Abstract
Introduction
Current international guidelines encourage cancer patients to engage in physical activity and recommend mind-body therapies (MBTs) as a method for treating cancer-related pain (CRP). However, the most effective MBTs for improving CRP in this population remain unknown. Therefore, this network meta-analysis (NMA) aimed to assess and rank the relative efficacy of different MBTs for CRP, and to conduct subgroup analyses according to different cancer types and stages of treatment.
Content
Eight electronic databases were searched for randomized controlled trials (RCTs) that compared different MBTs to improve pain in adults living with cancer. RCTs were evaluated using the Cochrane risk of bias tool. A random effects network meta-analysis was performed within a frequentist framework. Of the 4,916 articles retrieved and screened against the selection criteria. 36 studies with a total 2,387 participants were eligible to be included in the analysis. Qigong demonstrated significantly greater effects than Usual care (standardized mean difference [SMD] −0.85, 95 % confidence interval [CI] −1.46 to −0.24), Waitlist (SMD −0.93, −1.77 to −0.08), and Massage (SMD −1.71, −3.20 to −0.23), with the highest surface under the cumulative ranking value of 86.5 %, was ranked first. It was preceded by Conventional exercise (75.2 %), Taichi (74.9 %), with Massage having the lowest rank (7.2 %). In a subgroup analysis of breast cancer, Taichi (89.6 %), Conventional exercise (68.4 %), and Pilates (68.3 %) ranked as the top three.
Summary and outlook
This network meta-analysis indicates that Qigong and Tai Chi are among the most effective mind–body therapies (MBTs) for managing cancer-related pain and may serve as complementary adjuvant treatments for patients with cancer.
Introduction
Cancer significantly threatens global health, with projections estimating a 47 % increase in new cases to 28.4 million by 2040, compared to 2020. Breast cancer is notably the leading cancer type, accounting for 11.7 % of all diagnoses [1]. Cancer-related pain (CRP) is one of the most common, burdensome, and feared symptoms experienced by patients with cancer [2], 3]. During cancer treatment, up to 55 % of patients experienced severe pain. After treatment, 39.3 % of patients still suffered from pain [4]. More than 25 % of patients also reported clinically significant depression and anxiety symptoms [5]. This pain transcends mere physical sensation, evolving into a multidimensional syndrome that erodes patients’ quality of life. It simultaneously affects the sensory system, emotional state, cognitive function, and behavioral patterns [6], creating a vicious cycle of ‘pain-psychological distress-physical deterioration.
Contemporary management of CRP employs a dual-intervention approach combining pharmacologic and non-pharmacologic modalities [7]. Although opioids and antidepressants remain first-line pharmacotherapies, chemotherapy patients demonstrate significantly reduced adherence due to concerns about adverse effects, particularly addiction potential and gastrointestinal complications [8]. This paradigm has precipitated a transformative shift in clinical practice. The 2019 US. National Comprehensive Cancer Network (NCCN) guidelines notably incorporated mind-body therapies (MBTs) into standard CRP management protocols [9], [10], [11]. marking the evolution from a purely biomedical model to a biopsychosocial approach to pain care. This transition is grounded in seminal neuroscientific discoveries demonstrating dynamic bidirectional regulation between the brain and somatic systems through integrated neural networks [12]. However, MBTs primarily achieved a harmonious state of mind and body through the coordination of intention, respiration, and movement, thereby improving overall health conditions [13], and were considered potentially effective strategies for alleviating pain and its underlying psychosocial dynamics [14].
At present, studies have shown that psychologically oriented MBTs can effectively reduce CRP [15]. And compared with non-exercise or conventional nursing, cancer patients who engaged in exercise experienced more favorable pain outcomes, suggesting that MBTs may have offered superior analgesic effects on CRP [16], [17], [18]. However, critical knowledge gaps remain unresolved. While existing studies report inconsistent efficacy across various MBTs for CRP [19], [20], [21], systematic comparisons between different MBT modalities are notably absent. Significant research gaps persist in several clinically important subgroups, particularly among breast cancer patients who represent 11.7 % of all malignancies and constitute the largest subgroup in our network meta-analysis (NMA), yet optimal MBT approaches for this population remain undefined. Furthermore, with pain prevalence exceeding 30 % across both during treatment and post-treatment phases, the most effective MBT for different stages of cancer care have not been established. Thus, determining the most effective MBTs for CRP-afflicted cancer patients becomes paramount.
To address these challenges, conventional pairwise meta-analysis has proven insufficient, whereas NMA enables comparative efficacy ranking across multiple interventions by synthesizing both direct and indirect evidence. This innovative methodology constructs an interconnected intervention network and employs Bayesian statistical modeling to quantify hierarchy through surface under the cumulative ranking (SUCRA) probabilities [22], thereby generating objective efficacy rankings. Particularly suited for resolving the current MBTs research dilemma – identifying optimal interventions for CRP alleviation.
The summary of this article is presented in Figure 1.
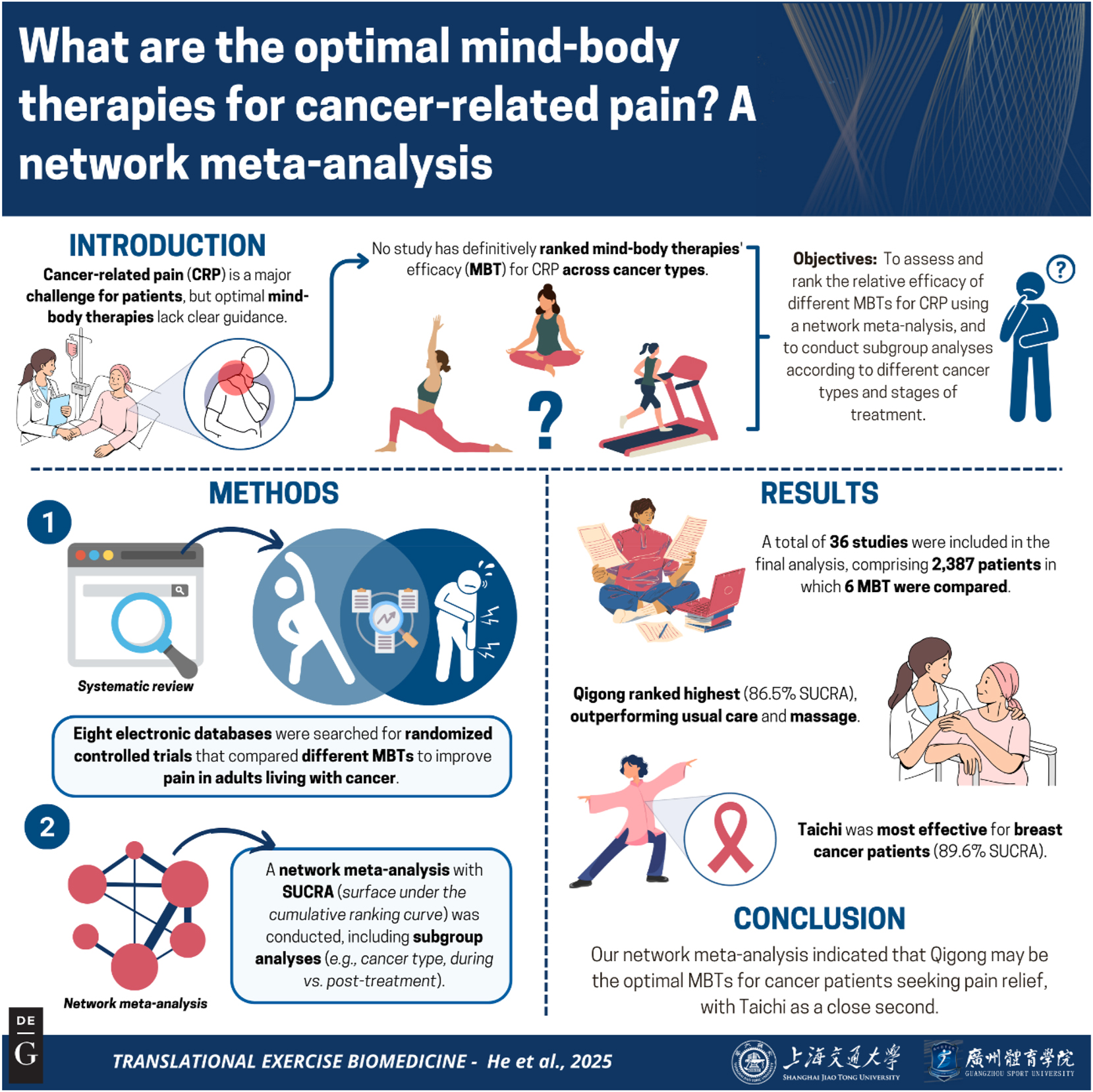
Graphical representation of this study. Key points: 1) Qigong demonstrated superior efficacy for cancer pain relief (SUCRA 86.5 %), providing an evidence-based complementary therapy for clinical adoption; 2) Taichi showed cancer-type specificity (89.6 % SUCRA in breast cancer), enabling precision pain management in oncology practice; 3) 36-trial evidence supports immediate integration of MBTs into: (a) palliative care protocols, (b) survivorship pain management programs. Figure created with BioRender.
Objectives
This study aimed to conduct a NMA of randomized controlled trials (RCTs) to (i) evaluate the comparative efficacy of different MBTs for CRP and establish their hierarchical ranking; and (ii) perform predefined subgroup analyses to identify optimal MBTs for breast cancer patients and patients at different treatment phases.
Methods
Registration
This NMA was registered with the International Prospective Register of Systematic Reviews (PROSPERO Reg No.–CRD42024508101). Further, the NMA was conducted with the following PRISMA statement guidelines (Supplementary Table 1) [23], 24].
Search strategy
Electronic searches were undertaken in the following databases: Cochrane Library, PubMed, Medline, Web of Science, ClinicalTrials.gov, SPORTDiscus, Embase and China National Knowledge Infrastructure (CNKI) published up to April 2024. The search strategy employed a blend of free-text words and subject heading terms for ‘pain’, ‘cancer’, ‘mind-body therapies’. The detailed search protocol for each database was available in Supplementary Table 2.
Eligibility criteria
Articles written in either Chinese or English were searched. The eligibility criteria were established using the Participant, Intervention, Comparison, Outcome, and Study design (PICOS) framework and are presented in Table 1. During screening, we identified Chinese-language theses meeting inclusion criteria; while acknowledging they undergo expert examination, we excluded them to maintain methodological consistency with peer-reviewed journal articles, which offer standardized reporting, verifiable methods, and greater accessibility. Additionally, review studies, abstracts from conferences, research protocols, book publications, and all peer-reviewed articles for which study data could not be acquired or translated were excluded. Non-randomized and single-group during/post intervention studies (with no comparison group) were ineligible.
Selection criteria.
| P – participants | Humans>18 years old who were diagnosed with any type of cancer and at any stage of treatment (either awaiting, undergoing or completed any form of cancer treatment) |
| I – interventions | Our analysis encompasses all forms of mind-body therapies (MBTs), including Yoga, Pilates, Taichi, Baduanjin, Qigong, Liuzijue, Wuqinxi, and dance therapy, with no restrictions imposed on intervention duration, intensity, or frequency. To prevent potential confounding effects from differential background treatments between MBTs and control groups on the network meta-analysis (NMA) outcomes, we systematically excluded studies that combined MBTs with other therapeutic interventions (e.g., pharmacotherapy or electrotherapy). |
| C – comparator |
|
| O – outcomes | The outcome of interest was pain. Any pain outcome assessed using any pain instrument or pain item/subscale on a non-pain instrument (e.g., pain subscale in a quality-of-life questionnaire) was eligible. |
| S –study | To investigate the efficacy of MBTs on pain intensity, we exclusively considered randomized controlled trials (RCTs) published in English or Chinese. |
Study selection
The Endnote X9 program for organizing research papers was employed to handle our search documentation. The process of picking relevant studies was split into three main steps. To start, two authors (X-YH and G-YL) looked over the collected papers just using their titles. If there was any uncertainty, the papers moved on to the next round where abstracts were considered. For the second step, every paper that made it past the initial screening was scrutinized by their abstracts and two separate authors decided if they were suitable. A consensus was reached between the two authors (X-YH and G-YL), and discrepancies were resolved by a third reviewer (X-HH). The agreement rate between reviewers was 0.94 (calculated using k statistics). Lastly, the papers that were still in the running were examined in full by the same two authors who had looked at the abstracts before, following the set rules for including studies. If there were any more disagreements, the whole group talked it through to find a solution.
Data extraction
Here, two authors (X-YH and G-YL) separately extracted data from each selected research, examined and amended by the corresponding author, including publishing details (e.g., author, year), research methodology (i.e., parallel or crossover trial), participant demographics (e.g., age, duration of pain, and sample size), treatment methods (e.g., Taichi, Yoga, Qigong), and outcome (i.e., pain intensity). Post-intervention mean and standard deviation (SD) were directly extracted from the result data of the publication. Notably, when the necessary information could not be sufficiently obtained, the study’s authors were contacted for the information. When we obtained standard errors (SEs), confidence intervals (Cls), p-value, or interquartile ranges (IQRs) instead of mean and SD, the RevMan 5.3 calculator was utilized to convert these to means and SDs. Besides, if data were presented solely in graphical form (rather than numerical data within the text), we used Engauge Digitizer software (version 10.8; developed by Mark Mitchell, USA) for data conversion.
Risk of bias assessment
Risk of bias was assessed independently by two authors (X-YH and G-YL) using version 2 of the Cochrane risk of bias tool (ROB 2) for RCTs [25]. The specific areas that were assessed for quality and bias appraisal were: (a) allocation, (b) performance, (c) follow-up, (d) measurement, (e) reporting, and (f) overall risk of bias. Each domain was categorized as presenting a “low,” “moderate,” or “high” risk of bias.
Certainty of evidence
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool was used to evaluate the quality of the evidence and make recommendations. The evidence base was classified into high, moderate, low, or very low-quality tiers, contingent upon factors such as study design, risk of bias, consistency of results, presence of indirect evidence, degree of imprecision, and potential publication bias.
Statistical analysis
The NMA was conducted when at least two studies exhibited consistency regarding population, intervention, and outcome characteristics [26], and was executed using Stata version 14.0, a frequentist statistical framework and following PRISMA guidelines for NMA [27]. A standardized mean difference (SMD) with a 95 % CI, along with random-effects models, were adopted. We employed a network map to illustrate the existing direct comparisons and furnish a visual summary of the data pertaining to the outcome. When encountering a closed loop, the split-node method was applied for a consistency check. A p-value > 0.05 indicated no considerable discrepancy between direct and indirect comparisons, and head-to-head comparisons were executed between various MBTs. The SUCRA value was determined to order the treatments for pain intensity. Two subgroup analyses were performed according to the patient’s cancer treatment stage and cancer type. This was done because breast cancer patients account for 11.7 % of all malignant tumors and constitute the largest subgroup in our NMA. Moreover, since pain incidence rates in both during-treatment and post-treatment phases exceed 30 % but the most effective MBT for these patients has not been established, we conducted these analyses. Sensitivity analyses were performed to evaluate the robustness of the research findings. Additionally, an adjusted funnel plot was constructed to detect potential publication bias.
Literature selection
We presented an overview of the extensive search and article selection process in Figure 2. After removing 1,798 duplicates from the initial 4,916 records, 3,118 unique articles remained for screening. Subsequently, two independent reviewers assessed the titles and abstracts, leading to the exclusion of 2,364 articles. Following a thorough examination of 754 articles. Ultimately, this study conducted a qualitative synthesis of 36 RCTs.
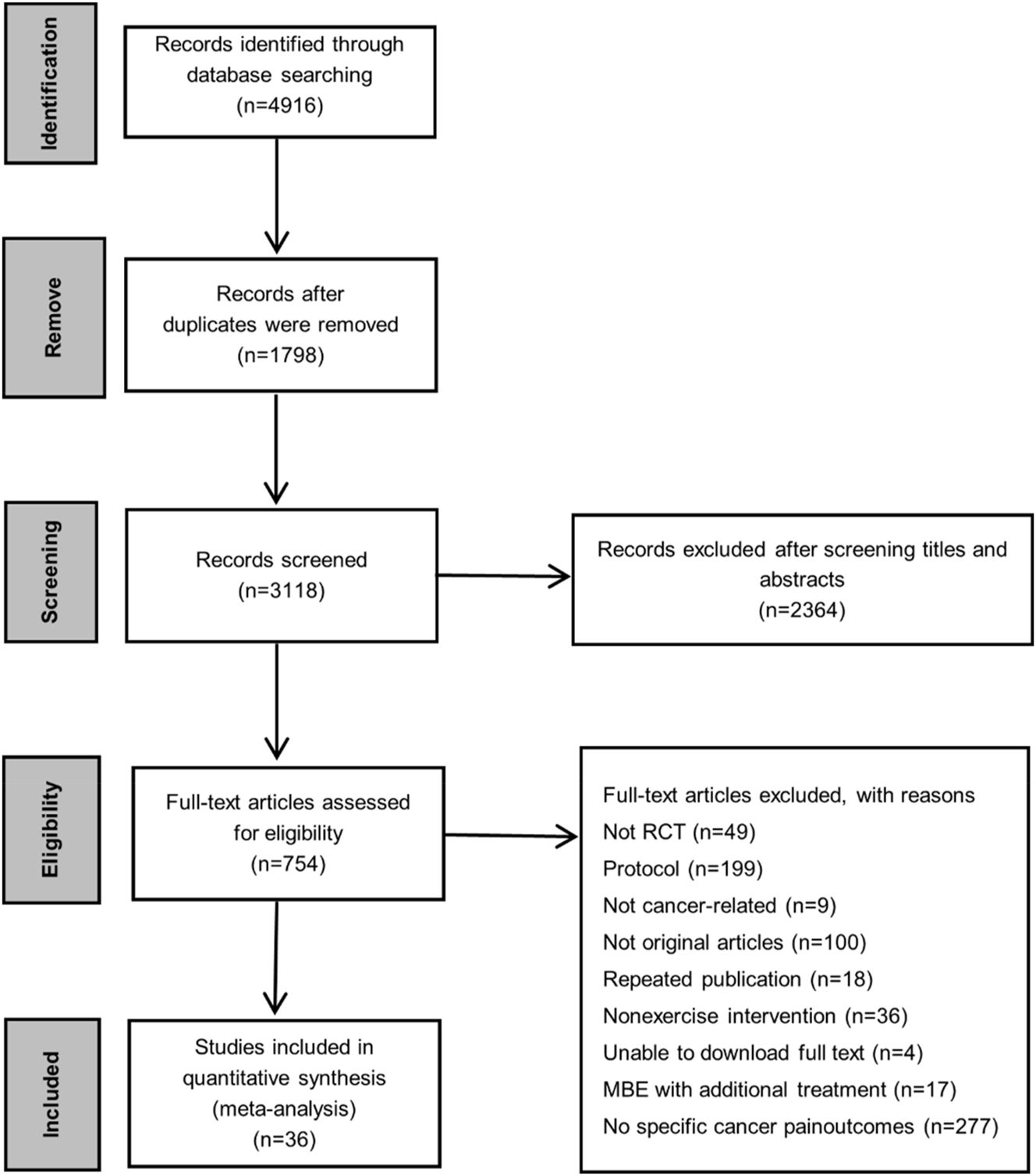
PRISMA flowchart of eligible studies selection. Abbreviations: RCT, randomized controlled trial; MBTs, mind-body therapies.
Characteristics of the included studies
The 36 research articles that were finally included spanned the period from 2008 to 2023 and involved a total of 2,387 cancer patients. The number of participants in each trial varied, with the smallest being 20 [28] and the largest 163 [29]. Among the various types of cancer, breast cancer was the predominant focus (n=25 studies). MBTs were performed during cancer treatment (n=20 studies) and after completion of cancer treatment (n=15 studies), respectively. A range of MBTs were implemented in the studies, encompassing Yoga [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48] (studies, n=20, participants, n=1,341), Qigong [49], [50], [51], [52], [53] (studies, n=5, participants, n=332), Taichi [28], 54], 55] (studies, n=3, participants, n=181), Pilates [56], [57], [58], [59] (studies, n=4, participants, n=227), Dance [60], [61], [62] (studies, n=3, participants, n=234), Baduanjin [63] (studies, n=1, participants, n=72). The control groups involved comparators such as Usual care, Conventional exercise, Massage, Waiting list, Health education. For further details, please refer to Table 2.
Characteristics of included studies.
| Author, year | Country | Sample size/ Age, year |
Type of cancer/ Stage of cancer/ Status of cancer |
Types of exercise (Intervention (I)/Control (C)) |
Exercise interventions |
Instrument for pain measurement |
Pain results (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| Tzu-Yun chuang et al. 2017 |
China | Control n=50 55.85 ± 16.78 Intervention n=50 64.54 ± 15.51 |
Non-hodgkin lymphoma/ II to IV/ During chemotherapy |
I: Qigong C: Usual care |
Length: 25 min/session Frequency: Two or three sessions/day Duration: Three weeks |
EORTC QLQ-C30 | Control: Pre=29.51 ± 9.25 Pos=30.9 ± 7.68 Intervention: Pre=25.00 ± 17.53 Pos=2.43 ± 10.87 |
| Chia‑LinTsai et al. 2021 |
China | Control n=30 52.8 ± 6.3 Intervention n=30 54.9 ± 6.4 |
Breast cancer/ I To III/ Only surgery±adjuvant therapy (radiation±chemotherapy ± plus both) |
I: Yoga C: Massage |
Length: 60 min/session Frequency: Two sessions/week Duration: Six weeks |
WOMAC | Control: Pre=9.7 ± 3.2 Pos=8.7 ± 4.2 Intervention: Pre=9.3 ± 2.8 Pos=4.2 ± 2.2 |
| Kavita D.Chandwani et al. 2014 | American | Waiting list n=54 52.11 ± 1.34 Conventional exercise n=56 51.14 ± 1.32 Intervention n=53 52.38 ± 1.35 |
Breast cancer/ 0 to III/ Scheduled to undergo daily Adjuvant XRT for 6 weeks |
I: Yoga C: Waiting list C: Conventional exercise |
Length: 60 min/session Frequency: Three sessions/week Duration: Six weeks |
SF-36 | Waiting list: Pre=44.6 ± 1.5 Pos=45.1 ± 1.4 Conventional exercise: Pre=44.8 ± 1.2 Pos=46.4 ± 1.3 Intervention: Pre=44.2 ± 1.4 Pos=44.3 ± 1.3 |
| Jingwen Liao et al. 2022 |
China | Control n=36 54.63 ± 8.44 Intervention n=36 53.12 ± 7.02 |
Breast cancer/ I To III/ Treated with AI More than 6 months |
I: Baduanjin C: Usual care |
Length: 90 min/session Frequency: Two sessions/week Duration: 12 weeks |
EORTC QLQ-C30 | Control: Pre =29.51 ± 23.17 Pos =23.37 ± 21.46 Intervention: Pre =28.36 ± 22.58 Pos =12.67 ± 13.12 |
| Sibel Eyigor et al. 2018 |
Turkey | Control n=20 51.5 ± 7.3 Intervention n=22 52.3 ± 9.5 |
Breast cancer/ No mention of staging/ Having completed surgical treatment, radio Therapy, and/or chemotherapy |
I: Yoga C: Usual care |
Length: 60 min/session Frequency: Two sessions/week Duration: 10 weeks |
VAS | Control: Pre =2.4 ± 3.2 Pos =1.1 ± 1.6 Intervention: Pre =2.7 ± 2.7 Pos =1.3 ± 1.8 |
| Ching‑I chang et al. 2023 |
China | Control n=33 52.77 ± 8.53 Intervention n=33 51.91 ± 10.51 |
Breast cancer/ II to III/ Completed chemotherapy after surgery |
I: Qigong C: Usual care |
Length: 55 min/session Frequency: Five sessions/week Duration: 15 weeks |
EORTC QLQ-C30 | Control: Pre =15 ± 18.23 Pos =12.22 ± 15.74 Intervention: Pre =20 ± 23.32 Pos =12.78 ± 23.03 |
| Miek C. Jong et al. 2018 |
Netherlands | Control n=36 51 ± 7.3 Intervention n=47 51 ± 8 |
Breast cancer/ I To III/ During chemotherapy |
I: Yoga C: Usual care |
Length: 75 min/session Frequency: One session/week Duration: 12 weeks |
EORTC QLQ-C30 | Control: Pre =26 ± 28 Pos =25 ± 25 Intervention: Pre =27 ± 25 Pos =25 ± 25 |
| Weimin Liu et al. 2022 |
China | Control n=68 NA Intervention n=68 NA |
Breast cancer/ I or II/ Receiving chemotherapy |
I: Yoga C: Usual care |
Length: 90 min/session Frequency: One session/week Duration: Eight weeks |
BPI | Control: Pre =2.31 ± 0.15 Pos =1.91 ± 0.17 Intervention: Pre =2.07 ± 0.17 Pos =1.69 ± 0.17 |
| Nilofar Pasyar et al. 2019 |
Iran | Control n=20 51.8 ± 11.4 Intervention n=20 51.6 ± 10.46 |
Breast cancer/ 0 to III/ At least 1-year had passed breast surgery |
I: Yoga C: Usual care |
Length: NA Frequency: Three sessions/week Duration: Eight weeks |
EORTC QLQ-C30 | Control: Pre=52.47 ± 33.89 Pos=23.31 ± 20.7 Intervention: Pre=81.59 ± 40.44 Pos=9.7 ± 14.99 |
| Hülya Özlem Şener et al. 2017 | Turkey | Control n=30 54.03 ± 12.57 Intervention n=30 53.17 ± 7.66 |
Breast cancer/ No mention of staging/ Completed Surgury+adjuvant therapy (radiotherapy±chemotherapy±hormonotherapy) |
I: Pilates C: Usual care |
Length: 60 min/session Frequency: Three sessions/week Duration: Eight weeks |
VAS | Control: Pre=2.3 ± 3.3 Pos=0.87 ± 1.43 Intervention: Pre=3.47 ± 3.18 Pos=0.67 ± 0.84 |
| S hosakote vadiraja et al. 2009 | India | Control n=44 NA Intervention n=44 NA |
Breast cancer/ I And III/ Receiving prescribed adjuvant radiotherapy |
I: Yoga C: Health education |
Length: 60 min/session Frequency: Three sessions/week Duration: Six weeks |
EORTC QLQ-C30 | Control: Pre=25.64 ± 20.13 Pos=26.92 ± 18.9 Intervention: Pre=32.64 ± 24.81 Pos=29.86 ± 24.07 |
| Laura S. Porter et al. 2020 | American | Control n=20 59.4 ± 11.3 Intervention n=43 56.3 ± 11.6 |
Breast cancer/ No mention of staging/ Receiving surgery±radiation ± chemotherapy |
I: Yoga C: Health education |
Length: 120 min/session Frequency: One session/week Duration: Eight weeks |
BPI | Control: Pre=3.4 ± 1.39 Pos=3.3 ± 1.82 Intervention: Pre=3.4 ± 2.11 Pos=2.7 ± 2.04 |
| Lisa K. Sprod et al. 2012 | American | Control n=9 52.70 ± 2.11 Intervention n=11 54.33 ± 3.55 |
Breast cancer/ 0 to III B/ Treatment completed more than 1 month prior |
I: Taichi C: Usual care |
Length: 60 min/session Frequency: Three sessions/week Duration: 12 weeks |
SF-36 | Control: Pre=9.1 ± 1.2 Pos=9.1 ± 1.74 Intervention: Pre=9.33 ± 1.32 Pos=9.11 ± 1.35 |
| Désirée Lötzke et al. 2016 | Germany | Control n=47 51.4 ± 11.1 Intervention n=45 51.0 ± 11.0 |
Breast cancer/ I To III/ Undergoing cytotoxic (neo)adjuvant or endocrine adjuvant therapy |
I: Yoga C: Conventional exercise |
Length: 60 min/session Frequency: One session/week Duration: 12 weeks |
EORTC QLQ-C30 | Control: Pre = −0.37 ± 30.46 Pos=1.41 ± 33.48 Intervention: Pre=1.13 ± 29.28 Pos=2.96 ± 30.41 |
| Jennifer huberty et al. 2019 | American | Control n=28 55.0 ± 11.4 Intervention n=34 58.3 ± 9.3 |
Myeloproliferative neoplasm/ No mention of staging/ Receiving Ruxolitinib/Other Janus Kinase-inhibitor treatment |
I: online Yoga C: Waiting list |
Length: 60 min/session Frequency: One session/week Duration: 12 weeks |
PROMIS | Control: Pre=40.4 ± 9 Pos=NA Intervention: Pre=45.1 ± 8.6 Pos=NA |
| Li-Hua Yang et al. 2021 | China | Control n=50 NA Intervention n=50 NA |
Gastrointestinal cancer/ II to IV/ Undergoing chemotherapy |
I: Qigong C: Usual care |
Length: 15–18 min/session Frequency: Five sessions/week Duration: Four weeks |
EORTC QLQ-C30 | Control: Pre=38.75 ± 16.18 Pos=53.91 ± 13.65 Intervention: Pre=43.33 ± 20.6 Pos=25.83 ± 15.07 |
| Naciye Vardar Yagli et al. 2015 | Turkey | Control n=10 68.88 ± 2.93 Intervention n=10 68.58 ± 6.17 |
Breast cancer/ I To II (A/B)/ At least 6 months had passed since Chemotherapy |
I: Yoga C: Conventional exercise |
Length: 60 min/session Frequency: One session/week Duration: Eight weeks |
VAS | Control: Pre=8.3 ± 1.01 Pos=2.16 ± 1 Intervention: Pre=7.93 ± 1.12 Pos=2.33 ± 0.98 |
| Robert Knoerl et al. 2022 | American | Median (range) Control n=16 60 (33–74) Intervention n=29 56.5 (40–79.0) |
Chemotherapy‑induced peripheral neuropathy pain/ I To IV/ At least 3 months post-taxane or Platinum-based chemotherapy |
I: Yoga C: Usual care |
Length: 45 min/session Frequency: One session/week Duration: Eight weeks |
PROMIS | Control: Pre=5.88 ± 1.27 Pos=4.48 ± 2.34 Intervention: Pre=5.94 ± 1.51 Pos=4.01 ± 2.1 |
| Annette Loudon et al. 2014 | Australia | Control n=11 60.5 ± 3.6 Intervention n=12 55.1 ± 2.5 |
Breast cancer/ I To III/ Completed treatment for breast cancer (surgery, radiotherapy and chemotherapy) at least 6 months previously |
I: Yoga C: Usual care |
Length: 90 min/session Frequency: One session/week Duration: Eight weeks |
VAS | Control: Pre=1.69 ± 2.31 Pos=1.44 ± 2.24 Intervention: Pre=0.99 ± 1.53 Pos=0.8 ± 1.48 |
| James W. Carson et al. 2009 | American | Control n=20 54.9 ± 6.2 Intervention n=17 53.9 ± 9.0 |
Breast cancer/ I A to II B/ Receiving at least one hot flash per day on four or more days per week |
I: Yoga C: Waiting list |
Length: 120 min/session Frequency: One session/week Duration: Eight weeks |
0–9 scale | Control: Pre=3.76 ± 0.05 Pos=4.35 ± 0.97 Intervention: Pre=3.69 ± 0.05 Pos=2.87 ± 0.97 |
| Keyla de Paula Barbosa et al. 2021 | Brazil | Median [CI 95 %] Conventional exercise n=20 54.0 [46.50; 60.75] Waiting list n=20 59.8 [46.0; 59.0] Intervention n=20 52.0 [47.25; 61.50] |
Breast cancer/ I To III/ Receiving surgery or Radiation or chemotherapy or hormonotherapy |
I: Pilates C: Conventional exercise C: Waiting list |
Length: 75 min/session Frequency: Two sessions/week Duration: Eight weeks |
NRS | Waiting list: Pre=5 ± 4.81 Pos=4.5 ± 5.88 Conventional exercise: Pre=5 ± 2.94 Pos=4.5 ± 4.27 Intervention: Pre=5 ± 3.74 Pos=3 ± 4 |
| A. Zengin Alpozgen et al. 2016 | Turkey | Conventional exercise n=19 51.94 ± 8.05 Usual care n=19 51.53 ± 13.81 Intervention n=19 46.22 ± 11.19 |
Breast cancer/ I To III/ Have completed radiotherapy or chemotherapy |
I: Pilates C: Conventional exercise C: Usual care |
Length: 45 min/session Frequency: Three sessions/week Duration: Eight weeks |
VAS | Conventional exercise: Pre=1.61 ± 1.82 Pos=0.22 ± 0.55 Usual care: Pre=1.97 ± 2.32 Pos=0.21 ± 0.71 Intervention: Pre=2.56 ± 2.53 Pos=0.5 ± 0.99 |
| Ting Bao et al. 2020 | American | Median (min, max) Control n=20 62.3 (42.4, 79.0) Intervention n=21 60.0 (35.5, 77.9) |
Breast cancer/ I To III/ Completed neurotoxic chemotherapy (e.g. paclitaxel, docetaxel, carboplatin) At least 3 months |
I: Yoga C: Usual care |
Length: 60 min/session Frequency: Seven sessions/week Two session face to face Five sessions online Duration: Eight weeks |
NRS | Control: Pre=3.4 ± 2.46 Pos=2.75 ± 1.81 Intervention: Pre=4.1 ± 2.45 Pos=2.15 ± 2.32 |
| Kamli Prakash et al. 2020 | India | Control n=52 NA Intervention n=48 NA |
Breast cancer/ No mention of staging/ Undergoing adjuvant chemotherapy Following surgery |
I: Yoga C: Usual care |
Length: NA Frequency: Two sessions/day Duration: 18 weeks |
EORTC QLQ-C30 | Intervention: Pre=22.693 ± 14.682 Pos =28.372 ± 22.658 Control: Pre=25.436 ± 13.94 Pos =35.757 ± 23.351 |
| B. L. Vanderbyl et al. 2017 | Canada | Control n=17 63.7 ± 7.7 Intervention n=19 66.1 ± 11.7 |
Advanced non-small Cell Lung cancer (NSCLC) or gastrointestinal (GI) cancer/ Ⅲ to Ⅳ/ Receiving chemotherapy |
I: Qigong C: Conventional exercise |
Length: 45 min/session Frequency: Two sessions/week Duration: Six weeks |
11-Point Likert-type scale (0–10) | Control: Pre=NA Pos=NA Intervention: Pre=NA Pos=NA |
| Maria Pisu et al. 2017 | American | Control n=16 59 ± 10 Intervention n=15 56.7 ± 8.6 |
Mostly breast cancer/ No mention of staging/ Surgery+chemotherapy±radiation±other |
I: Dance C: Waiting list |
Length: 45 min/session Frequency: One session/week Duration: 12 weeks |
SF-36 | Control: Pre=78.4 ± 23.4 Pos=71.5 ± 22.5 Intervention: Pre=77.1 ± 20.2 Pos=76.9 ± 15.2 |
| Rainbow T. H. Ho et al. 2015 | China | Control n=70 49.1 ± 8.7 Intervention n=69 48.6 ± 7.7 |
Breast cancer/ 0 to III/ Awaiting the initiation of RT or During the first week of adjuvant RT |
I: Dance C: Waiting list |
Length: 90 min/session Frequency: Two sessions/week Duration: Three weeks |
BPI | Control: Pre=4.9 ± 2.2 Pos=4.6 ± 2.3 Intervention: Pre=5.3 ± 2.3 Pos=4.9 ± 1.9 |
| Mohammad Namazinia et al. 2023 | Iran | Control n=39 45.2 ± 12.6 Intervention n=39 49.0 ± 9.6 |
No Restriction on the type/ No mention of staging/ Undergoing four sessions of chemotherapy per month |
I: Laughter Yoga C: Usual care |
Length: 20–30 min/session Frequency: Every week apart Duration: Eight weeks |
EORTC QLQ-C30 | Control: Pre=37.62 ± 33.66 Pos=38.57 ± 31.25 Intervention: Pre=27.45 ± 31.48 Pos=19.12 ± 26.31 |
| María Alejandra Rubio et al. 2023 | Colombia | Control n=33 55.84 ± 10.32 Intervention n=31 57.02 ± 8.70 |
Breast cancer/ No mention of staging/ Cancer-related treatment has been completed |
I: Dance C: Usual care |
Length:45–60 min/session Frequency: Three sessions/week Duration: Eight weeks |
EORTC QLQ-C30 | Control: Pre=12.64 ± 23.42 Pos=12.64 ± 23.42 Intervention: Pre=22.67 ± 19.77 Pos=22.67 ± 19.77 |
| Melissa Adair et al. 2018 | China | Control n=20 61.8 ± 9.2 Intervention n=15 65.0 ± 7.4 |
Head and neck cancer/ I To IV/ >3 months post HNC treatment |
I: Yoga C: Waiting list |
Length: 30–90 min/session Frequency: Three sessions/week Duration: Four weeks |
BPI | Control: Pre=2 ± 3.7 Pos=4.3 ± 6.5 Intervention: Pre=3.2 ± 5.9 Pos=1.5 ± 2.4 |
| Leonessa Boing et al. 2023 | Brazil | Control n=25 55.0 ± 9.9 Intervention n=25 54.3 ± 10.4 |
Breast cancer/ I To III/ Receiving hormone therapy |
I: Pilates C: Dance |
Length: 60 min/session Frequency: Three sessions/week Duration: 16 weeks |
VAS | Control: Pre=3.7 ± 0.5 Pos=3.4 ± 0.5 Intervention: Pre=3.2 ± 0.5 Pos=2.7 ± 0.5 |
| Byeongsang Oh et al. 2008 | Australia | Control n=15 NA Intervention n=15 NA |
No Restriction on the type/ Be at any stage of cancer/ Currently ongoing chemotherapy or Completed the cancer treatment |
I: Qigong C: Usual care |
Length: 90 min/session Frequency: One or two sessions/week Duration: Eight weeks |
EORTC QLQ-C30 | Control: Pre=NA Pos=NA Intervention: Pre=NA Pos=NA |
| Kavita D et al. 2010 | American | Control n=31 40.2 ± 9.96 Intervention n=30 51.39 ± 7.97 |
Breast cancer/ 0 to III/ Scheduled to undergo radiotherapy |
I: Yoga C: Waiting list |
Length: 60 min/session Frequency: Two sessions/week Duration: Six weeks |
SF-36 | Control: Pre=61.5 ± 4.4 Pos=58.5 ± 7.0 Intervention: Pre=62.3 ± 4.3 Pos=63.5 ± 6.8 |
| Rebecca A. Campo et al. 2013 | American | Mdn (LL, UL) Control n=31 65.64 (57–84) Intervention n=32 66.54 (55–89) |
Breast cancer/ I To III/ ≥3 months since completing treatment |
I: Taichi C: Health education |
Length: 60 min/session Frequency: Three sessions/week Duration: 12 weeks |
SF-36 | Control: Pre=53.45 ± 8.4 Pos=53.76 ± 9.64 Intervention: Pre=51.88 ± 5.18 Pos=62.12 ± 5.68 |
| Yuehua ding et al. 2020 | China | Control n=49 50.80 ± 7.60 Intervention n=49 53.20 ± 6.80 |
Tumor patients with PICC/ No mention of staging/ Receiving chemotherapy |
I: Tai chi C: Conventional exercise |
Length: 60 min/session Frequency: Five sessions/week Duration: Five weeks |
SF-36 | Control: Pre=62.83 ± 14.73 Pos=64.12 ± 13.4 Intervention: Pre=67.88 ± 12.52 Pos=68.83 ± 14.21 |
| Ryan Eckert et al. 2022 | American | Control n=39 NA Intervention n=33 NA |
Allogenic bone marrow transplant/ No mention of staging/ Bone marrow transplantation>6 months |
I: Yoga C: Health education |
Length: 60 min/session Frequency: One session/week Duration: 12 weeks |
PROMIS | Control: Pre=47.6 ± 18.2 Pos=48.1 ± 17.43 Intervention: Pre=47.9 ± 16.36 Pos=46.1 ± 15.51 |
-
VAS, visual analogue scale; NRS, numeric rating scale; BPI, brief pain inventory; EORTC QLQ-C30, European Organization for reasearch and treatment of cancer quality of life Questionnare-core 30; SF-36, The MOS item short fromhealth survey; PROMIS, patient-reported outcomes measurement information system; WOMAC, western ontario and mcMaster universities arthritis index; XRT, X-ray diffraction topography; NA, not available.
Results of ROB and GRADE
The findings from the ROB2 assessment for each study were shown in Supplementary Figures 1 and 2. Fourteen studies (38.9 %) possessed a high risk of bias, nine studies (25 %) had some concerns, and 13 studies (36.1 %) possessed a low risk of bias. Specifically, problems with randomization or assignment concealment, or differences in baselines between the two groups, three studies were classified as high risk of randomization process bias. Due to the presence of illness, death or unexplained withdrawal, the disengagement rate is too high, three studies were classified as high risk of missing outcome data bias.
The evidence quality for pairwise meta-analysis was appraised utilizing the GRADE framework, with the results detailed in Supplementary Table 3. The evidence quality was deemed high for four comparisons (10.1 %), moderate for seven comparisons (17.9 %), low for 10 comparisons (25.6 %), and very low for 18 comparisons (46.4 %).
Pairwise meta-analysis
A pairwise meta-analysis was conducted to assess the comparative efficacy of two interventions, utilizing pooled effect sizes. 16 direct comparisons were performed to use a random effect model. Pilates (three RCTs; SMD: −0.33, 95 % CI [−0.67, 0.00]; I 2<50 %) and Yoga (nine RCTs; SMD: −0.51, 95 % CI [−1.01 −0.01]; I 2≥50 %) were more efficacious than Usual care, but Qigong, Baduanjin, Dance and Taichi did not show a significant difference. Direct comparisons between yoga and Conventional exercise, Waiting list, Health education revealed no significant differences. Regarding breast cancer outcomes, 14 direct comparisons were established. Pilates showed superior efficacy compared to Usual care (three RCTs; SMD: −0.33, 95 % CI [−0.67, 0.00]), while Qigong, Yoga, Baduanjin, Dance, and Taichi did not significantly differ from Usual care. Supplementary Table 4 offered a detailed breakdown of direct comparisons for subgroups based on the stages of treatment.
Assessment of heterogeneity, transitivity, and inconsistency
Results of heterogeneity in direct pairwise comparisons are presented in Supplementary Table 4, along with the forest plot of network meta-analysis for pain intensity (Supplementary Figure 4). Overall, significant heterogeneity was observed in comparisons including Qigong vs. Usual care (I2=94 %, p=0.08), Yoga vs. Usual care (I2=86 %, p=0.04), and Yoga vs. Conventional exercise (I2=86 %, p=0.19), while most direct pairwise comparisons showed no statistically significant heterogeneity. We rigorously implemented predefined inclusion and exclusion criteria for eligible studies. Furthermore, Supplementary Table 5 comprehensively documents transferability considerations, including intervention duration, frequency, and population characteristics, supporting the validity of the transitivity assumption. No significant inconsistency was detected at either global or local levels in the network meta-analysis (Supplementary Table 6).
Network meta‐analysis
As depicted in Figure 3(a–d), each node signifies a distinct MBT, with the dimensions of the nodes reflecting the participant count. Direct comparisons between pairs of MBTs were marked by connecting lines, where the line’s thickness corresponds to the prevalence of studies comparing those two types. The analysis proceeded using a consistency model. For detailed information on the consistency tests, see Supplementary Table 6.
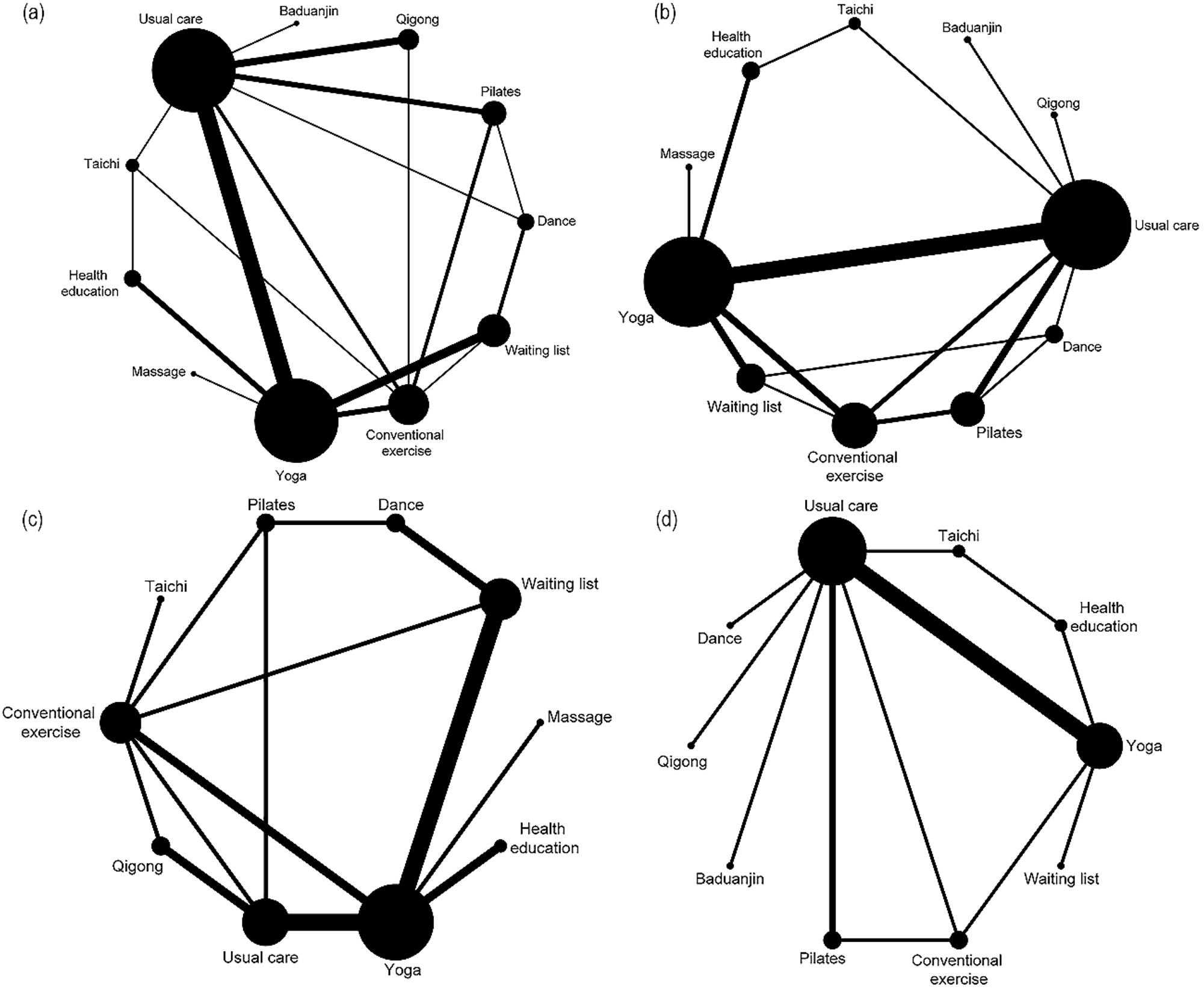
The comparative network diagram of various MBTs. Legend: (a) for various cancer types. (b) For the breast cancer subgroup. (c) During cancer treatment. (d) Post cancer treatment. The evidence network for pain intensity is such that the size of the nodes corresponds to the number of participants in each intervention type. Moreover, the thickness of the lines connecting different interventions indicates the number of studies conducted for that specific comparison.
As shown in Table 3(a), Qigong (SMD: −0.85, 95 % CI [−1.46, −0.24]), (SMD: −0.93, 95 % CI [−1.77, −0.08]), (SMD: −1.71, 95 % CI [−3.20, −0.23]), and Conventional exercise (SMD: −0.62, 95 % CI [−1.16, −0.07]), (SMD: −0.70, 95 % CI [−1.38, −0.01]), (SMD: −1.48, 95 % CI [−2.89, −0.07]), were found to be more efficacious than Usual care, Waiting list, Massage control group based on the results of consistency NMA.
Combine direct and indirect comparison results from the network meta-analysis.
| a | ||||||||||
| Qigong | 0.69 (−0.14,1.53) |
NA | NA | NA | NA | NA | NA | −1.02 (−2.17,0.13) |
NA | NA |
| −0.23 (−0.98,0.52) |
Conventional exercise |
0.02 (−0.37,0.42) |
−0.85 (−1.97,0.26) |
NA | 0.55 (−0.27,1.37) |
NA | NA | NA | NA | NA |
| −0.19 (−1.22,0.83) |
0.04 (−0.82,0.90) |
Taichi | NA | NA | NA | NA | −1.33 (−1.93,−0.74) |
0.14 ( −0.76,1.05) |
NA | NA |
| −0.37 (−1.25,0.51) |
−0.14 (−0.87,0.59) |
−0.18 (−1.21,0.85) |
Pilates | NA | NA | −0.08 (−0.73,0.58) |
NA | −0.33
(−0.67, 0.00) |
NA | NA |
| −0.40 (−1.81,1.01) |
−0.17 (−1.55,1.21) |
−0.21 (−1.74,1.32) |
−0.03 (−1.46,1.40) |
Baduanjin | NA | NA | NA | −0.45 (−0.93,0.03) |
NA | NA |
| −0.46 (−1.17,0.24) |
−0.23 (−0.77,0.30) |
−0.27 (−1.13,0.58) |
−0.09 (−0.81,0.62) | −0.06 (−1.39,1.27) |
Yoga | NA | −0.15 (−0.43,0.13) |
−0.51
(−1.01,−0.01) |
−0.43 (−0.98,0.12) |
−1.25 (−1.82,−0.68) |
| −0.77 (−1.73,0.18) |
−0.54 (−1.39,0.30) |
−0.58 (−1.67,0.51) |
−0.40 (−1.25,0.45) |
−0.37 (−1.85,1.11) |
−0.31 (−1.07,0.45) |
Dance | NA | 0.00 (−0.53,0.53) |
−0.32
(−0.63,−0.02) |
NA |
| −0.81 (−1.77,0.14) |
−0.58 (−1.40,0.24) |
−0.62 (−1.54,0.29) |
−0.44 (−1.40,0.52) | −0.41 (−1.89,1.06) |
−0.35 (−1.02,0.32) |
−0.04 (−1.05,0.96) |
Health education |
NA | NA | NA |
| −0.85 (−1.46,−0.24) |
−0.62
(−1.16,−0.07) |
−0.66 (−1.51,0.20) |
−0.48 (−1.13,0.18) |
−0.45 (−1.72,0.82) |
−0.38
(−0.77,0.00) |
−0.08 (−0.83,0.68) |
−0.03 (−0.79,0.72) |
Usual care | NA | NA |
| −0.93 (−1.77,−0.08) |
−0.70
(−1.38,−0.01) |
−0.74 (−1.71,0.24) | −0.56 (−1.37,0.26) |
−0.53 (−1.94,0.88) |
−0.46 (−1.00,0.07) |
−0.16 (−0.88,0.57) |
−0.11 (−0.96,0.74) | −0.08 (−0.69,0.53) | Waiting list | NA |
| −1.71 (−3.20,−0.23) |
−1.48
(−2.89,−0.07) |
−1.52 (−3.08,0.04) | −1.34 (−2.83,0.15) |
−1.31 (−3.18,0.55) |
−1.25 (−2.55,0.06) |
−0.94 (−2.45,0.57) |
−0.90 (−2.37,0.57) |
−0.86 (−2.23,0.50) |
−0.79 (−2.20,0.63) |
Massage |
| b | ||||||||||
| Taichi | NA | NA | NA | NA | NA | 0.14 (−0.76,1.05) |
−1.33 (1.93,−0.74) |
NA | NA | NA |
| −0.19 (−1.42,1.03) |
Conventional exercise |
−0.36 (−0.82,0.09) |
NA | 0.55 (−0.27,1.37) |
NA | NA | NA | NA | NA | NA |
| −0.18 (−1.46,1.09) |
0.01 (−0.75,0.77) |
Pilates | NA | NA | −0.08 (−0.73,0.58) |
−0.33
(−0.67,0.00) |
NA | NA | NA | NA |
| −0.18 (−1.86,1.50) |
0.01 (−1.40,1.42) |
0.00 (−1.43,1.43) |
Baduanjin | NA | NA | −0.45 (−0.93,0.03) |
NA | NA | NA | NA |
| −0.30 (−1.41,0.81) | −0.11 (−0.70,0.48) |
−0.11 (−0.85,0.62) | −0.12 (−1.45,1.22) | Yoga | NA | −0.58 (−1.22,0.06) |
−0.15 (−0.53,0.22) |
NA | −0.37 (−1.22,0.27) |
−4.10 (−5.77,−2.43) |
| −0.45 (−1.81,0.90) |
−0.26 (−1.20,0.67) |
−0.27 (−1.16,0.62) |
−0.27 (−1.78,1.24) |
−0.16 (−1.01,0.70) | Dance | 0.00 (−0.53,0.53) |
NA | NA | −0.32 (−0.66,0.01) |
NA |
| −0.63 (−1.73,0.47) |
−0.44 (−1.06,0.18) |
−0.45 (−1.11,0.22) | −0.45 (−1.71,0.82) |
−0.33 (−0.77,0.11) |
−0.18 (−1.00,0.65) |
Usual care | NA | 0.22 (−0.29,0.72) |
NA | NA |
| −0.72 (−1.78,0.34) |
−0.52 (−1.52,0.48) |
−0.53 (−1.62,0.55) |
−0.53 (−2.09,1.02) |
−0.42 (−1.24,0.41) | −0.26 (−1.43,0.91) |
−0.09 (−0.98,0.81) |
Health education |
NA | NA | NA |
| −0.84 (−2.52,0.84) |
−0.65 (−2.06,0.76) |
−0.66 (−2.09,0.78) |
−0.66 (−2.45,1.13) |
−0.54 (−1.89,0.80) |
−0.39 (−1.90,1.12) | −0.21 (−1.48,1.06) |
−0.13 (−1.68,1.43) |
Qigong | NA | NA |
| −0.78 (−2.05,0.50) |
−0.58 (−1.37,0.21) |
−0.59 (−1.49,0.31) |
−0.59 (−2.06,0.87) | −0.48 (−1.14,0.19) | −0.32 (−1.20,0.56) |
−0.14 (−0.88,0.59) | −0.06 (−1.11,0.99) |
0.07 (−1.40,1.53) |
Waiting list | NA |
| −1.55 (−3.26,0.16) |
−1.36 (−2.78,0.07) |
−1.36 (−2.86,0.13) | −1.36 (−3.23,0.50) |
−1.25 (−2.55,0.05) |
−1.09 (−2.65,0.46) |
−0.92 (−2.29,0.46) |
−0.83 (−2.37,0.71) |
−0.71 (−2.57,1.16) |
−0.77 (−2.23,0.69) |
Massage |
| c | |||||||||
| Qigong | 0.69 (−0.14,1.53) |
NA | NA | NA | NA | NA | NA | −1.92
(−2.28,−1.56) |
NA |
| −0.43 (−1.44,0.57) |
Conventional exercise |
0.02 (−0.37,0.42) |
−0.38 (−1.01,0.24) |
0.57 (−0.55,1.69) |
NA | NA | NA | NA | NA |
| −0.46 (−2.14,1.23) |
−0.02 (−1.38,1.33) |
Taichi | NA | NA | NA | NA | NA | NA | NA |
| −0.69 (−2.03,0.66) |
−0.26 (−1.38,0.87) |
−0.23 (−1.99,1.53) |
Pilates | NA | −0.08 (−0.73,0.58) |
NA | NA | −0.31 (−0.94,0.31) |
NA |
| −0.83 (−1.81,0.16) |
−0.39 (−1.14,0.35) |
−0.37 (−1.91,1.17) |
−0.14 (−1.26,0.98) |
Yoga | NA | −0.15 (−0.53,0.22) |
−0.37 (−1.01,0.27) |
−0.62 (−1.50,0.26) |
−1.25 (−1.82,−0.68) |
| −0.87 (−2.23,0.49) | −0.44 (−1.59,0.71) |
−0.42 (−2.19,1.36) |
−0.19 (−1.31,0.94) |
−0.05 (−1.09,1.00) |
Dance | NA | −0.32
(−0.63,−0.02) |
NA | NA |
| −0.95 (−2.35,0.44) | −0.52 (−1.75,0.71) |
−0.50 (−2.33,1.33) |
−0.27 (−1.76,1.22) |
−0.13 (−1.11,0.85) |
−0.08 (−1.52,1.36) |
Health education |
NA | NA | NA |
| −1.24 (−2.38,−0.11) |
−0.81 (−1.69,0.06) |
−0.79 (−2.40,0.83) |
−0.56 (−1.69,0.58) |
−0.42 (−1.08,0.25) |
−0.37 (−1.27,0.53) |
−0.29 (−1.48,0.90) |
Waiting list | NA | NA |
| −1.45 (−2.31,−0.59) | −1.02 (−1.82,−0.22) |
−1.00 (−2.57,0.58) |
−0.76 (−1.88,0.35) |
−0.63 (−1.24,−0.02) |
−0.58 (−1.71,0.56) |
−0.50 (−1.65,0.66) |
−0.21 (−1.06,0.64) |
Usual care | NA |
| −2.07 (−3.80,−0.35) | −1.64 (−3.24,−0.05) |
−1.62 (−3.71,0.48) |
−1.39 (−3.19,0.42) |
−1.25 (−2.66,0.17) |
−1.20 (−2.96,0.56) |
−1.12 (−2.84,0.60) |
−0.83 (−2.39,0.73) |
−0.62 (−2.16,0.92) |
Massage |
| d | |||||||||
| Taichi | NA | NA | NA | NA | NA | 0.14 (−0.76,1.05) |
NA | −1.33 (−1.93,−0.74) |
NA |
| −0.12 (−1.32,1.08) |
Pilates | NA | NA | −0.34 (−1.00,0.32) |
NA | −0.34 (−0.74,0.06) |
NA | NA | NA |
| −0.08 (−1.46,1.31) | 0.04 (−1.17,1.26) |
Baduanjin | NA | NA | NA | −0.45 (−0.93,0.03) |
NA | NA | NA |
| −0.31 (−1.30,0.68) |
−0.19 (−1.02,0.63) |
−0.23 (−1.32,0.85) |
Yoga | 0.50 (−0.39,1.39) |
NA | −0.40 (−0.82,0.02) |
NA | −0.13 (−0.60,0.33) |
−0.72 (−1.41,−0.03) |
| −0.30 (−1.55,0.94) |
−0.19 (−1.09,0.72) |
−0.23 (−1.50,1.04) |
0.01 (−0.82,0.84) |
Conventional exercise |
NA | NA | NA | NA | NA |
| −0.53 (−1.93,0.88) |
−0.41 (−1.64,0.83) |
−0.45 (−1.86,0.96) |
−0.21 (−1.33,0.90) |
−0.22 (−1.51,1.07) |
Dance | 0.00 (−0.53, 0.53) |
NA | NA | NA |
| −0.53 (−1.50,0.45) |
−0.41 (−1.12,0.31) |
−0.45 (−1.43,0.54) |
−0.21 (−0.68,0.25) |
−0.22 (−1.02,0.58) |
0.00 (−1.01,1.01) |
Usual care | 0.22 (−0.29, 0.72) |
NA | NA |
| −0.74 (−2.13,0.65) |
−0.62 (−1.85,0.60) |
−0.66 (−2.06,0.74) |
−0.43 (−1.53,0.67) |
−0.44 (−1.71,0.84) |
−0.21 (−1.63,1.20) |
−0.21 (−1.21,0.78) |
Qigong | NA | NA |
| −0.86 (−1.74,0.03) |
−0.74 (−1.88,0.40) |
−0.78 (−2.12,0.56) |
−0.55 (−1.40,0.31) |
−0.55 (−1.72,0.61) |
−0.33 (−1.69,1.03) |
−0.33 (−1.24,0.58) |
−0.12 (−1.47,1.23) |
Health education |
NA |
| −1.03 (−2.51,0.45) |
−0.91 (−2.29,0.47) |
−0.95 (−2.50,0.60) |
−0.72 (−1.82,0.38) |
−0.73 (−2.11,0.65) |
−0.50 (−2.07,1.06) |
−0.50 (−1.70,0.69) |
−0.29 (−1.85,1.27) |
−0.17 (−1.57,1.22) |
Waiting list |
-
(a) For various cancer types. (b) For the breast cancer subgroup. (c) During cancer treatment. (d) Post cancer treatment. Data are effect sizes (95 % confidence intervals). Effect sizes in bold are statistically significant. A positive value means the first pain – treatment intervention is better. The upper – right triangle shows pooled effect sizes from pairwise comparisons (column vs. row), and the lower – left from network meta – analysis (row vs. column). NA, not available.
As shown in Table 3(b–d), illustrated the pairwise comparison of interventions between the breast cancer subgroup, the during treatment subgroup, and the post-treatment subgroup, respectively. In Table 3(c), Qigong (SMD: −1.24, 95 % CI [−2.38, −0.01]), (SMD: −1.45, 95 % CI [−2.31, −0.59]), (SMD: −2.07, 95 % CI [−3.80, −0.35]) was found to be more effective than Usual care, Waiting list, Massage control group. In Table 3(b and d), the direct comparisons revealed no noteworthy disparities.
Rank probabilities
The comparative effectiveness of various MBTs in alleviating CRP was evaluated, with the SUCRA curve illustrating the rankings. Figure 4(a) shown as follows: Qigong (SMD=1.02, 95 % CI [−2.17, 0.13]) was ranked first (SUCRA=86.5 %)> Conventional exercise (SUCRA=75.2 %) > Taichi (SUCRA=74.9 %) > Pilates (SUCRA=64.6 %)> Baduanjin (SUCRA=59.5 %) > Yoga (SUCRA=59.3 %) > Dance (SUCRA=36.7 %) > Health education (SUCRA=33.5 %)> Usual care (SUCRA=28 %)> Waiting list (SUCRA=24.6 %)> Massage (SUCRA=7.2 %).
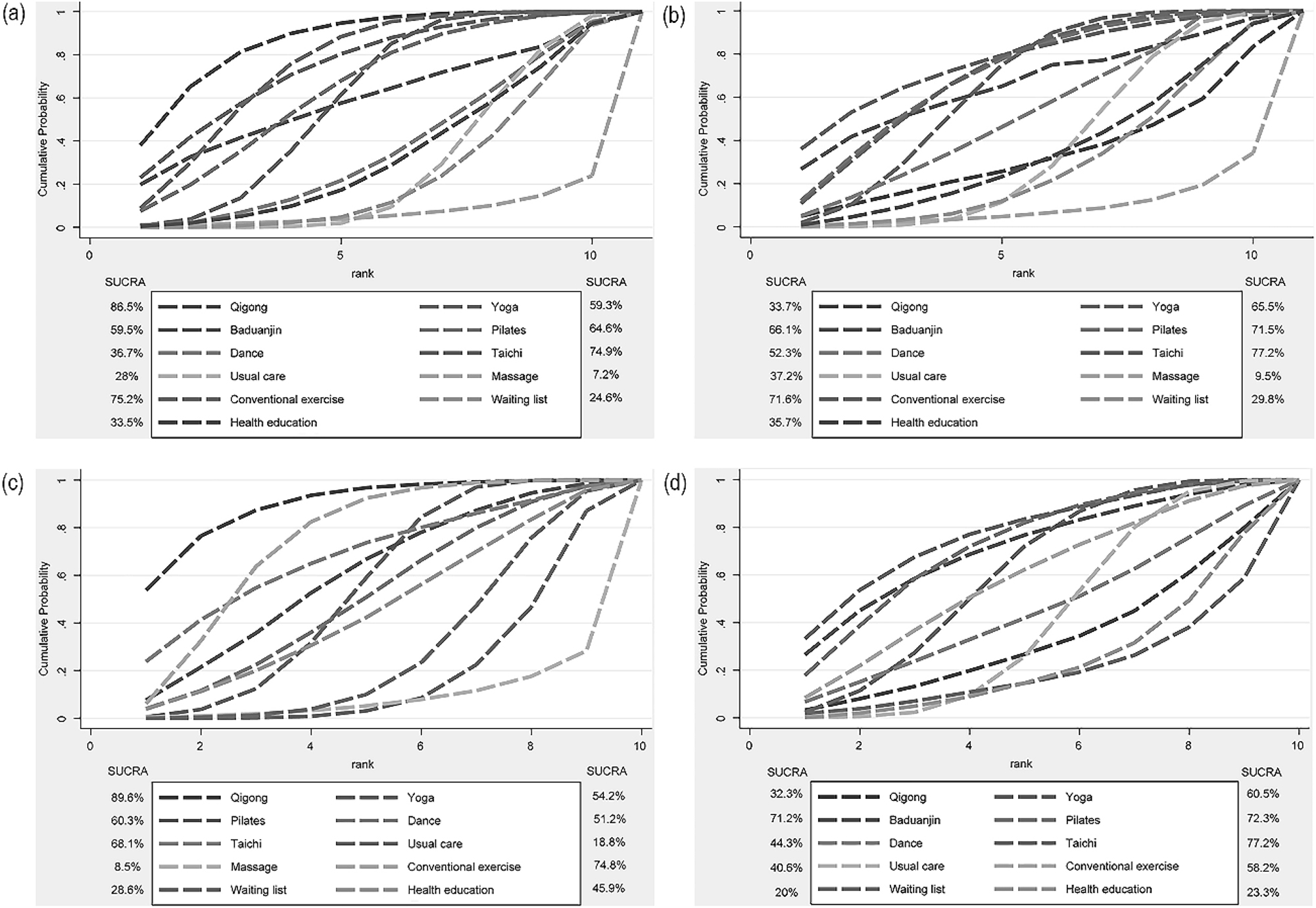
The ranking probability results of different MBTs in terms of improving pain intensity. Legend: (a) for various cancer types. (b) For the breast cancer subgroup. (c) During cancer treatment. (d) Post cancer treatment. A larger area under the curve indicated a more effective intervention.
The relative efficacy of various MBTs in reducing pain intensity in breast cancer patients and those during or post cancer treatment, were as follows: in breast cancer patients the highest ranked SUCRA was Taichi (SMD=0.14, 95 % CI [−0.76, 1.05]), ranked first (SUCRA=89.6 %), in patients during cancer treatment the highest ranked SUCRA was Qigong (SMD= −1.92, 95 % CI [−2.28, −1.56]), (SUCRA=89.6 %), and in patients post cancer treatment the highest ranked SUCRA was Taichi (SMD=0.14, 95 % CI [−0.76, 1.05]), (SUCRA=77.2 %). Further insights were presented in Figure 4(b–d).
Publication bias
We constructed and evaluated an adjusted funnel chart to identify any potential publication bias across all metrics. The results suggest the reliability of our findings. For additional information, refer to Figure 5(a–d). Moreover, the absence of small-study effects was confirmed by the Egger’s regression test, which yielded a non-significant p-value (In the four funnel plots, the corresponding p-values were p=0.732, p=0.648, p=0.611, p=0.956, respectively).
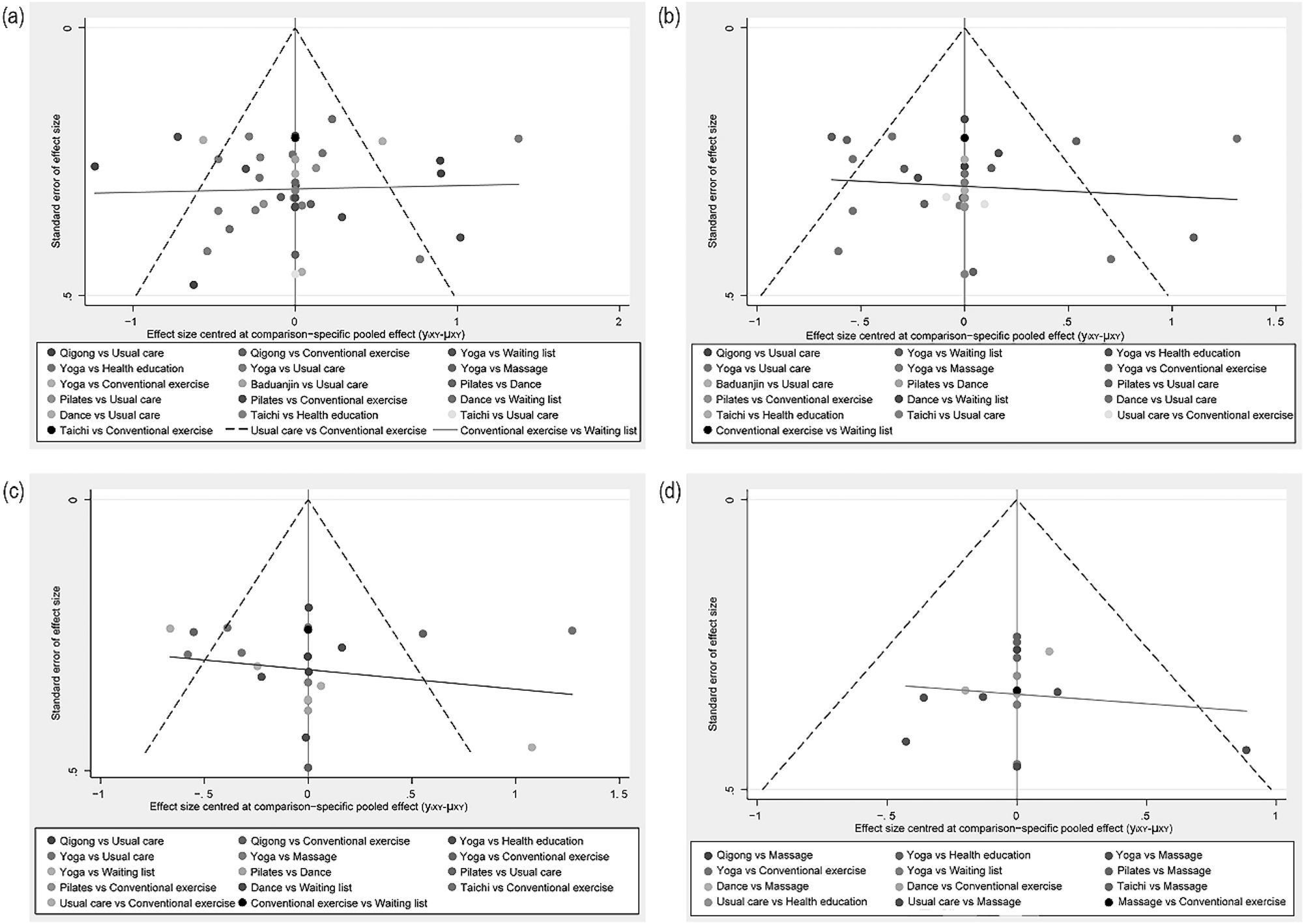
Comparative adjusted funnel plot for publication bias. Legend: (a) for various types of cancer. (b) For the breast cancer subgroup. (c) During cancer treatment. (d) Post cancer treatment. The red line represents the null hypothesis stating that the independent estimates of the effect size are not different from the comparison – specific pooled estimates.
Sensitivity analysis
To evaluate the robustness of our findings, we conducted leave-one-out sensitivity analyses by systematically excluding each included study from the network meta-analysis and recalculating the pooled effect sizes. The consistency of results was assessed by comparing original estimates with recalculated values, with all analyses performed using Stata/MP software (version 17.0; StataCorp LLC, College Station, TX, USA). Fixed-effects models were used for homogeneity testing and random-effects models for primary analyses. Upon sequential exclusion of each individual study, the resultant pooled estimates remained stable, demonstrating the absence of any single study exerting a significant influence on the aggregated outcomes. For visual representation of these sensitivity analyses, refer to Supplementary Fig. S3(a–d).
Discussion
In our study, we evaluated the pain-relieving effects of six MBTs to identify the optimal MBT for cancer patients. Our analysis suggested that Qigong was the most effective for pain reduction, followed by Taichi. Subgroup analyses focusing on breast cancer indicated Taichi’s superiority for CRP management. Furthermore, Qigong was most effective at reducing CRP during treatment, while Taichi proved to be more beneficial post treatment. Notably, conventional exercise also demonstrated significant benefits for pain management.
We found that Qigong was the most effective treatment for CRP, which was an unexpected outcome given the limited number of articles on this topic in our NMA. Our research findings echo previous studies regarding the efficacy of MBTs on CRP. Previous studies indicated that Qigong could serve as a beneficial adjunct treatment, significantly alleviating related symptoms in cancer survivors, such as CRP [64], 65]. Additionally, five studies with non-randomized designs were excluded from our NMA, two of which reported positive effects of Qigong on CRP [65], 66]. CRP is a complex disease that often leads to disruptions in patients’ physical and mental functioning. Qigong emphasizes a holistic approach to exercise that integrates “body regulation,” “breath regulation,” and “mind regulation,” focusing intently on the impact of psychological states on human health and adjusting physiological activities through proactive self-mental activities. Characterized by self-guided techniques that integrate posture, movement, breath regulation, and meditation, Qigong aims to promote the harmonious flow of the body’s energy, known as “Qi”. This practice elicits calming and relaxing effects, potentially alleviating the physical pain of cancer patients by reducing their psychological distress [67]. Recent studies have suggested that the potential mechanisms underlying the pain-relieving effects of Qigong can be partially explained by its ability to induce regional structural changes in the brain, such as increasing regional blood flow in the prefrontal cortex [68], and influencing its intrinsic functional architecture, which may potentially modulate the sympathetic nerves [69]. Another proposed mechanism may involve the hypothalamic-pituitary-adrenal (HPA) axis. The presence of pain stress could disrupt the endocrine system [70]. In contrast, the slow and gentle motions of Qigong combined with deep breathing may influence the sympathetic-adrenal-medullary axis through the HPA pathway, potentially reducing pain [71]. The neurophysiological changes associated with Qigong may facilitate its pain-relieving effects by influencing the brain’s processing of pain signals and emotional responses to pain.
Due to the majority of articles included focusing on breast cancer patients, we conducted subgroup analyses based on cancer type. We found that the most effective MBT for reducing pain among individuals with breast cancer was Taichi. Taichi was a recognized modality of gentle MBT, integrating physical, psychosocial, spiritual, and behavioral components [72]. Previous studies provided scientific evidence on the role of Taichi in alleviating CRP [73], 74], indicating that as a moderate physical activity, Taichi might positively impact the relief of CRP by improving musculoskeletal strength and joint stability, as well as by regulating emotional and autonomic nervous system functions. Notably, breast cancer patients often experienced anomalies in the structural and functional connectivity of various brain regions [75], including the anterior cingulate cortex and the insular cortex, which were closely related to the generation of pain [76]. Long-term Taichi training, associated with increased cortical density in the lower part of the insular sulcus [77] decreased functional uniformity within the left anterior cingulate cortex [78]. It is well established that the amygdala plays a significant role in the emotional regulation of pain [79], 80]. An earlier RCT also identified a moderate to high correlation between pre-post changes in the functional connectivity of the amygdala and medial prefrontal cortex associated with Taichi practice [81]. This further supported the notion that Taichi, as an MBT, might improve emotional states and thereby regulate pain experience.
Our study demonstrated that while Qigong emerged as the most effective intervention overall (ranked first), followed closely by Taichi (ranked second), subgroup analyses revealed Taichi exhibited particularly pronounced pain-relieving effects specifically in breast cancer patients. This discrepancy may be attributed to: 1) the distinct pathophysiological mechanisms underlying breast cancer-related pain (e.g., post-mastectomy pain syndrome, aromatase inhibitor-induced arthralgia) [82] and their differential responses to MBTs; and 2) the relatively higher proportion of Taichi studies in this subgroup compared to Qigong, whereas the overall analysis maintained a more balanced distribution of therapeutic modalities. Meanwhile, during our literature search, we found a substantial number of articles on the use of Yoga for treating CRP in breast cancer patients. Most previous reviews supported the benefits of Yoga in the treatment of breast cancer [83], 84]. Our NMA suggests that while Yoga helps alleviate pain in breast cancer patients, its effects are less pronounced compared to Qigong and Taichi. A recent trial also showed that Yoga did not significantly affect pain in breast cancer patients compared to a supportive control group [36]. Heterogeneity in the studies may be one of the most significant factors contributing to the limited effects we observed. Firstly, the duration of Yoga interventions varied across studies, with intervention times ranging from 30 min to 2 h and frequencies from 6 to 18 weeks. Additionally, Yoga was applied to breast cancer patients at different stages in the included articles, and patients with advanced breast cancer often experience more severe pain, which may result in a floor effect post-intervention. Furthermore, the prolonged analgesic effects of Taichi, which can last for an extended period [85], may also be a contributing factor. Moreover, compared to Taichi, Yoga has disadvantages such as the need for instructor-led classes and being limited by location, making the exploration of the potential of Qigong and Taichi in CRP management more cost-effective.
For numerous cancer survivors, pain has turned into a long – term chronic consequence of cancer treatment. In this context, the search for effective adjunctive treatments for pain relief was of utmost importance. To date, no studies had informed patients which MBTs they could choose to assist in managing pain during or after cancer treatment. Our study conducted subgroup analyses based on the different stages of cancer treatment that patients were in. Our study identified Qigong as the most efficacious MBT during cancer treatment, while Taichi was deemed most effective post-treatment. This distinction may stem from the limited number of studies in each subgroup. Our review included a single study on Taichi during cancer treatment and one on Qigong post-treatment. Despite this limitation, our findings align with the overall evidence, reinforcing the value of Qigong and Taichi as beneficial adjuncts to CRP management.
Our NMA demonstrated Conventional exercise’s efficacy among control conditions for pain management, yet the most clinically significant findings reveal MBTs distinctive psychophysiological mechanisms that extend beyond purely conventional exercise. Whereas Conventional exercise primarily influences circulating hormones (e.g., insulin, estrogens) and pro-inflammatory cytokines (e.g., IL-6, TNF-α) [86], 87]. MBTs achieve their effects through three synergistic pathways: the conscious integration of movement with breath regulation and mindfulness [88], reduce allostatic load and improve brain-networks connectivity [89], and stress-axis regulation via parasympathetic activation [90]. This multifaceted mechanism explains why Yoga and Taichi demonstrated superior outcomes to Massage (the lowest-ranked comparator) – MBTs’ combined cognitive-affective and physical dimensions prove essential for sustained cancer-related pain relief.
Our NMA possesses the following key advantages. It included only RCTs, which are considered the gold standard for assessing the efficacy of interventions. Our focus on CRP represents a key strength and innovation, as it is often overlooked in research assessing MBTs for cancer-related symptoms, despite its significant role as a correlate of quality of life. We are the first to explore which MBTs are most effective for treating CRP. MBTs possess unique advantages that other traditional exercises cannot match, owing to their gentle, safe, accessible, and sustainable characteristics, which make them suitable for individuals of any age with cancer and at any physical activity level, warranting further investigation.
The interpretation of these NMA results is subject to certain constraints. First, the absence of a standardized list of MBTs resulted in an ad hoc compilation for the NMA, which could have introduced bias through selective inclusion or exclusion of certain therapies. Furthermore, the distribution of studies among the six MBTs was uneven, with 20 articles focusing solely on Yoga, which may affect the validity and reliability of the findings. Lastly, most studies included in this analysis were centered on breast cancer, leading us to conduct a subgroup analysis specifically for this condition. Moreover, variations in intervention length, frequency, and duration across the included studies may have influenced treatment effects. Therefore, the findings of this NMA should be interpreted with caution.
Summary and outlook
This NMA indicates that Qigong and Taichi are among the most effective MBTs for CRP, and can be a complementary adjuvant treatment in cancer patients. We advocate for a multidisciplinary pain management strategy, encompassing pharmacological, physical, psychological, and supportive care interventions. Nonetheless, future research should adhere to stricter standards, with pain as the primary outcome measure, to validate these findings.
Funding source: the Research Foundation of Traditional Chinese Medicine Bureau of Guangdong Province
Award Identifier / Grant number: 20231,067
Funding source: the program of Guangdong Provincial Clinical Research Center for Rehabilitation Medicine
Award Identifier / Grant number: 2023B110003
Funding source: the Guangdong Hopson-Pearl River Education Development Foundation
Award Identifier / Grant number: No. H20190116202012724
Acknowledgments
We thank all authors for contributions to this article and we appreciate the reviewers’ valuable comments.
-
Research ethics: This study did not involve human or animal subjects, therefore no ethics approval was required.
-
Informed consent: Not applicable.
-
Author contributions: Xiaohui Hou and Yuling Wang: Conceptualization, investigation, supervision and design. Xiuyun He and Guangyuan Liang: conceptualization, methodology, software, data curation, and writing – original draft preparation. Zhi Zou and Siying Yu: writing – reviewing and editing. Youtian Lin,Yafei Wang, Yinhua Li: Analyzed and interpreted the data. All authors reviewed the drafts of the manuscript, and read and approved the final manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare no conflict of interest. Xiaohui Hou serves as an in-house Managing Editor for Translational Exercise Biomedicine but was not involved in the handling, editorial review, or decision-making process for this manuscript.
-
Research funding: This study was supported by the program of Guangdong Provincial Clinical Research Center for Rehabilitation Medicine (2023B110003), the Research Foundation of Traditional Chinese Medicine Bureau of Guangdong Province (20231,067), and the Guangdong Hopson-Pearl River Education Development Foundation (No. H20190116202012724).
-
Data availability: This is a review article and does not involve the collection of original data. All data referenced are publicly available in the published literature cited in the References section.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. https://doi.org/10.3322/caac.21660.Search in Google Scholar
2. Yoong, J, Poon, P. Principles of cancer pain management: an overview and focus on pharmacological and interventional strategies. Aust J Gen Pract 2018;47:758–62. https://doi.org/10.31128/AJGP-07-18-4629.Search in Google Scholar
3. Bhatnagar, S, Gupta, M. Integrated pain and palliative medicine model. Ann Palliat Med 2016;5:196–208. https://doi.org/10.21037/apm.2016.05.02.Search in Google Scholar
4. Van den Beuken-van Everdingen, MHJ, Hochstenbach, LMJ, Joosten, EAJ, Tjan-Heijnen, VCG, Janssen, DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag 2016;51:1070–90. https://doi.org/10.1016/j.jpainsymman.2015.12.340.Search in Google Scholar
5. Nipp, RD, El-Jawahri, A, Moran, SM, D’Arpino, SM, Johnson, PC, Lage, DE, et al.. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer 2017;123:4720–7. https://doi.org/10.1002/cncr.30912.Search in Google Scholar
6. Maindet, C, Burnod, A, Minello, C, George, B, Allano, G, Lemaire, A. Strategies of complementary and integrative therapies in cancer-related pain-attaining exhaustive cancer pain management. Support Care Cancer 2019;27:3119–32. https://doi.org/10.1007/s00520-019-04829-7.Search in Google Scholar
7. Paice, JA, Portenoy, R, Lacchetti, C, Campbell, T, Cheville, A, Citron, M, et al.. Management of chronic pain in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 2016;34:3325–45. https://doi.org/10.1200/JCO.2016.68.5206.Search in Google Scholar
8. Asciak, R, George, V, Rahman, NM. Update on biology and management of mesothelioma. Eur Respir Rev 2021;30:200226. https://doi.org/10.1183/16000617.0226-2020.Search in Google Scholar
9. Mao, JJ, Ismaila, N, Bao, T, Barton, D, Ben-Arye, E, Garland, EL, et al.. Integrative medicine for pain management in oncology: society for integrative oncology-ASCO guideline. J Clin Oncol 2022;40:3998–4024. https://doi.org/10.1200/JCO.22.01357.Search in Google Scholar
10. Hayes, SC, Newton, RU, Spence, RR, Galvão, DA. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport 2019;22:1175–99. https://doi.org/10.1016/j.jsams.2019.05.003.Search in Google Scholar
11. Syrjala, KL, Jensen, MP, Mendoza, ME, Yi, JC, Fisher, HM, Keefe, FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32:1703–11. https://doi.org/10.1200/JCO.2013.54.4825.Search in Google Scholar
12. Draguhn, A, Sauer, JF. Body and mind: how somatic feedback signals shape brain activity and cognition. Pflügers Archiv 2023;475:1–4. https://doi.org/10.1007/s00424-022-02778-5.Search in Google Scholar
13. Lin, X, Zheng, J, Zhang, Q, Li, Y. The effects of mind body exercise on anxiety: a systematic review and meta-analysis of randomized controlled trials. Ment Health Phys Act 2024;26:100587. https://doi.org/10.1016/j.mhpa.2024.100587.Search in Google Scholar
14. Shah, K, Ramos-Garcia, M, Bhavsar, J, Lehrer, P. Mind-body treatments of irritable bowel syndrome symptoms: an updated meta-analysis. Behav Res Ther 2020;128:103462. https://doi.org/10.1016/j.brat.2019.103462.Search in Google Scholar
15. Garland, EL, Brintz, CE, Hanley, AW, Roseen, EJ, Atchley, RM, Gaylord, SA, et al.. Mind-body therapies for opioid-treated pain: a systematic review and meta-analysis. JAMA Intern Med 2020;180:91–105. https://doi.org/10.1001/jamainternmed.2019.4917.Search in Google Scholar
16. Plinsinga, ML, Singh, B, Rose, GL, Clifford, B, Bailey, TG, Spence, RR, et al.. The effect of exercise on pain in people with cancer: a systematic review with meta-analysis. Sports Med 2023;53:1737–52. https://doi.org/10.1007/s40279-023-01862-9.Search in Google Scholar
17. Danon, N, Al-Gobari, M, Burnand, B, Rodondi, P-Y. Are mind-body therapies effective for relieving cancer-related pain in adults? A systematic review and meta-analysis. Psychooncology 2022;31:345–71. https://doi.org/10.1002/pon.5821.Search in Google Scholar
18. Wang, J, Lv, M, Li, H, Guo, D, Chu, X. Effects of exercise in adults with cancer pain: a systematic review and network meta-analysis. J Pain Symptom Manag 2025;69:82–101. https://doi.org/10.1016/j.jpainsymman.2024.08.033.Search in Google Scholar
19. Deng, G. Integrative medicine therapies for pain management in cancer patients. Cancer J 2019;25:343–8. https://doi.org/10.1097/PPO.0000000000000399.Search in Google Scholar
20. Deleemans, JM, Mather, H, Spiropoulos, A, Toivonen, K, Baydoun, M, Carlson, LE. Recent progress in mind-body therapies in cancer care. Curr Oncol Rep 2023;25:293–307. https://doi.org/10.1007/s11912-023-01373-w.Search in Google Scholar
21. Casuso-Holgado, MJ, Heredia-Rizo, AM, Gonzalez-Garcia, P, Muñoz-Fernández, MJ, Martinez-Calderon, J. Mind-body practices for cancer-related symptoms management: an overview of systematic reviews including one hundred twenty-nine meta-analyses. Support Care Cancer 2022;30:10335–57. https://doi.org/10.1007/s00520-022-07426-3.Search in Google Scholar
22. Bafeta, A, Trinquart, L, Seror, R, Ravaud, P. Reporting of results from network meta-analyses: methodological systematic review. BMJ 2014;348:g1741. https://doi.org/10.1136/bmj.g1741.Search in Google Scholar
23. Page, MJ, Moher, D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev 2017;6:263. https://doi.org/10.1186/s13643-017-0663-8.Search in Google Scholar
24. Ardern, CL, Büttner, F, Andrade, R, Weir, A, Ashe, MC, Holden, S, et al.. Implementing the 27 PRISMA 2020 statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: the PERSiST (implementing prisma in exercise, rehabilitation, sport medicine and sports science) guidance. Br J Sports Med 2022;56:175–95. https://doi.org/10.1136/bjsports-2021-103987.Search in Google Scholar
25. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al.. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.Search in Google Scholar
26. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al.. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev 2019;10:ED000142. https://doi.org/10.1002/14651858.ED000142.Search in Google Scholar
27. Shim, S, Yoon, B-H, Shin, I-S, Bae, J-M. Network meta-analysis: application and practice using stata. Epidemiol Health 2017;39:e2017047. https://doi.org/10.4178/epih.e2017047.Search in Google Scholar
28. Sprod, LK, Janelsins, MC, Palesh, OG, Carroll, JK, Heckler, CE, Peppone, LJ, et al.. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv 2012;6:146–54. https://doi.org/10.1007/s11764-011-0205-7.Search in Google Scholar
29. Chandwani, KD, Perkins, G, Nagendra, HR, Raghuram, NV, Spelman, A, Nagarathna, R, et al.. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol: Official J Am Soc Clin Oncol 2014;32:1058–65. https://doi.org/10.1200/JCO.2012.48.2752.Search in Google Scholar
30. Tsai, C-L, Liu, L-C, Liao, C-Y, Liao, W-L, Liu, Y-H, Hsieh, C-L. Yoga versus massage in the treatment of aromatase inhibitor-associated knee joint pain in breast cancer survivors: a randomized controlled trial. Sci Rep 2021;11:14843. https://doi.org/10.1038/s41598-021-94466-0.Search in Google Scholar
31. Jong, MC, Boers, I, Schouten van der Velden, AP, Svd, M, Göker, E, Timmer-Bonte, ANJH, et al.. A randomized study of yoga for fatigue and quality of life in women with breast cancer undergoing (Neo) adjuvant chemotherapy. J Alternative Compl Med 2018;24:942–53. https://doi.org/10.1089/acm.2018.0191.Search in Google Scholar
32. Eyigor, S, Uslu, R, Apaydın, S, Caramat, I, Yesil, H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Compl Ther Clin Pract 2018;32:40–5. https://doi.org/10.1016/j.ctcp.2018.04.010.Search in Google Scholar
33. Liu, W, Liu, J, Ma, L, Chen, J. Effect of mindfulness yoga on anxiety and depression in early breast cancer patients received adjuvant chemotherapy: a randomized clinical trial. J Cancer Res Clin Oncol 2022;148:2549–60. https://doi.org/10.1007/s00432-022-04167-y.Search in Google Scholar
34. Pasyar, N, Barshan Tashnizi, N, Mansouri, P, Tahmasebi, S. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: a pilot study. Eur J Oncol Nurs 2019;42:103–9. https://doi.org/10.1016/j.ejon.2019.08.008.Search in Google Scholar
35. Vadiraja, SH, Rao, MR, Nagendra, RH, Nagarathna, R, Rekha, M, Vanitha, N, et al.. Effects of yoga on symptom management in breast cancer patients: a randomized controlled trial. Int J Yoga 2009;2:73–9. https://doi.org/10.4103/0973-6131.60048.Search in Google Scholar
36. Porter, LS, Carson, JW, Olsen, M, Carson, KM, Sanders, L, Jones, L, et al.. Feasibility of a mindful yoga program for women with metastatic breast cancer: results of a randomized pilot study. Support Care Cancer 2019;27:4307–16. https://doi.org/10.1007/s00520-019-04710-7.Search in Google Scholar
37. Lötzke, D, Wiedemann, F, Rodrigues Recchia, D, Ostermann, T, Sattler, D, Ettl, J, et al.. Iyengar-yoga compared to exercise as a therapeutic intervention during (Neo)adjuvant therapy in women with stage i-iii breast cancer: health-related quality of life, mindfulness, spirituality, life satisfaction, and cancer-related fatigue. Evid Based Compl Alternat Med 2016;2016:5931816. https://doi.org/10.1155/2016/5931816.Search in Google Scholar
38. Huberty, J, Eckert, R, Dueck, A, Kosiorek, H, Larkey, L, Gowin, K, et al.. Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research. BMC Compl Alternat Med 2019;19:121. https://doi.org/10.1186/s12906-019-2530-8.Search in Google Scholar
39. Yagli, NV, Ulger, O. The effects of yoga on the quality of life and depression in elderly breast cancer patients. Compl Ther Clin Pract 2015;21:7–10. https://doi.org/10.1016/j.ctcp.2015.01.002.Search in Google Scholar
40. Knoerl, R, Giobbie-Hurder, A, Berfield, J, Berry, D, Meyerhardt, JA, Wright, AA, et al.. Yoga for chronic chemotherapy-induced peripheral neuropathy pain: a pilot, randomized controlled trial. J Cancer Surviv 2022;16:882–91. https://doi.org/10.1007/s11764-021-01081-z.Search in Google Scholar
41. Loudon, A, Barnett, T, Piller, N, Immink, MA, Williams, AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Compl Alternat Med 2014;14:214. https://doi.org/10.1186/1472-6882-14-214.Search in Google Scholar
42. Carson, JW, Carson, KM, Porter, LS, Keefe, FJ, Seewaldt, VL. Yoga of awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer 2009;17:1301–9. https://doi.org/10.1007/s00520-009-0587-5.Search in Google Scholar
43. Eckert, R, Huberty, J, Kurka, J, Laird, B, Mesa, R, Palmer, J. A randomized pilot study of online hatha yoga for physical and psychological symptoms among survivors of allogenic bone marrow transplant. Int J Yoga Therap 2022;32. https://doi.org/10.17761/2022-D-21-00047.Search in Google Scholar
44. Bao, T, Zhi, I, Baser, R, Hooper, M, Chen, C, Piulson, L, et al.. Yoga for chemotherapy-induced peripheral neuropathy and fall risk: a randomized controlled trial. JNCI Cancer Spectr 2020;4:pkaa048. https://doi.org/10.1093/jncics/pkaa048.Search in Google Scholar
45. Prakash, K, Saini, SK, Pugazhendi, S. Effectiveness of yoga on quality of life of breast cancer patients undergoing chemotherapy: a randomized clinical controlled study. Indian J Palliat Care 2020;26:323–31. https://doi.org/10.4103/IJPC.IJPC_192_19.Search in Google Scholar
46. Namazinia, M, Mazlum, SR, Mohajer, S, Lopez, V. Effects of laughter yoga on health-related quality of life in cancer patients undergoing chemotherapy: a randomized clinical trial. BMC Compl Med Ther 2023;23:192. https://doi.org/10.1186/s12906-023-04028-2.Search in Google Scholar
47. Adair, M, Murphy, B, Yarlagadda, S, Deng, J, Dietrich, MS, Ridner, SH. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther 2018;17:774–84. https://doi.org/10.1177/1534735417753540.Search in Google Scholar
48. Chandwani, KD, Thornton, B, Perkins, GH, Arun, B, Raghuram, NV, Nagendra, HR, et al.. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol 2010;8:43–55.Search in Google Scholar
49. Chuang, T-Y, Yeh, M-L, Chung, Y-C. A nurse facilitated mind-body interactive exercise (chan-chuang qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: randomised controlled trial. Int J Nurs Stud 2017;69:25–33. https://doi.org/10.1016/j.ijnurstu.2017.01.004.Search in Google Scholar
50. Chang, CI, Yeh, ML, Liao, J. Chan-chuang qigong with breathing meditation improves quality of life and interoceptive awareness in patients with breast cancer: a randomised controlled trial. Support Care Cancer 2023;31:140. https://doi.org/10.1007/s00520-023-07578-w.Search in Google Scholar
51. Yang, L-H, Duan, P-B, Hou, Q-M, Wang, X-Q. Qigong exercise for patients with gastrointestinal cancer undergoing chemotherapy and at high risk for depression: a randomized clinical trial. J Alternat Compl Med 2021;27:750–9. https://doi.org/10.1089/acm.2020.0531.Search in Google Scholar
52. Vanderbyl, BL, Mayer, MJ, Nash, C, Tran, AT, Windholz, T, Swanson, T, et al.. A comparison of the effects of medical qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer 2017;25:1749–58. https://doi.org/10.1007/s00520-017-3579-x.Search in Google Scholar
53. Oh, B, Butow, P, Mullan, B, Clarke, S. Medical qigong for cancer patients: pilot study of impact on quality of life, side effects of treatment and inflammation. Am J Chin Med 2008;36:459–72. https://doi.org/10.1142/s0192415x08005904.Search in Google Scholar
54. Campo, RA, O’Connor, K, Light, KC, Nakamura, Y, Lipschitz, DL, LaStayo, PC, et al.. Feasibility and acceptability of a tai chi chih randomized controlled trial in senior female cancer survivors. Integr Cancer Ther 2013;12:464–74. https://doi.org/10.1177/1534735413485418.Search in Google Scholar
55. Ding, Y, Ji, L, Hu, Y. Effects of tai chi on catheter management and quality of life in tumor patients with PICC at the intermission of chemotherapy: a non-inferiority randomized controlled trial. Ann Palliat Med 2020;9:3293–303. https://doi.org/10.21037/apm-20-1456.Search in Google Scholar
56. Şener, HÖ, Malkoç, M, Ergin, G, Karadibak, D, Yavuzşen, T. Effects of clinical pilates exercises on patients developing lymphedema after breast cancer treatment: a randomized clinical trial. J Breast Health 2017;13:16–22. https://doi.org/10.5152/tjbh.2016.3136.Search in Google Scholar
57. Barbosa, KP, da Silva, LGT, Garcia, PA, Freitas, CA, da Silva, ECF, Pereira, TV, et al.. Effectiveness of pilates and circuit-based exercise in reducing arthralgia in women during hormone therapy for breast cancer: a randomized, controlled trial. Support Care Cancer 2021;29:6051–9. https://doi.org/10.1007/s00520-021-06180-2.Search in Google Scholar
58. Zengin Alpozgen, A, Razak Ozdincler, A, Karanlik, H, Yaman Agaoglu, F, Narin, AN. Effectiveness of pilates-based exercises on upper extremity disorders related with breast cancer treatment. Eur J Cancer Care 2017;26. https://doi.org/10.1111/ecc.12532.Search in Google Scholar
59. Boing, L, Fretta, TB, Lynch, BM, Dias, M, Rosa, LM, Baptista, F, et al.. Mat pilates and belly dance: effects on patient-reported outcomes among breast cancer survivors receiving hormone therapy and adherence to exercise. Compl Ther Clin Pract 2023;50:101683. https://doi.org/10.1016/j.ctcp.2022.101683.Search in Google Scholar
60. Pisu, M, Demark-Wahnefried, W, Kenzik, KM, Oster, RA, Lin, CP, Manne, S, et al.. A dance intervention for cancer survivors and their partners (RHYTHM). J Cancer Surviv 2017;11:350–9. https://doi.org/10.1007/s11764-016-0593-9.Search in Google Scholar
61. Ho, RTH, Fong, TCT, Cheung, IKM, Yip, PSF, Luk, M-Y. Effects of a short-term dance movement therapy program on symptoms and stress in patients with breast cancer undergoing radiotherapy: a randomized, controlled, single-blind trial. J Pain Symptom Manag 2016;51:824–31. https://doi.org/10.1016/j.jpainsymman.2015.12.332.Search in Google Scholar
62. Rubio, MA, Mejía-Arbeláez, CM, Wilches-Mogollon, MA, Moreno, S, Finck, C, Rosas, LG, et al.. My body, my rhythm, my voice”: a community dance pilot intervention engaging breast cancer survivors in physical activity in a middle-income country. Pilot Feasibility Stud 2023;9:30. https://doi.org/10.1186/s40814-023-01253-x.Search in Google Scholar
63. Liao, J, Chen, Y, Cai, L, Wang, K, Wu, S, Wu, L, et al.. Baduanjin’s impact on quality of life and sleep quality in breast cancer survivors receiving aromatase inhibitor therapy: a randomized controlled trial. Front Oncol 2022;12:807531. https://doi.org/10.3389/fonc.2022.807531.Search in Google Scholar
64. Wayne, PM, Lee, MS, Novakowski, J, Osypiuk, K, Ligibel, J, Carlson, LE, et al.. Tai chi and qigong for cancer-related symptoms and quality of life: a systematic review and meta-analysis. J Cancer Surviv 2018;12:256–67. https://doi.org/10.1007/s11764-017-0665-5.Search in Google Scholar
65. Lee, TI, Chen, HH, Yeh, M-L. Effects of chan-chuang qigong on improving symptom and psychological distress in chemotherapy patients. Am J Chin Med 2006;34:37–46. https://doi.org/10.1142/s0192415x06003618.Search in Google Scholar
66. Fong, SS, Ng, SS, Luk, WS, Chung, LM, Wong, JY, Chung, JW. Effects of qigong training on health-related quality of life, functioning, and cancer-related symptoms in survivors of nasopharyngeal cancer: a pilot study. Evid Based Compl Alternat Med 2014;2014:495274. https://doi.org/10.1155/2014/495274.Search in Google Scholar
67. Holmberg, C, Rappenecker, J, Karner, JJ, Witt, CM. The perspectives of older women with chronic neck pain on perceived effects of qigong and exercise therapy on aging: a qualitative interview study. Clin Interv Aging 2014;3:403–10. https://doi.org/10.2147/CIA.S54249.Search in Google Scholar
68. Campo, RA, Agarwal, N, LaStayo, PC, O’Connor, K, Pappas, L, Boucher, KM, et al.. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of qigong. J Cancer Surviv 2014;8:60–9. https://doi.org/10.1007/s11764-013-0315-5.Search in Google Scholar
69. Xie, H, Zhang, M, Huo, C, Xu, G, Li, Z, Fan, Y. Tai chi chuan exercise related change in brain function as assessed by functional near-infrared spectroscopy. Sci Rep 2019;9:13198. https://doi.org/10.1038/s41598-019-49401-9.Search in Google Scholar
70. Feng, F, Tuchman, S, Denninger, JW, Fricchione, GL, Yeung, A. Qigong for the prevention, treatment, and rehabilitation of COVID-19 infection in older adults. Am J Geriatr Psychiatr 2020;28:812–9. https://doi.org/10.1016/j.jagp.2020.05.012.Search in Google Scholar
71. Thayer, JF, Lane, RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201-16. https://doi.org/10.1016/s0165-0327(00)00338-4.Search in Google Scholar
72. Wang, C, Schmid, CH, Fielding, RA, Harvey, WF, Reid, KF, Price, LL, et al.. Effect of tai chi versus aerobic exercise for fibromyalgia: comparative effectiveness randomized controlled trial. BMJ 2018;360:k851. https://doi.org/10.1136/bmj.k851.Search in Google Scholar
73. Kong, LJ, Lauche, R, Klose, P, Bu, JH, Yang, XC, Guo, CQ, et al.. Tai chi for chronic pain conditions: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2016;6:25325. https://doi.org/10.1038/srep25325.Search in Google Scholar
74. Gao, S, Ni, X, He, Z, Wang, Y, Sun, M, Liu, L, et al.. Tai chi for improving chronic primary musculoskeletal pain: protocol for a systematic review. Evid Based Compl Alternat Med 2021;2021:9932336. https://doi.org/10.1155/2021/9932336.Search in Google Scholar
75. Liu, R, Qiao, N, Shi, S, Li, S, Wang, Y, Song, J, et al.. Deficits in ascending pain modulation pathways in breast cancer survivors with chronic neuropathic pain: a resting-state fMRI study. Front Neurol 2022;13:959122. https://doi.org/10.3389/fneur.2022.959122.Search in Google Scholar
76. Starr, CJ, Sawaki, L, Wittenberg, GF, Burdette, JH, Oshiro, Y, Quevedo, AS, et al.. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 2009;29:2684–94. https://doi.org/10.1523/JNEUROSCI.5173-08.2009.Search in Google Scholar
77. Wei, G-X, Xu, T, Fan, F-M, Dong, H-M, Jiang, L-L, Li, H-J, et al.. Can taichi reshape the brain? A brain morphometry study. PLoS One 2013;8:e61038. https://doi.org/10.1371/journal.pone.0061038.Search in Google Scholar
78. Wei, G-X, Dong, H-M, Yang, Z, Luo, J, Zuo, X-N. Tai chi chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front Aging Neurosci 2014;6:74. https://doi.org/10.3389/fnagi.2014.00074.Search in Google Scholar
79. Rouwette, T, Vanelderen, P, Roubos, EW, Kozicz, T, Vissers, K. The amygdala, a relay station for switching on and off pain. Eur J Pain 2012;16:782–92. https://doi.org/10.1002/j.1532-2149.2011.00071.x.Search in Google Scholar
80. Gélébart, J, Garcia-Larrea, L, Frot, M. Amygdala and anterior insula control the passage from nociception to pain. Cerebr Cortex 2023;33:3538–47. https://doi.org/10.1093/cercor/bhac290.Search in Google Scholar
81. Shen, C-L, Watkins, BA, Kahathuduwa, C, Chyu, M-C, Zabet-Moghaddam, M, Elmassry, MM, et al.. Tai chi improves brain functional connectivity and plasma lysophosphatidylcholines in postmenopausal women with knee osteoarthritis: an exploratory pilot study. Front Med 2021;3:775344. https://doi.org/10.3389/fmed.2021.775344.Search in Google Scholar
82. Yuksel, SS, Chappell, AG, Jackson, BT, Wescott, AB, Ellis, MF. Post mastectomy pain syndrome: a systematic review of prevention modalities. JPRAS Open 2022;31:32–49. https://doi.org/10.1016/j.jpra.2021.10.009.Search in Google Scholar
83. Hsueh, E-J, Loh, E-W, Lin, JJ-A, Tam, K-W. Effects of yoga on improving quality of life in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer 2021;28:264–76. https://doi.org/10.1007/s12282-020-01209-6.Search in Google Scholar
84. Qi, Y, Li, H, Chan, DNS, Ma, X, Wong, CL. Effects of yoga interventions on the fatigue-pain-sleep disturbance symptom cluster in breast cancer patients: a systematic review. Eur J Oncol Nurs 2024;70:102594. https://doi.org/10.1016/j.ejon.2024.102594.Search in Google Scholar
85. Luo, XC, Liu, J, Fu, J, Yin, HY, Shen, L, Liu, ML, et al.. Effect of tai chi chuan in breast cancer patients: a systematic review and meta-analysis. Front Oncol 2020;10:607. https://doi.org/10.3389/fonc.2020.00607.Search in Google Scholar
86. Bouillet, T, Bigard, X, Brami, C, Chouahnia, K, Copel, L, Dauchy, S, et al.. Role of physical activity and sport in oncology: scientific commission of the national federation sport and cancer cami. Crit Rev Oncol Hematol 2015;94:74–86. https://doi.org/10.1016/j.critrevonc.2014.12.012.Search in Google Scholar
87. Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther 2016;23:47–56. https://doi.org/10.2147/ITT.S73223.Search in Google Scholar
88. Morgan, N, Irwin, MR, Chung, M, Wang, C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One 2014;9:e100903. https://doi.org/10.1371/journal.pone.0100903.Search in Google Scholar
89. Fialoke, S, Tripathi, V, Thakral, S, Dhawan, A, Majahan, V, Garg, R. Functional connectivity changes in meditators and novices during yoga nidra practice. Sci Rep 2024;14:12957. https://doi.org/10.1038/s41598-024-63765-7.Search in Google Scholar
90. Rogerson, O, Wilding, S, Prudenzi, A, O’Connor, DB. Effectiveness of stress management interventions to change cortisol levels: a systematic review and meta-analysis. Psychoneuroendocrinology 2024;159:106415. https://doi.org/10.1016/j.psyneuen.2023.106415.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/teb-2025-0009).
© 2025 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Is cardiac autonomic modulation influenced by beta blockers in adolescents with Duchenne Muscular Dystrophy?
- Association of circulating Notch1 and VEGF with flow-mediated dilation and aerobic fitness in healthy adults
- Acute partial sleep restriction attenuates working memory performance and does not affect BDNF in young adults
- Can we run away from the metabolic side effects of antipsychotics?
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- What are the optimal mind-body therapies for cancer-related pain? A network meta-analysis
- Section: Exercise and E-health, M-health, AI and technology
- Readiness, recovery, and strain: an evaluation of composite health scores in consumer wearables
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Is cardiac autonomic modulation influenced by beta blockers in adolescents with Duchenne Muscular Dystrophy?
- Association of circulating Notch1 and VEGF with flow-mediated dilation and aerobic fitness in healthy adults
- Acute partial sleep restriction attenuates working memory performance and does not affect BDNF in young adults
- Can we run away from the metabolic side effects of antipsychotics?
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- What are the optimal mind-body therapies for cancer-related pain? A network meta-analysis
- Section: Exercise and E-health, M-health, AI and technology
- Readiness, recovery, and strain: an evaluation of composite health scores in consumer wearables

