However, the contrary opinion still holds. Smith and colleagues mated the Glp 1r-Cre mouse line with the ROSA26EYFP transgenic mouse line. They hence introduced the fluorescent reporter into GLP-1R expressing cells. Brain GLP-1R+ cells were then FACS-purified and sequenced using single-cell RNA sequencing. They were able to provide the first profile of GLP-1R expression cells in the brain and further showed the detection of a population of arterial endothelial GLP-1R+ cells.[100]
In addition to the above controversies presented in PubMed by different research groups, a fundamental question that remains to be addressed is: although GLP-1 or GLP-1RAs may get access to the brain via a rapid and passive receptor-independent mechanism,[88,89] or by a receptor-mediated event,[95] does such access physiologically and pharmacologically relevant to their brain functions? It is also worth noting that the controversy generated could be because the detection method with fluorescence or radioisotope labelling being over-sensitive. Should such over-sensitive methods generate false positive results, technology advancement is required for our future investigations.
Can gut produced native GLP-1 or peripherally administrated GLP-1RAs access the brain via BCB
A few studies have shown that fluorescence labeled GLP-1RAs can interact with the choroid plexus, as well as certain CVOs.[97,98,101,102] However, clear labeling was virtually absent in mouse ependymal cells of the choroid plexus when high-resolution microscopy was applied.[103] Furthermore, choroid plexus labeling with peripheral fluorescence labelled GLP-1RA administration can occur in GLP-1R knockout mice as well, indicating that this event does not require GLP-1R, could be a false positive result as well. Finally, it is unlikely that fluorescence labeled GLP-1RA can enter the rat CSF.[95] Thus, peripheral GLP-1 and GLP-1RAs may not cross the rodent BCB, commented very recently by Buller and Blouet in their 2024 review article.[99] Consistently, a clinical study has reported that transfer of Liraglutide from blood to CSF was very minimal in patients with T2D who had received a long-term Liraglutide treatment.[104] In the study conducted by Christensen and colleagues, patients were treated with Liraglutide for 5–22 months, and the treatment has effectively induced the weight loss of 7–11 kg.[104] They reported that plasma Liraglutide level but not CSF Liraglutide level (at extremely low level) that tended to correlate with body weight loss in patients who has received the long-term Liraglutide treatment.[104]
Can gut produced native GLP-1 or peripherally administrated GLP-1RAs access brain via CVOS
A few recent studies have suggested that GLP-1RAs can access certain CVOs following their peripheral administration.[101,105] This may not occur for gut produced native GLP-1, as its half-life is very short. GLP-1R is likely expressed in ME in the hypothalamus and the AP in the hindbrain (Figure 1), while exact cell population or cell linage within these two CVOs that express GLP-1R needs further investigation and clarification.[102,106] The utilization of electron microscopy allowed the detection of GLP-1R immune-reactivity on ME dendrites and on the surface of axon varicosities and axon terminals.[106] However, functional consequences on ME or AP GLP-1/GLP-1R signaling activation are remaining controversial at the current stage. AP GLP-1 signaling is necessary and sufficient for producing taste avoidance following peripheral GLP-1RA administration, suggested by Zhang and colleagues recently.[107] However, an early study reported that electrolytic ablation of the AP, or another CVO, the SFO, or AP plus SFO, did not prevent the anorectic effects of Ex-4 peripheral administration.[108] Further cell linage studies are required to clarify GLP-1R expressing neurons in different CVOs, followed by the utilization of cell linage specific knockout approach to clarify their role in mediating the anorexic and other brain effect of peripheral GLP-1RA administration. It is worth mentioning that ME or AP GLP-1R-expressing neurons may mediate the anorexic effect of peripheral GLP-1RA administration directly, without allowing the drugs entering the brain region furtherly.
Conclusion
As we have discussed above whether gut-produced native GLP-1 or peripherally administrated GLP-1RA therapeutic agents access BBB remains controversial to date. The receptor-independent passive access theory presented by Kastin and colleagues[88,89] challenges the classical dogma that BBB only allows its paracellular access of molecules that are smaller than 500 Da, while native gut-produced GLP-1 and GLP-1RA therapeutic agents are much larger than 3000 Da. The receptor-mediated theory via a transcellular mechanism by Fu and colleagues[95] is not convincing yet as a few recent studies failed in the detection of GLP-1R in brain endothelia cells, the foundation of BBB.[97, 98, 99] Clear interaction of fluorescence labeled GLP-1RA with mouse ependymal cells of the choroid plexus was virtually absent when high-resolution microscopy was applied.[103] Importantly, the detected interaction also occurred in GLP-1R knockout mice. We hence intend to suggest that gut-produced native GLP-1 and peripherally administrated GLP- 1RA as therapeutic agent do not access the brain via BCB. Indeed, CSF GLP-1RA level is very minimal in patients with long-term Liraglutide treatment. Peripherally administrated GLP-1RAs may access the brain via ME and AP, as well as other selective CVOs. Key questions that remain to be addressed include exactly what neuronal GLP-1R producing cell population that mediates the anorexic function of peripheral GLP-1RA administration? The study by Smith and colleagues in profiling GLP-1R expression brain cell paved an avenue for our further investigations.[100] Will the access be sufficient in triggering PKA activity as well as c-FOS activation in the AP, the nucleus of the solitary tract (NTS), and elsewhere? As we have mentioned above that endogenous GLP-1 has a very short half-life (around 2 min) due to the existence of the degradation enzyme DPP-4.[2,109] A study has shown that only 25% of gut produced endogenous GLP-1 reaches the portal circulation intact, whereas further cleavage happens in the liver causes an additional 40%–50% reduction of intact GLP-1, leading to only 10%–15% of those enters the systemic circulation.[10] As illustrated in Figure 2, peripheral GLP-1 may send the signal to the brain through a neuroendocrine signaling mechanism, without entering the brain. This could be mediated by GLP-1R that are expressed on the portal vein that is heavily innervated by the vagal afferent nerve. This signal can be further sent to the nodose ganglion at the base of the skull, where a collection of neuronal cell bodies is located.[110] Portal vein GLP-1R expression has been demonstrated by several previous investigations.[111, 112, 113]
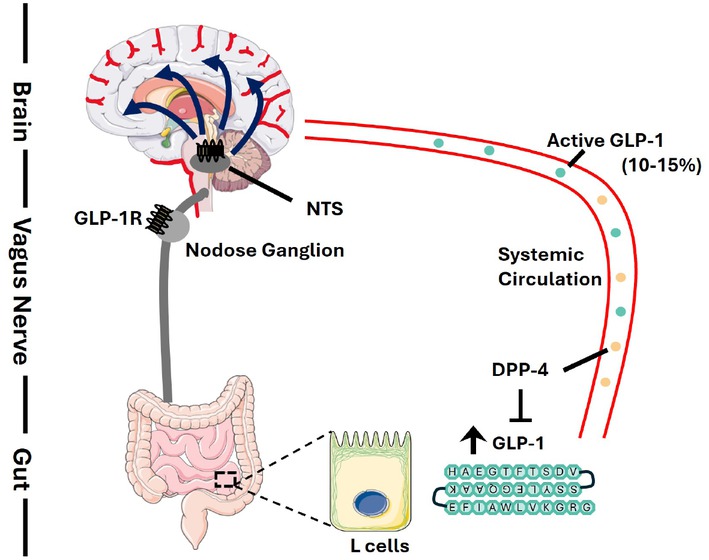
Proposed mechanism of brain access of endogenous GLP-1. Followed by nutritional stimulations, endogenous GLP-1 is secreted by enteroendocrine L cells located mainly in the distal intestine. Due to the existence of the degradation enzyme DPP-4 in the circulation, only 10%–15% of active GLP-1 will reach the systemic circulation and possibly exerts its effects centrally. Thus, it is possible that central effects of GLP-1 is mediated by a neuroendocrine signalling mechanism via the vagus nerve. Gut-produced GLP-1 activates GLP-1R in the portal vein where its concentration is the highest and sends the projection via vagal afferent neurons to their cell bodies called nodose ganglion, which also expresses GLP-1R. The nodose ganglion terminates in the nucleus tractus solitarius (NTS) in the brainstem and further transmit the signals to other brain regions to regulate metabolic and other responses. This evolutionarily developed mechanism via organ-organ communication, if exists, should also participate in mediating the anorexic and other brain functions of peripherally administrated GLP-1RAs.
The portal vein and vagus afferent nerve mediated effect of peripheral native GLP-17–36 amide administration on attenuating intestinal fat absorption has been reported recently by Hoffman and colleagues in two rodent models110. Briefly, Hoffman and colleagues have demonstrated that in obesogenic diet induced obesity and insulin resistance hamster as well as mouse models, portal vein injection of native GLP-17–36 amide effectively improved lipid homeostasis, reflected by reduced postprandial and fasting plasma lipid levels. Such inhibitory effects of portal vein GLP-17–36 amide injection were then shown, with the application of pharmacological and surgical denervation, to be dependent on intact afferent vagal singling cascade. Furthermore, such inhibitory effects were lost in the GLP-1R knockout hamster model.[110] It remains to be determined whether gut produced native GLP-1 or peripherally administrated GLP-1RAs exert their anorexic and other brain functions involves such organ-organ communication.
One may argue that with extended half-life, therapeutic agents GLP-1RAs are resistant to DPP-4 mediated degradation, allowing them to reach to the brain to exert their anorexic and other brain functions directly. However, if the portal vein system is evolutionarily developed for peripheral-central communications, including sending the signal to the brain for native GLP-1 to exert its anorexic and other brain functions, this existing system likely also mediates the pharmacological function of various GLP-1RAs. Finally, we have learned that brain not only express GLP-1R but also the hormone GLP-1 per se. The same proglucagon gene is expressed in rodent gut, pancreatic islet α-cells and the brain.[114] The same post-transcriptional machinery exists for processing the pre-hormone proglucagon in the gut and the brain. We are far away from understanding exactly why brain needs to express its own GLP-1 and how brain GLP-1, gut-produced GLP-1, as well as their receptor GLP-1R expressed in selected cell linages, orchestrate the anorectic program physiologically and patho-physiologically. Biomedical research enters the new era as we are now paying more and more attention to inter-organ crosstalk.[115] It appears that fundamental breakthroughs on organ-organ communications are needed in this area before we can advance our understanding on the anorectic effect and other brain functions of peripherally administrated GLP-1RAs.
Funding statement: This study was supported by the Canadian Institute of Health Research (CIHR, PJT159735 to T. J.).
Acknowledgements
We thank Banting & Best Diabetes Centre and Canadian Institute of Health Research for doctoral funding toward Jia Nuo Feng. Certain figures were created by using “Server Medical Art”.
-
Author Contributions
Jia Nuo Feng: Conceptualization, Writing—Original draft preparation, Writing—Reviewing and Editing. Tianru Jin: Conceptualization, Writing—Original draft preparation, Writing—Reviewing and Editing, Supervision, Project administration.
-
Ethical Approval
Not applicable.
-
Informed Consent
Not applicable.
-
Conflict of Interest
There is no conflict of interest among the authors.
-
Use of Large Language Models, AI and Machine Learning Tools
None declared.
-
Data Availability Statement
Not applicable.
References
1 Jin T, Weng J. Hepatic functions of GLP-1 and its based drugs: current disputes and perspectives. Am J Physiol Endocrinol Metab 2016;311:E620-E627.10.1152/ajpendo.00069.2016Search in Google Scholar PubMed
2 Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroen-terology 2007;132:2131–2157.10.1053/j.gastro.2007.03.054Search in Google Scholar PubMed
3 Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology 1994;107:1848–1855.10.1016/0016-5085(94)90831-1Search in Google Scholar PubMed
4 Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153– 165.10.1016/j.cmet.2006.01.004Search in Google Scholar PubMed
5 Drucker DJ. Discovery of GLP-1-Based Drugs for the Treatment of Obesity. N Engl J Med 2025;392:612–615.10.1056/NEJMcibr2409089Search in Google Scholar PubMed
6 Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705.10.1016/S0140-6736(06)69705-5Search in Google Scholar PubMed
7 Yu Z, Jin T. New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1). Cell Signal 2010;22:1–8.10.1016/j.cellsig.2009.09.032Search in Google Scholar PubMed
8 Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J Diabetes 2016;8:753–765.10.1111/1753-0407.12439Search in Google Scholar PubMed
9 Feng JN, Jin T. Hepatic function of glucagon-like peptide-1 and its based diabetes drugs. Med Rev (2021) 2024;4:312–325.10.1515/mr-2024-0018Search in Google Scholar PubMed PubMed Central
10 Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439.10.1152/physrev.00034.2006Search in Google Scholar PubMed
11 Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876–913.10.1210/edrv.20.6.0385Search in Google Scholar PubMed
12 Shao W, Wang D, Chiang YT, Ip W, Zhu L, Xu F, et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 2013;62:789–800.10.2337/db12-0365Search in Google Scholar PubMed PubMed Central
13 Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 2005;280:1457–1464.10.1074/jbc.M411487200Search in Google Scholar PubMed
14 Brown JC, Magee DF. Inhibitory action of cholecystokinin on acid secretion from Heidenhain pouches induced by endogenous gastrin. Gut 1967;8:29–31.10.1136/gut.8.1.29Search in Google Scholar PubMed PubMed Central
15 Brown JC. Presence of a gastric motor-stimulating property in duodenal extracts. Gastroenterology 1967;52:225-229.10.1016/S0016-5085(67)80011-8Search in Google Scholar
16 Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem 1971;49:867–872.10.1139/o71-122Search in Google Scholar PubMed
17 Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 1973;37:826–828.10.1210/jcem-37-5-826Search in Google Scholar PubMed
18 Pederson RA, Brown JC. The insulinotropic action of gastric inhibitory polypeptide in the perfused isolated rat pancreas. Endocrinology 1976;99:780–785.10.1210/endo-99-3-780Search in Google Scholar PubMed
19 Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes 1975;24:1050–1056.10.2337/diabetes.24.12.1050Search in Google Scholar
20 Brown JC, Pederson RA, Jorpes E, Mutt V. Preparation of highly active enterogastrone. Can J Physiol Pharmacol 1969;47:113–114.10.1139/y69-020Search in Google Scholar PubMed
21 Holst JJ, Rosenkilde MM. GIP as a Therapeutic Target in Diabetes and Obesity: Insight From Incretin Co-agonists. J Clin Endocrinol Metab 2020;105:e2710-e2716.10.1210/clinem/dgaa327Search in Google Scholar PubMed PubMed Central
22 Rosenkilde MM, Lindquist P, Kizilkaya HS, Gasbjerg LS. GIP-derived GIP receptor antagonists - a review of their role in GIP receptor pharmacology. Peptides 2024;177:171212.10.1016/j.peptides.2024.171212Search in Google Scholar PubMed
23 Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 2005;128:125–134.10.1016/j.regpep.2004.07.019Search in Google Scholar PubMed
24 Kieffer TJ. In Memoriam-John C. Brown, PhD, DSc, FRSC, 1938–2016: Discoverer of GIP and Motilin. Gastroenterology 2017;153:1169–1171.10.1053/j.gastro.2017.09.037Search in Google Scholar PubMed
25 Herman GA, Bergman A, Liu F, Stevens C, Wang AQ, Zeng W, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006;46:876–886.10.1177/0091270006289850Search in Google Scholar PubMed
26 Herman GA, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005;78:675–688.10.1016/j.clpt.2005.09.002Search in Google Scholar PubMed
27 Jin T. Tirzepatide, a new class of incretin-based drug for diabetes. Obesity Medicine 2023;39:100483.10.1016/j.obmed.2023.100483Search in Google Scholar
28 Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 1987;79:616–619.10.1172/JCI112855Search in Google Scholar PubMed PubMed Central
29 Heinrich G, Gros P, Lund PK, Bentley RC, Habener JF. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology 1984;115:2176–2181.10.1210/endo-115-6-2176Search in Google Scholar PubMed
30 Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986;261:1188011889.10.1016/S0021-9258(18)67324-7Search in Google Scholar
31 Orskov C, Holst JJ, Poulsen SS, Kirkegaard P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 1987;30:874–881.10.1007/BF00274797Search in Google Scholar PubMed
32 Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 1987;211:169–174.10.1016/0014-5793(87)81430-8Search in Google Scholar PubMed
33 Orskov C, Holst JJ. Radio-immunoassays for glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). Scand J Clin Lab Invest 1987;47:165-174.10.1080/00365518709168885Search in Google Scholar
34 Ip W, Shao W, Chiang YT, Jin T. GLP-1-derived nonapeptide GLP-1(28-36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 2013;305:E1348-E1358.10.1152/ajpendo.00376.2013Search in Google Scholar PubMed
35 Shao W, Wang Z, Ip W, Chiang YT, Xiong X, Chai T, et al. GLP-1(28-36) improves β-cell mass and glucose disposal in streptozotocin-induced diabetic mice and activates cAMP/PKA/β-catenin signaling in β-cells in vitro. Am J Physiol Endocrinol Metab 2013;304:E1263-E1272.10.1152/ajpendo.00600.2012Search in Google Scholar PubMed
36 Tomas E, Stanojevic V, McManus K, Khatri A, Everill P, Bachovchin WW, et al. GLP-1(32–36)amide Pentapeptide Increases Basal Energy Expenditure and Inhibits Weight Gain in Obese Mice. Diabetes 2015;64:2409–2419.10.2337/db14-1708Search in Google Scholar PubMed PubMed Central
37 Wang C, Gong B, Zhu Q, Han J, Sun L. Novel GLP-1(28–36) amide-derived hybrid peptide A3 with weight loss and hypoglycemic activities. Eur J Pharmacol 2023;961:176200.10.1016/j.ejphar.2023.176200Search in Google Scholar PubMed
38 Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept 2011;167:177-184.10.1016/j.regpep.2011.01.003Search in Google Scholar PubMed
39 Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regul Pept 2011;169:43-8.10.1016/j.regpep.2011.04.006Search in Google Scholar PubMed
40 Yap MKK, Misuan N. Exendin-4 from Heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant. Basic Clin Pharmacol Toxicol 2019;124:513–527.10.1111/bcpt.13169Search in Google Scholar PubMed
41 Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992;267:7402-7405.10.1016/S0021-9258(18)42531-8Search in Google Scholar
42 Malhotra R, Singh L, Eng J, Raufman JP. Exendin-4, a new peptide from Heloderma suspectum venom, potentiates cholecystokinin-induced amylase release from rat pancreatic acini. Regul Pept 1992;41:149–156.10.1016/0167-0115(92)90044-USearch in Google Scholar PubMed
43 Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 1993;268:19650–19655.10.1016/S0021-9258(19)36565-2Search in Google Scholar
44 Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013;19:567–575.10.1038/nm.3128Search in Google Scholar PubMed
45 Xiong X, Shao W, Jin T. New insight into the mechanisms underlying the function of the incretin hormone glucagon-like peptide-1 in pancreatic β-cells: the involvement of the Wnt signaling pathway effector β-catenin. Islets 2012;4:359–365.10.4161/isl.23345Search in Google Scholar PubMed PubMed Central
46 Islam D, Zhang N, Wang P, Li H, Brubaker PL, Gaisano HY, et al. Epac is involved in cAMP-stimulated proglucagon expression and hormone production but not hormone secretion in pancreatic alpha- and intestinal L-cell lines. Am J Physiol Endocrinol Metab 2009;296:E174-E181.10.1152/ajpendo.90419.2008Search in Google Scholar PubMed
47 Baggio LL, Drucker DJ. Harnessing the therapeutic potential of glucagon-like peptide-1: a critical review. Treat Endocrinol 2002;1:117–125.10.2165/00024677-200201020-00005Search in Google Scholar PubMed
48 Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004;145:2653–2659.10.1210/en.2004-0015Search in Google Scholar PubMed
49 Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187–215.10.1210/er.2011-1052Search in Google Scholar PubMed PubMed Central
50 Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130.10.1016/j.molmet.2019.09.010Search in Google Scholar PubMed PubMed Central
51 Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 2011;7:507–516.10.1038/nrendo.2011.77Search in Google Scholar PubMed PubMed Central
52 Pang J, Feng JN, Ling W, Jin T. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs-Therapeutic potential exploration in lung injury. Acta Pharm Sin B 2022;12:4040–4055.10.1016/j.apsb.2022.06.003Search in Google Scholar PubMed PubMed Central
53 Pang J, Liu M, Ling W, Jin T. Friend or foe? ACE2 inhibitors and GLP- 1R agonists in COVID-19 treatment. Obes Med 2021;22:100312.10.1016/j.obmed.2020.100312Search in Google Scholar PubMed PubMed Central
54 Chui ZSW, Xue Y, Xu A. Hormone-based pharmacotherapy for metabolic dysfunction-associated fatty liver disease. Med Rev (2021) 2024;4:158– 168.10.1515/mr-2024-0007Search in Google Scholar PubMed PubMed Central
55 Lu Y, Wang L, Luo F, Savani R, Rossi MA, Pang ZP. Dorsolateral septum GLP- 1R neurons regulate feeding via lateral hypothalamic projections. Mol Metab 2024;85:101960.10.1016/j.molmet.2024.101960Search in Google Scholar PubMed PubMed Central
56 Jin T, Chen YE. International Accolades for GLP-1 Research: Recognizing Pioneers in Diabetes and Obesity Treatment Across Five Prestigious Awards. Cardiovasc Drugs Ther 2025;39:9–12.10.1007/s10557-024-07630-9Search in Google Scholar PubMed
57 Lenharo M. Obesity-drug pioneers win prestigious Lasker Award for medical science. Nature 2024 Sep 19. Epub ahead of print.10.1038/d41586-024-03078-xSearch in Google Scholar PubMed
58 Cesta CE, Rotem R, Bateman BT, Chodick G, Cohen JM, Furu K, et al. Safety of GLP-1 Receptor Agonists and Other Second-Line Antidiabetics in Early Pregnancy. JAMA Intern Med 2024;184:144–152.10.1001/jamainternmed.2023.6663Search in Google Scholar PubMed PubMed Central
59 Ardehali H. Joel Habener, Svetlana Mojsov, and Lotte Bjerre Knudsen awarded Lasker prize for pioneering work on GLP- 1. J Clin Invest 2024;134:e186225.10.1172/JCI186225Search in Google Scholar PubMed PubMed Central
60 Jin, T. Three scientists on GLP-1 based obesity drug development are sharing 2024 Lasker-DeBakey Clinical Research Award. Obesity Medicine 2024;51:100560.10.1016/j.obmed.2024.100560Search in Google Scholar
61 Fei S, Feng X, Luo J, Guo L, Pan Q. Obesity and Coronavirus Disease 2019. J Transl Int Med 2022;10:207–218.10.2478/jtim-2022-0020Search in Google Scholar PubMed PubMed Central
62 Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007;116:1488–1496.10.1161/CIRCULATIONAHA.106.683243Search in Google Scholar PubMed
63 Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862–873.10.1016/j.jhep.2017.06.003Search in Google Scholar PubMed
64 Kernan WN, Dearborn JL. Obesity increases stroke risk in young adults: opportunity for prevention. Stroke 2015;46:1435–1436.10.1161/STROKEAHA.115.009347Search in Google Scholar PubMed
65 Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011;52:301–312.10.1093/cid/ciq152Search in Google Scholar PubMed
66 Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72.10.1038/379069a0Search in Google Scholar PubMed
67 Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 1999;140:244–250.10.1210/en.140.1.244Search in Google Scholar
68 Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest 2014;124:4223–4226.10.1172/JCI78371Search in Google Scholar PubMed PubMed Central
69 Trapp S, Brierley DI. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol 2022;179:557–570.10.1111/bph.15638Search in Google Scholar PubMed PubMed Central
70 Greenhill C. Brain-immune networks: a role for GLP1 receptor. Nat Rev Endocrinol 2024;20:125.10.1038/s41574-024-00952-6Search in Google Scholar PubMed
71 Chen B, Yu X, Horvath-Diano C, Ortuño MJ, Tschöp MH, Jastreboff AM, et al. GLP-1 programs the neurovascular landscape. Cell Metab 2024;36:2173–2189.10.1016/j.cmet.2024.09.003Search in Google Scholar PubMed
72 Drucker DJ. The benefits of GLP-1 drugs beyond obesity. Science 2024;385:258–260.10.1126/science.adn4128Search in Google Scholar PubMed
73 Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med 2015;373:11–22.10.1056/NEJMoa1411892Search in Google Scholar PubMed
74 Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021;384:989-1002.10.1056/NEJMoa2032183Search in Google Scholar PubMed
75 Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 2022;387:205-216.10.1056/NEJMoa2206038Search in Google Scholar PubMed
76 Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, Brar R, Baker C, Gluck-man TJ, et al. Semaglutide vs Tirzepatide for Weight Loss in Adults With Overweight or Obesity. JAMA Intern Med 2024;184:1056–1064.10.1001/jamainternmed.2024.2525Search in Google Scholar PubMed PubMed Central
77 Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med 2023;389:514–526.10.1056/NEJMoa2301972Search in Google Scholar PubMed
78 Wharton S, Blevins T, Connery L, Rosenstock J, Raha S, Liu R, et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N Engl J Med 2023;389:877–888.10.1056/NEJMoa2302392Search in Google Scholar PubMed
79 Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020;17:69.10.1186/s12987-020-00230-3Search in Google Scholar PubMed PubMed Central
80 Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 2010;518:459–476.10.1002/cne.22219Search in Google Scholar PubMed
81 Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996;17:305–311.10.1016/0196-9781(96)00025-3Search in Google Scholar PubMed
82 Butiaeva LI, Slutzki T, Swick HE, Bourguignon C, Robins SC, Liu X, et al. Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. Cell Metab 2021;33:1433–1448.10.1016/j.cmet.2021.05.017Search in Google Scholar PubMed
83 Johanson CE, Stopa EG, McMillan PN. in The Blood-Brain and Other Neural Barriers: Reviews and Protocols. Sukriti Nag ed, Humana Press, 2011:101–131.10.1007/978-1-60761-938-3_4Search in Google Scholar PubMed
84 Merino B, Díez-Fernández C, Ruiz-Gayo M, Somoza B. Choroid plexus epithelial cells co-express the long and short form of the leptin receptor. Neurosci Lett 2006;393:269-72.10.1016/j.neulet.2005.10.003Search in Google Scholar PubMed
85 Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 2013;521:3389–3405.10.1002/cne.23355Search in Google Scholar PubMed PubMed Central
86 Kaur C, Ling EA. The circumventricular organs. Histol Histopathol 2017;32:879-892.Search in Google Scholar
87 Jeong JK, Dow SA, Young CN. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites 2021;11:494.10.3390/metabo11080494Search in Google Scholar PubMed PubMed Central
88 Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 2002;18:7-14.10.1385/JMN:18:1-2:07Search in Google Scholar PubMed
89 Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 2003;27:313–318.10.1038/sj.ijo.0802206Search in Google Scholar PubMed
90 Salameh TS, Rhea EM, Talbot K, Banks WA. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics. Biochem Pharmacol 2020;180:114187.10.1016/j.bcp.2020.114187Search in Google Scholar PubMed PubMed Central
91 Salameh TS, Rhea EM, Talbot K, Banks WA. Corrigendum to "Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics"Search in Google Scholar
92 Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 2012;13:33.10.1186/1471-2202-13-33Search in Google Scholar PubMed PubMed Central
93 Gengler S, McClean PL, McCurtin R, Gault VA, Hölscher C. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging 2012;33:265–276.10.1016/j.neurobiolaging.2010.02.014Search in Google Scholar PubMed
94 McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci 2011;31:6587–6594.10.1523/JNEUROSCI.0529-11.2011Search in Google Scholar PubMed PubMed Central
95 Fu Z, Gong L, Liu J, Wu J, Barrett EJ, Aylor KW, et al. Brain Endothelial Cells Regulate Glucagon-Like Peptide 1 Entry Into the Brain via a Receptor-Mediated Process. Front Physiol 2020;11:555.10.3389/fphys.2020.00555Search in Google Scholar PubMed PubMed Central
96 Imbernon M, Saponaro C, Helms HCC, Duquenne M, Fernandois D, Deligia E, et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab 2022;34:1054–1063.10.1016/j.cmet.2022.06.002Search in Google Scholar PubMed PubMed Central
97 Skovbjerg G, Roostalu U, Salinas CG, Skytte JL, Perens J, Clemmensen C, et al. Uncovering CNS access of lipidated exendin-4 analogues by quantitative whole-brain 3D light sheet imaging. Neuropharmacology 2023;238:109637.10.1016/j.neuropharm.2023.109637Search in Google Scholar PubMed
98 Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020;5:e133429.10.1172/jci.insight.133429Search in Google Scholar PubMed PubMed Central
99 Buller S, Blouet C. Brain access of incretins and incretin receptor agonists to their central targets relevant for appetite suppression and weight loss. Am J Physiol Endocrinol Metab 2024;326:E472-E480.10.1152/ajpendo.00250.2023Search in Google Scholar PubMed PubMed Central
100 Smith C, Patterson-Cross R, Woodward O, Lewis J, Chiarugi D, Merkle F, et al. A comparative transcriptomic analysis of glucagon-like peptide-1 receptor- and glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus. Appetite 2022;174:106022.10.1016/j.appet.2022.106022Search in Google Scholar PubMed PubMed Central
101 Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488.10.1172/JCI75276Search in Google Scholar PubMed PubMed Central
102 Bakker W, Imbernon M, Salinas CG, Moro Chao DH, Hassouna R, Morel C, et al. Acute changes in systemic glycemia gate access and action of GLP-1R agonist on brain structures controlling energy homeostasis. Cell Rep 2022;41:111698.10.1016/j.celrep.2022.111698Search in Google Scholar PubMed PubMed Central
103 Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun 2020;11:467.10.1038/s41467-020-14309-wSearch in Google Scholar PubMed PubMed Central
104 Christensen M, Sparre-Ulrich AH, Hartmann B, Grevstad U, Rosenkilde MM, Holst JJ, et al. Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. Int J Obes (Lond) 2015;39:1651–1654.10.1038/ijo.2015.136Search in Google Scholar PubMed
105 Adriaenssens A, Broichhagen J, de Bray A, Ast J, Hasib A, Jones B, et al. Hypothalamic and brainstem glucose-dependent insulinotropic polypeptide receptor neurons employ distinct mechanisms to affect feeding. JCI Insight 2023;8:e164921.10.1172/jci.insight.164921Search in Google Scholar PubMed PubMed Central
106 Farkas E, Szilvásy-Szabó A, Ruska Y, Sinkó R, Rasch MG, Egebjerg T, et al. Distribution and ultrastructural localization of the glucagon-like peptide-1 receptor (GLP-1R) in the rat brain. Brain Struct Funct 2021;226:225–245.10.1007/s00429-020-02189-1Search in Google Scholar PubMed PubMed Central
107 Zhang Q, Delessa CT, Augustin R, Bakhti M, Colldén G, Drucker DJ, et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab 2021;33:833–844.10.1016/j.cmet.2021.01.015Search in Google Scholar PubMed PubMed Central
108 Baraboi ED, Smith P, Ferguson AV, Richard D. Lesions of area postrema and subfornical organ alter exendin-4-induced brain activation without preventing the hypophagic effect of the GLP-1 receptor agonist. Am J Physiol Regul Integr Comp Physiol 2010;298:R1098-R1110.10.1152/ajpregu.00326.2009Search in Google Scholar PubMed
109 McIntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl pepti-dase IV inhibitors: how do they work as new antidiabetic agents? Regul Pept 2005;128:159-65.10.1016/j.regpep.2004.06.001Search in Google Scholar PubMed
110 Hoffman S, Alvares D, Adeli K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol Metab 2022;65:101590.10.1016/j.molmet.2022.101590Search in Google Scholar PubMed PubMed Central
111 Aulinger BA, Perabo M, Seeley RJ, Parhofer KG, D'Alessio DA. Rapid hepatic metabolism blunts the endocrine action of portally infused GLP-1 in male rats. Am J Physiol Endocrinol Metab 2020;318:E189-E197.10.1152/ajpendo.00298.2019Search in Google Scholar PubMed PubMed Central
112 Andersen DB, Grunddal KV, Pedersen J, Kuhre RE, Lund ML, Holst JJ, et al. Using a Reporter Mouse to Map Known and Novel Sites of GLP-1 Receptor Expression in Peripheral Tissues of Male Mice. Endocrinology 2021;162:bqaa246.10.1210/endocr/bqaa246Search in Google Scholar PubMed
113 Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 1996;271:E808-E813.10.1152/ajpendo.1996.271.5.E808Search in Google Scholar PubMed
114 Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem 1988;263:13475-1347810.1016/S0021-9258(18)68261-4Search in Google Scholar
115 Jin T. Hormone based therapy and crosstalk beyond hormones. Med Rev (2021) 2024;4:257-261.10.1515/mr-2024-0052Search in Google Scholar PubMed PubMed Central
© 2025 Jianuo Feng, Tianru Jin, published by De Gruyter on behalf of the SMP
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Perspective
- Advances in the diagnosis and treatment of gastrointestinal tumors under the concept of super minimally invasive surgery
- Review Article
- Harnessing the potential of small extracellular vesicle biomarkers for cancer diagnosis and prognosis with advanced analytical technologies
- Current understanding and controversy on brain access of GLP-1 and GLP-1 receptor agonists
- Mitochondrial quality control as a therapeutic target in cardiovascular disease: Mechanistic insights and future directions
- Original Article
- Causal association between gut microbiota composition and the risk of atrial fibrillation
- Artificial intelligence-based predictive model for relapse in acute myeloid leukemia patients following haploidentical hematopoietic cell transplantation
- CircPLK1 upregulates ETS1 to confer anthracycline resistance in triple-negative breast cancer
- N-acetylglucosaminyltransferase V attenuates myocardial infarction by mediating the insulin-like growth factor 1 receptor signaling pathway
- A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial
Articles in the same Issue
- Perspective
- Advances in the diagnosis and treatment of gastrointestinal tumors under the concept of super minimally invasive surgery
- Review Article
- Harnessing the potential of small extracellular vesicle biomarkers for cancer diagnosis and prognosis with advanced analytical technologies
- Current understanding and controversy on brain access of GLP-1 and GLP-1 receptor agonists
- Mitochondrial quality control as a therapeutic target in cardiovascular disease: Mechanistic insights and future directions
- Original Article
- Causal association between gut microbiota composition and the risk of atrial fibrillation
- Artificial intelligence-based predictive model for relapse in acute myeloid leukemia patients following haploidentical hematopoietic cell transplantation
- CircPLK1 upregulates ETS1 to confer anthracycline resistance in triple-negative breast cancer
- N-acetylglucosaminyltransferase V attenuates myocardial infarction by mediating the insulin-like growth factor 1 receptor signaling pathway
- A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial


