Long-Term Stability Comparison between an Original and a Generic Version of Piperacillin/Tazobactam in Dextrose 5 % Infusion Polyolefin Bags at 5 ± 3 °C after Microwave Freeze-Thaw Treatment
-
Sophie Huvelle
Abstract
Background
Piperacillin-Tazobactam is frequently infused in hospitals. The use of a generic version was considered after the out of stock of the brand name Tazocin®. The stability of 4 g of Tazocin® in 120 mL of dextrose 5 % (D5) was demonstrated during 35 days at 5 °C ± 3 °C after freezing (−20 °C) and microwave thawing (FMT). The aim of the study was to investigate and compare the long-term stability of Tazocin® and a generic product in the same conditions.
Methods
Five polyolefin bags of 4 g of Piperacillin/Tazobactam® Sandoz and 5 bags of 4 g of Tazocin® were prepared under aseptic conditions in 120 mL of D5 and stored 3 months at 20 °C then thawed and stored 58 days at 5 ± 3 °C.
Spectrophotometric absorbance at different wavelengths, pH measurement, visual and microscopic observations were also performed.
The concentrations were measured by HPLC, at 211 nm for tazobactam and 230 nm for piperacilline.
Results
No significant change in pH values or optic densities, no crystals were detected. The lower confidence limit at 95 % of the concentration for the solutions remains superior to 90 % of the initial concentration until 58 days of storage at 5 ± 3 °C.
Conclusion
Under these conditions, 4 g/120 mL of Piperacillin/Tazobactam® Sandoz or Tazocin® in D5 infusion in polyolefin bags remains stable at least for 58 days at 5 ± 3 °C after FMT
Introduction
Piperacillin, an antibiotic of the penicillin family, is active against a wide range of Gram-negative organisms, including Klebsiella pneumonia, Pseudomonas aeruginosa and the enterobacteriacae. The combination of piperacillin with tazobactam, a beta-lactamase inhibitor, broadens the bactericidal activity [1].
This antibiotic may be infused in 30 min after dilution with NaCl 0.9 % or dextrose 5 % or by extended infusion [2].
The stability of piperacillin in combination with tazobactam has been investigated under a variety of conditions, including different concentrations (from 0.2 + 0.025 mg/mL to 200 + 25.0 mg/mL), different diluents (5 % dextrose in water, 0.9 sodium chloride, peritoneal dialysis solution,), containers (glass, plastic bags, syringes, portable pumps), temperatures (37 °C, 25 °C, 4 °C, −15 °C, −20 °C), and storage periods (from 18 hours to 98 days) [3, 4, 5, 6, 7, 8, 9, 10].
The preparation of intravenous treatments by a Centralized IntraVenous Admixtures Service (CIVAS) contributes to the global management of treatment by providing ready-to-use injectable drugs with acceptable physicochemical and bacteriological quality and by relieving nursing staff from the tasks of infusion preparation [11, 12, 13]. The advance preparation of this antibiotic has been taken in charge on 2004 by our CIVAS [7].
Drug shortage became a national, european and global problem [14, 15, 16] and the princeps product Tazocin® became unavailable for a long period. At the time of the study, only one generic product was available on the Belgian market but little long term chemical data were know. Previous study investigated long-term stability of the princeps product [7] but the stability of the generic version must be determinated and compared with its of princeps.
The aim of the study was to investigate the long term physico-chemical stability of this generic product of piperacillin/tazobactam in dextrose 5 % polyolefin bag after freezing, microwave thawing and final storage at 5 ± 3 °C.
Materials and methods
Solutions preparation
Ten polyolefin bags (Viaflo, co-extruded layers of polyethylene, polyamide, polypropylene,
Baxter, Lessines, Belgium, lot 12D27G61) of 100 mL of dextrose 5 % among which five have been supplemented with 4 g of Piperacillin/Tazobactam® (Sandoz, Vilvoorde, Belgium, lot CG2904) reconstituted in 20 mL of injectable water and 5 other supplemented with 4 g of Tazocin® (Pfizer, AGVG/11) reconstituted in 20 mL of injectable water were prepared under aseptic conditions to obtain a final concentration of 4 g/120 mL of piperacillin and 0.5 g/120 mL of tazobactam. The composition in excipient is not the same for the two products: Pfizer’s product contain edetate disodium and citric acid monohydrate, Sandoz’s product contain no excipients.
Standard solution
On each test day, a standard curve was prepared with the same commercially available powder reconstituted with purified water to obtain a 6 g/120 mL of piperacillin and 0.75 g/120 mL tazobactam stock solution. This solution is used to prepare standard curve with range concentrations of piperacillin from 0.75 g to 6 g/120 mL and tazobactam 0.09 g to 0.75 g/120 mL.
Internal standard solution
500 mg of Nafcillin (Sigma Aldrich, Germany, ref N-3269) were dissolved in a final volume of 10 mL purified water to obtain a stock solution of 50 mg/mL. Two hundred µl aliquots of this stock solution were stored at −20 °C and thawed each day of the test in order to prepare two work solutions 0.5 mg/mL (for piperacillin analysis) and 0.25 mg/mL (for tazobactam analysis).
Quality control solution
The first day of the study, two control solutions were prepared with the same powder reconstituted with purified water to obtain a 4 g/120 mL piperacillin and 0.5 g/120 mL tazobactam solution and a 0.75 g/120 mL and 0.09 g/120 mL tazobactam solution. Aliquots were stocked at −80 °C and one aliquot of each solution were thawed each day of the test.
Chromatographic conditions
Following a stability-indicating method as previously described [7], a high-performance liquid chromatography (Alliance, model 2695, Waters Association, Milford Massachusetts) using a reversed-phase pre-guard (Waters SunFireTM C18 Sentry Guard Cartridge, 5 µm, 4.6 mm X 20 mm, ref 186002684) and column C18 (Alltech Rocket EPS 100A, 3 µm, 53*7 mm maintained at 30 °C, and an isocratic mobile phase consisting of 45 % acetonitrile and 55 % potassium phosphate buffer (pH3, 0.05 mol/l) was used for the elution at a flow rate of 1.0 mL/min. The separations module was coupled to a photodiode array (model 996, Waters Association, Milford Massachusetts) with a wavelength set at 211 nm for tazobactam and 230 nm for piperacillin (Figures 1–5). The wavelength of detection of nafcillin is the same than for piperacillin and tazobactam, respectively.
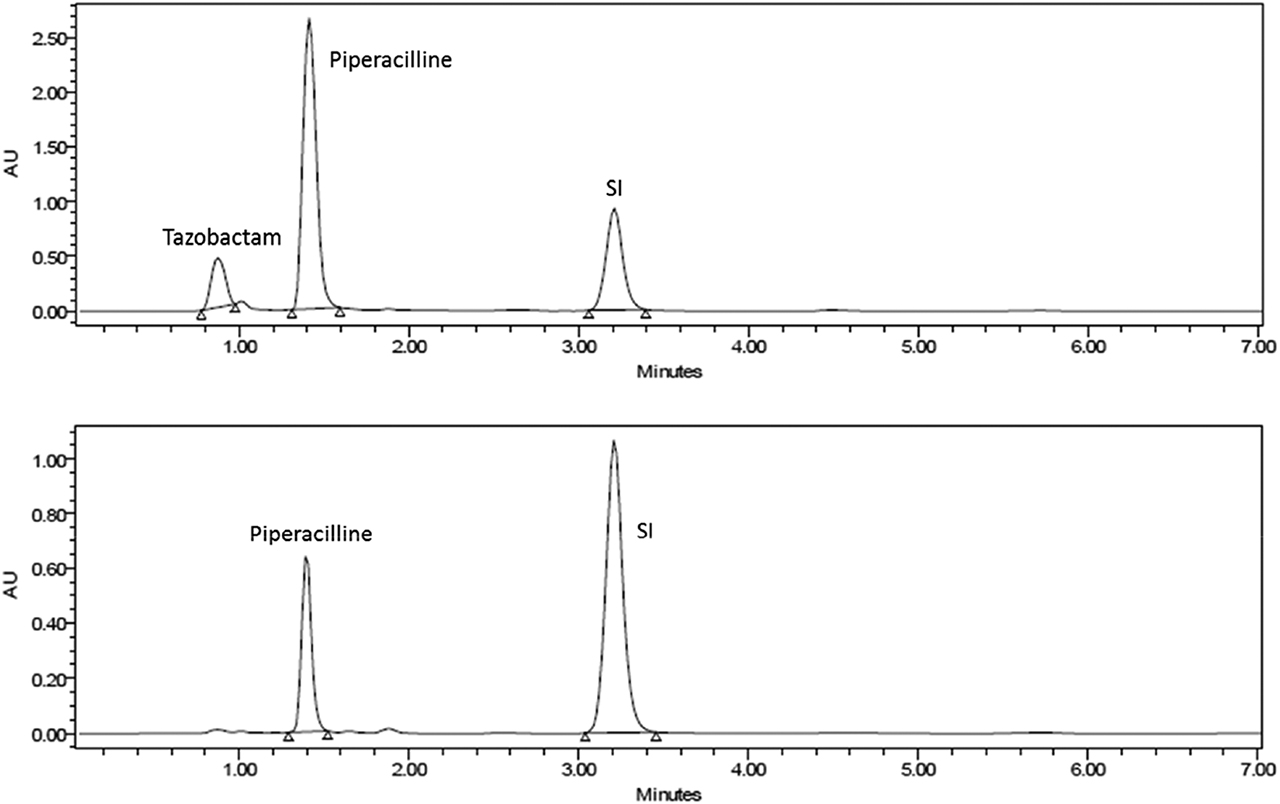
Chromatograms of tazobactam and piperacillin at normal conditions, at 230 nm and 211 nm respectively.
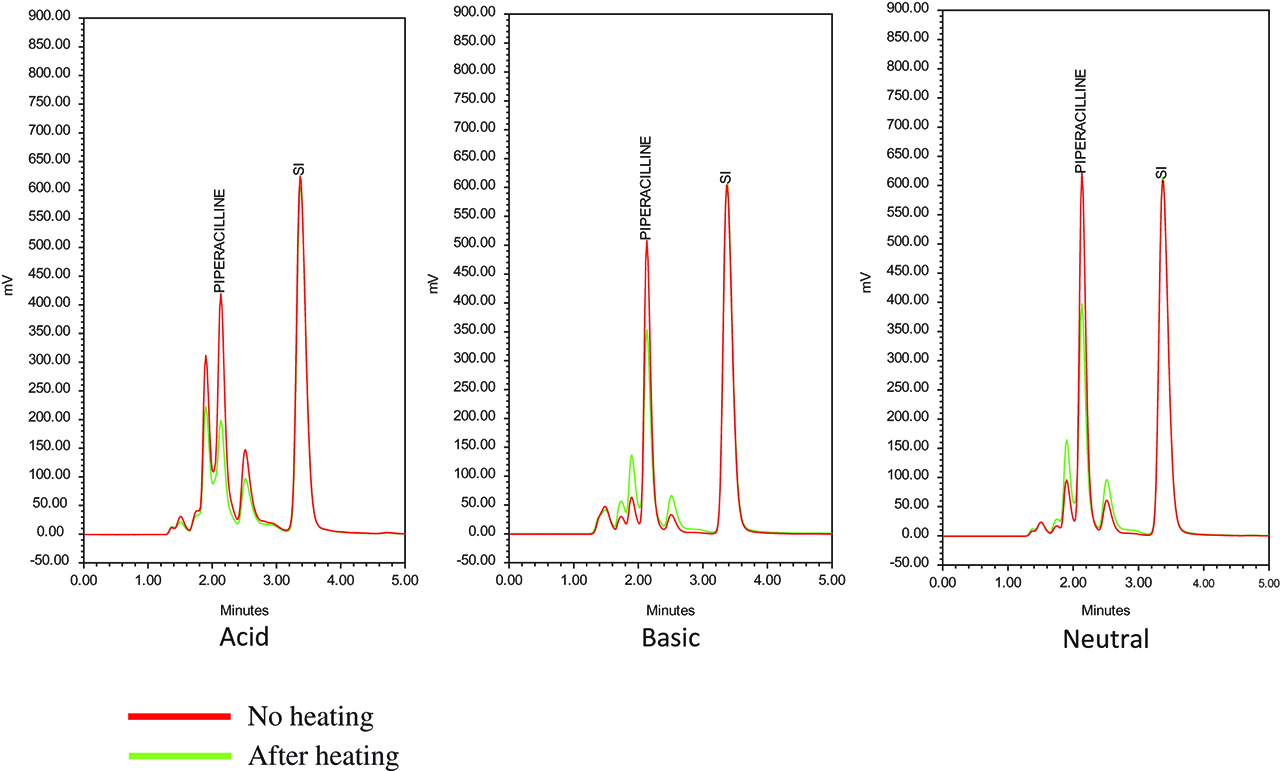
Chromatograms of generic piperacillin at different pH, without and after heating 30 min at 100 °C.
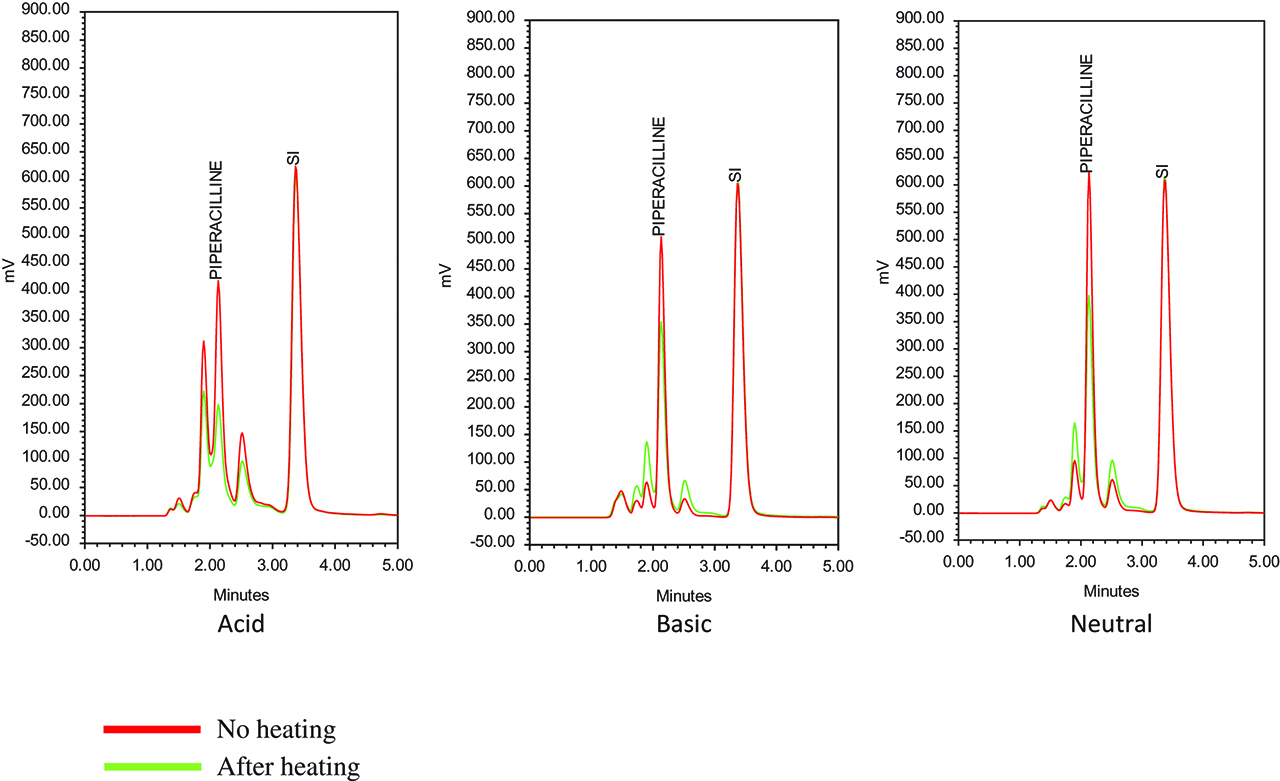
Chromatograms of piperacillin princeps at different pH, without and after 30 min at 100 °C.
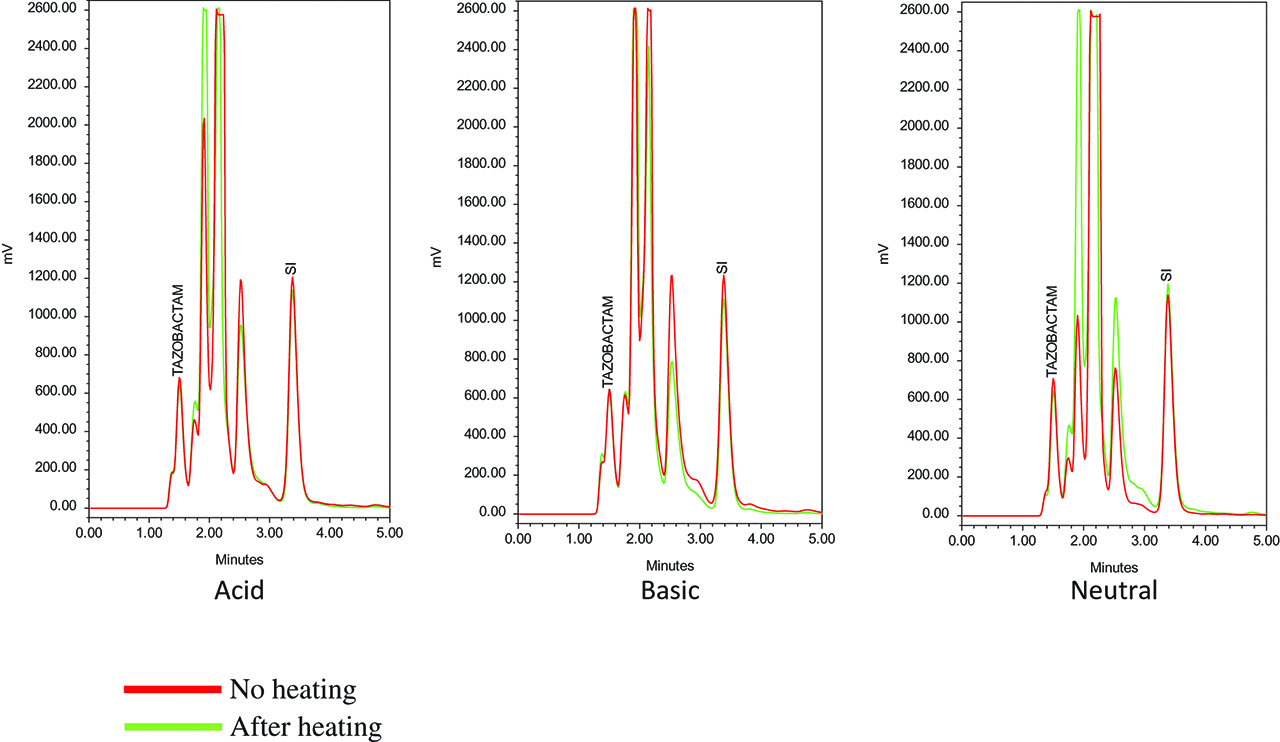
Chromatograms of generic tazobactam at different pH, without and after heating 30 min at 100 °C.
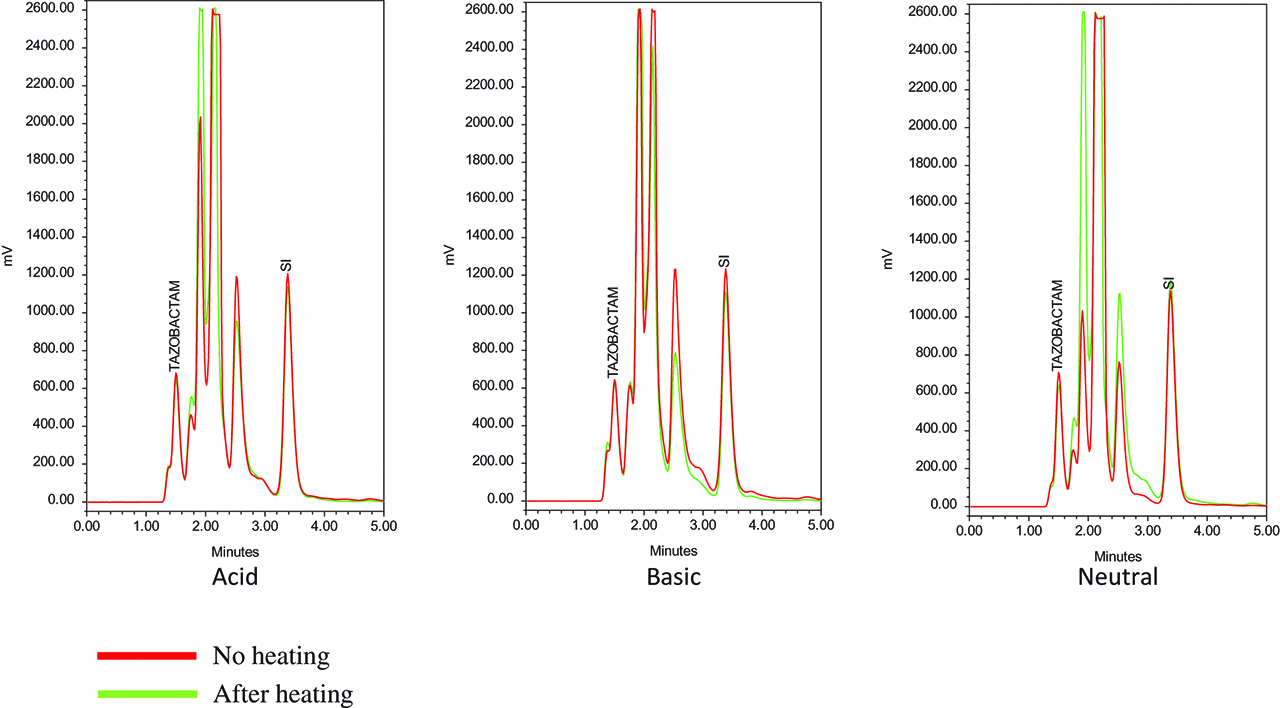
Chromatograms of tazobactam princeps at different pH, without and after heating 30 min at 100 °C.
pH determination, spectrophotometric analysis and microscope observation
Each day of the study, the pH of each solution was measured with a glass electrode pH meter (inoLab, WTW GmbH, Weilheim, Germany), the optic densities were measured with a spectrophotometer (Genesys 10 UV, Spectronic Unican) at 350 nm, 410 nm and 550 nm. Each sample was also centrifuged at 3000 rpm for 8 min to observe the pellet with an optic microscope 10 x (Carl Zeiss, Germany), looking for crystals.
Stability study
Five bags of 4 g of Piperacillin/Tazobactam® Sandoz in 120 mL of dextrose 5 % were prepared under aseptic conditions and stored 3 months at −20 °C then thawed and stored 58 days at 5 ± 3 °C. After 3 months, those bags were thawed in a microwave oven (NND998C/W, 230 V, 2450 MHz, 800W, Panasonic, Saint Denis, France) by a standardized and validated cycle, as described in a previous publication [17].
Briefly, these bags were hung to a carrousel, thawed for 13 min with an output power of 270 W then stirred and thawed again for seven minutes allowing to obtain a remaining ice volume between 1 and 8 cm3. The bags were then stored at 5 ± 3 °C for 30 days.
On each test day, 2 mL samples were withdrawn from each bag with a 2-mL polypropylene plastic syringe (Terumo, Haasrode, Belgium). The samples/controls/standards were then diluted in purified water (70-fold for piperacillin and 10-fold for tazobactam). Hundred µl of internal standard work solution 0.25 mg/mL were added to 100 µL of 70 fold diluted solution for the piperacillin analysis and 100 µL of internal standard work solution of 0.5 mg/mL were added to 100 µL of 10 – fold diluted solution.
Ten-microliter aliquots of the prepared mixture of samples with internal standard and standard solution were injected in triplicate into the chromatographic system under the conditions previously described.
Statistical analysis
The product is considered stable as long the lower limit of the 95 % unilateral confidence of the mean concentration remains above 90 % of the initial concentration [18] or 95 % of the initial concentration when any signs of physical instability exist as recently recommend [19].
The evolution of piperacillin and tazobactam concentrations over time where compared with linear mixed models with the molecule concentration as dependent variable, the version (generic vs princeps), the time and the time x version interaction as independent fixed variables and a random intercept for the bag. This model allows a comparison of baseline differences as well as degradation rates (or slopes) in both versions while taking into account the dependence between measures taken on the same bag.
All analyses were performed with R 3.3.2 (R Foundation of Statistical Computing, Vienna, Austria, 2016) and the ggplot2 and nlme package [20, 21].
Results
Physical stability
No significant change in pH values or optic density was observed during the study for the princeps infusion (pH values mean 6.13 ± 0.27) or for the generic infusion (pH values mean 4.99 ± 0.37). Any crystals were seen by microscopic analysis.
Chemical stability
There was no significant difference of degradation rate and concentration at any time between generic and princeps, and between the slopes after linear regression (p ≥ 0.795). (Table 1, Figure 6).
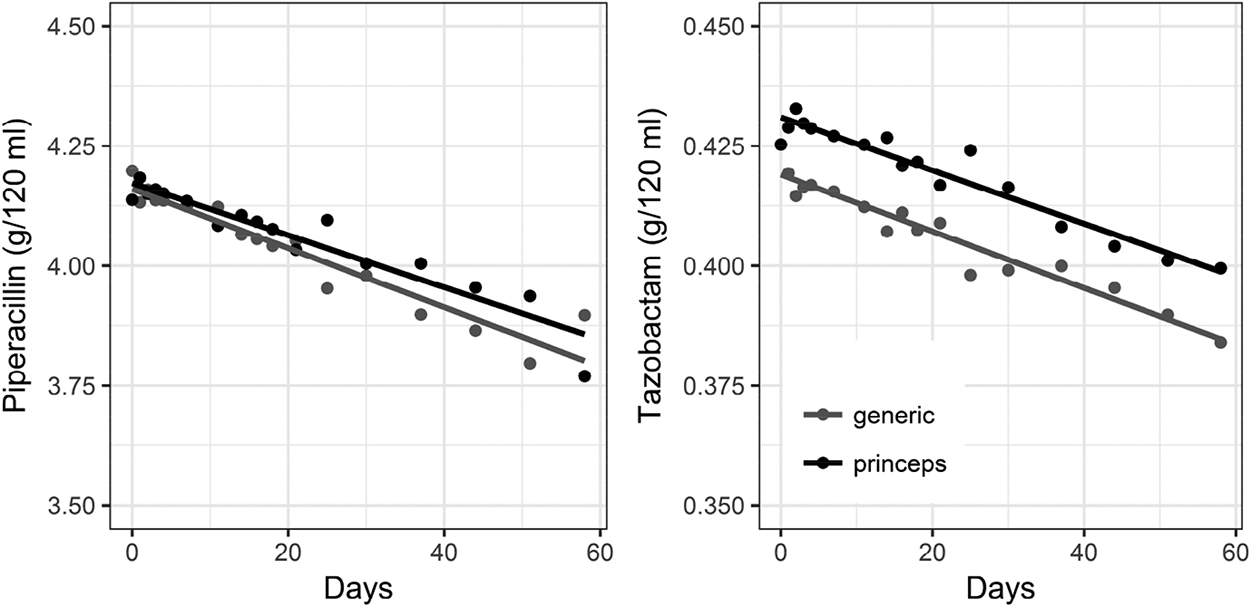
Evolution of piperacillin and tazobactam concentration over time in generic (grey) and princeps (black) versions. Each dot represent the mean concentration of five bags. Straight lines are linear regression lines.
Evolution of piperacillin and tazobactam concentrations over time.
| Piperacillin | Tazobactam | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Generic | Princeps | Generic | Princeps | ||||||||
| Day | Mean ± SD | Lower limit | Mean ± SD | Lower limit | Mean ± SD | Lower limit | Mean ± SD | Lower limit | |||
| 0 | 100.9 ± 0.4 | (99.7) | 99.2 ± 1.3 | (99.7) | 101.5 ± 1.0 | (99.6) | 98.7 ± 2.3 | (99.6) | |||
| 1 | 99.3 ± 0.7 | (99.5) | 100.3 ± 0.9 | (99.6) | 100.1 ± 0.6 | (99.5) | 99.5 ± 1.5 | (99.5) | |||
| 2 | 100.0 ± 0.9 | (99.4) | 99.5 ± 1.1 | (99.4) | 98.9 ± 0.7 | (99.4) | 100.4 ± 1.3 | (99.4) | |||
| 3 | 99.4 ± 0.5 | (99.3) | 99.7 ± 0.4 | (99.3) | 99.4 ± 0.9 | (99.2) | 99.7 ± 0.8 | (99.3) | |||
| 4 | 99.4 ± 0.4 | (99.1) | 99.5 ± 0.5 | (99.2) | 99.5 ± 0.5 | (99.1) | 99.5 ± 1.1 | (99.2) | |||
| 7 | 99.1 ± 0.8 | (98.7) | 99.1 ± 0.8 | (98.8) | 99.2 ± 0.8 | (98.7) | 99.1 ± 1.2 | (98.8) | |||
| 11 | 99.1 ± 0.4 | (98.1) | 97.9 ± 0.9 | (98.3) | 98.4 ± 0.7 | (98.2) | 98.7 ± 1.2 | (98.3) | |||
| 14 | 97.7 ± 1.3 | (97.7) | 98.4 ± 0.7 | (97.9) | 97.2 ± 1.6 | (97.8) | 99.0 ± 0.7 | (97.9) | |||
| 16 | 97.5 ± 0.6 | (97.4) | 98.1 ± 2.0 | (97.7) | 98.1 ± 2.9 | (97.5) | 97.7 ± 2.6 | (97.7) | |||
| 18 | 97.2 ± 0.6 | (97.1) | 97.7 ± 0.5 | (97.4) | 97.2 ± 0.4 | (97.2) | 97.8 ± 0.8 | (97.4) | |||
| 21 | 97.4 ± 0.6 | (96.7) | 96.7 ± 0.8 | (97.0) | 97.6 ± 1.1 | (96.8) | 96.7 ± 0.5 | (97.1) | |||
| 25 | 95.0 ± 2.1 | (96.1) | 98.1 ± 1.4 | (96.5) | 95.0 ± 1.7 | (96.2) | 98.4 ± 1.8 | (96.5) | |||
| 30 | 95.6 ± 1.6 | (95.3) | 96.0 ± 0.2 | (95.8) | 95.2 ± 1.7 | (95.5) | 96.6 ± 0.7 | (95.9) | |||
| 37 | 93.7 ± 1.1 | (94.2) | 96.0 ± 0.6 | (94.9) | 95.4 ± 1.9 | (94.4) | 94.7 ± 0.4 | (94.9) | |||
| 44 | 92.9 ± 0.3 | (93.1) | 94.8 ± 0.8 | (93.9) | 94.4 ± 0.7 | (93.4) | 93.8 ± 1.5 | (93.9) | |||
| 51 | 91.3 ± 0.9 | (92.0) | 94.3 ± 1.0 | (92.9) | 93.0 ± 0.7 | (92.3) | 93.1 ± 0.9 | (92.9) | |||
| 58 | 93.7 ± 1.0 | (90.9) | 90.3 ± 1.2 | (91.9) | 91.6 ± 1.0 | (91.2) | 92.7 ± 0.9 | (92.0) | |||
Mean and standard deviation (sd) of piperacillin and tazobactam in generic and princeps bags. Lower limit: lower limit of the unilateral 95 percent confidence interval on the mean. The mean, standard deviations and lower limit are expressed in percent of initial concentration. Initial concentration of piperacillin was nominally 4 g/120 mL (measured concentration 4.16 and 4.17 g/120 mL for generic and princeps respectively). Initial concentration of tazobactam was nominally 0.5 g/120 mL (measured concentration 0.42 and 0.43 g/120 mL for generic and princeps respectively).
As previously described in a first stability indicating study, quality control measured at each time of the study are correct [7].
The evolution of piperacillin and tazobactam concentration in princeps and generic versions are shown in Figure 3.
Initial concentration of piperacillin were similar between generic and princeps (4.16 vs 4.17 g/120 mL respectively, p=0.32) while initial concentration of tazobactam were slightly different (0.42 vs 0.43 g/120 mL respectively, p=0.0002).
The degradation rates of piperacillin and tazobactam were not significantly different between the generic and the princeps product (−0.15 %/day vs −0.13 %/day, p=0.09 and −0.14 %/day vs −0.13 %/day, p=0.44).
While the stability of penicillin is greatest at pH 7, and rapidly declines at pH 5.5 and below and at pH values above 8, the different values of pH for the princeps infusion (pH values mean 6.13 ± 0.27) or for the generic infusion (pH values mean 4.99 ± 0.37) does not influence the degradation rates [22].
For both versions and both molecules, the lower limit of the 95 % unilateral confidence interval on the mean concentration remained above 90 % of the initial concentration for at least 58 days (Table 1). These data support a stability of 58 days for both the generic and the princeps product. If one would use the more stringent limit of 95 % of the initial concentration that is sometimes recommended (REF), the stability would be of 30 days (Table 1).
Discussion
A previous study investigated long term-stability of piperacillin/tazobactam in dextrose 5 % in polyvinyl chloride bags [7]. Peaks for decomposition products could be observed on the chromatogram without interference from the peak corresponding to the intact drug. Observed overlap did not affect either peak shape or height.
The within-day and between day coefficients of variation for replicate analysis averaged 1.0 % (n=10) and 2.4 % (n=20), respectively, for tazobactam and 2.4 % (n=10) and 4.4 % (n=20) for piperacillin, respectively.
The results of stability in polyolefin bags look similar to the previous study after freezing and microwave thawing either for princeps or generic products.
In this experiment, no differences were detected in degradation rates between the princeps and the generic product. However, we cannot rule out the existence of small differences of degradation rates between princeps and generic product.
Nowadays, the definition of supported stability is criticized. The initial definition (ICH 2003) states that a product is considered stable as long as there was enough evidence that the mean concentration remains above the acceptable limit (90 % of the initial concentration). Some authors have proposed that the definition targets a certain proportion of the samples instead of the samples mean. A product would thus be considered stable as long as the concentration of a sufficiently high proportion (say 95 %) of samples remains above the acceptable limit [23]. Under this definition, and with the help of a 95 % unilateral prediction interval, a supported stability of 51 days was obtained.
Chemical stability was only evaluated and the microbiological aspects were not investigated. However, according to the chapter 797 of the United States Pharmacopeia (USP), this preparation can be assimilated to low-risk compounding [24].
Advance preparation of intravenous solutions by a centralized intravenous additive service might improve quality assurance, security, time management and be cost-saving for drug delivery [11, 12]. The preparation of intravenous treatments by a CIVAS contributes to the global management of treatment by providing ready-to-use injectable drugs with acceptable physicochemical and bacteriological quality and by relieving nursing staff from the tasks of infusion preparation [13].
As demonstrated for several other therapeutic infusions, freezing the solutions can extend long-term stability of ready-to-use injectable drugs [25] and microwave thawing allows to decrease the thawing time-consuming step.
Advance preparation of piperacillin/tazobactam generic infusion may be considered and added to the range of drugs reconstituted by a CIVAS [7, 17, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44].
Conclusion
Under the conditions of this study, Piperacillin/Tazobactam® Sandoz and Tazocin® Pfizer 4 g/120 mL of dextrose 5 % infusion in polyolefin bags remains both stable at least for 58 days at 5 ± 3 °C after freezing at −20 °C and microwave thawing.
Concerning the initial content or degradation rates, no substantial differences were detected between the princeps and the generic product.
This mode of preparation appears suitable for centralized pharmacy services, which could be of significant benefit to patients and of help to the nursing staff.
Advance preparation of piperacillin/tazobactam generic such as Piperacillin/Tazobactam® Sandoz infusion may be considered and added to the range of drugs reconstituted by a CIVAS.
The conclusions of this study are limited to the studied generic form only.
Conflict of interest statement: Authors state no conflict of interest. All authors have read the journal’s Publication ethics and publication malpractice statement available at the journal’s website and hereby confirm that they comply with all its parts applicable to the present scientific work.
References
1. Sweetman SC, editor. Martindale the complete drug reference, 36th ed. London, UK: Pharmaceutical Press, 2009.Suche in Google Scholar
2. Kauffman SE, Donnell RW, Hickey WS. Rationale and evidence for extended infusion of piperacillin-tazobactam. Am J Health Syst Pharm 2011;68:1521–6.10.2146/ajhp100694Suche in Google Scholar PubMed
3. Mathew M, Das Gupta V, Bethea C. Stability of piperacillin sodium sodium in the presence of tazobactam sodium in 5 % dextrose and normal saline injections. J Clin PharmTher 1994;19:397–9.10.1111/j.1365-2710.1994.tb00700.xSuche in Google Scholar PubMed
4. Park TW, Le-Bui LPK, Chung KC, Rho JP, Gill MA. Stability of piperacillin sodium – tazobactam sodium in peritoneal dialysis solutions. Am J Hosp Pharm 1995;52:2022–4.10.1093/ajhp/52.18.2022Suche in Google Scholar PubMed
5. Moon YSK, Chung KC, Chin A, Gill MA. Stability of piperacillin sodium – tazobactam sodium in polypropylene syringes and PVC minibags. Am J HealTh-SysT Pharm 1995;52:999–1001.10.1093/ajhp/52.9.999Suche in Google Scholar PubMed
6. Viaene E, Chanteux H, Servais H, Mingeot-Leclercq MP, Tulkens PM. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob Agents Chemother 2002;46:2327–32.10.1128/AAC.46.8.2327-2332.2002Suche in Google Scholar PubMed PubMed Central
7. Hecq J-D, Berlage V, Vanbeckbergen D, Jamart J, Galanti L. Effects of freezing, long-term storage, and microwave thawing on the stability of piperacillin plus tazobactam in 5 % dextrose for infusion. Can J Hosp Pharm 2004;5:276–82.Suche in Google Scholar
8. Rigge DC, Jones MF. Shelf lives of aseptically prepared medicines – stability of piperacillin/tazobactam in PVC and non-PVC bags. J Pharm Biomed Analy 2005;39:339–43.10.1016/j.jpba.2005.03.013Suche in Google Scholar PubMed
9. Arlicot N, Rochefort GY, Schlecht D, Lamoureux F, Marchand S, Antier D. Stability of antibiotics in portable pumps used for bronchial superinfection: guidelines for prescribers. Pediatrics 2007;120:1255–9.10.1542/peds.2007-0630Suche in Google Scholar PubMed
10. Donnelly RF. Stability of aseptically prepared Tazocin solutions in polyvinyl chloride bags. Can J Hosp Pharm 2009;62:226–31.10.4212/cjhp.v62i3.792Suche in Google Scholar PubMed PubMed Central
11. Koundalijan J. Setting up a CIVAS. In: Needle R, Sizer T., et al., editors. The CIVAS Handbook, 1st ed. London: Pharmaceutical Press, 1998: 1–5.Suche in Google Scholar
12. Hecq J-D. Centralized Intravenous Additive Services (CIVAS): the state of the art in 2010. Ann Pharm Fr 2011;69:30–7.10.1016/j.pharma.2010.09.002Suche in Google Scholar PubMed
13. Hecq J-D. Ten Years of European hospital pharmacy history: centralized intravenous additives services. Eur J Hosp Pharm 2004;10:47.Suche in Google Scholar
14. Claus B, Pauwels K, Baert M, Depoorter J, De Weerdt E, Boussery K, et al. Indisponibillité des médicaments dans les hôpitaux: gestion, causes et impact sur le budget. J Pharm Belg 2015;87:24–34.Suche in Google Scholar
15. Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc 2014;89:361–73.10.1016/j.mayocp.2013.11.014Suche in Google Scholar PubMed
16. Pauwels K, Simoens S, Casteels M, Huys I. Insights into European drug shortages: a survey of hospital pharmacists. Plos One 2015;10:e0119322.10.1371/journal.pone.0119322Suche in Google Scholar PubMed PubMed Central
17. Hecq J-D, Boitquin LP, Vanbeckbergen DF, Jamart J, Galanti L. Effect of the freezing conditions and microwave thawing power on the stability of cefuroxime in dextrose 5 % infusion polyolefin bags at 4 °C. Ann Pharmacother 2005;39:1244–8.10.1345/aph.1E686Suche in Google Scholar PubMed
18. Food and Drug Administration. Guideline for submitting documentation for stability studies of human drugs and biologics. Rockville, Maryland: Food and Drug Administration, 1987:551–79.Suche in Google Scholar
19. Bardin C, Astier A, Vulto A, Sewell G, Vigneron J, Trittler R, et al. Guidelines for the practical stability studies of anti-cancer drugs: a European consensus conference. Ann Pharm Fr 2011;69:221–31.10.1016/j.pharma.2011.07.002Suche in Google Scholar PubMed
20. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. Available at: https://www.R-project.org/Suche in Google Scholar
21. Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team. nlme: linear and nonlinear mixed effects models. R Package Version 2016;3:1–128.Suche in Google Scholar
22. Mc Evoy GK, editor. Handbook on injectable drugs, 19th ed. Bethesda, USA: ASHP, 2017.Suche in Google Scholar
23. Capen R, Christopher D, Forenzo P, Ireland C, Liu O, Lyapustina S, et al. On the shelf life of pharmaceutical products. AAPS Pharm Sci Tech 2012;13:911–18.10.1208/s12249-012-9815-2Suche in Google Scholar PubMed PubMed Central
24. Trissel LA. The new national standard for sterile preparation. Hosp Pharm 2004;39:900–04.Suche in Google Scholar
25. Hecq J-D, Godet M, Jamart J, Galanti L. Microwave freeze-thaw technique of injectable drugs A review from 1980 to 2014. Ann Pharm Fr 2015;73:436–41.10.1016/j.pharma.2015.04.009Suche in Google Scholar PubMed
26. Schlesser V, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long-term storage and microwave thawing on the stability of cefepime in 5 % dextrose infusion P. V. C. bags Int J Pharm Compd 2002;6:391–4.Suche in Google Scholar
27. Lebrun J, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long-term storage and microwave thawing on the stability of tramadol in 5 % dextrose infusion P. V. C. bags. Int J Pharm Compd 2004;2:156–9.Suche in Google Scholar
28. Boitquin L, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Stability of sufentanil citrate with levobupivacaine HCl in 0.9 % sodium chloride infusion after microwave freeze-thaw treatment. Ann Pharmacother 2004;38:1836–9.10.1345/aph.1D564Suche in Google Scholar PubMed
29. Hecq J-D, Schlesser V, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long-term storage, and microwave thawing on the stability of cefuroxime sodium in 5 % dextrose infusion polyvinyl chloride bags. Eur J Hosp Pharm Sci 2005;1:23–5.Suche in Google Scholar
30. Hecq J-D, Boitquin LP, Vanbeckbergen D, Jamart J, Galanti LM. Effect of freezing, long-term storage, and microwave thawing on the stability of ketorolac tromethamine. Ann Pharmacother 2005;39:1654–8.10.1345/aph.1G194Suche in Google Scholar PubMed
31. Rodenbach MP, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti G. Effect of freezing, long-term storage, and microwave thawing on the stability of vancomycine hydrochloride in 5 % dextrose infusions. Eur J Hosp Pharm Sci 2005;11:111–13.Suche in Google Scholar
32. Hecq J-D, Evrard JM, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long term storage and microwave thawing on the stability of ceftriaxone sodium in 5 % dextrose infusion polyolefin bags at 2-8 °C. Eur J Hosp Pharm Sci 2006;12:52–6.Suche in Google Scholar
33. Hecq J-D, Boitquin L, Lebrun C, Vanbeckbergen D, Evrard JM, Jamart J, et al. Freeze thaw treatment of ketorolac tromethamine in 5 % dextrose infusion polyolefine bags: effect of drug concentration and microwave power on the final long-term stability at 4 °C. Eur J Hosp Pharm Sci 2006;12:72–5.Suche in Google Scholar
34. Cadrobbi J, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long-term storage and microwave thawing on the stability of sodium folinate in dextrose 5 % polyolefin bags. Eur J Hosp Pharm Sci 2009;15:15–19.Suche in Google Scholar
35. Galanti L, Lebitasy MP, Hecq J-D, Vanbeckbergen D, Jamart J. Long-term stability of 5-Fluorouracil in 0.9 % sodium chloride after freezing, microwave thawing and refrigeration. Can J Hosp Pharm 2009;62:34–8.10.4212/cjhp.v62i1.115Suche in Google Scholar PubMed PubMed Central
36. Lebitasy M, Hecq J-D, Athanassopoulos A, Vanbeckbergen D, Jamart J, Galanti L. Effect of Freezing, microwave thawing, and long-term storage on the stability of calcium levofolinate in 5 % dextrose infusion polyolefin bags. J Clin Pharm Ther 2009;34:423–8.10.1111/j.1365-2710.2009.01043.xSuche in Google Scholar PubMed
37. François J, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Effect of freezing, long-term storage and microwave thawing on the stability of a mixture of diclofenac and sodium bicarbonate in dextrose 5 % polyolefin bags. Ann Pharm Fr 2009;67:427–32.10.1016/j.pharma.2009.09.004Suche in Google Scholar PubMed
38. Rollin C, Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Effects of freezing and microwave thawing on the stability an ondansetron/dexamethasone mixture stored in dextrose 5 % polyolefin bags. Ann Pharmacother 2011;45:130–1.Suche in Google Scholar
39. Hecq J-D, Vanbeckbergen D, Jamart J, Galanti L. Physico-chemical analysis of several injectable drug in ready-to-use infusion after microwave freeze-thaw treatment and final storage at 5 ± 3 °C. Ann Pharm Fr 2011;69:270–6.10.1016/j.pharma.2011.08.001Suche in Google Scholar PubMed
40. Rolin C, Hecq J-D, Tulkens P, Vanbeckbergen P, Jamart J, Galanti L. Long-term stability of temocillin in dextrose 5 % and in sodium chloride 0.9 % polyolefin bags at 5 ± 3 °C after freeze-thaw treatment. Ann Pharm Fr 2011;69:296–301.10.1016/j.pharma.2011.09.004Suche in Google Scholar PubMed
41. Hecq J-D, Jamart J, Galanti L. Microwave freeze-thaw treatment of dose-banded cytotoxics injectable drugs: a review of the literature from 1980 to 2011. Ann Pharm Fr 2012;70:227–35.10.1016/j.pharma.2012.04.003Suche in Google Scholar PubMed
42. Hecq J-D, Godet M, Gillet P, Jamart J, Galanti L. Long-term stability of morphine Hydrochoride in 0.9 % NaCl infusion polyolefin bags after freeze-thaw treatment and in polypropylene syringes at 5 °C ± 3 °C. Int J Pharm Compd 2014;18:78–82.Suche in Google Scholar
43. Dewulf J, Galanti L, Godet M, Gillet P, Jamart J, Hecq J-D. Long-term stability of acyclovir in 0.9 % NaCl infusion polyolefin bags at 5 °C ± 3 °C after freeze-thaw treatment: a generic product versus the brand name. Ann Pharm Fr 2015;73:108–13.10.1016/j.pharma.2014.10.003Suche in Google Scholar PubMed
44. Hecq J-D, Rolin C, Godet M, Gillet P, Jamart J, Galanti LM. Long term stability of esomeprazole in 5 % dextrose infusion polyolefin bags at 5 °C ± 3 °C after microwave freeze-thaw treatment. Int J Pharm Compd 2015;19:521–4.Suche in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- After Ten Issues Our Journal Has Found Its Audience and Main Topics
- Research Articles
- HPLC – Quality by Design Approach for Simultaneous Detection of Torsemide, Spironolactone and Their Degradant Impurities
- Physico-Chemical Stability of Sodium Thiosulfate Infusion Solutions in Polyolefin Bags at Room Temperature over a Period of 24 Hours
- Long-Term Stability Comparison between an Original and a Generic Version of Piperacillin/Tazobactam in Dextrose 5 % Infusion Polyolefin Bags at 5 ± 3 °C after Microwave Freeze-Thaw Treatment
- Environmental and Product Contamination during the Preparation of Antineoplastic Drugs with Robotic Systems
- Qualification and Performance Evaluation of an Automated System for Compounding Injectable Cytotoxic Drugs
- Short Communication
- Feedback on the Centralization of Intrathecal Analgesic Preparations in Hospital Pharmacy
- Opinion Paper
- Pharmaceutical Technology in Practice: A Personal View
Artikel in diesem Heft
- Frontmatter
- Editorial
- After Ten Issues Our Journal Has Found Its Audience and Main Topics
- Research Articles
- HPLC – Quality by Design Approach for Simultaneous Detection of Torsemide, Spironolactone and Their Degradant Impurities
- Physico-Chemical Stability of Sodium Thiosulfate Infusion Solutions in Polyolefin Bags at Room Temperature over a Period of 24 Hours
- Long-Term Stability Comparison between an Original and a Generic Version of Piperacillin/Tazobactam in Dextrose 5 % Infusion Polyolefin Bags at 5 ± 3 °C after Microwave Freeze-Thaw Treatment
- Environmental and Product Contamination during the Preparation of Antineoplastic Drugs with Robotic Systems
- Qualification and Performance Evaluation of an Automated System for Compounding Injectable Cytotoxic Drugs
- Short Communication
- Feedback on the Centralization of Intrathecal Analgesic Preparations in Hospital Pharmacy
- Opinion Paper
- Pharmaceutical Technology in Practice: A Personal View

