Abstract
The hydrazide-hydrazones 6a–d and 7a, b were obtained by treating isatin (1) or its Mannich base 2 with hydrazides incorporating piperidine, morpholine, and piperazine units. The reaction of 1 and 2 with hydrazides related to triazenes having piperidine, morpholine, and 1,2,3,4-tetrahydroisoquinoline moieties gave 12a–c and 13a–c. Treatment of 1 and 2 with iminodiacetohydrazide (14) and ethylenediamine tetraacetohydrazide (18) afforded 15–17 and 19a, b, respectively. The Mannich reaction of the Schiff base 20 with formaldehyde and the appropriate hydrazide or bis(hydrazide) gave 21a, b and 22a, b. The hydrazides related to Schiff bases 20 and 26 were used as precursors in synthesis of compounds incorporating two 2-oxoindoline units or formazan moiety.
1 Introduction
The chemistry and pharmacological activities of hydrazide-hydrazones have been the object of considerable synthetic effort, and a variety of biologically active hydrazide-hydrazones have been described [1], [2], [3], [4]. Variously substituted hydrazide-hydrazones have been extensively studied as ligands in a wide range of metal complexes, and are used as starting materials for the synthesis of biologically active compounds as well as for the construction of heterocyclic systems. Depending on the substitution pattern and functionalization, hydrazide-hydrazones have shown to be effective antitubercular [5], [6], [7], anticonvulsant [8], [9], and antimicrobial [10], [11] agents. In particular, considerable attention has been devoted to the reaction of isatin with hydrazine derivatives because of the potent anticonvulsant [12], [13], [14] and antitubercular activity [15], [16], [17], [18] of the products.
In view of this, and in connection with our studies in this area [19], [20], the reaction of isatin (1) and 1-(piperidinomethyl)isatin (2) with a variety of hydrazides was further investigated as a route to some new hydrazide-hydrazones of pharmaceutical interest.
2 Results and discussion
The interest in the pharmacological activities of compounds having a piperidine, morpholine, or piperazine moiety as a structural unit prompted us to prepare 2-oxo-2-(piperidin-1-yl)acetohydrazide (4a), the morpholino analog (4b), and the bis(hydrazide) 5b, as depicted in Scheme 1. The reaction of 1 with 4a and 4b gave 2-oxo-N′-(2-oxoindolin-3-ylidene)-2-(piperidin-1-yl)acetohydrazide (6a) and 2-morpholino-2-oxo-N′-(2-oxoindolin-3-ylidene)acetohydrazide (6b), respectively.
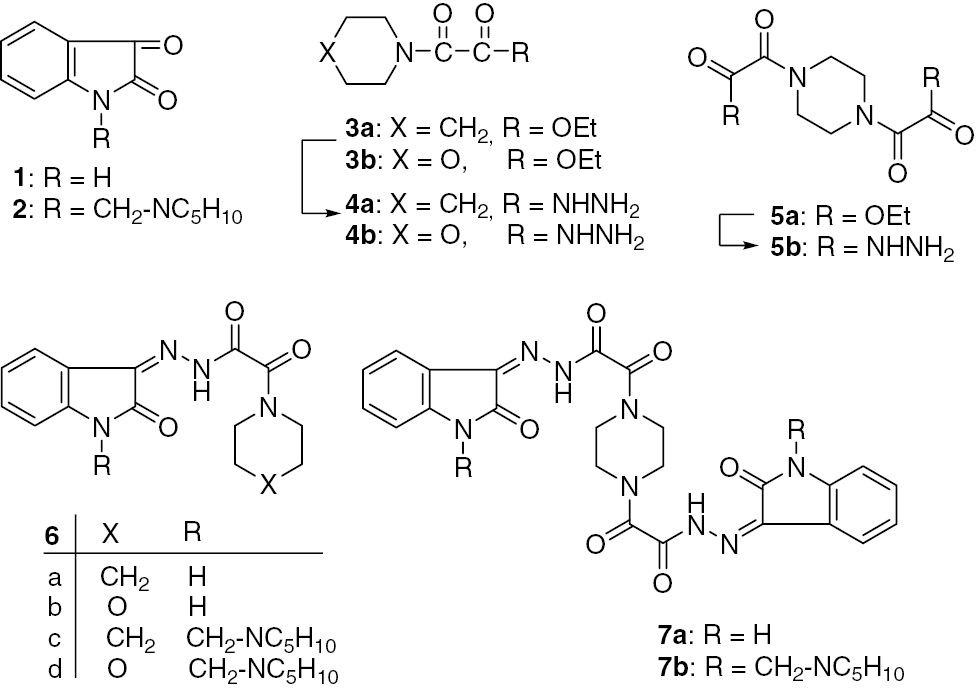
Synthesis of the hydrazide-hydrazones 6a–d and 7a, b.
A similar reaction of the Mannich base 2 with 4a and 4b afforded the corresponding hydrazide-hydrazones 6c and 6d. In line with this, treatment of 1 and 2 with the bis(hydrazide) 5b gave 2,2′-(piperazine-1,4-diyl)-bis(2-oxo-N′-(2-oxoindolin-3-ylidene)acetohydrazide) (7a) and the related bis(Mannich base) 7b, respectively (Scheme 1).
In connection with this study, we prepared a new series of hydrazides related to triazenes incorporating a piperidine, morpholine, and 1,2,3,4-tetrahydroisoquinoline moiety as a structural unit. This was achieved by a reaction sequence which involved the coupling of ethyl 4-(chlorodiazenyl)benzoate (8) with the appropriate sec-amine to give the ethyl 4-(amino-diazenyl)benzoates 9a–c, which were converted into the corresponding hydrazides 11a–c (Scheme 2).
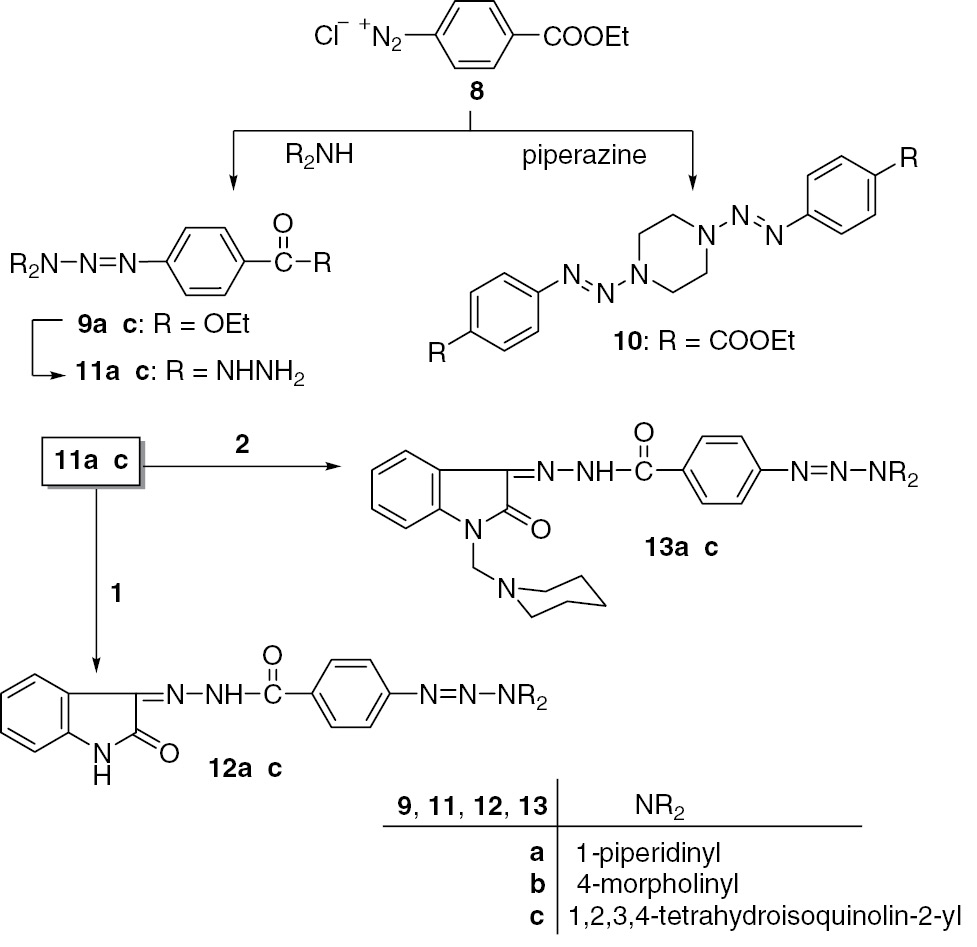
Synthesis of the hydrazide-hydrazones 12a–c and 13a–c.
The coupling of 8 with piperazine afforded diethyl 4,4′-(piperazine-1,4-diyl-bis(diazene-2,1-diyl))dibenzoate (10), which is sparingly soluble in most organic solvents. Treatment of 1 with 11a–c afforded the N′-(2-oxoindolin-3-ylidene)-benzohydrazides 12a–c. The synthesis of the corresponding Mannich bases 13a–c was achieved by the reaction of 2 with 11a–c.
Treatment of 1 and 2 with iminodiacetohydrazide (14) [21] afforded iminobis(N′-(2-oxoindolin-3-ylidene)acetohydrazide) (15) and the corresponding bis(Mannich base) (16), respectively (Scheme 3).
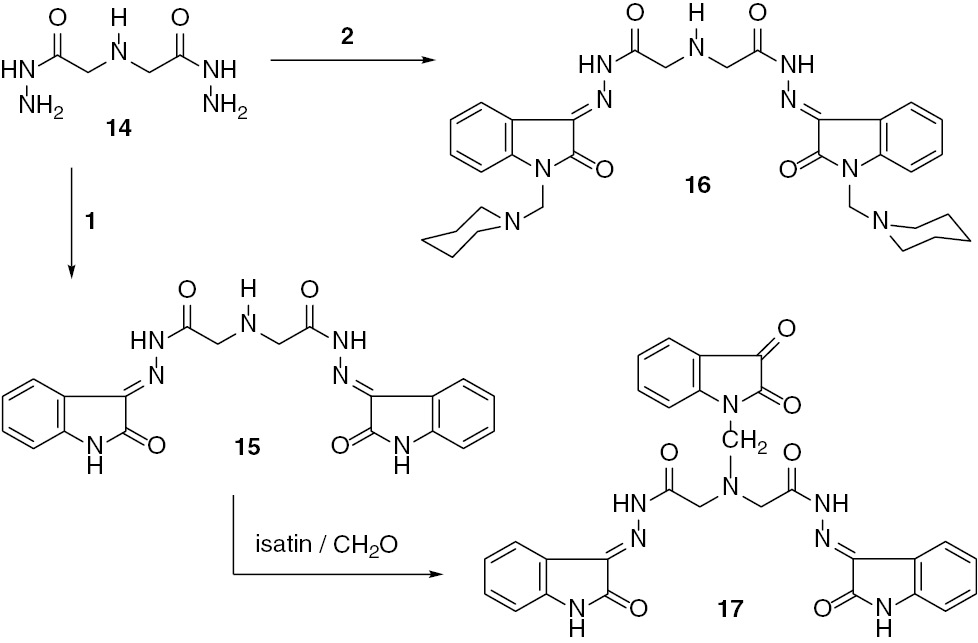
Reaction of 1 or its Mannich base 2 with iminodiacetohydrazide 14.
The Mannich reaction of the sec-amine moiety of compound 15 with isatin has been of considerable synthetic interest, because it provides access to bis(hydrazide-hydrazones) of isatin, incorporating isatin N-Mannich base as a structural unit. Thus, 15 was treated with formalin and isatin to afford 17. The mass spectra of compounds 15, 16, and 17 revealed molecular ion peaks at m/z=419 (36%), 612 (12%, [M–1]+), and 578 (51%), respectively, and fragmentation patterns which support their structures. The major fragmentation peaks can be accounted for cleavage in the hydrazide-hydrazone side chain.
In an extension of this study, the reaction of ethylenediamine tetraacetohydrazide (EDTA)-tetrahydrazide (18) [22] with isatin constitutes an interesting approach toward the synthesis of the corresponding tetra(hydrazide-hydrazones). This has been realized by treating isatin with 18 in a molar ratio of 4 to 1 to give the tetra(acetohydrazide) 19a. The analogous reaction of the Mannich base 2 with 18 afforded the tetrakis(Mannich base) 19b (Scheme 4).
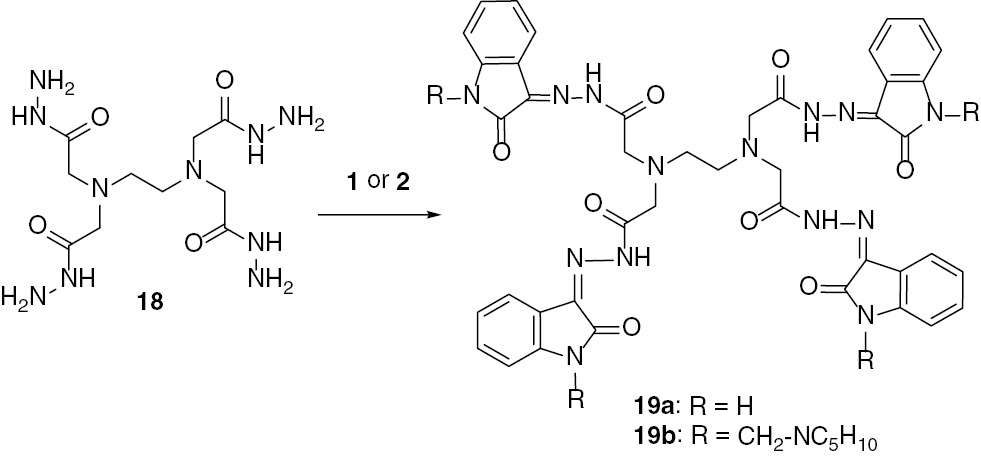
Reaction of 1 or its Mannich base 2 with EDTA-tetrahydrazide.
Although the synthetic utility of various amines in the Mannich reaction with isatin and its Schiff bases is well established, the use of hydrazides as the amine components in such reactions has not been reported. One of the specific objectives of this study was to investigate the possibility of using hydrazides as the amine components in the Mannich reaction with the Schiff bases of isatin. Therefore, the Schiff base 20 [13] was treated with isonicotinic hydrazide and formaldehyde to give the isonicotinohydrazide 21a (Scheme 5).
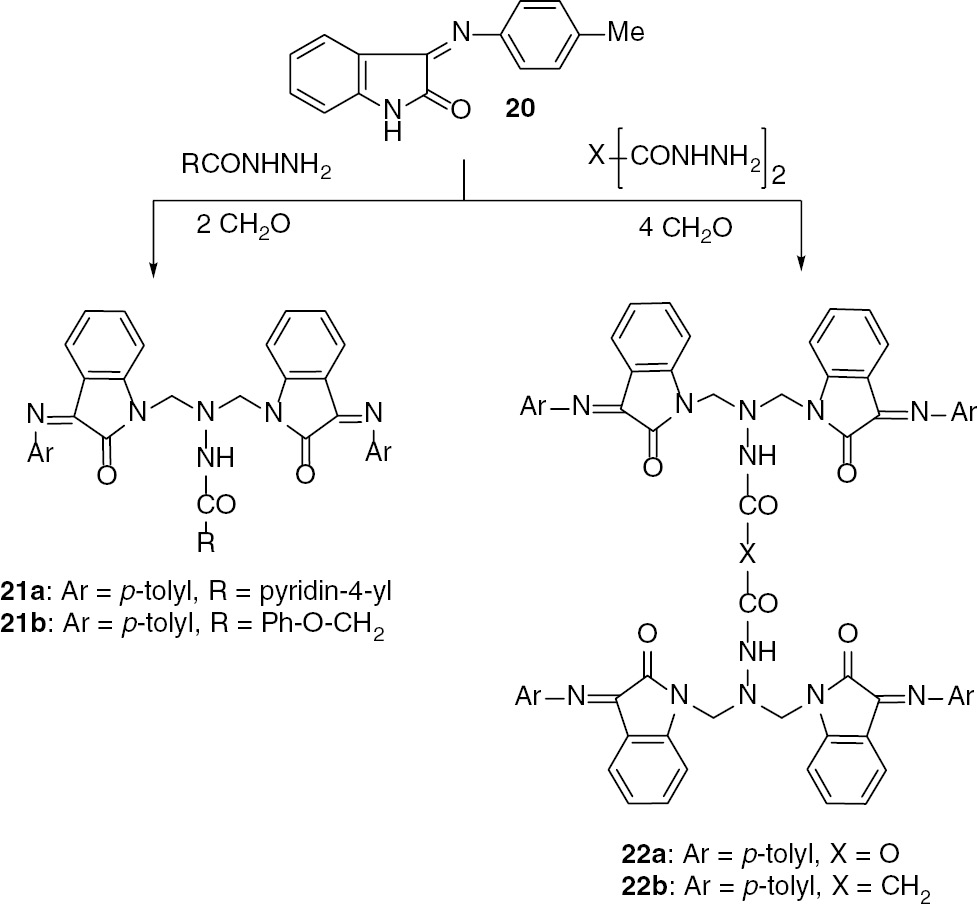
Mannich reaction of 20 with hydrazides and dihydrazides.
A similar reaction takes place with phenoxyacetohydrazide yielding 21b. The synthetic potential of Mannich reactions with bis(hydrazides) was established by its application to the synthesis of the Mannich bases 22a and 22b. This has been realized by treating 20 with formaldehyde and oxalohydrazide to afford the oxalohydrazide 22a. The similar reaction with malonohydrazide afforded 22b.
A variety of isatin Schiff bases with various substitution patterns have been synthesized and studied with interest centered on their potential pharmaceutical activities [12], [23], [24], [25], [26], [27]. In view of this, 2-(2-oxo-3-(p-tolylimino)indolin-1-yl)acetohydrazide (23b) was synthesized starting from the Schiff base 20. Treatment of isatin with 23b afforded the hydrazide-hydrazone 24, incorporating two 2-oxoindoline moieties, and this was subjected to the Mannich reaction with piperidine and formaldehyde to give the Mannich base 25 (Scheme 6).
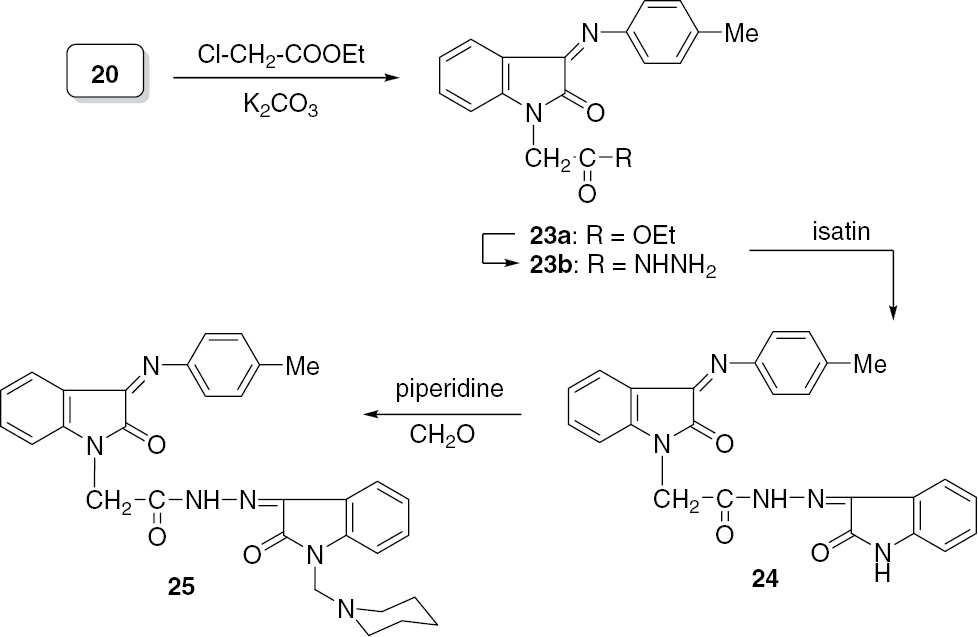
Synthesis of the hydrazide 23b and the hydrazide-hydrazones 24 and 25.
The mass spectrum of compound 24 indicated the molecular ion peak at m/z=437 (65%, [M]+), whereas the Mannich base 25 is unstable to electron impact. The molecular ion peak [M]+ was absent, and only fragment peaks were detected.
In the course of our study, the hydrazide-hydrazone 27 has been used as a precursor for the synthesis of isatin Schiff bases incorporating a formazan moiety as a structural unit. This has been achieved by coupling benzenediazonium chloride with 27 to afford the formazan 28 (Scheme 7). The formation of 28 is of interest because formazans have been extensively studied as ligands in a wide range of metal complexes [28], [29], [30] and a variety of biologically active formazans have been described [31], [32], [33].
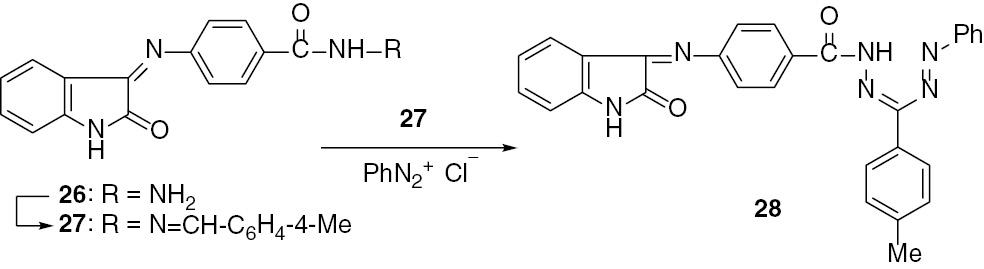
Reaction of the hydrazide-hydrazone 27 with benzenediazonium chloride.
3 Experimental section
All melting points (uncorrected) were determined on a Gallenkamp electric melting point apparatus (Sanyo Gallenkamp, Southborough, UK). Elemental microanalyses were carried out on a Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany) at the Microanalytical Unit, Faculty of Science, Cairo University. Infrared spectra were measured on a Mattson 5000 FTIR spectrometer (Mattson Instruments, Inc., Madison, WI, USA). 1H and 13C NMR data were obtained in [D6]DMSO solution on a Varian XL 300 MHz instrument (Varian, Inc., CA, USA) using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in ppm (δ) downfield from internal TMS. Mass spectra were recorded on a GC-MS QP-1000 EX Shimadzu instrument (Shimadzu, Tokyo, Japan). The course of the reaction and the purity of the synthesized compounds were monitored by TLC using EM science silica gel coated plates, 0.25 nm, 60 GF 254 (Merck, Darmstadt, Germany) with visualization by irradiation with an ultraviolet lamp. Compounds 7b, 12c, 13a–c, 19a, 19b, 21a, 21b, 22a, 22b, 24, and 25 are of limited solubility in common 1H NMR solvents. Compounds 2 [34], 5a [35], 14 [21], 18 [22], 20 [13], and 26 [36] were prepared as previously described.
3.1 Synthesis of the hydrazides 4a, b, and 5b
A solution of 3a (1.85 g, 10 mmol) or 3b (1.87 g, 10 mmol) or 5a (1.43 g, 5 mmol) and hydrazine hydrate (80%, 1.9 mL, 30 mmol) in ethanol (30 mL) was refluxed for 1 h. The product obtained was filtered and crystallized from ethanol to give 4a, b and from ethanol–water (2:1) to give 5b.
3.1.1 2-Oxo-2-(piperidin-1-yl)acetohydrazide (4a)
M.p. 242°C. Yield 82% (white powder). – IR (KBr): ν=3293–3210 (NH–NH2), 1696 (CO), 1619 (CO), 1541, 1210 cm−1. – 1H NMR ([D6]DMSO): δ=1.65–1.68 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 3.32 (m, 4H, 2-H2, 6-H2 of piperidine), 4.55 (s, 2H, NH2), 9.72 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=173 (1) [M+2]+, 172 (7) [M+1]+, 171 (100) [M]+, 153 (22), 127 (24), 100 (15), 84 (3), 82 (19), 72 (13), 56 (19). – C7H13N3O2 (171.20): calcd. C 49.11, H 7.65, N 24.54; found C 49.09, H 7.60, N 24.43.
3.1.2 2-Morpholino-2-oxoacetohydrazide (4b)
M.p. 214°C. Yield 80% (white powder). – IR (KBr): ν=3286–3217 (NH–NH2), 1681 (CO), 1649 (CO), 1587, 1215 cm−1. – 1H NMR ([D6]DMSO): δ=3.44 (m, 4H, CH2–N–CH2 of morpholine), 3.65 (m, 4H, CH2–O–CH2 of morpholine), 4.59 (s, 2H, NH2), 10.11 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=174 (2) [M+1]+, 173 (18) [M]+, 155 (3), 114 (7), 112 (100), 100 (23), 85 (46), 68 (14), 57 (34). – C6H11N3O3 (173.17): calcd. C 41.61, H 6.40, N 24.27; found C 41.59, H 6.44, N 24.20.
3.1.3 2,2′-(Piperazine-1,4-diyl)bis(2-oxoacetohydrazide) (5b)
M.p. 220°C. Yield 80% (white powder). – IR (KBr): ν=3291–3244 (NH–NH2), 1678 (CO), 1640 (CO), 1467, 1163 cm−1. – 1H NMR ([D6]DMSO): δ=3.49 [t, 8H, N(CH2CH2)2N], 4.49 [s, 4H, 2×(NH2)], 8.59 ppm [s, 2H, 2×(NH)]. – MS (EI, 70 eV): m/z (%)=258 (12) [M]+, 240 (2), 197 (2), 155 (8), 133 (32), 127 (69), 126 (99), 73 (33), 57 (28). – C8H14N6O4 (258.23): calcd. C 37.21, H 5.46, N 32.54; found C 37.19, H 5.40, N 32.48.
3.2 Synthesis of the hydrazide-hydrazones 6a–d
A mixture of 1 (1.47 g, 10 mmol) or the Mannich base 2 [34] (2.44 g, 10 mmol) and the appropriate hydrazide (10 mmol) in ethanol (70 mL) and acetic acid (0.3 mL) was refluxed for 45 min. The product obtained on cooling was filtered and crystallized from chloroform to give 6a–d.
3.2.1 2-Oxo-N′-(2-oxoindolin-3-ylidene)-2-(piperidin-1-yl)acetohydrazide (6a)
M.p. >300°C. Yield 61% (orange crystals). – IR (KBr): ν=3329 (NH), 3212 (NH), 1725 (CO), 1690 (CO), 1608, 1510, 1211 cm−1. – 1H NMR ([D6]DMSO): δ=1.70–1.74 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 331 (m, 4H, 2-H2, 6-H2 of piperidine), 9.17 [s, 1H, (=N–NH–)], 6.95–7.77 (m, 4.H, aromatic), 10.31 ppm (s, 1H, NH of oxoindoline).– 13C NMR ([D6]DMSO): δ=23.96 (C-3, C-4, C-5 of piperidine), 45.33 (C-2, C-6 of piperidine), 139.23 (C-3 of oxoindoline moiety), 120.57, 120.71, 121.21, 122.77, 132.23, 142.36 (all Ar C), 161.29 (NC=O) 162.44 (HNC=O), 167.17 ppm (C=O of oxoindoline moiety). – MS (EI, 70 eV): m/z (%)=301 (11) [M+1]+, 300 (51) [M]+, 298 (78), 261 (35), 217 (22), 204 (15), 193 (41), 168 (46), 147 (58), 116 (62), 99 (66), 80 (100). – C15H16N4O3 (300.31): calcd. C 59.99, H 5.37, N 18.66; found C 59.90, H 5.32, N 18.61.
3.2.2 2-Morpholino-2-oxo-N′-(2-oxoindolin-3-ylidene)acetohydrazide (6b)
M.p.>300°C. Yield 57% (orange crystals). – IR (KBr): ν=3358 (NH), 3242 (NH), 1676 (CO), 1644 (CO), 1610, 1513, 1142 cm−1. – 1H NMR ([D6]DMSO): δ=3.34 (m, 4H, CH2–N–CH2 of morpholine), 3.61 (m, 4H, CH2–O–CH2 of morpholine), 9.22 [s, 1H, (=N–NH–)], 6.91–7.76 (m, 4H, aromatic), 10.36 ppm (s, 1H, NH of oxoindoline). – 13C NMR ([D6]DMSO): δ=44.65 (C-3, C-5 of morpholine), 64.76 (C-2, C-6 of morpholine), 139.45 (C-3 of oxoindoline moiety), 120.53, 120.73, 121.20, 122.74, 132.23, 142.39 (all Ar C), 161.27 (NC=O) 162.42 (HNC=O), 167.58 ppm (C=O of oxoindoline moiety). – MS (EI, 70 eV): m/z (%)=302 (10) [M]+, 280 (96), 274 (46), 250 (61), 237 (100), 212 (88), 171 (71), 148 (13), 106 (96), 97 (35), 85 (31). – C14H14 N4O4 (302.29): calcd. C 55.63, H 4.67, N 18.53; found C 55.61, H 4.62, N 18.49.
3.2.3 2-Oxo-N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)-2-(piperidin-1-yl)acetohydrazide (6c)
M.p.>300°C. Yield 44% (yellow crystals). – IR (KBr): ν=3250 (NH), 1686 (CO), 1671 (CO), 1618, 1546, 1182, 1110 cm−1. – 1H NMR ([D6]DMSO): δ=1.68–1.72 [m, 12H, 2×(3-H2, 4-H2, 5-H2 of piperidine)], 2.43 (m, 4H, 2-H2, 6-H2 of piperidine), 3.34 (m, 4H, 2-H2, 6-H2 of piperidine), 4.12 (s. 2H, N–CH2–N of side chain), 6.88–7.73 (m, 4H, aromatic), 9.10 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=398 (15) [M+1]+, 397 (61) [M]+, 396 (35) [M–1]+, 395 (100), 369 (91), 322 (37), 289 (70), 277 (48), 182 (60), 142 (19), 112 (74), 97 (53). – C21H27N5O3 (397.47): calcd. C 63.46, H 6.85, N 17.62; found C 63.40, H 6.82, N 17.59.
3.2.4 2-Morpholino-2-oxo-N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)acetohydrazide (6d)
M.p.>300°C. Yield 51% (yellow crystals). – IR (KBr): ν=3421 (NH), 1681 (CO), 1668 (CO), 1614, 1522, 1127, 1102 cm−1. – 1H NMR ([D6]DMSO): δ=1.57–1.66 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 2.41 (m, 4H, 2-H2, 6-H2 of piperidine), 3.28 (m, 4H, CH2–N–CH2 of morpholine), 3.66 (m, 4H, CH2–O–CH2 of morpholine), 4.10 (s, 2H, N–CH2–N of side chain), 6.81–7.25 (m, 4H, aromatic), 9.13 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=400 (16) [M+1]+, 399 (56) [M]+, 383 (60), 323 (47), 260 (55), 235 (37), 222 (93), 149 (50), 134 (100). – C20H25N5O4 (399.44): calcd. C 60.14, H 6.31, N 17.53; found 60.03, H 6.29, N 17.50.
3.3 2,2′-(Piperazine-1,4-diyl)bis(2-oxo-N′-(2-oxoindolin-3-ylidene)acetohydrazide) (7a)
A mixture of 1 (0.88 g, 6 mmol) and 5b (0.77 g, 3 mmol) in ethanol (50 mL) and acetic acid (0.3 mL) was refluxed for 1 h. The product obtained on cooling was filtered and washed with boiling ethanol (3×15 mL) to give 7a. M.p.>300°C. Yield 45% (yellow powder). – IR (KBr): ν=3318 (NH), 3210 (NH), 1710 (CO), 1691 (CO), 1618, 1513, 1156 cm−1. – 1H NMR ([D6]DMSO): δ=3.36 [br. s, 8H, N(CH2CH2)2N], 9.06 [br. s, 2H, 2×(=N–NH–)], 6.91–7.56 (m, 8H, aromatic), 11.28 ppm [s, 2H, 2×(NH) of oxoindoline]. – 13C NMR ([D6]DMSO): δ=45.33 (4×CH2 of piperazine), 139.29 (C-3 of oxoindoline moiety), 120.73, 120.82, 121.24, 122.69, 132.29, 142.90 (all Ar C), 161.27 (NC=O) 162.35 (HNC=O), 166.35 ppm (C=O of oxoindoline moiety). – MS (EI, 70 eV): m/z (%)=517 (34) [M+1]+, 516 (29) [M]+, 167 (27), 149 (54), 148 (27), 98 (29), 93 (28). – C24H20N8O6 (516.47): calcd. C 55.81, H 3.90, N 21.70; found C 55.79, H 3.92, N 21.68.
3.4 2,2′-(Piperazine-1,4-diyl)bis(2-oxo-N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)acetohydrazide) (7b)
This compound was obtained from 2 (1.46 g, 6 mmol) and 5b (0.77 g, 3 mmol), following the procedure described for the synthesis of 7a. The product was washed with boiling ethanol (3×15 mL) to give 7b. M.p. 252°C. Yield 48% (yellow powder). – IR (KBr): ν=3418 (NH), 3310 (NH), 1698 (CO), 1684 (CO), 1641, 1466, 1173 cm−1. – MS (EI, 70 eV): m/z (%)=710 (33) [M]+, 636 (20), 567 (30), 444 (30), 306 (31), 203 (22), 188 (31), 160 (37), 145 (47), 113 (73), 97 (22), 84 (39). – C36H42 N10O6 (710.33): calcd. C 60.83, H 5.96, N 19.71; found C 60.79, H 5.90, N 19.68.
3.5 Synthesis of the triazenes 9a–c
Diazotized ethyl 4-aminobenzoate (28 mmol) was added dropwise with stirring to a cold solution at 0–5°C, of the appropriate amine (31 mmol) during 10 min. Then saturated K2CO3 solution was added until pH of 8 was reached and the solution was stirred for 30 min at 0–5°C, a pale-yellow suspension resulted. The product was filtered, washed with water (3×20 mL) and air dried. The crude product was crystallized from ethyl acetate to give 9a–c.
3.5.1 Ethyl 4-(piperidin-1-yldiazenyl)benzoate (9a)
M.p. 67°C. Yield 68% (pale-yellow crystals). – IR (KBr): ν=1712, 1599, 1272, 1098, 773 cm−1. – 1H NMR ([D6]DMSO): δ=1.36 (t, 3H, CH3), 1.63–1.72 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 3.38 (m, 4H, 2-H2, 6-H2 of piperidine), 4.36 (q, 2H, CH2CH3), 7.46 (d, 2H, aromatic), 8.01 ppm (d, 2H, aromatic). – MS (EI, 70 eV): m/z (%)=262 (9) [M+1]+, 261 (59) [M]+, 230 (4), 202 (67), 143 (65), 130 (15), 118 (8), 77 (100). – C14H19N3O2 (261.32): calcd. C 64.35, H 7.33, N 16.08; found C 64.29, H 7.30, N 16.14.
3.5.2 Ethyl 4-(morpholinodiazenyl)benzoate (9b)
M.p. 70°C. Yield 76% (pale-yellow crystals). – IR (KBr): ν=1718, 1591, 1266, 1110, 777 cm−1. – 1H NMR ([D6]DMSO): δ=1.33 (t, 3H, CH3), 3.04 (m, 4H, CH2–N–CH2 of morpholine), 3.48 (m, 4H, CH2–O–CH2 of morpholine), 4.30 (q, 2H, CH2CH3), 7.48 (d, 2H, aromatic), 8.11 ppm (d, 2H, aromatic). – MS (EI, 70 eV): m/z (%)=264 (7) [M+1]+, 263 (43) [M]+, 217 (94), 193 (7), 148 (6), 119 (4), 99 (100), 90 (13), 57 (46). – C13H17N3O3 (263.29): calcd. C 59.30, H 6.51, N 15.96; found C 59.27, H 6.49, N 15.83.
3.5.3 Ethyl 4-((3,4-dihydroisoquinolin-2(1H)-yl)diazenyl)benzoate (9c)
M.p. 85°C. Yield 60% (yellow crystals). – IR (KBr): ν=1707, 1598, 1271, 1105, 752 cm−1. – 1H NMR ([D6]DMSO): δ=1.41 (t, 3H, CH3), 3.09 (t, 2H, 4-H2 of tetrahydroisoquinoline), 4.15 (t, 2H, 3-H2 of tetrahydroisoquinoline), 4.37 (q, 2H, CH2CH3), 5.01 (s, 2H, 1-H2 of tetrahydroisoquinoline), 6.89–8.06 ppm (m, 8H, aromatic). – MS (EI, 70 eV): m/z (%)=311 (1) [M+2]+, 310 (9) [M+1]+, 309 (50) [M]+, 264 (18), 263 (100), 218 (14), 191 (47), 190 (52), 128 (4), 96 (7). – C18H19N3 O2 (309.36): calcd. C 69.88, H 6.19, N 13.58; found C 69.81, H 6.11, N 13.50.
3.6 Diethyl 4,4′-(piperazine-1,4-diylbis (diazene-2,1-diyl))dibenzoate (10)
This compound was obtained from 8 (28 mmol) and piperazine (1.20 g, 14 mmol), following the procedure described above for the synthesis of 9a–c. The product was washed with boiling ethanol (3×15 mL) to give 10. M.p. 181°C. Yield 72% (pale-yellow powder). – IR (KBr): ν=1700, 1602, 1278, 1138, 693 cm−1. – 1H NMR ([D6]DMSO): δ=1.43 (t, 6H, 2×CH3), 4.09 ([br. s, 8H, N(CH2CH2)2N], 4.39 (q, 4H, 2×CH2CH3), 7.49–8.06 ppm (m, 8H, aromatic). – MS (EI, 70 eV): m/z (%)=439 (4) [M+1]+, 438 (6) [M]+, 397 (4), 394 (27), 366 (12), 349 (63), 338 (12), 294 (8), 176 (13), 166 (14), 147 (20), 109 (100), 93 (68). – C22H26N6O4 (438.48): calcd. C 60.26, H 5.98, N 19.17; found C 60.21, H 5.92, N 19.10.
3.7 Synthesis of 4-(substituted-diazenyl)benzohydrazides 11a–c
A solution of 9a, 9b, or 9c (2 mmol) and hydrazine hydrate (80%, 0.5 mL, 8 mmol) in ethanol (20 mL) was refluxed on a steam bath for 3 h. The reaction mixture was cooled to give a solid product which was filtered and crystallized from ethyl acetate to give compounds 11a–c.
3.7.1 4-(Piperidin-1-yldiazenyl)benzohydrazide) (11a)
M.p. 140°C. Yield 60% (white powder). – IR (KBr): ν=3318, 3266 (NH–NH2), 1662 (CO), 1607, 1427, 1104, 670 cm−1. – 1H NMR ([D6]DMSO): δ=1.66–1.71 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 3.82 (m, 4H, 2-H2, 6-H2 of piperidine), 4.54 (s, 2H, NH2), 7.44 (d, 2H, aromatic), 7.90 (d, 2H, aromatic), 9.77 ppm (s, 1H, NH). – 13C NMR ([D6]DMSO): δ=23.55 (C-3, C-4, C-5 of piperidine), 43 (C-2, C-6 of piperidine), 119.66, 127.88, 129.76, 152.40 (all aromatic C), 165.67 ppm (C=O). – MS (EI, 70 eV): m/z (%)=248 (7) [M+1]+, 247 (49) [M]+, 215 (3), 201 (100), 163 (24), 144 (4), 133 (15), 105 (9), 89 (16), 63 (15). – C12H17N5O (247.30): calcd. C 58.28, H 6.93, N 28.32; found C 58.21, H 6.89, N 28.30.
3.7.2 4-(Morpholinodiazenyl)benzohydrazide (11b)
M.p. 169°C. Yield 68% (white powder). – IR (KBr): ν=3310–3214 ((NH–NH2), 1649 (CO), 1621, 1447, 1124, 677 cm−1. – 1H NMR ([D6]DMSO): δ=2.49 (m, 4H, CH2–N–CH2 of morpholine), 3.77 (m, 4H, CH2–O–CH2 of morpholine), 4.45 (s, 2H, NH2), 7.39 (d, 2H, aromatic), 7.82 (d, 2H, aromatic), 9.69 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=250 (5) [M+1]+, 249 (36) [M]+, 231 (16), 218 (100), 201 (7), 184 (5), 172 (27), 155 (8), 142 (11), 129 (6), 76 (6). – C11H15N5O2 (249.27): calcd. C 53.00, H 6.07, N 28.10; found C 52.96, H 6.01, N 28.02.
3.7.3 4-((3,4-Dihydroisoquinolin-2(1H)-yl)diazenyl)benzohydrazide (11c)
M.p. 178°C. Yield 66% (white powder). – IR (KBr): ν=3318–3219 ((NH–NH2), 1651 (CO), 1619, 1453, 1120, 676 cm−1. – 1H NMR ([D6]DMSO): δ=3.12 (t, 2H, 4-H2 of tetrahydroisoquinoline), 4.19 (t, 2H, 3-H2 of tetrahydroisoquinoline), 4.51 (s, 2H, NH2), 5.08 (s, 2H, 1-H2 of tetrahydroisoquinoline), 6.92–8.01 (m, 8H, aromatic), 9.83 ppm (s, 1H, NH). – MS (EI, 70 eV): m/z (%)=295 (2) [M]+, 263 (2), 249 (7), 222 (20) 194 (10) 180 (100), 176 (14), 164 (8), 150 (8), 106 (16), 91 (8). – C16H17N5 O (295.34): calcd. C 65.07, H 5.80, N 23.71; found C 65.01, H 5.83, N 23.69.
3.8 Synthesis of the hydrazide-hydrazones 12a–c
A solution of 1 (0.44 g, 3 mmol) and 11a (0.74 g, 3 mmol) or 11b (0.75 g, 3 mmol), or 11c (0.88 g, 3 mmol) in ethanol (30 mL) and acetic acid (0.3 mL) was refluxed for 30 min. The precipitated product was filtered and washed with boiling ethanol (3×15 mL) to give 12a–c.
3.8.1 N′-(2-Oxoindolin-3-ylidene)-4-(piperidin-1-yldiazenyl)benzohydrazide (12a)
M.p. 260°C. Yield 72% (yellow powder). – IR (KBr): ν=3437 (NH), 3212 (NH), 1714 (CO), 1676 (CO), 1609, 1519, 1262, 751 cm−1. – 1H NMR ([D6]DMSO): δ=1.67–1.71 (m, 6H, 3-H2, 4-H2, 5-H2 of piperidine), 3.84 (m, 4H, 2-H2, 6-H2 of piperidine), 6.90–7.99 (m, 8H, aromatic), 10.79 (s, 1H, NH of oxoindoline), 11.34 ppm (s, 1H, CONH). – MS (EI, 70 eV): m/z (%)=377 (2) [M+1]+, 376 (8) [M]+, 292 (18), 264 (28), 236 (22) 217 (15), 216 (100), 160 (21), 145 (18), 132 (10), 121 (16), 105 (31), 104 (45), 85 (19), 84 (55). – C20H20N6O2 (376.41): calcd. C 63.82, H 5.36, N 22.33; found C 63.80, H 5.32, N 22.30.
3.8.2 4-(Morpholinodiazenyl)-N′-(2-oxoindolin-3-ylidene)benzohydrazide (12b)
M.p. 248°C. Yield 53% (yellow powder). – IR (KBr): ν=3430 (NH), 3222 (NH), 1717 (CO), 1667 (CO), 1613, 1532, 747 cm−1. – 1H NMR ([D6]DMSO): δ=3.69 (m, 4H, CH2–N–CH2 of morpholine), 3.91 (m, 4H, CH2–O–CH2 of morpholine), 6.90–8,02 (m, 8H, aromatic), 10.79 (s, 1H, NH of oxoindoline), 11.35 ppm (s, 1H, CONH). – MS (EI, 70 eV): m/z (%)=379 (2) [M+1]+, 378 (5) [M]+, 293 (4), 264 (18), 237 (11), 236 (20), 218 (100), 200 (52), 160 (32), 157 (12), 133 (18), 121 (31), 105 (61), 86 (29). – C19H18N6O3 (378.38): calcd. C 60.31, H 4.79, N 22.21; found C 60.29, H 4.73, N 22.20.
3.8.3 4-((3,4-Dihydroisoquinolin-2(1H)-yl)diazenyl)-N′-(2-oxoindolin-3-ylidene)-benzohydrazide (12c)
M.p. 206°C. Yield 83% (yellow powder). – IR (KBr): ν=3443 (NH), 3237 (NH), 1710 (CO), 1661 (CO), 1609, 1535, 750 cm−1. – MS (EI, 70 eV): m/z (%)=426 (8) [M+2]+, 425 (5) [M+1]+, 224 (11) [M]+, 397 (9), 353 (10), 340 (11), 291 (10), 264 (8) 160 (11), 145 (10), 132 (19), 111 (20), 97 (31), 76 (18). – C24H20N6O2 (424.45): calcd. C 67.91, H 4.75, N 19.80; found C 67.89, H 4.73, N 19.82.
3.9 Synthesis of the hydrazide-hydrazones 13a–c
These compounds were obtained from the Mannich base 2 (0.73 g, 3 mmol) and 11a (0.74 g, 3 mmol) or 11b (0.75 g, 3 mmol) or 11c (0.88 g, 3 mmol) in the manner described for the synthesis of 12a–c. The product was filtered and washed with boiling ethanol (3×15 mL) to give 13a–c.
3.9.1 N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)-4-(piperidin-1-yldiazenyl)benzohydrazide (13a)
M.p. 251°C. Yield 58% (yellow powder). – IR (KBr): ν=3446 (NH), 1701 (CO), 1668 (CO), 1611 (C=N), 1531, 1105, 748 cm−1. – MS (EI, 70 eV): m/z (%)=474 (11) [M+1]+, 473 (30) [M]+, 455 (29), 389 (34), 363 (21), 347 (26), 295 (29), 222 (28), 192 (28), 188 (21), 175 (28), 131(28), 121 (31), 98 (31), 84 (33), 80 (100). – C26H31N7O2 (473.57): calcd. C 65.94, H 6.60, N 20.70; found C 65.90, H 6.62, N 20.69.
3.9.2 4-(Morpholinodiazenyl)-N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)benzohydrazide (13b)
M.p. 246°C. Yield 51% (yellow powder). – IR (KBr): ν=3449 (NH), 17I5 (CO), 1667 (CO), 1614 (C=N), 1528, 1109, 678 cm−1. – MS (EI, 70 eV): m/z (%)=476 (4) [M+1]+, 475 (6), [M]+, 445 (6), 388 (4), 327 (7), 295 (6), 175 (51), 168 (8) 148 (12), 147 (100), 119 (11), 104 (14), 87 (17), 86 (7), 77 (21), 76 (33). – C25H29N7O3 (475.54): calcd. C 63.14, H 6.15, N 20.62; found C 63.07, H 6.10, N 20.59.
3.9.3 4-((3,4-Dihydroisoquinolin-2(1H)-yl)diazenyl)-N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)benzohydrazide (13c)
M.p. 155°C. Yield 65% (yellow powder). – IR (KBr): ν=3445 (NH), 1723 (CO), 1655 (CO), 1608 (C=N), 1521, 1114, 749 cm−1. – MS (EI, 70 eV): m/z (%)=521 (92) [M]+, 520 (92) [M–1]+, 491 (53), 447 (65), 390 (53), 361 (80), 347 (65), 320 (72), 292 (62) 244 (84), 202 (59), 163 (86), 104 (100), 98 (11), 83 (5). – C30H31N7O2 (521.25): calcd. C 69.08, H 5.99, N 18.80; found C 69.00, H 5.92, N 18.79.
3.10 Synthesis of the bis(hydrazide-hydrazones) 15 and 16
A solution of 1 (1.17 g, 8 mmol) or the Mannich base 2 (1.95 g, 8 mmol) and iminodiacetohydrazide 14 (0.65 g, 4 mmol) in ethanol (50 mL) and acetic acid (0.3 mL) was refluxed for 40 min. The precipitated product was filtered and washed with boiling ethanol (3×15 mL) to give 15 and 16.
3.10.1 Iminobis[N′-(2-oxoindolin-3-ylidene)acetohydrazide] (15)
M.p. 202°C. Yield 47% (pale-yellow powder). – IR (KBr): ν=3365 (NH), 3244 (NH), 1683 (CO), 1664 (CO), 1621 (C=N), 1610, 1466, 1136, 791 cm−1. – 1H NMR ([D6]DMSO): δ=3.47 [s, 4H, 2×(CO–CH2–)], 6.88–7.52 (m, 8H, aromatic), 7.57 (s, 1H, CH2–NH–CH2), 10.75 [s, 2H, 2 × (NH of oxoindoline)], 11.12 ppm [s, 2H, 2×(CO–NH)]. – 13C NMR ([D6]DMSO): δ=54.67 (CH2–NH), 138.54 (C-3 of oxoindoline moiety), 120.51, 120.77, 121.28, 122.66, 132.21, 142.37 (all Ar C), 163.21 (HN–C=O), 167.71 ppm (C=O of oxoindoline moiety). – MS (EI, 70 eV): m/z (%)=421 (21) [M+2]+, 420 (23) [M+1]+, 419 (36) [M]+, 392 (31), 368 (23), 309 (27), 295 (25), 278 (31), 274 (23), 230 (27), 217 (36), 201 (23), 188 (23), 147 (41), 146 (23), 131 (29), 118 (22), 82 (29.7), 69 (100). – C20H17N7O4 (419.39): calcd. C 57.28, H 4.09, N 23.38; found C 57.21, H 4.00, N 23.30.
3.10.2 Iminobis[N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)acetohydrazide] (16)
M.p. 207°C. Yield 45% (dark-yellow powder). – IR (KBr): ν=3403 (NH), 1718 (CO), 1669 (CO), 1617 (C=N), 1609, 1470, 1346, 754 cm−1. – 1H NMR ([D6]DMSO): δ=1.35–1.47 [m, 12H, 2×(3-H2, 4-H2, 5-H2 of piperidine)], 2.44 (m, 4H, 2-H2, 6-H2 of piperidine), 2.51 (m, 4H, 2-H2, 6-H2 of piperidine), 3.49 [s, 4H, 2×(CO–CH2–)], 4.17 (s, 2H, N–CH2–N of side chain), 6.81–7.51 (m, 8H, aromatic), 7.59 (s, 1H, CH2–NH–CH2), 10.98 ppm [s, 2H, 2×(CO–NH)]. – 13C NMR ([D6]DMSO): δ=24.33 (C-3, C-4, C-5 of piperidine), 47.87 (C-2, C-6 of piperidine), 54.57 (CH2–NH), 67.45 (N–CH2–N of side chain), 138.77 (C-3 of oxoindoline moiety), 120.46, 120.68, 121.22, 122.59, 132.26, 142.47 (all Ar C), 163.44 (HN–C=O), 168.11 ppm (C=O of oxoindoline moiety). – MS (EI, 70 eV): m/z (%)=612 (12) [M–1]+, 556 (12), 503 (11), 478 (12), 247 (11), 242 (12), 221 (13), 167 (13), 155 (14), 149 (23), 145 (63), 144 (15), 130 (13), 118 (21), 98 (57), 84 (100), 77 (18). – C32H39N9O4 (613.71): calcd. C 62.63, H 6.41, N 20.54; found C 62.60, H 6.43, N 20.51.
3.11 2,2′-[(2,3-Dioxoindolin-1-yl)methylimino)bis(N′-(2-oxoindolin-3-ylidene)acetohydrazide] (17)
A mixture of 16 (1.25 g, 3 mmol), 1 (0.44 g, 3 mmol), and formalin (36%, 0.25 mL, 3 mmol) in pyridine–ethanol (1:4) (40 mL) was heated under reflux for 1 h. After cooling, the reaction mixture was poured on cold water and the product obtained was filtered and crystallized from chloroform to give compound 17. M.p. 240°C. Yield 63% (pale-yellow powder). – IR (KBr): ν=3422 (NH), 1693 (CO), 1655 (CO), 1619 (C=N), 1605, 1466, 1349, 757 cm−1. – 1H NMR ([D6]DMSO): δ=3.50 [s, 4H, 2 × (CO–CH2–)], 4.21 (s, 2H, N–CH2–N of side chain), 6.86–7.61 (m, 12H, aromatic), 10.71 [s, 2H, 2×(NH of oxoindoline)], 11.10 ppm [s, 2H, 2×(CO–NH)]. – MS (EI, 70 eV): m/z (%)=579 (57) [M+1]+, 578 (51) [M]+, 577 (52), 536 (85), 508 (55), 470 (68), 425 (58), 416 (48), 393 (64), 244 (46), 188 (49), 160 (68), 145 (56), 132 (47), 107 (100). – C29H22N8O6 (578.53): calcd. C 60.21, H 3.83, N 19.37; found C 60.11, H 3.80, N 19.32.
3.12 Synthesis of the tetrakis(hydrazide-hydrazones) 19a, b
A solution of EDTA-tetrahydrazide 18 [22] (1 g, 3 mmol) and 1 (1.76 g, 12 mmol) or the Mannich base 2 (2.93 g, 12 mmol) in aqueous ethanol (60%, 50 mL) and acetic acid (0.3 mL) was heated over a steam bath for 1 h. The product obtained on cooling was filtered and washed by boiling aqueous ethanol (60%, 3×15 mL) to give 19a, b.
3.12.1 2,2′,2″,2′″-(Ethane-1,2-diylbis(azanetriyl))tetrakis(N′-(2-oxoindolin-3-ylidene)acetohydrazide) (19a)
M.p. 215°C. Yield 72% (yellow powder). – IR (KBr): ν=3357 (NH), 3188 (NH), 1685 (CO), 1658 (CO), 1620 (C=N), 1588, 1464, 1195, 757 cm−1. – MS (EI, 70 eV): m/z (%)=866 (2) [M+2]+, 865 (2) [M+1]+, 848 (3), 815 (3), 599 (2), 532 (2), 360 (2), 232 (3) 173 (3), 162 (11), 161 (100), 149 (5), 133 (17), 118 (8), 116 (10), 105 (27), 104 (92), 90 (24), 80 (10), 77 (21). – C42H36N14O8 (864.82): calcd. C 58.33, H 4.20, N 22.67; found C 58.31, H 4.21, N 22.62.
3.12.2 2,2′,2″,2′″-(Ethane-1,2-diylbis(azanetriyl))tetrakis(N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)-acetohydrazide) (19b)
M.p. 208°C. Yield 40% (yellow powder). – IR (KBr): ν=3359 (NH), 1688 (CO), 1667 (CO), 1617 (C=N), 1588, 1444, 1163, 759 cm−1. – C66H80N18O8 (1253.46): calcd. C 63.24, H 6.43, N 20.11; found C 63.21, H 6.40, N 20.09.
3.13 Synthesis of the bis(Mannich bases) 21a, b
A solution of 20 (0.94 g, 4 mmol), isonicotinic hydrazide (0.27 g, 2 mmol) or phenoxyacetohydrazide (0.33 g, 2 mmol), and formalin (36%, 0.40 mL, 5 mmol) in ethanol (50 mL) was heated over a steam bath for 1 h. The product obtained on cooling was filtered and washed with boiling ethyl acetate (3×10 mL) to give 21a, b.
3.13.1 N′,N′-bis((2-oxo-3-(p-tolylimino)indolin-1-yl)methyl)isonicotinohydrazide (21a)
M.p. 287°C. Yield 57% (orange crystals). – IR (KBr): ν=3331 (NH), 1719 (CO), 1674 (CO), 1618 (C=N), 1596, 1274, 1150, 753 cm−1. – MS (EI, 70 eV): m/z (%)=632 (2) [M–1]+, 631 (1), 289 (5), 266 (22), 238 (19), 160 (68), 133 (98), 132 (79), 116 (36), 107 (85), 104 (52), 90 (7), 79 (58), 78 (100), 76 (18). – C38H31N7O3 (633.70): calcd. C 72.02, H 4.93, N 15.47; found C 71.98, H 4.90, N 15.41.
3.13.2 N′,N′-bis((2-oxo-3-(p-tolylimino)indolin-1-yl)methyl)-2-phenoxyacetohydrazide (21b)
M.p. 180°C. Yield 50% (brown crystals). – IR (KBr): ν=3426 (NH), 1721 (CO), 1667 (CO), 1610 (C=N), 1526, 1255, 1144, 755 cm−1. – MS (EI, 70 eV): m/z (%)=663 (4) [M+1]+, 662 (5) [M]+, 609 (4), 568 (5), 552 (6), 468 (6), 394 (6), 247 (4), 234 (18), 191 (25), 147 (33), 119 (100), 107 (12), 97 (11), 80 (10), 77 (3). – C40H34N6O4 (662.74): calcd. C 72.49, H 5.17, N 12.68; found 72.43, H 5.14, N 12.61.
3.14 Synthesis of the tetrakis(Mannich bases) 22a, b
These compounds were obtained from 20 (2.36 g, 10 mmol), formalin (36%, 0.85 mL, 10 mmol), and oxalohydrazide (0.29 g, 2.5 mmol) or malonohydrazide (0.33 g, 2.5 mmol) in the manner described for the synthesis of 21a, b. The product was filtered and washed with boiling ethyl acetate (3×15 mL) to give 22a, b.
3.14.1 N1′,N1′,N2′,N2′-tetrakis((2-oxo-3-(p-tolylimino)indolin-1-yl)methyl)oxalohydrazide (22a)
M.p.>300°C. Yield 40% (dark-red powder). – IR (KBr): ν=3396 (NH), 1699 (CO), 1665 (CO), 1615 (C=N), 1523, 1347, 1176, 745 cm−1. – C66H54N12O6 (1111.21): calcd. C 71.34, H 4.90, N 15.13; found C 71.30, H 4.89, N 15.11.
3.14.2 N1′,N1′,N2′,N2′-tetrakis((2-oxo-3-(p-tolylimino)indolin-1-yl)methyl)malonohydrazide (22b)
M.p.>300°C. Yield 44% (orange powder). – IR (KBr): ν=3377 (NH), 1694 (CO), 1660 (CO), 1611 (C=N), 1519, 1467, 1351, 1126, 757 cm−1. – C67H56N12O6 (1125.24): calcd. C 71.52, H 5.02, N 14.94; found C 71.50, H 5.05, N 14.91.
3.15 Synthesis of compounds 23a, b
3.15.1 Ethyl 2-(2-oxo-3-(p-tolylimino)indolin-1-yl)acetate (23a)
A mixture of 20 (1.42 g, 6 mmol), ethyl chloroacetate (0.80 g, 6.6 mmol), and K2CO3 (1.20 g) in DMF (20 mL) was heated in an oil bath at 120°C for 1 h. After cooling, the reaction mixture was poured in cold water. The product was filtered and crystallized from ethanol to give 23a. M.p. 190°C. Yield 40% (red crystals). – IR (KBr): ν=1742 (ester), 1657 (C=O), 1611 (C=N), 1500, 1462, 1202 cm−1. – MS (EI, 70 eV): m/z (%)=323 (1) [M+1]+, 322 (5) [M]+, 237 (12), 236 (65), 221 (18), 209 (16), 208 (100), 192 (5), 180 (10), 145 (5), 131 (2), 118 (13), 91 (58), 76 (8). – C19 H18N2O3 (322.36): calcd. C 70.79, H 5.63, N 8.69; found 70.74, H 5.60, N 8.61.
3.15.2 2-[2-Oxo-3-(p-tolylimino)indolin-1-yl]acetohydrazide (23b)
A mixture of 23a (0.8 g, 2.5 mmol) and hydrazine hydrate (80%, 0.20 mL, 3 mmol) in ethanol (30 mL) was refluxed for 90 min. The product obtained on cooling was filtered and crystallized from ethanol to give 23b. M.p. 236°C. Yield 60% (pale-brown crystals). – IR (KBr): ν=3557, 3158 (NH–NH2), 1682 (CO), 1594, 152, 1200, 746 cm−1. – MS (EI, 70 eV): m/z (%)=248 (6) [M–(CONHNH2+1)]+, 247 (8), 234[M–(CH2CONHNH2+1)]+ (27), 219 (6), 202 (16), 174 (59), 161 (57), 146 (61), 133 (33), 118 (100), 104 (86), 91 (69), 78 (24), 77 (45), 76 (30), 59 (16). – C17H16N4O2 (308.33): calcd. C 66.22, H 5.23, N 18.17; found C 66.20, H 5.21, N 18.13.
3.16 2-[2-Oxo-3-(p-tolylimino)indolin-1-yl]-N′-(2-oxoindolin-3-ylidene)acetohydrazide (24)
A solution of 1 (0.44 g, 3 mmol) and 23b (0.92 g, 3 mmol) in aqueous ethanol (50%, 40 mL) and acetic acid (0.2 mL) was refluxed for 40 min. After standing at room temperature for 24 h, the product obtained was filtered and washed with boiling ethanol to give 24. M.p. 268°C. Yield 45% (orange crystals). – IR (KBr): ν=3438 (NH), 1692 (CO), 1615, 1552, 1514, 1169, 751 cm−1. – MS (EI, 70 eV): m/z (%)=438 (50) [M+1]+, 437 (65) [M]+, 424 (58), 418 (50), 402 (62), 366 (57), 332 (68), 309 (50), 250 (54), 235 (47), 205 (68), 160 (2), 152 (100), 145 (45), 131 (57), 97 (29), 92 (62), 76 (33). – C25H19N5O3 (437.45): calcd. C 68.64, H 4.38, N 16.01; found C 68.60, H 4.34, N 15.92.
3.17 N′-(2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)-2-(2-oxo-3-(p-tolylimino)indolin-1-yl)acetohydrazide (25)
This compound was obtained from 23b (0.92 g, 3 mmol) and the Mannich base 2 (0.73 g, 3 mmol), following the procedure described for the synthesis of 24. The product was filtered and washed with boiling ethanol to give 25. M.p.>300°C. Yield 40% (yellow powder). – IR (KBr): ν=3410 (NH), 1690 (CO), 1609 (C=N), 1467, 1163, 745 cm−1. – C31H30N6O3 (534.61): calcd. C 69.65, H 5.66, N 15.72; found C 69.61, H 5.63, N 15.70.
3.18 N′-(4-methylbenzylidene)-4-(2-oxoindolin-3-ylideneamino)benzohydrazide (27)
A mixture of 26 [36] (1.12 g, 4 mmol) and p-tolualdehyde (0.48 g, 4 mmol) in ethanol (30 mL) and acetic acid (0.3 mL) was heated under reflux for 3 h. After standing at room temperature for 24 h, the product obtained was filtered and crystallized from ethanol to give 27. M.p. 212°C. Yield 65% (yellow powder). – IR (KBr): ν=3424 (NH), 1690 (CO), 1649 (CO), 1619 (C=N), 1544, 1133 cm−1. – 1H NMR ([D6]DMSO): δ=2.38 (s, 3H, Ar-CH3), 6.88–7.75 (m, 12H, aromatic), 8.43 (s, 1H, Ar-CH=N), 9.42 (s, 1H, CO–NH), 10.81 ppm (s, 1H, NH of oxoindoline). – C23H18N4O2 (382.40): calcd. C 72.24, H 4.74, N 14.65; found C 72.20, H 4.71, N 14.61.
3.19 1-[4-(2-Oxoindolin-3-ylideneamino)benzoyl]-5-phenyl-3-p-tolylformazan (28)
Benzenediazonium chloride (10 mmol, prepared by diazotization of aniline) (10 mmol) was added dropwise with stirring to a cold solution at 0–5°C, of 27 (2.67 g, 7 mmol) during 5 min. Then saturated K2CO3 solution was added until pH of 8 was reached and the solution was stirred for 30 min at 0–5°C, a dark brownish-red suspension resulted. The product was filtered, washed with water (3×20 mL) and air dried. The crude product was crystallized from DMF–ethanol (1:1) to give 28. M.p. 248°C. Yield 55% (brownish-red powder). – IR (KBr): ν=3422 (NH), 1708 (CO), 1666 (CO), 1617, 1546, 1204, 756 cm−1. – 1H NMR ([D6]DMSO): δ=2.35 (s, 3H, Ar-CH3), 6.91–8.05 (m, 17H, aromatic), 8.58 (s, 1H, NH of formazan), 10.85 ppm (s, 1H, NH of oxoindoline). – 13C NMR ([D6]DMSO): δ=21.24 (CH3), 116.46, 120.72, 122.47, 126.43, 128.35, 128.75, 128.85, 129.42, 129.75, 130.76, 131.16, 133.53, 141.22, 144.94, 146.45 (all aromatic C), 151.48 (N=C), 160.66 (C=O), 164.54 ppm (C=O). – MS (EI, 70 eV): m/z (%)=487 (51) [M+1]+, 486 (63) [M]+, 486 (58), 406 (48), 402 (62), 338 (60), 324 (69), 305 (67), 294 (60), 243 (60), 225 (50), 208 (9), 189 (48), 182 (46), 166 (100), 161 (65), 144 (70), 130 (60), 104 (6), 91 (28), 74 (50). – C29H22N6O2 (486.52): calcd. C 71.59, H 4.56, N 17.27; found C 71.54, H 4.53, N 17.22.
References
[1] S. Rollas, S. G. Küçükgüzel, Molecules2007, 12, 1910.10.3390/12081910Suche in Google Scholar
[2] R. Narang, B. Narasimhan, S. Sharma, Curr. Med. Chem.2012, 19, 569.10.2174/092986712798918789Suche in Google Scholar
[3] G. Verma, A. Marella, M. Shaquiquzzaman, M. Akhtar, M. R. Ali, M. M. Alam, J. Pharm. Bioallied Sci.2014, 6, 69.10.4103/0975-7406.129170Suche in Google Scholar
[4] Y. Janin, Bioorg. Med. Chem. 2007, 15, 2479.10.1016/j.bmc.2007.01.030Suche in Google Scholar
[5] K. Bedia, O. Elcin, U. Seda, K. Fatma, S. Nathaly, A. Dimoglo, Eur. J. Med. Chem.2006, 41, 1253.10.1016/j.ejmech.2006.06.009Suche in Google Scholar
[6] T. Aboul-Fadl, F. A. Bin-Jubair, O. Aboul-Wafa, Eur. J. Med. Chem.2010, 45, 4578.10.1016/j.ejmech.2010.07.020Suche in Google Scholar
[7] A. Jamadar, A. Duhme-Klair, K. Vemuri, M. Sritharan, P. Dandawatec, S. Padhye, Dalton Trans. 2012, 41, 9192.10.1039/c2dt30322aSuche in Google Scholar
[8] D. Kaushik, S. A. Khan, G. Chawla, S. Kumar, Eur. J. Med. Chem.2010, 45, 3943.10.1016/j.ejmech.2010.05.049Suche in Google Scholar
[9] S. K. Sridhar, S. N. Pandeya, J. P. Stables, A. Ramesh, Eur. J. Pharm. Sci.2002, 16, 129.10.1016/S0928-0987(02)00077-5Suche in Google Scholar
[10] G. Gurkok, N. Altanlar, S. Suzen, Chemotherapy2009, 55, 15.10.1159/000166999Suche in Google Scholar PubMed
[11] S. Rollas, N. Gulerman, H. Erdeniz, II Farmaco2002, 57, 171.10.1016/S0014-827X(01)01192-2Suche in Google Scholar
[12] S. N. Pandeya, S. Smitha, M. Jyoti, S. K. Sridhar, Acta Pharm. 2005, 55, 27.Suche in Google Scholar
[13] S. K. Sridhar, M. Saravanan, A. Ramesh, Eur. J. Med. Chem.2001, 36, 615.10.1016/S0223-5234(01)01255-7Suche in Google Scholar
[14] S. N. Pandeya, A. S. Raja, J. P. Stables, J. Pharm. Pharmaceut. Sci.2002, 5, 266.Suche in Google Scholar
[15] T. Aboul-Fadl, F. A. Mohammed, E. A. Hassan, Arch. Pharm. Res.2003, 26, 778.10.1007/BF02980020Suche in Google Scholar
[16] N. Karali, A. Gursoy, F. Kandemirli, N. Shvets, F. Betul Kaynak, S. Ozbey, V. Kovalishyn, A. Dimoglo, Bioorg. Med. Chem. 2007, 15, 5888.10.1016/j.bmc.2007.05.063Suche in Google Scholar
[17] O. Guzel, N. Karali, A. Salman, Bioorg. Med. Chem. 2008, 16, 8976.10.1016/j.bmc.2008.08.050Suche in Google Scholar
[18] H. Adibi, M. M. Khodaei, P. Pakravan, R. Abiri, Pharm. Chem. J.2010, 44, 219.10.1007/s11094-010-0436-3Suche in Google Scholar
[19] E. M. Afsah, S. S. Elmorsy, S. M. Abdelmageed, Z. E. Zaki, Z. Naturforsch.2015, 70b, 393.10.1515/znb-2014-0262Suche in Google Scholar
[20] W. S. Hamama, H. H. Zoorob, M. A. Gouda, E. M. Afsah, Pharm. Chem. J.2011, 45, 118.10.1007/s11094-011-0573-3Suche in Google Scholar
[21] T. Curtius, O. Hofmann, J. Prakt. Chem. 1917, 96, 218.10.1002/prac.19180960121Suche in Google Scholar
[22] F. Bermejo-Martinez, J. M. Graña-Molares, J. A. Rodriguez-Vazquez, Microchem. J.1976, 21, 261.10.1016/0026-265X(76)90005-9Suche in Google Scholar
[23] J. F. M. Da Silva, S. J. Garden, A. C. Pinto, J. Braz. Chem. Soc. 2001, 12, 273.10.1590/S0103-50532001000300002Suche in Google Scholar
[24] K. L. Vine, L. Matesic, J. M. Loke, M. Ranson, D. Skropeta, Anticancer Agents Med. Chem. 2009, 9, 397.10.2174/1871520610909040397Suche in Google Scholar
[25] T. Aboul-Fadl, F. A. Bin-Jubair, Int. J. Res. Pharm. Sci. 2010, 1, 113.Suche in Google Scholar
[26] B. Bhrigu, D. Pathak, N. Siddiqui, M. S. Alam, W. Ahsan, Int. J. Pharm. Sci. Drug Res.2010, 2, 229.Suche in Google Scholar
[27] P. Pakravan, S. Kashanian, M. M. Khodaei, F. Harding, J. Pharmcol. Rep.2013, 65, 313.10.1016/S1734-1140(13)71007-7Suche in Google Scholar
[28] R. Price in Comprehensive Coordination Chemistry, Vol. 6 (Ed.: G. Wilkinson), Pergamon Press, New York, 1987, p. 35.Suche in Google Scholar
[29] M. Szymczyk, A. El-Shafei, H. S. Freeman, Dyes Pigments2006, 71, 206.Suche in Google Scholar
[30] H.Tezcan, E. Uzluk, M. Levent Aksu, Spectrochemica Acta, Part A2008, 70, 973.10.1016/j.saa.2007.10.010Suche in Google Scholar PubMed
[31] A. S. Shawali, N. A. Samy, J. Adv. Res. 2015, 6, 241.10.1016/j.jare.2014.07.001Suche in Google Scholar PubMed PubMed Central
[32] S. S. Rajput, Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 376.Suche in Google Scholar
[33] G. Mariappan, R. Korim, N. M. Joshi, F. Alam, R. Hazarika, D. Kumar, T. Uriah, J. Adv. Pharm. Technol. Res. 2010, 1, 396.10.4103/0110-5558.76438Suche in Google Scholar PubMed PubMed Central
[34] H. Hellmann, I. Löschmann, Chem. Ber. 1954, 87, 1684.10.1002/cber.19540871112Suche in Google Scholar
[35] A. Schmidt, G. Wichmann, Chem.Ber. 1891, 24, 3240.10.1002/cber.189102402175Suche in Google Scholar
[36] T. I. EL-Emary, Polish J. Chem. 1996, 70, 1143.Suche in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry

