A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial
-
Lamia M’barek
Abstract
Background and Objectives
Hemostasis factors affecting clot patterns, particularly fibrinogen, may influence the effectiveness of intravenous thrombolysis (IVT). We aimed to investigate the impact of differences in fibrinogen plasma levels on the efficacy and safety of tenecteplase versus alteplase in an acute ischemic cerebrovascular events-II (TRACE-II) trial.
Methods
In a multi-center, prospective, open-label, end-point blinded, randomized, controlled trial. Adults with acute ischemic stroke (AIS) were enrolled. Patients received intravenous tenecteplase (0–25 mg/kg) or alteplase (0–9 mg/kg) within 4–5 h. Patients were divided into three groups according to their plasma fibrinogen level: low fibrinogen level (< 2 g/L), normal fibrinogen level (2–4 g/L), and high fibrinogen level (> 4 g/L). The Modified Rankin Score (mRS) from 2 to 6 was used to define the efficacy outcome. The safety outcomes were the occurrence of symptomatic intracranial hemorrhage (sICH) within 36 h and 90 days, parenchymal hematoma 2 (PH2) within 36 h, any intracranial hemorrhage (ICH), other significant hemorrhagic events, and death at 3 months. SAS software version 9.4 was used for statistical analysis. Binary logistic regression was used to evaluate the efficacy and safety outcomes differences between tenecteplase and alteplase in the three fibrinogen groups. The interaction between treatment and fibrinogen subgroups was used to assess the effect of fibrinogen levels on the efficacy and safety of different treatments. All P-values are two-tailed and significance was defined as P < 0.05.
Results
The trial enrolled 1409 patients with AIS. Among them, 705 patients received tenecteplase treatment and 704 patients received alteplase treatment. Six percent of all patients had a low plasma fibrinogen level ( < 2 g/L), 81% had a normal fibrinogen level (2–4 g/L), and 13% had a high plasma fibrinogen level ( > 4 g/L). The efficacy of tenecteplase compared to alteplase remained consistent across varying fibrinogen levels (interaction P = 0.30). Additionally, the safety outcomes were comparable between the two treatments across all fibrinogen levels [sICH at 36 h (interaction P = 0.94); sICH at 90 days (interaction P = 0.77); PH2ICH at 36 h (interaction P = 0.84); Other symptomatic hemorrhagic events within 90 days (interaction P = 0.54)]. Similarly, there was no significant difference in mortality rates between patients treated with tenecteplase and alteplase across different plasma fibrinogen levels (interaction P = 0.58).
Conclusion
The results of the study suggest that the efficacy and safety of tenecteplase in treated AIS patients within 4.5 h are comparable to those of alteplase, regardless of plasma fibrinogen levels.
Introduction
Stroke is the second leading cause of death and the third leading cause of death and disability in the world. In 2019, Stroke cost 143 million disability-adjusted life years and 6.5 million deaths worldwide.[1] In China, stroke is the first leading cause of mortality and disability. Acute ischemic stroke (AIS) and transient ischemic attack (TIA) account for the majority of strokes (69.6% to 70.8%). AIS continues to have a significant impact on individuals, families, and healthcare systems despite recent advances in the treatment of AIS. Although mechanical thrombectomy has transformed stroke care over the past decade.[2] Recombinant tissue plasminogen activator (also known as alteplase) remains the treatment of choice. It is widely used to treat AIS.[3] Recently, results from several clinical trials have shown that tenecteplase may have an alternative role to alteplase.[4,5] Intravenous thrombolysis (IVT) is an effective therapy for appropriate AIS patients. It is designed to dissolve the causative clot and recanalize the occluded vessel to restore blood flow to the affected brain area.[6] However, it may be associated with hemorrhage transformation, particularly symptomatic intracranial hemorrhage (sICH), which can be a complication in stroke patients.[7] Theoretically, the efficacy of IVT may depend on hemostasis factors that influence clot patterns. A complete list of hemostasis parameters about the outcome of thrombolysis is available.[8] Fibrinogen is the most important hemostatic protein. As mentioned above, fibrinogen is the substrate for thrombin, which is important in determining blood viscosity. It’s also an important factor in platelet activation.[9] In patients with AIS or TIA, high baseline fibrinogen levels have been associated with poor functional outcomes and dependence.[10] Moreover, fibrinogen has been shown to play a role in predicting bleeding[11] and poor functional outcomes[12] after IVT with alteplase. However, Studies investigating the effect of fibrinogen concentration in the tenecteplase group are limited. As well, the effect of the differences in fibrinogen levels between tenecteplase and alteplase has not been investigated. Given the important role of fibrinogen in the thrombolysis process, we aim to evaluate the effect of baseline plasma fibrinogen levels on the efficacy and safety of tenecteplase versus alteplase in patients with AIS within 4.5 h of onset included in Tenecteplase Reperfusion therapy in Acute ischemic Cerebrovascular Events-II trial (TRACE-II).
Patients and methods
Study design
The TRACE II trial was a phase 3, multicenter, prospective, open-label, end-point blinded, randomized, controlled trial conducted at 53 centers in China. The protocol of the trial was published in 2022.[13] Patients enrolled in the study were determined to receive thrombolytic therapy based on the recommendations of the Chinese Stroke Association,[14] which are followed in other countries such as the United States[15] and Europe,[16] were used to determine who should receive thrombolytic therapy in this trial. In addition, the trial was conducted following quality control and the Declaration of Helsinki. The Steering Committee designed and monitored the trial. This trial is registered on ClinicalTrials. gov under the number NCT04797013 and has been completed.
Participants
The TRACE II trial enrolled all patients 18 years of age and above who were eligible to receive an IVT within 4.5 h of the AIS. As well, had a modified Rankin Scale (mRS) score of 1 or less and a National Institutes of Health Stroke Scale (NIHSS) score of 5 to 25 between June 12, 2021, and May 29, 2022. Patients who underwent endovascular thrombectomy and patients without fibrinogen plasma level measurement were excluded from our study. Additional information on inclusion and exclusion criteria is provided in Table S1. All clinical endpoints were first identified by the investigator. They were then analyzed and determined by the medical team based on clinical examination, laboratory, and radiology information. All clinical endpoints will then be the subject of review by the independent Clinical Endpoints Committee.
Study designs and treatment
The study drug was promptly administered following randomization (within 4.5 h of disease onset for the initiation of thrombolysis, as previously described.[17] Patients were randomized in a 1:1 ratio to receive either intravenous tenecteplase (0.25 mg/kg) or alteplase (0.9 mg/kg). The central web-based randomization system (Randomization and Trial Supply Management version 3.1.2, Beijing Bioknow Information Technology, China) was used for block randomization, with four blocks without stratification. Local reviewers visited the web randomization system and obtained the random codes, and treatment assignment was done according to the random code. The standard of care for AIS was used for all other treatments.
Baseline measurement
On admission, patients with suspected AIS were assessed for IVT eligibility within 4.5 h of symptom onset. All patients underwent a CT scan with or without CT angiography (CTA) (or MRI/MR angiography [MRA]). Physical examination (e.g., NIHSS and mRS), vital signs, and combination therapy were determined by certified doctors with formal education and standardized training. Demographic (age, sex, body mass index [BMI]), behavioral (smoking, alcohol use), medical history (hypertension, diabetes, dyslipidemia, heart disease), and primary laboratory tests (thrombin time, prothrombin time, fibrinogen, platelet count) were collected.
Biochemical measurements
Measure plasma fibrinogen concentration at admission and before IVT. The usual morning plasma fibrinogen concentration in our laboratory was 2–4 g/L.
Patient follow-up
Data were collected through face-to-face interviews. Clinical assessments were performed at 24 h, 7 days, or hospital discharge (whichever came first), and 90 days by trained investigators at each center. In-person assessments or telephone interviews were used to determine the 90-day mRS score. A clinical events committee adjudicated endpoint events based on clinical judgment.
Outcomes
The primary efficacy outcome was the proportion of patients with a poor functional outcome, defined as an mRS score of 2 or greater at 90 days. The primary safety outcome was the rate of sICH. This was defined by brain CT within 36 h of IVT. According to the European Cooperative Acute Stroke Study III (ECASS III), sICH was defined as any apparent extravascular blood in the brain or skull associated with clinical deterioration as defined by an increase of 4 points or more in the NIHSS score or leading to death and identified as the predominant cause of neurological deterioration.[18] Other safety outcomes included parenchymal hematoma 2 (PH2), as defined by the Safe Implementation of Thrombolysis in Stroke-Monitoring trial, which was defined as the location of the hematoma that represents more than 30% of the infarct area and the hematoma has a major impact on the region,[19] any intracranial hemorrhage, other major hemorrhagic events as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria,[20] and death from any cause within 90 days of the onset of the disease.
Statistical analysis
Continuous data are presented as median and interquartile range (IQR). Demographic and clinical characteristics were compared using the Mann-Whitney U test for sustained differences between tenecteplase and alteplase in different fibrinogen subgroups. For categorical variables, the chi-squared test or Fisher’s exact test was used. To investigate its association with outcomes, fibrinogen level was examined as a categorical variable. Patients were divided into 3 groups according to plasma fibrinogen cutoff: low fibrinogen ( < 2 g/L), normal (2–4 g/L), and high fibrinogen ( > 4 g/L). The differences in the efficacy and safety outcomes between tenecteplase and alteplase among the three fibrinogen groups were evaluated using binary logistic regression, and the odds ratio (OR) as well as its 95% confidence interval (CI) were reported. The OR was adjusted for age, sex, and NIHSS score at the time of admission. The interaction between treatment and fibrinogen subgroups was used to evaluate the effect of fibrinogen levels on the efficacy and safety of different treatments (interaction p). SAS software, version 9.4 (SAS InstituteInc., Cary, NC) was used for all statistical analyses. All P values are two-tailed. P < 0.05 was considered to be statistically significant.
Results
A total of 1434 patients were screened after written informed consent and 4 were ineligible (one had uncontrolled hypertension despite antihypertensive treatment, one had been treated with heparin within 24 h; one had an NIHSS less than 4 and another one had sudden onset of severe headache). Of the 1430 AIS enrolled at 53 clinical sites in China, as shown in Figure 1, 716 were randomly assigned to receive tenecteplase, while 714 were assigned to receive alteplase. Following exclusions, 705 patients were allocated to tenecteplase and 704 to alteplase. There was no missing baseline variable of interest.

Flowchart of study.
Table 1 shows the baseline characteristics of patients treated with tenecteplase or alteplase stratified by fibrinogen plasma level, 6% of all patients had a low plasma fibrinogen level (< 2 g/L), 81% of patients with a normal fibrinogen level (2–4 g/L), and 13% for them with a high plasma fibrinogen level (< 4 g/L). The distribution of the fibrinogen group and different IVT treatments is almost equal
Baseline Characteristics Among Individuals Stratified by Fibrinogen Plasma Levels
| Characteristics | < 2 g/dL, n = 79 (6%) |
2-4 g/dL, n = 1149 (81%) |
> 4 g/dL. n = 181 (13%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tenecteplase 38. (48%) | Alteplase 41. (52%) | P | Tenecteplase 580. (50%) | Alteplase 569, (50%) | P | Tenecteplase 87, (48%) | Alteplase 94 (52%) | P | |
| Age (years), median (IQR) | 62 (54-72) | 59 (52-69) | 0.39 | 65 (58-73) | 65 (58-73) | 0.13 | 68 (59-75) | 66 (60-74) | 0.86 |
| Male, n (%) | 24 (63) | 35 (85) | 0.02 | 411 (71) | 384 (67) | 0.21 | 54 (62) | 58 (61) | 0.95 |
| BMI, kg/m2, median (IQR) | 23.5 (22-25) | 24 (24-21) | 0.46 | 24 (21-26) | 24 (22-27) | 0.06 | 23 (21-25) | 23 (21-26) | 0.78 |
| Smoking, n (%) | 15 (39) | 19 (46) | 0.53 | 275 (47) | 273 (48) | 0.84 | 39 (45) | 47 (50) | 0.48 |
| Drinking, n (%) | 18 (47) | 20 (49) | 0.90 | 194 (33) | 196 (34) | 0.72 | 25 (29) | 26 (28) | 0.87 |
| Hypertension, n (%) | 27 (71) | 29 (70) | 0.97 | 416 (72) | 412 (72) | 0.07 | 63 (72) | 68 (72) | 0.99 |
| Diabetes, n (%) | 12 (31) | 9 (22) | 0.33 | 131 (22) | 166 (29) | 0.01 | 26 (30) | 31 (33) | 0.65 |
| Dyslipidemia, n (%) | 2 (5) | 9 (22) | 0.05 | 110 (19) | 132 (23) | 0.07 | 16 (18) | 18 (19) | 0.89 |
| Coronary history, n (%) | 10 (26) | 9 (22) | 0.65 | 128 (22) | 131 (23) | 0.69 | 27 (31) | 26 (28) | 0.61 |
| NIHSS, median (IQR) | 9 (5-7) | 8 (5-7) | 0.87 | 7 (6-10) | 7 (6-10) | 0.03 | 7 (5-11) | 7 (5-11) | 0.62 |
| APTT, median (IQR) | 26 (25-24) | 28 (24-31) | 0.04 | 28 (25-32) | 28 (25-32) | 0.97 | 28 (25-32) | 30 (26-34) | 0.05 |
| Thrombin Times, median (IQR) | 20 (19-18) | 19 (18-20) | 0.67 | 17 (16-18) | 17 (16-18) | 0.79 | 16 (15-17) | 16 (15-17) | 0.13 |
| Prothrombin Time, median (IQR) | 11 (10-12) | 12 (11-13) | <0.001 | 12 (11-13) | 12 (10-13) | 0.25 | 12 (11-13) | 12 (11-13) | 0.07 |
| Platelet count, median (IQR) | 189 (158-222) | 200(149-235) | 0.69 | 204 (168-244) | 215 (178-248) | 0.02 | 233 (189-291) | 235 (192-288) | 0.93 |
n: number of patients enrolled in the study; IQR: interquartile range; APTT: activated partial thromboplastin time; P: significant if P < 0.05.
Table 2 shows the efficacy and safety of tenecteplase versus alteplase stratified according to the groups of plasma fibrinogen levels. In the first part, the efficacy analysis revealed that in low fibrinogen plasma level, the incidence of poor outcome at 90 days was 31% in the tenecteplase group and 35% in the alteplase group, with no significant difference between the two treated groups (OR: 0.92; 95%CI [0.60–1.42]; P = 0.71). In the normal range fibrinogen level, there were 37% and 40% of patients treated with tenecteplase and alteplase, respectively, with no significant difference between the two treated groups (OR: 0.70; 95%CI [0.45–1.09]; P = 0.11). The incidence of poor outcome was 45% in the tenecteplase group and 56% in the alteplase group (OR: 0.99; 95%CI [0.63–1.54]; P = 0.97) in the group with fibrinogen levels greater than 4 g/L. Furthermore, there was no interaction between the three fibrinogen level groups and IVT in the analysis of efficacy (P = 0.30). For further details, Figure 2 shows the distribution of mRS scores at 90 days for different fibrinogen plasma levels.
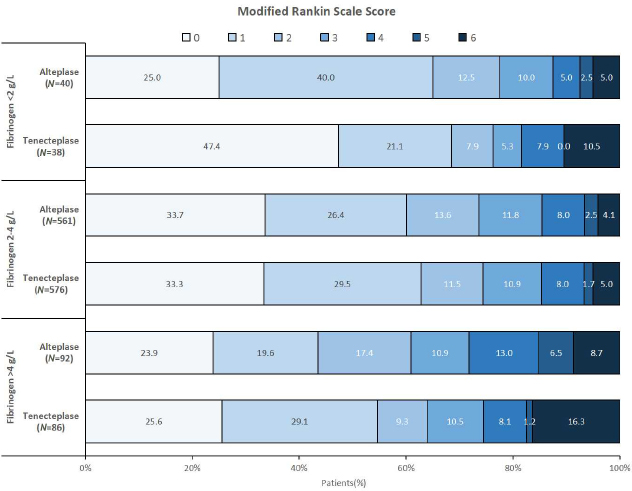
Distribution of modified Rankin Scale Score in patients treated with alteplase or tenecteplase according to fibrinogen plasma level.
Efficacy and safety of Tenecteplase vs. Alteplase stratified by fibrinogen plasma levels
| Items | FIB | Tenecteplase (%) | Alteplase (%) | OR | 95% Cl | P | Interaction P |
|---|---|---|---|---|---|---|---|
| Efficacy | |||||||
| Good outcome | < 2 | 26 (69) | 26 (65) | 1.16 | 0.45-2.99 | 0.74 | |
| 2-4 | 362 (63) | 337 (60) | 1.12 | 0.88-1.42 | 0.33 | 0.21 | |
| > 4 | 47 (55) | 40 (43) | 1.56 | 0.86-2.83 | 0.13 | ||
| Poor outcome | < 2 | 12 (31) | 14 (35) | 0.92 | 0.60 - 1.42 | 0.71 | 0.30 |
| 2-4 | 214 (37) | 224 (40) | 0.70 | 0.45 - 1.09 | 0.11 | ||
| > 4 | 39 (45) | 52 (56) | 0.99 | 0.63 - 1.54 | 0.97 | ||
| Safety | |||||||
| sICH within 36 h | < 2 | 2 (5.3) | 0(0) | - | - | - | 0.94 |
| 2-4 | 10 (1.7) | 9 (1.6) | 1.09 | 0.44 - 2.71 | 0.85 | ||
| > 4 | 3 (3.5) | 4 (4.3) | 0.80 | 0.18 - 3.70 | 0.79 | ||
| sICH within 90 days | < 2 | 2 (5) | 0 | - | - | 0.77 | |
| 2-4 | 12 (2) | 10 (2) | 1.18 | 0.50 - 2.75 | 0.70 | ||
| > 4 | 3 (3.4) | 5 (5.3) | 0.63 | 0.14 - 2.74 | 0.54 | ||
| PH2ICH within 36 h | < 2 | 1 (3) | 0 | - | - | - | 0.84 |
| 2-4 | 5 (1) | 1 (0.1) | 4.93 | 0.57 - 42.40 | 0.14 | ||
| > 4 | 4 (5) | 2 (2) | 2.21 | 0.39 - 12.41 | 0.36 | ||
| Any ICH within 90 days | < 2 | 3 (8) | 2 (5) | 1.67 | 0.26 - 10.59 | 0.58 | 0.52 |
| 2-4 | 30 (5) | 38 (7) | 0.76 | 0.46 - 1.24 | 0.28 | ||
| > 4 | 11 (13) | 10 (11) | 1.21 | 0.48 - 3.02 | 0.67 | ||
| Other SH events within 90 days | < 2 | 0 | 0 | - | - | - | 0.54 |
| 2-4 | 2 (0.34) | 3 (0.5) | 0.65 | 0.10 - 3.92 | 0.64 | ||
| > 4 | 3 (3.4) | 1 (1) | 3.32 | 0.33 - 32.53 | 0.30 | ||
| < 2 | 4 (10.5) | 2 (5) | 2.29 | 0.39 - 13.30 | 0.35 | 0.58 | |
| Mortality | 2-4 | 29 (5) | 23 (4) | 1.24 | 0.71 - 2.18 | 0.43 | |
| > 4 | 14 (16) | 8 (8.5) | 2.06 | 0.81 - 5.18 | 0.12 |
Poor outcome (mRS 2-6 within 90 days); FIB: Fibrinogen plasma level in admission; ICH: intracranial hemorrhage; h: hours; OR: odds ratio adjusted for (age, gender, NIHSS); SH: symptomatic hemorrhage; PH2: Parenchymal hematoma 2.
In the second part of the safety analysis, we found that in the low fibrinogen plasma level group, there was no sICH within 36 h and 90 days, PH2ICH within 36 h, and no other symptomatic hemorrhage within 90 days in patients treated with alteplase IVT. In the tenecteplase group, the rates of sICH within 36 h and 90 days were 5.3% and 5% respectively. In addition, the incidence of PH2ICH within 36 h was 3% in the tenecteplase group. After 90 days of IVT, ICH occurred in 8% and 5% of patients in the tenecteplase and alteplase groups, respectively. There was no significant difference between the two treatments (OR: 1.67; 95%CI [0.26–10.59]; P = 0.58). No further ICH occurred within 3 months in either treatment group. The proportions and the interaction between tenecteplase and alteplase were similar (interaction P = 0.52) for different plasma-level distributions. Furthermore, there is no significant difference between different fibrinogen groups and the safety of tenecteplase vs. alteplase [sICH at 36 h (interaction P = 0.94); sICH at 90 days (interaction P = 0.77); PH2ICH at 36 h (interaction P = 0.84); Other symptomatic hemorrhagic events within 90 days (interaction P = 0.54)].
Regarding mortality, the interaction between tenecteplase and alteplase at 3 months was comparable at different plasma fibrinogen levels (interaction P = 0.58). In the safety analysis, there was no interaction between the 3 groups with different fibrinogen levels and IVT treatment.
Discussion
This post-hoc study, based on the TRACE II trial, shows that the fibrinogen level on admission has a similar effect on the efficacy and safety of IVT in AIS patients receiving tenecteplase or alteplase within 4.5 h of first onset.
Markers of hemostasis, especially fibrinogen, are significantly associated with the efficacy of IVT and the prognosis of patients with AIS.[21] Therefore, several studies have reported plasma fibrinogen level as a risk factor predicting sICH and poor functional outcomes post alteplase IVT.[10,22] However, whether the fibrinogen level in admission affects the efficacy and safety of tenecteplase compared to alteplase in AIS patients has not been investigated.
This study evaluated the effect of fibrinogen on the efficacy and safety of tenecteplase versus alteplase in AIS within 4.5 h of onset. Our results showed in the first part that tenecteplase had the same effect on poor functional outcomes as alteplase at different ranges of plasma fibrinogen levels (interaction P = 0.30). The relationship between plasma fibrinogen levels and poor outcomes after IVT is not clear. Recently, a study by Bembenek et al. showed that fibrinogen levels at admission, 24 h, and 3 months after IVT in AIS patients with poor outcomes (mRS > 2) were not significantly different from those with favorable outcomes at 3 months[23]. Similarly, a study by Marti-Fabregas et al. included 83 AIS patients treated by alteplase indicating that fibrinogen levels at admission are not associated with long-term outcomes at 3 months.[24] The effect of fibrinogen on poor outcomes in patients treated with tenecteplase has not been reported in any study to date. The results of this part of the study may support the idea that the effect of plasma fibrinogen on functional disability is the same in patients who are treated with tenecteplase or alteplase.
The second part of this study focused on the effect of fibrinogen on the safety of tenecteplase versus alteplase in AIS. Hemorrhage is the most feared complication after IVT. A significant association between this complication and low plasma fibrinogen levels has been demonstrated in several studies. It has been known since 1995 that fibrinogen is one of the first coagulation proteins to decrease critically during major blood loss.[25] Fibrinogen is essential for effective clot formation and plays a critical role in achieving and maintaining hemostasis. It is also responsible for platelet aggregation and can be cleaved and activated by thrombin to form a stable clot. The normal range of fibrinogen levels is between 2 g/L and 4 g/L. When major bleeding occurs, fibrinogen levels decrease rapidly due to various ongoing activities such as factor consumption, dilution (by fluid therapy), fibrinolysis, and fibrinogenolytic. Numerous clinical studies suggest that fibrinogen is a vital target.[26,27] The association between low fibrinogen and hemorrhage transformation in AIS patients after alteplase IVT was reported in 2004 by the phenomena of “early fibrinogen degradation coagulopathy”, which describes a biological syndrome predicting cerebral hemorrhage after thrombolysis associated with the early loss of fibrinogen and an increase in fibrin degradation products.[28] Alteplase binds to thrombus fibrin and activates plasminogen to plasmin. Plasmin cleaves fibrin into fibrin degradation products, dissolves the thrombus, and provides recanalization. However, alteplase’s affinity is not limited to thrombus fibrin. It also binds to circulating fibrinogen, resulting in fibrinogen degradation.[22] However, the relationship between fibrinogen and tenecteplase is still limited. The Alteplase-Tenecteplase Trial Evaluation for Stroke Thrombolysis (ATTEST) study suggested that the fibrinolytic system was not impaired in patients treated with tenecteplase (0.25 mg/kg) compared with the alteplase (0.9 mg/kg) patient group.[29] To the best of our knowledge, Tenecteplase is a type of tissue plasminogen activator (tPA). Compared with alteplase, tenecteplase showed 14-fold higher fibrin specificity, 10-fold higher fibrinogen conservation, 80-fold higher resistance to plasminogen activator inhibitor-1 activity, faster thrombolysis, and lower plasma clearance.[30] Based on this information, we hypothesize that the effect of fibrinogen on the safety of tenecteplase is different from that of alteplase. However, our results show that the interaction between fibrinogen levels and drug is the same on sICH at 36 h (interaction P = 0.94); sICH at 90 days; (interaction P = 0.77); PH2ICH at 36 h (interaction P = 0.84); any ICH sICH at 90 days (interaction P = 0.52) and others symptomatic hemorrhage within 3 months (interaction P = 0.54). Also, for mortality risk within 90 days, there was no difference between the two IVTs at different fibrinogen levels (interaction P = 0.58).
Our results show that tenecteplase is as effective and safe as alteplase in patients with AIS at different levels of fibrinogen. Overall, these results are consistent with the study by Wang et al. which showed that tenecteplase was non-inferior (not superior) to alteplase in AIS within 4–5 h of symptom onset and support the introduction of intravenous tenecteplase 0–25 mg/kg as an alternative thrombolytic agent to the standard of care alteplase.[4] In addition, in 1600 patients randomized within 4.5 h of AIS onset, the Alteplase Compared to Tenecteplase (ACT) trial found no significant differences in any outcome measure between tenecteplase and alteplase.[5]
In clinical use, thrombolytic drugs are all plasminogen activators and trypsin-like serine proteases. Each has a specific function. Thrombolysis is also mediated by plasmin, which breaks down fibrin polymers. Fibrinogen provides a useful tool to monitor the effect of thrombolytic therapy. Under the influence of thrombin, fibrinogen can enter various protein bodies that promote platelet adhesion, release, and aggregation, and promote thrombosis in the human body.[12] In addition, fibrinogen can also repair the vascular penetration and damage caused by human low-density lipoprotein and cholesterol, promote the growth of muscle tissue, promote the migration of the fiber to the intima, and finally participate in the formation and development of atherosclerotic plaques, and play an auxiliary role in maintaining the human hypercoagulable state.
Given the importance of fibrinogen in predicting adverse events after thrombolysis, our study investigated the effect of fibrinogen on the efficacy and safety of tenecteplase versus alteplase in AIS patients. Therefore, this biomarker does not influence the physician’s choice of drug. Other studies on the composition of the thrombus also seem useful to increase our knowledge, which may be key to improving the treatment of AIS.
This study had several limitations. First, it is recommended to increase the number of patients to better understand the risk of sICH because the rate of sICH is low, so we cannot provide robust results. Second, the study is a post hoc analysis. The results need to be confirmed by future qualitative studies. Third, we examined plasma fibrinogen levels. Therefore, other biomarkers need to be included to understand thrombus resistance to thrombolysis better.
Conclusion
Our results suggest that the efficacy and safety of tenecteplase are comparable to those of alteplase across a range of fibrinogen levels in patients with AIS.
Funding statement: The National Science and Technology Major Project (2017ZX09304018), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), and the National Natural Science Foundation of China (81870905, U20A20358, 82111530203). China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou).
Acknowledgements
We thank all the participants and investigators in this study. We thank the National Science and Technology Major Project, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou) for funding support.
-
Author Contributions
L. M: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing; A. J: Formal analysis, Writing – original draft, Writing – review & editing; Y. P: Methodology, Writing – review & editing; S. L: Methodology, Resources, Writing – review & editing; H. L: Conceptualization, Writing – review & editing; Y. X: Conceptualization, Writing – review & editing; X. M: Investigation, Writing – review & editing; Y. W: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing.
-
Ethical Approval
The ethical standards of the institutional research committee and the tenets of the Declaration of Helsinki were followed for all procedures in studies involving human participants. All participants gave informed consent before becoming enrolled. Ethical approval for this study was obtained from the Institutional Review Board of the Beijing Tiantan Hospital, Capital Medical University with the number YW2020-046-04.
-
Informed Consent
Written informed consent was obtained from all subjects before they were enrolled.
-
Conflict of Interest
The authors declared that there is no conflicts of interest.
-
Use of Large Language Models, AI and Machine Learning Tools
None declared.
-
Data Availability Statement
All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
-
Trial Registration
The trial was registered with ClinicalTrials.gov, 10/03/2021; NCT04797013.
References
[1] GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–480.10.1016/S1474-4422(18)30499-XSearch in Google Scholar PubMed PubMed Central
[2] Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018;378:11–21.10.1056/NEJMoa1706442Search in Google Scholar PubMed
[3] Advani R, Naess H, Kurz MW. The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med 2017;25:54.10.1186/s13049-017-0398-5Search in Google Scholar PubMed PubMed Central
[4] Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet 2023;401:645–654.10.1016/S0140-6736(22)02600-9Search in Google Scholar PubMed
[5] Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022;400:161–169.10.1016/S0140-6736(22)01054-6Search in Google Scholar PubMed
[6] Urra X, Ariño H, Llull L, Amaro S, Obach V, Cervera Á, et al. The outcome of patients with mild stroke improves after treatment with systemic thrombolysis. PLoS One 2013;8:e59420.10.1371/journal.pone.0059420Search in Google Scholar PubMed PubMed Central
[7] Strbian D, Sairanen T, Meretoja A, Pitkäniemi J, Putaala J, Salonen O, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011;77:341–348.10.1212/WNL.0b013e3182267b8cSearch in Google Scholar PubMed
[8] Bagoly Z, Szegedi I, Kálmándi R, Tóth NK, Csiba L. Markers of Coagulation and Fibrinolysis Predicting the Outcome of Acute Ischemic Stroke Thrombolysis Treatment: A Review of the Literature. Front Neurol 2019;10:513.10.3389/fneur.2019.00513Search in Google Scholar PubMed PubMed Central
[9] Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler Thromb Vasc Biol 2017;37:e13–e21.10.1161/ATVBAHA.117.308564Search in Google Scholar PubMed PubMed Central
[10] Hou HQ, Xiang XL, Pan YS, Zhang QH, Li H, Meng X, et al. Baseline or 90-day fibrinogen levels and long-term outcomes after ischemic stroke or TIA: Results from the China national stroke registry III. 2021;337:35–41.10.1016/j.atherosclerosis.2021.10.002Search in Google Scholar PubMed
[11] Tanne D, Macko RF, Lin Y, Tilley BC, Levine SR; NINDS rtPA Stroke Study Group. Hemostatic activation and outcome after recombinant tissue plasminogen activator therapy for acute ischemic stroke. Stroke 2006;37:1798–1804.10.1161/01.STR.0000226897.43749.27Search in Google Scholar PubMed
[12] Li D, Xing C, Li Y, Zhu X. Elevated plasma fibrinogen indicates short-term poor outcome in patients with acute ischemic stroke after intravenous thrombolysis. J Stroke Cerebrovasc Dis 2020;29:104991.10.1016/j.jstrokecerebrovasdis.2020.104991Search in Google Scholar PubMed
[13] Li S, Pan Y, Wang Z, Liang Z, Chen H, Wang D, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 2022;7:47–53.10.1136/svn-2021-000978Search in Google Scholar PubMed PubMed Central
[14] Dong Q, Dong Y, Liu L, Xu A, Zhang Y, Zheng H, et al. The Chinese Stroke Association scientific statement: intravenous thrombolysis in acute ischaemic stroke. Stroke Vasc Neurol 2017;2:147–159.10.1136/svn-2017-000074Search in Google Scholar PubMed PubMed Central
[15] Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418.10.1161/STR.0000000000000211Search in Google Scholar PubMed
[16] Berge E, Whiteley W, Audebert H, De Marchis G, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021;6:I–LXII.10.1177/2396987321989865Search in Google Scholar PubMed PubMed Central
[17] Li S, Campbell BCV, Schwamm LH, Fisher M, Parsons M, Li H, et al. Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-II (TRACE II): rationale and design. Stroke Vasc Neurol 2022;7:71–76.10.1136/svn-2021-001074Search in Google Scholar PubMed PubMed Central
[18] Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329.10.1056/NEJMoa0804656Search in Google Scholar PubMed
[19] Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275–282.10.1016/S0140-6736(07)60149-4Search in Google Scholar PubMed
[20] GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–682.10.1056/NEJM199309023291001Search in Google Scholar PubMed
[21] Huang X, Cao K, Chen D. The relationship between the levels of fibrinogen, D-dimer and the efficacy of intravenous thrombolysis in patients with ischemic stroke. Minerva Surg 2022;77:490–492.10.23736/S2724-5691.21.09048-1Search in Google Scholar PubMed
[22] Romoli M, Vandelli L, Bigliardi G, Naccarato M, Moller J, Balestrino M, et al. Fibrinogen Depletion Coagulopathy Predicts Major Bleeding After Thrombolysis for Ischemic Stroke: A Multicenter Study. Stroke 2022;53:3671–3678.10.1161/STROKEAHA.122.039652Search in Google Scholar PubMed
[23] Bembenek JP, Niewada M, Siudut J, Plens K, Członkowska A, Undas A. Fibrin clot characteristics in acute ischaemic stroke patients treated with thrombolysis: the impact on clinical outcome. Thromb Haemost 2017;117:1440–1447.10.1160/TH16-12-0954Search in Google Scholar PubMed
[24] Martí-Fàbregas J, Borrell M, Cocho D, Belvís R, Castellanos M, Montaner J, et al. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-PA. Neurology 2005;65:366–370.10.1212/01.wnl.0000171704.50395.baSearch in Google Scholar PubMed
[25] Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg 1995;81:360–365.10.1097/00000539-199508000-00026Search in Google Scholar PubMed
[26] Bialkower M, Garnier G. Fibrinogen Diagnostics in Major Hemorrhage. Crit Rev Anal Chem 2022;52:194–209.10.1080/10408347.2020.1793098Search in Google Scholar PubMed
[27] Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014;54:1389–1388.10.1111/trf.12431Search in Google Scholar PubMed
[28] Jovin TG, Nogueira RG; DAWN Investigators. Thrombectomy 6 to 24 Hours after Stroke. N Engl J Med 2018;378:1161–1162.10.1056/NEJMc1801530Search in Google Scholar PubMed
[29] Huang X, Moreton FC, Kalladka D, Cheripelli BK, MacIsaac R, Tait RC, et al. Coagulation and Fibrinolytic Activity of Tenecteplase and Alteplase in Acute Ischemic Stroke. Stroke 2015;46:3543–3546.10.1161/STROKEAHA.115.011290Search in Google Scholar PubMed
[30] Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase Thrombolysis for Acute Ischemic Stroke. Stroke 2020;51:3440–3451.10.1161/STROKEAHA.120.029749Search in Google Scholar PubMed PubMed Central
© 2025 Lamia M’barek, Aoming Jin, Yuesong Pan, Shuya Li, Hao Li, Yunyun Xiong, Xia Meng, Yongjun Wang, published by De Gruyter on behalf of the SMP
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Perspective
- Advances in the diagnosis and treatment of gastrointestinal tumors under the concept of super minimally invasive surgery
- Review Article
- Harnessing the potential of small extracellular vesicle biomarkers for cancer diagnosis and prognosis with advanced analytical technologies
- Current understanding and controversy on brain access of GLP-1 and GLP-1 receptor agonists
- Mitochondrial quality control as a therapeutic target in cardiovascular disease: Mechanistic insights and future directions
- Original Article
- Causal association between gut microbiota composition and the risk of atrial fibrillation
- Artificial intelligence-based predictive model for relapse in acute myeloid leukemia patients following haploidentical hematopoietic cell transplantation
- CircPLK1 upregulates ETS1 to confer anthracycline resistance in triple-negative breast cancer
- N-acetylglucosaminyltransferase V attenuates myocardial infarction by mediating the insulin-like growth factor 1 receptor signaling pathway
- A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial
Articles in the same Issue
- Perspective
- Advances in the diagnosis and treatment of gastrointestinal tumors under the concept of super minimally invasive surgery
- Review Article
- Harnessing the potential of small extracellular vesicle biomarkers for cancer diagnosis and prognosis with advanced analytical technologies
- Current understanding and controversy on brain access of GLP-1 and GLP-1 receptor agonists
- Mitochondrial quality control as a therapeutic target in cardiovascular disease: Mechanistic insights and future directions
- Original Article
- Causal association between gut microbiota composition and the risk of atrial fibrillation
- Artificial intelligence-based predictive model for relapse in acute myeloid leukemia patients following haploidentical hematopoietic cell transplantation
- CircPLK1 upregulates ETS1 to confer anthracycline resistance in triple-negative breast cancer
- N-acetylglucosaminyltransferase V attenuates myocardial infarction by mediating the insulin-like growth factor 1 receptor signaling pathway
- A similar effect of fibrinogen on efficacy and safety of tenecteplase versus alteplase in acute ischemic cerebrovascular events (TRACE II) trial

