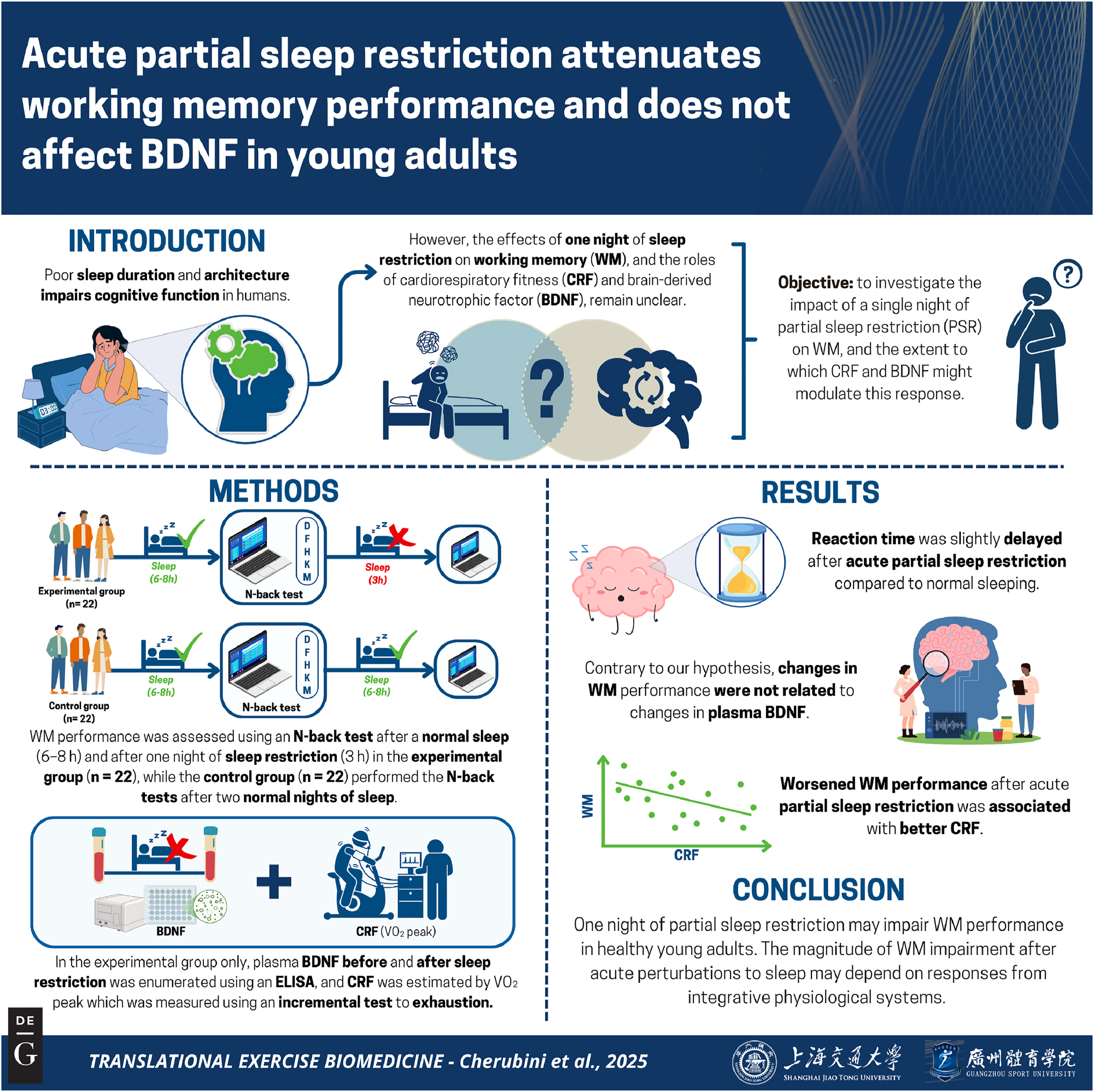
Abstract
Objectives
Sleep loss decreases working memory (WM) performance in young adults. The mechanisms that regulate WM performance during sleep loss are integrative and likely depend on the duration of perturbed sleep. The aim of this research was to investigate the impact of a single night of partial sleep restriction (PSR) on WM, and the extent to which factors such as cardiorespiratory fitness and brain-derived neurotrophic factor (BDNF) influence WM performance following one night of PSR.
Methods
We measured WM performance after one night of PSR in twenty-two young adults (n=13 females) with otherwise normal sleep habits. Participants completed an N-back test following one night of normal sleep (6–9 h sleep duration) and after one night of PSR (3 h sleep duration). To account for learning effects due to repeated cognitive testing, a time-matched normal sleep control group was also collected (n=22). Plasma BDNF was measured after normal sleep and PSR. Cardiorespiratory fitness was assessed via an incremental test to exhaustion on a cycle ergometer.
Results
3-back reaction time was slightly slower after PSR compared to the control group (P
Interaction=0.038;
Conclusions
Our data suggest that a single night of PSR may acutely impair WM in young adults and contribute to an understanding about the incipient cognitive consequences of shortened sleep.
Introduction
Sleep plays a critical role in modulating cognitive function, and short sleep durations impose a profound physiological stress that adversely impacts cognition in humans. Despite the importance of continuous and adequate sleep, contemporary societal demands shorten sleep duration [1] such that almost 10 % of the global population reports sleep durations of less than 6 h per night [2]. The impact of shortened sleep on cognitive function depends on numerous factors, including sleep quality, sleep duration, and frequent insomnia symptoms [3], [4], [5], [6]. Chronically shortened sleep associates with decreases in working memory (WM) performance, decreases in vigilance [7], 8], and increases in the risk of neurological disease [9]. Acute reductions in sleep durations, such as observed during total sleep deprivation (TSD) where sleep is eliminated for 24 h or more, also impairs both motor performance [10] and neurobehavioral performance which may increase rates of serious workplace errors [11].
Indeed, laboratory evidence demonstrates that acute TSD or partial sleep restriction (PSR) transiently impairs many aspects of cognition including WM, evidenced by decreases in accuracy on an N-back test of WM in healthy humans [12], [13], [14]. Not surprisingly, the magnitude of cognitive dysfunction increases as a function of greater accumulated sleep debt [13], such that total sleep deprivation results in exacerbated cognitive dysfunction compared to PSR across longer periods of wakefulness [14]. Indeed, total sleep deprivation represents an excellent model for investigating the deleterious physiological effects of prolonged wakefulness; however, humans may rarely miss an entire night of sleep. Conversely, PSR due to poor sleep hygiene and rigid lifestyle and occupational schedules is likely more common in society across longer timescales [15]. Although the negative impacts of chronic PSR on cognition are well described, the effects of acute PSR (e.g., one night of 3 h of sleep) on WM in males and females are comparatively less researched.
Factors that underlie the impaired cognition that is associated with shortened sleep may be integrative in nature. One such factor may involve changes in concentrations and signalling of the neurotrophin brain-derived neurotrophic factor (BDNF). BDNF is critical for cognitive processes including learning and memory. Further, BDNF orchestrates activity-dependent synaptic plasticity and is implicated in the regulation of sleep by increasing regional sleep pressure in activity-dependent manner during waking hours [16]. Circulating BDNF follows a biphasic response to sleep stressors, where chronic perturbations to sleep decrease concentrations of circulating BDNF, and acute total sleep deprivation increases BDNF levels [17], 18]. Increases in circulating BDNF, without the expected impairments to cognitive performance following acute total sleep deprivation, may reflect synaptic potentiation due to prolonged waking without appropriate synaptic rescaling during sleep and thereby uncouple the protective relationship between elevated circulating BDNF and cognitive performance [17]. The impact of acute PSR on circulating BDNF in healthy humans, however, remains unexplored. It is plausible that the early stress-associated elevations in circulating BDNF following sleep restriction may contribute to the preservation working memory, in accordance with the proposed adaptive response. However, domain-specific attenuations in attentional measures, which are sensitive to acute instances of PSR [13], may be impaired due to the increases in pressure to sleep prompted by BDNF [16] and decreases in vigilance characteristic of acute sleep restriction. Furthermore, adequate vascular function such as vasomotion of cerebral arterioles penetrating the brain parenchyma [19] – which may be in part facilitated by BDNF signaling [20] – is integral for sleep-dependent metabolic flux in the brin and the overall maintenance of cerebral health and cognitive function [21]. Research further indicates that maladaptive sleep causes significant perturbations to vascular function [22]. Examining associations between vascular function and WM performance during instances of restricted sleep presents an opportunity to highlight cognitive performance as the output of interactions between multiple physiological systems, all reliant on adequate sleep duration.
The objective of this study was to investigate the effect of PSR on circulating plasma BDNF and WM performance in young and healthy adults. We hypothesized that WM performance would decrease following a single night of PSR, and that this impairment would be associated with an increase in circulating plasma BDNF compared to when measured after one night of normal sleep. The summary of this article is presented in Figure 1.
Graphical representation of this study. Key points: (1) working memory assessments were conducted using an N-back task and plasma BDNF was measured after normal sleep and after acute partial sleep restriction in order to examine the relationships between acute perturbations to sleep and circulating BDNF dynamics in humans. (2) Sleep restriction slightly reduced reaction time during the N-back task relative to a normal sleeping control group. (3) Changes in reaction time were independent of changes in plasma BDNF.
Materials and methods
This paper presents an analysis of secondary outcomes from an experiment that was designed to examine the effects of PSR on vascular function [23]. Twenty-two male and female adults between the ages of 18–35 were recruited to participate in this experiment involving one night of PSR. A parallel-arm and normal sleeping time-control group, consisting of an additional sex-matched 22 participants, was used to account for the passage of time and potential learning effects due to repeated cognitive testing. Inclusion criteria for participation were the absence of any diagnosed sleeping disorders (e.g., obstructive sleep apnea, insomnia, narcolepsy), or a history of diagnosed medical disorders including any cardiovascular, musculoskeletal, and metabolic diseases. Data from female participants in the experimental group were collected during the early follicular phase of the menstrual cycle as determined by self-report, except for one participant who reported use of an intrauterine device. All participants provided written informed consent and all experimental procedures that were approved by the Hamilton Integrated Research Ethics Board (Project ID: 8270) and conformed to the Declaration of Helsinki, with the exception of preregistration.
Study protocol and data collection
To investigate the effect of acute PSR on WM and BDNF, experimental group participants completed two experimental visits consisting of normal sleep or PSR, in a fixed order. Prior to completing experimental visits, participants attended a familiarization session where they were introduced to study procedures, completed practice trials of the WM test, and completed a maximal exercise test to exhaustion on a cycle ergometer to characterize cardiorespiratory fitness (VO2 peak test). Participants were asked to arrive to the laboratory in a standardized fasted state (within 6 h preceding the visit), without consuming caffeine or alcohol (within 12 h preceding the visit), and without performing moderate-to-vigorous physical activity (within 24 h preceding the visit).
A habitual sleep baseline period was completed prior to commencement of the first experimental condition, where participants were instructed to maintain their habitual sleep behavior for at least three nights prior to undergoing testing for the normal sleep condition. Following the normal sleep condition, all participants completed the acute PSR condition, which was one night of 3 h sleep. For PSR participants were instructed to sleep for 3 h during the second half of the night (after 02:00 h) and returned to the laboratory the next morning. All data was collected at approximately the same time in the morning or early afternoon to avoid a diurnal influence on differences in outcome measures (NS collected at 11:12±02:02 hh:mm; PSR collected at 11:11±01:59 hh:mm). Sleep duration was recorded via an activity tracking device (Fitbit Charge HR, 2014; San Francisco, CA, USA), a mobile sleep recording application (Sleepcycle; Gothenburg, Sweden) and via a consensus sleep diary [24] that participants completed each morning during the experimental phase. In addition to sleep duration, the consensus sleep diary includes a subjective rating of sleep quality using a Likert scale ranging from 1 (very poor quality) to 5 (very good quality).
Experimental group data collection sessions began with of 10 min of supine rest. Heart rate variability (HRV) was recorded for an additional 5 min in the supine position. After HRV acquisition, participants underwent assessment of vascular structure and function [reported elsewhere; [23]]. A venous blood sample was also collected during the vascular testing sessions. Participants assumed an upright seated position and completed the N-back test of working memory [25] after the vascular function assessment. A three-lead ECG remained connected to the participants as they completed the N-back testing to monitor HRV.
A separate time-control group was included to account for potential learning effects associated with repeated cognitive testing. The time-control group was instructed to maintain their habitual sleep duration throughout the experimental period. Participants in the time-control group attended two sessions to the laboratory where they engaged only in an identical N-back assessment of working memory as the experimental group after 15 min of supine rest. The time control was only used to assess cognitive performance. Blood samples and hemodynamic assessment was not taken from participants in the time control group.
VO2 peak cardiopulmonary exercise test
Cardiorespiratory fitness moderates associations between sleep quality and memory performance in older adults [26]. We therefore measured VO2 peak to characterize cardiorespiratory fitness levels in our sample of young and healthy humans. Participants in the experimental group completed a VO2 peak to exhaustion test on a cycle ergometer during the familiarization visit. Participants were fitted with a face mask and a heart rate monitor (Model A300, Polar H9 heart rate sensor; Polar Electro Oy, Finland). Gas exchange was measured using a mixing chamber and metabolic cart (COSMED; Quark CPET). The ramp protocol involved a three-minute warmup at 50 W (W), thereafter followed by a progressive increase in resistance at a rate of 1 W every 2 s. Participants were instructed to maintain a pedaling cadence of 70–90 revolutions per minute (rpm) throughout the entire duration of the exercise test. VO2 peak was defined as plateau in VO2 despite an increase in work rate (W). We also defined VO2 peak according to secondary criteria if a plateau was not achieved, including a respiratory exchange ratio (RER)≥1.1, the participant reaching volitional exhaustion, or a HR within ±5 beats of their age-predicted HR maximum [27]. VO2 peak was calculated as the average VO2 in three 10-s bins that occurred concomitant to the criteria for VO2 peak and is reported relative to the participant’s body weight (mL/kg/min).
N-back test of working memory
To assess working memory, both experimental and control group participants completed the 1- and 3-back variants of the N-back test of WM on a laptop computer (Inquisit, by Millisecond Software; Version 6.4.2). Prior to the commencement of each test, a researcher read a standardized description of the N-back instructions and participants performed a brief practice trial of each test variant to minimize practice effects (Supplementary Script 1). Participants completed the N-back test in a quiet room free of auditory or visual distractions and completed three iterations of the 1-back and three iterations of the 3-back in a randomized order. Letters were presented on a computer screen to the participants individually with a stimulus onset asynchrony set to 2,500 ms and a stimulus presentation time of 500 ms. The match to mismatch ratio was 1:2 for each of the six blocks, and each block consisted of 15 trials. WM performance during the N-back task was quantified using three different metrics, including (i) mean hit reaction time (RT); (ii) hit rate; and (iii) d-prime sensitivity. Each of the performance metrics were quantified separately for the 1-back condition, the 3-back condition, and for the overall N-back test.
BDNF analysis
Venous blood was sampled from the antecubital vein in a subset of 16 participants in the experimental group. Blood was drawn from participants following a period of supine rest and at the start of the data collection session both before and after acute PSR. Plasma samples were collected into 4.0 mL K2 EDTA vacutainer tubes for plasma (BD Vacutainer; Franklin Lakes, NJ, USA) and centrifuged for 15 min at 4 °C at 4,000 rpm. The plasma supernatant was collected, and all samples were stored at −80 °C until use. Plasma BDNF represents the unbound and bioavailable pool in circulation [28] and was therefore analyzed using a commercially available enzyme-linked immunoassay kit (BEK-2211-2P; Biosensis, Thebarton, SA, Australia). An independent third-party has reported the intra- and inter-assay coefficients of variation for this immunoassay kit to be 1.0 % and 5.0 %, respectively [29], 30]. Plasma BDNF enzyme-linked immunoassays and standard curves were performed as per the manufacturer’s instruction.
Heart rate variability and vascular outcomes
HRV was measured to estimate cardiovagal control of heart rate and the autonomic response to acute PSR. HRV was quantified in the experimental group during supine rest and whilst seated during the N-back test after both the normal sleep and PSR data collection sessions. Participants were fitted with three-lead electrocardiography to collect continuous cardiac signals. The cardiac ECG data was recorded at a sampling frequency of 1,000 Hz for 5 min during supine rest, and during the last single minute of the N-back test after both the normal sleep and PSR data collection sessions. Only high frequency activity could be estimated from these recording durations [31]. HRV was thus represented in the time-domain as the root-mean square of successive RR intervals (RMSSD; m/s) using algorithms provided by LabChart8 Pro (ADInstruments Inc; Colorado Springs, CO, USA) analysis software. Vascular outcomes included resting hemodynamics such as heart rate and blood pressure, flow-mediated dilation at the brachial artery, and carotid-to-femoral pulse wave velocity, which estimate endothelial function and central arterial stiffness, respectively. These data were collected whilst adhering to published guidelines [32], 33], and the details pertaining to the collection of these outcomes were previously described in a derivative [34] of this experiment.
Sample size and statistical analyses
We had the capacity to detect large interaction effects (
Data were treated using R version 4.3.1 [35]. A linear mixed model with fixed terms group (experimental or time-control) × time (to denote visit 1 or visit 2) interaction and participant identification as a random effect was used to compare N-back WM performance. Tukey post-hoc penalties followed significant main effects. We used a paired t-test to compare plasma BDNF levels before and after acute PSR in a subset of 16 participants. Exploratory analyses were conducted in this study to generate hypotheses related to mechanisms that may underlie putative changes in WM acutely following a single night of PSR. N-back performance metrics of the experimental group were z-transformed relative to the mean and standard deviation of those metrics from the time-control group. These z-transformed performance metrics were then regressed against VO2 peak to describe the effects of cardiorespiratory fitness on changes in WM after acute PSR relative to normal-sleeping controls. We performed further correlations to examine the extent to which changes in circulating plasma BDNF and hemodynamic parameters related to changes in WM performance parameters measured after normal sleep and acute PSR. All data represents mean±standard deviation (SD) unless otherwise stated.
Results
Participant characteristics
Data from a total of forty-four participants (n=22 from the experimental group and n=22 from the time-control group) are included in this study. Participant characteristics are provided in Table 1. Sleep duration was significantly reduced with acute PSR in the experimental group across all modalities of assessment (Fitbit Δ=3.97 h; 95 % CI [2.94, 5.00], SleepCycle Δ=4.10 h; 95 % CI [3.53, 4.66], consensus sleep diary Δ=4.39; 95 % CI [4.16, 4.61]; all p<0.001) as per our intended experimental intervention (Table 1). Moreover, the perception of sleep quality as recorded by the consensus sleep diary significantly changed between conditions in the experimental group such that sleep quality was perceived as significantly poorer following PSR compared to NS (NS: 3.7±0.7; PSR: 2.5±1.0 arbitrary Likert scale units, p<0.001).
Participant anthropometrics and sleep characteristics.
| Variable | |||
|---|---|---|---|
| Total sample n | 44 | ||
| Experimental group | 9 M/13 F | ||
| Time control group | 9 M/13 F | ||
| Age, years | 21 (3) | ||
| BMI, kg/m2 | 24.0 (3.2) | ||
| VO2 peak, mL/kg/min | 41.3 (8.6) | ||
|
|
|||
| Experimental group sleep durations | NS | PSR | p-Value |
|
|
|||
| Fitbit sleep duration, h | 6.7 (2.3) | 2.7 (0.7) | <0.001 |
| SleepCycle sleep duration, h | 6.5 (1.1) | 2.4 (0.7) | <0.001 |
| Consensus diary sleep duration, h | 7.3 (0.5) | 2.9 (0.4) | <0.001 |
-
NS, normal sleep; PSR, partial sleep restriction; BMI, body mass index. Data represents means (±SD). p-values accepted as significant when p<0.05 using a paired t-test.
Working memory performance
Working memory performance scores assessed using the N-back test are presented in Table 2. We found a significant group by time interaction for the 3-back mean hit RT (p=0.038; β=−100.46; 95 % CI [−192.20, −8.73]), where the mean absolute magnitude of change in RT from Visit 1 to Visit 2 in the experimental group recorded a +36.13 ms difference (95 % CI [−33.87, 106.13], p=0.697), and the control group recorded a mean RT −64.34 ms difference (95 % CI [−132.15, 3.47], p=0.227; Figure 2). There was, however, no group by time interaction in the overall mean hit RT (p=0.070; β=−41.20; 95 % CI [−84.49, 2.09]), nor in the 1-back mean hit RT (p=0.972; β=0.61; 95 % CI [−33.41, 34.63]). We evaluated the overall proportion of correct responses and found no group by time interaction (p=0.534; β=0.606; 95 % CI [−1.29, 2.50]). The statistically insignificant group by time interaction persisted when analyzing the hit rate in the 1-back condition (p=0.542; β=−0.01; 95 % CI [−0.05, 0.02]) and 3-back condition (p=0.756; β=0.01; 95 % CI [−0.06, 0.09]) separately. There were no significant group by time interactions for overall d-prime scores (p=0.666; β=0.09; 95 % CI [−0.30, 0.48]), 1-back d-prime scores (p=0.731; β=−0.08; 95 % CI [−0.53, 0.37]), or 3-back d’ scores (p=0.614; β=0.14; 95 % CI [−0.39, 0.66]).
Working memory performance as evaluated by the N-back experimental paradigm.
| Time-control group (n=22) | Experimental group (n=22) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | NS | PSR | Condition | Group | Interaction | |
| 1-Back hit rate | 98.8 (3.3) | 96.4 (7.3) | 97.6 (6.4) | 96.4 (8.2) | 0.072 | 0.725 | 0.542 |
| 3-Back hit rate | 79.1 (14.3) | 83.0 (13.6) | 72.7 (16.1) | 75.5 (15.9) | 0.093 | 0.095 | 0.756 |
| 1-Back d-prime | 4.95 (0.52) | 4.67 (0.75) | 4.77 (0.77) | 4.56 (0.91) | 0.038a | 0.456 | 0.731 |
| 3-Back d-prime | 2.71 (1.10) | 3.04 (1.15) | 2.40 (0.94) | 2.60 (1.00) | 0.058 | 0.193 | 0.614 |
| 1-Back RT | 579 (121) | 587 (140) | 630 (135) | 638 (130) | 0.347 | 0.195 | 0.972 |
| 3-Back RT | 818 (191) | 754 (133) | 822 (247) | 858 (249) | 0.550 | 0.364 | 0.038a |
-
RT, reaction time (ms); LMEM, linear mixed effects model. Data from n=22 represent means (SD); aSignificantly different at p<0.05 using a linear mixed effects model.
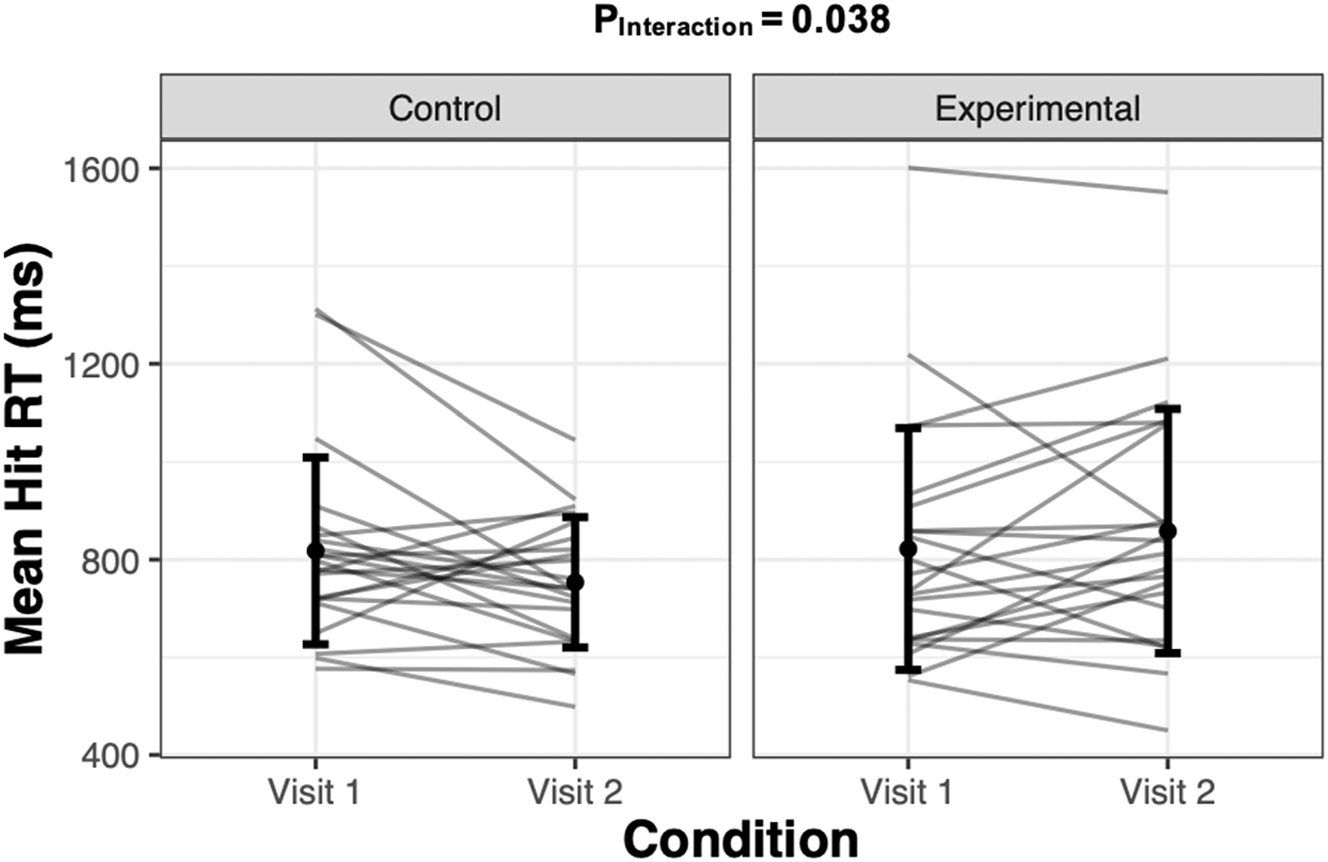
Mean hit reaction time (milliseconds; ms) after visit #1 and after visit #2 in the time-control group, and after normal sleep and partial sleep restriction in the experimental group. Grey lines represent individual responses, and the dot and err or bars represent mean±SD (n=22 per group).
Circulating plasma BDNF
Blood samples were obtained from fourteen participants in the experimental group who completed the WM tests, and blood samples were also obtained from an additional two participants who completed the sleep restriction intervention but without WM performance metrics. Some participants had completed the trial before blood samples were included in the protocol, explaining the discrepancy in the total number of participants vs. the number of participants with blood samples. Plasma BDNF failed tests of normality and were right-skewed based on positive Fisher skewness coefficients. We therefore log-transformed the plasma BDNF data which then subsequently passed normality testing. There was no change in plasma BDNF levels following PSR compared to NS (presented on the log scale; NS: 489.01±590.94 pg/mL vs. PSR: 516.58±691.00 pg/mL; p=0.720; Cohen’s d=−0.04; Figure 3A). Contrary to our hypothesis, changes in the impaired 3-back RT response did not relate to changes in circulating plasma BDNF (R2=−0.07; p=0.769; Figure 3B). Further, exploratory correlation analyses revealed that there were no associations between plasma BDNF and measures of WM N-back performance metrics (Figure 3B; all p>0.05).
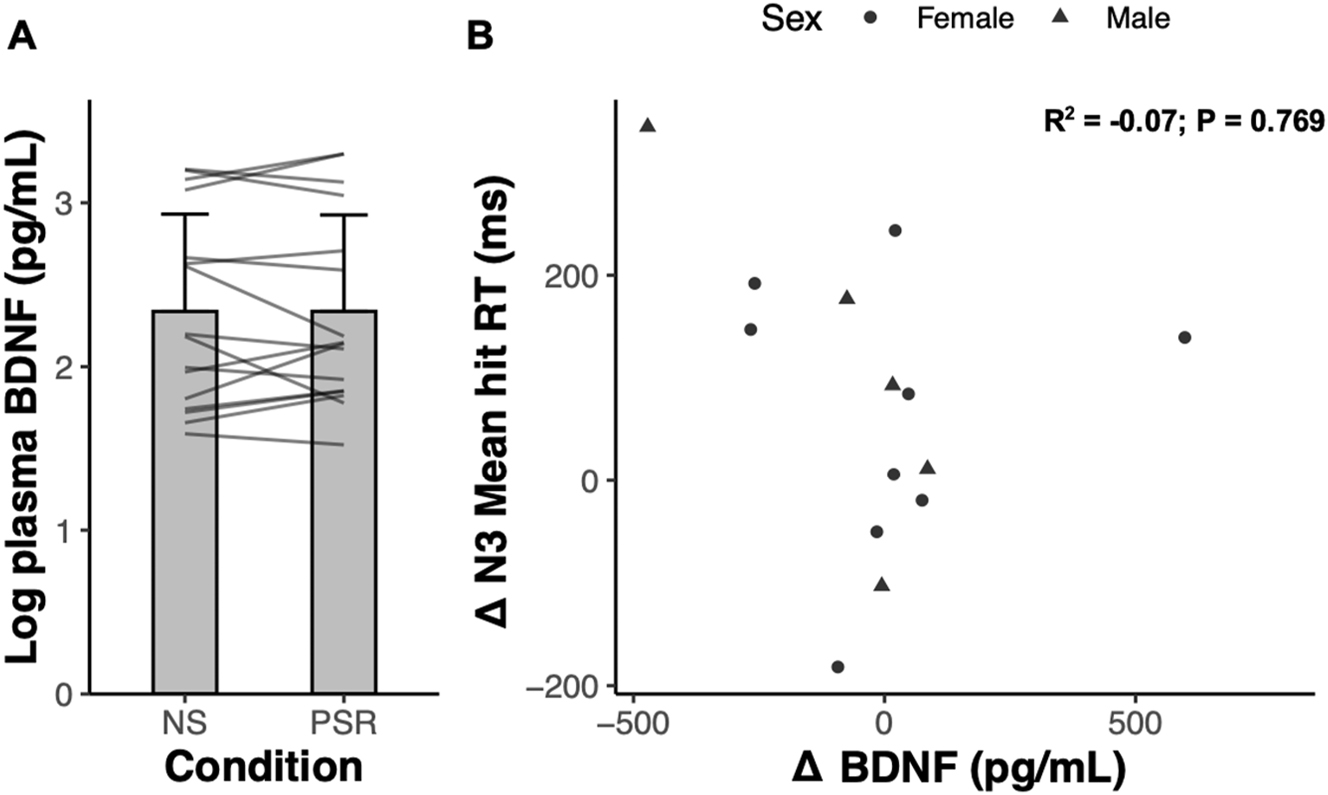
Plasma BDNF following normal sleep (NS) versus partial sleep restriction (PSR), and its relation to changes in working memory performance. (A) Log plasma BDNF (pg/mL) measured after NS and after acute PSR. Grey lines represent individual participant responses (n=16). Data represents mean±SD. (B) Relationship between the change in 3-back (N3) mean hit reaction time (RT) and the change in BDNF (pg/mL) between normal sleep and acute PSR (n=14).
Heart rate variability responses
We evaluated HRV at rest and during the last minute of the N-back test in the experimental group as a crude estimator of cardiovagal modulation to measure potential associations between changes in HRV and WM performance after acute PSR. No differences were found in HRV metrics including RMSSD, SDRR, and high-frequency spectral power during working memory testing following PSR compared to NS (all p>0.05; representative exposition of RMSSD included in Table 3). No associations were found between changes in HRV measured in the time-domain and changes in 3-back RT when HRV was recorded both at rest, nor during the last minute of the N-back test (all p>0.05).
Hemodynamic parameters measured after normal sleep and after acute partial sleep restriction in the experimental group.
| Normal sleep | Sleep restriction | p-Value | |
|---|---|---|---|
| SBP, mmHg | 113 (12) | 113 (10) | 0.607 |
| DBP, mmHg | 62 (6) | 62 (5) | 0.293 |
| MAP, mmHg | 82 (7) | 81 (6) | 0.365 |
| HR, bpm | 63 (10) | 62 (13) | 0.707 |
| FMD (% dilation) | 6.7 (3.2) | 6.4 (2.9) | 0.379 |
| PWV, m/s | 7.4 (1.4) | 7.7 (1.4) | 0.355 |
| Resting HRV: RMSSD | 62.2 (39.2) | 68.4 (50.9) | 0.228 |
| N-back HRV: RMSSD | 58.1 (48.7) | 50.2 (33.6) | 0.259 |
-
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; FMD, flow-mediated dilation; PWV, central artery pulse wave velocity; HRV, heart rate variability. Data represents means (SD).
Exploratory associations between working memory performance, cardiorespiratory fitness, and vascular measures
We were interested to see if cardiorespiratory fitness, estimated by VO2 peak, moderated the change in RT between conditions given prior research suggesting protective effects of cardiorespiratory fitness on WM performance during poor sleep [26]. The standardized change in RT during the 3-back condition was regressed against VO2 peak and, contrary to our exploratory hypothesis, we found a negative linear relationship between the standardized change in RT and VO2 peak (Pearson’s r=−0.48, p=0.025; Figure 4). This negative relationship persisted after adjusting for sex and the perceived impact of sleep restriction. Another exploratory analysis was conducted to examine associations between indices of peripheral vascular function and working memory between NS and acute PSR. No associations were detected between changes in any resting hemodynamic parameter (SBP, DBP, and HR) and measures of working memory performance (all p>0.05) between sleep conditions. Furthermore, no associations were found between changes in arterial stiffness estimated by pulse wave velocity and measures of working memory performance (all p>0.05). Interestingly, there was a significant negative correlation between changes in %FMD and changes in overall RT (Pearson’s r=−0.47, 95 % CI [−38.3, −2.3], p=0.028; Figure 5A), and changes in RT in the 3-back condition, such that larger %FMD values correlated with faster RT (Pearson’s r=−0.43, 95 % CI [−79.2, −1.3], p=0.044; Figure 5B).
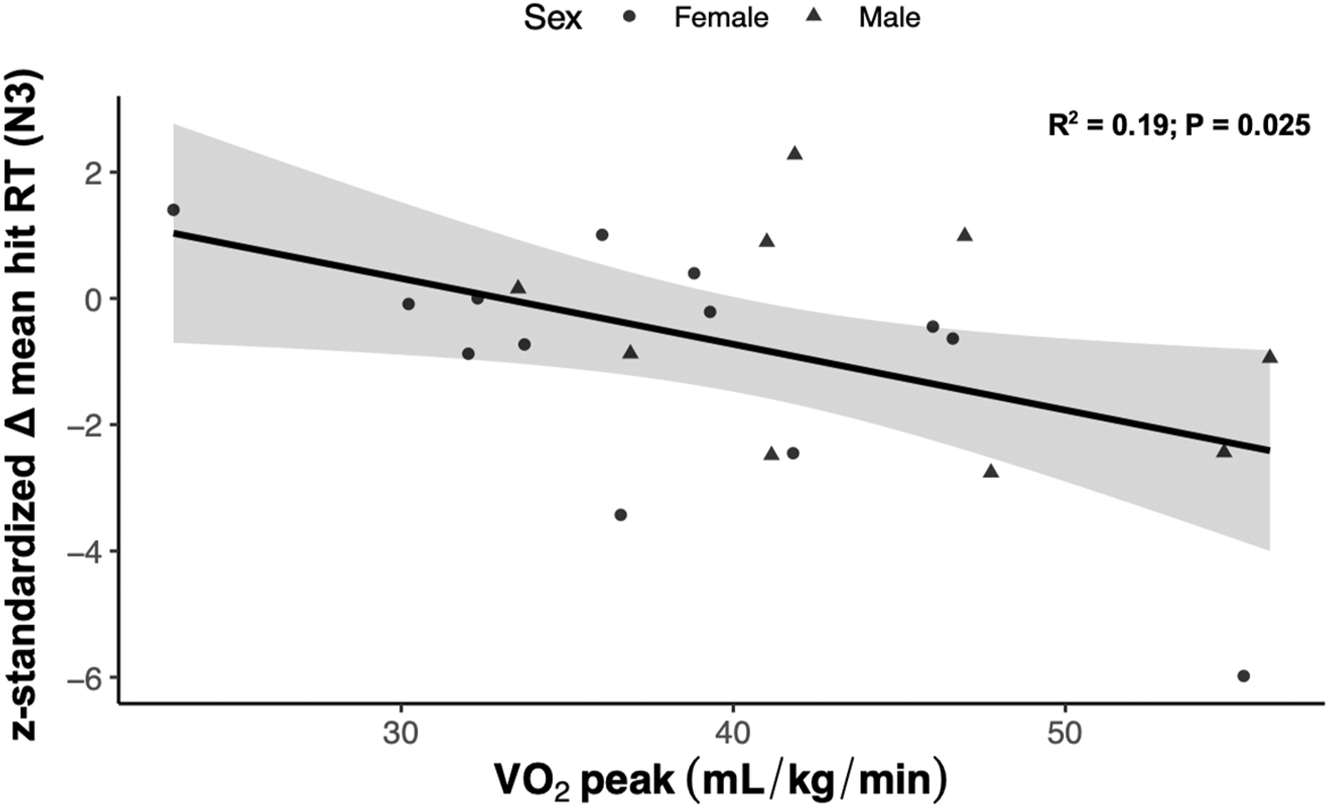
Correlation analysis depicting the standardized change in mean hit reaction time in the 3-back (N3) condition relative to a time-control as a function of cardiorespiratory fitness (VO2 peak) in the experimental group (n=22).
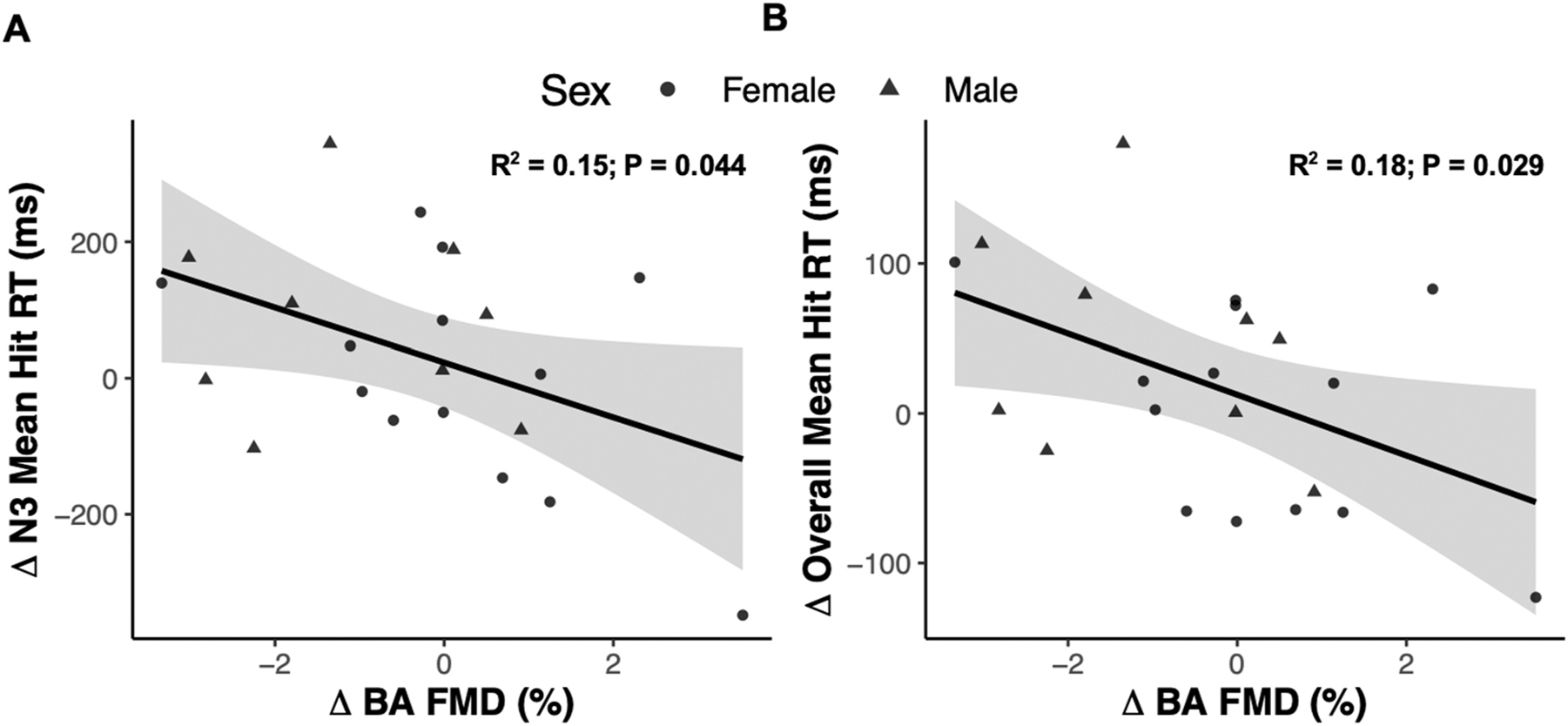
Correlation analysis of the association between the change in 3-back (N3) reaction time (A) and overall reaction time (B) as a function of the change in % flow-mediated dilation measured at the brachial artery (BA FMD) from the experimental group (n=22).
Discussion
The purpose of this study was to investigate the impact of acute PSR on circulating plasma BDNF and WM performance in young adults. A single night of PSR acutely worsened WM performance compared to a normal sleeping time-control group, such that there was no observed improvement due to repeated testing in the PSR group. This effect was mild in magnitude given the opposing directionality of the mean RT scores between conditions across the experimental and control groups, and did not persist following post-hoc testing. However, contrary to our hypothesis there was no effect of PSR on plasma BDNF. Interestingly, exploratory analysis revealed that higher cardiorespiratory fitness was associated with a greater decrement in WM after acute PSR. These data add to emerging findings that acute PSR may alter WM performance independent of plasma BDNF concentrations. These findings provide important insights into the integrated physiological and neurobiological consequences of acute sleep restriction.
The observed acute and mild impairment in RT during the 3-back condition is consistent with work showing slower processing speed in females after acute PSR [36]. We add to this burgeoning body of literature by showing that acute PSR mildly impacts reaction time in both males and females. We present novel findings that changes in WM occur without changes in plasma BDNF. A shortened sleep opportunity may limit the processes underlying memory consolidation and synaptic rescaling [37]. Reduced opportunities for memory consolidation due to acute PSR may, in part, have limited the learning effects due to repeated N-back testing that ostensibly accrued overnight in the time-control group. Consistent with the active systems consolidation theory [37], synaptic rescaling during sleep reorganizes synaptic connections within and between the hippocampus and neocortex. Accordingly, acute PSR may impair consolidation of synapses created during the preceding waking period. Our data suggest, however, a mild interaction between acute PSR and WM performance given a small RT increase during the high-cognitive load 3-back task in the experimental group relative to the control group. This observation aligns with the notion that WM decrements are exacerbated as a function of accruing short sleep [14], and highlights acute PSR as a relevant, but mild cognitive stressor. Future research using an acute PSR model may therefore be guided by our findings to include larger sample sizes that are statistically powered to detect the small effects that we report herein.
The neurotrophin BDNF is critical for orchestrating synaptic plasticity and memory formation and plays an important role in regulating sleep pressure [16]. Accordingly, we assessed changes in plasma BDNF in the morning after a single night of PSR to examine the extent to which changes in circulating BDNF, particularly in a context of elevated sleep pressure, related to changes in WM. Contrary to our hypothesis, there was no change in plasma BDNF following acute PSR compared to NS. Reducing sleep to 3 h on a single night may not be a sufficient stimulus to evoke a stress-induced adaptive response. Indeed, circulating BDNF increases following acute total sleep restriction and may represent an adaptive response to protect against cognitive impairment during waking hours and increase non-rapid eye movement sleep during subsequent convalescent sleep [38]. In agreement with others [13], 36], the current experiment shows mild effects of acute PSR on WM evidenced by a slowing of response time in the 3-back condition only, which represents a higher imposed WM processing challenge. Indeed, the 1-back performance was not different from baseline, nor from the time-control group, suggesting that the effects of acute sleep loss are limited to tasks with higher WM challenge. These results substantiate the speculation that sleep loss may first impact cellular neuronal signalling before progressing to affect broader physiological and cerebral processes as the duration of sleep restriction increases. Therefore, the impact of acute PSR on BDNF and other physiological markers may not be as pronounced as observed in more severe forms of acute sleep loss such as TSD.
Prior research indicates that higher levels of cardiorespiratory fitness may protect cognition following acute sleep deprivation [26]. Accordingly, we explored cardiorespiratory fitness as a candidate physiological factor that may buffer against impaired WM following PSR. However, we observed the opposite relationship, as higher VO2 peak was associated with slowed RT acutely following a single night of PSR. These data suggest that individuals with higher aerobic fitness in our sample may have placed a greater emphasis on lifestyle factors, such as exercise and sleep. The disturbance to sleep caused by our intervention may have been perceived as more disruptive for these individuals, which may have influenced working memory performance via expectancy biases. Indeed, individual perceptions of sleep duration have been shown to modulate RT, independent of objectively measured sleep duration [39]. However, the negative relationship between VO2 peak and WM RT was not impacted when we controlled for sleep perceptions in our sample. Future research may build on this exploratory finding to determine the extent to which the perceptions of sleep loss and associated expectancy biases modulate WM performance, particularly in individuals with high aerobic capacities.
We also explored the relationship between peripheral vascular function and cognition, as previous research indicates that vasomotion in the cerebral vasculature affects metabolic clearance from the brain during sleep [40]. We regressed changes in vascular endothelial cell function against changes in 3-back RT between sleep conditions and found that increased vascular function was associated with faster 3-back RT. Although exploratory, this preliminary result is conceptually consistent with importance of vascular dynamics for facilitating the flow and clearance of metabolic waste throughout the brain parenchyma during sleep [19]. A responsive endothelium, as inferred by improved conduit artery vasodilation following hyperemia, may confer a more robust cerebrovascular oscillation and therefore enhance clearance. This result, however, is exploratory and the associations between peripheral and cerebral endothelial function that are required to support this hypothesis are not yet sufficiently established [41].
Among the strengths of this study include the ecological relevance of the study design wherein sleep restriction occurred in free-living conditions familiar to participants. This enabled participants to partake in behaviours intrinsic to their daily routines within conditions, in contrast to the constrained array of activities inherent in a supervised sleep restriction setting. Furthermore, the N-back task was used to assess a spectrum of imposed cognitive processing demands that spanned from easier processing loads (1-back condition) to comparatively difficult processing loads (3-back condition) in a randomized order. That a discernable impairment in RT was observed in the 3-back condition underscores the discriminative capacity of the N-back task in delineating nuanced cognitive impairment.
Limitations of this experiment also warrant acknowledgment. First, BDNF has been proposed to follow a biphasic pattern such that circulating concentrations of BDNF increase concomitantly with the acute stressor, and then dissipate during the sleep opportunity [17], 18]. A nighttime measure of circulating BDNF during the sleep restriction period, while sleep pressure is building, would increase the temporal resolution of circulating BDNF levels and therefore provide a better index of the integrative stress that acute PSR imposed on the body. Second, we reconciled the observed impairment in RT by deferring to reductions in opportunities for active systems consolidation and synaptic rescaling. That our current study had no direct measures of either encephalography or synaptic scaling as a proxy for consolidation limits our mechanistic speculation and presents an intriguing opportunity for further research. Objective measures of sleep quality, such as polysomnography, may have also provided insight into sleep architecture and learning that was not captured with the subjective and objective methods used to measure sleep duration herein. Measures of sleep architecture during partial sleep would provide powerful evidence to substantiate the observed effects of acute PSR on WM to extend our findings. Outcomes involving the proportion of time spent in REM sleep, and the slow-wave amplitudes characteristic of NREM, should be described in subsequent PSR interventions. Sex differences in WM performance following acute PSR have not been investigated. Our research was underpowered to detect potential sex differences, however equal representation of sexes was included in each group and sensitivity analyses with participants separated by sex did not indicate that results would differ between sexes.
Conclusions
Acute PSR yielded a mild impairment to RT relative to a time-matched and normal sleeping control on the N-back test of WM independent of BDNF changes between NS and acute PSR. The subtle effects of PSR on RT observed in our data have broad implications for public health messaging given the prevalence of PSR, and highlights sleep duration as a relevant consideration prior to the assessment of WM in future investigations particularly where anticipated effects may be small. Furthermore, the slower RT response occurred in the absence of changes in plasma BDNF. Our exploratory analyses warrant follow-up experiments that measure integrative mechanisms, such as cardiorespiratory fitness and hemodynamic function, which may contribute to changes in cognitive processing during perturbed sleep. Our research characterizing the effects of acute PSR on WM performance and plasma BDNF levels in young adults contributes valuable insight into our understanding about the immediate consequences of short-term sleep insufficiency.
Funding source: Natural Engineering and Sciences Research Council of Canada
Award Identifier / Grant number: NSERC DG: RGPIN-2021-03522
Funding source: CFI John R. Evans Leader’s Fund
Funding source: Natural Engineering and Sciences Research Council of Canada
Award Identifier / Grant number: NSERC DG: RGPIN-2021-03522
Acknowledgments
We acknowledge our funding sources and acknowledge and thank the participants from whom the data presented herein was gathered.
-
Research ethics: All experimental procedures that were approved by the Hamilton Integrated Research Ethics Board (Project ID: 8270) and conformed to the Declaration of Helsinki, with the exception of preregistration.
-
Informed consent: All participants provided written informed consent.
-
Author contributions: JJW, MJM, and JMC designed the study. JMC, DW, JS, and LN collected data. JMC and JJW treated the data, interpreted results, and drafted the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None used.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: JMC is supported by an Ontario Graduate Scholarship (OGS). MJM is funded by the Natural Engineering and Sciences Research Council of Canada (NSERC DG: #20011033). JJW is funded by the Natural Engineering and Sciences Research Council of Canada (NSERC DG: RGPIN-2021-03522) and Canada Foundation for Innovation (CFI John R. Evans Leader’s Fund).
-
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
1. Basner, M, Spaeth, AM, Dinges, DF. Sociodemographic characteristics and waking activities and their role in the timing and duration of sleep. Sleep 2014;37:1889–906. https://doi.org/10.5665/sleep.4238.Suche in Google Scholar
2. Coutrot, A, Lazar, AS, Richards, M, Manley, E, Wiener, JM, Dalton, RC, et al.. Reported sleep duration reveals segmentation of the adult life-course into three phases. Nat Commun 2022;13:7697. https://doi.org/10.1038/s41467-022-34624-8.Suche in Google Scholar
3. Krause, AJ, Simon, EB, Mander, BA, Greer, SM, Saletin, JM, Goldstein-Piekarski, AN, et al.. The sleep-deprived human brain. Nat Rev Neurosci 2017;18:404–18. https://doi.org/10.1038/nrn.2017.55.Suche in Google Scholar
4. Lowe, CJ, Safati, A, Hall, PA. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci Biobehav Rev 2017;80:586–604. https://doi.org/10.1016/j.neubiorev.2017.07.010.Suche in Google Scholar
5. Tan, X, Åkerstedt, T, Lagerros, YT, Åkerstedt, AM, Bellocco, R, Adami, HO, et al.. Interactive association between insomnia symptoms and sleep duration for the risk of dementia – a prospective study in the Swedish National March Cohort. Age Ageing 2023;52:afad163. https://doi.org/10.1093/ageing/afad163.Suche in Google Scholar
6. Tan, X, Lebedeva, A, Åkerstedt, T, Wang, HX. Sleep mediates the association between stress at work and incident dementia: study from the survey of health, ageing and retirement in Europe. J Gerontol A Biol Sci Med Sci 2023;78:447–53. https://doi.org/10.1093/gerona/glac104.Suche in Google Scholar
7. Tai, XY, Chen, C, Manohar, S, Husain, M. Impact of sleep duration on executive function and brain structure. Commun Biol 2022;5:201. https://doi.org/10.1038/s42003-022-03123-3.Suche in Google Scholar
8. Dinges, DF, Pack, F, Williams, K, Gillen, KA, Powell, JW, Ott, GE, et al.. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997;20:267–77.Suche in Google Scholar
9. Sabia, S, Fayosse, A, Dumurgier, J, van Hees, VT, Paquet, C, Sommerlad, A, et al.. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun 2021;12:2289. https://doi.org/10.1038/s41467-021-22354-2.Suche in Google Scholar
10. Williamson, AM, Feyer, AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med 2000;57:649–55. https://doi.org/10.1136/oem.57.10.649.Suche in Google Scholar
11. Landrigan, CP, Rothschild, JM, Cronin, JW, Kaushal, R, Burdick, E, Katz, JT, et al.. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med 2004;351:1838–48. https://doi.org/10.1056/nejmoa041406.Suche in Google Scholar
12. Dai, C, Zhang, Y, Cai, X, Peng, Z, Zhang, L, Shao, Y, et al.. Effects of sleep deprivation on working memory: change in functional connectivity between the dorsal attention, default mode, and fronto-parietal networks. Front Hum Neurosci 2020;14:360. https://doi.org/10.3389/fnhum.2020.00360.Suche in Google Scholar
13. Lo, JC, Ong, JL, Leong, RL, Gooley, JJ, Chee, MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep 2016;39:687–98. https://doi.org/10.5665/sleep.5552.Suche in Google Scholar
14. Van Dongen, H, Maislin, G, Mullington, JM, Dinges, DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–26. https://doi.org/10.1093/sleep/26.2.117.Suche in Google Scholar
15. Hershner, SD, Chervin, RD. Causes and consequences of sleepiness among college students. Nat Sci Sleep 2014;6:73. https://doi.org/10.2147/nss.s62907.Suche in Google Scholar
16. Faraguna, U, Vyazovskiy, VV, Nelson, AB, Tononi, G, Cirelli, C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 2008;28:4088–95. https://doi.org/10.1523/jneurosci.5510-07.2008.Suche in Google Scholar
17. Schmitt, K, Holsboer-Trachsler, E, Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann Med 2016;48:42–51. https://doi.org/10.3109/07853890.2015.1131327.Suche in Google Scholar
18. Giacobbo, BL, Corrêa, MS, Vedovelli, K, de Souza, CE, Spitza, LM, Gonçalves, L, et al.. Could BDNF be involved in compensatory mechanisms to maintain cognitive performance despite acute sleep deprivation? An exploratory study. Int J Psychophysiol 2016;99:96–102. https://doi.org/10.1016/j.ijpsycho.2015.11.008.Suche in Google Scholar
19. Mortensen, KN, Sanggaard, S, Mestre, H, Lee, H, Kostrikov, S, Xavier, AL, et al.. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci 2019;39:6365–77. https://doi.org/10.1523/jneurosci.1974-18.2019.Suche in Google Scholar
20. Donovan, MJ, Lin, MI, Wiegn, P, Ringstedt, T, Kraemer, R, Hahn, R, et al.. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Devt 2000;127:4531–40. https://doi.org/10.1242/dev.127.21.4531.Suche in Google Scholar
21. Rasmussen, MK, Mestre, H, Nedergaard, M. Fluid transport in the brain. Physiol Rev 2022;102:1025–151. https://doi.org/10.1152/physrev.00031.2020.Suche in Google Scholar
22. Holmer, BJ, Lapierre, SS, Jake-Schoffman, DE, Christou, DD. Effects of sleep deprivation on endothelial function in adult humans: a systematic review. GeroScience 2021;43:137–58. https://doi.org/10.1007/s11357-020-00312-y.Suche in Google Scholar
23. Cherubini, JM, Cheng, JL, Armstrong, CM, Kamal, MJ, Parise, G, MacDonald, MJ. Acute partial sleep restriction does not impact arterial function in young and healthy humans. Exp Physiol 2024;109:1492–504. https://doi.org/10.1113/ep091699.Suche in Google Scholar
24. Carney, CE, Buysse, DJ, Ancoli-Israel, S, Edinger, JD, Krystal, AD, Lichstein, KL, et al.. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. https://doi.org/10.5665/sleep.1642.Suche in Google Scholar
25. Owen, AM, McMillan, KM, Laird, AR, Bullmore, E. N‐back working memory paradigm: a meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59. https://doi.org/10.1002/hbm.20131.Suche in Google Scholar
26. Kuhn, T, Heisz, J. Cardiorespiratory fitness may protect memory for poorer sleepers. Front Psychol 2022;13:793875. https://doi.org/10.3389/fpsyg.2022.793875.Suche in Google Scholar
27. Howley, ET, Bassett, DR, Welch, HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc 1995;27:1292–301. https://doi.org/10.1249/00005768-199509000-00009.Suche in Google Scholar
28. Walsh, JJ, Tschakovsky, ME. Exercise and circulating BDNF: mechanisms of release and implications for the design of exercise interventions. Appl Physiol Nutr Metabol 2018;43:1095–104. https://doi.org/10.1139/apnm-2018-0192.Suche in Google Scholar
29. Polacchini, A, Metelli, G, Francavilla, R, Baj, G, Florean, M, Mascaretti, LG, et al.. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep 2015;5:17989. https://doi.org/10.1038/srep17989.Suche in Google Scholar
30. Walsh, JJ, Caldwell, HG, Neudorf, H, Ainslie, PN, Little, JP. Short‐term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: a randomized cross‐over trial. J Physiol 2021;599:4763–78. https://doi.org/10.1113/jp281988.Suche in Google Scholar
31. Camm, AJ, Malik, M, Bigger, JT, Breithardt, G, Cerutti, S, Cohen, RJ, et al.. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–65.Suche in Google Scholar
32. Thijssen, DH, Bruno, RM, van Mil, AC, Holder, SM, Faita, F, Greyling, A, et al.. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019;40:2534–47. https://doi.org/10.1093/eurheartj/ehz350.Suche in Google Scholar
33. Van Bortel, LM, Laurent, S, Boutouyrie, P, Chowienczyk, P, Cruickshank, JK, De Backer, T, et al.. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–8. https://doi.org/10.1097/hjh.0b013e32834fa8b0.Suche in Google Scholar
34. Lakens, D, Caldwell, AR. Simulation-based power analysis for factorial analysis of variance designs. Adv Methods Pract Psychol Sci 2021;4:2515245920951503. https://doi.org/10.1177/2515245920951503.Suche in Google Scholar
35. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Available from: https://www.R-project.org/.Suche in Google Scholar
36. Cunningham, JE, Jones, SA, Eskes, GA, Rusak, B. Acute sleep restriction has differential effects on components of attention. Front Psychiatry 2018;9:499. https://doi.org/10.3389/fpsyt.2018.00499.Suche in Google Scholar
37. Klinzing, JG, Niethard, N, Born, J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 2019;22:1598–610. https://doi.org/10.1038/s41593-019-0467-3.Suche in Google Scholar
38. Rahmani, M, Rahmani, F, Rezaei, N. The brain-derived neurotrophic factor: missing link between sleep deprivation, insomnia, and depression. Neurochem Res 2020;45:221–31. https://doi.org/10.1007/s11064-019-02914-1.Suche in Google Scholar
39. Rahman, SA, Rood, D, Trent, N, Solet, J, Langer, EJ, Lockley, SW. Manipulating sleep duration perception changes cognitive performance – an exploratory analysis. J Psychosom Res 2020;132:109992. https://doi.org/10.1016/j.jpsychores.2020.109992.Suche in Google Scholar
40. Bojarskaite, L, Vallet, A, Bjørnstad, DM, Gullestad Binder, KM, Cunen, C, Heuser, K, et al.. Sleep cycle-dependent vascular dynamics in male mice and the predicted effects on perivascular cerebrospinal fluid flow and solute transport. Nat Commun 2023;14:953. https://doi.org/10.1038/s41467-023-36643-5.Suche in Google Scholar
41. Carr, JM, Hoiland, RL, Caldwell, HG, Coombs, GB, Howe, CA, Tremblay, JC, et al.. Internal carotid and brachial artery shear‐dependent vasodilator function in young healthy humans. J Physiol 2020;598:5333–50. https://doi.org/10.1113/jp280369.Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/teb-2025-0012).
© 2025 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Is cardiac autonomic modulation influenced by beta blockers in adolescents with Duchenne Muscular Dystrophy?
- Association of circulating Notch1 and VEGF with flow-mediated dilation and aerobic fitness in healthy adults
- Acute partial sleep restriction attenuates working memory performance and does not affect BDNF in young adults
- Can we run away from the metabolic side effects of antipsychotics?
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- What are the optimal mind-body therapies for cancer-related pain? A network meta-analysis
- Section: Exercise and E-health, M-health, AI and technology
- Readiness, recovery, and strain: an evaluation of composite health scores in consumer wearables
Artikel in diesem Heft
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Is cardiac autonomic modulation influenced by beta blockers in adolescents with Duchenne Muscular Dystrophy?
- Association of circulating Notch1 and VEGF with flow-mediated dilation and aerobic fitness in healthy adults
- Acute partial sleep restriction attenuates working memory performance and does not affect BDNF in young adults
- Can we run away from the metabolic side effects of antipsychotics?
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- What are the optimal mind-body therapies for cancer-related pain? A network meta-analysis
- Section: Exercise and E-health, M-health, AI and technology
- Readiness, recovery, and strain: an evaluation of composite health scores in consumer wearables


