Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
-
Amadou Dawé
, Benjamin Talom
Abstract
Termiglaucescin (1), a new triterpene glucoside, has been isolated from the ethyl acetate extract of the root bark of Terminalia glaucescens Planch. ex Benth, together with 11 known compounds, β-D-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate (2), arjunglucoside I (3), sericoside (4), arjungenin (5), sericic acid (6), arjunetin (7), chebuloside II (8), 3,3′,4-tri-O-methylelagic acid (9), 3,3′-di-O-methylelagic acid (10), β-sitosterol (11) and stigmasterol (12). Compounds 2, 3, 7, 8 and 9 are reported from the plant for the first time. The structures of the isolated compounds were characterized by spectroscopic data interpretations, especially 1D and 2D NMR. The triterpenic isolates showed potent antioxidant and anti-inflammatory activities.
1 Introduction
The genus Terminalia (Combretaceae) consists of 200 species distributed in the tropics and subtropics [1], [2]. About 30 species of this genus are found in Africa [1]. Terminalia glaucescens Planch. ex Benth is used in Central Africa for the treatment of dysentery and other microbial infections. Leaves of this plant are reported to be useful in the last phase of AIDS [3]. The extracts of leaves and roots of T. glaucescens have been found to be highly active against both Candida species and dermatophytes such as Trichophyton spp. [4]. T. glaucescens is used as chewing sticks in some countries of Western Africa, and extracts made from the sticks (the stem bark and wood) showed a wide spectrum of antibacterial activity against different bacterial species including methicillin-resistant Staphylococcus aureus and dentally relevant bacteria [5], [6], [7]. The antioxidant and antihyperglycemia effects of methanol-methylene chloride extract in streptozotocin-induced diabetes is also in agreement with the use of T. glaucescens for diabetes treatment in Cameroon [8], [9]. A number of terpenoids (arjunic acid, arjungenin, sericoside, frideline, glaucinoic and glaucescic acid), phytosterols (β-sitosterol and stigmasterol) and ellagic acids have previously been isolated from the stem bark and roots of T. glaucescens [10], [11], [12], but the identification of active principles and the mechanism of therapeutic activity of this species have not so far been established.
In the present study, we have isolated one new oleanane type triterpene glucoside named as termiglaucescin (1) from the ethyl acetate sub-fraction of the root bark of T. glaucescens along with 11 known compounds (2–12). The isolated triterpenes (1–8) showed significant antioxidant and anti-inflammatory activities (Figure 1). The potent activities of these compounds supplemented the previous literature reports and medicinal uses of this plant in the treatment of diabetes and dental caries.
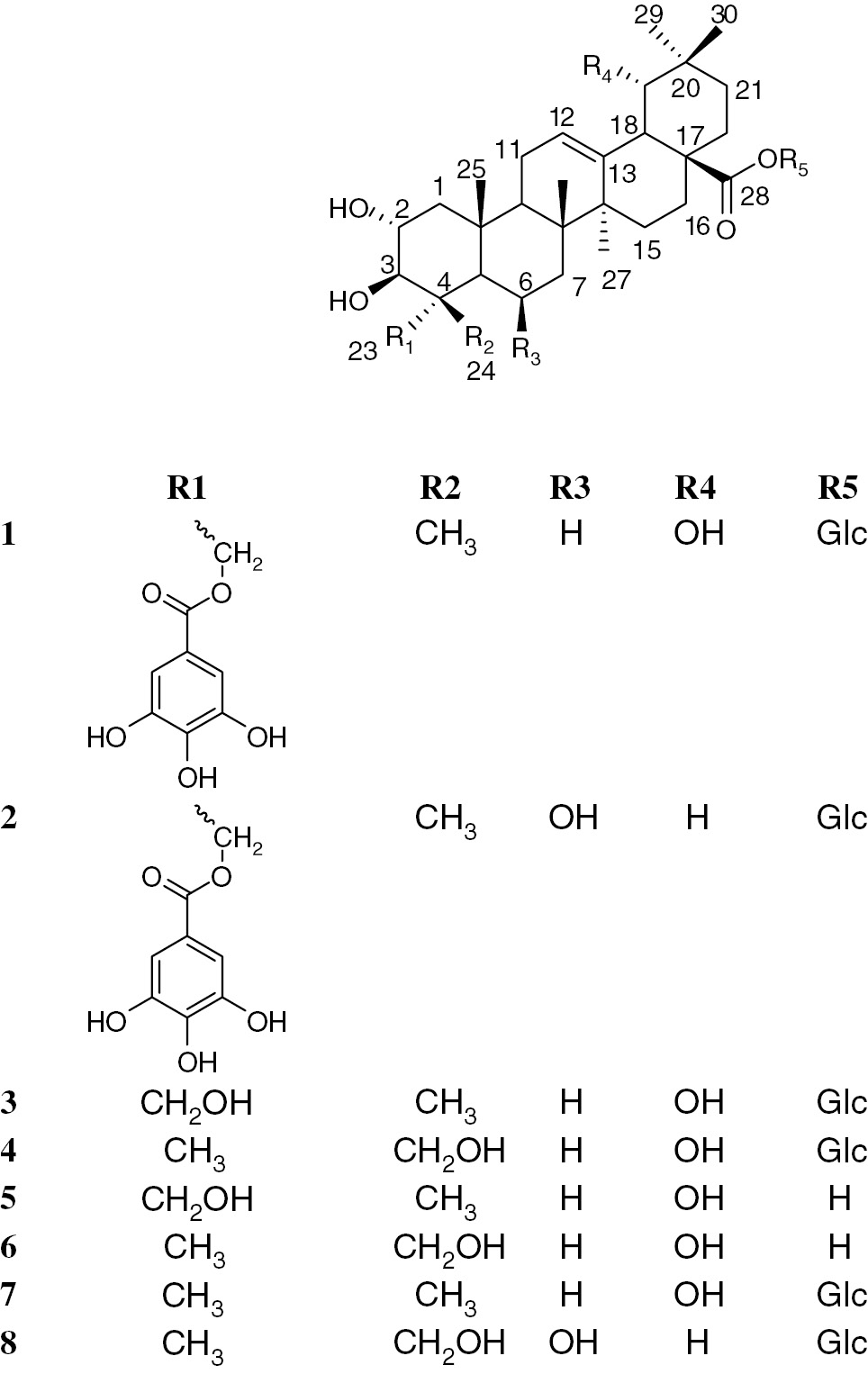
Structures of isolated triterpenes from Terminalia glaucescens.
2 Results and discussion
2.1 Structural elucidation
Termiglaucescin (1) was isolated as a colorless amorphous solid which gave similar color reactions as those of 1. The molecular ion peak at m/z 817.2631 [M-1]− in the high resolution-fast atom bombardement-mass spectroscopy (HR-FAB-MS) was consistent with the molecular formula C43H62O15. Absorption bands at 3417, 1696 and 1614 cm−1 in the IR spectrum suggested the presence of hydroxyl, ester and olefinic functionalities. The 1H-NMR spectrum of 1 displayed signals for six tertiary methyl groups (δH 0.73, 0.82, 0.92, 0.93, 1.05 and 1.16), an olefin proton (δH 5.31, br t), three oxygenated methines (δH 3.74, 3.33 and 3.25) and an oxygenated methylene (δH 4.03, 4.19, each d, J=11.0 Hz), together with six other oxygenated methine and methylene protons ascribable to a sugar unit (Table 1). The 13C-NMR spectrum showed 43 signals including 6 primary, 10 secondary, 14 tertiary, and 13 quaternary carbons (Table 1). The olefinic carbons at δC 124.8 and 144.6 were typical of oleanane-type triterpenes [13]. Comparison of NMR spectral data with those of 28-O-β-D-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-dien-28-oate (2) previously isolated from Combretum molle revealed a downfield shift of C-19 (δC 82.5) and an upfield shift of C-6 (δC 19.5) indicative of the absence of hydroxyl group at C-6 and its presence at C-19. Full assignments of proton and carbon resonances of the aglycone were achieved by analysis of the heteronuclear single quantum correlation (HSQC), correlation spectroscopy (COSY) and heteronuclear multiple quantum correlaation (HMBC) experiments (Table 1). Moreover, the presence of one singlet of two protons at δH 7.08 (H-2′′, H-6′′) on the 1H NMR spectrum and carbon atoms of one carbonyl at δC 168.3 (C-7′′), one aromatic quaternary carbon at δC 121.6 (H-1′′), two aromatic methines at δC 110.1 (C-2′′, C-6′′) and three quaternary oxygenated carbons at δC 139.9 (C-4′′) and 146.6 (C-3′′, C-5′′) on the 13C NMR spectrum evidenced the presence of the galloyl group in compound 1 [14]. The galloyl moiety could be assigned to C-23 on the basis of HMBC experiments in which the oxymethylene protons showed cross peak correlation with δ C-3 (δC 78.7), C-5 (δC 49.5), C-24 (δC 13.7) and C-7′′ (δC 168.3). The sugar moiety was determined as β-D-glucopyranoside on the basis of the characteristic J1, 2 coupling constant of its anomeric proton (J=8.0 Hz) and typical 1H and 13C NMR shifts [15]. The position of the glucose moiety was confirmed by correlation of the anomeric proton with C-28 (δ 178.0).
1H and 13C NMR data of compound 1 in methanol-d4.
| 1 | |||
|---|---|---|---|
| Position | δC | δH (J in Hz) | HMBC correlations |
| 1 | 47.1 | 0.98a | |
| 1.94 dd (12.5, 4.0) | C-2, C-5, C-10 | ||
| 2 | 69.3 | 3.74 m | C-3 |
| 3 | 78.7 | 3.33 d (7.0) | |
| 4 | 44.1 | ||
| 5 | 49.5 | 1.36a | |
| 6 | 19.5 | 1.44a | |
| 7 | 33.3 | 1.23a | |
| 1.39a | |||
| 8 | 40.9 | ||
| 9 | 48.5 | 1.82 t | C-8, C-10, C-11, C-25 |
| 10 | 39.1 | ||
| 11 | 24.9 | 1.99a | C-12, C-13 |
| 12 | 124.8 | 5.31 br t | |
| 13 | 144.6 | ||
| 14 | 42.7 | ||
| 15 | 29.5 | 0.96a 1.72a | |
| 16 | 28.4 | 1.66a | |
| 2.26 m | C-28 | ||
| 17 | 47.9 | ||
| 18 | 45.1 | 3.03 br s | C-12, C-13, C-19, C-28 |
| 19 | 82.5 | 3.25 d (3.5) | C-18, C-20, C-29, C-30 |
| 20 | 35.9 | ||
| 21 | 29.3 | 1.02a | |
| 1.29a | |||
| 22 | 33.5 | 1.66a | |
| 1.76a | |||
| 23 | 66.8 | 4.03 d (11.0) | C-3, C-4, C-5, C-24, C-7′′ |
| 4.19 d (11.0) | |||
| 24 | 13.7 | 0.82 s | C-3, C-4, C-5, C-23 |
| 25 | 17.8 | 1.05 s | C-9, C-10 |
| 26 | 17.4 | 0.73 s | C-6, C-8, C-9, C-14 |
| 27 | 25.2 | 1.16 s | C-8, C-13, C-14, C-15 |
| 28 | 178.6 | ||
| 29 | 25.2 | 0.93 s | C-19, C-20, C-30 |
| 30 | 28.5 | 0.92 s | C-10, C-20, C-29 |
| glc | |||
| 1′ | 95.8 | 5.35 d (8.0) | C-28 |
| 2′ | 73.9 | 3.30a | |
| 3′ | 78.1 | 3.42 d (9.5) | |
| 4′ | 71.1 | 3.36a | |
| 5′ | 78.7 | 3.38a | |
| 6′ | 62.4 | 3.66 dd (12.5, 4.0) | |
| 3.80 dd (12.5, 4.0) | |||
| Gal | |||
| 1′′ | 121.6 | ||
| 2′′ | 110.1 | 7.08 s | C-1′′, C-3′′, C-4′′, C-6′′, C-7′′ |
| 3′′ | 146.6 | ||
| 4′′ | 139.9 | ||
| 5′′ | 146.6 | ||
| 6′′ | 110.1 | 7.01 s | C-1′′, C-2′′, C-4′′, C-5′′, C-7′′ |
| 7′′ | 168.3 | ||
aOverlapped, assignment determined by COSY, HSQC and HMBC.
The stereochemistry at different stereocenters was established through nuclear Overhauser effect spectroscopy (NOESY) spectrum, which showed correlation of the oxymethines proton at δH 3.74 (H-2) with the angular methyl groups at δH 0.82 (H-24) and 1.05 (H-25) and the proton at δH 3.33 (H-3) with the oxymethylene protons at δH 4.08 and 4.19 (H-23). The proton at δH 3.03 (H-18) and δH 3.25 (H-19) also showed through space coupling with the methyl at δH 0.93 (H-30). On the basis of these evidence and comparison of NMR spectral data with related triterpenes, the structure of termiglaucescin (1) was assigned as 28-O-β-D-glucopyranosyl 2α,3β,19α-trihydroxy-23-galloylolean-12-dien-28-oate.
Among the isolated compounds, β-sitosterol, stigmasterol, arjungenin, sericoside and β-D-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate have previously been isolated from the roots and leaves of T. glaucescens [10], [11], [12], Combretum racemosum [16] and the stem bark of C. molle [17]. This reveals close taxanomical similarity between two species of Combretaceae family. T. glaucescens extracts have previously been evaluated for their antioxidant activity and showed promising activities [8], [9]. Moreover, arjungenin and β-D-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate have previously been reported as potent anti-inflammatory compounds against carrageenan-induced paw edema in rats [17]. In general, the results obtained (Table 2) showed that all the isolated triterpenes exhibit significant antioxidant and potent anti-inflammatory activities. The isolated compounds were subsequently assessed for 15-LOX (lipoxygenase) inhibitory activity and showed promising activity. They also showed potential scavenging ability against 1,1-diphenyl-2-picryl-hydrazil (DPPH) radicals. While being the active inhibitor of LOX, these compounds were also analyzed for anti-inflammatory prospective. From the results, it was concluded that all compounds exhibited potential inhibition of membrane lyses and albumin denaturation. These results are in good agreement with previous reported results. Thus, the potent antioxidant and anti-inflammatory activities of different extracts from T. glaucescens could be attributed to these compounds. Exposure of red blood cell (RBC) to injurious substances such as hypotonic medium and phenylhydrazine results in lysis of its membrane accompanied by hemolysis and oxidation of hemoglobin [18]. The hemolytic effect of hypotonic solution is related to excessive accumulation of fluid within the cell resulting in the rupturing of its membrane. Such injury to RBC membrane will further render the cell more susceptible to secondary damage through free radical-induced lipid peroxidation [19]. Since our result showed a marked attenuation of heat-induced protein denaturation by compounds, these compounds can be useful in treatment of diseases such as rheumatic disorders, cataract and Alzheimer’s diseases [20], and all the tested compounds presented their strong potential to be developed as anti-inflammatory drug.
Antioxidant and anti-inflammatory activities of compounds (1–8).
| Compound | Antioxidant | Anti-inflammatory | ||
|---|---|---|---|---|
| DPPH scavenging IC 50 μM | LOX inhibition IC 50μM | % Inhibition of albumin denaturation | % Inhibition of hemolysis | |
| 1 | 24.0±0.11 | 79.0±1.10 | 88±4.10 | 76.33±7.91 |
| 2 | 101±0.98 | 61±2.23 | 55±3.44 | 61.55±3.22 |
| 3 | 61.32 ±0.19 | 77.0±3.50 | 71±1.42 | 55.14±3.51 |
| 4 | 102.4±0.15 | 61±2.11 | 44± 1.58 | 46.53±2.52 |
| 5 | 56.7±0.05 | 78.0±4.22 | 68±2.78 | 56.0±3.10 |
| 6 | 46.11±0.21 | 71.65±2.15 | 79±0.98 | 76.46±2.89 |
| 7 | 37.32±0.03 | 84.0±2.5 | 76±1.22 | 65.14±4.58 |
| 8 | 83.11±0.73 | 69.45±2.41 | 65±1.28 | 85.36±2.85 |
| BHA | 44.2±0.06 | – | – | – |
| Baicalein | – | 22.1±0.03 | – | – |
| Aspirin | – | – | 78±0.55 | 75.32±3.51 |
3 Experimental
3.1 General experimental procedure
Column chromatography (CC): silica gel (SiO2; 250–400 mesh; E. Merk, D-Darmstadt). Thin-layer chromatography: SiO2 F254 plates (E. Merk, D-Darmstadt). Melting point: Buchi M-560 melting point. Optical rotations: Jasco DIP-360 digital polarimeter. UV Spectra: Hitachi UV-3200 spectrophotometer; λmax (log ε) in nm. IR Spectra: Jasco 302-A spectrophotometer; in KBr; ϋ in cm−1. NMR Spectra: Bruker 600 MHz instrument; δ in ppm rel. to Me4Si as internal standard, J in Hz. EI-, and HR-FAB-MS: Joel JMS-HX-110 and JMS-DA-500 mass spectrometers with glycerol as matrix; in m/z (rel, %). All the reagents, devices and software were supplied through ICCBS Karachi, Pakistan.
3.2 Plant material
The root bark of T. glaucescens was collected in Ngaoundéré (Adamaoua region of Cameroon) in October 2015 and authentified by Dr G. Achoundong of the Cameroon National Herbarium, Yaoundé, where specimens documenting the collection are deposited (22126/SRF/CAM).
3.3 Extraction and isolation
The dried and powdered roots bark of T. glaucescens (1.45 kg) was macerated for 24 h with 10 L of MeOH. The filtrate obtained was concentrated under reduced pressure to yield a dark residue (155 g). Part of this extract (133 g) was suspended in water (500 mL) and successively extracted with EtOAc and n-BuOH, yielding 45 and 88 g of extracts after evaporation to dryness, respectively. One part of the EtOAc extract (40 g) was subjected to silica gel (63–200 mm) CC, using a gradient of EtOAc in n-hexane, then with EtOAc–MeOH (90:10) to give six main fractions (A–F). Fraction A (n-hex-EtOAc 90: 10) was subjected to CC using silica gel (32–63 mm) and eluted successively with 0%, 5%, 10%, 15% and 20% of EtOAc in n-hexane to afford compounds 9 (18.2 mg), 10 (13.7 mg), 11 (36,4 mg) and 12 (67.0 mg). Fraction C (n-hex-EtOAc 80:20–60:40) was chromatographed on a silica gel column with n-hex-EtOAc gradient system to yield compound 4 (124 mg), 5 (68 mg) and 6 (42 mg). Further purification of two sub-fractions of fraction C with successive silica gel and sephadex LH-20 CC and elution with DCM-MeOH gradient system gave compounds 2 (34 mg), 7 (18 mg) and 8 (11 mg). Other sub-fractions of C were subjected to reversed-phase high-performance liquid chromatography using a 10×250 nm Reliasil C18 column, eluting with an isocratic system on MeOH-H2O (75:25) at the flow rate of 0.5 mL/min led to the isolation of compounds 1 (Rt 22 min, 14 mg) and 3 (Rt 26 min, 16 mg).
3.4 In vitro anti-inflammatory activities
3.4.1 Inhibition of albumin denaturation
Method of Labu et al. [19] followed with minor modifications. The reaction mixture consists of test and 1% aqueous solution of bovine albumin fraction; pH of the reaction mixture was adjusted using small amount of HCl. The reaction mixture was incubated at 37°C for 20 min and then heated to 51°C for 20 min. after cooling the samples; the turbidity was measured at 660 nm. The experiment was performed in triplicate. Percent inhibition of protein denaturation was calculated as follows:
where Abs control is the absorbance without sample and Abstest is the absorbance of test.
3.4.2 Membrane stabilization test
Preparation of RBCs suspension: fresh whole human blood (10 mL) was collected and transferred to the anticoagulant-containing centrifuge tubes. The tubes were centrifuged at 3000 rpm for 10min and were washed three times with equal volume of normal saline. The volume of blood was measured and reconstituted as 10% v/v suspension with normal saline.
Heat-induced hemolysis: The reaction mixture (2 mL) consisted of 1 mL of test sample (1 mg/mL) solution and 1 mL of 10% RBCs suspension; instead of test sample only, saline was added to the control test tube. Aspirin was used as a standard drug. All the centrifuge tubes containing reaction mixture were incubated in water bath at 56°C for 30 min, and then tubes were cooled under running tap water. The reaction mixture was centrifuged at 2500 rpm for 5 min, and the absorbance of the released hemoglobin in the supernatant was read at 560 nm.
The experiment was performed in triplicates for all the test samples. The percentage membrane stability was estimated using the expression:
The percentage of membrane stabilization or protection was calculated by using the following formula:
3.5 Antioxidant
3.5.1 DPPH radical scavenging assay
The free radical scavenging activity was carried out by DPPH [21]. The solution of DPPH (0.3 μM) was prepared in ethanol. The solution of each sample was prepared in methanol. Five microliters of solution of each sample (with concentration range 50–500 μg) was added to 95 μL of DPPH solution; the mixture was then dispersed in 96-well plates and placed for 30 min into the incubator at 37°C. Then absorbance was recorded at 515nm by Elisa plate reader (Spectramax plus 384 Molecular Device, CA, USA), and percent radical scavenging activity was assessed in contrast to methanol-treated control. BHA (butylated hydroxylanisole) was used as standard.
3.5.2 Lipoxygenase inhibition assay
One hundred and sixty microliters of 100 mM sodium phosphate buffer (pH 8.0) and 10 μL of sample in methanol (concentration range 50–500μM) was added to each labeled well. Twenty microliters of LOX solution (enzyme 130 units per well) was added, mixed and incubated for 10 min at 25°C. The reaction was then initiated by the addition of 10 μL substrate solution (linoleic acid, 0.5mM, 0.12% w/v tween 20 in the ratio of 1:2) to each well, and the absorbance was measured after 15 min at 234 nm. Baicalein was used as standard [22].
Termiglaucescin (28-O-β-D-glucopyranoside-2α,3β,19α- dihydroxy-23-galloylolean-12-dien-28-oate (1): Colorless amorphous solid, mp 234–236°C and
Acknowledgments
This work was supported by a TWAS-ICCBS research grant number: 3240280473 from the University of Karachi, Pakistan.
References
1. Wickens GE. Combretaceae. In: Polhill RM, editor. Flora of Tropical East Africa. London: Crown Agents for Overseas Governments and Administrations, 1973.Suche in Google Scholar
2. Tan F, Shi S, Zhong Y, Gong X, Wang Y. Phylogenetic relationships of Combretoideae (Combretaceae) inferred from plastid, nuclear gene and spacer sequences. J Plant R 2002;115:475–81.10.1007/s10265-002-0059-1Suche in Google Scholar
3. Koudou J, Roblot G, Wylde R. Tannin constituents of Terminalia glaucescens. Planta Med 1995;61:490–1.10.1055/s-2006-958153Suche in Google Scholar
4. Batawila K, Kokou K, Koumaglo M, Gbeassor M, De Foucalt B, Bouchet P, et al. Antifungal activities of five Combretaceae used in Togolese traditional medicine. Fitoterapia 2005;76:264–8.10.1016/j.fitote.2004.12.007Suche in Google Scholar
5. Taiwo O, Xu HX, Lee SF. Antibacterial activities of extracts from Nigerian chewing sticks. Phytother Res 1999;13:675–9.10.1002/(SICI)1099-1573(199912)13:8<675::AID-PTR513>3.0.CO;2-XSuche in Google Scholar
6. Oshomo EO, Idu M. Isolation and characterization of beta-sitosterol from ethyl acetate extract of root bark of Terminalia glaucescens. Int J Med Arom Plants 2011;1:287–93.Suche in Google Scholar
7. Bulama JS, Dangoggo SM, Bwala YA, Abah JO. Phytochemicals and antibacterial evaluation of root bark extract of Terminalia glaucescens. J App Pharm Sci 2014;4:129–32.10.7324/JAPS.2014.40221Suche in Google Scholar
8. Njomen GB, Kamgang R, Soua PR, Oyono JL, Njikam N. Protective effect of methanol-methylene chloride extract of Terminalia glaucescens leaves on streptozotocin-induced diabetes in mice. Trop J Pharm Res 2009;8:19–26.10.4314/tjpr.v8i1.14708Suche in Google Scholar
9. Njomen GB, Kamgang R, Soua PR, Oyono JL, Njikam N. Antioxidant potential of the methanol-methylene chloride extract of Terminalia glaucescens leaves on mice liver in streptozotocin-induced stress. Indian J Pharmacol 2008;40:266–70.10.4103/0253-7613.45153Suche in Google Scholar
10. Atta-ur-Rahman, Seema Z, Iqbal MC, Nadeem MA, Shahida S, Ngounou FN. Some chemical constituents of Terminalia glaucescens and their enzymes inhibition activity. Z Naturforsch 2005;60:347–50.10.1515/znb-2005-0320Suche in Google Scholar
11. Olapeju A, Olaoluwa O, Oladosu I, Gibbons S. A new triterpenoid from Terminalia glaucescens (Planch. ex Benth). Rec Nat Prod 2014;8:7–11.Suche in Google Scholar
12. Bulama JS, Dangoggo SM, Mathias SN. Isolation and characterization of beta-sitosterol from ethyl acetate extract of root bark of Terminalia glaucescens. Inter J Sc Res Pub 2015;5: 1–3.Suche in Google Scholar
13. Adnyana IK, Tezuka Y, Banskota AH, Tran KQ, Kadota S. Three new triterpenes from the seeds of Combretum quadrangulare and their hepatoprotective activity. J Nat Prod 2001;64:360–3.10.1021/np000486xSuche in Google Scholar
14. Adnyana IK, Tezuka Y, Awale S, Banskota AH, Tran KQ, Kadota S. Quadranosides VI-XI, six new triterpene glucosides from the seeds of Combretum quadrangulare. Chem Pharm Bull 2000;48:1114–20.10.1248/cpb.48.1114Suche in Google Scholar
15. Agrawal PK. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992;31:3307–30.10.1016/0031-9422(92)83678-RSuche in Google Scholar
16. Gossan DP, Magid AA, Yao-Kouassi PA, Josse J, Gangloff SC, Morjani H, et al. Antibacterial and cytotoxic triterpenoids from the roots of Combretum racemosum. Fitoterapia 2016;110:89–95.10.1016/j.fitote.2016.03.002Suche in Google Scholar
17. Ponou KB, Barboni L, Teponno RB, Mbiantcha M, Nguelefack TB, Park HJ, et al. Polyhydroxyoleanane-type triterpenoids from Combretum molle and their anti-inflammatory activity. Phytochem Lett 2008;1:183–7.10.1016/j.phytol.2008.09.002Suche in Google Scholar
18. Augusto O, Kunze KL, Montellano PR. N-phenyl protoporphyrin formation in the haemoglobin-phenylhydrazine reaction. J Biol Chem 1982;257:6231–41.10.1016/S0021-9258(20)65129-8Suche in Google Scholar
19. Labu ZK, Laboni FR, Tarafdar M, Howlader MS, Rashid MH. Membrane stabilization as a mechanism of anti-inflammatory and thrombolytic activities of ethanolic extract of arial part of Spondiasis pinanata. Pharm Online 46 Arch 2015;2:44–51.Suche in Google Scholar
20. Saso L, Valentini G, Casini ML, Silvestrini B. Inhibition of heat-induced denaturation of albumin by nonstereoidal anti-inflammatory drugs (NSAIDs): pharmacological implications. Arch Pharm Res 2001;24:150–8.10.1007/BF02976483Suche in Google Scholar PubMed
21. Ferheen S, Afza N, Malik A, Iqbal L, Azam Rasool M, Irfan Ali M, et al. Galinsosides A and B, bioactive flavanone glucosides from Galinsoga parviflora. J Enzyme Inhib Med Chem 2009;24:1128–32.10.1080/14756360802667688Suche in Google Scholar PubMed
22. Aslam M, Itrat A, Nighat A, Lubna I, Samina I, Hussain AS, et al. Biological evaluation of potent antioxidant, lipoxygenase inhibitor and antibacterial: a comparative study. J Saudi Chem Soc 2016;20:45–8.10.1016/j.jscs.2012.09.009Suche in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol


