Abstract
This review paper explores the ongoing challenge of internal corrosion in oil and gas pipelines, specifically focusing on the damage caused by hydrogen sulfide (H2S). It highlights the superior performance of coating technologies such as chemical resistance, long-term durability, and resistance to high temperatures, including epoxy and other nonmetallic coatings, which effectively protect pipelines against H2S-induced corrosion. The review covers the practical application of coating technologies to improve pipeline durability and operational efficiency, beginning with an examination of the corrosive impact of H2S on pipelines. It reviews existing mitigation strategies, highlighting their advantages and limitations, and then analyzes nonmetallic coatings as a promising solution to H2S-induced corrosion. The paper demonstrates the benefits of these advanced coatings. It concludes with a summary of key findings and provides industry recommendations for selecting and implementing effective coating technologies, alongside suggestions for future research in this field.
1 Introduction
Pipelines are essential in the oil and gas industry for moving products to processing and storage facilities (Muthukumar 2014). These pipelines transport hazardous substances, making failures costly and dangerous, potentially leading to severe economic and environmental damage (Soomro et al. 2022). Failures can result from various sources such as corrosion, mechanical defects, external interference, operational errors, and natural disasters (Senouci et al. 2014). Between 1990 and 2005, corrosion was the major cause of pipeline failures, responsible for 46.6 % of raw natural gas and 70.7 % of crude oil pipelines (Pournara et al. 2014). The financial impact of corrosion is substantial, with a notable oil and gas company spending around USD 900 million in 2003 alone, and the global annual cost estimated at USD 60 billion (Obot et al. 2023). This highlights the necessity for effective risk management strategies to ensure pipeline safety and cost efficiency. Corrosion, especially internal corrosion caused by substances like hydrogen sulfide and carbon dioxide, presents a significant risk, leading to most pipeline leaks and failures (Zhao et al. 2018). Effective management of internal corrosion is crucial for maintaining the safety and integrity of the industry (Pournara et al. 2014).
Figure 1 illustrates the pipelines in the oil and gas industry that are most affected by corrosion. The crude oil and raw natural gas extracted from the extraction unit contain toxic gases, including high levels of hydrogen sulfide. Consequently, the pipelines most susceptible to corrosion are those transporting raw natural gas and crude oil to the processing units and refineries. Even when raw natural gas is converted to liquid form and liquefied petroleum gas (LPG), all of these substances become less toxic, reducing their corrosive impact on the pipelines.
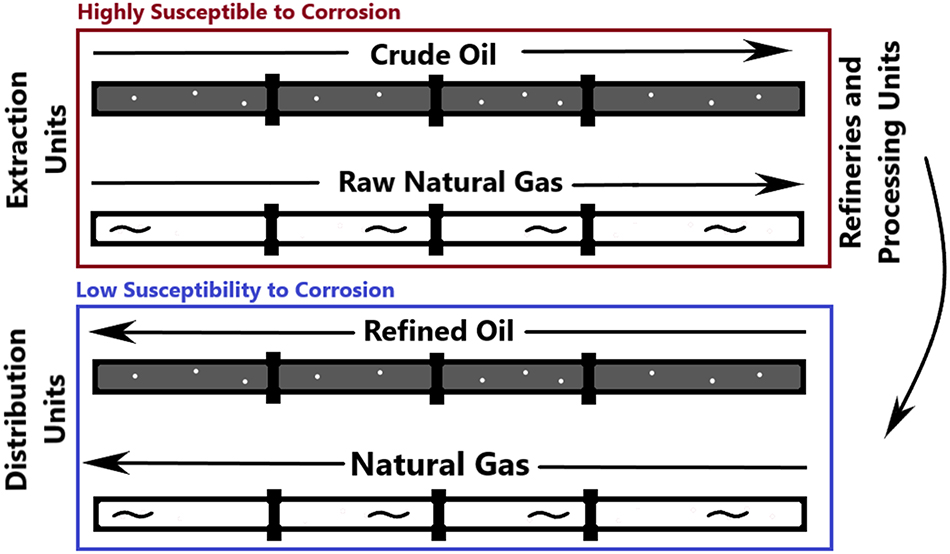
Corrosion susceptibility of oil and gas pipelines from extraction to distribution.
Corrosion-related material failures in sour and sweet environments present significant safety, economic, and environmental issues. Figure 2 displays the percentage distribution of different corrosion failures in the 1970s (Obot et al. 2023). Hydrogen sulfide (H2S)-induced sour corrosion is the leading cause of such failures in the petroleum industry, with its incidence increasing over the years. It is crucial to proactively manage and implement preventive strategies to mitigate sour corrosion risks in this sector.
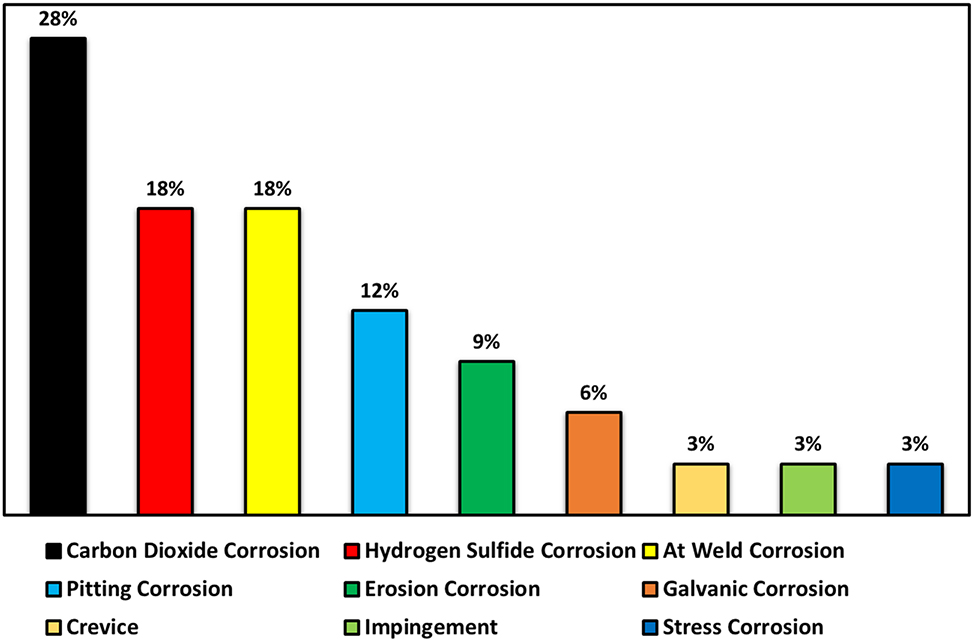
Percentage distribution of corrosion failure types in the industry during the 1970s (Obot et al. 2023).
In the oil and gas industry, corrosion is mainly divided into two types: sweet and sour, commonly found in environments with high partial pressures of H2S and CO2 (
Categories of corrosion (Shi et al. 2021).
| Pressure ratio | Corrosion category |
|---|---|
|
|
Sweet corrosion |
|
|
Sweet–sour corrosion |
|
|
Sour corrosion |
Ultra-sour corrosion will also occur due to high levels of hydrogen sulfide (H2S) concentration, which exceeds 5 mol% in the raw gas (Jariwala 2019). Key determinants of corrosion include partial pressures of H2S and CO2, along with temperature and pH levels. These factors greatly impact the solubility of corrosive gases and the development of corrosion byproducts in both sweet and sour environments. Elevated temperatures accelerate reaction rates and reduce gas solubility, increasing corrosion rates. High pressure, on the other hand, can increase gas solubility, enhancing corrosive attack, especially in confined environments like pipelines or pressure vessels, which in turn raises corrosion rates (Sharrad 2018). The pH of an environment influences its acidity or alkalinity; lower pH levels speed up corrosion, while higher pH levels can trigger localized corrosion. The presence of dissolved CO2 and H2S results in acidic conditions that lead to the formation of compounds that are less protective than the passive films of metal surfaces, thus accelerating corrosion. Sweet corrosion typically involves the formation of metal carbonates (MeCO3), whereas sour corrosion is characterized by the production of various metal sulfides (Obot et al. 2019). Handling and processing materials containing H2S are major challenges in the oil and gas industry due to the severe damage it can cause to equipment and infrastructure. Understanding H2S corrosion is critical, as it can lead to significant equipment degradation, increase maintenance costs, reduce operational efficiency, and elevate the risk of failures and accidents, ultimately impacting production and energy usage.
Figure 3 presents the results of a comprehensive Scopus search conducted using the intersection of synonymous terms related to internal corrosion, sour gas, and pipelines. Combinations of terms from each group were utilized to precisely identify relevant literature, highlighting fluctuating research on corrosion mitigation due to H2S over the period from 1982 to 2024. The data indicate that there are only limited number of papers available on this exact topic. This highlights the need for further studies to better understand current mitigation solutions and their challenges. This review paper aims to provide up-to-date information on pipeline corrosion issues caused by sour gas and hydrogen sulfide. It discusses the sources of corrosion, current mitigation techniques, and future research directions. Implementing various corrosion mitigation methods could potentially reduce corrosion costs by 15–35 %, or up to $875 billion annually (McMahon et al. 2019).
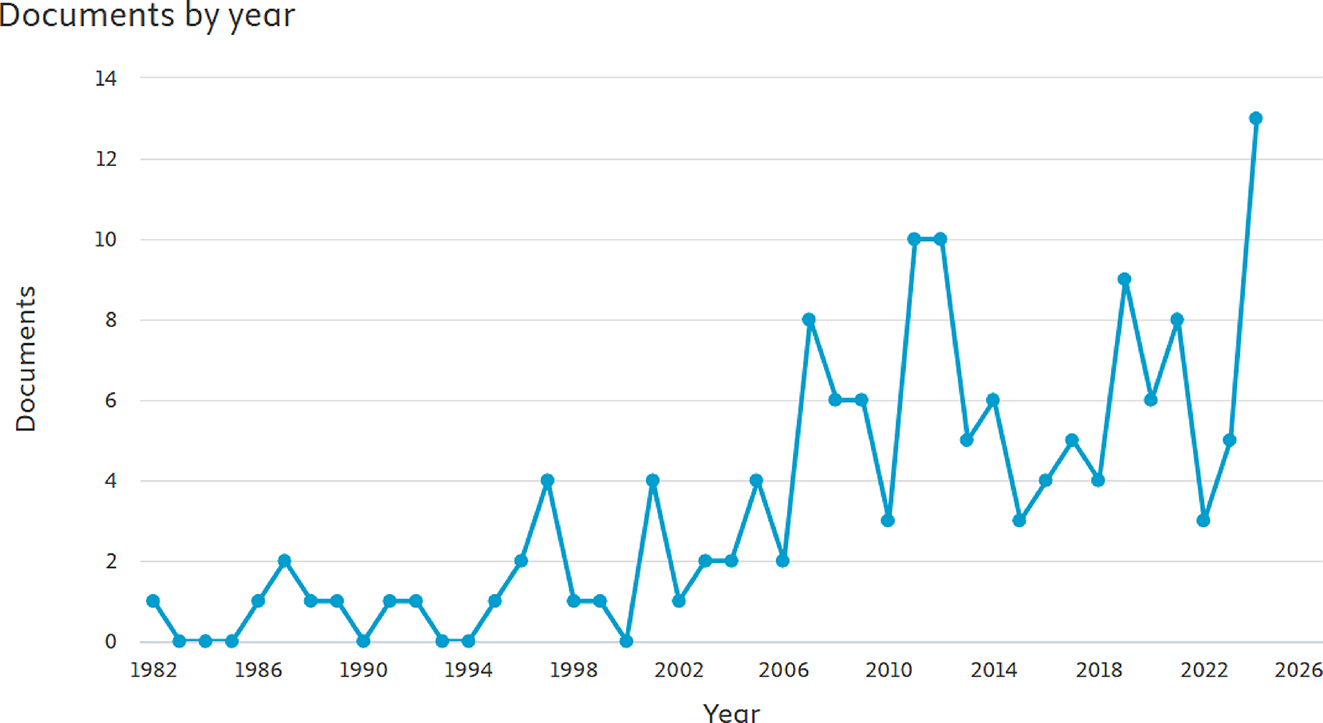
Number of publications per year from 1982 to 2024 as retrieved from Scopus.
2 Sour gas and hydrogen sulfide (H2S)
Sour gas is natural gas with hydrogen sulfide (H2S) and sulfur compounds, known for its rotten egg smell. Despite the stench, it’s valuable in global gas fields. Yet, its toxicity and corrosiveness create major challenges. Even in small amounts, hydrogen sulfide is harmful to health. It also corrodes equipment and pipelines, leading to safety issues and expensive maintenance (Albiter 2020). Table 2 shows the physicochemical properties of H2S.
Physicochemical properties of hydrogen sulfide (H2S) (Ahmad et al. 2021).
| Chemical structure | Molar weight | Odor | Color | Taste | Density | Specific gravity | Boiling point |
|---|---|---|---|---|---|---|---|
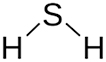 |
34.08 g/mol | Strong, rotten eggs smell | Colorless | Sweetish | 1.5392 g/L | 1.189 | −60.25 °C |
|
|
|||||||
| Melti ng point | Physical state | Upper explosive limit (UEL) | Lower explosive limit (LEL) | Auto-ignition temperature | Henry’s law constant at 25 °C | Vapor pressure at 25 °C | Solubility in water (H2O) |
|
|
|||||||
| −82 °C | Gas | 44 % | 4 % | 260 °C (500 °F) | 0.0098 atm m3/mol | 13,600 mmHg | 4 g/L at 20 °C |
Despite these obstacles, techniques like sulfur recovery and gas sweetening are employed to manage sour gas safely, ensuring its continued use as a vital energy resource while minimizing health and environmental risks. Hydrogen sulfide (H2S) is a valuable resource in producing sulfur and sulfuric acid, essential for fertilizers, chemicals, and pharmaceuticals. It also serves as a precursor in refining processes and indicates methane-rich natural gas reserves. However, its highly corrosive nature and associated risks make it a significant nuisance in corrosion-related challenges. Sour gas corrosion is caused by the presence of acidic materials in the gaseous state. When the partial pressure of H2S exceeds 0.05 psi, or the concentrations of these two surpass 0.1 % by volume, an acid gas is classified as sour gas (Albiter 2020). When H2S dissolves in water, it forms hydrosulfuric acid, creating a mildly acidic solution. In aqueous solutions, H2S dissociates into hydronium ions and hydrosulfide ions, with the form present depending on the pH. At higher pH levels, HS− can further dissociate into sulfide ions (Guidotti 2015). Due to its heavier density compared to air, H2S often collects in low areas and depressions. Its ignition point is generally recognized at 518 °F (270 °C) (Ausma and De Kok 2019). In the presence of air, H2S quickly oxidizes to produce sulfate and sulfur dioxide (SO2), facilitated by oxidizing agents such as airborne radicals.
In the Middle East, many natural gas reserves are rich in sulfur, with some classified as ultra-sour for having over 20 % acid gas. The complexity and cost of processing these reserves have limited their development until recently. Economic and population expansions in regions like the UAE and China have spurred the need for more energy, leading to the exploration of these ultra-sour gas fields (Manuel et al. 2019). Sour and ultra-sour gas fields make up over 40 % of the world’s natural gas reserves, mostly concentrated in the Middle East (Spatolisano et al. 2023). These fields are also rich in CO2, with concentrations ranging from 15–80 % [13]. The Shah gas plant in the UAE, operational since 2016, processes over one billion cubic feet per day of ultra-sour gas containing more than 23 % hydrogen sulfide. In Abu Dhabi’s Ghasha field, the gas layers vary, with the Hail reservoir having about 15 % H2S and the Dalma zone up to 25 % H2S (Spatolisano et al. 2023). In China, 38 significant gas fields in the Sichuan Basin account for about 80 % of the national output, with sulfur levels in fields like Puguang reaching up to 17 % and Zhaolanzhuang in Bohai Bay as high as 60 % (Jin et al. 2010). Kuwait is also tapping into its northern and western sour gas reserves to meet domestic demands, notably at the Jurassic production facilities and West Kuwait areas (Al-Ghanem et al. 2019). Sour gas corrosion remains a major challenge, posing significant risks in oil and gas production (Al-Janabi 2020). Table 3 provides an overview of the distribution of several sour gas fields currently in production.
Examples of sour natural gas composition (vol %) (Spatolisano et al. 2023).
| Location\gas components | N2 | CO2 | H2S | CH4 | C2H6 | C3H8 | C4H10 | C5+ |
|---|---|---|---|---|---|---|---|---|
| Ram river (Alberta) | 2.53 | 8.22 | 35.79 | 52.34 | 0.41 | 0.14 | 0.16 | 0.41 |
| Burnt timber facility (Alberta) | 0.5 | 9 | 8 | 80 | 0.8 | 0.2 | – | 1.5 |
| Süd-Oldenburg (Germany) | 7 | 8 | 8 | 77 | 0.1 | – | – | – |
| Tengiz (Kazakhstan) | 0.8 | 2.6 | 16 | 42 | 8.5 | 5.2 | 3.3 | 22 |
| Bearberry (Canada) | 1 | 5 | 90 | 4 | – | – | – | – |
| Lacq (France) | 0.4 | 9.6 | 15.2 | 69.3 | 3.1 | 1.1 | 0.6 | 0.7 |
| Qilibei (China) | 0.5 | 3.73 | 16.3 | 77.9 | 0.5 | – | – | – |
| Kashagan (Kazakhstan) | 1.02 | 5.06 | 17.69 | 58.83 | 9.1 | 4.69 | 2.28 | 0.95 |
| Puguang, Sichuan Basin (China) | – | 10 | 14 | 76 | – | – | – | – |
| Uthmaniyah (KSA) | 7–14 | 0.5–8 | 8–14 | 60–64 | – | – | – | – |
| Shahb (UAE) | – | 10 | 23 | – | – | – | – | – |
| Astrakhan (Russia) | 3 | 19 | 23 | 55 | – | – | – | – |
Within these industries, it presents a serious risk to system reliability and operational integrity. Because pressure containment systems are crucial to process operations in oil and gas facilities, this issue is especially concerning as sour gas is highly corrosive, which increases the risk of equipment failure and damage, making it a major problem for maintaining safe and effective operations in the oil and gas industry (Al-Janabi 2020). In the oil and gas industry, when the temperature and pressure levels shift, the composition of natural gas in a reservoir can change suddenly. A well can produce a certain gas of H2S content under different temperature and pressure levels, and it can also change the composition as it is produced (ZareNezhad and Ziaee 2013). Predicting and controlling corrosion behavior requires an understanding of the conditions in which a gas will be generated and treated. Having understood the challenges that the industry faces with sour gas, particularly hydrogen sulfide, the following subsection will address the associated problems.
2.1 Material degradation and crack formation
In their dry state, neither CO2 nor H2S exhibit corrosive properties; however, upon dissolution in water, they precipitate the formation of corrosive solutions. This corrosion process initiates the thinning of pipes and induces metal embrittlement. Over time, this degradation can progress to pipe ruptures, with severe consequences including explosions as the weakened metal fails to withstand the operational pipeline’s elevated pressures (Goodwin et al. 2015). Carbon dioxide corrosion primarily occurs through pitting, where localized areas of corrosion develop rather than a uniform coating across the entire pipe surface. The resultant FeCO3 can form a protective scale layer, effectively shielding the pipe from further corrosion. However, this protective layer may degrade over time, leading to the exposure of the underlying metal and subsequent corrosion. This phenomenon, known as mesa corrosion, results in the formation of deep, sharply edged pits in the pipe; the term “mesa” is adopted from the same terminology used to characterize widely recognized geological formations (Han et al. 2010).
Due to hydrogen sulfide, pipelines typically experience two common types of failures: hydrogen-induced cracking (HIC) and sulfide stress cracking (SSC) (Mohtadi-Bonab et al. 2013). When H2S dissolves in water, it dissociates to release hydrogen ions (H+), which contribute to the formation of an acidic environment. These hydrogen ions then undergo a reduction reaction at the metal surface, where they are reduced to atomic hydrogen. The atomic hydrogen penetrates the metal and causes hydrogen embrittlement at defect sites, significantly reducing the pipeline’s service life. While the corrosion product layer formed on pipelines can decrease the penetration rate of corrosive agents, thereby slowing down further corrosion of steel (Castaneda et al. 2009), the corrosive ions H+, HS−, and S2− generated from H2S dissociation can permeate this protective layer and reach the substrate, continuing the corrosion process. Research by Lei et al. (2018) indicates that the growth of the corrosion product layer can be hindered by the accumulation of hydrogen blisters at the interface between the substrate and the barrier film, which subsequently reduces the corrosion resistance of the pipeline. Figure 4 illustrates the impact of hydrogen on the passivation behavior of metals, with the hydrolysis equilibrium reaction of H2S shown below.
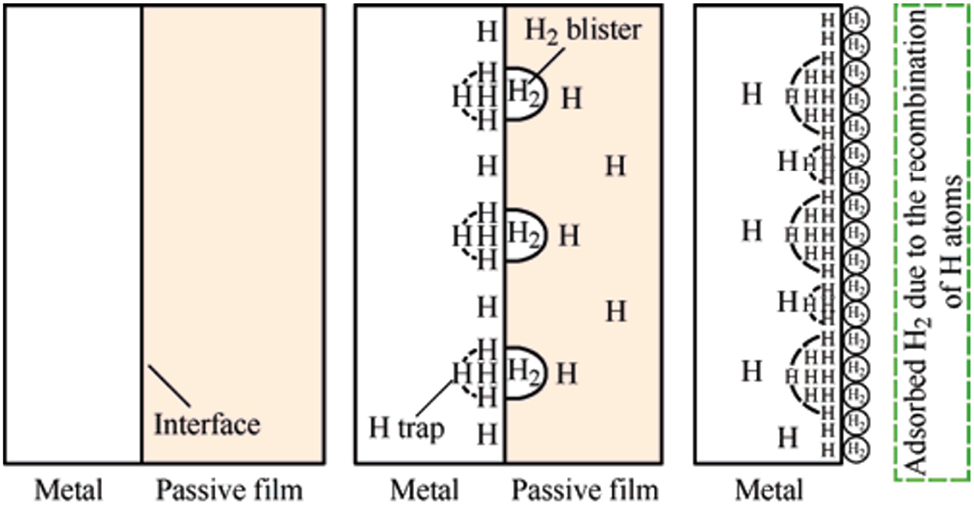
Impact of hydrogen on metal passivation. Reprinted with permission from (Lei et al. 2018). Copyright@2018, Elsevier.
Sulfide stress cracking (SSC) is exclusively observed in aqueous environments containing H2S. Typically, failure is a brittle fracture occurring in regions under applied or residual tensile stress. The formation of atomic hydrogen, which is required for SSC, arises during the corrosion reaction between iron and H2S. High-strength steels (yield strength ≥ 550 MPa) and hardened heat-affected zones in welded joints have been documented to experience SSC failure at room temperature. Elevated environmental temperatures have been identified as advantageous in reducing the susceptibility of steels to SSC (Steinberg and Kane 1982).
Both stress corrosion cracking (SCC) and sulfide stress cracking (SSC) involve the degradation of metal due to chemical processes. SCC arises from the combined effect of corrosion and mechanical stress, which is primarily stress-controlled, while SSC occurs in the presence of hydrogen sulfide (H2S) and strain, being primarily strain-controlled. Both types of corrosion result in cracking of the material, leading to structural integrity issues and potential equipment failure. SSC is characterized by hydrogen embrittlement caused by stress and the penetration of atomic hydrogen into metal lattice structures due to corrosion by wet H2S. The conditions necessary for SSC involve tensile stress, a material with structural imperfections, and when the hydrogen atoms enter and contact with a corrosive atmosphere containing hydrogen sulfide. These may lead to the formation of cracks. Hydrogen atoms are encouraged to enter the metal’s lattice structure when H2S is present, and the protective layer against corrosion frequently breaks and separates from the metal surface. This process occurs even in the absence of plastic strain, as hydrogen permeation and trapping can cause embrittlement independently of localized deformation (Tale et al. 2021).
Sour gas has harmful effects on metallic materials, including components not intended for primary structural support and low alloy steels. In sour gas systems, corrosion is the main cause of material degradation. It is electrochemical in nature, and it is initiated by the presence of H2S. It depends on different factors such as the partial pressure, pH, and temperature. Even a low amount of H2S, as little as a 0.82 (ppm), can cause SSC (Shimamura et al. 2022). When sour gas contains elemental sulfur or iron sulfides formed from the interaction of corrosion, H2S, and steel, it increases the chance of localized corrosion such as pitting or crevice corrosion in the material. These substances accelerate corrosion as catalysts and diminish the quality of steel by interacting with preexisting inclusions, which form during steel elaboration rather than during service. Sour gas corrosion processes tend to accelerate at high temperatures and help the material to degrade. This degradation reduces mechanical properties like strength and ductility, ultimately leading to equipment failure and material loss (Li et al. 2021a).
3 Existing mitigation strategies
In a sour gas environment, protecting pipelines from internal corrosion is important. Mitigation methods include applying protective coatings or injecting corrosion inhibitors. However, the use of coatings in sour environments is subject to restrictions outlined in industry standards such as ISO 15156. This standard specifically prohibits the use of certain coatings as a stand-alone solution for reducing sulfide stress cracking (SSC) in sour service conditions. This caution derives from a number of intrinsic limits of coatings. These limitations include compatibility with H2S exposure, resistance to blistering, and delamination under high-pressure conditions and ensuring that the coatings do not introduce contaminants that could exacerbate corrosion. Coatings can provide a barrier against corrosive chemicals; however, defects like pores, cracks, or partial coverage can lead to localized exposure to hydrogen sulfide (H2S). This concentrated exposure can accelerate hydrogen penetration into the underlying metal, increasing the risk of SSC. Coatings can trap hydrogen sulfide (H2S) at metal-coating interfaces, leading to increased cracking risk. These drawbacks emphasize the importance of carefully considering and validating coatings for specific sour service applications. Figure 5 presents the latest state-of-the-art internal corrosion protection methods for pipelines.
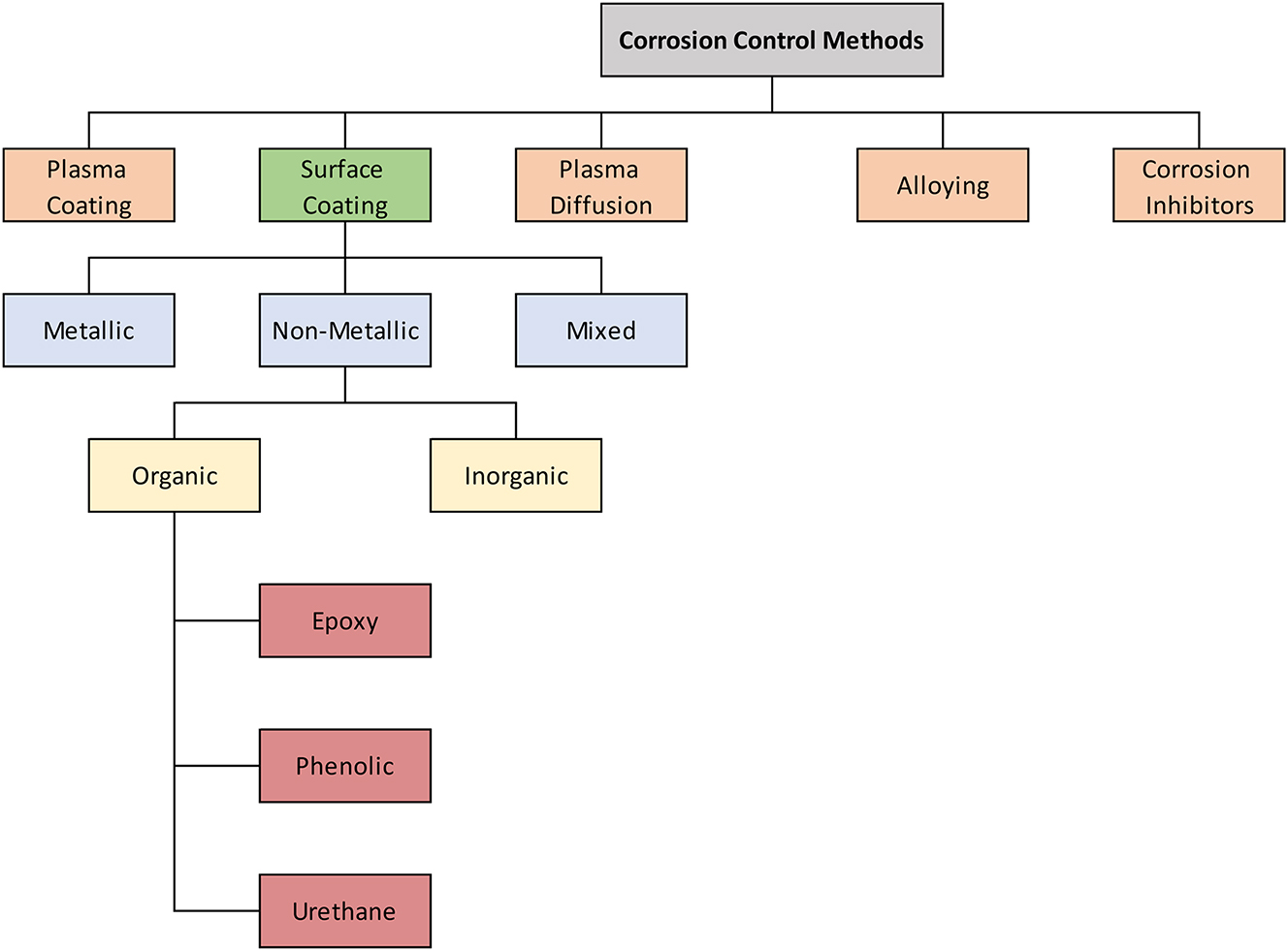
Summary of internal corrosion control methods.
3.1 Plasma diffusion techniques
Nitriding has traditionally been used to harden the surfaces of austenitic stainless steels, but this process at temperatures above 500 °C typically reduces the material’s corrosion resistance due to chromium nitride precipitate formation. Recently, it has been found that carbon can also effectively alloy with these steels, leading to the development of a low-temperature plasma carburizing process that enhances both wear and corrosion resistance. Plasma-based chemical heat treatments such as carburizing, nitriding, or carbonitriding enhance the corrosion resistance of metals in CO2/H2S environments embedding interstitial atoms into the metal surface, which slows the corrosion rate (Bell and Sun 2002). Li et al. (2018) employed low-temperature liquid nitriding on 304 austenitic stainless steel, creating a triple-layered structure consisting of an oxide layer on top, a nitrogen-enriched middle layer, and a carbon-rich bottom layer, as shown in Figure 6.
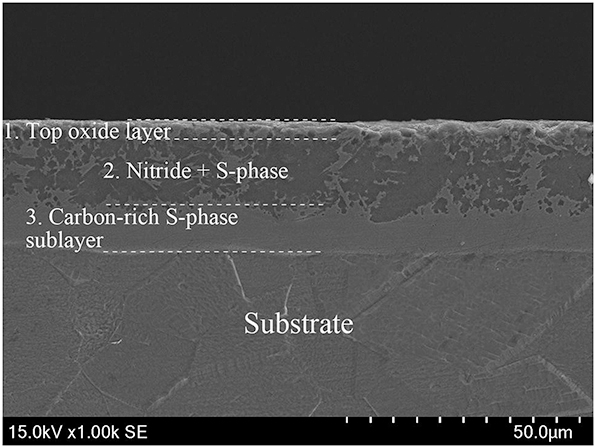
Layered structure and corrosion resistance of nitride 304 stainless steel. Reprinted with permission from (Li et al. 2018). Copyright@2018, Elsevier.
This layered structure reduces hydrogen diffusion and decreases the number of atoms penetrating the material, enhancing its resistance to hydrogen embrittlement. Additionally, the nitride layer reduces the formation of corrosive products, and the active nitrogen atoms can combine with H+ to prevent a drop in pH, thereby slowing the corrosion rate from H2S (Li et al. 2018). 304 stainless steel demonstrates improved resistance to hydrogen embrittlement due to its austenitic structure, which impedes hydrogen absorption and diffusion. The addition of the nitride layer further strengthens this resistance by acting as a physical barrier against hydrogen ingress (Martin et al. 2011). After soaking in a saturated hydrogen sulfide (H2S) solution prepared according to NACE TM0177-2005 standard (Solution A) for 720 h, and without applying external stress, the cross section of the untreated sample displayed numerous pits and cracks with extensive corrosion product formation (Li et al. 2018). In contrast, the nitrided sample’s cross section showed no pits or cracks, and only minimal corrosion products were formed, indicating that nitriding significantly improves the corrosion resistance of 304 stainless steel in H2S environments. To further enhance the resistance of stainless steel against crevice and pitting corrosion in chloride-containing solutions, low-temperature gaseous nitriding introduced a 26 μm thick S-phase (expanded austenite) layer (Li et al. 2021c), raising the pitting potential and improving crevice corrosion performance. The Fe3O4 layer formed effectively blocks corrosive ions, making it difficult for the corrosion medium to penetrate the steel. Plasma carburizing and nitriding not only significantly increase the hardness and fatigue resistance of stainless steel but also enhance its inherent corrosion resistance, making these techniques excellent options for surface treatment (Borgioli et al. 2024).
3.2 Surface modifications coating technologies
Plasma-assisted carburizing, nitriding, or carbonitriding significantly boosts the corrosion resistance of metals in environments rich in CO2 and H2S. However, these treatments can increase the brittleness of pipelines, making them less suited to withstand the abrasive conditions encountered in oil and gas extraction. Pipeline coating technologies not only shield the substrate from corrosive agents but also reduce wear on the inner walls, proving to be highly effective protective measures in oil and gas extraction processes.
Ni–P coatings have garnered significant attention in the oil and gas corrosion field due to their superior corrosion resistance in acidic environments, facilitated by the formation of hypophosphite ions (Sun et al. 2019). Uncoated L360 steel, a linepipe-grade material commonly used in oil and gas pipelines, exposed to an H2S/Cl− environment (>2,300 ppm H2S, 5 wt% NaCl, and 0.5 wt% CH3COOH) for 1,440 h undergoes severe corrosion, forming pits over 50 μm on the surface as shown in Figure 7.
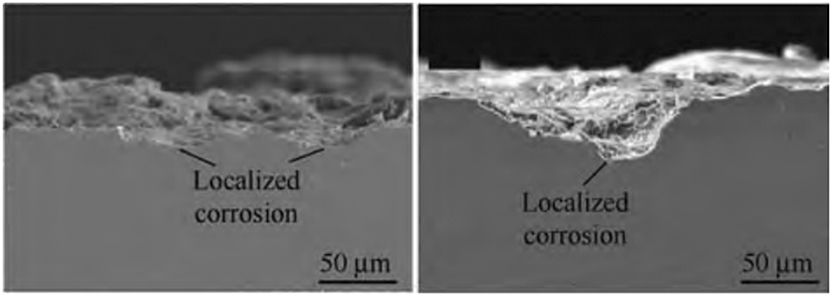
Corrosion impact on uncoated L360 steel in H2S/Cl− environment. Reprinted with permission from (Li et al. 2021b). Copyright@2021, Elsevier.
However, the application of Ni–P coatings drastically reduces the corrosion rate, with nickel dissolution forming corrosion products such as NiS and Ni3S2, which adhere to the outer layer of the coating. This prevents direct contact between the corrosive medium and the chemical plating. Stable NiO and Ni(OH)2 passivation layers act as barriers, effectively slowing down the corrosion rate. After 720 h in an H2S/Cl− environment, only micrometric pitting is observed, and uniform corrosion develops only after 1,440 h (Li et al. 2021b). Even under high-temperature, high-pressure CO2/H2S/Cl− conditions, Ni–P coatings continue to exhibit excellent corrosion resistance (Sun et al. 2019). Sulfides react with the coating during the mass transfer process of corrosion, depleting the sulfides and allowing only a minimal number of corrosive agents to penetrate the protective corrosion product layer and induce pitting. The dense corrosion product layer combined with the extended corrosion pathway greatly slows down the corrosion rate, enhancing the pipeline’s durability in oil and gas extraction environments.
Coatings serve a similar function to corrosion products in slowing the substrate’s corrosion rate by blocking corrosive agents from penetrating the base material. Moreover, coatings, compared to corrosion product layers, are denser and provide a better barrier effect. Some coatings, like Ni–P, can also absorb some corrosive ions, offering more effective protection of the substrate from both aspects. Beyond Ni–P coatings, Wang et al. (2016) have shown that Ni–Cr–Mo coatings prepared using a high-power diode laser on an X70 substrate exhibit stable anticorrosion properties in simulated H2S and CO2 solutions. Dushik et al. (2016) have identified hard W–C coatings prepared via chemical vapor deposition as promising due to their extremely low porosity of 0.02 % and excellent corrosion resistance from tungsten. These coatings were tested in 25 % hydrochloric acid (HCl) solution and 25 % HCl solution saturated with hydrogen sulfide (H2S), environments that simulate conditions in chemical and oil and gas industries.
3.3 Plasma coating technologies
While coating technologies have shown exceptional corrosion resistance in CO2/H2S environments, applying these coatings on the inner surfaces of pipelines during oil and gas extraction remains a challenge. Using hollow cathode plasma enhanced chemical vapor deposition (HC-PECVD), diamond-like carbon (DLC) films can be deposited inside pipelines. These DLC coatings are highly chemically inert and do not react with acids, bases, or salts (Wei et al. 2019; Wei et al. 2021a; Zhang et al. 2018), providing effective protection for the inner walls of pipelines under high-temperature, high-pressure gas environments containing both CO2 and H2S. Wang et al. (2013) have successfully created ultra-thick DLC coatings exceeding 50 μm through a multilayer structure design that manages compressive and tensile stresses. These coatings have been successfully applied to various pipe materials and diameters, including 304 stainless steel, aluminum, cast iron, and U-shaped pipelines (Wei et al. 2021b), as illustrated in Figure 8. Corrosion tests conducted under neutral salt spray for 480 h and 720 h, and high-temperature, high-pressure CO2 environments showed no significant damage to the coatings, as depicted in Figure 9. The corrosion morphology after salt spray tests indicates that DLC coatings have excellent corrosion resistance and long-term stability. Enhancements in corrosion resistance can be further achieved by doping the films with elements such as fluorine and nitrogen (Wei et al. 2022). The application of DLC films using HC-PECVD to pipeline interiors offers a promising new method to mitigate corrosion in oil and gas field environments.
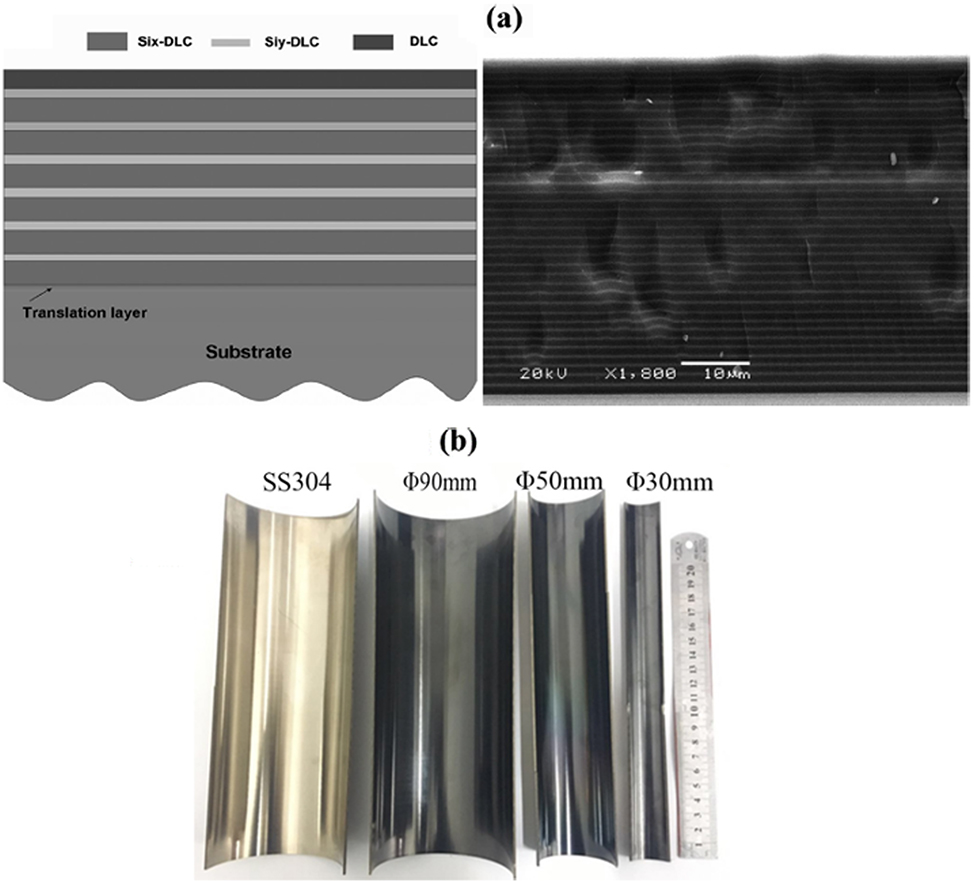
Applications of ultra-thick DLC coatings on various pipeline materials. (a) Schematic view and SEM image of the (Six-DLC/Siy-DLC)n/DLC film with a thickness of 52.8 μm. Reprinted with permission from (Wang et al. 2013). Copyright @ 2013 by American Chemical Society. (b) Si-DLC films deposited on the internal surface of SS304 pipes with various diameters. Reprinted with permission from (Zhang et al. 2018). Copyright @ 2018 by IOP publishing Ltd. All rights reserved.
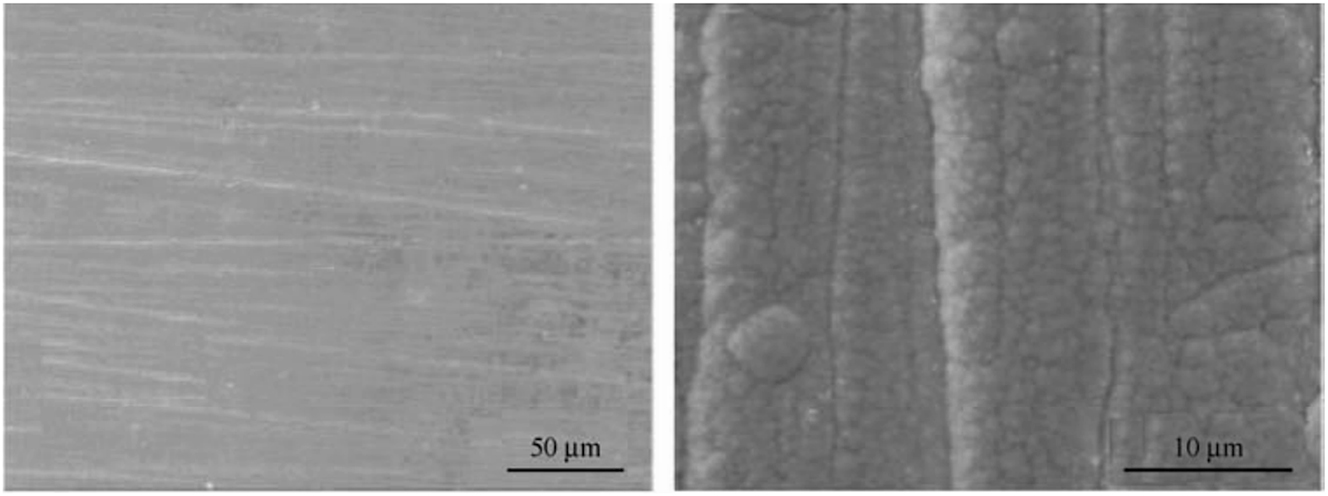
Durability of DLC coatings under high-temperature, high-pressure CO2 exposure (coating morphology on the inner surface of stainless pipe). Reprinted with permission from (Zhang et al. 2018). Copyright @ 2018 by IOP Publishing Ltd. All rights reserved.
3.4 Alloying of metal pipelines
Enhancing the corrosion resistance of metal pipelines in the oil and gas industry can be achieved through alloying with elements. These elements effectively reduce pitting and stress corrosion cracking by altering the morphology of corrosion products to form denser layers, which improve resistance (Davoodi et al. 2011; He et al. 2009). Researchers have conducted a study on the alloying of Ni, Cr, Mo, and Fe alloys in H2S–Cl− environments. Their findings indicate that the alloyed molybdenum reduces stress corrosion cracking (SCC) and pitting corrosion susceptibilities of the Ni, Cr, Mo, and Fe alloy in these conditions. The study specifically used alloys with compositions of 60 % Ni, 16 % Cr, and 8–16 % Mo with Fe as the balance (Tomio et al. 2015). Research has shown that in CO2/H2S environments, Cr-rich corrosion products like Cr(OH)3 enhance the protective barrier (Dong et al. 2020), and Ni-based alloys form protective layers of NiS and Ni oxides (Zhao et al. 2011). Titanium alloyed low-carbon steels have been noted to produce very dense and uniform protective films, substantially increasing their resistance to corrosive media (Yu et al. 2020). The introduction of Mo in Ni–Cr–Mo–Fe alloys has been particularly effective; it forms molybdenum sulfide during corrosion, which has excellent cation selectivity that helps reduce the activity of corrosive agents like H2S and promotes the formation of protective chromium oxide layers, thus mitigating both pitting and stress corrosion cracking (Clayton and Lu 1986; Tomio et al. 2015). The effectiveness of these alloying strategies is illustrated in Figure 10, which shows the significant reduction in corrosion rates and the durable nature of the developed alloys. These innovations underscore the ongoing research focus on developing alloy steel pipelines that balance corrosion resistance with mechanical strength and cost-efficiency for harsh oil and gas environments. The microstructure of Ni, Cr, Mo, and Fe alloys features a two layer surface film, consisting of an outer molybdenum sulfide layer and an inner chromium oxide layer. This arrangement greatly enhances SCC resistance by reinforcing the protective oxide film in H2S–Cl− conditions (Tomio et al. 2015).
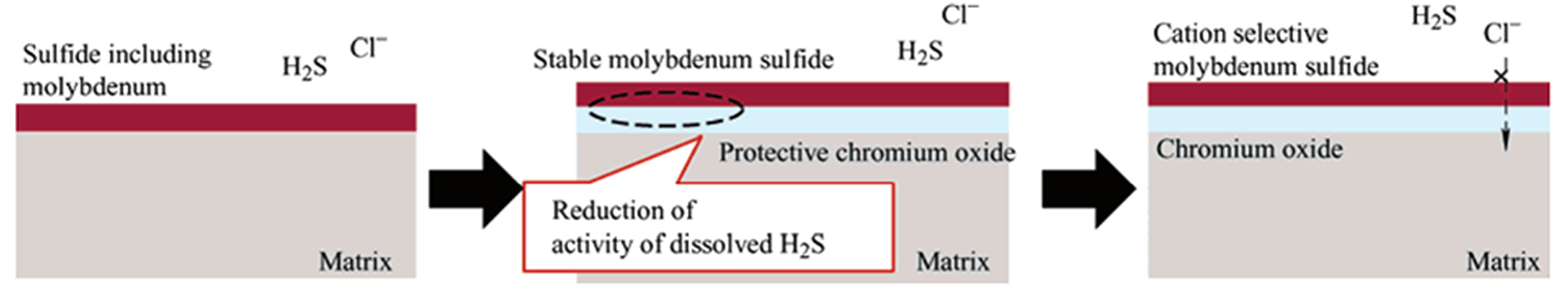
Impact of alloying elements on corrosion resistance in Ni–Cr–Mo–Fe alloys. Reprinted with permission from (Tomio et al. 2015). Copyright@2015, Elsevier.
3.5 Corrosion inhibitors
Injecting corrosion inhibitors is a cost-effective and simple method to control corrosion in oil and gas extraction. Liu et al. (2013) conducted a comprehensive study on four bis(benzimidazole) derivatives containing different heteroatoms and their corrosion inhibition effects on N80 steel in H2S solutions. They found that a stable adsorption monolayer of inhibitors on the inner walls of pipelines could slow down both anodic dissolution and cathodic reduction reactions. The effectiveness of corrosion inhibitors is closely linked to their adsorption properties, as they prevent corrosive media from contacting the pipeline walls, thus mitigating corrosion (Abboud et al. 2012). Consequently, Zhuoke et al. (2021) undertook a series of experiments to enhance the adsorptive capabilities of inhibitors and synthesized bis-Manich base TZBM containing a thiazole ring. It is an organic compound synthesized to enhance the adsorption properties of corrosion inhibitors. It incorporates a thiazole ring and functional groups capable of forming coordinate bonds with metal surfaces, thus improving its corrosion inhibition performance. The N and O atoms in TZBM molecules feature lone pairs of electrons that can enter the iron’s hybrid orbitals to form coordinate bonds, creating a stable six-membered ring structure that firmly adheres TZBM to the pipeline surface. An illustration of TZBM adsorption on Fe surfaces is shown in Figure 11.
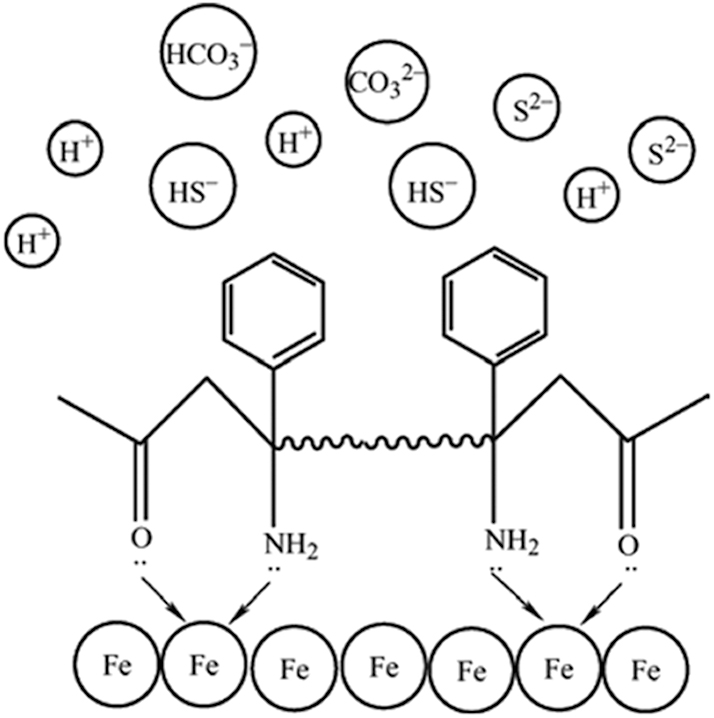
Adsorption mechanism of TZBM on Fe surfaces. Reprinted with permission from (Zhuoke et al. 2021). Copyright@2021, Elsevier.
However, due to the potential environmental pollution from corrosion inhibitors, their widespread use is limited. Thus, nontoxic, environmentally friendly corrosion inhibitors have received significant attention. Researchers exploring the corrosion inhibition properties of plant extracts such as banana peel, pomegranate peel, and citrus peel have found them effective in acidic environments containing HCl and H2S(O)4 (Ibrahim et al. 2012; Pradeep Kumar and Mohana 2014). Building on this, Zhang and Zhao (2018) further discovered the corrosion inhibitory effect of citrus peel in brine solutions containing CO2 and H2S. While various environmentally friendly inhibitors have been identified that are effective in acidic CO2/H2S environments, there is still a lack of research on their performance under the high temperature and pressure conditions of simulated oil and gas field environments. Additionally, the effects of external factors like temperature, pH, moisture content, and flow rate on their inhibition performance are not well-studied. Therefore, the development of eco-friendly corrosion inhibitors and their effectiveness in simulated real-world oil and gas field conditions will be a prominent area of future research.
4 Nonmetallic coatings
In the previous section, various mitigation solutions for hydrogen sulfide were discussed. Surface coatings represent one of the key approaches to mitigating corrosion risks in hydrogen sulfide environments, as they offer significant resistance to harsh conditions. Surface coatings are divided into metallic and nonmetallic categories. Nonmetallic coatings, in particular, are known for their resistance to corrosion, chemical stability, and adaptability to harsh environments, making them a reliable solution. However, due to the complexity and variability of nonmetallic coatings, a dedicated section is necessary to thoroughly examine their properties, performance, and potential applications. In the oil and gas industry, ensuring the durability and integrity of infrastructure is crucial, which emphasizes the importance of studying these coatings in detail. Nonmetallic coatings play a critical role in this context, safeguarding equipment against corrosion and environmental wear. These coatings are categorized into two main types: organic and inorganic (Harris et al. 1985). Organic coatings, primarily consisting of various polymers like epoxy and phenolic resins, are favored for their barrier protection against corrosive elements. In contrast, inorganic coatings include materials such as oxides and carbides that provide robust physical and chemical resistance.
Epoxy resins are noted for their straightforward application process and have demonstrated significant benefits in protective efficacy. For instance, studies show that epoxy coated J55 casing tubes exhibit wear resistance five times greater than that of their uncoated counterparts (Sangaj and Malshe 2004). Phenolic coatings, historically valued for their durability and environmental resistance, also continue to play a substantial role in the industry (Alibakhshi et al. 2014; Cui et al. 2019).
Advanced coatings such as Fusion Bonded Epoxy (FBE) and Amine-Cured Novolac Epoxy further illustrate the technological evolution in this field. FBE coatings are particularly renowned for their effectiveness in protecting steel pipelines from corrosion, capable of enduring the coating process where the resin powder is heated and chemically bonded to the pipeline (Bobby and Samad 2017; Farshad and Garber 1999). Similarly, Amine-Cured Novolac Epoxy coatings are known for their robust corrosion resistance and are commonly used as linings in chemical storage tanks due to their hard and cohesive film formation (Alkordy 2015; Heidersbach 2010).
Additionally, Bredero Shaw’s contributions to pipeline coating technologies highlight significant advancements in this sector. Their development of the High-Performance Composite Coating system demonstrates how multilayered coatings can enhance pipeline integrity and operational longevity (Guan et al. 2005).
4.1 FBE epoxy coating
FBE, a thermoset compound, represents a high-performance anticorrosion coating tailored for protecting both small and large diameter pipelines operating at moderate temperatures, offering exceptional protection (Kehr and Enos 2000). The FBE coating systems deliver effective protection within a moderate temperature range of −40 °C to 85 °C. Applied either in wet or dry powder form, typically at thicknesses ranging from 400 to 600 μm, onto the preheated steel pipe surface. Upon application and curing, the epoxy film forms an exceptionally robust surface with strong adherence to the steel substrate. Characterized by its homogeneous nature, the FBE protective layer demonstrates outstanding resistance to chemical reactions (Gbarnjah 2014). In general, standalone FBE coatings prove to be an optimal choice when accompanied by efficient construction and installation practices, along with controlled backfill aggregate, particularly in scenarios where operating temperatures remain below 65 °C (150 °F). For more demanding environmental conditions, such as higher temperatures or extreme installation circumstances, options like dual and multilayer FBE systems are available. Alternatively, in certain cases, a three-layer system comprising an FBE base layer, an adhesive, and a thick polyolefin layer may be specified. While these systems offer enhanced damage tolerance and an expanded high-temperature operating range, there are concerns among some researchers regarding their compatibility with cathodic protection measures. It is effective against general corrosion but is less effective in preventing stress corrosion cracking (SCC) due to its inability to address localized stresses and microstructural factors driving SCC (Kehr and Enos 2000).
FBE coating is predominantly employed as a unified corrosion protection layer. Fusion-bonded epoxy coatings demonstrate exceptional adhesion to steel, possess commendable chemical resistance, and importantly, do not inhibit the effectiveness of cathodic protection measures applied to the pipe. Despite its toughness, when installation damage arises, it can be promptly identified and resolved. FBE coating systems have a commendable history of installation with minimal damage. Considering both the extent of damage and repair costs, FBE coatings may offer the most economically viable solution to address field and construction damage. The application of FBE coating follows a straightforward process, which is one of its key advantages (3M 2015):
Cleaning: The pipe undergoes a thermal pickling process at 725 °F (385 °C), followed by blast cleaning according to NACE No. 1/SSPC-SP 5 standards, utilizing hardened steel grit or an appropriate mineral abrasive (Davis 1994).
Priming: In corrosive pipelines with high pressure, temperature, and CO2 or H2S, a primer is essential. For both phenolic and water-based systems, the primer is applied before heating. With phenolic primers, preheating removes volatile solvents and initiates partial curing. Precise control of this step is crucial for proper coating performance; overheating or full curing before FBE application can lead to inadequate adhesion. Water-based primers require application at temperatures below water’s boiling point. To prevent flash rusting, pipes are preheated to around 150 °F (66 °C) or undergo oil-free, compressed-air drying after application. If using induction coils for heating, the primer should be almost dry before entering the coil (Nayyar et al. 2000).
Heating: The pipe is heated to the temperature range recommended by the coating material supplier, typically around 350 °F (177 °C) for internal coatings.
Applying FBE: Various application techniques can be employed depending on the configuration of the component to be coated (Nayyar et al. 2000):
In the fluidized bed method, powder is aerated in a chamber using airflow, and the heated component is then dipped into the aerated powder.
In the flocking method, powder is sprayed onto the component using compressed air.
In the electrostatic spray technique, powder is sprayed onto the preheated component using compressed air. An electrostatic charge is applied to enhance powder usage efficiency.
Curing: For heavy-walled items, typically 1⁄8 in (3.2 mm) thickness or more, some FBE systems utilize the metal’s heat retention to achieve curing without requiring postcure facilities. Phenolic primer systems usually necessitate a postcure heating step to complete the primer’s chemical reaction. Water-based primer systems rely on the fusion-bonded epoxy for postcure conditions, typically aligning with the application temperature range. However, for metal prone to outgassing like cast iron, it’s vital to postcure at a temperature below the application temperature. For instance, a component lined at 400 °F (204 °C) might undergo postcuring at 350 °F (177 °C). In extreme cases, a preheating outgassing step may be necessary, involving several h at 660–725 °F (350–385 °C), before cooling to the application temperature and applying the lining (Nayyar et al. 2000).
Cooling: Cooling is accomplished either by spraying water on the outside and/or inside of the pipe or by letting it cool in ambient air (3M 2015).
Inspection: After cooling is finished, but before storage, the pipe must undergo continuity inspection following NACE Standard RP0490-01. A steel spring or conductive rubber search electrode is used for this purpose. Additionally, the coating thickness is measured using calibrated gauges to ensure it meets the specified minimum thickness (3M 2015).
In summary, FBE coating is a protective layer applied to pipelines to combat corrosion and ensure their long-term functionality, especially in transporting various fluids like oil, gas, and water. Its significance lies in preserving the integrity of pipelines. FBE offers multiple benefits, including corrosion resistance, increased durability, improved flow efficiency, and decreased maintenance expenses. Moreover, it aids in environmental protection by preventing leaks and spills. The lifespan of FBE coating on pipelines varies based on factors such as environmental conditions, usage, and application quality, but it can endure for decades with proper application and upkeep. Although highly durable, FBE coatings may require periodic inspections and touch-up repairs to address damage or wear, thereby prolonging their effectiveness against corrosion. Regular maintenance is crucial for extending the coating’s lifespan and ensuring continued pipeline protection.
4.2 Phenolic coatings
Phenolic coatings, particularly those based on novolac phenolic resins, have proven to be highly effective in combating internal corrosion in pipelines exposed to hydrogen sulfide (H2S). These coatings are well-suited for the harsh conditions found in oil and gas environments, where high temperatures and corrosive elements like H2S and CO2 are prevalent. The inherent chemical stability, high-temperature resistance, and excellent barrier properties of phenolic coatings make them a reliable choice for protecting pipeline infrastructure from the detrimental effects of H2S. Studies have shown that phenolic coatings not only resist the permeation of corrosive ions but also maintain their integrity in extreme conditions, significantly reducing the risk of corrosion-related failures in pipelines (Hao et al. 2020; Jiang et al. 2023). A coating is referred to as phenolic coating if it contains phenolic acids, which are identified by the presence of carboxylic acid in their molecular structure (Altemimi et al. 2017). These are further broken down into hydroxycinnamic and hydroxybenzoic acids, of which 3-hydroxy, 4-hydroxy, 2,4-dihydroxy, and gallic acids are typical examples. The two primary classes of phenolic acids are hydroxybenzoic acids, which don’t have a side chain, and hydroxycinnamic acids, which have a double bond in the side chain. P-coumaric, caffeic, ferulic, and sinapic acids are the primary components of hydroxycinnamic acids (Altemimi et al. 2017). Phenolic compounds possess distinct beneficial properties that are attributed to their molecular structure, specifically the quantity and location of hydroxyl groups on the ring. The chemical characteristics of phenolic compounds can be altered through substitution, which will also affect their solubility, stability, and reactivity (Mu’azu et al. 2017). Figure 12 shows the chemical structure of the phenolic acid substitution patterns of phenolic compounds (Mu’azu et al. 2017).
Phenolic acid structure. Originally published in (Mu’azu et al. 2017) under the terms CC BY 4.0 license. Available from: doi.org/10.3390/ijerph14101094.
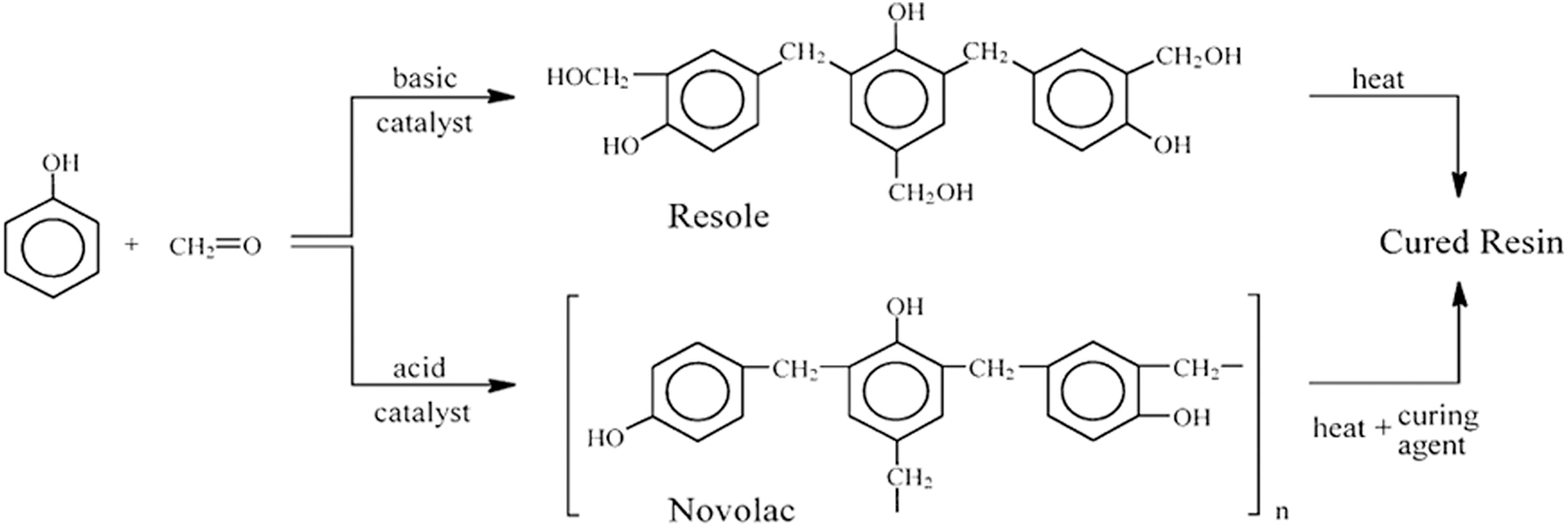
Phenol is obtained by several synthesis procedures and fractional coal tar extraction methods. The industrial synthesis process for producing phenols uses a variety of commercial methods. Below is a detailed explanation of some of the highly typical processes seen in industries like Dow, Raschig, and Cumene (Dodiuk and Goodman 2014). Phenolic coatings have limited flame propagation, stability at high temperatures, and are quite inexpensive (Gourichon et al. 2008; Guilleminot et al. 2008). They are utilized as flame-retardant coatings in a variety of industries, including electronics, aircraft, automotive, and oil and gas industry. In the coatings industry, phenolic coatings are frequently referred to as a “workhorse,” this is because of their ability to offer resistance against substances like chemicals, indirect moisture, and even wet conditions (Gourichon et al. 2008; Guilleminot et al. 2008). Phenolic coatings can be categorized into two main types: novolac phenolic coatings and resole phenolic coatings.
4.2.1 Novolac phenolic coating
Novolac phenolic coatings, as mentioned in the previous section, are well-known for their effectiveness in combating internal corrosion in pipelines when they are exposed to hydrogen sulfide (H2S). These coatings are also known as two-stage phenolics, and they are created when formaldehyde and phenol react in a very acidic environment (Ismail et al. 2020). Formaldehyde content in the reaction mixture is typically low, ranging from 0.75 to 0.85 mol per mole of phenol (Allen and Ishida 2001). The most often utilized acids in the production of novolac phenolic resins include toluene sulfonic, hydrochloric, sulfuric, and oxalic acids. Because they are thermoplastic, novolacs melt in the presence of heat but do not cross-link. Novolac resins, in contrast to resoles, need to have a hardener added in order to cure into an insoluble, infusible product (Allen and Ishida 2001). These coating have high chemical resistance and are used where low heat is required (Allen and Ishida 2001).
4.2.2 Resol phenolic coatings
Resol phenolic coatings have demonstrated significant effectiveness in combating internal corrosion in pipelines exposed to hydrogen sulfide (H2S). Due to their robust chemical resistance, thermal stability, and strong adhesive properties, these coatings provide a reliable barrier against the harsh corrosive environments typically found in the oil and gas industry (Pilato 2013). The ability of resol phenolic coatings to form a highly cross-linked polymer network under various curing conditions enhances their durability, making them well-suited for protecting pipeline infrastructure from the detrimental effects of H2S (Zaldivar et al. 2022). Their widespread applicability in demanding environments underscores their importance in maintaining the integrity of pipelines subjected to corrosive agents like hydrogen sulfide (Grenier-Loustalot et al. 1996). Resoles, also known as single-stage phenolics, are created when phenol reacts excessively with formaldehyde in the presence of an alkaline catalyst as shown in Figure 13, such as sodium hydroxide, ammonia, or sodium carbonate (Allen and Ishida 2001; Ismail et al. 2020). A phenol to formaldehyde molar ratio of between 1: 1 and 1: 1.3 is typically utilized (Allen and Ishida 2001). Although reaction products are typically liquids, they can be vacuum-dried at low temperatures to produce a solid intermediate if needed. Resoles can have a broad variety of molecular weights and compositions, depending on the kind and quantity of catalyst, molar ratio, and reaction conditions (Allen and Ishida 2001). As a result of their ability to cure in a heated mold without the need for an extra catalyst or curing agent, resoles are regarded as single-stage phenolic resins. Water is released as a byproduct of the polycondensation reaction known as thermal polymerization, which needs temperatures between 130 and 200 °C. They are also highly cross linked, which helps them excel high temperature applications (Allen and Ishida 2001). Resol are usually liquid while novolac are solid (Pizzi and Ibeh 2014).
Phenol and formaldehyde reaction with an acid and base catalyst. Reprinted with permission from (Allen and Ishida 2001). Copyright@2001, Elsevier.
One of the earliest low-cost synthetic resins, resole phenolic resin has excellent mechanical, solvent, and weather resistance qualities (Sandhya et al. 2019). These qualities make it a great option for a wide range of applications in the automotive, aerospace, and thermal insulation industries. They are also very advantageous for composite preparation due to their fire smoke, low toxicity characteristics (Sandhya et al. 2019). These coatings are used where high temperatures are required.
One of the primary disadvantages associated with these coatings is their inherent lack of durability, particularly noticeable during their curing process (Kandola and Horrocks 2001). A significant issue arises as water is generated during this stage, which poses a challenge due to the potential for this water to become trapped within the composite material (Kandola and Horrocks 2001). In the unfortunate event of a fire, the presence of this trapped water can lead to the generation of steam, creating internal pressure that induces microcracks and delamination, compromising the material’s structural integrity (Kandola and Horrocks 2001). After undergoing the curing process, phenolic coatings may experience a transformation, becoming brittle and stiff. This change in their physical properties can lead to issues such as cracking or chipping when subjected to mechanical stress (Allen and Ishida 2001). Therefore, to enhance the durability and structural integrity of phenolic resins, it is common practice to incorporate fillers or other reinforcements into the formulation. Such measures help to mitigate the brittleness associated with phenolic coatings, ensuring better overall performance and longevity (Allen and Ishida 2001). Various factors, including temperature, humidity levels, and the duration of the curing process, all play significant roles in influencing the extent of cross-linking reactions and, consequently, impact the overall effectiveness and quality of the coating (Ebnesajjad 2011).
4.3 Epoxy–phenolic coatings
Epoxy–phenolic coatings are hybrid coatings that combine the properties of both epoxy resins and phenolic resins. Epoxy resins are widely used in various industries; however, they are known for their limitations, including poor heat resistance and toughness. To address these issues and enhance overall performance, numerous research studies have been conducted to develop new formulations, additives, and manufacturing techniques, aimed at mitigating these weaknesses and optimizing the properties of epoxy resins (Zhao et al. 2022). However, because the phenolic resin contains a high concentration of aromatic cycles, which are known for their stability and heat resistance, the material exhibits excellent thermal properties when exposed to elevated temperatures (El Gazzani et al. 2016).
Aldehyde groups present in phenolic resin play a significant role in enhancing the intermolecular cross-linking of the epoxy resin by forming strong chemical bonds with the epoxy groups (Zhao et al. 2022). This reaction not only boosts the overall structural integrity but also contributes to the enhanced durability and performance of the resulting composite material. However, epoxy–phenolic curing is done at high temperature around 120–240 °C. In a recent study, the performance of phenol-epoxy coatings was improved through postcuring, which involved exposure to 120 °C for up to 40 days (Zhao et al. 2022).
The findings imply that the presence of hydrogen, chloride, hydrogen sulfide (H2S), and carbon dioxide (CO2) ions exerts minimal influence on the degradation of epoxy–phenolic inner coating tubing as the concentration of ions increases (Deng et al. 2022). Despite this observation, it is noteworthy that the detrition of organic coatings can experience a notable escalation due to elevated flow rates when subjected to 120 °C conditions. This suggests that while the ion concentrations themselves have limited impact on the failure mechanisms observed, environmental factors such as temperature and flow play a crucial role in accelerating the breakdown of protective coatings (Wu et al. 2020). Therefore, it becomes apparent that a combination of ion concentration and external conditions contributes to the overall deterioration of the coatings, highlighting the complex interplay of various factors in the degradation process.
The rate at which ions and water penetrate the coating is crucial in determining its durability and longevity. This process plays a significant role in the overall performance of the coating, as the speed at which it fails is directly influenced by how quickly these substances can permeate through its protective layers. When exposed or damaged holes are present, this penetration process is accelerated (Wu et al. 2020).
4.4 Urethane
Urethane coatings have proven to be effective barriers against the corrosive effects of hydrogen sulfide (H2S). The application of urethane coatings provides a protective layer that resists the permeation of H2S, thereby preventing its interaction with the metal surface and mitigating corrosion (Vakili et al. 2024). This protective capability makes urethane coatings a valuable solution for prolonging the lifespan of pipelines exposed to H2S, ensuring safety and reducing maintenance costs. Studies have shown that urethane coatings exhibit excellent adhesion, chemical resistance, and durability, making them suitable for use in harsh environments where H2S is present (Mohtadi et al. 2003). These properties underscore the potential of urethane coatings as an effective measure to combat internal corrosion caused by H2S in pipelines and other industrial systems. They are essential in environments where H2S, a highly corrosive gas often present in oil and gas extraction and processing, poses significant material degradation risks (Chikezie Nwaoha 2017).
Polyurethane coatings are employed primarily due to their excellent chemical resistance, mechanical strength, and flexibility, which make them ideal for the harsh conditions inside pipelines (Polyurethane Coating: Uses, Types, Advantages, and Disadvantages 2024). The coatings form a barrier that prevents H2S and other corrosive substances from coming into contact with the metal surfaces of pipelines, thereby prolonging the infrastructure’s operational life and enhancing safety (Bickham 2020).
In the oil and gas sector, PU coatings are specifically applied within the internal surfaces of pipelines that transport crude oil or natural gas. These pipelines are often located in extraction units and refineries where the presence of H2S is a common challenge (Solovyeva et al. 2023). The internal coating of these pipelines is critical as it directly interacts with the transported materials, which may contain corrosive elements like H2S (Samimi 2012).
To ensure that polyurethane coatings effectively protect against H2S corrosion, they must comply with industry standards such as ASTM D16, which provides guidelines for the classification, requirements, and testing of protective coatings in the oil and gas industry (D16 Standard Terminology for Paint, Related Coatings, Materials, and Applications 2024). Following such standards ensures that the coatings are capable of withstanding specific environmental conditions, including the presence of H2S (Standard Recommended Practice Liquid-Applied Internal Protective Coatings for Oilfield Production Equipment 1994).
Polyurethane coatings can be classified based on type and additive content. Additives are typically included to reduce costs, but it is important to note that cost-reducing additives can also decrease quality. Adding 10–20 % filler material (such as tar) can effectively lower costs with minimal impact on coating properties. However, increasing filler content to 40 % or more significantly reduces costs but also substantially degrades the coating’s performance. Common fillers in 100 % solid polyurethane include raw oil, asphalt, or tar pitch. It is crucial to note that tar pitch is carcinogenic (Samimi 2012). Table 4 shows a comparison of effectiveness tests for different coating types, highlighting the differences in performance and cost between epoxy and polyurethane coatings.
Comparison of epoxy and polyurethane coatings based on various tests (Samimi 2012).
| Property | Epoxy | Polyurethane | Cost comparison |
|---|---|---|---|
| Impact resistance | 1/8 J | 2/3 J | Higher |
| Flexibility | 2 mandrills | 1 mandrill | Lower |
| Abrasion resistance | 120 mg loss | 52 mg loss | Lower |
| Adhesion strength | 9/7 cm2 | 3/2 cm2 | Lower |
| Tensile strength | 705 N/cm2 | 1,410 N/cm2 | Higher |
| Water vapor permeability | 0.0041 perm cm | 0.0041 perm cm | Lower |
Urethane, a key component in polyurethane, contributes significantly to the coating’s performance due to its robustness and chemical resistance (Polyurethane Coating: Uses, Types, Advantages, and Disadvantages 2024). Researchers and manufacturers have experimented with various formulations to enhance the efficacy and durability of PU coatings:
4.4.1 Rapeseed oil-based polyurethane
Rapeseed oil-based polyurethane coatings are increasingly utilized in the industry due to their strong resistance to hydrogen sulfide (H2S)-induced corrosion, particularly in pipelines (Beauchamp et al. 1984). These coatings, derived from renewable resources, offer effective protection by creating a durable barrier against corrosive agents, extending the lifespan of infrastructure while maintaining environmental sustainability. Innovations include using bio-based polyols derived from rapeseed oil, offering an eco-friendlier alternative while maintaining or even enhancing the protective qualities against H2S (Fridrihsone-Girone et al. 2016). These coatings are particularly valued in environments where VOC (Volatile Organic Compound) emissions are a concern, providing a sustainable solution without compromising on performance (Stirna et al. 2013).
4.4.2 Hybrid systems
Combining polyurethane with other materials such as epoxy (as seen in products like Eton-SAP) can leverage the benefits of both materials (Keresten et al. 2021). Epoxy provides excellent adhesion and corrosion resistance, while polyurethane offers flexibility and durability, making the hybrid coating well-suited for dynamic environments where pipelines experience temperature fluctuations and physical stresses (Guan et al. 2005). Table 5 presents the results of Polykron-R compared to Forpol-60 and Forpol-80, tested under DIN EN 10290. Polykron-R shows lower adhesion (12 MPa vs. 14.9 MPa and 15.3 MPa) and tensile strength (25 MPa vs. 35 MPa for both), but it has higher tensile elongation (31 % vs. 23.2 % and 21 %). In cathodic disbondment resistance, Polykron-R shows 5.7 mm2 at 60 °C, slightly higher than Forpol-60 (4.5 mm2) and Forpol-80 (4.9 mm2). Despite slightly lower mechanical properties, Polykron-R offers competitive performance for manual application.
Comparative test results of Polykron-R and typical PU coatings (Keresten et al. 2021).
| Material | Polykron-R (hybrid system) | Forpol-60 | Forpol-80 |
|---|---|---|---|
| Application | Manual | Airless | Airless |
| Set up time (min) | 45 | 1 | 1 |
| Adhesion to steel (MPa) | 12 | 14.9 | 15.3 |
| Tensile strength (MPa) | 25 | 35 | 35 |
| Tensile elongation (%) | 31 | 23.2 | 21 |
| Area of cathodic disbondment after 1,000 h in 3 % NaCl | 5.7 (60 °C) | 4.5 (60 °C) | 4.9 (80 °C) |
4.4.3 Surface modification techniques
Advanced techniques such as adding fluorinated alkylsilane and barium titanate nanospheres or employing chemical vapor deposition (CVlD) to deposit hydrophobic materials like polydimethylsiloxane (PDMS) are explored to enhance the hydrophobic properties of PU coatings (Ballauff et al. 2023). It is a widely used silicon-based polymer known for its excellent chemical and thermal stability (Dalla Monta et al. 2018; Giri et al. 2012; Ren et al. 2020; Zeng and Taylor 2020), biocompatibility (Montazerian et al. 2019; Rao et al. 2013; Souza et al. 2020), corrosion resistance (Eduok et al. 2017; Salazar-Hernández et al. 2019), flexibility (An et al. 2017; Pinho et al. 2019; Wolf et al. 2018), repeatability (Amjadi et al. 2014), low cost (Yun et al. 2017), ease of use, chemical inertness, hyperplastic properties, and gas permeability (Adiguzel et al. 2017; Rodrigues et al. 2015). However, its applications can be limited by low mechanical properties, such as low elastic modulus and strength. To address this, bulk modifications can be made to create PDMS composites with improved properties. This can be achieved by incorporating free molecules (nano or microparticles) or altering the prepolymer composition (Wolf et al. 2018).
5 Summary and industry recommendations
This study addresses the ongoing issue of internal corrosion in oil and gas pipelines, focusing specifically on the corrosive effects of hydrogen sulfide (H2S). Traditional methods like corrosion-resistant alloys and inhibitors often fail under the high-pressure, high-temperature conditions typical in the oil and gas industry. This research highlights the effectiveness of coating technologies, such as epoxy and other nonmetallic coatings, in providing superior protection against H2S-induced corrosion.
When considering the best approach to combat internal corrosion in oil and gas pipelines, especially due to hydrogen sulfide (H2S), it is important to understand the strengths and limitations of various techniques. Table 6 shows the strengths and limitations of different mitigation methods such as plasma diffusion techniques, plasma coating technologies, alloying of metal pipelines, and corrosion inhibitors.
Strengths and limitations of different mitigation solutions.
| Method | Strengths | Limitations |
|---|---|---|
| Plasma diffusion techniques | Enhance the corrosion and wear resistance of austenitic stainless steels by embedding interstitial atoms into the metal surface | High-temperature nitriding can lead to chromium nitride precipitation, which reduces corrosion resistance. Careful control of process parameters is needed to avoid this issue |
| Surface metallic coating technologies | Ni–P provide excellent protection against corrosion, significantly extending the durability of pipelines in harsh environments | Some surface coatings can induce residual stresses or microstructural changes that may lead to increased brittleness. This makes the pipelines more prone to cracking or failure under mechanical stress in abrasive conditions |
| Plasma coating technologies | DLC coatings provide excellent protection against corrosion in CO2 and H2S environments, enhancing pipeline durability | Coating the inner surfaces of pipelines is technically challenging and requires advanced techniques like HC-PECVD |
| Alloying of metal pipelines | Alloying pipelines with elements like Ni, Cr, Mo, and Ti significantly improves their corrosion resistance by forming dense, protective layers that reduce pitting and stress corrosion cracking. Higher strength in low alloy steels minimizes localized deformation, enhancing SCC resistance | Adding alloying elements can increase the cost of pipeline materials, making them more expensive to produce and implement in large-scale oil and gas operations |
| Corrosion inhibitors | Injecting corrosion inhibitors is a simple and cost-effective method to control corrosion in oil and gas pipelines | Some corrosion inhibitors can cause environmental pollution, limiting their widespread use. There is a need for more research on environmentally friendly alternatives and their performance under real-world conditions |
Having discussed the strengths and limitations of the mitigation solutions above, Table 7 will show the strengths and limitations for the nonmetallic coatings: FBE, phenolics, epoxy–phenolics, and polyurethane.
Strengths and limitations of different nonmetallic coatings.
| Method | Strengths | Limitations |
|---|---|---|
| FBE | Fusion-bonded epoxy (FBE) coatings provide exceptional protection against corrosion for pipelines, with strong adhesion to steel and high chemical resistance, ensuring long-term durability | There are concerns about the compatibility of some FBE coating systems with cathodic protection measures, which could affect their overall effectiveness in certain conditions |
| Phenolics | Phenolic coatings offer excellent resistance to chemicals, high temperatures, and flame propagation, making them suitable for various industrial applications, including electronics, automotive, and oil and gas | Phenolic coatings can become brittle and prone to cracking or chipping after curing, particularly when subjected to mechanical stress, requiring additional reinforcement to enhance durability |
| Epoxy–phenolics | Epoxy–phenolic coatings combine the strengths of both epoxy and phenolic resins, providing excellent thermal stability and chemical resistance, making them suitable for harsh environments | Despite their strong resistance to ion concentration, epoxy–phenolic coatings can deteriorate under high temperatures and flow rates, which can accelerate the breakdown of the protective layers |
| Polyurethane | Polyurethane (PU) coatings provide strong chemical resistance and mechanical strength, making them ideal for protecting pipeline interiors from hydrogen sulfide (H2S) and other corrosive substances | Adding cost-reducing fillers can degrade the performance of PU coatings. High filler content can compromise the coating’s durability and protective properties, necessitating a balance between cost and quality |
This study identifies nonmetallic coatings, especially epoxy-based ones, as the best solution for mitigating internal corrosion in oil and gas pipelines caused by hydrogen sulfide (H2S). These coatings excel in chemical resistance, durability, and ease of application compared to traditional methods. Despite varying strengths and limitations among different types, the overall performance of nonmetallic coatings makes them highly recommended for industry use.
Adopting nonmetallic coatings offers the oil and gas industry a reliable and cost-effective way to extend pipeline lifespan, reduce maintenance costs, and enhance safety. Continued research is crucial to optimize these coatings for diverse conditions and to develop environmentally friendly alternatives that maintain high performance.
Acknowledgments
The authors would like to acknowledge the support of King Fahd University of Petroleum and Minerals (KFUPM), Dhahran, 31261 for funding this work.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Research funding: Not applicable.
-
Data availability: Not applicable.
References
3M, 2015 3M (2015). 3M TM scotchkote TM fusion-bonded epoxy coating 6233P. July, pp. 1–6.Search in Google Scholar
Abboud, Y., Hammouti, B., Abourriche, A., Ihssane, B., Bennamara, A., Charrouf, M., and Al-Deyab, S.S. (2012). 2-(o-Hydroxyphenyl)benzimidazole as a new corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 7: 2543–2551, https://doi.org/10.1016/s1452-3981(23)13900-9.Search in Google Scholar
Adiguzel, Z., Sagnic, S.A., and Aroguz, A.Z. (2017). Preparation and characterization of polymers based on PDMS and PEG-DMA as potential scaffold for cell growth. Mater. Sci. Eng.: C 78: 942–948, https://doi.org/10.1016/J.MSEC.2017.04.077.Search in Google Scholar
Ahmad, W., Sethupathi, S., Kanadasan, G., Lau, L.C., and Kanthasamy, R. (2021). A review on the removal of hydrogen sulfide from biogas by adsorption using sorbents derived from waste. Rev. Chem. Eng. 37: 407–431, https://doi.org/10.1515/revce-2018-0048.Search in Google Scholar
Albiter, A. (2020). Sulfide stress cracking assessment of carbon steel welding with high content of H2S and CO2 at high temperature: a case study. Engineering 12: 863–885, https://doi.org/10.4236/ENG.2020.1212061.Search in Google Scholar
Al-Ghanem, F., Al-Jabri, S., Abdul Hameed, M.R., Al-Saeed, M., and Al-Otaibi, M. (2019) Cutting-edge solutions for sour gas treatment. A retrospective study by Kuwait oil company. In: Society of Petroleum Engineers - Abu Dhabi International Petroleum Exhibition and Conference 2018, ADIPEC 2018.10.2118/192783-MSSearch in Google Scholar
Alibakhshi, E., Ghasemi, E., and Mahdavian, M. (2014). The influence of surface modification of lithium zinc phosphate pigment on corrosion inhibition of mild steel and adhesion strength of epoxy coating. J. Sol-Gel Sci. Technol. 72: 359–368, https://doi.org/10.1007/S10971-014-3441-2/METRICS.Search in Google Scholar
Al-Janabi, Y.T. (2020) An overview of corrosion in oil and gas industry: upstream, midstream, and downstream sectors. In: Corrosion inhibitors in the oil and gas industry, pp. 3–39.10.1002/9783527822140.ch1Search in Google Scholar
Alkordy, F.M. (2015). Evaluation of organic protective coatings as corrosion prevention for the interior of subsea pipelines in sour gas service, FIU electronic theses and dissertations.Search in Google Scholar
Allen, D.J. and Ishida, H. (2001). Thermosets: phenolics, novolacs, and benzoxazine. In: Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., and Veyssière, P. (Eds.). Encyclopedia of materials: science and technology. Elsevier, Amsterdam, Netherlands, pp. 9226–9229.10.1016/B0-08-043152-6/01662-4Search in Google Scholar
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D., and Lightfoot, D. (2017). Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6: 42, https://doi.org/10.3390/plants6040042.Search in Google Scholar PubMed PubMed Central
Amjadi, M., Pichitpajongkit, A., Lee, S., Ryu, S., and Park, I. (2014). Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 8: 5154–5163, https://doi.org/10.1021/NN501204T/SUPPL_FILE/NN501204T_SI_003.AVI.Search in Google Scholar
An, A.K., Guo, J., Lee, E.J., Jeong, S., Zhao, Y., Wang, Z., and Leiknes, T.O. (2017). PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 525: 57–67, https://doi.org/10.1016/J.MEMSCI.2016.10.028.Search in Google Scholar
Ausma, T. and De Kok, L.J. (2019). Atmospheric H2S: impact on plant functioning. Front. Plant Sci. 10, https://doi.org/10.3389/fpls.2019.00743.Search in Google Scholar PubMed PubMed Central
Ballauff, M., Maschke, U., Alamo, R.G., Białkowska, A., Bakar, M., Kucharczyk, W., and Zarzyka, I. (2023). Hybrid epoxy nanocomposites: improvement in mechanical properties and toughening mechanisms. A review. Polymers 15: 1398, https://doi.org/10.3390/POLYM15061398.Search in Google Scholar PubMed PubMed Central
Beauchamp, R.O., Bus, J.S., Popp, J.A., Boreiko, C.J., Andjelkovich, D.A., and Leber, P. (1984). A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 13: 25–97, https://doi.org/10.3109/10408448409029321.Search in Google Scholar PubMed
Bell, T. and Sun, Y. (2002). Low-temperature plasma nitriding and carburising of austenitic stainless steels. Heat Treat. Met. 29: 57–64.Search in Google Scholar
Bickham, R.A. (2020). Battling internal corrosion in oil and gas pipelines, https://doi.org/10.5006/mp2020_59_4-16, https://www.materialsperformance.com/articles/material-selection-design/2020/05/battling-internal-corrosion-in-oil-and-gas-pipelines.Search in Google Scholar
Bobby, S. and Samad, M.A. (2017). Enhancement of tribological performance of epoxy bulk composites and composite coatings using micro/nano fillers: a review. Polym. Adv. Technol. 28: 633–644, https://doi.org/10.1002/PAT.3961.Search in Google Scholar
Borgioli, F., Adachi, S., and Lindner, T. (2024). Advances in low-temperature nitriding and carburizing of stainless steels and metallic materials: formation and properties. Metals 14: 1179, https://doi.org/10.3390/MET14101179.Search in Google Scholar
Castaneda, H., Sosa, E., and Espinosa-Medina, M.A. (2009). Film properties and stability influence on impedance distribution during the dissolution process of low-carbon steel exposed to modified alkaline sour environment. Corros. Sci. 51: 799–806, https://doi.org/10.1016/j.corsci.2009.02.002.Search in Google Scholar
Chikezie, Nwaoha (2017). The science behind oil and natural gas pipeline corrosion and coatings, https://www.corrosionpedia.com/the-science-behind-oil-and-natural-gas-pipeline-corrosion-and-coatings/2/6682.Search in Google Scholar
Clayton, C.R. and Lu, Y.C. (1986). A bipolar model of the passivity of stainless steel: the role of Mo addition. J. Electrochem. Soc. 133: 2465–2473, https://doi.org/10.1149/1.2108451.Search in Google Scholar
Cui, M., Ren, S., Pu, J., Wang, Y., Zhao, H., and Wang, L. (2019). Poly(o-phenylenediamine) modified graphene toward the reinforcement in corrosion protection of epoxy coatings. Corros. Sci. 159: 108131, https://doi.org/10.1016/J.CORSCI.2019.108131.Search in Google Scholar
D16 Standard Terminology for Paint, Related Coatings, Materials, and Applications (2024), https://www.astm.org/standards/d16.Search in Google Scholar
Dalla Monta, A., Razan, F., Le Cam, J.B., and Chagnon, G. (2018). Using thickness-shear mode quartz resonator for characterizing the viscoelastic properties of PDMS during cross-linking, from the liquid to the solid state and at different temperatures. Sens. Actuators A: Phys. 280: 107–113, https://doi.org/10.1016/J.SNA.2018.07.003.Search in Google Scholar
Davis, R.H. (1994). The use of internal plastic coatings to mitigate CO2 corrosion in downhole tubulars. In: National Association of Corrosion Engineers (NACE) international annual conference, 28 Feb – 4 March 1994, OSTI.GOV. Baltimore, MD, USA.10.5006/C1994-94023Search in Google Scholar
Davoodi, A., Pakshir, M., Babaiee, M., and Ebrahimi, G.R. (2011). A comparative H2S corrosion study of 304L and 316L stainless steels in acidic media. Corros. Sci. 53: 399–408, https://doi.org/10.1016/j.corsci.2010.09.050.Search in Google Scholar
Deng, C.-M., Zhu, Y., Sun, S., Wei, J., and Xia, D.-H. (2022). Analysis of failure causes of epoxy-phenolic coated tinplate after boiling sterilization. Eng. Fail. Anal. 135: 106129, https://doi.org/10.1016/j.engfailanal.2022.106129.Search in Google Scholar
Dodiuk, H. and Goodman, S. (2014). Handbook of thermoset plastics, Vol. 254, https://www.researchgate.net/publication/270957167_Handbook_of_Thermoset_Plastics.Search in Google Scholar
Dong, B., Liu, W., Zhang, Y., Banthukul, W., Zhao, Y., Zhang, T., Fan, Y., and Li, X. (2020). Comparison of the characteristics of corrosion scales covering 3Cr steel and X60 steel in CO2-H2S coexistence environment. J. Nat. Gas Sci. Eng. 80, https://doi.org/10.1016/j.jngse.2020.103371.Search in Google Scholar
Dushik, V.V., Lakhotkin, Y.V., Kuzmin, V.P., and Rozhanskii, N.V. (2016). The corrosion behavior of hard W–C system chemical vapor deposition layers in HCl and H2S aqueous solutions. Protect. Met. Phys. Chem. Surface 52: 1153–1156, https://doi.org/10.1134/S2070205116070042.Search in Google Scholar
Ebnesajjad, S. (2011) 8 - characteristics of adhesive materials. In: Ebnesajjad, S. (Ed.). Handbook of adhesives and surface preparation. William Andrew Publishing, pp. 137–183, https://www.sciencedirect.com/science/article/pii/B9781437744613100082.10.1016/B978-1-4377-4461-3.10008-2Search in Google Scholar
Eduok, U., Faye, O., and Szpunar, J. (2017). Recent developments and applications of protective silicone coatings: a review of PDMS functional materials. Prog. Org. Coat. 111: 124–163, https://doi.org/10.1016/J.PORGCOAT.2017.05.012.Search in Google Scholar
El Gazzani, S., Nassiet, V., Habas, J.-P., Freydier, C., and Hilleshein, A. (2016). High temperature epoxy foam: optimization of process parameters. Polymers 8: 215, https://doi.org/10.3390/polym8060215.Search in Google Scholar PubMed PubMed Central
Farshad, F. and Garber, J. (1999) Relative roughness chart for internally coated pipes (OCTG). In: Proceedings - SPE Annual Technical Conference and Exhibition, Vol. 1 (PI), pp. 579–586.10.2118/56587-MSSearch in Google Scholar
Fridrihsone-Girone, A., Stirna, U., Misane, M., Lazdiņa, B., and Deme, L. (2016). Spray-applied 100% volatile organic compounds free two component polyurethane coatings based on rapeseed oil polyols. Prog. Org. Coat. 94: 90–97, https://doi.org/10.1016/J.PORGCOAT.2015.11.022.Search in Google Scholar
Gbarnjah, E. (2014). Corrosion behavior of epoxy (sigmaline) coated X65 steel in some selected corrosive media. Vol. 58.Search in Google Scholar
Giri, R., Naskar, K., and Nando, G.B. (2012). Effect of electron beam irradiation on dynamic mechanical, thermal and morphological properties of LLDPE and PDMS rubber blends. Radiat. Phys. Chem. 81: 1930–1942, https://doi.org/10.1016/J.RADPHYSCHEM.2012.08.004.Search in Google Scholar
Goodwin, M.J., Musa, O.M., and Steed, J.W. (2015). Problems associated with sour gas in the oilfield industry and their solutions. Energy Fuel. 29: 4667–4682, https://doi.org/10.1021/acs.energyfuels.5b00952.Search in Google Scholar
Gourichon, B., Deléglise, M., Binetruy, C., and Krawczak, P. (2008). Dynamic void content prediction during radial injection in liquid composite molding. Compos. Part A: Appl. Sci. Manuf. 39: 46–55, https://doi.org/10.1016/j.compositesa.2007.09.008.Search in Google Scholar
Grenier-Loustalot, M.F., Larroque, S., Grande, D., Grenier, P., and Bedel, D. (1996). Phenolic resins. 2. Influence of catalyst type on reaction mechanisms and kinetics. Polymer 37: 1363–1369, https://doi.org/10.1016/0032-3861(96)81133-5.Search in Google Scholar
Guan, S.W., Gritis, N., Jackson, A., Singh, P. (2005). Advanced onshore and offshore pipeline coating technologies. In: 2005 China International Oil & Gas Pipeline Technology (Integrity) Conference & Expo, September 14–17, 2005, Shanghai, China.Search in Google Scholar
Guidotti, T.L. (2015) Hydrogen sulfide intoxication. In: Handbook of clinical neurology, Vol. 131, pp. 111–133, https://doi.org/10.1016/b978-0-444-62627-1.00008-1.Search in Google Scholar PubMed
Guilleminot, J., Comas-Cardona, S., Kondo, D., Binetruy, C., and Krawczak, P. (2008). Multiscale modelling of the composite reinforced foam core of a 3D sandwich structure. Compos. Sci. Technol. 68: 1777–1786, https://doi.org/10.1016/j.compscitech.2008.02.005.Search in Google Scholar
Han, J., Brown, B.N., and Nešić, S. (2010). Investigation of the galvanic mechanism for localized carbon dioxide corrosion propagation using the artificial pit technique. Corrosion 66: 0950031–09500312, https://doi.org/10.5006/1.3490308.Search in Google Scholar
Hao, Y., Zhao, Y., Li, B., Song, L., and Guo, Z. (2020). Self-healing effect of graphene@PANI loaded with benzotriazole for carbon steel. Corros. Sci. 163: 108246, https://doi.org/10.1016/J.CORSCI.2019.108246.Search in Google Scholar
Harris, S.J., Overs, M.P., and Gould, A.J. (1985). The use of coatings to control fretting wear at ambient and elevated temperatures. Wear 106: 35–52, https://doi.org/10.1016/0043-1648(85)90102-4.Search in Google Scholar
He, W., Knudsen, O.Ø., and Diplas, S. (2009). Corrosion of stainless steel 316L in simulated formation water environment with CO2-H2S-Cl-. Corros. Sci. 51: 2811–2819, https://doi.org/10.1016/j.corsci.2009.08.010.Search in Google Scholar
Heidersbach, R. (2010) Metallurgy and corrosion control in oil and gas production. In: Metallurgy and corrosion control in oil and gas production.10.1002/9780470925782Search in Google Scholar
Ibrahim, T., Alayan, H., and Mowaqet, Y.A. (2012). The effect of thyme leaves extract on corrosion of mild steel in HCl. Prog. Org. Coat. 75: 456–462, https://doi.org/10.1016/j.porgcoat.2012.06.009.Search in Google Scholar
Ismail, M., Hawa, A., and Norrrahim, M. (2020). Nanofibrillated cellulose based bio-phenolic composites. 139–151. https://www.researchgate.net/profile/Ainil-Hawa-2/publication/346096282_Nanofibrillated_Cellulose_Based_Bio-phenolic_Composites/links/5fd87067a6fdccdcb8c9dba1/Nanofibrillated-Cellulose-Based-Bio-phenolic-Composites.pdf.10.1007/978-981-15-8932-4_9Search in Google Scholar
Jariwala, A. (2019). High H2S gas field monetization: a novel approach. In: Proceedings of the Annual Offshore Technology Conference, 2019-May.10.4043/29370-MSSearch in Google Scholar
Jiang, C.Y., Liu, X.Z., Li, X.J., Fu, G.W., Zhang, K.l., Wang, J., and Xie, C.D. (2023). Preparation and anti-corrosion performance of temperature-resistant self-healing coating for L80 pipe. Int. J. Electrochem. Sci. 18: 100041, https://doi.org/10.1016/J.IJOES.2023.100041.Search in Google Scholar
Jin, S., Shuichang, Z., Guangyou, Z., Zhi, Y., Bin, Z., Anguo, F., and Debin, Y. (2010). Geological reserves of sulfur in China’s sour gas fields and the strategy of sulfur markets. Petrol. Explor. Dev. 37: 369–377, https://doi.org/10.1016/S1876-3804(10)60039-0.Search in Google Scholar
Kandola, B.K. and Horrocks, A.R. (2001). 5 - composites. In: Horrocks, A.R. and Price, D. (Eds.). Fire retardant materials. Woodhead Publishing, Cambridge, UK, pp. 182–203. https://www.sciencedirect.com/science/article/pii/B9781855734197500093.10.1533/9781855737464.182Search in Google Scholar
Kehr, J.A. and Enos, D.G. (2000). FBE, a Foundation for Pipeline Corrosion Coatings, Vol. 00129, pp. 0–94.10.5006/C2000-00757Search in Google Scholar
Keresten, A., Ostanin, S., and Zuev, V. (2021). Advanced liquid epoxy and polyurethane materials: internal and external coatings for pipeline and tubing protection. E3S Web of conferences, Vol. 225, https://doi.org/10.1051/E3SCONF/202122505004.Search in Google Scholar
Lei, X., Wang, H., Mao, F., Zhang, J., Zhao, M., Fu, A., Feng, Y., and Macdonald, D.D. (2018). Electrochemical behaviour of martensitic stainless steel after immersion in a H2S-saturated solution. Corros. Sci. 131: 164–173, https://doi.org/10.1016/j.corsci.2017.10.015.Search in Google Scholar
Li, L., Wang, J., Yan, J., Duan, L., Li, X., and Dong, H. (2018). The effect of liquid nitriding on the corrosion resistance of AISI 304 austenitic stainless steel in H2S environments. Metall. Mater. Trans. A: Phys.Metall. Mater. Sci. 49: 6521–6532, https://doi.org/10.1007/S11661-018-4920-9/FIGURES/12.Search in Google Scholar
Li, K., Zeng, Y., and Luo, J.-L. (2021a). Influence of H2S on the general corrosion and sulfide stress cracking of pipelines steels for supercritical CO2 transportation. Corros. Sci. 190, https://doi.org/10.1016/j.corsci.2021.109639.Search in Google Scholar
Li, L., Wang, J., Xiao, J., Yan, J., Fan, H., Sun, L., Xue, L., and Tang, Z. (2021b). Time-dependent corrosion behavior of electroless Ni–P coating in H2S/Cl− environment. Int. J. Hydrogen Energy 46: 11849–11864, https://doi.org/10.1016/j.ijhydene.2021.01.053.Search in Google Scholar
Li, L., Yan, J., Xiao, J., Sun, L., Fan, H., and Wang, J. (2021c). A comparative study of corrosion behavior of S-phase with AISI 304 austenitic stainless steel in H2S/CO2/Cl- media. Corros. Sci. 187, https://doi.org/10.1016/j.corsci.2021.109472.Search in Google Scholar
Liu, L., Zhang, Y., Yin, X., and Qian, J. (2013). Anti-corrosion properties of bis(benzimidazole) derivatives for N80 steel in H2S solution. Surf. Interface Anal. 45: 949–954, https://doi.org/10.1002/SIA.5187.Search in Google Scholar
Manuel, J., Damien, M., and Christian, B. (2019). Highly sour gas: the best options to process it. In: Society of Petroleum Engineers - Abu Dhabi International Petroleum Exhibition and Conference 2019, ADIP 2019.10.2118/197966-MSSearch in Google Scholar
Martin, M., Weber, S., Theisen, W., Michler, T., and Naumann, J. (2011). Effect of alloying elements on hydrogen environment embrittlement of AISI type 304 austenitic stainless steel. Int. J. Hydrogen Energy 36: 15888–15898, https://doi.org/10.1016/J.IJHYDENE.2011.09.013.Search in Google Scholar
McMahon, M.E., Santucci, R.J., Glover, C.F., Kannan, B., Walsh, Z.R., and Scully, J.R. (2019). A review of modern assessment methods for metal and metal-oxide based primers for substrate corrosion protection. Front. Mater. 6, https://doi.org/10.3389/FMATS.2019.00190.Search in Google Scholar
Mohtadi, R., Lee, W.K., Cowan, S., Van Zee, J.W., and Murthy, M. (2003). Effects of hydrogen sulfide on the performance of a PEMFc. Electrochem. Solid-State Lett. 6: A272, https://doi.org/10.1149/1.1621831/XML.Search in Google Scholar
Mohtadi-Bonab, M.A., Szpunar, J.A., and Razavi-Tousi, S.S. (2013). Hydrogen induced cracking susceptibility in different layers of a hot rolled X70 pipeline steel. Int. J. Hydrogen Energy 38: 13831–13841, https://doi.org/10.1016/j.ijhydene.2013.08.046.Search in Google Scholar
Montazerian, H., Mohamed, M.G.A., Montazeri, M.M., Kheiri, S., Milani, A.S., Kim, K., and Hoorfar, M. (2019). Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater. 96: 149–160, https://doi.org/10.1016/J.ACTBIO.2019.06.040.Search in Google Scholar PubMed
Mu’azu, N.D., Jarrah, N., Zubair, M., and Alagha, O. (2017). Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: a review. Int. J. Environ. Res. Publ. Health 14: 1094, https://doi.org/10.3390/IJERPH14101094.Search in Google Scholar PubMed PubMed Central
Muthukumar, N. (2014). Petroleum products transporting pipeline corrosion. A review. In: The Role of Colloidal Systems in Environmental Protection.10.1016/B978-0-444-63283-8.00021-1Search in Google Scholar
Nayyar, M., King, R., and Crocker, S. (2000). Piping handbook. In: Slurry and sludge piping.Search in Google Scholar
Obot, I.B., Solomon, M.M., Umoren, S.A., Suleiman, R., Elanany, M., Alanazi, N.M., and Sorour, A.A. (2019). Progress in the development of sour corrosion inhibitors: past, present, and future perspectives. J. Ind. Eng. Chem. 79: 1–18, https://doi.org/10.1016/j.jiec.2019.06.046.Search in Google Scholar
Obot, I.B., Sorour, A.A., Verma, C., Al-Khaldi, T.A., and Rushaid, A.S. (2023). Key parameters affecting sweet and sour corrosion: impact on corrosion risk assessment and inhibition. Eng. Fail. Anal. 145, https://doi.org/10.1016/j.engfailanal.2022.107008.Search in Google Scholar
Pilato, L. (2013). Phenolic resins: 100 years and still going strong. React. Funct. Polym. 73: 270–277, https://doi.org/10.1016/J.REACTFUNCTPOLYM.2012.07.008.Search in Google Scholar
Pinho, D., Muñoz-Sánchez, B.N., Anes, C.F., Vega, E.J., and Lima, R. (2019). Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 100: 103399, https://doi.org/10.1016/J.MECHRESCOM.2019.103399.Search in Google Scholar
Pizzi, A. and Ibeh, C.C. (2014). 2 - phenol–formaldehydes. In: Dodiuk, H., and Goodman, S.H. (Eds.). Handbook of thermoset plastics, 3rd ed. William Andrew Publishing, pp. 13–44, https://www.sciencedirect.com/science/article/pii/B9781455731077000026.10.1016/B978-1-4557-3107-7.00002-6Search in Google Scholar
Polyurethane Coating: Uses, Types, Advantages, and Disadvantages (2024). https://www.thomasnet.com/articles/chemicals/what-is-polyurethane-coating/.Search in Google Scholar
Pournara, A.E., Karamanos, S.A., Papatheocharis, T., and Perdikaris, P.C. (2014). Structural integrity of steel hydrocarbon pipelines with local wall distortions. In: Proceedings of the Biennial International Pipeline Conference, IPC, 2.10.1115/IPC2014-33210Search in Google Scholar
Pradeep Kumar, C.B. and Mohana, K.N. (2014). Phytochemical screening and corrosion inhibitive behavior of Pterolobium hexapetalum and Celosia argentea plant extracts on mild steel in industrial water medium. Egypt. J. Pet. 23: 201–211, https://doi.org/10.1016/j.ejpe.2014.05.007.Search in Google Scholar
Rao, H., Zhang, Z., and Liu, F. (2013). Enhanced mechanical properties and blood compatibility of PDMS/liquid crystal cross-linked membrane materials. J. Mech. Behav. Biomed. Mater. 20: 347–353, https://doi.org/10.1016/J.JMBBM.2013.01.010.Search in Google Scholar
Ren, L.F., Liu, C., Xu, Y., Zhang, X., Shao, J., and He, Y. (2020). High-performance electrospinning-phase inversion composite PDMS membrane for extractive membrane bioreactor: fabrication, characterization, optimization and application. J. Membr. Sci. 597: 117624, https://doi.org/10.1016/J.MEMSCI.2019.117624.Search in Google Scholar
Rodrigues, R.O., Lima, R., Gomes, H.T., and Silva, A.M.T. (2015). Polymer microfluidic devices: an overview of fabrication methods. U. Porto J. Eng. 1: 67–79, https://doi.org/10.24840/2183-6493_001.001_0007.Search in Google Scholar
Salazar-Hernández, C., Salazar-Hernández, M., Carrera-Cerritos, R., Mendoza-Miranda, J.M., Elorza-Rodríguez, E., Miranda-Avilés, R., and Mocada-Sánchez, C.D. (2019). Anticorrosive properties of PDMS-Silica coatings: effect of methyl, phenyl and amino groups. Prog. Org. Coat. 136: 105220, https://doi.org/10.1016/J.PORGCOAT.2019.105220.Search in Google Scholar
Samimi, A. (2012). Use of polyurethane coating to prevent corrosion in oil and gas pipelines transfer. Int. J. Innovat. Appl. Stud.: 186–193.Search in Google Scholar
Sandhya, P.K., Sreekala, M.S., Padmanabhan, M., Jesitha, K., and Thomas, S. (2019). Effect of starch reduced graphene oxide on thermal and mechanical properties of phenol formaldehyde resin nanocomposites. Composites, Part B: Eng. 167: 83–92, https://doi.org/10.1016/j.compositesb.2018.12.009.Search in Google Scholar
Sangaj, N.S. and Malshe, V.C. (2004). Permeability of polymers in protective organic coatings. Prog. Org. Coat. 50: 28–39, https://doi.org/10.1016/J.PORGCOAT.2003.09.015.Search in Google Scholar
Senouci, A., Elabbasy, M., Elwakil, E., Abdrabou, B., and Zayed, T. (2014). A model for predicting failure of oil pipelines. Struct. Infrastruct. Eng. 10: 375–387, https://doi.org/10.1080/15732479.2012.756918.Search in Google Scholar
Sharrad, M. (2018). Validation study of gas solubility correlations at bubble point pressure for some Libyan crude oils using three chosen correlations, https://www.researchgate.net/publication/326914674_Validation_Study_of_Gas_Solubility_Correlations_at_bubble_point_pressure_for_Some_Libyan_Crude_Oils_Using_Three_chosen_Correlations.Search in Google Scholar
Shi, X., Zhang, Z., Wu, L., Li, X., and Zhang, Z. (2021). Corrosion law of metal pipeline in tahe oilfield and application of new materials. Coatings 11, https://doi.org/10.3390/coatings11111269.Search in Google Scholar
Shimamura, J., Morikawa, T., Yamasaki, S., and Tanaka, M. (2022). Sulfide stress cracking (SSC) of low alloy linepipe steels in low H2S content sour environment. ISIJ Int. 62: 2095–2106, https://doi.org/10.2355/ISIJINTERNATIONAL.ISIJINT-2022-236.Search in Google Scholar
Solovyeva, V.A., Almuhammadi, K.H., and Badeghaish, W.O. (2023). Current downhole corrosion control solutions and trends in the oil and gas industry: a review. Materials 16: 1795. https://doi.org/10.3390/MA16051795.Search in Google Scholar PubMed PubMed Central
Soomro, A.A., Mokhtar, A.A., Kurnia, J.C., Lashari, N., Lu, H., and Sambo, C. (2022). Integrity assessment of corroded oil and gas pipelines using machine learning: a systematic review. Eng. Fail. Anal. 131, https://doi.org/10.1016/j.engfailanal.2021.105810.Search in Google Scholar
Souza, A., Marques, E., Balsa, C., and Ribeiro, J. (2020). Characterization of shear strain on PDMS: numerical and experimental approaches. Appl. Sci. 10: 3322. https://doi.org/10.3390/APP10093322.Search in Google Scholar
Spatolisano, E., Pellegrini, L.A., Finocchi, A., and de Angelis, A.R. (2023). Selecting technologies for sour and ultra-sour gas treating. ChemBioEng Rev. 10: 801–816, https://doi.org/10.1002/cben.202300030.Search in Google Scholar
Standard Recommended Practice Liquid-Applied Internal Protective Coatings for Oilfield Production Equipment. (1994), NACE International, https://relisleeve.com/wp-content/uploads/2021/12/rp-018194.pdf.Search in Google Scholar
Steinberg, B.G. and Kane, R.D. (1982) Stepwise cracking of line Pipe steels in simulated oilfield environments. In: Proceedings of the Annual Offshore Technology Conference, 1982-May, pp. 369–373.10.4043/4330-MSSearch in Google Scholar
Stirna, U., Fridrihsone, A., Lazdiņa, B., Misāne, M., and Vilsone, D. (2013). Biobased polyurethanes from rapeseed oil polyols: structure, mechanical and thermal properties. J. Polym. Environ. 21: 952–962, https://doi.org/10.1007/S10924-012-0560-0.Search in Google Scholar
Sun, C., Zeng, H., and Luo, J.-L. (2019). Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2/S/Cl– environments at high temperature and high pressure. Corros. Sci. 148: 317–330, https://doi.org/10.1016/j.corsci.2018.12.022.Search in Google Scholar
Tale, S., Ahmed, R., Elgaddafi, R., and Teodoriu, C. (2021). Sulfide stress cracking of C-110 steel in a sour environment. Corros. Mater. Degrad. 2: 376–396, https://doi.org/10.3390/cmd2030020.Search in Google Scholar
Tomio, A., Sagara, M., Doi, T., Amaya, H., Otsuka, N., and Kudo, T. (2015). Role of alloyed molybdenum on corrosion resistance of austenitic Ni-Cr-Mo-Fe alloys in H2S-Cl- environments. Corros. Sci. 98: 391–398, https://doi.org/10.1016/j.corsci.2015.05.053.Search in Google Scholar
Vakili, M., Koutník, P., and Kohout, J. (2024). Addressing hydrogen sulfide corrosion in oil and gas industries: a sustainable perspective. Sustainability 16: 1661, https://doi.org/10.3390/SU16041661.Search in Google Scholar
Wang, J., Pu, J., Zhang, G., and Wang, L. (2013). Interface architecture for superthick carbon-based films toward low internal stress and ultrahigh load-bearing capacity. ACS Appl. Mater. Interfaces 5: 5015–5024, https://doi.org/10.1021/am400778p.Search in Google Scholar PubMed
Wang, Q.-Y., Wang, X.-Z., Luo, H., and Luo, J.-L. (2016). A study on corrosion behaviors of Ni-Cr-Mo laser coating, 316 stainless steel and X70 steel in simulated solutions with H2S and CO2. Surf. Coat. Technol. 291: 250–257, https://doi.org/10.1016/j.surfcoat.2016.02.017.Search in Google Scholar
Wei, X., Zhang, M., Shang, L., Wang, Y., Lu, Z., and Zhang, G. (2019). Enhancement in the corrosive and tribological properties of the inner wall of 6063Al and CI pipes by thick multilayer Si-DLC coatings. Mater. Res. Express 6, https://doi.org/10.1088/2053-1591/ab28f1.Search in Google Scholar
Wei, X., Qi, S., Wu, J., Nie, X., Lu, Z., and Zhang, G. (2021a). Tuning pulse frequency for improvement in the corrosion resistance of 304SS pipes inner DLC coatings deposited by hollow cathode PECVD. Diamond Relat. Mater. 118, https://doi.org/10.1016/j.diamond.2021.108552.Search in Google Scholar
Wei, X., Yin, P., Wu, J., Nie, X., Lu, Z., and Zhang, G. (2021b). Deposition of DLC films on the inner wall of U-type pipes by hollow cathode PECVD. Diamond Relat. Mater. 114, https://doi.org/10.1016/j.diamond.2021.108308.Search in Google Scholar
Wei, X., Ning, C., Lu, Z., and Zhang, G. (2022). Si and N incorporated hydrogenated diamond like carbon film with excellent performance for marine corrosion resistance. Ceram. Int. 48: 8440–8450, https://doi.org/10.1016/j.ceramint.2021.12.052.Search in Google Scholar
Wolf, M.P., Salieb-Beugelaar, G.B., and Hunziker, P. (2018). PDMS with designer functionalities. Properties, modifications strategies, and applications. Prog. Polym. Sci. 83: 97–134, https://doi.org/10.1016/J.PROGPOLYMSCI.2018.06.001.Search in Google Scholar
Wu, G., Zhang, Q., and Zhang, N. (2020). The failure reason of epoxy-phenolic coating on the internal surface of BG90S steel tubing under sour gas environment. Pigm. Resin Technol. 49: 181–187, https://doi.org/10.1108/PRT-08-2019-0069.Search in Google Scholar
Yu, C., Wang, H., Gao, X., and Wang, H. (2020). Effect of Ti microalloying on the corrosion behavior of low-carbon steel in H2S/CO2 environment. J. Mater. Eng. Perform. 29: 6118–6129, https://doi.org/10.1007/s11665-020-05077-1.Search in Google Scholar
Yun, Y.J., Ju, J., Lee, J.H., Moon, S.H., Park, S.J., Kim, Y.H., Hong, W.G., Ha, D.H., Jang, H., Lee, G.H., et al.. (2017). Highly elastic graphene-based electronics toward electronic skin. Adv. Funct. Mater. 27: 1701513, https://doi.org/10.1002/ADFM.201701513.Search in Google Scholar
Zaldivar, R.J., Ferrelli, G.L., Arredondo, V., and Kim, H.I. (2022). Effect of cure temperature on the thermal degradation, mechanical, microstructural and moisture absorption behavior of vacuum-only, carbon-fiber reinforced phenolic composites. J. Appl. Polym. Sci. 139, https://doi.org/10.1002/APP.52819.Search in Google Scholar
ZareNezhad, B. and Ziaee, M. (2013). Accurate prediction of H2S and CO2 containing sour gas hydrates formation conditions considering hydrolytic and hydrogen bonding association effects. Fluid Phase Equilib. 356: 321–328, https://doi.org/10.1016/j.fluid.2013.07.055.Search in Google Scholar
Zeng, Z.-w. S. and Taylor, S.E. (2020). Facile preparation of superhydrophobic melamine sponge for efficient underwater oil-water separation. Sep. Purif. Technol. 247: 116996, https://doi.org/10.1016/J.SEPPUR.2020.116996.Search in Google Scholar
Zhang, C. and Zhao, J. (2018). Inhibition effects of orange peel extract on the corrosion of Q235 steel in CO2-saturated and CO2/H2S coexistent brine solutions. Res. Chem. Intermed. 44: 1275–1293, https://doi.org/10.1007/s11164-017-3166-2.Search in Google Scholar
Zhang, M., Wu, G., Lu, Z., Shang, L., and Zhang, G. (2018). Corrosion and wear behaviors of Si-DLC films coated on inner surface of SS304 pipes by hollow cathode PECVD. Surf. Topogr.: Metrol. Prop. 6: 034010, https://doi.org/10.1088/2051-672X/AAC45E.Search in Google Scholar
Zhao, X.H., Han, Y., Bai, Z.Q., and Wei, B. (2011). The experiment research of corrosion behaviour about Ni-based alloys in simulant solution containing H2S/CO2. Electrochim. Acta 56: 7725–7731, https://doi.org/10.1016/j.electacta.2011.05.116.Search in Google Scholar
Zhao, W., Zhang, T., Wang, Y., Qiao, J., and Wang, Z. (2018). Corrosion failure mechanism of associated gas transmission pipeline. Materials 11, https://doi.org/10.3390/ma11101935.Search in Google Scholar PubMed PubMed Central
Zhao, Y., Xu, R., Xiao, Y., Wang, H., Zhang, W., and Zhang, G. (2022). Mechanical performances of phenolic modified epoxy resins at room and high temperatures. Coatings 12: 643, https://doi.org/10.3390/coatings12050643.Search in Google Scholar
Zhuoke, L., Jun, C., Ting, M., and Dan, N. (2021). Synthesis of bimannich base with thiazole and its corrosion inhibition effect on H2S and CO2 at high temperature. BMC Chem. 15, https://doi.org/10.1186/s13065-021-00784-9.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Recent findings on lignin-based wear and corrosion resistance coatings
- Progress in the study of micro-arc oxidation film layers on biomedical metal surfaces
- A review on internal corrosion of pipelines in the oil and gas industry due to hydrogen sulfide and the role of coatings as a solution
- Cathodic protection and sulphate-reducing bacteria: a complex interaction in offshore steel structures
- Original Articles
- Correlation between synthesis conditions and corrosion behavior of nickel–aluminum layered double hydroxide (LDH)-sealed anodized aluminum
- Impact of plastic deformation on the corrosion behavior of S32304 duplex steel in a simulated industrial solution
- Corrigendum
- Corrigendum to: Corrosion of stainless steels and corrosion protection strategies in the semiconductor manufacturing industry: a review
Articles in the same Issue
- Frontmatter
- Reviews
- Recent findings on lignin-based wear and corrosion resistance coatings
- Progress in the study of micro-arc oxidation film layers on biomedical metal surfaces
- A review on internal corrosion of pipelines in the oil and gas industry due to hydrogen sulfide and the role of coatings as a solution
- Cathodic protection and sulphate-reducing bacteria: a complex interaction in offshore steel structures
- Original Articles
- Correlation between synthesis conditions and corrosion behavior of nickel–aluminum layered double hydroxide (LDH)-sealed anodized aluminum
- Impact of plastic deformation on the corrosion behavior of S32304 duplex steel in a simulated industrial solution
- Corrigendum
- Corrigendum to: Corrosion of stainless steels and corrosion protection strategies in the semiconductor manufacturing industry: a review

