Abstract
Glycerol acetals were prepared from glycerol and aromatic aldehydes (benzaldehyde, anisaldehyde, or furfural) using a solid acid (Amberlyst-15) catalyst. The acetals show promising antioxidant activity using the DPPH test with the glycerol/anisaldehyde acetal showing the best antioxidant properties, attributed to the additional radical stabilization by the electron-releasing methoxy group. The acetals formed have a wide range of potential industrial applications particularly enabling integration of the biodiesel manufacturing chain.
Introduction
The transesterification of fats and oils to biodiesel produces approximately 10 wt % of glycerol as a byproduct [1]. With the increasing demand for biodiesel, especially in Europe and the Americas, there is the potential for glycerol to become an important feedstock for the chemical industry. The chemical transformation of glycerol into value-added products has therefore attracted interest, with a variety of routes available [1–3]. Starting materials for polymers can be formed via dehydration and oxydehydration over heterogeneous catalysts to yield acrolein and acrylic acid [4], whilst glycerol reforming produces syn gas which can be coupled with Fischer-Tropsch processes to produce hydrocarbons [5]. Glycerol hydrogenolysis affords mainly propylene glycol [6], however, modifying the catalyst formulation and reaction conditions can produce propene with a high selectivity [7].
Alternatively, glycerol can be transformed into fuel additives. This is particularly beneficial as it integrates the whole industrial chain enabling biodiesel plants to add value and utilise the glycerol co-product on-site to improve one or more of the biodiesel properties. Biodiesel can be produced from many different sources including vegetable oils, such as soybean, canola and palm, waste cooking oils and sewage sludge. The chemical structure of the fatty esters formed affects the properties of the biodiesel [8]. Saturated chains, common in palm oil and tallow, generally increase the pour point, requiring the use of additives to improve the cold flow properties [9]. On the other hand, unsaturated chains, especially with two or more double bonds and bis-allylic sites [10], favors oxidation and formation of degradable products, requiring the addition of antioxidants to the biodiesel [11]. This is the case for soybean oil, the main starting material for biodiesel production in both Brazil and the US [12], which is composed of 50 % linoleic and linolenic acids, having two and three double bonds, respectively [13]. Thus, normally, an antioxidant needs to be added to soybean biodiesel to slow down the oxidation process.
Oxidation of biodiesel occurs through the abstraction of one methylene hydrogen atom, with those adjacent to the double bond more favorable, forming a free radical [10]. The presence of two or more double bonds favors the process, due to additional delocalization and stabilization (Scheme 1). An antioxidant slows down the methylene hydrogen atom abstraction, by preferentially reacting with the source of free radicals to form a highly delocalized and stable species (Scheme 2) [14]. Aromatic phenols and amines are among the most commonly used commercial antioxidants [11, 14]. The presence of electron donating groups in the aromatic ring generally increases the antioxidant character. For instance, BHT (butyl hydroxy toluene), which is one of the most widely used antioxidants, has a phenolic structure with tert-butyl and methyl group substituents.

Initial oxidation step, showing the formation of delocalized free radical. (R = alkyl radical; X = alkylcarboxyl moiety; Y = radical source).
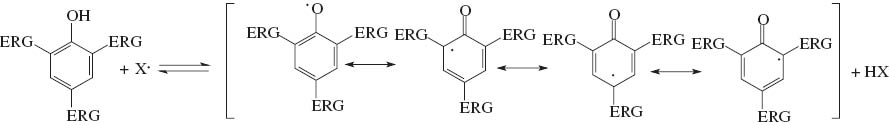
Antioxidant activity through the formation of highly delocalized free radical (ERG = electron releasing group).
About half of the glycerol weight corresponds to oxygen atoms and makes this molecule a good raw material for the synthesis of ethers, acetals, ketals and esters via acid catalysed reactions with alcohols, aldehydes, ketones and carboxylic acids or methyl esters respectively [1, 15–18]. We have previously demonstrated that solketal, the product of the reaction of acetone with glycerol, improves the octane number and reduces gum formation on addition to gasoline [17], whilst glycerol acetals improve the cold flow properties [16]. Here we continue these investigations, studying the antioxidant properties of acetals from the reaction of aromatic aldehydes (benzaldehyde, anisaldehyde and furfural) and glycerol and their potential as biodiesel fuel additives. Carefully selected substituents on the aromatic ring may enhance the antioxidant properties of these substances. This work highlights the potential for integrating the whole biodiesel manufacturing cycle by valorizing the byproduct glycerol, and potentially reducing the cost of the added antioxidants to biodiesel.
Results and discussion
Preparation of the acetals
The acetals were prepared by reacting glycerol and aromatic aldehydes in the presence of an Amberlyst-15 acid resin. To improve the yield, toluene and a Dean-Stark apparatus were used to remove the water formed. This procedure was successfully applied for both benzaldehyde and anisaldehyde. However, for furfural the reaction medium darkened after a few minutes and attempts to characterize and isolate the products were laborious. Furfural may undergo oligomerization in the presence of acidic catalysts [19], therefore to reduce this problem a smaller mass of catalyst was used along with cyclohexane, which has a lower azeotrope boiling temperature of 70 °C [20].
The products were characterized by GC/MS and NMR with four isomers of each acetal observed by both techniques. Table 1 shows the yields of the combined isomers products after isolation. The NMR spectra display multiplet bands between 4.7 and 3.4 ppm due to the methine, methylene and hydroxyl protons of the dioxacycle, with multiplets at higher chemical shifts from protons on the furan and benzene rings. Four singlet peaks between 6.0 and 5.4 ppm are characteristic of the H-2 protons of the Z + E isomers of the five-membered ring and six-membered ring compounds (Fig. 1 and Table 1) [21]. In addition, enantiomers of these compounds will be present which could not be separated on the GC column used. We did not attempt to isolate the isomers, assuming that they should present similar antioxidant activities. For simplicity, the mixture of isomers will be referred to as glycerol/aldehyde acetals. The yields of all three acetals were high (Table 1) and similar to the yields found for the C3-C6 aldehydes reacted with glycerol previously [16]. Following distillation the products all had a purity of 98–99 %.
Isolated yields of the glycerol acetals.
| Acetal | Yield (%) | |
|---|---|---|
| Glycerol/benzaldehyde (GB) | 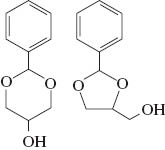 | 70 |
| Glycerol/anisaldehyde (GA) | 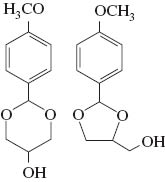 | 75 |
| Glycerol/furfural (GF) | 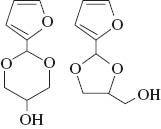 | 80 |
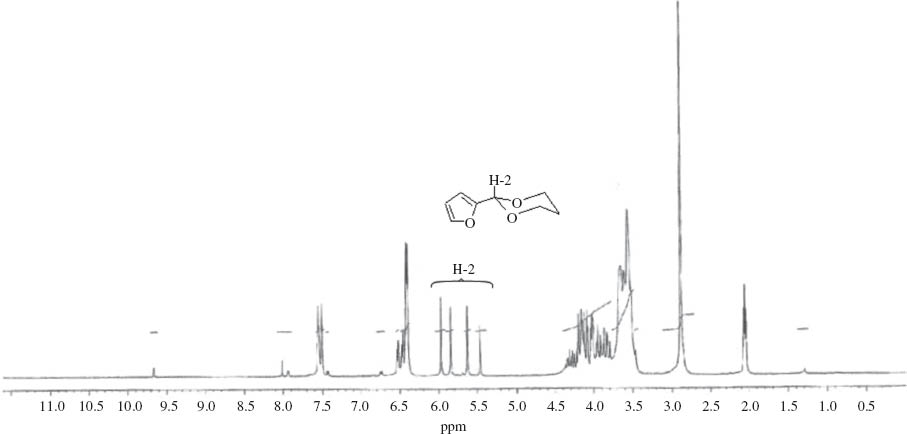
1H NMR spectra of the acetal isomers from the reaction of furfural and glycerol highlighting the characteristic singlet peaks from the H-2 protons.
Antioxidant activity
The antioxidant activity of the acetals was initially tested following a standard procedure using DPPH· [22]. During the test the spectrometric decay of the diphenyl-picryl-hydrazine radical (DPPH·) is followed in the presence of the potential antioxidant under controlled conditions. Scheme 3 shows the reaction that is monitored. The DPPH· radical shows a strong absorption at 516 nm, giving the solution a deep violet color [22]. Upon abstraction of a hydrogen atom and formation of diphenyl-picryl-hydrazine, the violet color is lost with a residual pale yellow color remaining. The kinetics of the radical decay due to the antioxidant can be monitored with a UV/visible spectrometer and a comparative behavior among several compounds can be established [23].
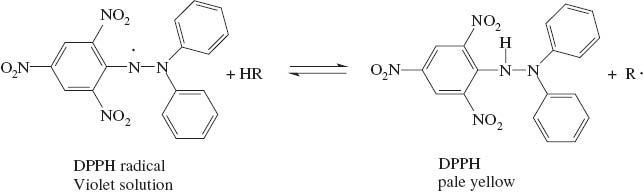
DPPH test of antioxidant activity (H-R stands for a potential antioxidant molecule).
The decay of the DPPH· radical over time was studied using various concentrations of the three acetals formed in this study, with the percentage of DPPH· remaining at the steady state observed (Fig. 2). With addition of sufficient concentration of the acetal, a sharp initial drop in the DPPH· concentration is observed which reaches the steady-state over 2 h.
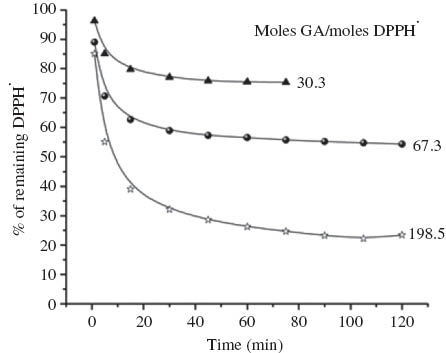
Antioxidant activity of glycerol/anisaldehyde acetal (GA) with varying ratios of GA/DPPH·.
The antiradical activity was then determined as the amount of antioxidant necessary to decrease the initial DPPH· radical concentration by 50 %, EC50 (Fig. 3). Only the glycerol/anisaldehyde acetal (GA) displayed a significant antioxidant activity with an EC50 = 80. This is similar to coumaric acid or vanillin, but higher than that of BHT [23]. With an acetal/DPPH molar ratio of 32 351 the glycerol/furfural acetal was able to reduce the DPPH· concentration to 50 % at the steady state (Fig. 4). However, even at this high molar ratio the DPPH· radical concentration was only reduced to 70.4 % at the steady state using the glycerol/benzaldehyde acetal. Higher ratios could not be achieved due to the viscosity of the acetal and poor miscibility with the DPPH· solution. This therefore indicates significant differences in the antioxidant activities between the three acetals in the order GA >> GF > GB.
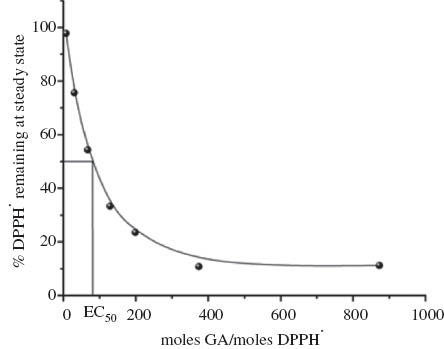
Determination of EC50 for glycerol/anisaldehyde acetal, the ratio of GA/DPPH· at which the percentage of DPPH· remaining at the steady state is 50 %.
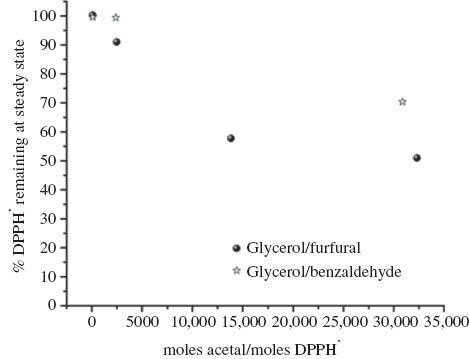
Change in the percentage of DPPH· remaining at the steady state with acetal/ DPPH· molar ratio using glycerol/furfural and glycerol/benzaldehyde acetals.
The antioxidant activities and the kinetics of DPPH· radical abstraction indicate that the antioxidant capabilities of the glycerol acetals studied rely on the abstraction of their benzylic hydrogen atom to form a delocalized radical (Scheme 4). The presence of a methoxy group in the para position of the glycerol/anisaldehyde acetal will greatly increase the stability of the radical formed, as well as the two oxygen atoms of the dioxane and dioxolan ring (oxygen atoms of the acetal function), thereby contributing to its increased antioxidant activity.
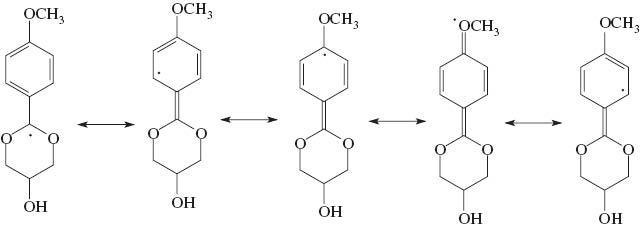
Free radical formed upon the abstraction of the benzylic hydrogen atom, showing electron delocalization in the aromatic ring.
Conclusion
Glycerol acetals were successfully formed in high yields and purity from the reaction of glycerol with aromatic aldehydes. These acetals were shown to have antioxidant activity by a standard DPPH standard test in the order anisaldehyde > furfural > benzaldehyde. The stability of the glycerol/anisaldehyde product was attributed to the additional radical stabilization by the electron releasing methoxy group.
Experimental
Amberlyst-15 was purchased from Sigma-Aldrich, benzaldehyde from Reagen, cyclohexane from Isofar and all other reagents from Vetec.
Acetals
Cyclic acetals of glycerol were synthesised by mixing glycerol (62 g), an activated Amberlyst-15 catalyst (3.8 g) and either a toluene or cyclohexane solvent (70 mL) in a Dean-Stark apparatus. The solution was stirred and once reflux was reached the required aldehyde (0.75 mol) was added. The reaction mixture was heated under reflux for 3–4 h. Once cooled the product was filtered and the solution transferred to a separation funnel. The residual glycerin was separated by washing with 0.1 M NaHCO3 and water. Finally, the acetals were recovered by distillation under reduced pressure with the products stored in a freezer until use.
Analysis of samples
All of the samples were analyzed by GC-MS and NMR. An Agilent 6850 GC was used fitted with a HP-5MS 5 % Phenyl Methyl Siloxane column with a nominal size of 30 m × 250 μm × 0.25 μm. 0.2 μL of sample was injected onto a column heated to 120 °C, with a split of 25:1 with the temperature heated linearly to 220 °C. 1H NMR was carried out on a Bruker DPX 200 using CDCl3 or deuterated acetone as a solvent.
The antioxidant activity of the acetals was analyzed using a UV-vis spectrophotometer Biospectro SP-22. Solutions containing DPPH (3.9 mL, 0.00006 M) and acetals of various concentrations (0.1 mL) were analyzed at a wavelength of 516 nm after 5–120 min reaction. The absorbance of the initial DPPH· solutions were also analyzed. The percentage of DPPH· remaining was calculated.
Article note: A collection of invited papers based on presentations on the Clean Energy Through Chemistry theme at the 44th IUPAC Congress, Istanbul, Turkey, 11–16 August 2013.
Acknowledgments
The authors would like to acknowledge financial support and fellowships from CNPq, CAPES, FAPERJ and IBP.
References
[1] A. Behr, J. Eilting, K. Irawadi, J. Leschinski, F. Lindner. Green Chem. 10, 13 (2008).Suche in Google Scholar
[2] F. Jérôme, Y. Pouilloux, J. Barrault. ChemSusChem. 1, 586 (2008).Suche in Google Scholar
[3] M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi, C. D. Pina. Angew. Chem. Int. Ed. 46, 4434 (2007).Suche in Google Scholar
[4] J. Deleplanque, J.-L. Dubois, J.-F. Devaux, W. Ueda. Catalysis Today. 157, 351 (2010).Suche in Google Scholar
[5] D. A. Simonetti, J. Rass-Hansen, E. L. Kunkes, R. R. Soares, J. A. Dumesic. Green Chem. 9, 1073 (2007).Suche in Google Scholar
[6] M. A. Dasari, P. P. Kiatsimkul, W. R. Sutterlin, G. J. Suppes. Appl. Catal. A. 281, 225 (2005).Suche in Google Scholar
[7] C. J. A. Mota, V. L. C. Gonçalves, J. C. Fadigas, R. Gambetta. US patent 0184 216 A1 (2011).Suche in Google Scholar
[8] G. Knothe. Fuel Process. Tech. 86, 1059 (2005).10.1016/j.fuproc.2004.11.002Suche in Google Scholar
[9] P. Lv, Y. Cheng, L. Yang, Z. Yuan, H. Li, W. Luo. Fuel Process. Tech. 110, 61 (2013).Suche in Google Scholar
[10] R. L. McCornick, M. Ratcliffe, L. Moens, R. Lawrence. Fuel Process. Tech. 88, 651 (2007).Suche in Google Scholar
[11] R. O. Dunn. Fuel Process. Tech. 86, 1071 (2005).10.1016/j.fuproc.2004.11.003Suche in Google Scholar
[12] A. C. Pinto, L. L. N. Gaurieiro, M. J. C. Rezende, N. M. Ribeiro, E. A. Torres, W. A. Lopes, P. A. P Pereira, J. B. Andrade. J. Braz. Chem. Soc. 16, 1313 (2005).Suche in Google Scholar
[13] H. Tang, R. C. De Guzman, S. O. Salley, S. K. Y. Ng. Lipid Technology. 20, 249 (2008).Suche in Google Scholar
[14] G. Knoethe. Fuel Process. Tech. 88, 669 (2007).10.1016/j.fuproc.2007.01.005Suche in Google Scholar
[15] C. R. B. da Silva, V. L. C. Gonçalves, E. R. Lachter, C. J. A. Mota. J. Braz. Chem. Soc. 20, 201 (2009).Suche in Google Scholar
[16] P. H. R. Silva, V. L. C. Gonçalves, C. J.A. Mota. Bioresour. Technol. 101, 6225 (2010).Suche in Google Scholar
[17] C. J. A. Mota, C. X. A. da Silva, N. Rosenbach, J. Costa, F. da Silva. Energ. Fuel. 24, 2733 (2010).Suche in Google Scholar
[18] J. Barrault, S. Bancquart, Y. Pouilloux. C. R. Chimie. 7, 593 (2004).Suche in Google Scholar
[19] R. Roque-Malherbe, J. Oñate-Martinez, E. Navarro. J. Mat. Sci. Letters. 12, 1037 (1993).Suche in Google Scholar
[20] R. C. Weast (Ed.). Handbook of Chemistry and Physics, 49th Edition. CRC, Cleveland, (1969).10.1097/00000441-196906000-00020Suche in Google Scholar
[21] E. V. Gromachevskaya, F. V. Kvitkovsky, E. B. Usova, V. G. Kulnevich. Chemistry of Heterocyclic Compounds 40, 8 (2004).10.1023/B:COHC.0000046685.17653.c4Suche in Google Scholar
[22] P. Molyneux. Songklanakarin J. Sci. Technol. 26, 211 (2004).Suche in Google Scholar
[23] W. Brand-Williams, M. E. Cuvelier, C. Berset. Lebensm.-Wiss. u.-Technol., 28, 30 (1995).Suche in Google Scholar
©2014 IUPAC & De Gruyter Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Preface
- 44th IUPAC World Chemistry Congress: Clean Energy Through Chemistry
- Conference papers
- The energy landscape concept and its implications for synthesis planning
- Near-IR absorbing Bodipy functionalized SPIONs: a potential magnetic nanoplatform for diagnosis and therapy
- Glycerol acetals with antioxidant properties
- Lithiated oxazolinyloxiranes and oxazolinylaziridines: key players in organic synthesis
- Access to pyrrole-based heterocyclic compounds via addition of pyrrole to C=C and C=N bonds
- Hydrogenation of carboxylic acid derivatives with bifunctional ruthenium catalysts
- Generation of singlet oxygen (1O2) from hydrogen peroxide decomposition by in situ generated hypervalent iodoarene reagents
- Organometallic macrocycles and cages based on bis(amidinate) ligands
- Anisotropic core-shell Fe3 O4 @Au magnetic nanoparticles and the effect of the immunomagnetic separation volume on the capture efficiency
- Recent investigations of bioactive natural products from endophytic, marine-derived, insect pathogenic fungi and Thai medicinal plants
- Chemoecological studies on marine natural products: terpene chemistry from marine mollusks
- IUPAC Recommendations
- Abbreviations of polymer names and guidelines for abbreviating polymer names (IUPAC Recommendations 2014)
- IUPAC Technical Report
- Toward a comprehensive definition of oxidation state (IUPAC Technical Report)
Artikel in diesem Heft
- Frontmatter
- Preface
- 44th IUPAC World Chemistry Congress: Clean Energy Through Chemistry
- Conference papers
- The energy landscape concept and its implications for synthesis planning
- Near-IR absorbing Bodipy functionalized SPIONs: a potential magnetic nanoplatform for diagnosis and therapy
- Glycerol acetals with antioxidant properties
- Lithiated oxazolinyloxiranes and oxazolinylaziridines: key players in organic synthesis
- Access to pyrrole-based heterocyclic compounds via addition of pyrrole to C=C and C=N bonds
- Hydrogenation of carboxylic acid derivatives with bifunctional ruthenium catalysts
- Generation of singlet oxygen (1O2) from hydrogen peroxide decomposition by in situ generated hypervalent iodoarene reagents
- Organometallic macrocycles and cages based on bis(amidinate) ligands
- Anisotropic core-shell Fe3 O4 @Au magnetic nanoparticles and the effect of the immunomagnetic separation volume on the capture efficiency
- Recent investigations of bioactive natural products from endophytic, marine-derived, insect pathogenic fungi and Thai medicinal plants
- Chemoecological studies on marine natural products: terpene chemistry from marine mollusks
- IUPAC Recommendations
- Abbreviations of polymer names and guidelines for abbreviating polymer names (IUPAC Recommendations 2014)
- IUPAC Technical Report
- Toward a comprehensive definition of oxidation state (IUPAC Technical Report)

