Morphology, genetics and echolocation calls of the genus Kerivoula (Chiroptera: Vespertilionidae: Kerivoulinae) in Thailand
-
Bounsavane Douangboubpha
, Sara Bumrungsri
Abstract
Following extensive field work in Thailand (2010–2013) and the examination of 155 museum specimens, this paper reviews and examines the taxonomy of the genus Kerivoula in Thailand, based on morphology, genetics, and echolocation call characteristics. Seven species (as currently understood), Kerivoula papillosa, K. kachinensis, K. hardwickii, K. titania, K. pellucida, K. krauensis and K. minuta, were analysed in detail. Thai specimens of two species, K. picta and K. whiteheadi, were not available for study. Morphological data suggested a complex pattern of possible cryptic species, with at least five morphotypes, based on cranial data for K. papillosa, and nine for K. hardwickii, as currently understood. An analysis of the mitochondrial DNA (COI) from Thai specimens identified three genetic lineages in K. papillosa and K. hardwickii, respectively. The echolocation calls data differed significantly, albeit with individual acoustic parameters overlapping considerably, among genetic lineages. The taxonomic status of the various lineages and morphotypes are discussed.
Introduction
Southeast Asia is one of the world’s biodiversity hotspots (Myers et al. 2000). Currently, nearly 500 species of mammals are recognised from mainland Southeast Asia (Francis 2008) of which approximately one third are bats (Kingston 2010). However, the results of a recent DNA barcoding study, based on specimens from both mainland Southeast Asia and Borneo and Java, suggest that bat diversity may be twice what it is thought to be today. Many species that were described on the basis of morphological characters were found to contain multiple, distinct genetic lineages. Some of these lineages may prove to be valid species (Francis et al. 2010).
Recent studies by Francis and Eger (2012) and Soisook et al. (2013a,b) and others have shown the value of combining multiple data sets, i.e., morphology and genetics, for understanding the true diversity of species in the more complex, small vespertilionid groups, including Murina Gray, 1842 and Kerivoula Gray, 1842. This is especially the case where the species are primarily cryptic (Helversen et al. 2001, Mayer and von Helversen 2001, Kiefer et al. 2002), and there is a lack of material for comparison (Anwarali et al. 2010).
The genus Kerivoula is a large genus in the family Vespertilionidae, which is considered currently to include 22 species (Bates et al. 2004, 2007, Simmons 2005, Francis et al. 2007). Its disjunct distribution comprises sub-Sahara Africa and in Asia, from India to the Philippines and New Guinea (Corbet and Hill 1992). Nine species are currently recorded from Thailand (Table 1) (Bumrungsri et al. 2006, Bates et al. 2007, Soisook et al. 2007, Francis 2008, Douangboubpha et al. 2014).
List of Kerivoula species and their synonyms currently known from Thailand, based on the taxonomy of Simmons (2005); type localities are included for each taxon.
| No | Species (sensu Simmons 2005) | Synonym | Type locality |
|---|---|---|---|
| 1 | K. papillosa (Temminck, 1840) | Bantam, Western Java | |
| K. p. malayana Chasen, 1940 | Ginting Bedai, Selangor-Pahang boundary, Malaysia | ||
| 2 | K. kachinensis Bates et al., 2004 | Namdee Forest, Bhamo Township, Kachin State, Myanmar | |
| 3 | K. titania Bates et al., 2007 | Seima Biodiversity Conservation Area, Mondul Kiri Province, Cambodia | |
| 4 | K. hardwickii (Horsfield, 1824) | Java | |
| K. fusca Dobson, 1871 | unknown | ||
| K. depressa Miller, 1906a | Biapo, Carin Hill, North-east of Tounghoo, South-eastern Myanmar | ||
| K. engana Miller, 1906b | Dua Island, about 1 mile from Enggano Island, South-west of Sumatra | ||
| K. crypta Wroughton and Ryley, 1913 | Karbidetta forest, Shimoga, Southern India | ||
| K. malpasi Phillips, 1932 | Kumbalgamuwa, Mulhalkelle district (c. 30 miles east of Kandy), Central Province, Sri Lanka | ||
| 5 | K. pellucida (Waterhouse, 1845) | Philippines | |
| K.bombifrons Lyon, 1911 | Along Matam River, Western Borneo | ||
| 6 | K. krauensis Francis et al., 2007 | Kuala Lompat, Krau Wildlife Reserve, Pahang, peninsular Malaysia | |
| 7 | K. whiteheadi Thomas, 1894 | Molino, Isabella, North-eastern Luzon Island, Philippines | |
| K. pusilla Thomas, 1894 | Mount Mulu, Eastern Sarawak, Borneo | ||
| K. bicolor Thomas, 1904 | Biserat, Jalor (=Yala), Southern Thailand | ||
| 8 | K. picta (Pallas, 1767) | Ternate Island, Northern Moluccas | |
| K. p. rubellus Kerr, 1792 | unknown | ||
| K. p. bellissima Thomas, 1906 | Pak-hoi, Guangdong, Southern China | ||
| 9 | K. minuta Miller, 1898 | Lay Song Hong, Trang, Southern Thailand |
Recent genetic analyses reveal that bats referred to Kerivoula papillosa (Temminck, 1840) and K. lenis Thomas, 1916 include at least four monophyletic types (Francis et al. 2010). Previously, Ellerman and Morrison-Scott (1951) and Hill (1965) had included lenis as a distinct race of K. papillosa, with a distribution restricted to India. The nominative form, K. p. papillosa, was restricted to Java and K. p. malayana Chasen, 1940 restricted to Indochina, peninsular Malaysia and Borneo. Hill (1983) referred specimens from Sulawesi to K. p. malayana.
Subsequently, Payne and Francis (1985) noted that the bats referred to K. papillosa from Borneo included more than one species. This view was not followed by Corbet and Hill (1992). However, Kingston et al. (1999) found that K. papillosa from peninsular Malaysia comprised two size classes: K. papillosa S[mall] (forearm length (FA): 37.8–40.0 mm) and K. papillosa L[arge] (FA: 40.5–45.0 mm). They also noted that they exhibited average differences in acoustic characters. Subsequently, Vanitharani et al. (2003) elevated the taxon K. lenis from the synonymy of K. papillosa to species level and compared it to K. papillosa from Southeast Asia. Simmons (2005) listed K. lenis as a separate species and the taxon malayana as a valid subspecies of K. papillosa.
Anwarali et al. (2010) concluded that bats previously referred to K. papillosa included more than one genetic lineage and morphotype. On the basis of size, they identified three forms, K. lenis (FA: 38.7–38.9 mm) from peninsular Malaysia and southwestern Borneo, K. p. papillosa (FA: 39.5–43.0 mm) from peninsular Malaysia and central Borneo and Kerivoula sp. (45.0–46.5 mm) from central Borneo. The taxon Kerivoula sp. was considered to be of similar size to K. p. malayana but was not included in this taxon as “all specimens…were collected from outside the known distribution of K. p. malayana” (Anwarali et al. 2010). Therefore, the specimens were referred to “an undescribed species pending genetic data from K. p. malayana”. However the taxonomic basis for this conclusion is not clear as Chasen (1940) had already referred two specimens from Sarawak, northern Borneo to malayana.
Hasan and Abdullah (2011) studied the morphology of Malaysian Kerivoula. Like Anwarali et al. (2010), they also found three distinct morphotypes within the papillosa complex. The smallest, from Sarawak, they referred to K. lenis (FA: 39.9–40.9 mm). The other two were assigned to K. papillosa type large [type L] (FA: 44.7–47.3 mm), based on specimens from Sarawak and Sabah, and K. papillosa type small [type S] (FA: 42.2–43.7 mm), based on specimens from Sarawak. These latter two taxa were, respectively, referred to Kerivoula indet. (“a yet to be described species”) and K. p. malayana.
The literature concerning K. hardwickii (Horsfield, 1824) (sensu Simmons 2005) is extensive but confusing and often contradictory. Ellerman and Morrison-Scott (1951) recognised four subspecies, namely: the nominative form from Darjeeling (India), Malaysia, Borneo, Java, Bali and Celebes; depressa Miller, 1906 a from southern Myanmar and Szechuan and Fukien in China; crypta Wroughton and Ryley, 1913 from Southern India and Upper Myanmar; and malpasi Phillips, 1932 from Sri Lanka. Hill (1965) included comments, descriptive characters, and/or measurements for K. h. hardwickii (including fusca Dobson, 1871 – type locality unknown) and four subspecies, namely: crypta, depressa, malpasi and engana Miller, 1906b from Western Sumatra. He noted that depressa could be distinguished from hardwickii by its “relatively flattened braincase”. Subsequently, Hill (1975) noted that a specimen of K. hardwickii from Chiang Mai, northern Thailand had “a relatively full, high braincase”. He referred it to K. h. hardwickii, which he considered to have a predominantly Malaysian distribution. In contrast, he also noted that a specimen from Phetchabun Province, central Thailand, was “in excellent agreement with the low crowned subspecies K. h. depressa from Burma and southern China”. Lekagul and McNeely (1988) suggested that the taxon depressa is found in the Tenasserim Range of western Thailand, whereas hardwickii is found in the remainder of the country. Meanwhile, Corbet and Hill (1992) stated that specimens from the western part of the range of K. hardwickii (from India to Vietnam) have relatively flattened skulls. Previously, Taylor (1934) had remarked that specimens from the Philippines were generally smaller than those from more western parts of the range. Sinha (1999) based on research in India, included depressa and crypta as synonyms of the nominative form.
Simmons (2005), following Corbet and Hill (1992) and Sinha (1999), did not recognise any subspecies of K. hardwickii. Bates et al. (2007) suggested that the smaller, flat-headed taxon (BH<5.1 mm) may prove to be K. depressa, whereas that with the slightly larger, domed-skull taxon (BH>5.1 mm) may be referable to the true K. hardwickii. They did not include information on the possible distributional ranges of these two taxa. Francis et al. (2007) included a detailed discussion of the cranial and dental morphology and pelage colour of K. h. hardwickii, based on the literature, the damaged holotype from Java and three specimens referred to this taxon from peninsular Malaysia. They also provided some information about the pelage colour and cranial characters of engana and depressa. Genetic analyses of 26 specimens of “K. hardwickii” (geographical origin of specimens not given) suggested that there were multiple clusters of barcodes, which were referred to “K. cf. hardwickii-1”, “K. cf. hardwickii-2” and “K. cf. hardwickii-3” respectively. A comparable neighbour-joining dendrogram in Francis et al. (2010), based on 54 specimens, also indicated multiple barcode lineages, which may equate to three or more distinct species. In contrast, Anwarali et al. (2010) documented a single genotype of K. hardwickii from specimens collected in central, eastern and southwestern Borneo, based on cytochrome b (Cytb) (see Figure 2 in Anwarali et al. 2010). They also found a single genotype, based on a comparison with the COI gene tree of Francis et al. (2007), for specimens from peninsular Malaysia, Borneo, Thailand, Laos and Vietnam. They reported that mtDNA haplotypes were similar even for specimens with different karyotypes. Similarly Hasan and Abdullah (2011) found only a single morphotype for specimens referred to K. hardwickii from Sarawak, Borneo.
K. minuta Miller, 1898 was described from southern Thailand. Descriptive characters and external, cranial and dental measurements were included. Hill (1965) referred two specimens from Selangor, peninsular Malaysia, to this species and provided further details concerning its morphology and size. Subsequently, Yenbutra and Felten (1986) referred material to K. minuta from Chiang Mai and Phetchabun Provinces, in northern and central Thailand respectively. However, this geographical addition to the species’ range was not accepted by Corbet and Hill (1992), Simmons (2005) or Francis (2008). It is unclear whether this apparently geographically isolated material from northern Thailand is referable to an undescribed species. A recent genetic study, based on seven specimens, showed two monophyletic clades of K. minuta (Francis et al. 2010). However, a subsequent study of the Cytb 1140bp and AFLP 481 bands of K. minuta, based on specimens from Borneo and peninsular Malaysia, showed only a single clade (Anwarali et al. 2010). Hasan and Abdullah (2011) considered K. minuta to be a single morphotype, based on specimens from Sarawak. Both these latter papers noted that there was some confusion between K. minuta and the slightly larger K. intermedia Hill and Francis, 1984 from Borneo.
In the recent years, several new species were described from the region, including K. kachinensis Bates et al., 2004, K. titania Bates et al., 2007 and K. krauensis Francis et al., 2007. In addition, there have been some changes to the rank of individual taxa, for example, with the elevation of K. lenis to specific status (Vanitharani et al. 2003). However, despite these recent reviews, it still appears that the species diversity of the genus Kerivoula is underestimated, and further taxonomic revision is needed.
The present study aims to examine the taxonomic status of bats in the genus Kerivoula in Thailand based on morphology, genetics and echolocation calls. It is based on extensive field work (2010–2013), a thorough examination of museum specimens and a detailed review of the literature.
Materials and methods
Field work
In the present study, bats were captured at 16 localities in six provinces of Thailand (Figure 1) using four-bank harp traps (Francis 1989) and mist nets. The harp traps were set on natural trails, over small streams and across paths in forest understorey. Harp traps were erected before sunset and left over night. Mist nets were used to capture bats in similar locations to the harp traps, but were more often used in open spaces. The mist nets were set at the same time as the harp traps and taken down at 22.00 h.
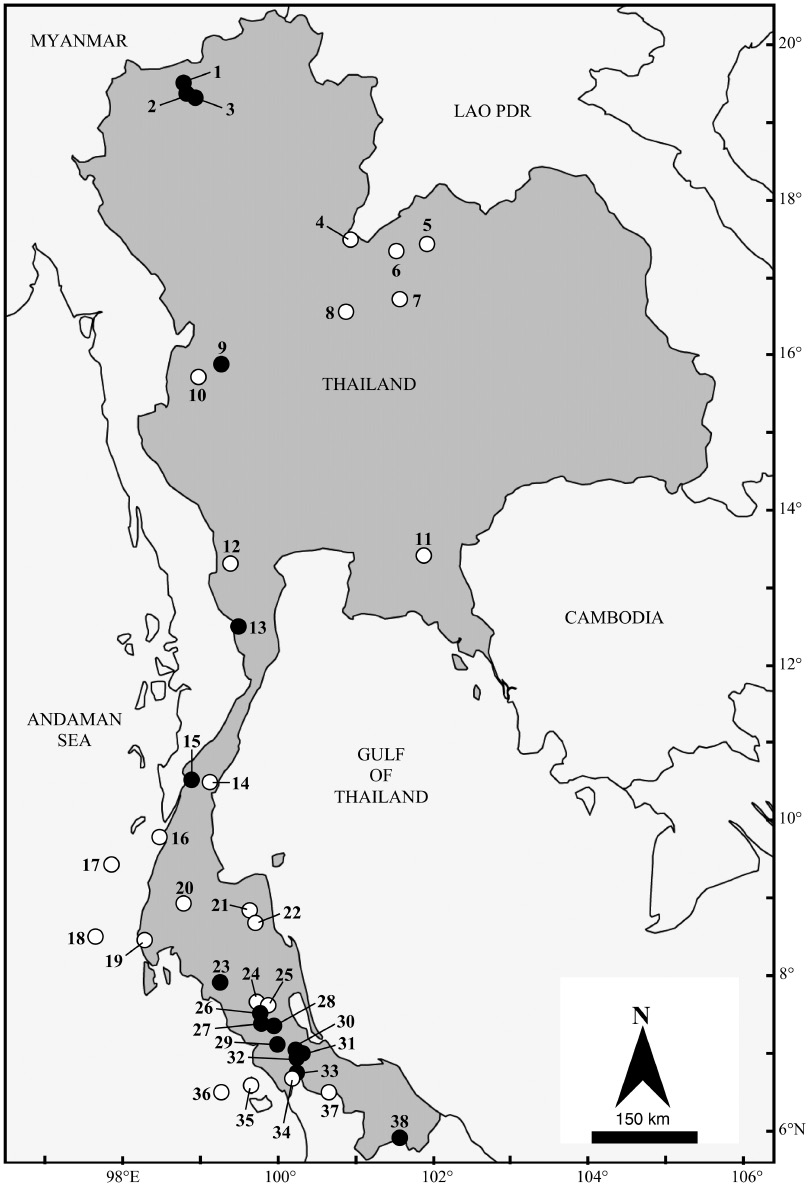
Localities in Thailand from which Kerivoula specimens were examined; black symbols for visited localities; open symbols for museum specimens. For detail of location see Appendix 1.
The species, sex, age (adult or juvenile) and the reproductive condition (pregnant or lactating) were determined in the field. Initial species identification was made in the field following Payne and Francis (1985), Corbet and Hill (1992) and Francis (2008). Juveniles were identified by the presence of unfused epiphyses of phalanges and metacarpal joints (Brunet-Rossinni and Wilkinson 2009). The reproductive status of female bats was determined by examining the nipples (Racey 2009).
One to three specimens were taken from each locality for the study of morphometrics, especially cranial and dental characters, and as vouchers to support genetic and echolocation call studies. The body mass and some external characters were measured, including head and body, forearm, ear, tail, tibia and foot. In addition, coordinates were recorded using a GPS, and habitats were described.
Measurements
In this study, 155 voucher specimens were measured. They are held in the collections of the Princess Maha Chakri Sirindhorn Natural History Museum, Prince of Songkla University, Thailand (PSUZC) and the Hala-Bala Wildlife Research Station, Thailand (HBWRS).
External and skull measurements were taken with a digital caliper to the nearest 0.01 mm, following Bates and Harrison (1997) and Bates et al. (2004). Body mass was measured with a Pesola Spring balance. Measurements included: HB: head and body – from the tip of the snout to the anus, ventrally; FA: forearm length – from the extremity of the elbow to the extremity of the carpus with the wings folded; E: ear length – from the lower border of the external auditory meatus to the tip of the pinna; TL: tail length – from the tip of the tail to its base adjacent to the anus; TIB: tibia length – from the knee joint to the extremity of the heel behind the os calcis; HF: foot length – from the extremity of the heel behind the os calcis to the extremity of the longest digit, not including the hairs or claws; 3MT, 4MT, 5MT: third, fourth, fifth metacarpal lengths, respectively – from the extremity of the carpus to the distal extremity of the third, fourth and fifth metacarpals, respectively; 3D1P, 3D2P, 4D1P, 4D2P: first and second phalanges of the third and fourth digits, respectively – from the proximal to the distal extremity of the phalanges; W: body mass (g); GLS: greatest length of skull – the greatest antero-posterior diameter of the skull, from the most projecting point at each extremity regardless of what structure forms these points; CCL: condylo-canine length – from the exoccipital condyle to the alveolus of the canine; CBL: condylo-basal length – from the exoccipital condyle to the alveolus of the anterior incisor; MW: mastoid width – the greatest distance across the mastoid region; ZB: zygomatic breadth – the greatest width of the skull across the zygomata; BB: breadth of braincase – greatest breadth of the braincase at the posterior roots of the zygomatic arches; BH: braincase height – taken from the basisphenoid to the highest part of the skull; PC: postorbital constriction – the narrowest width across the constriction posterior to the orbits; ML: mandible length – from the most posterior part of the condyle to the most anterior part of the mandible, including the lower incisors; C1-C1: anterior palatal width – taken across the outer borders of the upper canine; M3-M3: posterior palatal width – taken across the outer borders of the upper third molar; C-M3: upper toothrow length – from the front of the upper canine to the back of the crown of the third molar; C-M3: lower toothrow length – from the front of the lower canine to the back of the crown of the third lower molar.
Morphological analysis
To determine whether there were significant differences in metric characters between taxa, a series of Mann-Whitney U-tests was conducted using SPSS version 14.1 (SPSS 2005) for Windows. Each test was run at a confidence limit of 95%. For multivariate comparisons, principal component analysis (PCA) was performed on the correlative matrix and tested using the software PCORD version 5 (McCune and Mefford 2006) for Windows.
Genetic analysis
Sequence analysis was carried out at the Department of Biotechnology and Bioinformatics, Prince of Songkla University, Thailand and the Canadian Centre for DNA Barcoding, Canada.
For sequence analysis in Thailand, the deoxyribonucleic acid (DNA) was extracted from wing punches, and 657 base pairs (bp) of the cytochrome c oxidase subunit I (COI) was amplified and sequenced following the standard protocols published in Anwarali et al. (2010). For the tissue sent to the Canadian Centre for DNA Barcoding, DNA was extracted, and 657 bp of COI was amplified and sequenced following standard protocols published in Ivanova et al. (2006, 2012).
Additional, published sequences were included in the analysis, namely for K. papillosa (CMF920706-03 – HM540730, ROM MAM 113056 – HM540726, ROM MAM 113110 – HM540729, ROM MAM 117932 – HM540724, ROM MAM 117933 – HM540725, ROM MAM 117934 – HM540723, S300091 – HM540727, S300721 – HM540728, TK 125619 – GU585624, TK 125620 – GU585623, TK 152020 – GU585632, TK 152023 – GU585619, TK 152403 – GU585625, TK 152061 – GU585618, TK 152062 – GU585622, TK 152994 – GU585620, TK 156001 – GU585627, TK 156004 – GU585628, TK 156036 – GU585626), K. lenis (ROM MAM 113049 – HM540719, ROM MAM 113053 – HM540718, ROM MAM 117931 – HM540717, TK 152052 – GU585595 and TK 152178 – GU585596), K. cf. lenis (ROM MAM 110520 – HM540721, ROM MAM 110527 – HM540722, ROM MAM 110589 – HM540720, ROM MAM 110850 – JF443954, ZMMU139-09 – HM914930), K. kachinensis (EBD 25122 – HM540735), K. picta (Pallas, 1767) (ROM MAM 106371 – HM540755), K. krauensis (SMF 83824 – HM540742), K. intermedia (TK 153621 – GU585601), K. hardwickii (INECOL M0071 – HM540715 and INECOL M0108 – HM540716) and K. minuta (INECOL M0111 – HM540749 and INECOL M0112 –HM540745). Sequences of Murina suilla (Temminck, 1840) (ROM MAM 117936 – HM540991), M. peninsularis Hill, 1964 (CMF920703-03 – HM540973) and Myotis cf. muricola (Gray, 1846) (ROM MAM 117943 – HM541064) were used as outgroups. These are available in the Barcode of Life Database (www.boldsystems.org) and are published in Anwarali et al. (2010) and/or Francis et al. (2010).
Phylogenetic reconstruction was undertaken using neighbor joining (NJ) with a Kimura 2-parameter (K2) model in the software MEGA version 5.1 (Kimura 1980, Tamura et al. 2011) at bootstrap method for 1000 replications. The divergent distance between and within taxa was generated using the Kimura 2-parameter model in the software MEGA version 5.1 (Kimura 1980, Tamura et al. 2011).
Echolocation call records and analysis
Echolocation calls were recorded from free flying bats in one room (4 m long×4 m wide×3 m high). Otherwise, they were recorded when the bats were free flying in an artificial enclosure comprising mosquito nets for a roof and walls (4 m long×3 m wide×2.5 m high). The calls were detected with a Pettersson Ultrasound Detector D 1000x.
For each individual, 2–50 high-quality calls were analyzed using BatSound Pro v3.1 (Pettersson Elektronik AB) software. Call parameters comprised minimum frequency (MinF) and maximum frequency (MaxF) – both values measured from the spectrogram; maximum energy frequency (MaxEF) and middle frequency (MidF) – both values measured using the power spectrum function. These four parameters are comparable to those of Preatoni et al. (2005). Start frequency (SF) and end frequency (EF) data were omitted in the current study due to equipment constraints. For technical reasons, call duration, an additional parameter in Kingston et al. (1999), was also not included here.
To determine whether there were significant differences in each parameter between taxa, a series of Kruskal-Wallis tests were run at a confidence limit of 95%.
Results
External, cranial and dental characters
External measurements were available for 154 specimens from the study area. PCA based on nine external characters shows that the bats in the genus Kerivoula from the study area are divided into two main groups (Figure 2, Table 2), comprising a “large-size” group (K. papillosa [sensu Corbet and Hill 1992=sensu lato] and K. kachinensis) and a “medium-small” size group (K. titania, K. pellucida, K. hardwickii [sensu Corbet and Hill 1992=sensu lato], K. kraunensis and K. minuta).
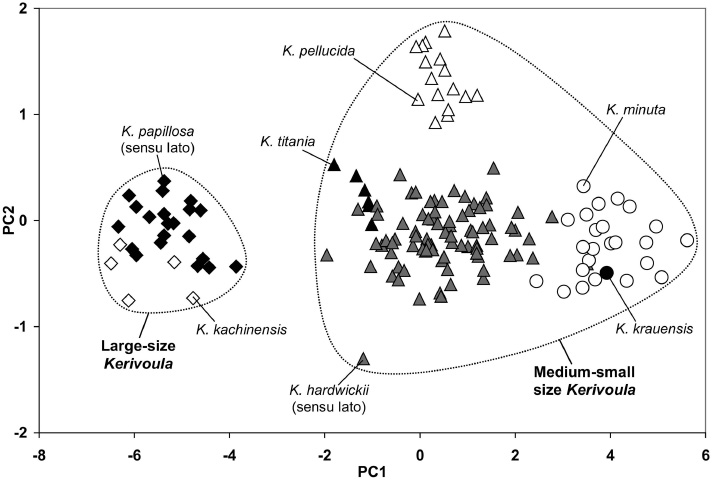
First and second principal components of the PCA computed on nine external measurements. Black diamonds: K. papillosa (sensu lato), open diamonds: K. kachinensis, black triangles: K. titania, grey triangles: K. hardwickii (sensu lato), open triangles: K. pellucida, black circle: K. krauensis and open circles: K. minuta.
Eigenvectors and eigenvalues of principal component analysis of nine external measurements of 154 specimens of seven species of Kerivoula from Thailand.
| Character | Eigenvector | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| FA | –0.34 | –0.27 | –0.25 |
| TIB | –0.33 | 0.17 | 0.04 |
| 3MT | –0.34 | –0.16 | –0.31 |
| 4MT | –0.34 | –0.09 | –0.31 |
| 5MT | –0.34 | –0.06 | –0.28 |
| 3D1P | –0.34 | –0.08 | 0.00 |
| 3D2P | –0.33 | 0.42 | 0.31 |
| 4D1P | –0.32 | 0.62 | 0.16 |
| 4D2P | –0.31 | –0.54 | 0.74 |
| Eigenvalue | 8.23 | 3.27 | 2.34 |
| % of total variation explained | 91.40 | 94.66 | 97.01 |
The external measurements of K. papillosa (sensu lato) and K. kachinensis are similar in size, except for forearm, tail, tibia, third and fourth metacarpal lengths, which average significantly longer in K. kachinensis (Tables 3 and 4).
External measurements (mm) and body mass (g) of seven species of Kerivoula from Thailand.
| n | Sex | HB | FA | E | TL | TIB | HF | 3MT | 4MT | 5MT | 3D1P | 3D2P | 4D1P | 4D2P | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kerivoula papillosa (sensu lato) | |||||||||||||||
| 21 | ♂♂♀♀ | 40.3–52.2 | 39.1–42.3 | 13.1–17.4 | 47.7–56.4 | 18.8–23.2 | 7.9–9.9 | 42.3–46.2 | 40.9–45.5 | 39.4–43.5 | 18.9–22.8 | 18.6–22.6 | 12.5–13.9 | 9.1–10.4 | 6.5–9.0 |
| 45.6, 2.8 | 40.6, 0.9 | 14.4, 0.9 | 51.1, 2.5 | 20.8, 1.2 | 9.2, 0.5 | 43.8, 0.9 | 42.6, 1.0 | 41.1, 1.0 | 21.0, 1.0 | 21.1, 0.9 | 13.2, 0.5 | 9.7, 0.4 | 7.9, 0.7 (11) | ||
| Kerivoula kachinensis | |||||||||||||||
| 5 | ♂♂♀ | 44.7–49.9 | 41.0–42.1 | 12.9–14.8 | 52.7–59.4 | 21.7–23.6 | 8.7–9.7 | 44.5–45.5 | 43.2–44.5 | 41.3–42.8 | 19.7–22.2 | 19.1–21.4 | 11.8–13.3 | 9.0–10.5 | 6.3–8.0 |
| 48.0, 2.5 | 41.5, 0.5 | 14.2, 0.8 | 56.0, 2.7 | 22.9, 0.7 | 9.1, 0.4 | 45.0, 0.5 | 43.7, 0.5 | 41.9, 0.6 | 21.4, 1.0 | 20.5, 1.0 | 12.7, 0.7 | 9.9, 0.7 | 7.1, 0.7 | ||
| Kerivoula titania | |||||||||||||||
| 6 | ♂♂♀♀ | 38.4–41.0 | 34.1–35.9 | 12.5–13.8 | 48.1–53.5 | 18.8–19.8 | 7.3–7.8 | 35.2–36.4 | 34.7–35.9 | 33.4–34.4 | 18.1–19.4 | 17.4–19.0 | 11.0–12.3 | 8.3–8.8 | 4.0–5.0 |
| 39.5, 1.0 | 34.8, 0.7 | 13.1, 0.5 | 50.1, 2.0 | 19.4, 0.3 | 7.6, 0.2 | 35.7, 0.5 | 35.2, 0.4 | 34.0, 0.4 | 18.7, 0.5 | 18.0, 0.6 | 11.7, 0.5 | 8.6, 0.2 | 4.5, 0.5 | ||
| Kerivoula hardwickii (sensu lato) | |||||||||||||||
| 81 | ♂♂♀♀ | 32.0–42.5 | 28.9–35.7 | 10.6–15.0 | 33.8–47.9 | 15.2–19.4 | 6.5–8.4 | 31.6–38.1 | 30.1–37.6 | 28.3–35.9 | 14.7–18.8 | 13.3–18.0 | 8.7–11.9 | 6.4–10.5 | 3.0–5.5 |
| 36.9, 2.0 | 32.8, 1.4 | 12.8, 0.9 | 41.3, 3.0 | 17.4, 0.8 | 7.6, 0.4 | 35.0, 1.4 | 34.0, 1.4 | 32.7, 1.4 | 16.6, 0.8 | 16.1, 1.0 | 10.5, 0.6 | 7.9, 0.6 | 4.1, 0.6 (44) | ||
| Kerivoula pellucida | |||||||||||||||
| 16 | ♂♂♀♀ | 35.4–41.4 | 29.2–32.1 | 13.2–16.3 | 44.0–51.2 | 16.3–19.5 | 6.7–7.8 | 33.6–35.6 | 32.8–34.6 | 31.5–33.6 | 15.0–16.7 | 17.0–19.3 | 11.1–12.4 | 6.7–8.6 | 3.7–5.5 |
| 37.4, 1.7 | 30.9, 0.8 | 14.8, 0.9 | 47.2, 2.1 | 17.9, 0.8 | 7.3, 0.3 | 34.3, 0.5 | 33.6, 0.5 | 32.5, 0.5 | 15.8, 0.5 | 18.0, 0.8 | 11.8, 0.4 | 7.3, 0.4 | 4.6, 0.6 (11) | ||
| Kerivoula krauensis | |||||||||||||||
| 1 | ♂ | 31.6 | 30.8 | 12.1 | 33.1 | 14.6 | 7.5 | 31.5 | 29.9 | 28.3 | 13.6 | 13 | 8.3 | 6.3 | |
| Kerivoula minuta | |||||||||||||||
| 24 | ♂♂♀♀ | 28.2–39.0 | 25.3–29.4 | 7.9–11.6 | 30.6–42.3 | 12.0–14.8 | 6.1–7.1 | 27.9–32.1 | 26.4–30.8 | 25.3–30.3 | 12.5–14.9 | 12.8–15.8 | 7.9–9.4 | 6.3–8.4 | 2.5–3.5 |
| 31.4, 2.4 | 28.0, 1.1 | 9.8, 0.4 | 37.0, 3.0 | 13.9, 0.6 | 6.5, 0.2 | 30.2, 1.2 | 28.9, 1.1 | 27.9, 1.2 | 13.9, 0.7 | 14.2, 0.7 | 8.9, 0.4 | 7.1, 0.5 | 2.6, 0.3 (16) | ||
HB, head and body; FA, forearm; E, ear; TL, tail; TIB, tibia; HF, foot; 3MT, 4MT, 5MT, third, fourth, fifth metacarpals; 3D1P, 3D2P, 4D1P, 4D2P, first and second phalanges of third and fourth digits; W, body mass. Mean, range and standard deviation. Sample sizes differing from those reported under n are given in parentheses.
Comparison between K. papillosa (sensu lato) and K. kachinensis from Thailand.
| Characters | K. papillosa (sensu lato) | K. kachinensis | p-Value |
|---|---|---|---|
| External characters | |||
| HB | 45.6±2.8 | 48.0±2.5 | ns |
| FA | 40.6±0.9 | 41.5±0.5 | <0.04 |
| E | 14.4±0.9 | 14.2±0.8 | ns |
| TL | 51.1±2.5 | 56.0±2.7 | <0.01 |
| TIB | 20.8±1.2 | 22.9±0.7 | <0.01 |
| HF | 9.2±0.5 | 9.1±0.4 | ns |
| 3MT | 43.8±0.9 | 45.0±0.5 | <0.01 |
| 4MT | 42.6±1.0 | 43.7±0.5 | <0.01 |
| 5MT | 41.1±1.0 | 41.9±0.6 | ns |
| 3D1P | 21.0±1.0 | 21.4±1.0 | ns |
| 3D2P | 21.1±0.9 | 20.5±1.0 | ns |
| 4D1P | 13.2±0.5 | 12.7±0.7 | ns |
| 4D2P | 9.7±0.4 | 9.9±0.7 | ns |
| Cranio-dental characters | |||
| GLS | 17.3±0.3 | 17.5±0.2 | ns |
| CCL | 15.4±0.3 | 15.6±0.2 | ns |
| CBL | 15.9±0.3 | 16.1±0.2 | ns |
| MW | 8.4±0.2 | 8.7±0.1 | <0.02 |
| ZB | 10.5±0.3 | 10.4±0.1 | ns |
| BB | 7.9±0.2 | 8.3±0.1 | <0.01 |
| BH | 6.9±0.3 | 5.6±0.1 | <0.01 |
| PC | 3.4±0.2 | 3.6±0.1 | <0.03 |
| ML | 12.6±0.2 | 12.3±0.2 | <0.02 |
| C1-C1 | 4.3±0.1 | 4.2±0.1 | ns |
| M3-M3 | 6.5±0.2 | 6.5±0.1 | ns |
| C-M3 | 7.0±0.1 | 6.8±0.1 | <0.03 |
| C-M3 | 7.5±0.2 | 7.2±0.1 | <0.01 |
Levels of significance (p-value) based on a series of Mann-Whitney U-tests. External and cranio-dental measurements (mm) presented as mean±SD; ns, not significant.
In the “medium-small” size group, specimens referred to K. hardwickii (sensu lato) average significantly smaller than those referred to K. titania, except for ear, foot and third metacarpal lengths, which are not significantly different (Tables 3 and 5). K. hardwickii (sensu lato) is significantly smaller in some features and larger in others compared to K. pellucida; the exceptions are the lengths of head and body, and fourth and fifth metacarpals. The measurements of K. titania are significantly larger than those of K. pellucida, except the second phalanx of third digit and the first phalanx of fourth digit. External measurements were available for only one specimen of K. krauensis, which is smaller than K. hardwickii, K. titania and K. pellucida (Table 3). K. minuta is significantly smaller than those of the other species in the “medium-small” group.
Comparison between K. hardwickii (sensu lato), K. titania and K. pellucida from Thailand.
| Characters | K. hardwickii (sensu lato) | K. titania | p-Value | K. hardwickii (sensu lato) | K. pellucida | p-Value | K. titania | K. pellucida | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| External characters | |||||||||
| HB | 36.9±2.0 | 39.5±1.0 | <0.01 | 36.9±2.0 | 37.4±1.7 | ns | 39.5±1.0 | 37.4±1.7 | <0.01 |
| FA | 32.8±1.4 | 34.8±0.7 | <0.01 | 32.8±1.4 | 30.9±0.8 | <0.01 | 34.8±0.7 | 30.9±0.8 | <0.01 |
| E | 12.8±0.9 | 13.1±0.5 | ns | 12.8±0.9 | 14.8±0.9 | <0.01 | 13.1±0.5 | 14.8±0.9 | <0.01 |
| TL | 41.3±3.0 | 50.1±2.0 | <0.01 | 41.3±3.0 | 47.2±2.1 | <0.01 | 50.1±2.0 | 47.2±2.1 | <0.01 |
| TIB | 17.4±0.8 | 19.4±0.3 | <0.01 | 17.4±0.8 | 17.9±0.8 | <0.02 | 19.4±0.3 | 17.9±0.8 | <0.01 |
| HF | 7.6±0.4 | 7.6±0.2 | ns | 7.6±0.4 | 7.3±0.3 | <0.04 | 7.6±0.2 | 7.3±0.3 | 0.04 |
| 3MT | 35.0±1.4 | 35.7±0.5 | ns | 35.0±1.4 | 34.3±0.5 | <0.04 | 35.7±0.5 | 34.3±0.5 | <0.01 |
| 4MT | 34.0±1.4 | 35.2±0.4 | <0.02 | 34.0±1.4 | 33.6±0.5 | ns | 35.2±0.4 | 33.6±0.5 | <0.01 |
| 5MT | 32.7±1.4 | 34.0±0.4 | <0.02 | 32.7±1.4 | 32.5±0.5 | ns | 34.0±0.4 | 32.5±0.5 | <0.01 |
| 3D1P | 16.6±0.8 | 18.7±0.5 | <0.01 | 16.6±0.8 | 15.8±0.5 | <0.01 | 18.7±0.5 | 15.8±0.5 | <0.01 |
| 3D2P | 16.1±1.0 | 18.0±0.6 | <0.01 | 16.1±1.0 | 18.0±0.8 | <0.01 | 18.0±0.6 | 18.0±0.8 | ns |
| 4D1P | 10.5±0.6 | 11.7±0.5 | <0.01 | 10.5±0.6 | 11.8±0.4 | <0.01 | 11.7±0.5 | 11.8±0.4 | ns |
| 4D2P | 7.9±0.6 | 8.6±0.2 | <0.01 | 7.9±0.6 | 7.3±0.4 | <0.01 | 8.6±0.2 | 7.3±0.4 | <0.01 |
| Cranio-dental characters | |||||||||
| GLS | 14.6±0.5 | 15.5±0.1 | <0.01 | 14.6±0.5 | 14.4±0.3 | ns | 15.5±0.1 | 14.4±0.3 | <0.01 |
| CCL | 12.9±0.4 | 13.7±0.1 | <0.01 | 12.9±0.4 | 12.7±0.2 | <0.05 | 13.7±0.1 | 12.7±0.2 | <0.01 |
| CBL | 13.4±0.5 | 14.2±0.1 | <0.01 | 13.4±0.5 | 13.2±0.3 | ns | 14.2±0.1 | 13.2±0.3 | <0.01 |
| MW | 7.4±0.2 | 7.8±0.2 | <0.02 | 7.4±0.2 | 7.1±0.1 | <0.01 | 7.8±0.2 | 7.1±0.1 | <0.01 |
| ZB | 8.6±0.3 | 9.1±0.1 | <0.01 | 8.6±0.3 | 8.1±0.1 | <0.01 | 9.1±0.1 | 8.1±0.1 | <0.01 |
| BB | 7.3±0.2 | 7.7±0.2 | <0.01 | 7.3±0.2 | 6.9±0.1 | <0.01 | 7.7±0.2 | 6.9±0.1 | <0.01 |
| BH | 5.5±0.4 | 5.4±0.1 | ns | 5.5±0.4 | 5.9±0.1 | <0.01 | 5.4±0.1 | 5.9±0.1 | <0.01 |
| PC | 3.3±0.1 | 3.3±0.1 | ns | 3.3±0.1 | 3.0±0.1 | <0.01 | 3.3±0.1 | 3.0±0.1 | <0.01 |
| ML | 10.1±0.4 | 10.7±0.1 | <0.01 | 10.1±0.4 | 9.9±0.2 | <0.03 | 10.7±0.1 | 9.9±0.2 | <0.01 |
| C1-C1 | 3.5±0.2 | 3.6±0.1 | ns | 3.5±0.2 | 3.1±0.1 | <0.01 | 3.6±0.1 | 3.1±0.1 | <0.01 |
| M3-M3 | 5.4±0.2 | 5.6±0.1 | <0.02 | 5.4±0.2 | 5.1±0.1 | <0.01 | 5.6±0.1 | 5.1±0.1 | <0.01 |
| C-M3 | 5.5±0.2 | 6.0±0.1 | <0.01 | 5.5±0.2 | 5.5±0.1 | ns | 6.0±0.1 | 5.5±0.1 | <0.01 |
| C-M3 | 5.8±0.2 | 6.3±0.1 | <0.01 | 5.8±0.2 | 5.8±0.2 | ns | 6.3±0.1 | 5.8±0.2 | <0.01 |
Levels of significance (p-value) based on a series of Mann-Whitney U-tests. External and cranio-dental measurements (mm) presented as mean±SD; ns, not significant.
The pelage colour of K. pellucida is distinctive; pale orange on the dorsal surface and creamy orange on the ventral surface (Figure 3B). K. krauensis is also distinctive, with golden hair tips on its dorsal surface (Figure 3C). Both species can be distinguished from the predominantly grey-brown dorsal surfaces and grey belly fur of all K. hardwicki (sensu lato) (Figure 3A). The pelage of K. minuta is brown above and below with very dark brown hair bases (Figure 3D).
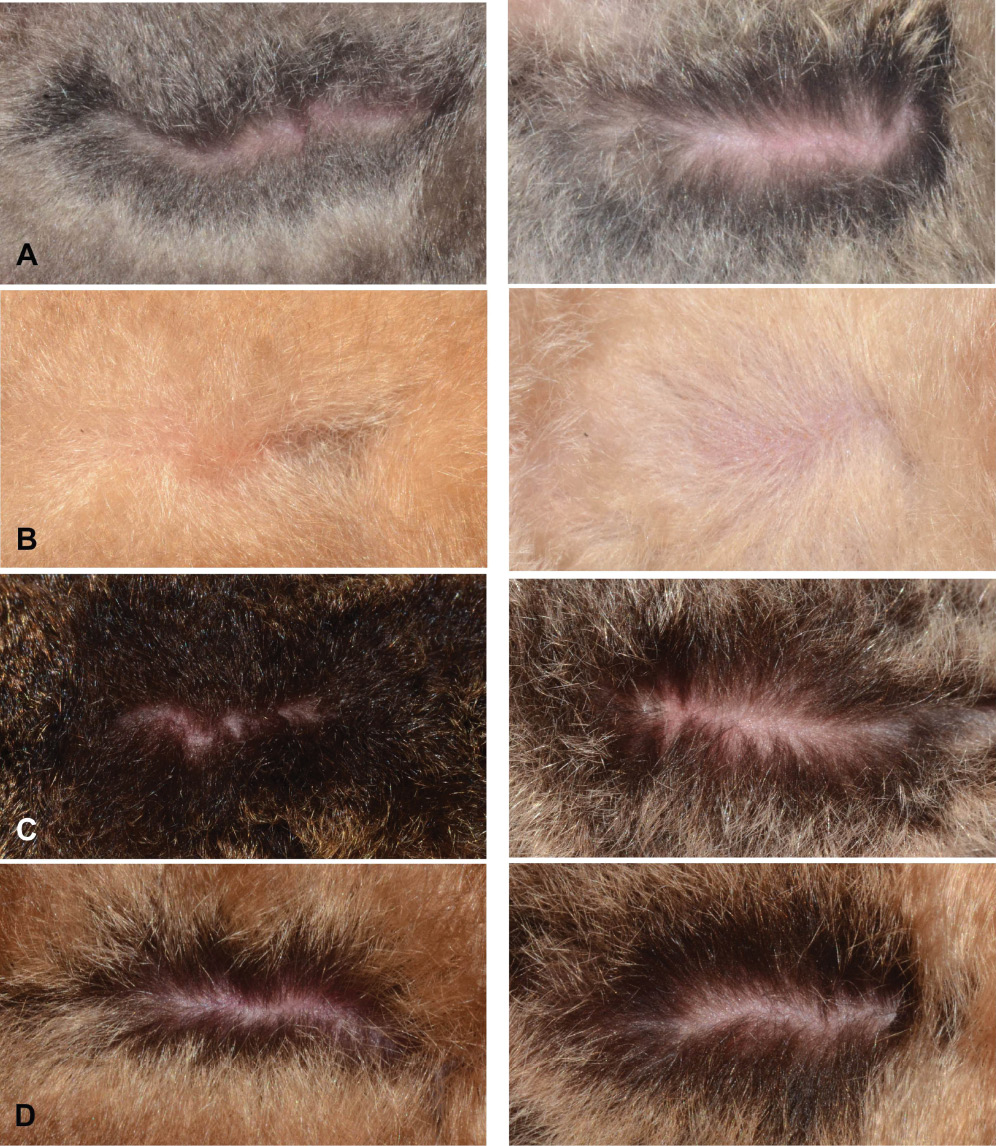
Dorsal (left) and ventral (right) pelage of four species of Kerivoula. (A) K. hardwickii C, PSUZC-MM2013.22, ♂, Phu Pha Phet Ranger Station, Khao Bantad Wildlife Sanctuary, Satun Province; (B) K. pellucida, PSUZC-MM2013.24, ♂, Phu Pha Phet Ranger Station, Khao Bantad Wildlife Sanctuary, Satun Province; (C) K. krauensis, PSUZC-MM2013.25, ♂, Hala-Bala Wildlife Research Station, Narathiwat Province; (D) K. minuta, BD130825.3, ♂, Phu Pha Phet Ranger Station, Khao Bantad Wildlife Sanctuary, Satun Province. No scale.
A multivariate analysis based on 12 cranial and dental characters of the 155 skulls shows that the bats in this genus are divided into three groups, including “large-size” (K. papillosa [sensu lato] and K. kachinensis), “medium-size” (K. hardwickii [sensu lato], K. titania, K. pellucida and K. krauensis) and “small-size” (K. minuta) (Figure 4, Tables 6 and 7).
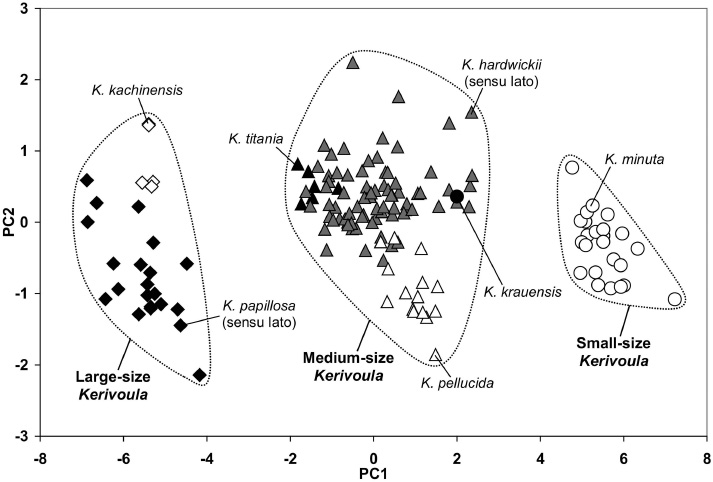
First and second principal components of the PCA computed on 12 cranial and dental measurements. Black diamonds: K. papillosa (sensu lato), open diamonds: K. kachinensis, black triangles: K. titania, grey triangles: K. hardwickii (sensu lato), open triangles: K. pellucida, black circle: K. krauensis and open circles: K. minuta.
Eigenvectors and eigenvalues of the principal component analysis of 12 cranio-dental measurements of 151 specimens of seven species of Kerivoula from Thailand.
| Characters | Eigenvector | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| GLS | –0.30 | –0.06 | –0.04 |
| CCL | –0.30 | –0.05 | 0.01 |
| CBL | –0.30 | –0.06 | 0.02 |
| MW | –0.30 | 0.13 | –0.01 |
| BB | –0.28 | 0.30 | –0.43 |
| BH | –0.26 | –0.42 | –0.69 |
| PC | –0.24 | 0.79 | –0.09 |
| ML | –0.30 | –0.13 | 0.17 |
| C1-C1 | –0.29 | 0.01 | 0.31 |
| M3-M3 | –0.29 | –0.08 | 0.42 |
| C-M3 | –0.30 | –0.17 | 0.15 |
| C-M3 | –0.30 | –0.13 | 0.08 |
| Eigenvalue | 10.90 | 4.50 | 1.94 |
| % of total variation explained | 90.86 | 95.36 | 97.30 |
Cranio-dental measurements of seven species of Kerivoula from Thailand.
| n | Sex | GLS | CCL | CBL | MW | ZB | BB | BH | PC | ML | C1–C1 | M3–M3 | C–M3 | C–M3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kerivoula papillosa (sensu lato) | ||||||||||||||
| 21 | ♂♂♀♀ | 16.6–17.8 | 14.9–16.0 | 15.4–16.5 | 8.1–9.0 | 10.0–11.1 | 7.4–8.2 | 6.3–7.4 | 3.0–3.8 | 12.1–13.0 | 4.1–4.6 | 6.2–6.9 | 6.8–7.2 | 7.1–7.8 |
| 17.3, 0.3 | 15.4, 0.3 | 15.9, 0.3 | 8.4, 0.2 | 10.5, 0.3 | 7.9, 0.2 | 6.9, 0.3 | 3.4, 0.2 | 12.6, 0.2 | 4.3, 0.1 | 6.5, 0.2 | 7.0, 0.1 | 7.5, 0.2 | ||
| Kerivoula kachinensis | ||||||||||||||
| 5 | ♂♂♀ | 17.2–17.7 | 15.3–15.9 | 15.9–16.4 | 8.5–8.7 | 10.3–10.4 | 8.2–8.4 | 5.5–5.7 | 3.5–3.7 | 12.1–12.6 | 4.1–4.3 | 6.4–6.6 | 6.7–7.0 | 7.1–7.3 |
| 17.5, 0.2 | 15.6, 0.2 | 16.1, 0.2 | 8.7, 0.1 | 10.4, 0.1 | 8.3, 0.1 | 5.6, 0.1 | 3.6, 0.1 | 12.3, 0.2 | 4.2, 0.1 | 6.5, 0.1 | 6.8, 0.1 | 7.2, 0.1 | ||
| Kerivoula titania | ||||||||||||||
| 6 | ♂♂♀♀ | 15.4–15.6 | 13.5–13.9 | 14.1–14.4 | 7.5–7.9 | 9.0–9.2 | 7.5–7.9 | 5.2–5.6 | 3.3–3.4 | 10.6–10.8 | 3.5–3.6 | 5.4–5.7 | 5.9–6.1 | 6.1–6.5 |
| 15.5, 0.1 | 13.7, 0.1 | 14.2, 0.1 | 7.8, 0.2 | 9.1, 0.1 | 7.7, 0.2 | 5.4, 0.1 | 3.3, 0.1 | 10.7, 0.1 | 3.6, 0.1 | 5.6, 0.1 | 6.0, 0.1 | 6.3, 0.1 | ||
| Kerivoula hardwickii (sensu lato) | ||||||||||||||
| 82 | ♂♂♀♀ | 13.4–15.6 | 11.8–13.7 | 12.1–14.3 | 6.9–7.9 | 8.0–9.2 | 6.7–7.8 | 4.3–6.2 | 3.1–3.7 | 9.1–10.8 | 3.1–3.9 | 4.9–5.9 | 4.9–6.0 | 5.1–6.3 |
| 14.6, 0.5 (81) | 12.9, 0.4 (80) | 13.4, 0.5 (80) | 7.4, 0.2 (81) | 8.6, 0.3 (74) | 7.3, 0.2 | 5.5, 0.4 (81) | 3.3, 0.1 | 10.1, 0.4 | 3.5, 0.2 (80) | 5.4, 0.2 | 5.5, 0.2 | 5.8, 0.2 | ||
| Kerivoula pellucida | ||||||||||||||
| 16 | ♂♂♀♀ | 14.0–14.9 | 12.3–13.1 | 12.8–13.7 | 6.9–7.4 | 7.9–8.4 | 6.6–7.0 | 5.7–6.1 | 2.8–3.2 | 9.6–10.2 | 2.8–3.3 | 4.9–5.3 | 5.2–5.7 | 5.4–6.1 |
| 14.4, 0.3 (15) | 12.7, 0.2 | 13.2, 0.3 (15) | 7.1, 0.1 | 8.1, 0.1 (15) | 6.9, 0.1 | 5.9, 0.1 | 3.0, 0.1 | 9.9, 0.2 | 3.1, 0.1 (15) | 5.1, 0.1 | 5.5, 0.1 | 5.8, 0.2 | ||
| Kerivoula krauensis | ||||||||||||||
| 1 | ♂ | 13.3 | 12.0 | 12.5 | 7.0 | 8.1 | 7.0 | 5.5 | 3.2 | 9.4 | 3.0 | 5.1 | 5.0 | 5.2 |
| Kerivoula minuta | ||||||||||||||
| 24 | ♂♂♀♀ | 11.1–12.3 | 9.9–10.8 | 10.3–11.4 | 5.8–6.5 | 6.7–7.5 | 5.3–6.1 | 4.0–4.5 | 2.6–3.2 | 7.8–8.6 | 2.6–2.9 | 4.3–5.0 | 4.1–4.7 | 4.2–4.8 |
| 11.8, 0.3 | 10.5, 0.2 | 11.0, 0.3 | 6.3, 0.1 | 7.2, 0.2 (21) | 5.6, 0.2 | 4.3, 0.1 | 2.9, 0.1 | 8.2, 0.2 | 2.8, 0.1 | 4.7, 0.1 | 4.5, 0.1 | 4.6, 0.1 | ||
GLS, greatest skull length; CCL, condylo-canine length; CBL, condylo-basal length; MW, mastoid width; ZB, zygomatic breadth; BB, breadth of braincase; BH, braincase height; PC, postorbital constriction; ML, mandible length; C1–C1, anterior palatal width; M3–M3, posterior palatal width; C–M3, upper toothrow length; C–M3, lower toothrow length. Mean, range and standard deviation. Sample sizes differing from those reported under n are given in parentheses.
Specimens of K. papillosa (sensu lato) are similar in skull length to those of K. kachinensis except for the mandible, and upper and lower toothrows, which are significantly longer in K. papillosa (sensu lato) (Tables 4 and 7). K. papillosa (sensu lato) is narrower in some features, such as mastoid width, breadth of braincase and postorbital constriction. Most characteristically, the skulls of K. papillosa (sensu lato) have a significantly higher and more domed braincase (BH>6.3 mm) (Figure 5A), whereas it is flattened (BH<5.7 mm) in K. kachinensis (Figure 5B). However, interestingly, the skulls of specimens referred to K. papillosa (sensu lato) also vary from markedly domed in some specimens to more flattened in others. Here, they are considered to conform to five morphotypes (Figure 6), which also show some difference in greatest skull length.
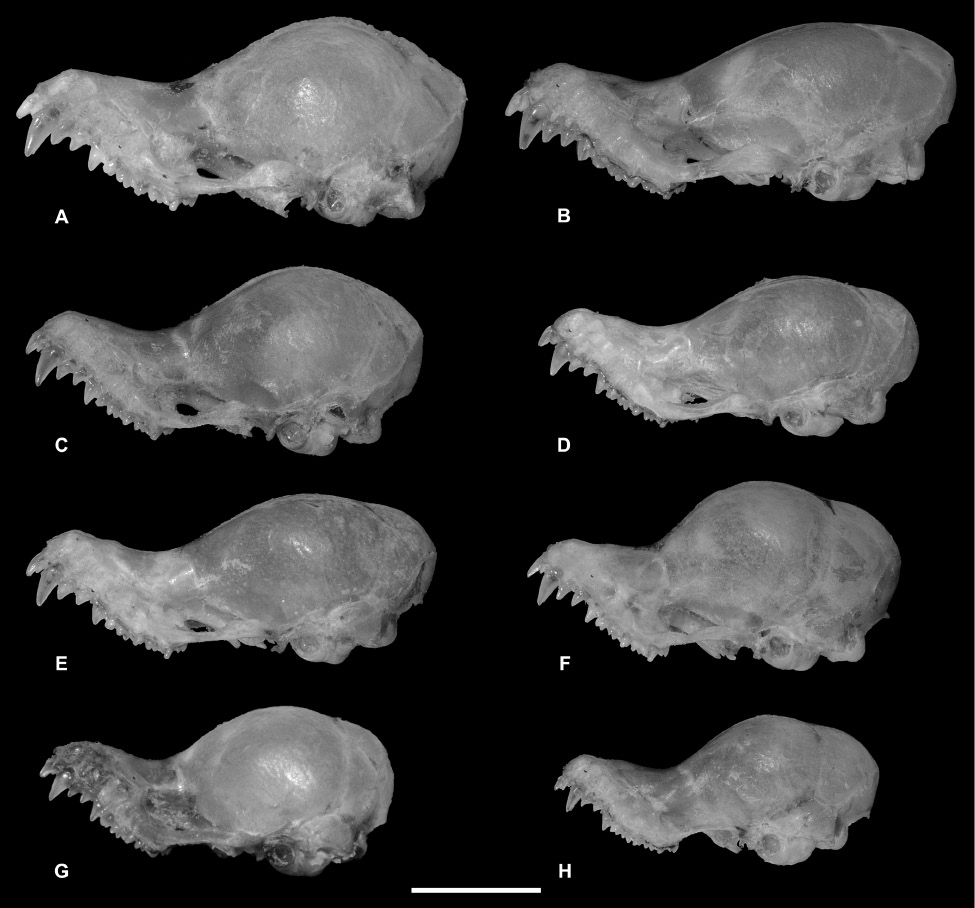
Skull of seven species of Kerivoula. (A) K. papillosa (K. papillosa C), PSUZC-MM2011.2, ♂, Khao Chong Wildlife Education Centre, Khao Bantad Wildlife Sanctuary, Trang Province; (B) K. kachinensis, PSUZC-MM2007.243, ♂, Phou Suan Sai National Park, Loei Province; (C) K. hardwickii (“domed-skull” type; K. hardwickii C), PSUZC-MM2012.160, ♂, Phu Pha Phet Ranger Station, Khao Bantad Wildlife Sanctuary, Satun Province; (D) K. hardwickii (“flat-skulled” type; K. hardwickii A), PSUZC-MM2011.19, ♀, Chiang Dao Wildlife Research Station, Chiang Mai Province; (E) – K. titania, PSUZC-MM2011.18, ♂, Khun Mae Ngai Guard Station, Chiang Dao Wildlife Sanctuary, Chiang Mai Province; (F) K. pellucida, PSUZC-MM2011.12, ♀, Hala-Bala Wildlife Research Station, Narathiwat Province; (G) K. krauensis, PSUZC-MM2013.25, ♂, Hala-Bala Wildlife Research Station, Narathiwat Province; and (H) K. minuta, PSUZC-MM2011.5, ♀, Hala-Bala Wildlife Research Station, Narathiwat Province. Scale: 5 mm.
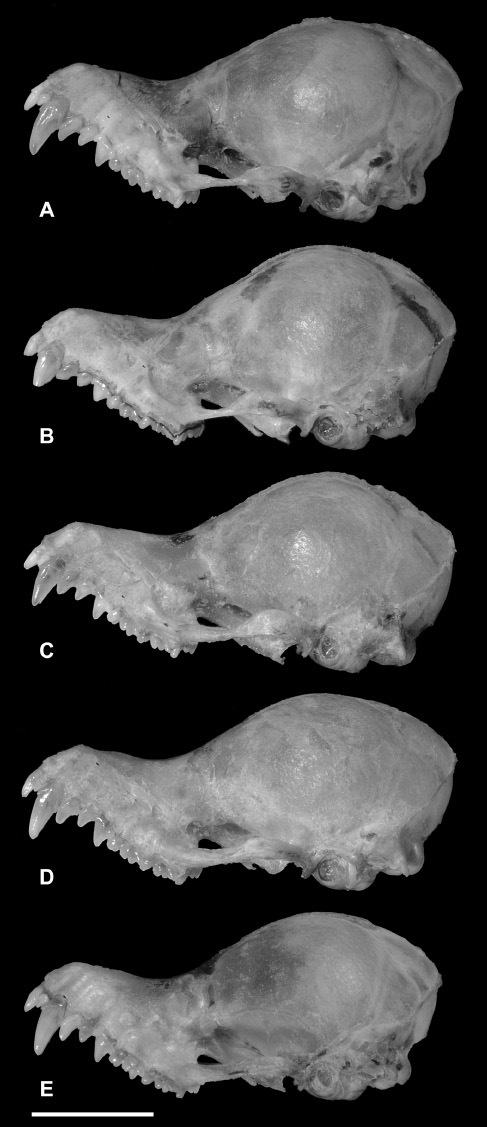
Variation in skull morphology of K. papillosa. (A) K. papillosa A, HBWRS2010.3, ♂, Khao Nam Khang National Park, Songkhla Province; (B) K. papillosa C, PSUZC-MM2011.10, ♀, Hala-Bala Wildlife Research Station, Narathiwat Province; (C) K. papillosa C, PSUZC-MM2011.2, ♂, Khao Chong Wildlife Education Centre, Khao Bantad Wildlife Sanctuary, Trang Province; (D) K. papillosa B, PSUZC-MM2008.57, ♂, Pha Chi Wildlife Sanctuary, Ratchaburi Province; and (E) K. papillosa B, PSUZC-MM2011.53, ♀, South Klom Luang Chumporn Wildlife Sanctuary, Ranong Province. Scale: 5 mm.
The skull measurements of K. hardwickii (sensu lato) average significantly smaller than those of K. titania except for braincase height, postorbital constriction and anterior palatal width (Tables 5 and 7). The skulls of K. hardwickii (sensu lato) are similar in length to those of K. pellucida but are broader and not as high compared to the latter species. The specimens of K. titania are larger than those of K. pellucida. Cranio-dental measurements of K. krauensis were available for only one specimen and are mostly smaller than other species in this group (Table 7).
The skulls of specimens referred to K. hardwickii (sensu lato) include several forms, but can be divided into “flat” and “domed” morphotypes (Figures 5C and D, 7 and 8), with variable skull lengths. The “domed-skull” morphotype clearly differs from the flat skull of K. titania (Figure 5E). However, this difference is less apparent when the “flat-skull” morphotype of K. hardwickii is compared to K. titania (Figure 5D and E). In the case of K. pellucida, the skull is more bulbous, and the braincase rises abruptly and characteristically from the rostrum (Figure 5F). The skull of K. krauensis (Figure 5G) is more flattened than those of the “domed-skull” K. hardwickii (Figure 5C) and higher than those of the “flat-skull” morphotype (Figure 5D).
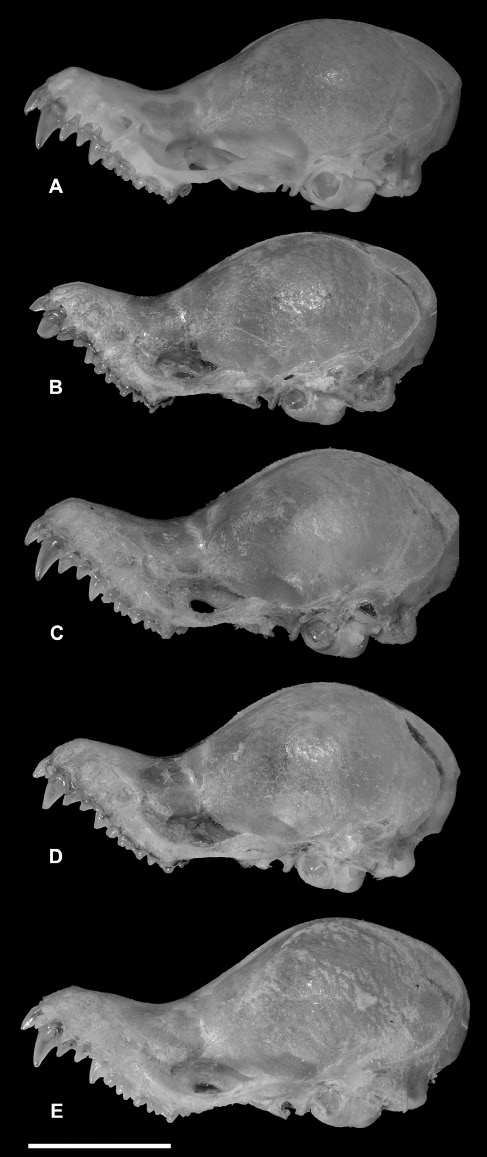
Variation in morphology of the “domed-skull” type of K. hardwickii (K. hardwickii C). (A) PSUZC-MM2005. 204, ♀, Ton Nga Chang Wildlife Sanctuary, Songkhla Province; (B) PSUZC-MM2012.161, ♂, Khao Pra-Bang Kram Wildlife Sanctuary, Krabi Province; (C) PSUZC-MM2012.160, ♂, Phu Pha Phet Ranger Station, Khao Bantad Wildlife Sanctuary, Satun Province; (D) PSUZC-MM2012.162, ♂, Khao Pra-Bang Kram Wildlife Sanctuary, Krabi Province; and (E) PSUZC-MM2008.131, ♀, Tarutao Island, Tarutao National Park, Satun Province. Scale: 5 mm.
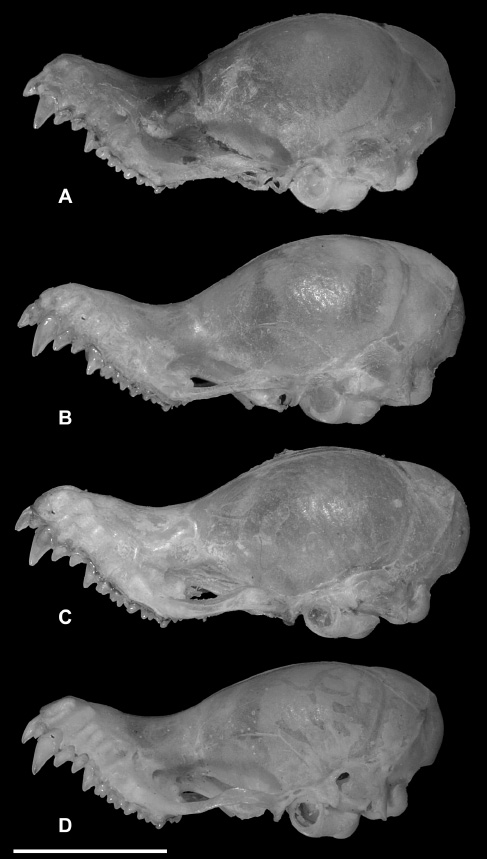
Variation in the morphology of the “flat-skull” type of K. hardwickii (K. hardwickii A and K. hardwickii B). (A) K. hardwickii A, PSUZC-MM2012.166, ♂, Khao Luang National Park, Nakhon Si Thammarat Province; (B) K. hardwickii B, PSUZC-MM2011.17, ♂, Khun Mae Ngai Suard Station, Chiang Dao Wildlife Sanctuary, Chiang Mai Province; (C) K. hardwickii A, PSUZC-MM2011.52, ♂, Pa La-U Ranger Station, Kaeng Krachan National Park, Prachuap Khiri Khan Province; (D) K. hardwickii B, PSUZC-MM2005.201, ♂, Hauy Nam Chan, Phu Laung Wildlife Sanctuary, Loei Province. Scale: 5 mm.
Bats within the “small-skull” size group are all referable to K. minuta, which is differentiated from all other local Kerivoula species by its smaller size (Table 7). The braincase is intermediate between flattened and domed; it is elevated but not abruptly so, only rising slightly above the rostrum (Figure 5H).
Genetics
Twenty-eight sequences are available for Thai specimens, 10 for K. papillosa (sensu lato), 12 for K. hardwickii (sensu lato), two for K. titania, one for K. pellucida and three for K. minuta. Forty sequences for 10 taxa of Kerivoula, in the Barcode of Life Database, were included in the analysis, namely: K. papillosa S (13), K. papillosa L (6), K. lenis (5), K. cf. lenis (5), K. kachinensis (1), K. hardwickii (sensu lato) (2), K. krauensis (1), K. picta (1), K. minuta (2) and K. intermedia (1). The phylogeny shows that the sequences of material of Kerivoula from the study area, together with others from the region, can be divided into 16 monophyletic clades (Figure 9), with an average divergence of 17.71%. The nine clades of Thai material have an average 16.75% divergence from each other.
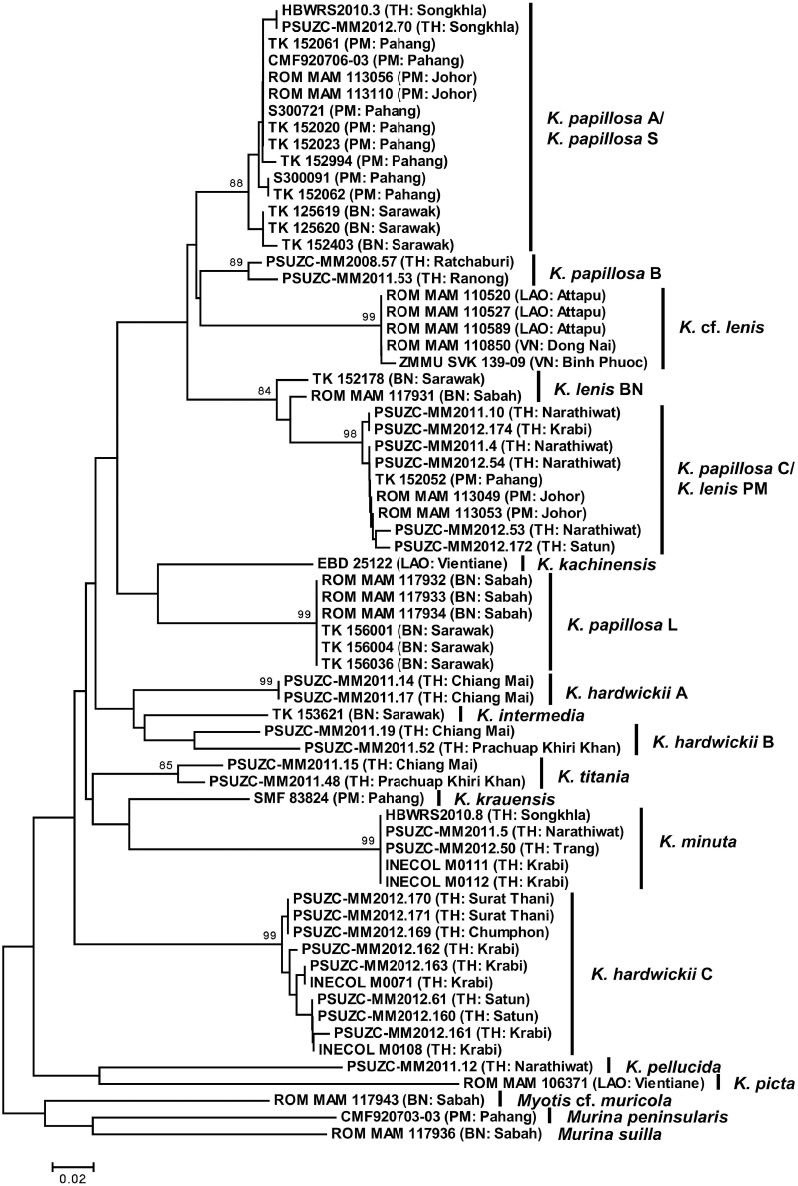
Phylogeny based on cytochrome c oxidase subunit I (COI) of 28 sequences of five species of Kerivoula from Thailand together with 37 sequences of 10 species from the South-east Asia in GenBank. Origin of the clade names are explained in the text: K. papillosa S and L after Anwarali et al. (2010), K. cf. lenis after Francis et al. (2010), K. lenis BN and PM refer to Borneo and Malaysia, respectively.
The sequences referable to K. papillosa (sensu lato) from Thailand are divided into three monophyletic clades, with an average divergence of 10.36% from each other (Table 8). The clade of K. papillosa A has an average divergence of 6.97% from K. papillosa B; the clade K. papillosa C has an average divergence of 13.06% and 14.86%, respectively, from clades K. papillosa A and K. papillosa B (Table 9).
Average percentage of Kimura 2-parameter distance values within and between taxa of Kerivoula based on cytochrome c oxidase subunit I (COI).
| No | Taxon | n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K. papillosa (sensu lato) | 32 | 10.36/2.04 | 2.30 | 3.88 | 4.05 | 4.10 | 3.90 | 5.67 | 4.26 | 7.01 | 5.58 | 4.55 |
| 2 | K. lenis BN | 2 | 10.17 | 2.72/1.40 | 4.11 | 5.30 | 4.68 | 4.14 | 6.08 | 4.02 | 7.08 | 5.76 | 4.77 |
| 3 | K. cf. lenis | 5 | 16.85 | 14.94 | 0.26/0.25 | 6.04 | 5.15 | 4.65 | 7.28 | 4.26 | 7.72 | 7.72 | 5.42 |
| 4 | K. kachinensis | 1 | 18.18 | 20.94 | 23.22 | NA | 4.61 | 4.23 | 7.65 | 4.88 | 6.56 | 7.55 | 4.51 |
| 5 | K. hardwickii (sensu lato) | 14 | 20.09 | 20.79 | 22.07 | 19.45 | 11.17/2.42 | 3.83 | 6.48 | 4.29 | 6.04 | 5.85 | 4.09 |
| 6 | K. titania | 2 | 17.61 | 15.71 | 18.11 | 15.50 | 16.55 | 3.41/1.52 | 6.90 | 4.17 | 6.87 | 4.82 | 3.87 |
| 7 | K. pellucida | 1 | 26.37 | 26.23 | 31.01 | 31.64 | 29.37 | 29.62 | NA | 5.04 | 6.85 | 6.52 | 7.59 |
| 8 | K. krauensis | 1 | 19.09 | 15.05 | 16.22 | 18.34 | 18.42 | 15.87 | 20.95 | NA | 8.31 | 5.18 | 4.31 |
| 9 | K. picta | 1 | 33.21 | 30.75 | 31.95 | 28.15 | 27.73 | 30.16 | 29.32 | 34.12 | NA | 10.39 | 6.12 |
| 10 | K. minuta | 5 | 24.88 | 22.96 | 30.30 | 28.83 | 25.26 | 17.46 | 25.89 | 18.05 | 42.11 | 0.00/0.00 | 5.32 |
| 11 | K. intermedia | 1 | 20.49 | 18.53 | 20.68 | 16.49 | 17.35 | 13.59 | 30.78 | 15.41 | 25.49 | 19.94 | NA |
The left scores in bold represent the percentage difference between taxa, and the right scores in bold are the standard deviation percentages.
Average percentage of Kimura 2-parameter distance values within and between taxa of K. papillosa (sensu lato) and K. lenis based on cytochrome c oxidase subunit I (COI).
| No | Taxon | n | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1 | K. papillosa A (K. papillosa S) | 15 | 0.96/0.43 | 2.19 | 3.58 | 2.87 | 3.52 | 4.38 |
| 2 | K. papillosa B | 2 | 6.97 | 1.99/1.13 | 4.00 | 2.62 | 3.36 | 5.41 |
| 3 | K. papillosa C (K. lenis PM) | 9 | 13.06 | 14.86 | 0.55/0.32 | 1.96 | 5.32 | 5.32 |
| 4 | K. lenis BN | 2 | 9.60 | 8.94 | 5.33 | 2.72/1.40 | 4.11 | 5.15 |
| 5 | K. cf. lenis | 5 | 12.64 | 12.32 | 19.50 | 14.94 | 0.26/0.25 | 6.84 |
| 6 | K. papillosa L | 6 | 16.24 | 20.47 | 19.80 | 19.25 | 24.89 | 0.00/0.00 |
The left scores in bold represent percentage difference between taxa, and the right scores in bold are the standard deviation percentage. Clade names are explained in the caption to Figure 9.
Similarly, bats referable to K. hardwickii (sensu lato) are also divided into three monophyletic clades, with an average divergence of 11.17% between each other (Table 8). K. hardwickii A has an average divergence of 14.99% from K. hardwickii B and 16.37% from K. hardwickii C; and K. hardwickii B has an average divergence of 20.02% from K. hardwickii C (Table 10). Moreover, there are two subclades within the clade of K. hardwickii A, with an average divergent of 8.75%. In contrast, the intra-clade sequence divergence in K. hardwickii B and K. hardwickii C are <0.01% and 1.04%, respectively.
Average percentage of Kimura 2-parameter distance values within and between taxa of K. hardwickii based on cytochrome c oxidase subunit I (COI).
| No | Taxon | n | 1 | 2 | 3 |
|---|---|---|---|---|---|
| 1 | K. hardwickii A | 2 | 8.75/1.54 | 2.14 | 2.24 |
| 2 | K. hardwickii B | 2 | 14.99 | 0.00/0.00 | 2.81 |
| 3 | K. hardwickii C | 10 | 16.37 | 20.02 | 1.04/0.28 |
The left scores in bold represent percentage difference between taxa, and the right scores in bold are the standard deviation percentage. Clade names explained in text.
The sequences of K. titania and K. minuta from Thailand have an average divergence of 3.41% and <0.01%, respectively, within the clade. Only one sequence from Thailand is available for K. pellucida, and no sequence is available for K. kachinensis and K. krauensis.
Echolocation calls
Echolocation call data are available for 20 individuals of six species including K. papillosa (sensu lato) (6), K. hardwickii (sensu lato) (6), K. titania (2), K. pellucida (1), K. krauensis (1) and K. minuta (5). A Kruskal-Wallis test showed that all measurement parameters of the calls, on average, differ significantly from each other (Table 11). The minimum frequency of all available species shows a large overlap in range, except for K. krauensis and K. pellucida, which are slightly lower than the others. The maximum frequency is slightly lower in K. papillosa (sensu lato) and K. minuta, while it is relatively higher in K. hardwickii (sensu lato), K. titania, K. pellucida and K. krauensis. The maximum energy frequency and middle frequency show a large overlap among species.
Four parameters of echolocation calls (kHz) of six species of Kerivoula from Thailand.
| Parameter | K. papillosa (sensu lato) (6, 138) | K. hardwickii (sensu lato) (6, 89) | K. titania(2, 15) | K. pellucida(1, 14) | K. krauensis(1, 67) | K. minuta(5, 80) | p-Value |
|---|---|---|---|---|---|---|---|
| MinF | 60.0–89.8 | 62.0–87.0 | 74.8–90.0 | 35.0–49.0 | 44.0–62.0 | 54.0–97.2 | |
| 73.5±5.8 | 75.3±5.3 | 82.6±4.2 | 40.6±5.3 | 51.3, 4.4 | 81.4±10.3 | <0.01 | |
| MaxF | 169.0–207.7 | 194.0–245.0 | 217.0–243.0 | 210.0–235.0 | 205.0–241.0 | 160.1–193.5 | |
| 189.9±8.1 | 220.4±11.6 | 227.0±7.4 | 220.4±8.5 | 224.8, 9.3 | 178.4±8.9 | <0.01 | |
| MaxEF | 109.9–161.4 | 129.5–186.4 | 137.7–168.7 | 130.1–161.1 | 136.8–166.8 | 108.2–137.6 | |
| 138.8±10.6 | 151.1±13.3 | 157.0±7.6 | 142.4±8.0 | 146.7, 8.7 | 126.0±6.3 | <0.01 | |
| MidF | 97.8–147.8 | 102.0–150.6 | 113.1–148.4 | 102.0–138.0 | 114.3–140.2 | 89.4–125.1 | |
| 116.0±11.5 | 129.1±9.9 | 133.1±10.8 | 126.0±9.2 | 134.6, 4.5 | 113.2±7.5 | <0.01 |
MinF, minimum frequency; MaxF, maximum frequency; MaxEF, maximum energy frequency; MidF, middle frequency. Range, mean and standard deviation are given for each parameter. Numbers in parentheses under species names represent the numbers of bats and pulses of echolocation calls, respectively. Levels of significance (p-value) based on a series of Kruskal-Wallis tests.
Within K. papillosa (sensu lato), the frequency of material referred to K. papillosa B averages significantly lower than those referred to K. papillosa C in three of the measured parameters, except maximum frequency, which is not significantly different (Table 12). However, there is a large overlap in range between these two taxa. There is no call available for the present study for K. papillosa A.
Four parameters of echolocation calls (kHz) of K. papillosa and K. hardwickii.
| Parameter | K. papillosa B (1, 30) | K. papillosa C (5, 108) | P-value | K. hardwickii A (2, 14) | K. hardwickii C (3, 72) | p-Value |
|---|---|---|---|---|---|---|
| MaxF | 180.0–196.0 | 169.0–207.7 | ns | 222.0–234.0 | 194.0–245.0 | <0.01 |
| 190.3, 3.8 | 189.8, 8.9 | 228.1, 3.6 | 218.4, 11.8 | |||
| MinF | 60.0–69.0 | 63.5–89.8 | <0.01 | 74.0–87.0 | 62.0–86.0 | <0.01 |
| 65.5, 2.4 | 75.7, 4.4 | 81.9, 3.6 | 73.8, 4.4 | |||
| MaxEF | 109.9–157.6 | 112.1–161.4 | <0.01 | 145.9–180.7 | 129.5–186.4 | <0.01 |
| 131.6, 8.6 | 140.8, 10.2 | 160.9, 13.0 | 149.0, 12.8 | |||
| MidF | 98.2–119.4 | 97.8–147.8 | <0.01 | 121.9–145.0 | 102.0–150.6 | <0.01 |
| 108.2, 5.9 | 118.2, 11.7 | 135.0, 6.1 | 128.0, 10.3 |
MinF, minimum frequency; MaxF, maximum frequency; MaxEF, maximum energy frequency; MidF, middle frequency. Range, mean and standard deviation are given for each parameter. Numbers in parentheses under species names represent the numbers of bats and pulses of echolocation calls, respectively. Levels of significance (p-value) based on a series of Mann-Whitney U-tests.
In the case of K. hardwickii (sensu lato), the frequencies of calls for K. hardwickii A average significantly higher than those for K. hardwickii C in all measured parameters (Table 12). However, once again, there is a large overlap in ranges between the taxa. There is no call of K. hardwickii B available for the present study.
Discussion
The results of the present study indicate that the bats in the genus Kerivoula from Thailand are taxonomically complex: both K. papillosa (sensu lato) and K. hardwickii (sensu lato) include a number of cryptic species.
In terms of morphology, there appear to be five morphotypes amongst specimens referred to K. papillosa (Figure 6), whereas the genetics results indicate only three monophyletic clades (Figure 9). However, the genetic analysis in this study is only based on one mitochondrial gene (COI). Further study, including cytochrome b (Cytb) and/or nuclear genes may help considerably in resolving the taxonomy of this species complex (Anwarali et al. 2010, Nesi et al. 2011, Francis and Eger 2012, Thong et al. 2012, Lin et al. 2014). As, currently, there are no additional supporting data, the specimens referred to K. papillosa are here separated into three distinct taxa or monophyletic clades.
Genetically, K. papillosa A is separated from K. papillosa B, with an average divergence of 6.97%. K. papillosa A (Figure 6A) also has a larger skull and a higher braincase than K. papillosa B (Figure 6D & E).
Specimens of K. papillosa A, which are restricted within Thailand to the south of the peninsula (Figure 10), are here assigned to the taxon malayana on the basis of size. In a comparison with specimens of malayana listed in the type description by Chasen (1940), the four specimens from peninsular Thailand have a similar forearm length (40.7–43.4 mm) and a slightly smaller skull length (18.2–18.4 mm), mastoid width (8.7 mm), breadth of braincase (8.4 mm) and upper toothrow length (7.6 mm). In terms of genetics, the Thai specimens of this taxon cluster together with those referred to K. papillosa S in Anwarali et al. (2010) from peninsular Malaysia (some of which are collected close to the type locality of malayana) and Borneo (Figure 9).
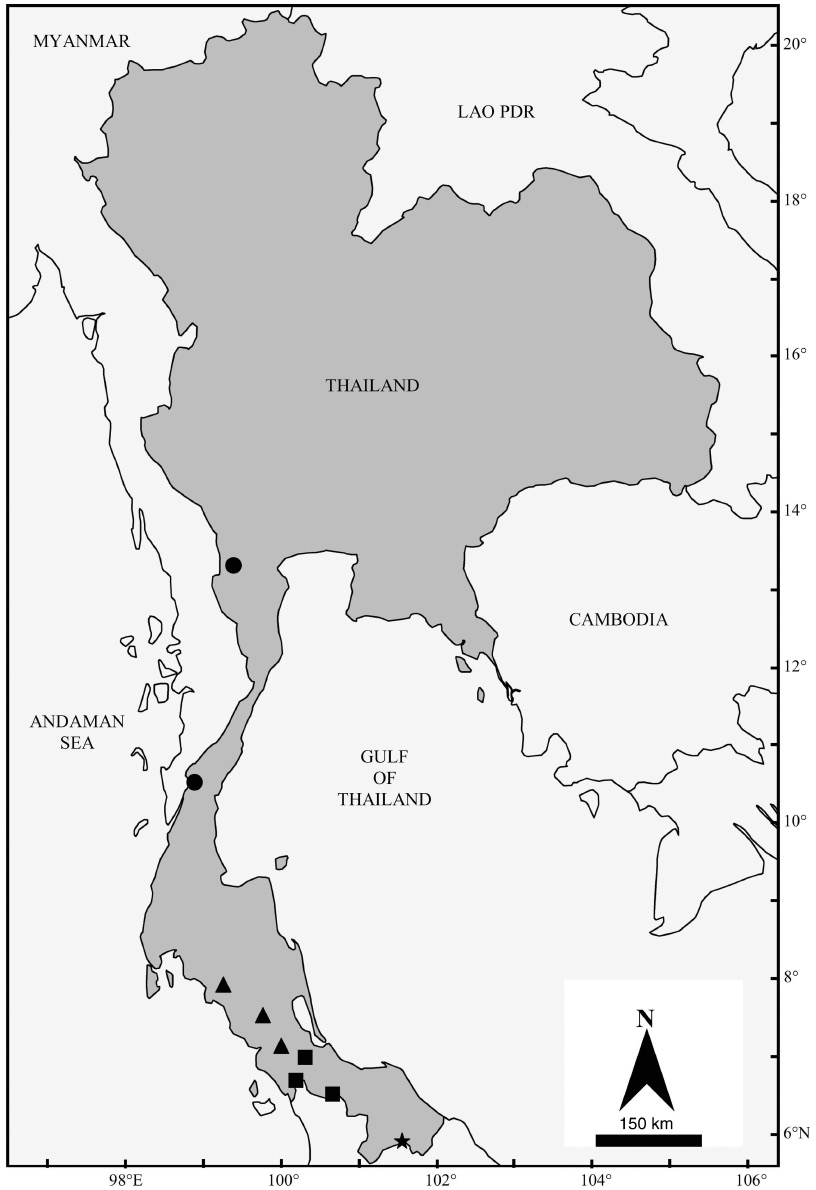
Distribution map of K. papillosa (sensu lato) in Thailand. Black squares: K. papillosa A, black circles: K. papillosa B, black triangles: K. papillosa C and black star: location where the three taxa are found sympatrically.
The clade of Kerivoula papillosa B, which is found throughout peninsular Thailand (Figure 10), includes two different morphotypes (Figures 6D and E), which are relatively larger (FA: 42.1–42.3 mm and GLS: 17.0–17.1 mm) and relatively smaller (FA: 39.4–40.2 mm and GLS: 16.6–17.0 mm). Genetically, there is only 1.99% divergence within the clade. On the basis of genetics, they are regarded herewith as a single taxon, until there is more evidence to support a separation. Specimens of K. papillosa B from throughout the study area are difficult to assign to any particular named taxon. Only two pre-existing names are available, K. p. papillosa from Bantam, northwestern Java and K. lenis from Calcutta, Bengal, India. Others, such as K. flora Thomas, 1914 from Flores, Lesser Sunda Islands have similar forearm length (39.5 mm) but a smaller skull (GLS: 16.0 mm; C-M3: 6.2 mm).
K. papillosa B has a smaller forearm length than the 43.2 mm and 44.5 mm of the holotype and co-type (“a”) of papillosa listed by Dobson (1876) and Tate (1941), but has a similar skull length to those from Java (<17.0 mm) examined by Chasen (1940). The Thai specimens of this clade have a similar forearm length (37.2–40.2 mm) and skull length (16.4–17.1 mm) to those of K. lenis examined by Vanitharani et al. (2003). However, genetically, they are separated from those K. lenis and K. papillosa reported by Anwarali et al. (2010) and Francis et al. (2010) in the Barcode of Life Database (Figure 9). Clearly, further research is required in Southeast Asia and India, to help assign K. papillosa B with certainty to its correct taxon. It is also possible that it may represent a new and, as yet, undescribed species. However, without additional studies, especially in the type localities of papillosa and lenis, it will be difficult to prove that it is not referable to one or other of these two taxa.
Specimens within the clade of K. papillosa C, which in Thailand is restricted to the south of the peninsula (Figure 10), show morphological variation (Figure 6B and C), but are similar in size. Genetically, material of this clade is clustered together, with a 0.55% divergence within the clade. Thai specimens have smaller forearm length than those of the holotype and co-type of K. papillosa listed by Dobson (1876) and Tate (1941), but a longer skull than that examined by Chasen (1940). The specimens average larger in external and cranio-dental measurements than those of K. lenis (Vanitharani et al. 2003). Specimens within the K. papillosa C/K. lenis PM clade from Thailand and peninsular Malaysia differ by a 5.33% divergence from K. lenis BN from Borneo (Table 9). However although within the same genetic lineage, the specimens from peninsular Malaysia and Borneo included in Anwarali et al. (2010) are smaller in external and cranial measurements compared to the Thai specimens of K. papillosa C. In addition, the taxonomic status of K. lenis from throughout its range is unclear. Genetically, K. cf. lenis from Lao PDR does not cluster with the K. lenis from peninsular Malaysia and Borneo, as there is >14% divergence (Anwarali et al. 2010, Francis et al. 2010) (Table 9). As there is currently a lack of comparative material and genetic information from India, it is difficult to assign the correct name to this taxon.
The specimens referred to K. kachinensis on the basis of their large size and flat skull are clearly different from other species in the genus and are comparable to the description and measurements of K. kachinensis in Bates et al. (2004), Thong et al. (2006) and Soisook et al. (2007).
Material referred to K. hardwickii (sensu lato) includes several forms, which can be divided into two main morphotypes, “domed” and “flat” skulls (Figure 5C and D). The specimens with “domed-skull” vary in both size and morphology (Figure 7), but the result of genetic analysis shows that all available sequences of specimens with domed skulls cluster together in a monophyletic clade (K. hardwickii C), with a 1.04% divergence from each other. Specimens of this clade are restricted in Thailand to the east and peninsula (Figure 11). However, this study is only based on COI. A further study with Cytb and a nuclear gene could be of considerable interest to clarify whether there are cryptic species “hidden” within this taxon (Anwarali et al. 2010, Nesi et al. 2011, Francis and Eger 2012, Thong et al. 2012, Lin et al. 2014). As there is no additional supporting information, specimens with domed skulls are here assigned to K. hardwickii. Other taxa, such as fusca and engana, are considered to be synonyms of hardwickii on the basis of similar skull morphology with only minor differences in colour of the pelage (Dobson 1876, Chasen 1940, Hill 1965).
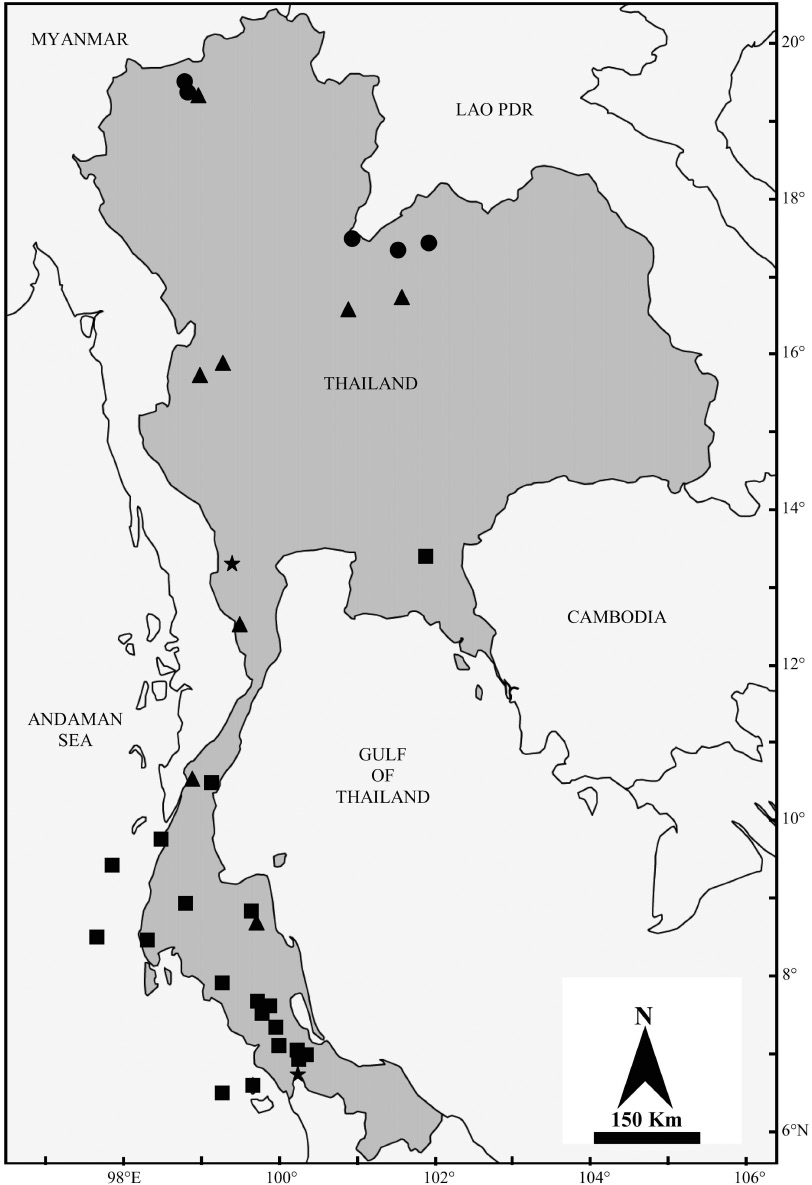
Distribution map of K. hardwickii (sensu lato) in Thailand. Black triangles: K. hardwickii A, black circles: K. hardwickii B, black squares: K. hardwickii C and black stars: locations where K. hardwickii A and K. hardwickii C are found sympatrically.
The specimens with “flat-skulls” are essentially similar to each other in both size and morphology (Figure 8), but differ genetically and cluster into two monophyletic clades (K. hardwickii A and K. hardwickii B) (Figure 9), which may represent two distinct species. The clade K. hardwickii A is widely distributed in Thailand from the north of the country to the south of the peninsula, whereas K. hardwickii B is only found in northern Thailand (Figure 11). The most geographically adjacent available name is depressa described from southeast Myanmar. Currently the taxa, crypta and malpasi, are considered to be synonyms of depressa. The taxon crypta, which has a relatively flattened braincase was described from western peninsular India, and the taxon malpasi, which differs only in pelage colour, is geographically isolated in Sri Lanka (Bates and Harrison 1997). Bates et al. (2007) suggest that the correct name for the bats with smaller, flat-headed skulls (BH<5.1 mm) may be K. depressa. However, as the genetic analysis shows there are potentially two cryptic species within the taxon, without additional study, especially in the type locality of depressa (and possibly crypta, although geographically this seems unlikely), it will be very difficult to prove which one is referable to the taxon depressa and which is possibly referable to an undescribed species.
The specimens referred to K. titania on the basis of their slightly larger, flat-skulls are comparable to the description of K. titania by Bates et al. (2007).
Specimens referred to K. pellucida are distinguished from other species in the genus by their orange-brown pelage and high and bulbous skull, the braincase of which rises abruptly from the rostrum (Figure 5F); this corresponds to descriptions given in Hill (1965), Corbet and Hill (1992) and Francis (2008). In the case of K. krauensis, it distinguished from all other species by its dorsal pelage (shiny golden hair tips with dark brown bases) and slightly flattened skull (Figure 5G) (Francis et al. 2007, Douangboubpha et al. 2014). Specimens here referred to K. minuta have small, bulbous skulls (Figure 5H), averaging smaller and more inflated than those of K. intermedia; this agrees with the findings of Miller (1898), Hill and Francis (1984), Corbet and Hill (1992) and Francis (2008). It also agrees with the genetic results in which the Thai material is separated from K. intermedia from Borneo (Figure 9).
Kingston et al. (1999) suggested that the echolocation calls of six Kerivoula forms in Malaysia (K. intermedia, K. minuta, K. papillosa L[arge], K. papillosa S[mall], K. pellucida and Kerivoula sp. [=K. krauensis in Francis et al. 2007]) can be used to assist with their identification. Analysis showed that there is a significant difference in average call characters among species, although there was a large overlap in the ranges of all measured parameters. The present study supports the findings of Kingston et al. (1999).
The results also support the need of genetic data for recognising cryptic species, as well as for differentiating intra-specific from inter-specific morphological variation (Francis and Eger 2012). Thus, combining morphometric, genetic and acoustic data is particularly valuable, even though the interpretation of the results from a taxonomic perspective may be challenging. This is particularly the case where there is a lack of congruence between the different character states. Further studies, including type specimen of each known taxon and specimens from mainland Southeast Asia (Myanmar, Lao PDR, Vietnam and Cambodia), Sumatra, Java, Borneo, Sulawesi and Philippines would help to find out their diagnosis characters.
Acknowledgments
In Lao PDR, we would like to thank the staff of the Faculty of Environmental Sciences, National University of Laos, especially Assoc. Prof. Souphab Khouangvichit, Daosavanh Sanamxay, Oley Phearkeo for their help and support of bat research in Lao PDR. In Thailand, many thanks are due to the students of the Small Mammal and Bird Research Unit, Department of Biology and Department of Biotechnology and Bioinformatics, Faculty of Science and the staff of the Princess Maha Chakri Sirindhorn Natural History Museum, Prince of Songkla University for the help. We also thank the staff of Hala-Bala Wildlife Research Station, especially Mr. Sunate Karapan, for their help in organising and participating in a range of the field surveys. In UK, we would like to thank David Harrison, Malcolm Pearch, Nikky Thomas and Beatrix Lanzinger of Harrison Institute for their help, support and suggestions. We thank Tigga Kingston of the Texas Tech University and SEABCRU (Southeast Asian Bat Conservation and Research Unit) for promoting networking amongst bat taxonomists in Southeast Asia. Finally, we would like to thank the National Research University Project of Thailand’s Office of the Higher Education Commission and Graduate School, Prince of Songkla University, Thailand, the Darwin Initiative (Project No: 18002) of the UK Government for their financial support. Without all of the above and the collaboration of many others, this project would not have been possible.
Appendix
Gazetteer.
| No | Location | Province | Coordinate | ||
|---|---|---|---|---|---|
| 1 | Khun Mae Ngai GS., Chiang Dao WS. | Chiang Mai | 19°30′33″N, 98°49′57″E | ||
| 2 | Hui Mae Kok RS, Chiang Dao WS. | Chiang Mai | 19°22′25″N, 98°50′05″E | ||
| 3 | Chiang Dao WRS. | Chiang Mai | 19°21′51″N, 98°55′23″E | ||
| 4 | Phou Suan Sai NP. | Loei | 17°30′39″N, 100°57′07″E | ||
| 5 | Na Haew, Phu Suan Sai NP. | Loei | 17°28′00″N, 101°58′00″E | ||
| 6 | Hauy Nam Chan, Phu Loung WS. | Loei | 17°20′34″N, 101°33′49″E | ||
| 7 | Namnao NP. | Phetchabun | 16°45′06″N, 101°33′44″E | ||
| 8 | Thung Salang Luang NP. | Phisanoulok | 16°34′16″N, 100°52′34″E | ||
| 9 | Mae Wong NP. | Tak | c.o. 15°54′N, 99°12′E | ||
| 10 | East Thung Yai Naresuan WS. | Tak | 15°44′00″N, 98°59′06″E | ||
| 11 | Angruenai WS. | Chachuengsao | 13°24′44″N, 101°52′44″E | ||
| 12 | Pha Chi WS. | Ratchaburi | 13°18′30″N, 99°24′51″E | ||
| 13 | Pa La-U RS., Kaeng Krachan NP. | Prachuap Khiri Khan | 12°32′17″N, 99°27′48″E | ||
| 14 | Khao Kaew, Muang District | Chumphon | 10°30′18″N, 99°07′09″E | ||
| 15 | South Klom Luang Chumporn WS. | Ranong | 10°31′02″N, 98°54′09″E | ||
| 16 | Koh Chang, Mo Koh Payam NP. | Ranong | 9°48′41″N, 98°26′08″E | ||
| 17 | North Surin Island, Surin Islands NP. | Phang Nga | 9°26′45″N, 97°52′18″E | ||
| 18 | Koh No. 8, Mu Koh SimilanNP. | Phang Nga | 8°39′46″N, 97°39′10″E | ||
| 19 | Lumpee Waterfall, Khao Lumpee-Had Thaymaung NP. | Phang Nga | 8°27′55″N, 98°17′34″E | ||
| 20 | Khao Sok NP. | Surat Thani | c.o. 8°57′N, 98°48′E | ||
| 21 | Khao Nan NP. | Nakhon Si Thammarat | 8°51′56″N, 99°37′25″E | ||
| 22 | Krungching, Khao Luang NP. | Nakhon Si Thammarat | c.o. 8°42′N, 99°41′E | ||
| 23 | Khao Pra-Bang Kram WS. | Krabi | 7°55′31″N, 99°15′47″E | ||
| 24 | Ban Nai Khao, Hui Yod District | Trang | 7°42′28″N, 99°41′10″E | ||
| 25 | Khao Pu-Khao Ya NP. | Patthalung | c.o. 7°40″N, 99°52′E | ||
| 26 | Khao Chong WEC., Khao Bantad WS. | Trang | 7°33′02″N, 99°46′54″E | ||
| 27 | Sai Rung Waterfall, Khao Bantad WS. | Trang | 7°25′27″N, 99°46′10″E | ||
| 28 | Priwan Waterfall, Khao Bantad WS. | Phatthalung | 7°23′48″N, 99°58′40″E | ||
| 29 | Phu Pha Phet RS., Khao Bantad WS. | Satun | 7°07′56″N, 100°00′27″E | ||
| 30 | Had Sai Khao Waterfall, Rattaphum District | Songkhla | 7°02′40″N, 100°12′31″E | ||
| 31 | Kuan Khao Wang FP. | Songkhla | 7°00′28″N, 100°18′45″E | ||
| 32 | Ton Nga Chang WS. | Songkhla | 6°56′44″N, 100°14′28″E | ||
| 33 | Pha Dam Ranger Station, Ton Nga Chang WS. | Songkhla | 6°47′13″N, 100°13′34″E | ||
| 34 | Taleban NP. | Satun | 6°39′25″N, 100°09′03″E | ||
| 35 | Tarutao Island, Tarutao NP. | Satun | 6°39′30″N, 99°40′00″E | ||
| 36 | Adang-Rawi Islands, Tarutao NP. | Satun | 6°32′57″N, 99°16′52″E | ||
| 37 | Khao Nam Khang NP. | Songkhla | 6°06′30″N, 101°04′28″E | ||
| 38 | Hala-Bala WS. | Narathiwat | 5°48′01″N, 101°50′00″E |
List of specimens examined in the present study for morphometrics, COI sequence (in bold) and echolocation data with the superscript (i).
| Location | Total number | Specimen number |
|---|---|---|
| Kerivoula papillosa A | ||
| Kuan Khao Wang FP. | ♀ | PSUZC-MM2012.70 |
| Khao Nam Khang NP. | ♂ | HBWRS2010.3 |
| Taleban NP. | ♂ | PSUZC-MM2013.8 |
| Hala-Bala WS. | ♀ | PSUZC-MM2013.28 |
| Kerivoula papillosa B | ||
| Pha Chi WS. | 2♂♂ | PSUZC-MM2008.56, 2008.57 |
| South Klom Luang Chumporn WS. | ♀ | PSUZC-MM2011.53(i) |
| Hala-Bala WS. | 2♀♀ | PSUZC-MM2013.10, 2013.11 |
| Kerivoula papillosa C | ||
| Khao Pra-Bang Kram WS. | 2♀♀ | PSUZC-MM2012.173(i), 2012.174 |
| Khao Chong, Khao Bantad WS. | 2♂♂♀ | PSUZC-MM2011.2, 2012.175, 2013.9 |
| Phu Pha Phet, Khao Bantad WS. | ♀ | PSUZC-MM2012.172 |
| Hala-Bala WS. | 2♂♂4♀♀ | PSUZC-MM2005.123, 2011.4(i), 2011.10(i), 2012.53(i), 2012.54(i), 2013.12 |
| Kerivoula kachinensis | ||
| Khun Mae Ngai., Chiang Dao WS. | ♀ | PSUZC-MM2011.16 |
| Phou Suan Sai NP. | 3♂♂ | PSUZC-MM2006.66, 2007.242, 2007.243 |
| Namnao NP. | ♂ | PSUZC-MM2007.244 |
| Kerivoula titania | ||
| Khun Mae Ngai., Chiang Dao WS. | ♂ | PSUZC-MM2011.18 |
| Chiang Dao WRS. | ♀ | PSUZC-MM2011.15(i) |
| Phou Suan Sai NP. | ♂♀ | PSUZC-MM2007.302, 2007.303 |
| Namnao NP. | ♀ | PSUZC-MM2007.304 |
| Pa La-U, Kaeng Krachan NP. | ♂ | PSUZC-MM2011.48(i) |
| Kerivoula hardwickii A | ||
| Chiang Dao WRS. | ♀ | PSUZC-MM2011.19(i) |
| Namnao NP. | ♂♀ | PSUZC-MM2007.274, 2007.275 |
| Thung Salang Luang NP. | ♀ | PSUZC-MM2006.165 |
| Mae Wong NP. | 2♀♀ | PSUZC-MM2013.26, 2013.27 |
| East Thung Yai Naresuan WS. | ♀ | PSUZC-MM2008.75 |
| South Klom Luang Chumporn WS. | ♀ | PSUZC-MM2011.51 |
| Khao Luang NP. | ♂ | PSUZC-MM2012.166 |
| Pha Dam, Ton Nga Chang WS. | ♀ | PSUZC-MM2007.281 |
| Pha Chi WS. | ♀ | PSUZC-MM2007.345 |
| Pa La-U, Kaeng Krachan NP. | ♂ | PSUZC-MM2011.52(i) |
| Kerivoula hardwickii B | ||
| Khun Mae Ngai, Chiang Dao WS. | ♂ | PSUZC-MM2011.17(i) |
| Hui Mae Kok, Chiang Dao WS. | ♀ | PSUZC-MM2011.14 |
| Phu Saun Sai NP. | 2♂♂♀ | PSUZC-MM2007.272, 2007.273 |
| Na Haew, Phu Suan Sai NP. | PSUZC-MM2006.166 | |
| Hauy Nam Chan, Phu Loung WS. | ♂♀ | PSUZC-MM2005.201, 2005.202 |
| Kerivoula hardwickii C | ||
| Angruenai WS. | ♂ | PSUZC-MM2005.200 |
| Pha Chi WS. | ♂♀ | PSUZC-MM2007.343, 2007.344 |
| Khao Kaew, Muang District | ♀ | PSUZC-MM2012.169 |
| Koh Chang, Mo Koh Payam NP. | ♂ | PSUZC-MM2011.20 |
| North Surin Island, Surin Islands NP. | 2♂♂♀ | PSUZC-MM2006.152, 2006.187, 2008.74 |
| Lumpee Waterfall, Khao Lumpee-Had Thaymaung NP. | ♂ | PSUZC-MM2013.4 |
| Koh No. 8, Mu Koh Similan NP. | 2♂♂ | PSUZC-MM2008.77, 2008.78 |
| Khao Sok NP. | 3♂♂♀ | PSUZC-MM2012.167, 2012.170, 2012.171, 2013.6 |
| Khao Nan NP. | ♂3♀♀ | PSUZC-MM2007.286, 2007.287, 2007.311, 2007.312 |
| Khao Pra-Bang Kram WS. | 4♂♂♀ | PSUZC-MM2012.161, 2012.162(i), 2012.163, 2012.164, 2012.168(i) |
| Ban Nai Khao, Hui Yod District | ♀ | PSUZC-MM2007.285 |
| Khao Chong, Khao Bantad WS. | 2♂♂♀ | PSUZC-MM2007.282, 2007.284, 2012.113 |
| Khao Pu-Khao Ya NP. | 2♂♂ | HBWRS2010.7, 2010.11 |
| Priwan Waterfall, Khao Bantad WS. | ♂ | PSUZC-MM2012.165 |
| Phu Pha Phet, Khao Bantad WS. | 2♂♂2♀♀ | PSUZC-MM2012.160 |
| Hin Sam Kon Waterfall, Rattaphum District | ♀ | PSUZC-MM2012.59 |
| Kuan Khao Wang Forest Park | ♂♀ | PSUZC-MM2011.49(i), 2011.50 |
| Ton Nga Chang WS. | 6♂♂3♀♀ | PSUZC-MM2007.270, 2007.271, 2007.276, 2007.277, 2007.280, 2007.283, 2007.289, 2012.62 |
| Pha Dam, Ton Nga Chang WS. | 2♂♂2♀♀ | PSUZC-MM2007.152, 2007.279, 2012.57, 2012.58 |
| Tarutao Island, Tarutao NP. | 4♂♂2♀♀ | PSUZC-MM2005.187, 2007.147, 2008.131, 2008.132, 2009.62, 2013.3 |
| Adang-Rawi Islands, Tarutao NP. | 4♂♂2♀♀ | PSUZC-MM2008.79, 2009.40, 2009.41, 2012.60, 2012.61, 2013.5 |
| Kerivoula pellucida | ||
| Khao Pra-Bang Kram WS. | ♀ | PSUZC-MM2012.176 |
| Khao Pu Khao Ya NP. | ♂♀ | HBWRS2010.6, 2010.10 |
| Phu Pha Phet, Khao Bantad WS. | ♂ | PSUZC-MM2013.24 |
| Kuan Khao Wang FP. | ♂♀ | PSUZC-MM2011.46(i), 2011.47 |
| Ton Nga Chang WS. | 3♂♂2♀♀ | PSUZC-MM2012.66, 2012.69, 2013.2 |
| Khao Nam Khang NP. | ♀ | HBWRS2010.9 |
| Hala-Bala WS. | 2♂♂2♀♀ | PSUZC-MM2011.12, 2011.13, 2012.55, 2012.56 |
| Kerivoula krauensis | ||
| Hala-Bala WS. | ♂ | PSUZC-MM2013.25(i) |
| Kerivoula minuta | ||
| Khao Chong, Khao Bantad WS. | ♂ | PSUZC-MM2011.1 |
| Sai Rung Waterfall, Khao Bantad WS. | ♀ | PSUZC-MM2012.50 |
| Phu Pha Phet, Khao Bantad WS. | 2♂♂ | PSUZC-MM2013.19-20 |
| Ton Nga Chang WS. | 5♂♂2♀♀ | PSUZC-MM2007.153, 2007.278, 2007.310, 2012.63, 2012.65, 2013.7 |
| Khao Nam Khang NP. | 2♂♂♀ | HBWRS2010.4, 2010.5, 2010.8 |
| Hala-Bala WS. | 9♂♂♀ | PSUZC-MM2011.3, 2011.5(i), 2011.6(i), 2011.7, 2011.8(i), 2011.9(i), 2012.51(i), 2011.52, 2012.108, 2012.110 |
List of sequences in the Barcode of Life Database used in the present study.
| Location | Specimen number | GenBank number |
|---|---|---|
| Kerivoula papillosa Large | ||
| Sepilok Forest Reserve, Borneo | ROM MAM 117932, 117933, 117934 | HM540724, 540725, 540723 |
| Niah NP., Borneo | TK 156001, 156004, 156036 | GU585627, 585628, 585626 |
| Kerivoula papillosa Small | ||
| Kuala Lompat, Malaysia | CMF920706-03 | HM540730 |
| Endau Rompin NP., Malaysia | ROM MAM 113056, 113110 | HM540726, 540729 |
| Krau Wildlife Reserve, Malaysia | S300091, 300721, TK 152020, 152023, 152061, 152062 | HM540727, 540728, GU585632, 585619, 585618, 585622 |
| Similajau NP., Borneo | TK 125619, 125620 | GU585624, 585623 |
| Niah NP., Borneo | TK 152403 | GU585625 |
| Taman Negara, Malaysia | TK 152994 | GU585620 |
| Kerivoula lenis | ||
| Endau Rompin NP., Malaysia | ROM MAM 113049, 113053 | HM540719, 540718 |
| Sepilok Forest Reserve, Borneo | ROM MAM 117931 | HM540717 |
| Krau Wildlife Reserve, Malaysia | TK 152052 | GU585595 |
| Kubah NP., Borneo | TK 152178 | GU585596 |
| Kerivoula cf. lenis | ||
| Xe Kaman, Dong Amphan NBCA, Laos | ROM MAM 110520, 110527, 110589 | HM540721, 540722, 540720 |
| Cat Tien NP., Vietnam | ROM MAM 110850 | JF443954 |
| Bu Gia Map NR., Vietnam | ZMMU139-09 | HM914930 |
| Kerivoula kachinensis | ||
| Phou Khao Khouay NBCA, Laos | EBD 25122 | HM540735 |
| Kerivoula kardwickii | ||
| Khao Nor Chuchi Reserve | INECOL M0071, 0108 | HM540715, 540716 |
| Kerivoula krauensis | ||
| Krau Wildlife Reserve, Malaysia | SMF 83824 | HM540742 |
| Kerivoula picta | ||
| Houi Nhang NR, Laos | ROM MAM 106371 | HM540755 |
| Kerivoula intermedia | ||
| Lanjak Entimau WS., Borneo | TK 153621 | GU585601 |
| Kerivoula minuta | ||
| Khao Nor Chuchi Reserve | INECOL M0111, 0112 | HM540749, 540745 |
| Murina suilla | ||
| Sepilok FR, Malaysia | ROM MAM 117936 | HM540991 |
| Murina peninsularis | ||
| Krau Wildlife Reserve, Malaysia | CMF920703-03 | HM540973 |
| Myotis cf. muricola | ||
| Tuaran, Borneo | ROM MAM 117943 | HM541064 |
References
Anwarali, F.A., S. Solari, V.J. Swier, P.A. Larsen, M.T. Abdullah and R.J. Baker. 2010. Systematics of Malaysian woolly bats (Vespertilionidae: Kerivoula) inferred from mitochondrial, nuclear, karyotypic, and morphological data. J. Mammal. 91: 1058–1072.Suche in Google Scholar
Bates, P.J.J. and D.L. Harrison. 1997. Bats of the Indian Subcontinent. Harrison Zoological Museum, Sevenoaks, England. pp. xvi + 258.Suche in Google Scholar
Bates, P.J.J., M.J. Struebig, S.J. Rossiter, T. Kingston, S.S. Lin Oo and K. Mya Mya. 2004. A new species of Kerivoula (Chiroptera: Vespertilionidae) from Myanmar (Burma). Acta Chiropterol. 6: 219–226.10.3161/001.006.0203Suche in Google Scholar
Bates, P.J.J., M.J. Struebig, B.D. Hayes, N.M. Furey, K. Mya Mya, V.D. Thong, P.D. Tien, N.T. Son, D.L. Harrison, C.M. Francis and G. Csorba. 2007. A new species of Kerivoula (Chiroptera: Vespertilionidae) from Southeast Asia. Acta Chiropterol. 9: 323–337.10.3161/1733-5329(2007)9[323:ANSOKC]2.0.CO;2Suche in Google Scholar
Brunet-Rossinni, A.K. and G.S. Wilkinson. 2009. Methods for age estimation and the study of senescence in bats. In: (T.H. Kunz and S. Parsons, eds.) Ecological and behavioral methods for the study of bats. 2nd edition. The Johns Hopkins University Press, Baltimore, MD. pp. 315–325.Suche in Google Scholar
Bumrungsri, S., D.L. Harrison, C. Satasook, A. Prajukjitr, S. Thong-Aree and P.J.J. Bates. 2006. A review of bat research in Thailand with eight new species records for the country. Acta Chiropterol. 8: 325–359.10.3161/1733-5329(2006)8[325:AROBRI]2.0.CO;2Suche in Google Scholar
Chasen, F.N. 1940. A handlist of Malaysian mammals: a systematic list of the mammals of the Malay Peninsula, Sumatra, Borneo and Java, including the adjacent small islands. Bull. Raffles Mus., Singapore, Straits Settlements 15: i-xx + 1–209.Suche in Google Scholar
Corbet, G.B. and J.E. Hill. 1992. The mammals of the Indomalayan region: a systematic review. Natural History Museum Publications and Oxford University Press, London, England. pp. 488.Suche in Google Scholar
Dobson, G.E. 1876. Monograph of the Asiatic Chiroptera, and catalogue of the species of bats in the collection of the Indian Museum, Calcutta. Trustees of the Indian Museum, London, England. pp. viii + 228.Suche in Google Scholar
Douangboubpha, B., S. Bumrungsri, P. Soisook, S. Karapan and P.J.J. Bates. 2014. The discovery of Kerivoula krauensis (Chiroptera: Vespertilionidae) from southern peninsular Thailand provides new information on the distribution and conservation status of this data deficient species. Songklanakarin J. Sci. Technol. 36: 577–582.Suche in Google Scholar
Ellerman, J.R. and T.C.S. Morrison-Scott. 1951. Checklist of Palaearctic and Indian mammals 1758 to 1946. Trustees of the British Museum, London, England. pp. 810.Suche in Google Scholar
Francis, C.M. 1989. A comparison of mist nets and two designs of harp traps for capturing bats. J. Mammal. 70: 865–870.10.2307/1381730Suche in Google Scholar
Francis, C.M. 2008. A field guide to the mammals of Thailand and South-east Asia. New Holland Publishers (UK) Ltd, London, England. pp. 392.Suche in Google Scholar
Francis, C.M. and J.L. Eger. 2012. A review of tube-nosed bats (Murina) from Laos with a description of two new species. Acta Chiropterol. 14: 15–38.10.3161/150811012X654231Suche in Google Scholar
Francis, C.M., T. Kingston and A. Zubaid. 2007. A new species of Kerivoula (Chiroptera: Vespertilionidae) from peninsular Malaysia. Acta Chiropterol. 9: 1–12.10.3161/1733-5329(2007)9[1:ANSOKC]2.0.CO;2Suche in Google Scholar
Francis, C.M., A.V. Borisenko, N.V. Ivanova, J.L. Eger, B.K. Lim, A. Guillén-Servent, S.V. Kruskop, I. Mackie and P.D.N. Hebert. 2010. The role of DNA barcodes in understanding and conservation of mammal diversity in Southeast Asia. PLoS One 5: e12575.10.1371/journal.pone.0012575Suche in Google Scholar
Hasan, N.H. and M.T. Abdullah. 2011. A morphological analysis of Malaysian Kerivoula (Chiroptera: Vespertilionidae). Mammal Study 36: 87–97.10.3106/041.036.0207Suche in Google Scholar
Helversen, O. von, K.G. Heller, F. Mayer, A. Nemeth, M. Volleth and P. Gombkötö. 2001. Cryptic mammalian species: a new species of whisker bat (Myotis alcathoe n. sp.) in Europe. Naturwissenschaften 88: 217–223.10.1007/s001140100225Suche in Google Scholar
Hill, J.E. 1965. Asiatic bats of the genera Kerivoula and Phoniscus (Vespertilionidae), with a note on Kerivoula aerosa Tomes. Mammalia 29: 524–556.Suche in Google Scholar
Hill, J.E. 1975. Taxonomic background, systematic section, summary of systematic work, bibliography, gazetteer. In: (CTNRT Staffs, J.E. Hill and J.A. McNeely, eds.) The bats and bat parasites of Thailand. U.S. Army Res. Devel. Group Far East, Report No. FE-516-1. pp. 3–40, 79–84.Suche in Google Scholar
Hill, J.E. 1983. Bats (Mammalia: Chiroptera) from Indo-Australia. Bull. Brit. Mus. (Nat. Hist.) 45: 103–208.10.5962/p.27997Suche in Google Scholar
Hill, J.E. and C.M. Francis. 1984. New bats (Mammalia: Chiroptera) and new records of bats from Borneo and Malaya. Bull. Brit. Mus. (Nat. Hist.) 47: 305–329.10.5962/p.21841Suche in Google Scholar
Ivanova, N.V., J.R. Dewaard and P.D.N. Hebert. 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 6: 998–1002.Suche in Google Scholar
Ivanova, N.V., E.L. Clare and A.V. Borisenko. 2012. DNA barcoding in mammals. In: (W.J. Kress and D.L. Erickson, eds.) DNA barcodes: methods and protocol. Met. Mol. Biol. 856: 153–182.10.1007/978-1-61779-591-6_8Suche in Google Scholar
Kiefer, A., F. Mayer, J. Kosuch, O. von Helversen and M. Vieth. 2002. Conflicting molecular phylogenies of European long-eared bats (Plecotus) can be explained by cryptic diversity. Mol. Phylogenet. Evol. 25: 557–556.10.1016/S1055-7903(02)00299-3Suche in Google Scholar
Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120.10.1007/BF01731581Suche in Google Scholar
Kingston, T. 2010. Research priorities for bat conservation in Southeast Asia: a consensus approach. Biodiv. Conserv. 19: 471–484.10.1007/s10531-008-9458-5Suche in Google Scholar
Kingston, T., G. Jones, Z. Akbar and T.H. Kunz. 1999. Echolocation signal design in Kerivoulinae and Murininae (Chiroptera: Vespertilionidae) from Malaysia. J. Zool. (Lond.) 249: 359–374.10.1111/j.1469-7998.1999.tb00771.xSuche in Google Scholar
Lekagul, B. and J.A. McNeely. 1988. Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, Thailand. pp. 758.Suche in Google Scholar
Lin, A.Q., G. Csorba, L.F. Li, T.L. Jiang, G.J. Lu, V.D. Thong, P. Soisook, K.P. Sum and J. Feng. 2014. Phylogeography of Hipposideros armiger (Chiroptera: Hipposideridae) in the Oriental Region: the contribution of multiple Pleistocene glacial refugia and intrinsic factors to contemporary population genetic structure. J. Biogeogr. 41: 317–327.10.1111/jbi.12163Suche in Google Scholar
Mayer, F. and O. von Helversen. 2001. Cryptic diversity in European bats. Proc. R. Soc. Lond. Biol. Sci. 268: 1825–1832.10.1098/rspb.2001.1744Suche in Google Scholar
McCune, B. and M.J. Mefford. 2006. PC-ORD. Multivariate Analysis of Ecological Data. Version 5.10. MjM Software, Gleneden Beach, Oregon, USA.Suche in Google Scholar
Miller, G.S. 1898. List of bats collected by Dr. W. L. Abbott in Siam. Proc. Acad. Nat. Sci. 316–325.Suche in Google Scholar
Myers, N., R.A. Mittermeier, C.G. Mittermeier, G.A.B. da Fonseca and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.10.1038/35002501Suche in Google Scholar
Nesi, N., E. Nakouné, C. Cruaud and A. Hassanin. 2011. DNA barcoding of African fruit bats (Mammalia, Pteropodidae). The mitochondrial genome does not provide a reliable discrimination between Epomophorus gambianus and Micropteropus pusillus. C. R. Biol. 334: 544–554.Suche in Google Scholar
Payne, J. and C.M. Francis. 1985. A field guide to the mammals of Borneo. The Sabah Soc., Kota Kinabalu, Sabah, Malaysia. pp. 332.Suche in Google Scholar
Preatoni, D.G., M. Nodari, R. Chirichella, G. Tosi, L.A. Wauters and A. Martinoli. 2005. Identifying bats from time-expanded recordings of search calls: comparing classification methods. J. Wildl. Manage. 69: 1601–1614.10.2193/0022-541X(2005)69[1601:IBFTRO]2.0.CO;2Suche in Google Scholar
Racey, P.A. 2009. Reproductive assessment of bats. In: (T.H. Kunz and S. Parsons, eds.) Ecological and behavioral methods for the study of bats. 2nd edition. The Johns Hopkins University Press, Baltimore, MD. pp. 249–264.Suche in Google Scholar
Simmons, N.B. 2005. Order Chiroptera. In: (D.E. Wilson and D.M. Reeder, eds.) Mammal species of the World: a taxonomic and geographic reference. 3rd edition. The Johns Hopkins University Press, Baltimore, MD. pp. 312–529.Suche in Google Scholar
Sinha, Y.R. 1999. Records of the zoological survey of India: contribution to the knowledge of bats (Mammalia: Chiroptera) from North East Hills, India. Zool. Surv. India, Calcutta, India. pp. 52.Suche in Google Scholar
Soisook, P., S. Bumrungsri, A. Dejtaradol, C.M. Francis, G. Csorba, A. Guillén-Servent and P.J.J. Bates. 2007. First records of Kerivoula kachinensis (Chiroptera: Vespertilionidae) from Cambodia, Lao PDR and Thailand. Acta Chiropterol. 9: 339–345.Suche in Google Scholar
Soisook, P., S. Karapan, C. Satasook and P.J.J. Bates. 2013a. A new species of Murina (Mammalia: Chiroptera: Vespertilionidae) from peninsular Thailand. Zootaxa 3746: 567–579.10.11646/zootaxa.3746.4.4Suche in Google Scholar
Soisook, P., S. Karapan, C. Satasook, V.D. Thong, F.A.A. Khan, I. Maryanto, G. Csorba, N. Furey, B. Aul and P.J.J. Bates. 2013b. A review of the Murina cyclotis complex (Chiroptera: Vespertilionidae) with descriptions of a new species and subspecies. Acta Chiropterol. 15: 271–292.10.3161/150811013X678928Suche in Google Scholar
SPSS. 2005. SPSS for Windows, Rel. 14.1.0.0. Chicago: SPSS Incorporation.10.1007/978-3-663-05757-4_1Suche in Google Scholar
Tamura, K., D. Peterson, N. Peterson, G. Stecher, M. Nei and S. Kumar. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739.Suche in Google Scholar
Tate, G.H.H. 1941. Notes on vespertilionid bats of the subfamilies Miniopterinae, Murininae, Kerivoulinae, and Nyctophilinae. Bull. Am. Mus. Nat. Hist. 78: 567–597.Suche in Google Scholar
Taylor, E.H. 1934. Philippine land mammals. Monogr. Philippine Bur. Sci., Manila 30: 1–548 + 25 pl.Suche in Google Scholar
Thong, V.D., S. Bumrungsri, D.L. Harrison, M.J. Pearch, K.M. Helgen and P.J.J. Bates. 2006. New records of Microchiroptera (Rhinolophidae and Kerivoulinae) from Vietnam and Thailand. Acta Chiropterol. 8: 83–93.10.3161/1733-5329(2006)8[83:NROMRA]2.0.CO;2Suche in Google Scholar
Thong, V.D., S.J. Puechmaille, A. Denzinger, P.J.J. Bates, C. Dietz, G. Csorba, P. Soisook, E.C. Teeling, S. Matsuura, N.M. Furey and H.U. Schnitzler. 2012. Systematics of the Hipposideros turpis complex and a description of a new subspecies from Vietnam. Mammal Rev. 42: 166–192.10.1111/j.1365-2907.2011.00202.xSuche in Google Scholar
Vanitharani, J., A. Rajendran, P.J.J. Bates, D.L. Harrison and M.J. Pearch. 2003. A taxonomic reassessment of Kerivoula lenis Thomas, 1916 (Chiroptera: Vespertilionidae) including a first record from peninsular India. Acta Chiropterol. 5: 49–60.Suche in Google Scholar
Yenbutra, S. and H. Felten. 1986. Bat species and their distribution in Thailand according to the collections in TISTR and SMF. In: (H. Felten, ed.) Contributions to the knowledge of bats of Thailand. Cour. Forschungs. Senckenberg 87: 9–45.Suche in Google Scholar
©2016 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Original Studies
- Summer temperature and precipitation govern bat diversity at northern latitudes in Norway
- Structure of three subtropical bat assemblages (Chiroptera) in the Andean rainforests of Argentina
- Morphology, genetics and echolocation calls of the genus Kerivoula (Chiroptera: Vespertilionidae: Kerivoulinae) in Thailand
- Status and population structure of a hunted population of Marco Polo Argali Ovis ammon polii (Cetartiodactyla, Bovidae) in Southeastern Tajikistan
- Habitat use of Himalayan grey goral in relation to livestock grazing in Machiara National Park, Pakistan
- Characterization and selection of microhabitat of Microcavia australis (Rodentia: Caviidae): first data in a rocky habitat in the hyperarid Monte Desert of Argentina
- Pregastric and caecal fermentation pattern in Syrian hamsters
- Short Notes
- Barn owl pellets collected in coastal savannas yield two additional species of small mammals for French Guiana
- Wiedomys cerradensis (Gonçalves, Almeida, Bonvicino, 2003) (Rodentia, Cricetidae): first record from the state of Maranhão, Brazil
- Morpho-anatomical characteristics of Indian pangolin (Manis crassicaudata) from Potohar Plateau, Pakistan
- First confirmed records of two bat species for Iraq: Rhinolophus euryale and Myotis emarginatus (Chiroptera)
- An efficient timing device to record activity patterns of small mammals in the field
- First record of Leontopithecus chrysopygus (Primates: Callitrichidae) in Carlos Botelho State Park, São Miguel Arcanjo, São Paulo, Brazil
Artikel in diesem Heft
- Frontmatter
- Original Studies
- Summer temperature and precipitation govern bat diversity at northern latitudes in Norway
- Structure of three subtropical bat assemblages (Chiroptera) in the Andean rainforests of Argentina
- Morphology, genetics and echolocation calls of the genus Kerivoula (Chiroptera: Vespertilionidae: Kerivoulinae) in Thailand
- Status and population structure of a hunted population of Marco Polo Argali Ovis ammon polii (Cetartiodactyla, Bovidae) in Southeastern Tajikistan
- Habitat use of Himalayan grey goral in relation to livestock grazing in Machiara National Park, Pakistan
- Characterization and selection of microhabitat of Microcavia australis (Rodentia: Caviidae): first data in a rocky habitat in the hyperarid Monte Desert of Argentina
- Pregastric and caecal fermentation pattern in Syrian hamsters
- Short Notes
- Barn owl pellets collected in coastal savannas yield two additional species of small mammals for French Guiana
- Wiedomys cerradensis (Gonçalves, Almeida, Bonvicino, 2003) (Rodentia, Cricetidae): first record from the state of Maranhão, Brazil
- Morpho-anatomical characteristics of Indian pangolin (Manis crassicaudata) from Potohar Plateau, Pakistan
- First confirmed records of two bat species for Iraq: Rhinolophus euryale and Myotis emarginatus (Chiroptera)
- An efficient timing device to record activity patterns of small mammals in the field
- First record of Leontopithecus chrysopygus (Primates: Callitrichidae) in Carlos Botelho State Park, São Miguel Arcanjo, São Paulo, Brazil

