Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA)
-
Ana-Maria Simundic
, Michael Cornes
Abstract
Background: European questionnaire survey was conducted by the European Federation of Clinical Chemistry and Laboratory Medicine Working Group for the Preanalytical Phase (EFLM WG-PA) to assess how phlebotomy is performed in EFLM countries, including differences in personnel, level of education and skills, and to investigate the presence and compliance of national phlebotomy guidelines on this matter.
Methods: A questionnaire was constructed containing questions elucidating different aspects of the organization behind the phlebotomy praxis on a national basis, including questions on the staff performing phlebotomy, the education of these staff members, and the existence of and adherence to national guidelines. All 39 EFLM member countries were invited to participate.
Results: In total 28/39 (72%) EFLM member countries responded. Seven out of the 28 (25%) have national phlebotomy guidelines and five have implemented other guidelines. The estimated compliance with phlebotomy guidance for the laboratories in the countries that have national guidelines available is poor, regardless to whether the phlebotomy was under the laboratory control or not. Most countries were interested in EFLM guidelines and to participate in a pilot EFLM preanalytical phase external quality assessment (EQA) scheme. In the responding EFLM member countries, the majority of phlebotomy is performed by nurses and laboratory technicians. Their basic education is generally 4–5 years of high school, followed by 2–5 years of colleague or university studies. Only a third (10/28; 36%) of the participating member countries has any specific training available as a continuous educational resource. A specific training for phlebotomy is not part of the education required to become qualified in 6/28 (21%) and 9/28 (32%) of countries for nurses and laboratory technicians, respectively. In countries and professions where training is required, most require more than 5 h of training.
Conclusions: Based on the results of this survey we conclude the following: 1) There is a need to assess the quality of current practices, compliance to the CLSI H3-A6 guidelines and to identify some most critical steps which occur during phlebotomy, in different healthcare settings, across Europe; 2) Existing CLSI H3-A6 phlebotomy guidelines should be adapted and used locally in all European countries which do not have their own guidelines; 3) National EFLM societies need to be engaged in basic training program development and continuous education of healthcare phlebotomy staff (implementing the certification of competence).
Introduction
The vast majority of all errors in the total testing process are most often attributable to human mistakes within the preanalytical phase, i.e., before the sample is analyzed in a laboratory, when the specimen is collected and handled locally at primary healthcare centers and hospital wards [1–6]. Phlebotomy is one of the most common procedures in healthcare [1, 2] and is a base for diagnosis and treatment [7, 8]. Errors in phlebotomy may lead to patient suffering and compromised patient safety [1, 2]. Phlebotomy errors are in addition latent and distant from direct control, and therefore often go unrecognized. Controlling phlebotomy variables is therefore challenging as the influence of the quality of a sample on test results depends on the biomolecule analyzed as well as the robustness of the analytical method. Variations in phlebotomy practices also jeopardize the clinical value of the test analysis as comparisons using reference change value calculations or reference interval comparisons are based on the analysis of the results from specimens collected under controlled preanalytical practices [9].
Phlebotomy is, in agreement with other healthcare practical skills, a complex procedure which demands theoretical knowledge and manual skills, as well as accuracy, responsibility, ability, good caring conduct and good interaction between the phlebotomist and patient. For high quality patient outcomes, it is important for the phlebotomist to stay abreast of the latest laboratory sampling procedures, since laboratory methods change and phlebotomy instructions with them [2, 4]. Knowledge of how to search relevant information thus helps phlebotomy staff to preserve stringent preanalytical practices [10, 11] which leads to more reliable test results [2, 4, 12]. Overall, the phlebotomist must be well educated and trained to fulfil all these qualifications [2].
There are only a few official international recommendations or guidelines on phlebotomy, such as the H3-A6 guideline issued by the Clinical Laboratory Standards Institute (CLSI) in 2007 [10], or the guidelines on drawing blood published by the World Health Organization in 2010 [13]. These international guidelines are based on systematic reviewing of evidence, are comprehensive and extensive, and consist of several pages. As such, they are not really suited for daily healthcare practice. It is therefore of national as well as local interest to adapt an international phlebotomy guideline, i.e., to modify it for application in the cultural and organizational setting (including language, education and training of operators, legislation, values, healthcare settings). Local guidelines may give a practical sequence of steps to follow and provide an explanation of the background for steps with reference to the original (inter-)national guideline and an explanation of the adaptations of original recommendations [14, 15].
Studies assessing adherence to venous blood specimen collection guideline practices including phlebotomy are few [16]. Staff self-reported adherence to national guideline phlebotomy practices (identical to the CLSI H3-A6 guideline) using a validated questionnaire [17] was poor [18–22]. A short educational intervention demonstrated, however, that adherence to guideline practices regarding patient identification, tourniquet release, test tube labeling and information search procedures were substantially improved [23], whereas venous blood sample quality per se as assessed by low-grade hemolysis was essentially unaffected [23]. The reasons why healthcare staff do not adhere to practical procedures of guidelines are manifold and include the lack of theoretical knowledge, not being familiar with guideline content, poor attitudes towards guidelines, work overload or lack of time [24]. Environmental characteristics such as lack of support from the clinic or superiors, insufficient staff and time [25] and, even more importantly, the way they are implemented [11] also appear to be the main impediments.
Accreditation of a clinical laboratory demonstrates that it has a quality management system and ensured reliable analytical test results [26–28]. With the high error rate in the total testing process it seems obvious that preanalytical activities should also be included in the accreditation. The ISO 15189:2012 standard is considered the most relevant by all International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) societies as well as the European national accreditation bodies. The standard contains paragraphs specifically focused on the preanalytical phase including phlebotomy [29]. It also regulates that the laboratory is responsible for producing adequate instructions and possibility for training and that it is responsible for the conditions of the samples at arrival too. The preanalytical conditions are regularly reviewed by the laboratory and the national accreditation bodies regularly assess the laboratory’s adherence to good practice [27]. In some countries phlebotomy is regulated to be accredited, and in other countries the phlebotomy service is supervised by the medical laboratory but in most countries the sampling is outside the jurisdiction of the laboratory. The laboratories should ideally engage themselves in all processes in healthcare that affect the total testing process including the clinical applicability of the analysis results also outside the laboratory walls [30–32]. Preanalytical errors will of course still be prevalent in an accredited laboratory [33], although a decrease in the number of non-conformities was observed in accredited laboratories over time, suggesting that ISO 15189 accreditation does contribute to laboratory quality improvement [34].
According to ISO 15189, external quality assessment (EQA) programs should provide clinically relevant challenges that mimic patient samples and have the effect of checking the entire examination process, including pre- and post-examination procedures [35]. Few initiatives have hitherto been taken to incorporate preanalytical phase issues into EQAs. When incorporated it is usually done by circulating questionnaires or asking participants to register onto a web site to report their practices and errors [36]. Important initiatives have, however, recently been undertaken to increase awareness and establish a governance of the preanalytical aspect of the total testing process, including: the “Working Group on Laboratory Errors and Patient Safety (WG-LEPS)” instituted by the Division of Education and Management (EMD) of the IFCC. Its primary goal is to identify and evaluate reliable quality indicators and related quality specifications for addressing all the stages of the total testing process [37, 38] and establish EQA programs to encourage investigations into every kind of error (including the preanalytical phase) in laboratory medicine, collect data available and recommend strategies and procedures for improving patient safety [37, 38]. “Specimencare.com” is an online resource designed to identify, evaluate and promote the application of best practices in all aspects of the preanalytical phase of laboratory testing in clinical medicine [39]. Similar EQA programs covering the preanalytical phase have also been developed on national basis in, e.g., Spain, Croatia, Australia and New Zealand [40–42].
The recently established EFLM Working Group on the Preanalytical Phase (WG-PA) aims to identify some of the most critical elements and make recommendations to reduce the impact of preanalytical phase throughout the diagnostic process [43]. Several educational and scientific activities are already ongoing. An international, educational meeting supported by the EFLM has already been successfully organized in Parma, Italy in 2011 [9] and the second edition (available at: http://www.preanalytical-phase.org)has just recently been held in Zagreb, Croatia in March 2013 [44]. The third meeting has already been scheduled, to be held in Porto, Portugal, in spring 2014. Another important initiative is to present the result of this European survey assessing the presence and compliance of national phlebotomy guidelines, the national societies interest for an EFLM phlebotomy guideline as well as to identify by whom phlebotomy is done and what level of education is required for this specific task.
Materials and methods
In order to assess how phlebotomy is performed in the EFLM countries, including differences in personnel, level of education and skills, and also to investigate the presence and compliance of national phlebotomy guidelines on this matter, a European survey was conducted by the EFLM WG-PA. The survey was performed during April–August 2012.
A questionnaire was constructed containing questions elucidating different aspects of the organization behind the phlebotomy praxis on a national basis. As shown in Supplementary Figure 1 (Supplementary Data, which accompanies the article at http://www.degruyter.com/view/j/cclm.2013.51.issue-8/issue-files/cclm.2013.51.issue-8.xml)the questionnaire included questions on the staff performing phlebotomy, the education of these staff members, and the existence of and adherence to national guidelines concerning this issue. As additional information to the participants it was noted that the term “routine phlebotomy” was strictly considered as the procedure of drawing venous blood, i.e., patient preparation, test requesting, sample handling and sample delivery to the laboratory was not considered as part of routine phlebotomy in this survey.
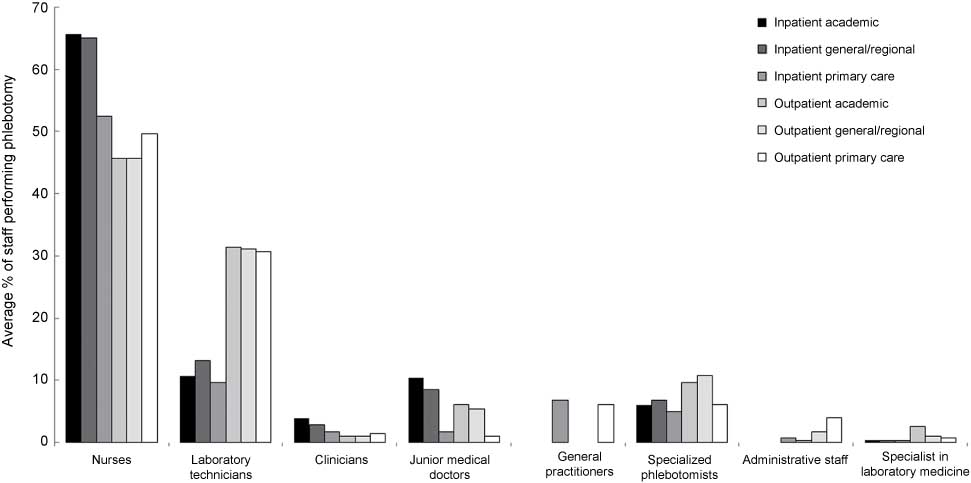
Data showing the responses to questions about who performs phlebotomy in different inpatient and outpatient groups.
All EFLM member countries (n=39) were invited to participate; to ensure a proper participation percentage, follow-up requests were performed by members of the working group. Answers were sent to and collected by the chairman of the working group, Professor Ana-Maria Simundic. Interpretation of the assembled answers obtained through the questionnaire was performed by all members of the working group.
Results
Out of 39 countries approached, 28 (response rate 72%) have responded. Responding countries were Albania, Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Italy, Ireland, The Netherlands, Norway, Poland, Portugal, Romania, Russia, Serbia, Slovenia, Spain, Sweden, Switzerland, Ukraine and UK.
National guidelines and interest in European guideline for routine phlebotomy
Only seven out of the 28 responding countries (25%) have national guidelines for phlebotomy. For the countries with national guidance, 54 were issued by national societies and two were driven by government. The estimated compliance with phlebotomy guidance for the laboratories in the countries that have national guidelines available is poor, regardless to whether the phlebotomy was under the laboratory control or not.
The predominant reasons for countries not having implemented national guidelines seemed to be the lack of time or leadership to take on this work (14/21; 67%), or attributable to the implementation of other – mostly CLSI – guidelines (5/21; 24%).
Twenty-one of the 28 countries (75%) were extremely interested and six (22%) moderately interested in EFLM guidelines whereas even 24/28 (86%) responding member countries expressed an interest in being involved in a pilot EFLM preanalytical phase external quality assessment (EQA) scheme.
Healthcare personnel categories and level of education of phlebotomists
In the responding EFLM member countries, the majority of phlebotomy is performed by nurses and laboratory technicians regardless of the patient population (Figure 1). Only 5%–11% of the venipunctures are performed by specialist phlebotomists, whereas 45%–65% are performed by nurses and 10%–32% by laboratory technicians.
Generally, nurses perform phlebotomy less often in outpatient than in inpatient settings, whereas laboratory technicians perform phlebotomy more often in outpatient than in inpatient settings.
In the majority of countries like Bosnia and Herzegovina, Albania, Portugal, Romania, Ukraine, Spain, Switzerland, Hungary, Slovenia, Sweden, Czech Republic, Serbia, Greece, Italy, Croatia and Poland, nurses are almost exclusively responsible for performing phlebotomy for hospital inpatients. However, phlebotomy for inpatients is performed most often by junior medical doctors in general and academic hospitals in Austria (100%) and Germany (≥50%).
Quite uniquely, laboratory technicians in Denmark are responsible for phlebotomy both for inpatients and outpatients in the majority of the healthcare facilities (academic hospitals, general hospitals, primary care facilities).
Specialized phlebotomists are involved in phlebotomy only in Netherlands, Belgium, Ireland and UK. There are no specialized phlebotomists in other countries.
The level of education varied throughout Europe. In most of the countries nurses and laboratory technicians attend 4–5 years high school, followed by 2–5 years of colleague or university education. In a quarter of the countries nurses and laboratory technicians become qualified after high school.
Specific training for phlebotomy
Only a third (10/28; 36%) of the participating member countries has any specific training for phlebotomy available as a continuous educational resource. A specific training for phlebotomy is not part of the education required to become qualified in 6/28 (21%) and 9/28 (32%) of countries for nurses and laboratory technicians, respectively.
Strikingly enough, a specific training for phlebotomy is not provided in some EFLM member societies, neither as a part of the education required to become qualified as a nurse or laboratory technician, nor as a separate training or educational resource.
Whether or not phlebotomy is a standard part of the training in each country varies depending on the profession. As shown in Figure 2, in 22 out of the 28 countries (79%) phlebotomy training is required for nurses, whereas only one country has training for administrative staff as part of the education. For the most staff groups performing phlebotomy more than half of the countries had specific training.
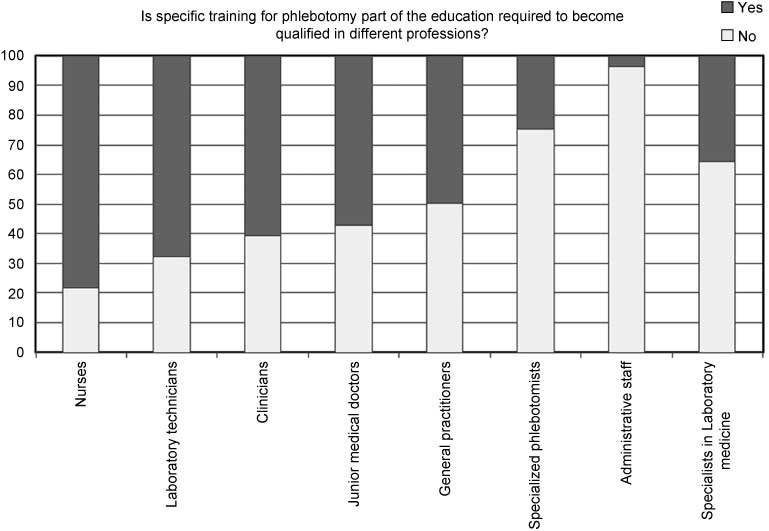
The percentage of countries in each staffing group for which there is specific training in phlebotomy as part of the training.
Training time to qualify as phlebotomist in each profession
The data in Figure 3 illustrates how many hours of training are required to become qualified phlebotomist. In countries and professions where training is required, most require more than 5 h of training, with a significant amount requiring 1–5 h and only a minority of countries stating that only 1 h of training is required.
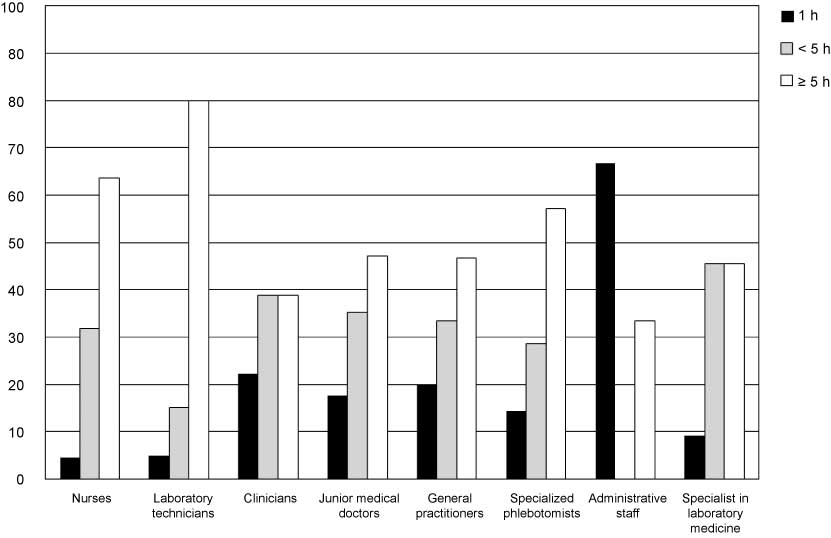
The percentage of countries in each staffing group for which there is specific training in phlebotomy and how many hours of training is typically included.
Duration and provider of phlebotomy course
More than half of the responding countries do not have a separate phlebotomy course for many professions. For those that do have a separate phlebotomy course, it tends to be mandatory for nurses (8/13; 61%), laboratory technicians (6/10; 60%) and for specialized phlebotomists (4/4; 100%). The course tends to be optional for all other professions.
The length of the course tends to be longer for nurses, laboratory technicians and specialized phlebotomists than for other professions, ranging from half a day to a week. For the other professions, it is generally 1 day or less.
The course is almost always provided by the healthcare institution regardless of the profession, with only few countries indicating that blood collection system suppliers perform the training.
Discussion
There is mounting evidence that the various steps of the preanalytical phase, especially the most manually intensive activities related to collection and handling of biological specimens, are those most vulnerable to errors throughout the total testing process. When inaccurate, inappropriate or even mishandled procedures are followed for drawing venous and arterial blood, test results may be seriously compromised and patient safety may be contextually jeopardized [32, 43]. It is thereby essential that laboratory medicine – in hierarchical order international organizations, national societies and professionals – recognizes that more focus should be placed on education, training and performance monitoring of phlebotomists, since these efforts are likely to produce the most favorable outcomes on the quality of in vitro diagnostics, much greater than subsidiary benefits arising from actions specifically design to target the analytical and post-analytical phases. This paradigm has prompted the EFLM WG-PA to design and disseminate a specific questionnaire aimed at establishing the state-of-the-art of phlebotomy practices across Europe, and which is supposed to pave the way to further actions such as filling educational gaps and – even more importantly – harmonize practices by developing official guidelines or recommendations on this topic.
Regardless of an official “call for” involving delegates and society members, the response rate of the questionnaire has been lower than expected, since one quarter of the countries did not reply to the invitation. This is an important issue that further confirms the participation of some European countries in the economic, political and organizational destiny of the Old Continent can be improved. As regards the specific issues, the compelling need to develop specific guidelines is clearly emphasized by the low rate (i.e., one quarter) of countries that have developed national guidelines. The great interest around this issue is confirmed by the fact that most of the responders were interested (78% extremely interested) in EFLM guidelines and the vast majority of them were also interested in participating in a pilot EQA scheme for the preanalytical phase promoted by the EFLM.
As regards the specific questions about education and training of phlebotomists, marked differences appeared when compared with other medical procedures, wherein phlebotomy practice seems almost exclusively the domain of nurses and laboratory technicians in Europe (i.e., approx. 90%, especially for inpatients), whereas the profession of phlebotomist is already well established or predominantly emerging in other countries, similar to the US situation [2]. The Netherlands, Belgium, Ireland and UK are the few but notable exceptions to this rule in Europe. Although a high degree of education characterizes nurses and laboratory technicians with blood collection responsibility (in most of countries the high school, College or University education lasts not <6 years), it is however surprising that specific training on phlebotomy is only offered in one third of the European countries and – even more unexpectedly – specific phlebotomy training is not part of the education in more than 50% of cases. In line with these findings, the training time dedicated to blood drawing is not always satisfactory for both nurses and laboratory technicians, being <5 h in more than one-third of responders. For those countries where a separate phlebotomy course is available (ranging from half a day to a week for nurses and laboratory technicians), this tends to be mandatory in more than two thirds of cases.
It has to be pointed that the validity of this study might be somewhat uncertain due to the nature of the way responses were collected. For a single country there was only one individual who was responsible for answering the questionnaire on behalf of several different professions. It could be that his/her estimations are not fully correct and accurate and that our results are at least to a certain degree not fully representative for the entire population of that National Society. We are aware of that potential limitation, but also hope that these results are the closest possible estimation for the real situation in each National Society.
Taken together, the results of this survey raise at least three major issues. First, a high degree of heterogeneity was found around the title of phlebotomist throughout Europe. This is a straightforward clinical practice, which does not require specific interventions provided that the procedure is conducted by an appropriately educated healthcare professional, regardless of the professional figure that is finally in charge of this specific activity. The need for developing simple practice recommendations, auspiciously provided by the EFLM, is however strongly reaffirmed by most countries, especially by those which have not developed specific national guidelines. It is also noteworthy that the degree and length of training required to draw blood was found to be rather variable across Europe. This evidence appears particularly concerning, since insufficient training for puncturing a vein or an artery not only may compromise the quality of diagnostic samples, but can especially produce serious (and preventable) harm to patients.
It is then obvious from the results of this survey that establishing international, national or local phlebotomy, accreditation of biochemical laboratories, quality indicators or EQA programs does not guarantee an improvement of phlebotomy performance in itself. The guidelines and tools already exist, but lack of effective methods for implementation and support appear to be the main obstacles for success. In the existing venous specimen collection guidelines and accreditation statutes there is only, at best, rudimentary information on how to implement and sustain guideline practices. There are few evaluations of how guidelines should be effectively implemented and the responsibility for implementation and sustaining guideline knowledge and practices are in addition often unclear [11]. Some general suggestions of how to best implement phlebotomy practices have however been proposed [23].
The structure and content of the guidelines themselves also affect their adoption and actual use. Guidelines that are easy to understand and that can easily be tried out and do not require specific resources have a greater chance of implementation [25, 45], and this will be indeed a driving paradigm when the EFLM embarks on drafting supranational recommendations. Accreditation of healthcare and clinical laboratories has been increasingly suggested as a tool to enhance total quality [26–28]. Even if the activities of the preanalytical phase are included in the accreditation, the problems with implementing and sustaining correct procedures seem to be similar to those of phlebotomy guidelines. In short, the EFLM WG-PA intend to perform a survey to assess phlebotomy staff adherence to phlebotomy practices, identify crucial steps in phlebotomy such as use of inappropriate or mishandled practices [46] and to present evidence-based suggestions on the best ways to implement and sustain phlebotomy guideline adherence.
Conclusions and recommendations
Based on the results of this survey we conclude the following: 1) There is a need to assess the quality of current practices, compliance to the CLSI H3-A6 guidelines and to identify some most critical steps which occur during phlebotomy, in different healthcare settings, across Europe; 2) Existing CLSI H3-A6 phlebotomy guidelines should be adapted and used locally in all European countries which do not have their own guidelines; 3) National EFLM societies need to be engaged in basic training program development and continuous education of healthcare phlebotomy staff (implementing the certification of competence).
The authors of this article wish to express their thanks to National representatives of EFLM National Societies for their precious time spent in providing the answers to this survey: Avram Sanja (BiH), Agnes Ivanov (Estonia), Mathias M Mueller (Austria), Edmée Van Dongen-Lases (The Netherlands), Krasneci Bulo Anyla (Albania), Langlois Michel (Belgium), Orla Maguire (Ireland), Joao Tiago Guimaraes (Portugal), Simona Berbecar (Romania), Ganna Lunyova (Ukraine), Fabian Sanchis-Goamar (Spain), Karl Lackner (Germany), Wolfgang Korte (Switzerland), Evgenija Homsak (Slovenija), Kamen Tzatchev (Bulgaria), Peter Antal-Szalmas (Hungary), Timo Kouri (Finland), Gustav Mikkelsen (Sweden), Nada Majkic-Singh (Serbia), Kroupis Christos (Greece), Tomasz Anyszek (Poland).
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
References
1. Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab 2006;52:217–30.Search in Google Scholar PubMed
2. Lippi G, Mattiuzzi C, Guidi GC. Laboratory quality improvement by implementation of phlebotomy guidelines. Med Lab Obs 2006;38:6–7.Search in Google Scholar
3. Fang L, Fang SH, Chung YH, Chien ST. Collecting factors related to the haemolysis of blood specimens. J Clin Nurs 2008;17:2343–51.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000258405800014&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1111/j.1365-2702.2006.02057.xSearch in Google Scholar PubMed
4. Becan-McBride K. Laboratory sampling: does the process affect the outcome? J Intraven Nurs 1999;22:137–42.Search in Google Scholar
5. Boone DJ. How can we make laboratory testing safer? Clin Chem Lab Med 2007;45:708–11.10.1515/CCLM.2007.169Search in Google Scholar PubMed
6. Lippi G, Avanzini P, Cervellin G. Prevention of hemolysis in blood samples collected from intravenous catheters. Clin Biochem 2013;46:561–4.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000317878100001&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1016/j.clinbiochem.2013.01.021Search in Google Scholar PubMed
7. Kalra J. Medical errors: an introduction to concepts. Clin Biochem 2004;37:1043–51.10.1016/j.clinbiochem.2004.08.007Search in Google Scholar PubMed
8. Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med 2006;44:750–9.10.1515/CCLM.2006.123Search in Google Scholar PubMed
9. Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med 2011;49:1113–26.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000292538800004&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
10. Clinical Laboratory Standards Institute. Procedures for collection of diagnostic blood specimens by venipuncture; approved guideline, 6th ed. CLSI document H3-A6. Wayne, PA: Clinical and Laboratory Standards Institute, 2007.Search in Google Scholar
11. Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we improve guideline use? A conceptual framework of implementability. Implement Sci 2011;6:26.10.1186/1748-5908-6-26http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000289293800001&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed PubMed Central
12. Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost 2012;38: 565–75.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000308401300004&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1055/s-0032-1315961Search in Google Scholar PubMed
13. World Heath Organization. WHO guidelines on drawing blood. Available from: http://whqlibdoc.who.int/publications/2010/9789241599221_eng.pdf. Accessed on January 11, 2013.Search in Google Scholar
14. Oosterhuis WP, Bruns DE, Watine J, Sandberg S, Horvath AR. Evidence-based guidelines in laboratory medicine: principles and methods. Clin Chem 2004;50:806–18.10.1373/clinchem.2003.025528Search in Google Scholar PubMed
15. Watine J, Oosterhuis W, Nagy E, Bunting PS, Horvath AR. Formulating and using evidence-based guidelines. In: Price CP, Christenson RH, editors. Evidence-based laboratory medicine. Washington, DC: AACC Press, 2007:275–94.Search in Google Scholar
16. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03-A6 – procedures for the collection of diagnostic blood. Biochemia Med (Zagreb) 2012;22:342–51.10.11613/BM.2012.036Search in Google Scholar
17. Bolenius K, Brulin C, Grankvist K, Lindkvist M, Soderberg J. A content validated questionnaire for assessment of self reported venous blood sampling practices. BMC Res Notes 2012;5:39.10.1186/1756-0500-5-39Search in Google Scholar PubMed PubMed Central
18. Soderberg J, Brulin C, Grankvist K, Wallin O. Preanalytical errors in primary healthcare: a questionnaire study of information search procedures, test request management and test tube labelling. Clin Chem Lab Med 2009;47:195–201.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000263656400013&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
19. Soderberg J, Wallin O, Grankvist K, Brulin C. Is the test result correct? A questionnaire study of blood collection practices in primary health care. J Eval Clin Practice 2010;16:707–11.10.1111/j.1365-2753.2009.01179.xhttp://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000279901700007&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar
20. Wallin O, Soderberg J, Van Guelpen B, Brulin C, Grankvist K. Patient-centred care – preanalytical factors demand attention: a questionnaire study of venous blood sampling and specimen handling. Scand J Clin Lab Invest 2007;67:836–47.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000251144000006&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1080/00365510701370675Search in Google Scholar PubMed
21. Wallin O, Soderberg J, Van Guelpen B, Stenlund H, Grankvist K, Brulin C. Preanalytical venous blood sampling practices demand improvement – A survey of test-request management, test-tube labelling and information search procedures. Clin Chim Acta 2008;391:91–7.10.1016/j.cca.2008.02.016http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000255846000016&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
22. Wallin O, Soderberg J, Van Guelpen B, Stenlund H, Grankvist K, Brulin C. Blood sample collection and patient identification demand improvement: a questionnaire study of preanalytical practices in hospital wards and laboratories. Scand J Caring Sci 2010;24:581–91.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000281000800020&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1111/j.1471-6712.2009.00753.xSearch in Google Scholar PubMed
23. Bolenius K, Soderberg J, Hultdin J, Lindkvist M, Brulin C, Grankvist K. Minor improvement of venous blood specimen collection practices in primary health care after a large-scale educational intervention. Clin Chem Lab Med 2013;51:303–10.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000314999000019&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1515/cclm-2012-0159Search in Google Scholar PubMed
24. Amon E. Communication strategies for reducing hospital error and professional liability. Obstet Gynecol Surv 2002;57:713–4.10.1097/00006254-200211000-00001Search in Google Scholar PubMed
25. Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak 2008;8:38.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000259819400001&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1186/1472-6947-8-38Search in Google Scholar PubMed
26. Zima T. Accreditation in clinical laboratories. Biochem Med (Zagreb) 2010;20:215–20.10.11613/BM.2010.026Search in Google Scholar
27. Huisman W. European Communities Confederation of Clinical Chemistry Working Group on Accreditation: past, present and future. Clin Chim Acta 2001;309:111–4.10.1016/S0009-8981(01)00505-8Search in Google Scholar
28. Huisman W, Horvath AR, Burnett D, Blaton V, Czikkely R, Jansen RT, et al. Accreditation of medical laboratories in the European union. Clin Chem Lab Med 2007;45:268–75.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000244866900029&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
29. Jansen RT, Kenny D, Blaton V, Burnett D, Huisman W, Plebani M, et al. Usefulness of EC4 essential criteria for quality systems of medical laboratories as guideline to the ISO 15189 and ISO 17025 documents. European Community Confederation of Clinical Chemistry (EC4) working group on harmonisation of quality systems and accreditation. Clin Chem Lab Med 2000;38:1057–64.10.1515/CCLM.2000.158Search in Google Scholar
30. Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med 2007;45:720–7.10.1515/CCLM.2007.167Search in Google Scholar PubMed
31. Lippi G, Fostini R, Guidi GC. Quality improvement in laboratory medicine: extra-analytical issues. Clin Chem Lab Med 2008;28:285–94.10.1016/j.cll.2007.12.007Search in Google Scholar PubMed
32. Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006;44:358–65.10.1515/CCLM.2006.073Search in Google Scholar PubMed
33. Simundic AM, Nikolac N, Vukasovic I, Vrkic N. The prevalence of preanalytical errors in a Croatian ISO 15189 accredited laboratory. Clin Chem Lab Med 2010;48:1009–14.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000279341900016&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1515/CCLM.2010.221Search in Google Scholar PubMed
34. Ho B, Ho E. The most common nonconformities encountered during the assessments of medical laboratories in Hong Kong using ISO 15189 as accreditation criteria. Biochem Med 2012;22:247–57.10.11613/BM.2012.027Search in Google Scholar
35. Ricós C, Biosca C, Ibarz M, Minchinela J, Llopis M, Perich C, et al. Quality indicators and specifications for strategic and support processes in laboratory medicine. Clin Chem Lab Med 2008;46:1189–94.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000259147200023&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
36. Lippi G, Montagnana M, Giavarina D. National survey on the pre-analytical variability in a representative cohort of Italian laboratories. Clin Chem Lab Med 2006:44;1491–4.10.1515/CCLM.2006.274Search in Google Scholar PubMed
37. Sciacovelli L, O’ Kane M, Skaik YA, Caciagli P, Pellegrini C, Da Rin G, et al. Quality indicators in laboratory medicine: from theory to practice. Preliminary data from the IFCC Working Group Project “Laboratory errors and patient safety”. Clin Chem Lab Med 2011;49:835–44.10.1515/CCLM.2011.128Search in Google Scholar
38. Sciacovelli L, Plebani M. The IFCC Working Group on laboratory errors and patient safety. Clin Chim Acta 2009;404:79–85.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000267005800017&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1016/j.cca.2009.03.025Search in Google Scholar PubMed
39. Specimencare.com. Available from: http://www.specimencare.com. Accessed on April 11, 2013.Search in Google Scholar
40. Alsina MJ, Alvarez V, Barba N, Bullich S, Cortés M, Escoda I, et al. Preanalytical quality control program – an overview of results (2001–2005 summary). Clin Chem Lab Med 2008;46:849–54.10.1515/CCLM.2008.168Search in Google Scholar PubMed
41. Bilic-Zulle L, Simundic AM, Supak Smolcic V, Nikolac N, Honovic L. Self reported routines and procedures for the extraanalytical phase of laboratory practice in Croatia – cross-sectional survey study. Biochem Med 2010;20:64–74.10.11613/BM.2010.008Search in Google Scholar
42. Khoury M, Burnett L, Mackay M. Error rates in Australian chemical pathology laboratories. Med J Aust 1996;165:128–30.10.5694/j.1326-5377.1996.tb124883.xSearch in Google Scholar
43. Simundic AM, Lippi G. Preanalytical phase – a continuous challenge for laboratory professionals. Biochem Med (Zagreb) 2012;22:145–9.10.11613/BM.2012.017Search in Google Scholar PubMed PubMed Central
44. Lippi G, Becan-McBride K, Behúlová D, Bowen RA, Church S, Delanghe J, et al. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med 2013;51:229–41.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000312497600023&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f3Search in Google Scholar PubMed
45. Ploeg J, Davies B, Edwards N, Gifford W, Miller PE. Factors influencing best-practice guideline implementation: lessons learned from administrators, nursing staff, and project leaders. Worldviews Evid Based Nurs 2007;4:210–9.http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcApp=PARTNER_APP&SrcAuth=LinksAMR&KeyUT=000251436100005&DestLinkType=FullRecord&DestApp=ALL_WOS&UsrCustomerID=b7bc2757938ac7a7a821505f8243d9f310.1111/j.1741-6787.2007.00106.xSearch in Google Scholar PubMed
46. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Pitanguiera Manguera CL, Sumita NM, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med (Zagreb) 2011;21:152–9.10.11613/BM.2011.024Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
Articles in the same Issue
- Letters to the Editors
- Clinical utility of serum tumor markers and cytokines in cervical cancer and neoplasia
- Establishing reference intervals for LDL subfractions in a Korean population using the Lipoprint LDL system
- Measurement imprecision of common urinary biochemical analytes on the Roche Cobas 6000 system
- A comparison between turbidimetric inhibition immunoassay and capillary electrophoresis in glycated hemoglobin (HbA1c) measurement
- Commutability: a peculiar property of calibration and control materials. Definition and evaluation
- Diagnostic sensitivity of a panel of tests to detect monoclonal protein in Korean multiple myeloma patients
- Commutability of proficiency testing (PT): status of the matrix-related bias in general clinical chemistry
- Masthead
- Masthead
- Editorial
- Why specifications for allowable glucose meter errors should include 100% of the data
- Reviews
- ABO blood group: old dogma, new perspectives
- Trace elements and bone health
- Mini Review
- The role of transcription factors in laboratory medicine
- Opinion Papers
- Nobelitis: a common disease among Nobel laureates?
- The syndrome of the “obsessive-compulsory scientist”: a new mental disorder?
- Can current analytical quality performance of UK clinical laboratories support evidence-based guidelines for diabetes and ischaemic heart disease? – A pilot study and a proposal
- Guidelines and Recommendations
- Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA)
- General Clinical Chemistry and Laboratory Medicine
- Red cell indices: differentiation between β-thalassemia trait and iron deficiency anemia and application to sickle cell disease and sickle cell thalassemia
- Problems in determining thalassemia carrier status in a program for prevention and control of severe thalassemia syndromes: a lesson from Thailand
- An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype
- Blood loss from laboratory diagnostic tests in children
- Hematocrit correction does not improve glucose monitor accuracy in the assessment of neonatal hypoglycemia
- Evaluation of a mobile clinical pathology laboratory developed for the home care of pediatric patients following transplantation of peripheral blood precursor cells
- Folic acid supplementation does not reduce intracellular homocysteine, and may disturb intracellular one-carbon metabolism
- Influence of spurious hemolysis on blood gas analysis
- Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection
- Reference Values and Biological Variations
- Nationwide multicenter study aimed at the establishment of common reference intervals for standardized clinical laboratory tests in Japan
- Cancer Diagnostics
- Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis
- Comparative evaluation of the My5-FU™ immunoassay and LC-MS/MS in monitoring the 5-fluorouracil plasma levels in cancer patients
Articles in the same Issue
- Letters to the Editors
- Clinical utility of serum tumor markers and cytokines in cervical cancer and neoplasia
- Establishing reference intervals for LDL subfractions in a Korean population using the Lipoprint LDL system
- Measurement imprecision of common urinary biochemical analytes on the Roche Cobas 6000 system
- A comparison between turbidimetric inhibition immunoassay and capillary electrophoresis in glycated hemoglobin (HbA1c) measurement
- Commutability: a peculiar property of calibration and control materials. Definition and evaluation
- Diagnostic sensitivity of a panel of tests to detect monoclonal protein in Korean multiple myeloma patients
- Commutability of proficiency testing (PT): status of the matrix-related bias in general clinical chemistry
- Masthead
- Masthead
- Editorial
- Why specifications for allowable glucose meter errors should include 100% of the data
- Reviews
- ABO blood group: old dogma, new perspectives
- Trace elements and bone health
- Mini Review
- The role of transcription factors in laboratory medicine
- Opinion Papers
- Nobelitis: a common disease among Nobel laureates?
- The syndrome of the “obsessive-compulsory scientist”: a new mental disorder?
- Can current analytical quality performance of UK clinical laboratories support evidence-based guidelines for diabetes and ischaemic heart disease? – A pilot study and a proposal
- Guidelines and Recommendations
- Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA)
- General Clinical Chemistry and Laboratory Medicine
- Red cell indices: differentiation between β-thalassemia trait and iron deficiency anemia and application to sickle cell disease and sickle cell thalassemia
- Problems in determining thalassemia carrier status in a program for prevention and control of severe thalassemia syndromes: a lesson from Thailand
- An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype
- Blood loss from laboratory diagnostic tests in children
- Hematocrit correction does not improve glucose monitor accuracy in the assessment of neonatal hypoglycemia
- Evaluation of a mobile clinical pathology laboratory developed for the home care of pediatric patients following transplantation of peripheral blood precursor cells
- Folic acid supplementation does not reduce intracellular homocysteine, and may disturb intracellular one-carbon metabolism
- Influence of spurious hemolysis on blood gas analysis
- Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection
- Reference Values and Biological Variations
- Nationwide multicenter study aimed at the establishment of common reference intervals for standardized clinical laboratory tests in Japan
- Cancer Diagnostics
- Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis
- Comparative evaluation of the My5-FU™ immunoassay and LC-MS/MS in monitoring the 5-fluorouracil plasma levels in cancer patients

