Abstract
The rare earth fluoride borates RE4B4O11F2 (RE = Sm, Tb, Ho, Er) were synthesized in a Walker-type multianvil apparatus from the corresponding rare earth oxides and fluorides with boron oxide. Sm4B4O11F2 was obtained under high-pressure/high-temperature conditions of 6 GPa/1100°C, Tb4B4O11F2 at 7.5 GPa/1200°C, and Ho4B4O11F2 and Er4B4O11F2 at 9.5 GPa/1300°C. The single-crystal structure determinations showed that all compounds are isotypic to the known rare earth fluoride borates RE4B4O11F2 (RE = Pr, Nd, Eu, Gd, Dy). They crystallize in the monoclinic space group C2/c (Z = 4). The structure is built up from BO4 tetrahedra as well as BO3 groups connected via common corners. Here, we report about the crystallographic characterization of these new compounds in comparison to the isotypic phases RE4B4O11F2 (RE = Pr, Nd, Eu, Gd, Dy).
1 Introduction
Borates are well known for their extraordinary optical properties with a very high transparency into the deep UV. Therefore, they may be used as host materials for luminescent and nonlinear-optical applications. Some fluoride borates exhibit an even larger optical gap due to the incorporation of fluorine atoms [1], [2]. Rare earth fluoride borates also show very interesting luminescence, fluorescence, and dielectric properties [3], [4], [5], [6]. These compounds can be prepared with remarkable complexity. Borates in general form some of the most diverse structures because both planar BO3 as well as tetrahedral BO4 groups can be present and interconnected via common corners and/or edges. Recently, the structural motive of linear BO2 groups was confirmed by Höppe in the gadolinium borate fluoride oxide Gd4(BO2)O5F [7]. In combination with the wide range of possible coordination numbers of lanthanides, these compounds are very interesting for ongoing investigations.
For the synthesis of new rare earth fluoride borates, we apply high-pressure/high-temperature conditions. This has led to the finding of more than 20 new rare earth fluoride borates crystallizing in various structure types. A summary of the achievements reached so far can be found in Refs. [8], [9].
For the chemical composition RE4B4O11F2, two different structure types were obtained by high-pressure/high-temperature syntheses. In 2010, Haberer et al. presented the compound La4B4O11F2 [10] crystallizing in space group P21/c with the lattice parameters a = 778.1(2), b = 3573.3(7), c = 765.7(2) pm, and β = 113.92(3)° (Z = 8). The crystal structure consists of BO3 groups (Δ) which are either isolated (Δ), connected via common corners (ΔΔ), or connected via BO4 tetrahedra forming the fundamental building block (FBB) 2Δ□:Δ□Δ (after Burns et al. [11]).
Earlier in 2010, Haberer et al. discovered the fluoride borate Gd4B4O11F2 [12] showing the same atomic composition RE4B4O11F2 but a completely different crystal structure in space group C2/c. The crystal structure of Gd4B4O11F2 contains BO3 groups and BO4 tetrahedra connected via common corners. The structural motif consists of two BO3 groups (Δ) and two BO4 tetrahedra (□), and can be described with the fundamental building block 2Δ2□:Δ□□Δ, which represented a novelty in borate chemistry. Later in 2010, Haberer et al. were able to synthesize two compounds crystallizing isotypically to Gd4B4O11F2, namely Eu4B4O11F2 and Dy4B4O11F2 [13]. With the syntheses of Pr4B4O11F2 and Nd4B4O11F2 [14], we succeeded in the synthesis of two more isotypic compounds in 2013. To discover possibly all compounds RE4B4O11F2 that are isotypic to Gd4B4O11F2, we continued our investigations in synthesizing all other rare earth fluoride borates with this composition not described in literature so far. Finally, we succeeded in the synthesis of RE4B4O11F2 with RE = Sm, Tb, Ho, Er.
In the following, we report about the four newly synthesized compounds RE4B4O11F2 (RE = Sm, Tb, Ho, Er), which are all isotypic to RE4B4O11F2 (RE = Pr, Nd, Eu, Gd, Dy) [12], [13]. The syntheses and structural properties of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) are discussed in comparison to the isotypic compounds.
2 Experimental part
2.1 Syntheses
All new compounds RE4B4O11F2 (RE = Sm, Tb, Ho, Er) were synthesized under high-pressure/high-temperature conditions in a Walker-type multianvil apparatus. Reactions of the oxides Sm2O3, Tb4O7, Ho2O3, and Er2O3 with the corresponding rare earth fluorides REF3 (RE = Sm, Tb, Ho, Er) and B2O3 led to the formation of the products according to the following Eqs. 1–4.
Stoichiometric mixtures of Sm2O3 (Strem Chemicals, Newburyport, MA, USA, 99.9%), Tb4O7 (Smart Elements, Vienna, Austria, 99.99%), Ho2O3 (Strem Chemicals, Newburyport, MA, USA, 99.9%), or Er2O3 (Strem Chemicals, Newburyport, MA, USA, 99.9%) with the corresponding rare earth fluoride SmF3, TbF3, HoF3, or ErF3 (each from Strem Chemicals, Newburyport, MA, USA, 99.9%) and B2O3 (Strem Chemicals, Newburyport, MA, USA, 99.9+%) were finely ground inside a glove box under argon inert gas atmosphere and filled into boron nitride crucibles (Henze Boron Nitride Products GmbH, HeBoSint® P100, Kempten, Germany). These crucibles were placed into the center of 14/8 or 18/11 assemblies and compressed by eight tungsten carbide cubes (Hawedia, ha-7%Co, Marklkofen, Germany). Pressure was applied via a 1000 ton press with a modified multianvil Walker module (both devices from the company Voggenreiter, Mainleus, Germany). A detailed description of the construction of the assembly is given in Refs. [15], [16], [17], [18], [19].
For the synthesis of Sm4B4O11F2, the educt mixture inside the 18/11 assembly was compressed up to 6 GPa in 150 min, keeping it at this pressure for the following heating period. The temperature was then increased to 1100°C in 10 min, kept there for 15 min, and decreased to 500°C in 35 min and finally to room temperature by switching off the heating. This program was followed by a decompression period of 7.5 h. Tb4B4O11F2 was synthesized by compressing the 14/8 assembly up to 7.5 GPa within 160 min, heating it up to 1200°C in the following 10 min, holding the temperature for another 15 min and cooling it down to 500°C within 20 min. After natural cooling down to room temperature by switching off the heating, the sample was decompressed in about 8 h. For the syntheses of Ho4B4O11F2 and Er4B4O11F2, the educt mixtures in the 14/8 assemblies were compressed to 9.5 GPa in 210 min. Afterwards, the samples were heated up to 1300°C within 10 min, kept there for 8 min and cooled down to 650°C in 25 min at constant pressure. The temperature was decreased to room temperature by switching off the heating, and the samples were decompressed within 11 h.
The recovered MgO octahedra (Ceramic Substrates & Components Ltd., Newport, Isle of Wight, UK) were broken apart and the samples carefully separated from the surrounding boron nitride crucibles. While Sm4B4O11F2 and Tb4B4O11F2 were obtained as colorless, air- and water-resistant crystals, and Er4B4O11F2 as pink crystals, Ho4B4O11F2 showed a very intense alexandrite effect (daylight: yellow, incandescent light: pink).
2.2 Crystal structure analyses
The isotypic compounds RE4B4O11F2 (RE = Sm, Tb, Ho, Er) were characterized by powder X-ray diffraction. The powder diffraction patterns were obtained in transmission geometry from a flat sample of the reaction products, using a Stoe Stadi P powder diffractometer with MoKα1 radiation [Ge(111)-monochromatized, λ = 70.93 pm]. The powder diffraction patterns showed the reflections of the new rare earth fluoride borates as the main products in all cases. While the powder patterns of the samples of Tb4B4O11F2 and Ho4B4O11F2 contained only very weak reflections caused by a still unknown phase, the side products of the syntheses of Sm4B4O11F2 and Er4B4O11F2 could be identified by reflection patterns of α-Sm2B4O9 [20] and Er3B5O12 [21], respectively. Figure 1 shows the powder pattern of Ho4B4O11F2 with some weak reflections of the unknown side product (marked with asterisks). The experimental powder pattern (top) is in good agreement with the theoretical pattern (bottom), simulated from the single-crystal data. By indexing the reflections of the samarium fluoride borate, the parameters a = 1373.83(7), b = 466.53(3), c = 1380.82(8) pm, β = 91.09(1)°, and a volume of 0.88486(6) nm3 were obtained. The indexing of the corresponding terbium fluoride borate powder diffraction pattern led to the parameters a = 1355.35(7), b = 461.99(3), c = 1367.17(9) pm, β = 91.20(1)°, and a volume of 0.85588(6) nm3. For Ho4B4O11F2, the indexing of the powder diffraction pattern resulted in the parameters a = 1342.93(6), b = 459.84(3), c = 1359.08(7) pm, β = 91.38(1)°, and a volume of 0.83903(5) nm3, and for Er4B4O11F2, the parameters a = 1337.4(9), b = 458.9(3), c = 1352.9(8) pm, β = 91.4(1)°, and a volume of 0.8301(7) nm3 were obtained. These data validated the lattice parameters obtained from the single-crystal X-ray diffraction data for RE4B4O11F2 (RE = Sm, Tb, Ho, Er) (Table 1).
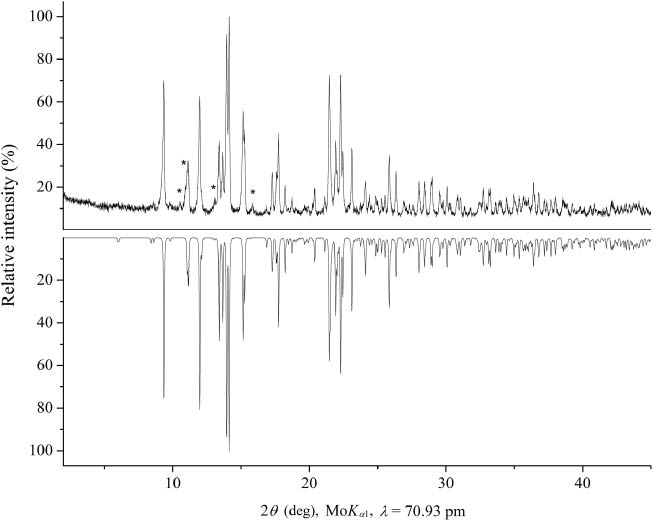
Top: experimental powder X-ray diffraction pattern of Ho4B4O11F2; the reflections of an unknown side product are indicated with asterisks. Bottom: theoretical powder pattern of Ho4B4O11F2, based on single-crystal diffraction data.
Crystal data and numbers pertinent to data collection and structure refinement of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) (standard deviations in parentheses).
| Empirical formula | Sm4B4O11F2 | Tb4B4O11F2 | Ho4B4O11F2 | Er4B4O11F2 |
| Mr | 858.64 | 892.92 | 916.96 | 926.28 |
| Crystal system | Monoclinic | |||
| Space group | C2/c | |||
| Powder diffractometer | Stoe Stadi P | |||
| Radiation; λ, pm | MoKα1; 70.93 (Ge(111) monochromator) | |||
| Powder data | ||||
| a, pm | 1373.83(7) | 1355.35(7) | 1342.93(6) | 1337.4(9) |
| b, pm | 466.53(3) | 461.99(3) | 459.84(3) | 458.9(3) |
| c, pm | 1380.82(8) | 1367.17(9) | 1359.08(7) | 1352.9(8) |
| β, deg | 91.09(1) | 91.20(1) | 91.38(1) | 91.4(1) |
| V, nm3 | 0.88486(6) | 0.85588(6) | 0.83903(5) | 0.8301(7) |
| Single-crystal diffractometer | Nonius Kappa CCD | |||
| Radiation; λ, pm | MoKα; 71.073 (graphite monochromator) | |||
| Single-crystal data | ||||
| a, pm | 1375.06(1) | 1356.0(3) | 1343.3(3) | 1337.0(3) |
| b, pm | 466.95(2) | 462.20(9) | 459.99(9) | 458.82(9) |
| c, pm | 1381.66(4) | 1368.1(3) | 1359.2(3) | 1355.4(3) |
| β, deg | 91.1(1) | 91.2(1) | 91.4(1) | 91.4(1) |
| V, nm3 | 0.88698(5) | 0.8573(3) | 0.8396(3) | 0.8312(3) |
| Formula units per cell | Z = 4 | |||
| Calculated density, g cm−3 | 6.43 | 6.92 | 7.25 | 7.40 |
| Crystal size, mm3 | 0.04 × 0.03 × 0.01 | 0.03 × 0.03 × 0.02 | 0.03 × 0.02 × 0.01 | 0.03 × 0.02 × 0.01 |
| Temperature, K | 293(2) | 293(2) | 293(2) | 293(2) |
| Absorption coefficient, mm−1 | 26.2 | 32.7 | 37.4 | 40.1 |
| F(000), e | 1496 | 1544 | 1576 | 1592 |
| θ range, deg | 1.00 37.79 | 1.00 37.79 | 1.00 37.79 | 1.00 37.79 |
| Range in hkl | ±20, ±7, ±20 | –20:19, ±6, ±20 | ±22, ±7, –21:23 | ±22, ±7, ±23 |
| Total no. of reflections | 17093 | 13407 | 15732 | 13199 |
| Independent reflections/Rint | 5884/0.0690 | 4893/0.0570 | 7576/0.0613 | 8003/0.0643 |
| Reflections with I > 2σ(I)/Rσ | 1476/0.0448 | 1266/0.0445 | 1853/0.0452 | 1723/0.0461 |
| Data/ref. parameters | 1594/97 | 1546/97 | 2235/97 | 2220/97 |
| Absorption correction | Multi-scan (Scalepack [22]) | |||
| Goodness-of-fit on F2 | 1.098 | 1.077 | 1.067 | 1.037 |
| Final indices R1/wR2 [I > 2 σ(I)] | 0.0240/0.0638 | 0.0288/0.0657 | 0.0304/0.0765 | 0.0299/0.0693 |
| Indices R1/wR2 (all data) | 0.0259/0.0650 | 0.0392/0.0696 | 0.0381/0.0808 | 0.0425/0.0767 |
| Largest diff. peak/hole, × 10−6e pm−3 | 2.94/–2.24 | 3.07/–2.25 | 2.70/–3.53 | 3.60/–3.98 |
For the single-crystal structure analyses, small single crystals of all four new compounds were isolated by mechanical fragmentation. The intensity data of the single-crystals were collected at room temperature with a Kappa CCD diffractometer (Bruker AXS/Nonius, Karlsruhe, Germany) equipped with a Miracol fiber optics collimator and a Nonius FR590 generator (graphite-monochromatized MoKα radiation, λ = 71.073 pm). Semiempirical absorption corrections based on equivalent and redundant intensities were applied with the program Scalepack [22]. According to the systematic extinctions, the monoclinic spacegroup C2/c was derived for the four isotypic compounds. Because of the fact that all four new compounds crystallize isotypic to Gd4B4O11F2, the positional parameters of Gd4B4O11F2 were used as starting values for the structural refinement [12]. The parameter refinements (full-matrix least-squares on F2) were achieved by using the Shelxs/l-97 software suite [23], [24]. All atoms could be refined with anisotropic displacement parameters. Final difference Fourier syntheses did not reveal any significant peaks in the refinements. All relevant details of the data collections and evaluations are given in Table 1. The positional parameters, interatomic distances, and interatomic angles are listed in the Tables 2–4.
Atomic coordinates and isotropic equivalent displacement parameters (Ueq in Å2) for RE4B4O11F2(RE = Sm, Tb, Ho, Er) (space group: C2/c) standard deviations in parentheses. Ueq is defined as one third of the trace of the orthogonalized Uij tensor.
| Atom | Wyckoff position | x | y | z | Ueq |
|---|---|---|---|---|---|
| Sm1 | 8f | 0.058848(16) | 0.52088(4) | 0.370591(13) | 0.00573(9) |
| Sm2 | 8f | 0.279341(15) | 0.01801(4) | 0.370945(14) | 0.00559(9) |
| B1 | 8f | 0.9071(3) | 0.9789(8) | 0.2856(3) | 0.0056(7) |
| B2 | 8f | 0.0948(3) | 0.9543(9) | 0.5254(3) | 0.0070(7) |
| F1 | 8f | 0.2306(2) | 0.5243(5) | 0.4252(2) | 0.0126(5) |
| O1 | 8f | 0.91191(18) | 0.8687(6) | 0.39347(17) | 0.0070(4) |
| O2 | 8f | 0.17286(19) | 0.8147(5) | 0.25790(17) | 0.0075(4) |
| O3 | 8f | 0.07902(18) | 0.6653(6) | 0.53520(17) | 0.0075(4) |
| O4 | 4e | 0 | 0.8487(8) | ¼ | 0.0061(6) |
| O5 | 8f | 0.1140(2) | 0.0592(6) | 0.43552(19) | 0.0091(5) |
| O6 | 8f | 0.90074(19) | 0.2799(6) | 0.27480(17) | 0.0082(5) |
| Tb1 | 8f | 0.05869(2) | 0.51813(6) | 0.370427(19) | 0.00679(10) |
| Tb2 | 8f | 0.279965(19) | 0.01583(6) | 0.370537(19) | 0.00617(10) |
| B1 | 8f | 0.9073(5) | 0.9764(13) | 0.2862(5) | 0.0082(11) |
| B2 | 8f | 0.0954(5) | 0.9561(14) | 0.5238(5) | 0.0083(11) |
| F1 | 8f | 0.2311(3) | 0.5271(7) | 0.4241(3) | 0.0144(7) |
| O1 | 8f | 0.9132(3) | 0.8646(8) | 0.3947(3) | 0.0061(7) |
| O2 | 8f | 0.1749(3) | 0.8139(8) | 0.2569(3) | 0.0071(7) |
| O3 | 8f | 0.0786(3) | 0.6669(8) | 0.5339(3) | 0.0072(7) |
| O4 | 4e | 0 | 0.8436(11) | ¼ | 0.0059(10) |
| O5 | 8f | 0.1154(3) | 0.0645(9) | 0.4331(3) | 0.0103(8) |
| O6 | 8f | 0.9014(3) | 0.2800(8) | 0.2760(3) | 0.0069(7) |
| Ho1 | 8f | 0.05840(2) | 0.51584(4) | 0.37029(2) | 0.00782(7) |
| Ho2 | 8f | 0.28056(2) | 0.01404(4) | 0.37002(2) | 0.00723(7) |
| B1 | 8f | 0.9066(4) | 0.9744(9) | 0.2867(4) | 0.0092(8) |
| B2 | 8f | 0.0964(4) | 0.9602(10) | 0.5227(4) | 0.0087(7) |
| F1 | 8f | 0.2316(2) | 0.5281(6) | 0.4237(3) | 0.0155(6) |
| O1 | 8f | 0.9126(2) | 0.8591(6) | 0.3954(2) | 0.0071(5) |
| O2 | 8f | 0.1768(2) | 0.8137(7) | 0.2569(2) | 0.0097(5) |
| O3 | 8f | 0.0791(2) | 0.6670(6) | 0.5330(2) | 0.0094(5) |
| O4 | 4e | 0 | 0.8381(9) | ¼ | 0.0064(6) |
| O5 | 8f | 0.1163(2) | 0.0659(7) | 0.4312(2) | 0.0107(5) |
| O6 | 8f | 0.9026(2) | 0.2811(6) | 0.2768(2) | 0.0094(5) |
| Er1 | 8f | 0.05858(2) | 0.51476(4) | 0.37005(2) | 0.00688(7) |
| Er2 | 8f | 0.28092(2) | 0.01168(4) | 0.36986(2) | 0.00611(7) |
| B1 | 8f | 0.9066(4) | 0.972(1) | 0.2864(4) | 0.0077(8) |
| B2 | 8f | 0.0966(4) | 0.962(2) | 0.5212(5) | 0.0101(9) |
| F1 | 8f | 0.2314(3) | 0.5297(6) | 0.4244(3) | 0.0144(6) |
| O1 | 8f | 0.9124(2) | 0.8566(7) | 0.3960(2) | 0.0065(5) |
| O2 | 8f | 0.1773(2) | 0.8114(7) | 0.2565(3) | 0.0081(6) |
| O3 | 8f | 0.0779(3) | 0.6661(7) | 0.5319(3) | 0.0085(6) |
| O4 | 4e | 0 | 0.8355(9) | ¼ | 0.0071(8) |
| O5 | 8f | 0.1165(3) | 0.0677(7) | 0.4299(3) | 0.0086(6) |
| O6 | 8f | 0.9027(3) | 0.2801(7) | 0.2773(3) | 0.0082(6) |
Interatomic distances (pm) in RE4B4O11F2 (RE = Sm, Tb, Ho, Er) (space group: C2/c), calculated with the single-crystal lattice parameters (standard deviations in parentheses).
| Sm1–O6a | 237.8(2) | Sm2–O2a | 232.3(3) | B1–O6 | 141.6(4) |
| Sm1–O3a | 238.3(2) | Sm2–O2b | 235.9(2) | B1–O2 | 146.0(5) |
| Sm1–O4 | 239.2(2) | Sm2–O6 | 242.3(2) | B1–O4 | 150.6(5) |
| Sm1–O5a | 245.0(3) | Sm2–O1 | 246.6(3) | B1–O1 | 157.6(5) |
| Sm1–F1 | 246.5(3) | Sm2–O5 | 246.5(3) | ø = 149.0 | |
| Sm1–O3b | 247.8(2) | Sm2–O3 | 247.2(2) | ||
| Sm1–O1 | 261.6(3) | Sm2–F1a | 251.9(2) | B2–O5 | 136.5(5) |
| Sm1–O2 | 261.9(3) | Sm2–F1b | 257.3(2) | B2–O3 | 137.4(5) |
| Sm1–O6b | 276.3(3) | Sm2–F1c | 282.9(3) | B2–O1 | 139.8(5) |
| Sm1–O5b | 277.0(3) | ø = 249.2 | ø = 137.9 | ||
| ø = 253.1 | |||||
| Tb1–O3a | 234.9(4) | Tb2–O2a | 228.5(4) | B1–O6 | 141.2(7) |
| Tb1–O4 | 235.7(3) | Tb2–O2b | 231.5(4) | B1–O2 | 145.8(8) |
| Tb1–O6a | 235.9(4) | Tb2–O6 | 238.0(4) | B1–O4 | 149.3(7) |
| Tb1–O5a | 238.6(4) | Tb2–O5 | 241.7(4) | B1–O1 | 157.2(8) |
| Tb1–F1 | 243.6(4) | Tb2–O1 | 243.9(4) | ø = 148.4 | |
| Tb1–O3b | 245.3(4) | Tb2–O3 | 244.8(4) | ||
| Tb1–O1 | 256.8(4) | Tb2–F1a | 247.0(3) | B2–O3 | 136.3(7) |
| Tb1–O2 | 262.1(4) | Tb2–F1b | 256.5(3) | B2–O5 | 137.1(8) |
| Tb1–O6b | 270.5(4) | Tb2–F1c | 282.4(4) | B2–O1 | 139.6(7) |
| Tb1–O5b | 277.0(4) | ø = 246.0 | ø = 137.7 | ||
| ø = 250.0 | |||||
| Ho1–O3a | 232.8(3) | Ho2–O2a | 224.8(3) | B1–O6 | 141.8(5) |
| Ho1–O4 | 232.9(3) | Ho2–O2b | 229.2(3) | B1–O2 | 145.6(6) |
| Ho1–O6a | 234.3(3) | Ho2–O6 | 235.3(3) | B1–O4 | 149.9(5) |
| Ho1–O5a | 235.4(3) | Ho2–O5 | 238.9(3) | B1–O1 | 157.1(6) |
| Ho1–F1 | 242.1(3) | Ho2–O1 | 239.9(3) | ø = 148.6 | |
| Ho1–O3b | 244.2(3) | Ho2–O3 | 242.2(3) | ||
| Ho1–O1 | 254.6(3) | Ho2–F1a | 244.6(3) | B2–O5 | 136.8(6) |
| Ho1–O2 | 262.6(3) | Ho2–F1b | 256.5(3) | B2–O3 | 137.6(5) |
| Ho1–O6b | 265.2(3) | Ho2–F1c | 281.9(4) | B2–O1 | 139.6(6) |
| Ho1–O5b | 276.8(3) | ø = 243.7 | ø = 138.0 | ||
| ø = 248.1 | |||||
| Er1–O3a | 231.0(3) | Er2–O2a | 224.0(3) | B1–O6 | 142.0(5) |
| Er1–O4 | 231.6(3) | Er2–O2b | 227.7(3) | B1–O2 | 145.2(7) |
| Er1–O5a | 233.1(3) | Er2–O6 | 233.6(3) | B1–O4 | 149.2(6) |
| Er1–O6a | 233.8(4) | Er2–O5 | 237.7(4) | B1–O1 | 157.7(7) |
| Er1–F1 | 240.9(4) | Er2–O1 | 238.6(3) | ø = 148.5 | |
| Er1–O3b | 242.9(3) | Er2–O3 | 242.4(4) | ||
| Er1–O1 | 253.7(3) | Er2–F1a | 242.9(3) | B2–O5 | 136.2(7) |
| Er1–O2 | 262.0(3) | Er2–F1b | 258.0(3) | B2–O3 | 138.9(6) |
| Er1–O6b | 263.7(4) | Er2–F1c | 280.4(4) | B2–O1 | 140.5(7) |
| Er1–O5b | 276.8(4) | ø = 242.8 | ø = 138.5 | ||
| ø = 247.0 |
Interatomic angles (deg) in RE4B4O11F2 (RE = Sm, Tb, Ho, Er) (space group: C2/c), calculated with the single-crystal lattice parameters (standard deviations in parentheses).
| O6–B1–O2 | 115.7(3) | O5–B2–O3 | 118.4(3) | Sm1–F1–Sm2a | 100.0(1) |
| O6–B1–O4 | 114.7(3) | O5–B2–O1 | 122.3(3) | Sm1–F1–Sm2b | 99.1(1) |
| O2–B1–O4 | 106.9(3) | O3–B2–O1 | 119.2(3) | Sm1–F1–Sm2c | 103.9(1) |
| O6–B1–O1 | 115.2(3) | ø = 120.0 | Sm2a–F1–Sm2b | 133.0(2) | |
| O2–B1–O1 | 103.6(3) | Sm2a–F1–Sm2c | 112.2(1) | ||
| O4–B1–O1 | 99.0(3) | Sm2b–F1–Sm2c | 104.1(1) | ||
| ø = 109.2 | ø = 108.7 | ||||
| O6–B1–O2 | 115.5(5) | O5–B2–O3 | 119.1(5) | Tb1–F1–Tb2a | 100.8(2) |
| O6–B1–O4 | 115.0(5) | O5–B2–O1 | 121.8(5) | Tb1–F1–Tb2b | 98.7(2) |
| O2–B1–O4 | 107.2(4) | O3–B2–O1 | 119.0(5) | Tb1–F1–Tb2c | 103.2(2) |
| O6–B1–O1 | 114.9(5) | ø = 120.0 | Tb2a–F1–Tb2b | 133.2(2) | |
| O2–B1–O1 | 103.6(4) | Tb2a–F1–Tb2c | 112.3(2) | ||
| O4–B1–O1 | 98.7(4) | Tb2b–F1–Tb2c | 103.8(2) | ||
| ø = 109.2 | ø = 108.7 | ||||
| O6–B1–O2 | 116.1(4) | O5–B2–O3 | 118.5(4) | Ho1–F1–Ho2a | 101.3(2) |
| O6–B1–O4 | 114.5(4) | O5–B2–O1 | 122.4(4) | Ho1–F1–Ho2b | 98.3(2) |
| O2–B1–O4 | 107.1(3) | O3–B2–O1 | 119.0(4) | Ho1–F1–Ho2c | 102.6(2) |
| O6–B1–O1 | 115.2(4) | ø = 120.0 | Ho2a–F1–Ho2b | 133.2(2) | |
| O2–B1–O1 | 103.5(3) | Ho2a–F1–Ho2c | 112.5(2) | ||
| O4–B1–O1 | 98.5(3) | Ho2b–F1–Ho2c | 103.9(1) | ||
| ø = 109.2 | ø = 108.6 | ||||
| O6–B1–O2 | 116.4(4) | O5–B2–O3 | 118.9(5) | Er1–F1–Er2a | 101.5(2) |
| O6–B1–O4 | 114.7(4) | O5–B2–O1 | 122.5(4) | Er1–F1–Er2b | 97.9(2) |
| O2–B1–O4 | 107.4(4) | O3–B2–O1 | 118.5(5) | Er1–F1–Er2c | 102.9(2) |
| O6–B1–O1 | 114.6(4) | ø = 120.0 | Er2a–F1–Er2b | 132.7(2) | |
| O2–B1–O1 | 103.1(4) | Er2a–F1–Er2c | 112.8(2) | ||
| O4–B1–O1 | 98.5(3) | Er2b–F1–Er2c | 104.1(2) | ||
| ø = 109.1 | ø = 108.6 |
Additional details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: +49-7247-808-666; e-mail: crysdata@fiz-karlsruhe.de, https://icsd.fiz-karlsruhe.de/search/basic.xhtml) on quoting the deposition numbers CSD-427586 for Sm4B4O11F2, CSD-427587 for Tb4B4O11F2, CSD-427588 for Ho4B4O11F2, and CSD-427589 for Er4B4O11F2.
3 Results and discussion
As reported for the previously published isotypic phases RE4B4O11F2 (RE = Pr, Nd, Eu, Gd, Dy) [12], [13], [14], these compounds are formed in a wide pressure and temperature range. Therefore, we were interested to investigate the possibility to synthesize more compounds with the same composition and crystal structure using different rare earth metal cations. While various syntheses applying different pressure and temperature conditions yielded the desired new phases RE4B4O11F2 (RE = Sm, Tb) as the main products, the syntheses of Ho4B4O11F2 and Er4B4O11F2 were only successful in a small pressure and temperature range, namely 9–10 GPa at about 1300°C. At lower pressure conditions, the main products of the syntheses were the rare earth oxides and rare earth fluoride oxides. Higher pressure conditions resulted in the formation of a new and not yet completely characterized product. While Tb4B4O11F2 and Ho4B4O11F2 could be obtained as phase pure products, the synthesis of Sm4B4O11F2 led to the formation of small amounts of α-Sm2B4O9 [20] as a byproduct. The reaction products of the syntheses of Er4B4O11F2 also contained significant amounts of Er3B5O12 [21] and a yet unknown byproduct.
As reported last year [14], the synthesis of “Ce4B4O11F2” has been attempted several times in our group using various reaction conditions, but without any success. Likewise, the synthesis of the compounds “RE4B4O11F2 (RE = Tm, Yb, Lu)” could not be achieved up to now. Interestingly, La4B4O11F2[10] is still the only known rare earth fluoride borate with the same composition but a completely different crystal structure.
The structure of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) contains planar BO3 groups as well as BO4 tetrahedra connected via common corners, as it is shown in Fig. 2. Two BO3 groups (Δ) and two BO4 tetrahedra (□) build up the main structural motif. This has first been discovered in Gd4B4O11F2 and can be described with the fundamental building block 2Δ2□:Δ□□Δ. A detailed depiction of the crystal structure of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) can be found in the description of the isotypic compound Gd4B4O11F2 [12]. This paper gives a comparison and overview of the structural properties of all nine isotypic compounds RE4B4O11F2 (RE = Pr, Nd, Sm–Er).
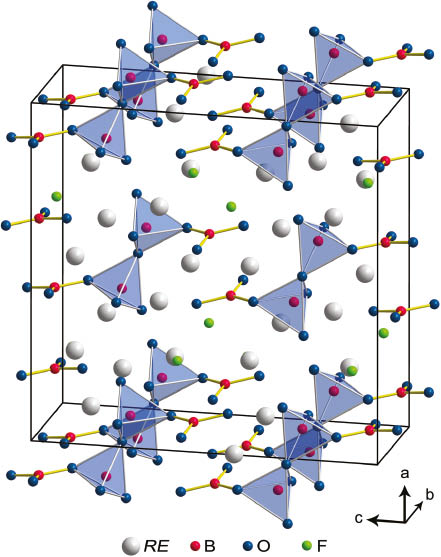
Crystal structure of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) showing the fundamental building block 2Δ2□:Δ□□Δ.
Figure 3 shows a comparison of the lattice parameters of Pr4B4O11F2 [14], Nd4B4O11F2 [14], Sm4B4O11F2, Eu4B4O11F2 [13], Gd4B4O11F2 [12], Tb4B4O11F2, Dy4B4O11F2 [13], Ho4B4O11F2, and Er4B4O11F2. The exact values are given in Table 5. The difference of the lattice parameters corresponds to the decreasing ionic radii of the rare earth cations, well known as the lanthanide contraction. The values for the ionic radii of ninefold coordinated lanthanide cations as given in literature [25] are as follows: Pr3+ (131.9 pm), Nd3+ (130.3 pm), Sm3+ (127.2 pm), Eu3+ (126.0 pm), Gd3+ (124.7 pm), Tb3+ (123.5 pm), Dy3+ (122.3 pm), Ho3+ (121.2 pm), and Er3+ (120.2 pm). Since the differences in size are not too large, the bond lengths and angles of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) are comparable to the values found in the other isotypic compounds [12], [13]. As expected, the RE–O/F distances in RE4B4O11F2 (RE = Pr, Nd, Sm–Er) decrease slightly but still significantly from values within 237.9(3)–285.5(3) pm in Pr4B4O11F2 and 231.0(3)–276.8(4) pm in Er4B4O11F2. The crystal structure of RE4B4O11F2 (RE = Pr, Nd, Sm–Er) contains a distorted tetrahedron that was interpreted as a BO3 group, in which the boron atom is drawn towards a fourth oxygen atom, resulting in a long B–O bond [12]. This long B1–O1 bond hardly varies in all nine isotypic compounds. The shortest B1–O1 bond measures 156.6(8) pm in Dy4B4O11F2 [13], the longest B1–O1 bond with a value of 159.0(6) pm is found in Gd4B4O11F2 [12]. The B1–O1 bond lengths in RE4B4O11F2 (RE = Sm, Tb, Ho, Er) all lie within these values. Obviously, the changing ionic radii of the rare earth cations have no influence on the B1–O1 bond length. The BO3 groups in RE4B4O11F2 (RE = Sm, Tb, Ho, Er) have average B–O distances between 137.7 and 138.5 pm – in good agreement with the literature value of 137.0 pm [26].
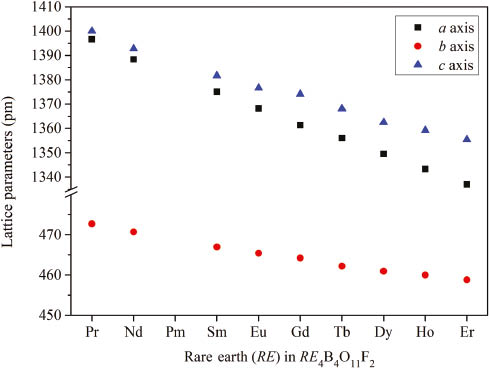
Visualization of the progression of the lattice parameters (in pm) of RE4B4O11F2 (RE = Pr, Nd, Sm–Er) with the typical decrease due to the lanthanoid contraction.
Comparison of the single-crystal lattice parameters (pm, deg) and unit cell volumes (nm3) of RE4B4O11F2 (RE = Pr, Nd, Sm–Er) (standard deviations in parentheses).
| Compound | a | b | c | β | V |
|---|---|---|---|---|---|
| Pr4B4O11F2 | 1396.7(5) | 472.7(2) | 1400.0(3) | 91.1(1) | 0.9242(3) |
| Nd4B4O11F2 | 1388.4(4) | 470.7(2) | 1392.8(5) | 91.1(1) | 0.9100(3) |
| Sm4B4O11F2 | 1375.06(1) | 466.95(2) | 1381.66(4) | 91.1(1) | 0.88698(5) |
| Eu4B4O11F2 | 1368.2(3) | 465.4(1) | 1376.6(3) | 91.2(1) | 0.8765(3) |
| Gd4B4O11F2 | 1361.3(3) | 464.2(2) | 1374.1(3) | 91.3(1) | 0.8681(3) |
| Tb4B4O11F2 | 1356.0(3) | 462.20(9) | 1368.1(3) | 91.2(1) | 0.8573(3) |
| Dy4B4O11F2 | 1349.5(3) | 460.9(1) | 1362.5(3) | 91.3(1) | 0.8472(3) |
| Ho4B4O11F2 | 1343.3(3) | 459.99(9) | 1359.2(3) | 91.4(1) | 0.8396(3) |
| Er4B4O11F2 | 1337.0(3) | 458.82(9) | 1355.4(3) | 91.4(1) | 0.8312(3) |
The bond valence sums of all atoms in RE4B4O11F2 (RE = Sm, Tb, Ho, Er) were calculated according to the BLBS (bond length/bond strength, ΣV) [27], [28], [29], [30], [31] and the CHARDI (charge distribution in solids, ΣQ) concept [29], [30], [32]. The results of both calculations verify the formal valence states in the fluoride borates. Table 6 shows the formal ionic charges received from the calculations, which fit well to the expected values.
Charge distribution in RE4B4O11F2 (RE = Sm, Tb, Ho, Er), calculated according to the BLBS (ΣV) [27], [28], [29], [30], [31] and the CHARDI concept (ΣQ) [29], [30], [32].
| Sm1 | Sm2 | B1 | B2 | Tb1 | Tb2 | B1 | B2 | |
| ΣV | 3.14 | 3.02 | 2.94 | 2.94 | 3.08 | 2.99 | 2.99 | 2.96 |
| ΣQ | 2.98 | 3.03 | 2.98 | 3.01 | 2.97 | 3.05 | 2.97 | 3.01 |
| O1 | O2 | O3 | O4 | O1 | O2 | O3 | O4 | |
| ΣV | –2.10 | –2.03 | –2.15 | –2.27 | –2.11 | –2.02 | –2.14 | –2.31 |
| ΣQ | –1.98 | –1.99 | –2.12 | –2.20 | –1.97 | –1.98 | –2.14 | –2.26 |
| O5 | O6 | F1 | O5 | O6 | F1 | |||
| ΣV | –1.91 | –1.91 | –0.80 | –1.91 | –1.91 | –0.77 | ||
| ΣQ | –1.89 | –1.93 | –0.99 | –1.88 | –1.93 | –0.96 | ||
| Ho1 | Ho2 | B1 | B2 | Er1 | Er2 | B1 | B2 | |
| ΣV | 3.03 | 3.00 | 2.97 | 2.93 | 3.04 | 2.99 | 2.97 | 2.89 |
| ΣQ | 3.04 | 3.09 | 2.90 | 2.97 | 2.99 | 3.07 | 2.94 | 3.00 |
| O1 | O2 | O3 | O4 | O1 | O2 | O3 | O4 | |
| ΣV | –2.12 | –2.02 | –2.09 | –2.29 | –2.09 | –2.02 | –2.05 | –2.32 |
| ΣQ | –2.25 | –1.99 | –2.12 | –2.25 | –1.98 | –2.00 | –2.06 | –2.29 |
| O5 | O6 | F1 | O5 | O6 | F1 | |||
| ΣV | –1.92 | –1.89 | –0.74 | –1.94 | –1.89 | –0.75 | ||
| ΣQ | –1.65 | –1.94 | –0.92 | –1.97 | –1.94 | –0.91 | ||
Furthermore, we calculated the MAPLE values (Madelung Part of Lattice Energy) [33], [34], [35] of RE4B4O11F2 (RE = Sm, Tb, Ho, Er) and compared them with the values for the binary components. We obtained a value of 72 178 kJ mol−1 for Sm4B4O11F2, to be compared with 72 227 kJ mol−1 (deviation: 0.07%) starting from the binary components [5/3 × Sm2O3([36], 14 767 kJ mol−1) + 2 × B2O3-II ([37], 21 938 kJ mol−1) +2/3 × SmF3 ([38], 5608 kJ mol−1)]. For Tb4B4O11F2, the resulting value is 72 667 kJ mol−1 compared to 72 723 kJ mol−1 (deviation: 0.08%) based on the binary components [5/3 × Tb2O3([39], 15 053 kJ mol−1) +2 × B2O3-II ([37], 21 938 kJ mol−1) +2/3 × TbF3 ([40], 5636 kJ mol−1)]. For Ho4B4O11F2, we obtained a value of 72 824 kJ mol−1, to be compared with 72 901 kJ mol−1 (deviation: 0.11%) starting from the binary components [5/3 × Ho2O3([41], 15 134 kJ mol−1) +2 × B2O3-II ([37], 21 938 kJ mol−1) +2/3 × HoF3 ([42], 5703 kJ mol−1)]. For Er4B4O11F2, the resulting value is 72 912 kJ mol−1 compared to 73 199 kJ mol−1 (deviation: 0.39%) based on the binary components [5/3 × Er2O3([43], 15 283 kJ mol−1) +2 × B2O3-II ([37], 21 938 kJ mol−1) +2/3 × ErF3 ([44], 5777 kJ mol−1)].
4 Conclusion
With the syntheses of RE4B4O11F2 (RE = Sm, Tb, Ho, Er), the possible formation range of compounds with the composition RE4B4O11F2 has been extensively investigated and the number of isotypic compounds with the constitution RE4B4O11F2 has been extended to nine. The existence of the compound “Ce4B4O11F2” could still not be proven but would be of great interest as it is the missing link between the different crystal structures of La4B4O11F2 and Pr4B4O11F2.
Acknowledgement
We thank Dr. G. Heymann (Leopold-Franzens-Universität Innsbruck, Austria) for collecting the single-crystal data. The research was funded by the Austrian Science Fund (FWF): P 23212-N19.
References
[1] G. Su, H. Toratani, Jpn. Kokai Tokkyo Koho1997, 6.Search in Google Scholar
[2] T. Suzuki, M. Hirano, H. Hosono, J. Appl. Phys.2002, 91, 4149.10.1063/1.1456946Search in Google Scholar
[3] L. R. P. Kassab, L. C. Courrol, A. S. Morais, S. H. Tatumi, N. U. Wetter. L. Gomes, J. Opt. Soc. Am. B: Opt. Phys.2002, 19, 2921.10.1364/JOSAB.19.002921Search in Google Scholar
[4] C. K. Jayasankar, V. Venkatramu, P. Babu, Th. Troster, W. Sievers, G. Wortmann, W. B. Holzapfel, J. Appl. Phys.2005, 97, 093523-1.10.1063/1.1890448Search in Google Scholar
[5] W. A. Pisarski, J. Pisarska, M. Mączka, W. Ryba-Romanowski, J. Mol. Struct.2006, 792–293, 207.10.1016/j.molstruc.2006.01.062Search in Google Scholar
[6] A. V. Ravi Kumar, B. Apparao, N. Veeraiah, Bull. Mater. Sci.1998, 21, 341.10.1007/BF02744964Search in Google Scholar
[7] H. A. Höppe, Z. Naturforsch.2015, 70b, 769.10.1515/znb-2015-0112Search in Google Scholar
[8] H. Huppertz, Chem. Commun.2011, 47, 131.10.1039/C0CC02715DSearch in Google Scholar PubMed
[9] A. Pitscheider, PhD thesis, Universität Innsbruck, Innsbruck, 2011.Search in Google Scholar
[10] A. Haberer, R. Kaindl, O. Oeckler, H. Huppertz, J. Solid State Chem.2010, 183, 1970.10.1016/j.jssc.2010.06.019Search in Google Scholar
[11] P. C. Burns, J. D. Grice, F. C. Hawthorne, Can. Mineral.1995, 33, 1131.Search in Google Scholar
[12] A. Haberer, R. Kaindl, H. Huppertz, J. Solid State Chem. 2010, 183, 471.10.1016/j.jssc.2009.12.003Search in Google Scholar
[13] A. Pitscheider, M. Enders, H. Huppertz, Z. Naturforsch. 2010, 65b, 1439.10.1515/znb-2010-1205Search in Google Scholar
[14] M. Glätzle, H. Huppertz, Z. Naturforsch. 2013, 68b, 635.10.5560/ZNB.2013-3086Search in Google Scholar
[15] D. Walker, M. A. Carpenter, C. M. Hitch, Am. Mineral.1990, 75, 1020.Search in Google Scholar
[16] D. Walker, Am. Mineral.1991, 76, 1092.10.1007/978-1-4615-3968-1_10Search in Google Scholar
[17] H. Huppertz, Z. Kristallogr.2004, 219, 330.10.1524/zkri.219.6.330.34633Search in Google Scholar
[18] D. C. Rubie, Phase Transitions1999, 68, 431.10.1080/01411599908224526Search in Google Scholar
[19] N. Kawai, S. Endo, Rev. Sci. Instrum.1970, 8, 1178.10.1063/1.1684753Search in Google Scholar
[20] H. Emme, H. Huppertz, Acta Crystallogr. 2005, C61, 29.10.1107/S0108270104030446Search in Google Scholar
[21] H. Emme, M. Valldor, R. Pöttgen, H. Huppertz, Chem. Mater. 2005, 17, 2707.10.1021/cm047741+Search in Google Scholar
[22] Z. Scalepack, W. Otwinowski, Minor in Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A (Eds.: C. W. Carter Jr., R. M. Sweet), Academic Press, New York, 1997, p. 307.10.1016/S0076-6879(97)76066-XSearch in Google Scholar
[23] G. M. Sheldrick, Shelxs/l-97, Programm Suite for the Solution and Refinement of Crystal Structures, University of Göttingen, Göttingen (Germany) 1997.Search in Google Scholar
[24] G. M. Sheldrick, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar PubMed
[25] R. D. Shannon, Acta Crystallogr.1976, A32, 751.10.1107/S0567739476001551Search in Google Scholar
[26] E. Zobetz, Z. Kristallogr.1982, 160, 81.10.1524/zkri.1982.160.1-2.81Search in Google Scholar
[27] L. Pauling, J. Am. Chem. Soc.1947, 69, 542.10.1021/ja01195a024Search in Google Scholar
[28] A. Byström, K.-A. Wilhelmi, Acta Chem. Scand.1951, 5, 1003.10.3891/acta.chem.scand.05-1003Search in Google Scholar
[29] I. D. Brown, D. Altermatt, Acta Crystallogr.1985, B41, 244.10.1107/S0108768185002063Search in Google Scholar
[30] N. E. Brese, M. O’Keeffe, Acta Crystallogr.1991, B47, 192.10.1107/S0108768190011041Search in Google Scholar
[31] N. E. Brese, M. O’Keeffe, Structure and Bonding, Springer-Verlag, Berlin, 1989.Search in Google Scholar
[32] R. Hoppe, S. Voigt, H. Glaum, J. Kissel, H. P. Müller, K. J. Bernet, J. Less-Common Met.1989, 156, 105.10.1016/0022-5088(89)90411-6Search in Google Scholar
[33] R. Hoppe, Angew. Chem., Int. Ed. Engl.1966, 5, 96.10.1002/anie.196600951Search in Google Scholar
[34] R. Hoppe, Angew. Chem., Int. Ed. Engl.1970, 9, 25.10.1002/anie.197000251Search in Google Scholar
[35] R. Hübenthal, M. Serafin, R. Hoppe, Maple (version 4.0), Program for the Calculation of Distances, Angles, Effective Coordination Numbers, Coordination Spheres, and Lattice Energies, University of Gießen, Gießen (Germany) 1993.Search in Google Scholar
[36] Y. Tabira, R. L. Withers, J. Solid State Chem. 1999, 148, 205.10.1006/jssc.1999.8433Search in Google Scholar
[37] C. T. Prewitt, R. D. Shannon, Acta Crystallogr.1968, B24, 869.10.1107/S0567740868003304Search in Google Scholar
[38] G.-Q. Wu, R. Hoppe, Z. Anorg. Allg. Chem. 1984, 514, 99.10.1002/zaac.19845140713Search in Google Scholar
[39] E. Hubbert-Paletta, Hk. Müller-Buschbaum, Z. Anorg. Allg. Chem. 1968, 363, 145.10.1002/zaac.19683630306Search in Google Scholar
[40] M. Piotrowski, A. Murasik, Phys. Stat. Sol.1985, A89, 571.10.1002/pssa.2210890218Search in Google Scholar
[41] S. A. Hering, H. Huppertz, Z. Naturforsch.2009, 64b, 1032.10.1515/znb-2009-0907Search in Google Scholar
[42] M. Piotrowski, H. Ptasiewicz-bak, A. Murasik, Phys. Stat. Sol. 1979, A55, K163.10.1002/pssa.2210550260Search in Google Scholar
[43] H. R. Hoekstra, Inorg. Chem.1966, 5, 754.10.1021/ic50039a013Search in Google Scholar
[44] A. Zalkin, D. H. Templeton, J. Am. Chem. Soc.1953, 75, 2453.10.1021/ja01106a052Search in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry
Articles in the same Issue
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry

